Introduction
As in many areas of biomedical research, “translational” and “preclinical” are now commonplace terminology in the neurosciences. Spinal cord injury (SCI) is a more recent area in which an active dialogue on translation has evolved (Sipski, 2003; Steeves et al., 2004; Blight and Tuszynski, 2006; Fawcett et al., 2007; Longbrake et al., 2007), following impressive advances in our understanding of the cellular and molecular biology of spinal cord trauma, the models and functional outcomes, and the therapeutic targets that have been identified (Tator, 2002; Hall and Springer, 2004; Kleitman, 2004; Kwon et al., 2005; Tsai and Tator, 2005; Rossignol et al., 2007; Eftekharpour et al., 2008). As with other neurological conditions, translational emphasis in SCI research underscores a growing sense of urgency for moving experimental strategies forward (Tator, 2006).
Inherent in bench-to-bedside translational research has been the premise that preclinical (i.e., laboratory animal) studies can provide predictive indices of therapeutic potential in human subjects, although this issue has been contested for many years (for reviews see Bracken, 2009a; van der Worp et al., 2010). An often cited example is the clinical disappointment of neuroprotective strategies which showed significant benefits in animal studies but not in human subjects (Hugenholtz, 2003; Lammertse, 2004; Hawryluk et al., 2008). Translational difficulties, however, are not unique to the SCI field and have been experienced with far more extensive endeavors in other neurological disorders, including stroke, traumatic brain injury, and amyotrophic lateral sclerosis (Tolias and Bullock, 2004; Kazanis, 2005; O'Collins et al., 2006; Benatar, 2007; Walmsley and Mir, 2007; Margulies and Hicks, 2009). A common message is that experimental designs must be improved to obtain the most relevant data possible to warrant future clinical applications (Bracken, 2009b; van der Worp et al., 2010). A recent survey of opinions from SCI investigators indicates the field is still defining what constitutes ideal preclinical–translational designs and the level of evidence required to justify advancement of novel treatments to humans (Kwon et al., 2010a, 2011a).
The following discussion expands upon several salient considerations related to translational–preclinical experimentation in acute and chronic SCI as presented in this volume by Dietz and Curt (see Chapter 29) and elsewhere by others (Steeves et al., 2004; Dobkin, 2007; Kwon et al., 2010a, 2011a, b; Tetzlaff et al., 2011). In that respect, this review is not to prescribe a specific roadmap for designing clinically relevant laboratory investigations. Rather, the objective is to raise further awareness of the fundamental challenges and complexities of translational SCI research by exploring general preclinical design issues, as well as others more specific to pharmacological and cellular interventions for acute and chronic SCI. In this review, discussion is limited to single treatment approaches though it is widely recognized that optimal benefits are more likely to result from multiple strategies combined with rehabilitation. Periodic reappraisal of the preclinical process is vital for further refinement and improved implementation of bench-to-beside, as well as beside-to-bench, experiences in SCI.
The Translational Path
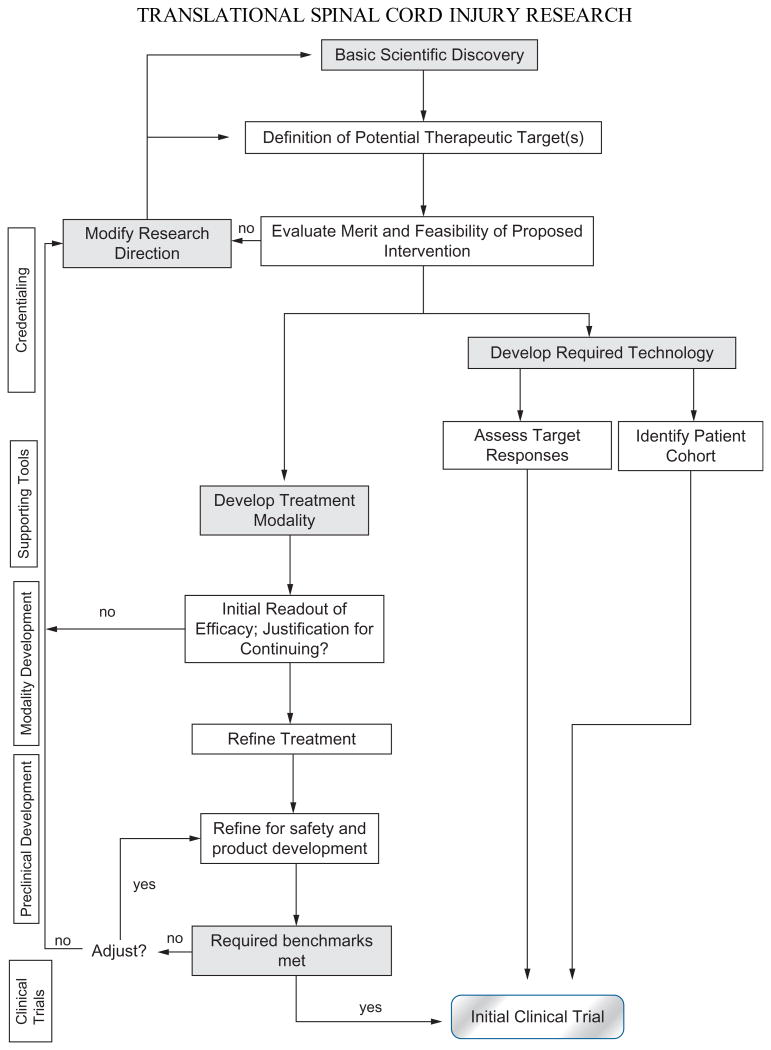
The descriptives “translational” and “preclinical” are frequently used interchangeably, as in this review. However, it is important to appreciate the terms have distinct meanings in different programmatic contexts. In principle, translation represents an evolving and flexible process that is rooted in basic scientific discovery with defined intention to seek specific clinical application (Fig. 26.1). Preclinical studies become an integral part of an applied basic science continuum in which new laboratory findings are incrementally transformed into clinical investigations (Hawk et al., 2008) by specific in vivo tests of efficacy and safety, and lastly, final “product” development and manufacturing when required. By its very nature, translational research is a bidirectional dynamic since clinical insights are critical for shaping basic laboratory studies (Lane et al., 2008a; see also Chapter 29). In that regard, “bench-to-bedside and back” should be the operative at many levels of biomedical scientific pursuit. While translation is generally viewed relative to the human as the ultimate endpoint, the process also provides license to incrementally advance studies of novel interventions from one species to another at various preclinical milestones.
Fig. 26.1.
An adaptation of the National Cancer Institute's translational path flowchart illustrates major phases in the transition from basic science discovery to early clinical trials. The main feature of this algorithm is that decision points are present at various levels of preclinical development; these allow for periodic evaluation of the status of a new treatment modality and the choice to move forward, or to return to an earlier stage for additional refinements. The development of supporting tools or technologies runs in parallel with the establishment of a new treatment modality. Suggestions for enhanced preclinical design discussed in this chapter identify with those two paths. Presently, such a model for translational spinal cord injury (SCI) research does not exist, and clinical trials – past and present – evolve far less systematically. (Adapted from Schilsky RL, Gordon G, Gilmer TM et al. (2008). The Translational Research Working Group developmental pathway for anticancer agents (drugs or biologics). Clin Cancer Res 14: 5685.)
Translational bottlenecks and challenges have been addressed in several areas of biomedical research. For example, the U.S. National Cancer Advisory Board established a working group to review the National Cancer Institute's translational enterprise and define where improvements could be made to facilitate more effective progression from discovery to clinical trials (Hawk et al., 2008). Evolving from this endeavor was a flowchart, the Developmental Pathways Concept (DPC), which incorporates decision points and feedback loops that could be systematically engaged to achieve the final objective for cancer and other clinical indications (Cheever et al., 2008; Dorfman et al., 2008a, b; Schilsky et al., 2008; Srivastava et al., 2008). An adaptation of the DPC is presented in Figure 26.1 to underscore challenges of SCI translation. In some respects, preclinical design improvements assume even greater significance in SCI than perhaps in many other areas of biomedical research (see figure legend for discussion).
Overview of Core Translational Spinal Cord Injury Research Guidelines
Although issues related to translational research were discussed in early workshops dealing with acute and chronic SCI (Hsu, 1992; Reier et al., 1994), the first formal white paper (Anderson et al., 2005) was modeled after guidelines (Redmond and Freeman, 2001) mostly related to neurodegenerative diseases and previously published by the practice committee of the American Society for Neural Transplantation and Repair (renamed American Society for Neural Therapy and Repair, www.asntr.org). The recommendations in the Anderson et al. (2005) document, which have been elaborated upon by Blight and Tuszynski (2006), centered on: (1) appropriate selection of animal models of SCI; (2) employment of experimental approaches consistent with the treatment modality (e.g., sensory versus motor) to be tested; (3) attention to safety issues; (4) definition of meaningful therapeutic outcomes; and (5) the importance of manuscript peer review. A sixth item on that list is the need for independent replication of promising therapies which identifies with the befuddling problem of verification in SCI research being the exception rather than the rule (Pinzon et al., 2008a, b; Steward et al., 2008; Lee et al., 2010b; Mann et al., 2010; Steward et al., 2012).
The following section is an overview of major considerations relating to the basic elements of preclinical experimental design.
Basic Preclinical Experimental Design: Guideline Considerations
Species selection
The rat or mouse is frequently chosen for initial studies of SCI mechanisms and novel therapeutics. Transgenic mice are unquestionably useful for investigating cellular, molecular, and immunological responses to SCI (Steward et al., 1999; Kwon et al., 2002a; Sroga et al., 2003; Rosenzweig and McDonald, 2004; Kigerl et al., 2006; Donnelly and Popovich, 2008). However, unlike SCI in rats and other species, including the human, contusion of the mouse spinal cord does not typically result in the formation of fluid-filled cavities at the site of injury (Steward et al., 1999; Ma et al., 2001; Inman and Steward, 2003). For perhaps that reason alone, rats and higher species are viewed as offering more relevant preclinical foundations (Blesch and Tuszynski, 2008; Kwon et al., 2011b).
One point of general agreement is that therapeutics showing efficacy in rodents, especially those involving invasive procedures, must be validated in large animal models before proceeding to clinical trial (Blight and Tuszynski, 2006). It has also been recognized that rodent data often overestimate anticipated outcomes in human trials (Pritchard et al., 2010). Even in the case of a noninvasive pharmacological agent, efficacy in the rodent alone is thought to be insufficient grounds for initiating clinical tests in humans.
Whether nonhuman primate studies represent an absolute translational prerequisite is still a matter of debate (Kwon et al., 2010a). One view argues the importance of scaling experimental results with regenerative therapies relative to the distances of repair that would be required in the human spinal cord (Watson et al., 2009), which measures 43–45 cm in length (http://faculty.washington.edu/chudler/facts.html). In the case of in vivo gene or intrathecal delivery of neurotrophic factors and other agents, diffusion distances in different tissue microenvironments must also be carefully considered (Hendriks et al., 2004). Consideration must also be given to the differences between rodents and nonhuman primates have also been considered with regard to the location and function of the corticospinal tract, which are similar in humans and macaque monkeys (Darian-Smith, 2007). Other studies further underscore the valuable contribution these animals can provide to the translational process (Glendinning et al., 1992; Leonard et al., 1992; Vierck et al., 2000; Weidner and Tuszynski, 2002; Yang et al., 2006; Rosenzweig et al., 2009, 2010).
Depending upon the specific questions being addressed, other large animal models, such as the cat (Tester and How-land, 2008; Cote et al., 2010) and mini-pig (Zurita et al., 2008; Lim et al., 2010; Kuluz et al., 2010; Navarro et al., 2012; Guest et al., 2011; Wewetzer et al., 2011), may serve as alternatives to nonhuman primates. Most notably, cat models of contusion and compression injuries have a long preclinical history related to neuroprotection and cell replacement therapies (Anderson et al., 1985; Hall and Braughler, 1986; Anderson et al., 1995) as well as an extensive background of spinal neurophysiology and neuroanatomical characterization. From a comparative perspective, the cat spinal cord is 34 cm, whereas that of the macaque is 17.5–28 cm in length. The locations of major intraspinal pathways also are similar in these species, and the cat has forelimb behaviors for which a neural circuitry has been identified (Pettersson et al., 2007).
Although the preclinical need for large animal model demonstrations of therapeutic efficacy seems inescapable, there are critical issues to keep in mind. One is that absolute length of the spinal cord may be less relevant than the distance and cross-sectional areas of individual segments (Ko et al., 2004), as well as comparative features regarding spinal circuitry that may affect therapeutic outcomes (Konya et al., 2008). To conclude that rodent studies have no predictive value may be an overstatement because there are basic behaviors, such as breathing, that have physiological and neuroanatomical signatures seen in other species (Kastner and Gauthier, 2008; Lane et al., 2008a, b, 2009, 2011; Qiu et al., 2010). Furthermore, many treatments shown to be effective in mice have later worked in humans (e.g., enzastaurin, an anticancer drug), whereas others (e.g., Accutane) have worked in rabbits, monkeys, and humans but not in rats and mice (Bracken, 2009a). Such examples underscore dangers of generalizations when other fundamental anatomical features or dynamics (e.g., pharmacokinetics, discussed below) may be more critical determinants of translational outcomes than size or functional–neuroanatomical features alone.
Lastly, real-life SCI in cats and dogs can present translational opportunities beyond traditional laboratory experiments (Laverty et al., 2004; Shapiro et al., 2005; Jeffery et al., 2006; Smith and Jeffery, 2006). While the feasibility of performing veterinary tests of experimental therapies on a large scale basis appears daunting, such studies are likely to be more in the translational pipeline in the future. They will most likely require a substantial consortium in order to achieve optimal fiscal and infrastructural conditions, multidisciplinary expertise, and an adequate veterinary “patient” population for rigorous statistical evaluations of outcomes.
Choice of injury model
The merits and limitations of different lesion models have been extensively reviewed, and it is difficult to rank one above the other without significant qualifications (Kwon et al., 2002b; Courtine et al., 2007; Rossignol et al., 2009). In general, complete or partial spinal transections are useful for demonstrating basic cellular and molecular principles that may lead to new interventions (e.g., Liu et al., 2010). Selective lesions of spinal white matter, for example, dorsal column or dorsolateral pathways and rat forelimb function (Schrimsher and Reier, 1993; McKenna and Whishaw, 1999; Anderson et al., 2007), also offer opportunities to explore spontaneous and therapeutically enhanced anatomical and functional plasticity (e.g., Garcia-Alias et al., 2009). Such guided “knife-cut” lesions, however, are rarely encountered in the clinical domain. Under such injury conditions, axonal growth through regions of fibroglial scarring may be more demanding than in the case of anatomically incomplete, contusion/compression SCIs.
As illustrated elsewhere in this volume, three spinal contusion devices have been shown to produce graded injuries in rats (Gruner, 1992; Stokes et al., 1992; Scheff et al., 2003) and mice (Jakeman et al., 2000; Hill et al., 2009). In addition to the clip-compression injury (Rivlin and Tator, 1978), many SCI researchers consider contusion and compression injuries to be effective preclinical models, although these injuries still do not fully reproduce the complex tissue dynamics, peripheral (i.e., root avulsions), and central pathologies associated with human SCI (Dietz and Curt, 2006; Akhtar et al., 2008). The same may be said of more selective lesion models (photochemical (Prado et al., 1987; Cameron et al., 1990; Bunge et al., 1994; Piao et al., 2009); excitotoxic (Magnuson et al., 1999; Courtine et al., 2008); and demyelinative (Loy et al., 2002a, b)) which have nonetheless been informative, especially regarding motor and sensory consequences of spinal level-related gray matter loss and regional primary demyelination. Irrespective of the injury chosen, many inherent experimental variables (Table 26.1) make correspondence to human injuries difficult to optimally obtain, although new approaches are being developed (Choo et al., 2009). Beyond injury modeling, it is difficult to overlook many aspects of post-SCI care, bladder, respiratory and endocrine complications that have general somatic consequences, surgical intervention (e.g., spine stabilization, decompression), and management (e.g., medications) that are rarely, if ever, incorporated into preclinical studies.
Table 26.1. Three challenging areas associated with preclinical research*.
| A. Injury considerations contributing to different pathophysiologies and conditions | ||
|---|---|---|
|
| ||
| Animal model | Human subject | |
| Type and extent of injury Pathology |
Controlled injury, often targeted Lack of cavitation in mice |
Uncontrolled, multitrauma and comorbidities Cavitation, root avulsion, spine fracture/dislocation, bone fragment |
| Initial lesion location Laminectomy |
Dorsal impact, vascular damage “Pretreatment” as occurs prior to SCI In some sense mimics decompression |
Ventral impact, vascular damage Decompression post injury |
| Anesthesia and pharmaceuticals | Present before, during and after injury Affects physiological parameters including blood pH, arterial pO2 | None |
| Likely to impact lesion size and thus functional outcomes | ||
| Analgesics | ||
| Environmental stresses | Handling, crede, cage conditions | Hospital environment, immobilization, psychological considerations |
| Patient care | Animal Ethics Committee-driven | Best practice, individualized care |
| B. Interpretational considerations | ||
|---|---|---|
|
| ||
| Animal model | Human subject | |
| Surrogate markers of function | Apparent tissue-sparing does not provide a full picture of neuronal and axonal compromise, and therefore functional ability | Limited CSF or blood biomarkers MRI is a poor predictor of outcome |
| Anatomical plasticity may not necessarily lead to positive functional change | ||
| Assessments of functional ability | Incomplete representation of function Often have low clinical relevance (poor surrogate of human behavior) |
ASIA scale reflects loss of function Often focus on task completion or speed Rarely differentiate between compensation and recovery |
| Comparative functional/behavioral differences | Use of tail in performing motor tasks Quadrupedal or inconsistent bipedal locomotion |
Bipedal locomotion |
| Other comparative species differences | Comparative anatomical, physiological, pathological and genetic differences | |
| C. Additional experimental considerations |
|---|
| Design and implementation is highly variable between and within laboratories |
| Randomization is often inconsistent and investigator blinding absent |
| Monitoring of vitals (e.g., blood pressure) is rare during experimental injuries |
| Functional assessments and periodic testing are not consistent across laboratories |
| Post-injury management of animals with regards to housing, support care, handling is different across laboratories |
Based on reviews published by Akhtar et al. (2008, 2009). Many inherent and inescapable differences exist between experimental and human spinal cord injury that place significant demand on the robustness of preclinical data. Attention to other experimental considerations could lead to greater standardization of preclinical studies between laboratories.
MRI, magnetic resonance imaging; SCI, spinal cord injury; CSF, cerebrospinal fluid.
Functional outcomes, spinal level of interest, and safety
Novel treatments can be assessed with a variety of morphological, molecular, and neurophysiological methods to complement behavioral outcomes, which are the principal preclinical benchmarks of treatment efficacy. Accordingly, the choice of spinal level in preclinical investigations is often decided by the primary functional modality being targeted.
Reflecting a history of emphasis on paraplegia and locomotor deficits, thoracic SCI has been the predominant experimental model which has led to significant advances in post-SCI rehabilitation based on the concept of activity-dependent neuroplasticity and the potential for activating circuits below the level of injury (for reviews, see Wolpaw and Tennissen, 2001; Edgerton et al., 2004; Behrman et al., 2005; Teng et al., 2006; Harkema, 2008; Dietz, 2009). Furthermore, innovative bioengineering technologies to exploit and enhance the function of these spinal circuitries are emerging (Edgerton and Roy, 2009; Gerasimenko et al., 2010; Bamford and Mushahwark, 2011). The history of this bench-to-bedside transition (reviewed in Wolpaw and Tennissen, 2001) reflects an interesting exception to two fundamental translational considerations discussed above – species selection and use of clinically relevant injuries. Specifically, treadmill walking following partial or complete spinalization essentially began with studies of a higher-order species, the cat, which remains an important, preclinical model for locomotor training (Frigon and Rossignol, 2008). Furthermore, the clinical advance of locomotor training was largely based upon complete spinal cord transection, though human application has focused mostly on adults with anatomically and functionally incomplete spinal injuries (Dobkin et al., 2007). While these investigations were in motion prior to the formulation of any specific guideline considerations, it serves as an important example of consistency with what might be regarded as basic requirements for moving forward novel, noninvasive interventions with minimal risks.
Other experimental design issues regarding spinal levels of interest and motor outcomes have emerged due especially to increasing attention on what is of greatest functional importance to those with spinal injuries (Anderson, 2004). First, the spinal level at which a treatment approach is studied should be consistent with the SCI population to which it will be directed (see Fig. 26.1). This relates to functional modalities of interest as well as evaluation of potential therapeutic risks. The thoracic spinal cord has been proposed to be the most prudent region for initial clinical trials of invasive procedures (Fawcett, 2002) since unforeseen complications are less likely to result in further major consequences. This view assumes, however, that cough and posture, which are controlled by thoracic spinal motor circuits, are unlikely to be placed at risk by an invasive approach. Few experimental studies (Jefferson et al., 2010) have considered such vital aspects of thoracic SCI. In contrast, cervical SCI models offer greater opportunities for investigating efficacy and safety (Lee et al., 2010b) in terms of segmental functions such as forelimb behavior (Anderson et al., 2009) and respiration (Lane et al., 2008a). In addition, pathways and neural circuits associated with these functions have been described (McKenna et al., 2000; Anderson et al., 2007; Lane et al., 2008b; Lane, 2011). A more generic safety concern relevant to all spinal levels is the risk of inducing or enhancing pain (e.g., Hofstetter et al., 2005). Therefore, preclinical studies in which procedural and biological safety are rigorously evaluated have as much, if not more, scientific importance as investigations exploring treatment efficacy and underlying mechanisms.
Injury severity
Because the overall magnitude of many human SCI cases exceeds experimental lesion intensities, tests applied to severe contusion injuries are recommended for preclinical testing of novel therapies (Blesch and Tuszynski, 2008). While some investigators are developing a nonhuman primate spinal contusion model, this may be problematic if severe contusion or compression lesions are ultimately deemed necessary. Such injuries in monkeys are rare (Bresnahan et al., 1976, 1991; Iwanami et al., 2005) and further nonhuman primate SCI model development and application will most certainly require demanding protocols, extensive collaboration, considerable oversight, and major investments of fiscal and infrastructural resources (Courtine et al., 2007; Blesch and Tuszynski, 2008).
Modeling severe bilateral contusion injuries, especially at mid- to upper cervical levels, even in rats and mice, is challenging given potential consequences to vital functions. On the other hand, less severe injuries will not fully replicate the magnitude of functional losses seen in humans. For example, neither bladder nor major sensory (i.e., pain) complications have been reported thus far following moderate C5–7/8 bilateral contusions in rats (Anderson et al., 2009) and reproducing ventilator dependency in any animal model for long-term study is not logistically or ethically practical. Differences in injury severity or symmetry, as well as contusion versus compression injuries, may further dictate histopathological features of SCI. Notably, demyelination and remyelination of spared fibers has been observed with moderate midline thoracic contusions, but to our knowledge, no demonstration of extensive post-SCI primary demyelination has been reported after less severe bilateral cervical contusions. Such pathology, however, could be more likely following severe unilateral cervical contusions or compression-type injuries, as in some examples of human SCI pathology (Guest et al., 2005).
Clinical heterogeneity and experimental therapeutic robustness
One of many clinically relevant issues to factor into laboratory experiments is the tremendous heterogeneity of the human SCI population in terms of genetics, sex, age, pre-existing health conditions, clinical logistics, and required medications (Dobkin, 2007; Blesch and Tuszynski, 2008). By default logic, preclinical designs should build upon clinical realities as much as possible (Kleitman, 2004; Blesch and Tuszynski, 2008). One of the deficiencies in SCI research is that such clinical-to-basic science feedback is still far from optimal.
A related demand on preclinical modeling is to provide compelling evidence for efficacy in the form of functional outcomes that can retain significance in the face of tremendous variables in the human SCI population. Such measures of therapeutic robustness are often lacking in the basic science literature (Kwon et al., 2011c). Papers published in high impact journals often report a p ≤ 0.05 level of statistical significance for treatment effects observed. Yet, the degree of actual behavioral improvement may be minimal or relatively meaningless to the animal's species-specific functions. Reasons for failed replications are likely to be numerous (e.g., Bunge and Pearse, 2012; Guth, 2012; Simard and Gerzanich, 2012). One possible explanation is that originally observed therapeutic effects or proposed mechanisms (Kim et al., 2003; Simonen et al., 2003; Zheng et al., 2003; Lee et al., 2010c) may not have been sufficient to endure other biological variables, even in the same species under analogous experimental conditions.
Therefore, central to preclinical design is the question to what degree statistically significant efficacy data from a controlled animal study effectively reflect the meaningful biological significance and reproducibility one would like to obtain in a diverse human population (Kwon et al., 2010a)? Ideally, translation at any level of preclinical investigation should demonstrate, in a double-blinded fashion, therapeutic efficacy in more than one type of injury severity and in different strains or species, with attention given to age, gender, dose-responses, and rates or persistence of therapeutic improvement (Kleitman, 2004; Anderson et al., 2005). Such data, however, are not mandated by the U.S. Food and Drug Adminstration (FDA). Efficacy related to thoracic injuries and hindlimb locomotion may not result when applied to cervical injuries and evaluated relative to functional modalities with significant upper and lower motor neuron components (Lee et al., 2010b).
Postinjury treatment intervals
In general, the distinctions between acute and chronic SCI are somewhat arbitrary and often dependent upon the type of intervention and pathophysiological mechanism (s) being addressed. As a rule, acute injuries are targeted by pharmacological and cellular strategies aimed at cell and tissue rescue. Often, however, other experimental approaches to promote tissue repair are introduced at the time of injury when opportunities for rendering the lesion environment more permissive to axonal growth seem greatest (Houle and Tessler, 2003). Some early post-injury approaches, however, are not ones that can be realistically applied to humans.
The acute injury neuroprotection treatment window in animals and humans (not considering spine stabilization and decompression procedures) can range from 8 to 12 hours and even as much as 1–3 days after injury (Fawcett et al., 2007; Kwon et al., 2010a). Identification of definitive biomarkers, as some laboratories have begun to pursue (Shaw et al., 2005; Chen and Springer, 2009; Kwon et al., 2010b), may provide even greater resolution.
Intermediate to the acute and chronic post-SCI milestones is the “subacute” or “recovery” phase (Young, 1996), to which the term “early chronic” has been added (e.g., Salazar et al., 2010). By most standards, subacute treatments are ones initiated beyond 3 days after injury, such as those addressing progressive cyst formation, demyelination, protracted inflammatory responses, and axonal die-back among others. The greater challenge, however, is to pinpoint when an injury transitions from subacute to chronic in order to appropriately scale preclinical studies to the human condition. Some maintain from experimental and clinical perspectives that “chronic” is when a stable lesion environment is established and spontaneous behavioral recovery plateaus (Houle and Tessler, 2003). Some microarray data support that view (Velardo et al., 2004). These criteria are satisfied beginning 4–5 weeks post contusion injury in rats (Noble and Wrathall, 1989; Basso et al., 1996). One report (Hill et al., 2001) has been cited, however, as suggesting even longer postoperative intervals before “chronic” is truly applicable. In humans, however, the timing of conversions from ASIA A to improved ASIA levels is variable and may occur over a year or more (Fawcett et al., 2007). Accordingly, many favor a minimum postinjury delay of 6–12 weeks for testing treatments directed at chronic SCI. Some even suggest delays of a year may be necessary (Kwon et al., 2010a). The latter would seem more reasonable for studies involving higher order species with a longer lifespan than that of the rodent. Otherwise aging would become a significant variable.
Definition of therapeutic mechanism
From a regulatory perspective, demonstration of mechanism is not generally required for Investigational New Drug approvals. Nevertheless, it is important for preclinical studies to identify as much as possible the basis for any therapeutic effect to advance beyond what otherwise would represent phenomenology. Without such background information, the outcome of an initial clinical study would be difficult to interpret. This concern would only be offset by extraordinary and unequivocal positive results. Appreciation for potential mechanisms also would define how likely it is the preclinical evidence will serve as a predictor of clinical relevance (Kleitman, 2004).
Special Design Considerations for Acute/Subacute Preclinical Spinal Cord Injury Studies
Reflections on early translation in spinal cord injury
The National Acute Spinal Cord Injury Study (NASCIS I-III) (Bracken et al., 1984, 1990, 1997) and subsequent Sygen Multicenter Acute Spinal Cord Injury Study (SMASCIS) (Geisler et al., 1991, 2001) are two ground-breaking experiences in clinical SCI research (for detailed discussions of the designs and outcomes, see Hall and Springer, 2004; Lammertse, 2004; Hawryluk et al., 2008) that provide important opportunities for exploring how the above preclinical issues may relate to clinical outcomes. An immediate translational consideration is that NASCIS I (Bracken et al., 1984), which tested the neuroprotective efficacy of two doses of methylprednisolone sodium succinate (MP), was largely based on extrapolations of clinical data showing glucocorticoid-mediated reductions in peritumoral brain edema and the prevalent use of corticosteroids for treating acute SCI. Likewise, the rationale for a pilot Sygen (Fidia Parmaceutical, Abano Terme, Italy) study (Geisler et al., 1990), testing the benefits of GM-1 (monosialote-trahexosylganglioside), was derived mostly from peripheral nerve and brain injury experiments and in vitro observations indicating gangliosides could enhance axonal growth (Kalia and DiPalma, 1982; Toffano et al., 1984). Only one peer-reviewed study, reporting a remarkable effect of ganglioside treatment following complete transection of the rat spinal cord (Bose et al., 1986), preceded the Sygen trial. Only four peer-reviewed experimental studies of ganglioside effects after SCI appeared up to 2001 when the Sygen trial ended.
Experimental SCI models played a more prominent role in the designs of NASCIS II and III. Prior to initiation of NASCIS II, animal studies were showing that much higher MP doses than used in NASCIS I were required to obtain meaningful levels of efficacy (Hall and Braughler, 1981, 1982; Braughler and Hall, 1983; Hall et al., 1992). NASCIS II was thus designed in accordance with effective experimental dosing regimens which demonstrated (in contrast with NASCIS I) a statistically significant, albeit relatively modest, therapeutic effect.
Early preclinical benchmarks
Species
In contrast with mice and rats, as now being the primary animal models in most current SCI studies, cats, dogs, and monkeys were typically employed prior to NASCIS II (Means et al., 1981). The first contusion SCI rat models did not begin appearing until the mid- to late 1980s (Noble and Wrathall, 1985, 1989), between NASCIS II and III. Variability in experimental outcomes between laboratories was already recognized as a major problem (reviewed in Means et al., 1981) due to several design issues still being discussed today.
Injury Model and Severity
The bulk of acute SCI data supporting NASCIS II and III derived primarily from contusion or static-load compression injuries in the cat. Unfortunately, detailed anatomical assessments of the injuries were rare (Eidelberg et al., 1976a, b; Means et al., 1981). It is possible that comparable experiments today would require far more detailed longitudinal characterization and standardization of lesion consistency within and between groups.
Functional Outcomes, Spinal Level of Interest, and Safety
With locomotion being the sole functional emphasis, investigators used either general neurological assessments or a Tarlov scoring method which only provided a gross impression of neurological status. The most definitive demonstrations of MP efficacy were obtained from two studies in which vertebral L2 level compression injuries were made in cats and locomotor recovery was evaluated using a scoring method that rated eight measures of general standing or walking, three of running, and five for stair climbing (Means et al., 1981; Braughler et al., 1987). Both studies were conducted in a blinded fashion and showed an MP treatment effect, but only the Braughler et al. study involved randomization.
Experimental Therapeutic Robustness
Of 10 papers published up to 1980, seven showed some indication of improved tissue sparing or functional recovery following corticosteroid treatment. Due to differences in hindlimb functional assessments, the type of injury models used and other experimental variables, no definitive conclusions could be reached (Means et al., 1981). Later studies (Demopoulos et al., 1982; Anderson et al., 1985; Braughler et al., 1987; Young et al., 1988; Yang et al., 2003) provided more consistent demonstrations of neuroprotective effects.
Postinjury Treatment Intervals
One striking deficiency in the preclinical MP process was that no definitive treatment window was established experimentally. Based on animal studies of SCI pathophysiology, however, it was appreciated that optimal efficacy was most likely dependent on rapid initiation of MP administration.
Lessons from NASCIS
Important perspectives can be derived from the preclinical process contributing to the NASCIS trials. First, a theoretical basis was established supporting the potential benefits of MP. There is no question that membrane lipid peroxidation is a major, though not sole, contributor to secondary tissue damage post-SCI. As such, it is a biologically robust mechanism that has been independently demonstrated by many laboratories. The fact that a single intervention was not optimal may reflect the need to identify a “gatekeeper” for paths leading to apoptosis or, as many now recognize, a rational combinatorial approach. Although often challenged (Fehlings, 2001; Hurlbert, 2001; Hugenholtz, 2003), the fact that an MP effect was even detected in NASCIS II was the product of an important dialogue between basic scientists and clinicians that groomed the design of that study in which higher doses of MP were used to approximate the theoretical threshold for efficacy (Bracken et al., 1990). In that regard, it is hard to deny that animal studies at the time had some positive impact on a human application. It is equally difficult to categorically refute that the NASCIS outcomes, though modest, were not in register with experimental data. The failure of NASCIS II and III to show profoundly improved recoveries in patients may reflect that neurological recovery indicated in the cat (Means et al., 1981; Braughler et al., 1987) was relatively modest when compared to the level of treatment efficacy a human would require.
Another factor that may have limited the degree of protective efficacy was the fact that decompression/spinal stabilization surgery was not undertaken in patients enrolled in NASCIS II or NASCIS III until days to weeks after injury. This could be critical, since it is now increasingly believed that early surgery to decompress the spinal cord, if needed, should be done within the first 24 hours (Fehlings and Perrin, 2005). Indeed, if the injured spinal cord remains compressed, thus reducing spinal cord blood flow, this would impair delivery of the neuroprotective drug to the injured tissue as well as continuing the mechanical injury to the cord, both of which would make it difficult at best for a neuroprotective drug to have the best chance to exert an effect.
Raising the bar in acute spinal cord injury experimental design
Studies of MP did not halt with conclusion of NASCIS III, and more recent experiments are the subject of an extensive literature review addressing problems in translating animal data to human applications (Akhtar et al., 2009). This review is commendable in showing the mix of conflicting results obtained in recent MP acute SCI studies along with many cogent problems in study designs coupled with other issues the author raised in a previous review on animal modeling (Akhtar et al., 2008) (summarized in Table 26.1). However, other essential design considerations that warrant significant attention in order to enhance the translational fidelity of preclinical data are not mentioned.
Pharmacological Dynamics and Drug Delivery
A need exists to more critically assess human and animal pharmacological dynamics, especially under normal and injury/disease conditions, in order to circumvent false negatives in experimental and clinical trials. Accordingly, another significant strength of the experimental data contributing to the NASCIS II and III trials was the focus on pharmacokinetics (Hall and Springer, 2004). Unfortunately, such analyses of the pharmacodynamics of MP and many other candidate neuroprotective substances are lacking for many species. This issue has been cited as one of the probable reasons for failed translations in stroke (Braeuninger and Kleinschnitz, 2009) and thereby illustrates the universal need for detailed pharmacological evaluations, including dose-response studies, as an intricate part of the overall preclinical design process. Many of the acute SCI studies with MP in rats subsequent to NASCIS II have simply extrapolated the cat doses without considering the fact that the pharmacokinetics and dose-response could be substantially different in these species. In addition, some rat studies have used one or two doses instead of the 24- or 48-hour dosing regimen used in the cat. Lastly, some studies gave the drug IP instead of IV, with the former resulting in much lower peak plasma and tissue levels than what occurs with an IV bolus.
Functional–Anatomical Outcome Measures
A publication by Goldberger et al. (1990) represents the earliest recognition of the need for more rigorous behavioral outcome measures in SCI research. Unfortunately, it can still be critically argued that existing indices of neuroprotective success are not ideal by being mostly limited to ratios of white versus gray matter sparing and the popular Basso-Bresnahan-Beattie (BBB) or Basso Mouse Scale (BMS) locomotor scoring systems (Basso, 2004; Basso et al., 1995, 2006). Many physiological, neurophysiological, neurochemical, histological, imaging and behavioral outcome measures are presently available that can provide more comprehensive, quantitative assessments of motor, autonomic, and sensory functions at different spinal levels. There are also methods available for assessing neuroprotection relative to specific functions and their underlying motor neuron and intraspinal substrate circuits using conventional (McKenna et al., 2000) and, when feasible, transneuronal (Bareyre et al., 2004; Lane et al., 2008b, 2009; Duale et al., 2009, 2010) retrograde tracing in combination with anterograde tracing.
It is generally viewed that post-SCI function is largely dependent upon anatomical sparing and the degree to which ascending and descending white matter tract continuity is preserved, as well as oligodendroglial survival and the integrity of myelin sheaths (Blight, 1985; Kakulas, 2004; McTigue and Tripathi, 2008). White matter rescue in terms of axonal and/or myelin integrity seems logically critical to improved ambulatory outcomes after thoracic SCI. However, this perspective does not hold entirely true when considering the combined upper- and lower motor neuron pathology following injuries at the level of the cervical and lumbar enlargements along with other potential substrates for neuroplasticity (Bareyre et al., 2004) that could be affected by segmental gray matter (i.e., motor neurons and related interneuronal circuits) damage (Steencken et al., 2009). Relative sparing of white and gray matter can provide a useful screening index of neuroprotective efficacy, but its value can become greatly diminished when employing this approach to interpret results in a specific functional context (see below).
Subacute/Chronic Injury Experimental Design Issues
The special design considerations for neuroprotection also apply to single or combinatorial interventions for subacute and chronic SCI. For example, pharmacokinetic analyses are equally essential for testing the efficacy of compounds that can reinstate the growth potential of surviving neurons, induce recruitment of endogenous neural stem cells, or interfere with molecular mechanisms that impede regeneration or plasticity (Hall and Traystman, 2009). Other approaches involving invasive procedures lead to a spectrum of preclinical issues ranging from underlying rationales for the treatments being tested to a host of intraoperative logistics, including attention to tissue biomechanical properties (Guest et al., 2011). This section will briefly highlight some immediate biological and technical considerations with selected focus on stem cell applications and modulation of the inhibitory glial scar microenvironment. Both are areas of current interest and at different phases of the translational pipeline.
Transplantation and preclinical guideline issues
Rationales
Therapeutic targets for cell transplantation therapies include: (1) neuroprotection (Kocsis, 2009); (2) providing compatible substrates for axonal growth (Bunge and Pearse, 2003; Bunge, 2008); (3) presentation of genetically modified or naïve cells expressing neurotrophic factors (Sasaki et al., 2009; Brock et al., 2010); (4) introduction of myelinogenic cells or their precursors (Kocsis et al., 2004; Sharp and Keirstead, 2009; Cao et al., 2010); and (5) neuronal replacement (Reier, 2004). Among many neural and non-neural cell types available, Schwann cells (SCs), olfactory ensheathing cells (OECs), autologous macrophages, mesenchymal precursor cells (MPCs), embryonic stem cells (ESCs), and embryonic or adult neural precursor cells (NPCs) have been the more extensively investigated. Unfortunately, compelling preclinical demonstrations of functionally significant and reproducible efficacy are lacking for all these cell types after in vivo testing either as monotherapies or in combination with other treatments (Tetzlaff et al., 2011; Snyder and Teng, 2012).
Although sound rationales underlie the therapeutic goals for each cell type, some lines of reasoning are more compelling than others. One that is still the subject of debate is the extent and frequency of post-SCI primary demyelination in the human (Bunge et al., 1993; Kakulas, 1999; Norenberg et al., 2004; Guest et al., 2005). Advanced magnetic resonance (MR) protocols (e.g., diffusor tensor imaging) may ultimately resolve this question (Cohen-Adad et al., 2011) and thus establish a more substantial basis for testing cells with the explicit purpose of promoting remyelination of spared axons.
Likewise, neuronal replacement is often raised as a generic goal of stem cell applications. Yet, as noted earlier, the relevance of gray matter loss and repair is poorly appreciated (Reier et al., 2002; Reier, 2004). Specific references to stem cells for neuronal replacement after SCI frequently default to motor neuron loss. However, an extensive literature demonstrates that interneurons are vital to shaping motor neuron outputs and rhythm generation (Brownstone and Wilson, 2008; Jankowska, 2008; Hart and Giszter, 2010). Discrete excitotoxic lesions of spinal gray matter can also lead to chronic pain as well as severe locomotor deficits, even when white matter and specific motor neuron pools are substantially preserved (Magnuson et al., 1999; Vierck et al., 2000). Restoration of inter-/intrasegmental circuits or reestablishment of gray matter continuity via interneuronal replacement may thus be necessary for optimal functional recoveries even when significant white matter repair is achieved.
To repair a functionally defined intraspinal circuit requires knowing what cell types it is made up of, along with their location, distribution, and patterns of connectivity. With rare exceptions discussed later, little is known about the composition and organization of functionally defined intraspinal circuits. Even the extensively investigated hindlimb spinal locomotor pattern generator circuitry is still largely theoretical in terms of its pharmacological and neuroanatomical configuration (Rossignol et al., 2009). To convincingly demonstrate functional contributions of neuronal precursors will require characterizations of donor cells at various molecular and pharmacological levels, along with demonstration of their afferent and efferent connectivity with an identified intraspinal circuitry. Such details, while seemingly beyond regulatory consideration, may be essential for appreciating how different neuronal populations can affect functional recovery or plasticity (White et al., 2010).
Species and Cell Sources
Inherent to cell-based therapies is tremendous variability in cell sources, their intrinsic properties, and tissue culture methods, among many other variables. Autologous cells are the ideal and could include adult MPCs, SCs, and OECs, not to mention inducible pluripotent stem cell derivatives. To assume, however, that the “same” general cell type in each individual shares equivalent properties would be unrealistic given genetic and other variables. This consideration clearly applies to MPCs, which offer many potential therapeutic benefits and delivery advantages. Unfortunately, the experimental and human data are disparate and difficult to interpret (Tetzlaff et al., 2011). Before the clinical potential of these cells can be fully determined, investigations of MPCs will need to incorporate more rigorous characterizations of these complex cells under conditions reflected by the above preclinical design discussion.
It is now becoming more questionable whether findings obtained in rodents can be extrapolated to humans and whether donor cells derived from rodents are similar in their therapeutic properties to same cell type(s) in the human. A recent review has indicated that OECs and SCs display species-specific properties and requirements for expansion in vitro. Porcine and primate SCs and OECs are more equivalent to those in the human than their rodent counterparts (Wewetzer et al., 2011). The predictive merits of rodent studies may thus need to be reevaluated. As in the case of promising neuroprotective agents, comparative examinations of different cells in the same animal/injury model are needed to determine whether one cellular therapeutic is better than another.
While human-to-rat or mouse xenograft studies are commonly accepted as indices of therapeutic efficacy, how such donor cells behave in the rodent may be significantly different in a human injury setting, especially when considering the substantial immunosuppression required. One basic issue to be more systematically investigated is to what extent human neurons establish morphologically and electrophysiologically bona fide synaptic interactions with nonhuman neurons in a preclinical small or large animal xenograft model (Ishibashi et al., 2004; Cummings et al., 2005; Gao et al., 2007; Yan et al., 2007; Nasonkin et al., 2009). Otherwise, functional outcomes involving human-to-nonhuman xenograft approaches have to default mostly to neurotrophic and/or anti-inflammatory effects.
Immunosuppression
Graft rejection in any region of the CNS is often a major concern since many stem cell applications are based on allogeneic sources. Presently, there are no established standards for defining the best immunosuppression protocols with the fewest possible contraindications (Barker and Widner, 2004). Some reports suggest neural precursors are not highly immunogenic and do not require immunosuppression (Lim et al., 2010). In fact, a recently initiated trial (Pilot Investigation of Stem Cells in Stroke) in Glasgow (ReNeuron, Ltd.) is not employing any form of immunosuppression for that reason. Yet, there are other lines of evidence showing that modulation of the immune system can lead to improved NSC survival (Saino et al., 2010). Intermediate to these observations are findings suggesting only temporary immunosuppression is necessary (Wennersten et al., 2006). At another extreme are MSCs which some publications demonstrate as having endogenous immunosuppressive capabilities affecting both T and B cell activation (Uccelli et al., 2007).
Current clinical trials for amyotrophic lateral sclerosis (NeuralStem, Inc.) and SCI (Geron Corp.) are said to be employing two different immunosuppression protocols (i.e., number and type of drugs used, doses, and duration of treatment) based on solid organ transplantation experiences which may not apply to certain neural or non-neural cellular grafts within the CNS. Systematic comparative studies to optimize graft survival via immunomodulation are necessary preclinical investigations with high impact on translational applications of cell-based therapies.
Transplantation Logistics
In addition to many biological considerations, preclinical designs should also attend to technical aspects of intraspinal transplantation (Guest et al., 2011). Cellular delivery dynamics and locations, however, will depend upon the therapeutic goals of the procedure. Likewise, the mode of delivery will identify with the intervention's objective(s) and where cells are to be introduced. A significant delivery emphasis is on combining cells with various biomaterial scaffolds (Teng et al., 2002; Stokols and Tuszynski, 2004; Straley et al., 2010). Given all the experimental variability already noted, the challenge will be to identify the most effective cell-matrix combination and perhaps to further augment donor cell survivability via controlled drug release (Yu et al., 2009).
Circumventing the growth-inhibitory molecular and cellular microenvironments
As discussed elsewhere in this volume (see Chapter 35), chondroitin sulfate proteoglycans (CSPGs) are associated with the scar that develops following stab (McKeon et al., 1991; Tang et al., 2003), partial transection (Jones et al., 2003; Tester and Howland, 2008), and contusion-type injuries (Lemons et al., 1999; Iaci et al., 2007; Iseda et al., 2008). CSPGs are arguably among the more potent growth-inhibiting molecules associated with CNS scar formation (Busch and Silver, 2007). Accordingly, use of chondroitinase ABC (ChABC) to cleave chondroitin sulfate glycosaminoglycans (CS-GAGs) following SCI is receiving significant attention as an approach for translation. The growing literature on the use of chondroitinase following SCI shows a robust effect with respect to testing chondroitinase across laboratories, multiple partial transection models, and evidence of translation to a larger mammalian species. A recent review (Bradbury and Carter, 2010) nicely summarizes anatomical and functional evidence from experimental animal work suggesting this bacterial enzyme from Proteus vulgaris has significant therapeutic potential. Studies, however, have been conducted primarily using partial transection models in rats and many assess anatomical changes only. Although outcomes associated with ChABC application in these models are promising and consistent, few studies use contusion-type lesions and the results from those reports are mixed (Caggiano et al., 2005; Iseda et al., 2008; Harris et al., 2010; Jakeman et al., 2011). Confounding interpretation of discrepancies in the literature relate to several factors, including dosing and the delivery route of ChABC. Reported doses range from 0.006 U to 0.60 U and from single to continuous application across multiple weeks. Further, due to ChABC's thermal sensitivity at body temperature (Tester et al., 2007; Lee et al., 2010a), studies using mini-pumps unintentionally deliver relatively inactive enzyme after 1–3 days. Studies using repeated doses through externalized or subcutaneous injectable ports avoid this issue (Bradbury et al., 2002; Tester and Howland, 2008). Standard dose-response studies have yet to be done. Recent reports of temperature stabilization of ChABC by trehalose (Lee et al., 2010a), and use of other biocompatible materials for stabilization and release (Hyatt et al., 2010; Lee et al., 2010a), appear to reveal important new tools, but these are virtually untested. Further, identification of protein modifications to enhance ChABC secretion from mammalian cells (Muir et al., 2010), as well as a CS hydrolase in higher organisms, have been identified (Kaneiwa et al., 2010).
Despite various delivery methods, little is known about the areal extent of cleavage and how it impacts anatomical or functional plasticity. The importance of delivery location is suggested by the promotion of anatomical, but not functional, plasticity following intraspinal injections of ChABC rostral or caudal to the site of injury (Tom and Houle, 2008) and local axonal sprouting following injection into distant denervated targets (Massey et al., 2008).
With few exceptions (Garcia-Alias et al., 2008; Tom et al., 2009; Karimi-Abdolrezaee et al., 2010; Carter et al., 2011), most studies have investigated the impact of chondroitinase ABC by initiating delivery immediately post-injury. Understanding delayed treatment effects is important not only for individuals with chronic injuries, but also to mimic any short period (e.g., a day) that might be necessary to initiate a surgical intervention following injury (Garcia-Alias et al., 2008). Surgical intervention timing may also impact outcome, as the immune and blood–spinal cord barrier environment of the injured spinal cord may affect ChABC repair (Kigerl et al., 2009).
To better understand functional benefits that may be derived from anatomical plasticity ChABC treatment has been scaled up to a larger mammal – the cat (Tester and Howland, 2008) – which offers a glimpse at translation across species. A feature of this work, not dissimilar to other studies, is the extensive training required for some behavioral assessments which can likely introduce a “training effect” (Kigerl et al., 2009). This could be critical for translation as a combination of training and chondroitinase may enhance recovery over either alone (Garcia-Alias et al., 2009).
Preclinical Guidelines and Clinical Trials Past and Present
It is daunting to envision a single laboratory or even a consortium being capable of achieving all the benchmarks discussed without substantially slowing the translational process. However, an overview of past clinical translations raises questions as to whether earlier efforts may have fared better and been more informative had some experimental design objectives discussed here been adopted. The same view applies to more recent studies directed at neuroprotection and repair (for review, see Kwon et al., 2011c).
All the more recently ended or ongoing trials listed in Table 26.2 share similar shortcomings in their preclinical foundations relative to the design issues discussed. All but one went to clinical trial without a large animal study. The majority of preclinical investigations relied on the BBB locomotor scale as the primary outcome measure, and in some cases when other measures were used, they were grossly substandard. Prior to or shortly after formal initiation of the Phase I Novartis trial in 2006, different lines of evidence (Anderson et al., 1985; Hall and Braughler, 1986; Schnell and Schwab, 1990, 1993; Bregman et al., 1995; Brosamle et al., 2000; Merkler et al., 2001; Fiedler et al., 2002; Raineteau et al., 2002; Liebscher et al., 2005; Pettersson et al., 2007) indicated, with one exception (Bareyre et al., 2004), that functional blocking of Nogo-A could lead to increased axonal growth and motor improvements (Zorner and Schwab, 2010). Other trials were launched on the basis of far fewer preclinical demonstrations of a therapeutic benefit. Given that the target human SCI population would largely represent individuals who have sustained cervical contusion/compression injuries, it is noteworthy that such SCIs were not employed in pre-Novartis trial studies. The same was the case with the Cethrin trial, for which the only published contusion study prior to its initiation showed negative results. With the exception of a recent article (Lee et al., 2010b), neuroprotection in an experimental cervical SCI model has not been investigated. The reader is referred to Kwon et al. (2010a) for more detailed discussion of these trials. A concern beyond preclinical design is that clinical trials presently focus on one therapeutic intervention, despite universal recognition that combinatorial therapies are likely to be necessary (e.g., van den Brand et al., 2012).
Table 26.2. General overview of recently completed and ongoing clinical trials*.
| Clinical trial | Major guideline considerations | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Species (no. of studies) | Injury model (no. of studies) | Spinal level | Behavioral outcomes (no. studies showing comparable outcomes); safety | Independent replication | Patient spinal level of injury | ||||
|
| |||||||||
| Rats (R)/mice (M) | Nonhuman primate | CT | PT | C/BC | |||||
| Minocycline1 | 1 (M) 9 (R) |
2 | 8 | C7/8 or T13 (PT) T9/10 (C) |
Sensory improvements (1); locomotor improvements (7); no effect (2) | Cervical and thoracic | |||
| Riluzole1 | 8 (R) | 1 | 7 | S2 (CT) and high or mid-thoracic (C) | No data for three studies; reduced spasticity (1); locomotor improvements (3); effect only with combined MP (1); qualitative improvement in rats' ability to sit upright (1) | Cervical and thoracic | |||
| Rho pathway antagonist (Cethrin and related compounds2) | 1 (M) 3 (R) |
2 | 2 | T7 (dorsal Hx) T3/4 (dorsal Hx) T9/10 contusion |
Corticospinal (CST) axonal regeneration/sprouting and locomotor recovery within 1 day (1) | Corroborative findings (Fournier et al., 2003) | Cervical and thoracic | ||
| C3 transferase treatment delayed locomotor recovery; Y27632 resulted in 3 pt BBB improvement 2 weeks post injury (1) | Negative effects of C3 and Y-27632 (Sung, 2003) | ||||||||
| C3 led to “poor rat response” Y-27632 delayed locomotor recovery (p < 0.05) Fasudil improved BBB (p < 0.05) (1) | |||||||||
| Significant BBB improvement by 5 weeks post-contusion SCI3 (1) | |||||||||
| Novartis anti-Nogo immunotherapy4 | 85 (R) | 36 | Pyramidal lesions and partial spinal transections | Thoracic | Anatomical and functional data showing axonal growth and improved locomotion | Yes/no7 | Cervical and thoracic | ||
| Proneuron | 1 (R) | 1 | Thoracic | Behavioral and electrophysiological functional improvements; axonal growth through the transection site; retransection led to loss of recovery | Yes (-) | ||||
| Geron | 1 (R) | 1 | Thoracic | Grafted oligodendrocyte progenitors enhanced remyelination post-SCI and improved locomotor recovery | No | ||||
See text for discussion.
Based on data presented in Kwon et al., 2010.
Based on review presented in McKerracher, 2006.
Contusion data presented in an abstract only.
Based Rossignol et al., 2007.
Primary references prior to 2006; some experiments involved spinal lesions in early postnatal rats.
Two studies published prior to and one subsequent to the start of the Phase I Novartis trial in 2006.
Proof-of-concept studies from different labs corroborated the potential importance of myelin inhibition of axonal growth; specific IN-1 independent confirmation does not appear to have been pursued.
CT, complete transection; PT, partial transection; C, contusion, clip compression; BC, balloon compression; Hx, hemisection.
Summary and Conclusions
With increasing emphasis on accelerating SCI translational research, the issue thus becomes one of striking a balance between ideal versus optimized preclinical progressions and timely bench-to-bedside translations. Currently, there are no satisfactory solutions; however, demonstration of therapeutic robustness is a preclinical design feature which the SCI research community recognizes as being critical to judicious translation (Kwon et al., 2011b) and which can be adopted to preclinical studies in both the academic and corporate sectors.
As discussed, “robustness” has broad implications, and is therefore inherent to all other guideline considerations reviewed in this chapter. A basic example, as noted above, would be demonstration of functionally meaningful, not just statistical, degrees of efficacy. Showing reproducibility of a therapeutic effect under different injury conditions (e.g., spinal level, lesion severities, and multiple lesion types) would also increase the predictability of a treatment being effective in the clinical setting. In addition, while results in mice and rats can still provide important proofs of principle, showing efficacy in a large animal model of SCI is necessary to establish a measure of therapeutic benefit and, perhaps even more importantly, another measure of therapeutic robustness. Independent verification of promising therapies is in many respects the most important of all the suggested preclinical criteria for translation, given the limited success in formal replications thus far. Again, successful replications, though very difficult to perform precisely, would provide another compelling indication of therapeutic robustness.
Aside from the basic design issues reviewed, we have taken the liberty of exploring other experimental variables relating to acute and chronic SCI experimentation that could significantly strengthen future pre-clinical studies. Using neuroprotection as an example, conclusions about a drug's efficacy without careful pharmacokinetic analyses are debatable. Furthermore, with many sophisticated functional measures now available, there is reason to move beyond locomotor screening data and white-gray matter ratios to showing a drug's contribution to sparing of specific functions with more specific anatomical correlates. Some of the better outcome measures, anatomical and functional, are laborious and time-consuming. Many experimental studies is they often resort to the most expeditious outcome measures. Unfortunately, much of this is driven by publication and funding pressures.
Where shortcomings in preclinical foundations are most apparent is revealed by review of past and current clinical trials with cell replacement therapies. While cellular transplantation offers viable therapeutic options, there are many practical and biological issues that have yet to be resolved in a rigorous manner. Significant interest is in using these cells to promote remyelination of spared white matter, yet the magnitude of primary demyelination is poorly understood both in animal experiments and human SCI. Advanced imaging and other approaches are likely to shed important light on this issue. In terms of neuronal replacement, nothing is known, for example, about the number of neurotransmitter phenotypes that are necessary for reconstructing intraspinal circuits. In fact, little attention has been given to the fundamental issue of gray matter repair. Initial clinical studies have or are currently testing stem cells of various types during the subacute post-SCI interval. Neuroprotection is an underlying rationale that has not been adequately proven in reported preclinical studies. Independent replications are also lacking. Lastly, the didactics of cell delivery and immunosuppression are as important as the biology of the repair intervention. These aspects of cellular replacement are very much in their infancy.
What is self-evident from this review is that “translation” is easier said than done. While emphasis is on improving preclinical design, it would be short-sighted to conclude that this alone is the problem, since the impact of preclinical data also depends on the design and conduct of the resulting clinical study. The decision to move forward with a novel therapy requires multiple considerations, not the least of which should be the desire to improve the quality of life for individuals who have sustained SCI. Unquestionably, it is the responsibility of the scientific community at large, whether in the academic, federal/private funding, or corporate sectors, to ensure that efforts are made to establish the strongest preclinical foundations possible. Otherwise, a history of disappointing translational experiences is likely to be perpetuated.
The design issues presented in this review are intended to provide a framework for further evaluation of the preclinical translational process. Without more concerted efforts to implement widely accepted suggestions for improved preclinical design with consideration of clinical realities, the general perception in translational neuroscience that “everything works in animals and nothing in humans” will remain more of a conceptual postulate rather than a statement of fact or testable hypothesis.
References
- Akhtar AZ, Pippin JJ, Sandusky CB. Animal models in spinal cord injury: a review. Rev Neurosci. 2008;19:47–60. doi: 10.1515/revneuro.2008.19.1.47. [DOI] [PubMed] [Google Scholar]
- Akhtar AZ, Pippin JJ, Sandusky CB. Animal studies in spinal cord injury: a systematic review of methylprednisolone. Altern Lab Anim. 2009;37:43–62. doi: 10.1177/026119290903700108. [DOI] [PubMed] [Google Scholar]
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Anderson DK, Saunders RD, Demediuk P, et al. Lipid hydrolysis and peroxidation in injured spinal cord: partial protection with methylprednisolone or vitamin E and selenium. Cent Nerv Syst Trauma. 1985;2:257–267. doi: 10.1089/cns.1985.2.257. [DOI] [PubMed] [Google Scholar]
- Anderson DK, Howland DR, Reier PJ. Fetal neural grafts and repair of the injured spinal cord. Brain Pathol. 1995;5:451–457. doi: 10.1111/j.1750-3639.1995.tb00624.x. [DOI] [PubMed] [Google Scholar]
- Anderson DK, Beattie M, Blesch A, et al. Recommended guidelines for studies of human subjects with spinal cord injury. Spinal Cord. 2005;43:453–458. doi: 10.1038/sj.sc.3101746. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Gunawan A, Steward O. Spinal pathways involved in the control of forelimb motor function in rats. Exp Neurol. 2007;206:318–331. doi: 10.1016/j.expneurol.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Sharp KG, Steward O. Bilateral cervical contusion spinal cord injury in rats. Exp Neurol. 2009;220:9–22. doi: 10.1016/j.expneurol.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford JA, Mushahwar VK. Intraspinal microstimulation for the recovery of function following spinal cord injury. Prog Brain Res. 2011;194:227–239. doi: 10.1016/B978-0-444-53815-4.00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Barker RA, Widner H. Immune problems in central nervous system cell therapy. NeuroRx. 2004;1:472–481. doi: 10.1602/neurorx.1.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM. Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J Neurotrauma. 2004;21:395–404. doi: 10.1089/089771504323004548. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, et al. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Behrman AL, Lawless-Dixon AR, Davis SB, et al. Locomotor training progression and outcomes after incomplete spinal cord injury. Phys Ther. 2005;85:1356–1371. [PubMed] [Google Scholar]
- Benatar M. Lost in translation: treatment trials in the SOD1 mouse and in human ALS. Neurobiol Dis. 2007;26:1–13. doi: 10.1016/j.nbd.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Blesch A, Tuszynski MH. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2008;32:41–47. doi: 10.1016/j.tins.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Blight AR. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Cent Nerv Syst Trauma. 1985;2:299–315. doi: 10.1089/cns.1985.2.299. [DOI] [PubMed] [Google Scholar]
- Blight AR, Tuszynski MH. Clinical trials in spinal cord injury. J Neurotrauma. 2006;23:586–593. doi: 10.1089/neu.2006.23.586. [DOI] [PubMed] [Google Scholar]
- Bose B, Osterholm JL, Kalia M. Ganglioside-induced regeneration and reestablishment of axonal continuity in spinal cord-transected rats. Neurosci Lett. 1986;63:165–169. doi: 10.1016/0304-3940(86)90055-8. [DOI] [PubMed] [Google Scholar]
- Bracken MB. Why animal studies are often poor predictors of human reactions to exposure. J R Soc Med. 2009a;102:120–122. doi: 10.1258/jrsm.2008.08k033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken MB. Why are so many epidemiology associations inflated or wrong? Does poorly conducted animal research suggest implausible hypotheses? Ann Epidemiol. 2009b;19:220–224. doi: 10.1016/j.annepidem.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Collins WF, Freeman DF, et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984;251:45–52. [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- Bradbury EJ, Carter LM. Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res Bull. 2010;84:306–316. doi: 10.1016/j.brainresbull.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Braeuninger S, Kleinschnitz C. Rodent models of focal cerebral ischemia: procedural pitfalls and translational problems. Exp Transl Stroke Med. 2009;1:8. doi: 10.1186/2040-7378-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braughler JM, Hall ED. Lactate and pyruvate metabolism in injured cat spinal cord before and after a single large intravenous dose of methylprednisolone. J Neurosurg. 1983;59:256–261. doi: 10.3171/jns.1983.59.2.0256. [DOI] [PubMed] [Google Scholar]
- Braughler JM, Hall ED, Means ED, et al. Evaluation of an intensive methylprednisolone sodium succinate dosing regimen in experimental spinal cord injury. J Neurosurg. 1987;67:102–105. doi: 10.3171/jns.1987.67.1.0102. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Kunkel-Bagden E, Schnell L, et al. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- Bresnahan JC, King JS, Martin GF, et al. A neuroanatomical analysis of spinal cord injury in the rhesus monkey (Macaca mulatta) J Neurol Sci. 1976;28:521–542. doi: 10.1016/0022-510x(76)90122-2. [DOI] [PubMed] [Google Scholar]
- Bresnahan JC, Beattie MS, Stokes BT, et al. Three-dimensional computer-assisted analysis of graded contusion lesions in the spinal cord of the rat. J Neurotrauma. 1991;8:91–101. doi: 10.1089/neu.1991.8.91. [DOI] [PubMed] [Google Scholar]
- Brock JH, Rosenzweig ES, Blesch A, et al. Local and remote growth factor effects after primate spinal cord injury. J Neurosci. 2010;30:9728–9737. doi: 10.1523/JNEUROSCI.1924-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosamle C, Huber AB, Fiedler M, et al. Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. J Neurosci. 2000;20:8061–8068. doi: 10.1523/JNEUROSCI.20-21-08061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstone RM, Wilson JM. Strategies for delineating spinal locomotor rhythm-generating networks and the possible role of Hb9 interneurones in rhythmogenesis. Brain Res Brain Res Rev. 2008;57:64–76. doi: 10.1016/j.brainresrev.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge MB. Novel combination strategies to repair the injured mammalian spinal cord. J Spinal Cord Med. 2008;31:262–269. doi: 10.1080/10790268.2008.11760720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge MB, Pearse DD. Transplantation strategies to promote repair of the injured spinal cord. J Rehabil Res Dev. 2003;40:55–62. doi: 10.1682/jrrd.2003.08.0055. [DOI] [PubMed] [Google Scholar]
- Bunge MB, Pearse DD. Response to the report, “A re-assessment of a combinatorial treatment involving Schwann cell transplants and elevation of cyclic AMP on recovery of motor function following thoracic spinal cord injury in rats” by Sharp et al. (this volume) Exp Neurol. 2012;233:645–648. doi: 10.1016/j.expneurol.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Bunge RP, Puckett WR, Becerra JL, et al. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993;59:75–89. [PubMed] [Google Scholar]
- Bunge MB, Holets VR, Bates ML, et al. Characterization of photochemically induced spinal cord injury in the rat by light and electron microscopy. Exp Neurol. 1994;127:76–93. doi: 10.1006/exnr.1994.1082. [DOI] [PubMed] [Google Scholar]
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Caggiano AO, Zimber MP, Ganguly A, et al. Chondroitinase ABCI improves locomotion and bladder function following contusion injury of the rat spinal cord. J Neurotrauma. 2005;22:226–239. doi: 10.1089/neu.2005.22.226. [DOI] [PubMed] [Google Scholar]
- Cameron T, Prado R, Watson BD, et al. Photochemically induced cystic lesion in the rat spinal cord. I. Behavioral and morphological analysis. Exp Neurol. 1990;109:214–223. doi: 10.1016/0014-4886(90)90076-5. [DOI] [PubMed] [Google Scholar]
- Cao Q, He Q, Wang Y, et al. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J Neurosci. 2010;30:2989–3001. doi: 10.1523/JNEUROSCI.3174-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LM, McMahon SB, Bradbury EJ. Delayed treatment with chondroitinase ABC reverses chronic atrophy of rubrospinal neurons following spinal cord injury. Exp Neurol. 2011;228:149–156. doi: 10.1016/j.expneurol.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Cheever MA, Schlom J, Weiner LM, et al. Translational Research Working Group developmental pathway for immune response modifiers. Clin Cancer Res. 2008;14:5692–5699. doi: 10.1158/1078-0432.CCR-08-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Springer JE. Neuroproteomic methods in spinal cord injury. Methods Mol Biol. 2009;566:57–67. doi: 10.1007/978-1-59745-562-6_4. [DOI] [PubMed] [Google Scholar]
- Choo AM, Liu J, Liu Z, et al. Modeling spinal cord contusion, dislocation, and distraction: characterization of vertebral clamps, injury severities, and node of Ranvier deformations. J Neurosci Methods. 2009;181:6–17. doi: 10.1016/j.jneumeth.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Cohen-Adad J, El Mendili MM, Lehericy S, et al. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage. 2011;55:1024–1033. doi: 10.1016/j.neuroimage.2010.11.089. [DOI] [PubMed] [Google Scholar]
- Cote MP, Hanna A, Lemay MA, et al. Peripheral nerve grafts after cervical spinal cord injury in adult cats. Exp Neurol. 2010;225:173–182. doi: 10.1016/j.expneurol.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Bunge MB, Fawcett JW, et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat Med. 2007;13:561–566. doi: 10.1038/nm1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BJ, Uchida N, Tamaki SJ, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith C. Monkey models of recovery of voluntary hand movement after spinal cord and dorsal root injury. ILAR J. 2007;48:396–410. doi: 10.1093/ilar.48.4.396. [DOI] [PubMed] [Google Scholar]
- Demopoulos HB, Flamm ES, Seligman ML, et al. Further studies on free-radical pathology in the major central nervous system disorders: effect of very high doses of methylprednisolone on the functional outcome, morphology, and chemistry of experimental spinal cord impact injury. Can J Physiol Pharmacol. 1982;60:1415–1424. doi: 10.1139/y82-210. [DOI] [PubMed] [Google Scholar]
- Dietz V. Body weight supported gait training: from laboratory to clinical setting. Brain Res Bull. 2009;78:I–VI. doi: 10.1016/S0361-9230(08)00410-3. [DOI] [PubMed] [Google Scholar]
- Dietz V, Curt A. Neurological aspects of spinal-cord repair: promises and challenges. Lancet Neurol. 2006;5:688–694. doi: 10.1016/S1474-4422(06)70522-1. [DOI] [PubMed] [Google Scholar]
- Dobkin BH. Curiosity and cure: translational research strategies for neural repair-mediated rehabilitation. Dev Neurobiol. 2007;67:1133–1147. doi: 10.1002/dneu.20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin B, Barbeau H, Deforge D, et al. The evolution of walking-related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: the multicenter randomized Spinal Cord Injury Locomotor Trial. Neurorehabil Neural Repair. 2007;21:25–35. doi: 10.1177/1545968306295556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman GS, Lawrence TS, Matrisian LM. The Translational Research Working Group developmental pathway for interventive devices. Clin Cancer Res. 2008a;14:5700–5706. doi: 10.1158/1078-0432.CCR-08-1263. [DOI] [PubMed] [Google Scholar]
- Dorfman GS, Sullivan DC, Schnall MD, et al. The Translational Research Working Group developmental pathway for image-based assessment modalities. Clin Cancer Res. 2008b;14:5678–5684. doi: 10.1158/1078-0432.CCR-08-1264. [DOI] [PubMed] [Google Scholar]
- Duale H, Hou S, Derbenev AV, et al. Spinal cord injury reduces the efficacy of pseudorabies virus labeling of sympathetic preganglionic neurons. J Neuropathol Exp Neurol. 2009;68:168–178. doi: 10.1097/NEN.0b013e3181967df7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duale H, Lyttle TS, Smith BN, et al. Noxious colorectal distention in spinalized rats reduces pseudorabies virus labeling of sympathetic neurons. J Neurotrauma. 2010;27:1369–1378. doi: 10.1089/neu.2010.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Roy RR. Robotic training and spinal cord plasticity. Brain Res Bull. 2009;78:4–12. doi: 10.1016/j.brainresbull.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, et al. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Eftekharpour E, Karimi-Abdolrezaee S, Fehlings MG. Current status of experimental cell replacement approaches to spinal cord injury. Neurosurg Focus. 2008;24:E19. doi: 10.3171/FOC/2008/24/3-4/E18. [DOI] [PubMed] [Google Scholar]
- Eidelberg E, Staten E, Watkins CJ, et al. Treatment of experimental spinal cord injury in ferrets. Surg Neurol. 1976a;6:243–246. [PubMed] [Google Scholar]
- Eidelberg E, Staten E, Watkins JC, et al. A model of spinal cord injury. Surg Neurol. 1976b;6:35–38. [PubMed] [Google Scholar]
- Fawcett J. Repair of spinal cord injuries: where are we, where are we going? Spinal Cord. 2002;40:615–623. doi: 10.1038/sj.sc.3101328. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Curt A, Steeves JD, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. doi: 10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
- Fehlings MG. Editorial: recommendations regarding the use of methylprednisolone in acute spinal cord injury: making sense out of the controversy. Spine (Phila Pa 1976) 2001;26:S56–S57. doi: 10.1097/00007632-200112151-00012. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Perrin RG. The role and timing of early decompression for cervical spinal cord injury: update with a review of recent clinical evidence. Injury. 2005;36:B13–B26. doi: 10.1016/j.injury.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Fiedler M, Horn C, Bandtlow C, et al. An engineered IN-1 F(ab) fragment with improved affinity for the Nogo-A axonal growth inhibitor permits immunochemical detection and shows enhanced neutralizing activity. Protein Eng. 2002;15:931–941. doi: 10.1093/protein/15.11.931. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Adaptive changes of the locomotor pattern and cutaneous reflexes during locomotion studied in the same cats before and after spinalization. J Physiol. 2008;586:2927–2945. doi: 10.1113/jphysiol.2008.152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Coggeshall RE, Chung JM, et al. Functional motoneurons develop from human neural stem cell transplants in adult rats. NeuroReport. 2007;18:565–569. doi: 10.1097/WNR.0b013e3280b10c2c. [DOI] [PubMed] [Google Scholar]
- Garcia-Alias G, Lin R, Akrimi SF, et al. Therapeutic time window for the application of chondroitinase ABC after spinal cord injury. Exp Neurol. 2008;210:331–338. doi: 10.1016/j.expneurol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Garcia-Alias G, Barkhuysen S, Buckle M, et al. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- Geisler FH, Dorsey FC, Coleman WP. GM1 gangliosides in the treatment of spinal cord injury: report of preliminary data analysis. Acta Neurobiol Exp (Wars) 1990;50:515–521. [PubMed] [Google Scholar]
- Geisler FH, Dorsey FC, Coleman WP. Recovery of motor function after spinal-cord injury – a randomized, placebo-controlled trial with GM-1 ganglioside. N Engl J Med. 1991;324:1829–1838. doi: 10.1056/NEJM199106273242601. [DOI] [PubMed] [Google Scholar]
- Geisler FH, Coleman WP, Grieco G, et al. The Sygen multicenter acute spinal cord injury study. Spine (Phila Pa 1976) 2001;26:S87–S98. doi: 10.1097/00007632-200112151-00015. [DOI] [PubMed] [Google Scholar]
- Gerasimenko Y, Gorodnichev R, Machueva E, et al. Novel and direct access to the human locomotor spinal circuitry. J Neurosci. 2010;30:3700–3708. doi: 10.1523/JNEUROSCI.4751-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning DS, Cooper BY, Vierck CJ, Jr, et al. Altered precision grasping in stumptail macaques after fasciculus cuneatus lesions. Somatosens Mot Res. 1992;9:61–73. doi: 10.3109/08990229209144763. [DOI] [PubMed] [Google Scholar]
- Goldberger ME, Bregman BS, Vierck CJ, Jr, et al. Criteria for assessing recovery of function after spinal cord injury: behavioral methods. Exp Neurol. 1990;107:113–117. doi: 10.1016/0014-4886(90)90149-m. [DOI] [PubMed] [Google Scholar]
- Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. discussion 126–128. [DOI] [PubMed] [Google Scholar]
- Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol. 2005;192:384–393. doi: 10.1016/j.expneurol.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Guest J, Benavides F, Padgett K, et al. Technical aspects of spinal cord injections for cell transplantation. Clinical and translational considerations. Brain Res Bull. 2011;84:267–279. doi: 10.1016/j.brainresbull.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Guth L. A reassessment of LPS/indomethacin/pregnenolone combination therapy after spinal cord injury in rats. Exp Neurol. 2012;233:686. doi: 10.1016/j.expneurol.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Hall ED, Braughler JM. Acute effects of intravenous glucocorticoid pretreatment on the in vitro peroxidation of cat spinal cord tissue. Exp Neurol. 1981;73:321–324. doi: 10.1016/0014-4886(81)90067-4. [DOI] [PubMed] [Google Scholar]
- Hall ED, Braughler JM. Glucocorticoid mechanisms in acute spinal cord injury: a review and therapeutic rationale. Surg Neurol. 1982;18:320–327. doi: 10.1016/0090-3019(82)90140-9. [DOI] [PubMed] [Google Scholar]
- Hall ED, Braughler JM. Role of lipid peroxidation in post-traumatic spinal cord degeneration: a review. Cent Nerv Syst Trauma. 1986;3:281–294. doi: 10.1089/cns.1986.3.281. [DOI] [PubMed] [Google Scholar]
- Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Traystman RJ. Role of animal studies in the design of clinical trials. Front Neurol Neurosci. 2009;25:10–33. doi: 10.1159/000209470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Yonkers PA, Andrus PK, et al. Biochemistry and pharmacology of lipid antioxidants in acute brain and spinal cord injury. J Neurotrauma. 1992;9:S425–S442. [PubMed] [Google Scholar]
- Harkema SJ. Plasticity of interneuronal networks of the functionally isolated human spinal cord. Brain Res Brain Res Rev. 2008;57:255–264. doi: 10.1016/j.brainresrev.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NG, Mironova YA, Hovda DA, et al. Chondroitinase ABC enhances pericontusion axonal sprouting but does not confer robust improvements in behavioral recovery. J Neurotrauma. 2010;27:1971–1982. doi: 10.1089/neu.2010.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CB, Giszter SF. A neural basis for motor primitives in the spinal cord. J Neurosci. 2010;30:1322–1336. doi: 10.1523/JNEUROSCI.5894-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk ET, Matrisian LM, Nelson WG, et al. The Translational Research Working Group developmental pathways: introduction and overview. Clin Cancer Res. 2008;14:5664–5671. doi: 10.1158/1078-0432.CCR-08-1268. [DOI] [PubMed] [Google Scholar]
- Hawryluk GW, Rowland J, Kwon BK, et al. Protection and repair of the injured spinal cord: a review of completed, ongoing, and planned clinical trials for acute spinal cord injury. Neurosurg Focus. 2008;25:E14. doi: 10.3171/FOC.2008.25.11.E14. [DOI] [PubMed] [Google Scholar]
- Hendriks WT, Ruitenberg MJ, Blits B, et al. Viral vector-mediated gene transfer of neurotrophins to promote regeneration of the injured spinal cord. Prog Brain Res. 2004;146:451–476. doi: 10.1016/S0079-6123(03)46029-9. [DOI] [PubMed] [Google Scholar]
- Hill CE, Beattie MS, Bresnahan JC. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp Neurol. 2001;171:153–169. doi: 10.1006/exnr.2001.7734. [DOI] [PubMed] [Google Scholar]
- Hill RL, Zhang YP, Burke DA, et al. Anatomical and functional outcomes following a precise, graded, dorsal laceration spinal cord injury in C57BL/6 mice. J Neurotrauma. 2009;26:1–15. doi: 10.1089/neu.2008.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter CP, Holmstrom NA, Lilja JA, et al. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- Houle JD, Tessler A. Repair of chronic spinal cord injury. Exp Neurol. 2003;182:247–260. doi: 10.1016/s0014-4886(03)00029-3. [DOI] [PubMed] [Google Scholar]
- Hsu CY. Criteria for valid preclinical trials. J Neurotrauma. 1992;9:177–179. doi: 10.1089/neu.1992.9.177. discussion 179–181. [DOI] [PubMed] [Google Scholar]
- Hugenholtz H. Methylprednisolone for acute spinal cord injury: not a standard of care. CMAJ. 2003;168:1145–1146. [PMC free article] [PubMed] [Google Scholar]
- Hurlbert RJ. The role of steroids in acute spinal cord injury: an evidence-based analysis. Spine (Phila Pa 1976) 2001;26:S39–S46. doi: 10.1097/00007632-200112151-00009. [DOI] [PubMed] [Google Scholar]
- Hyatt AJ, Wang D, Kwok JC, et al. Controlled release of chondroitinase ABC from fibrin gel reduces the level of inhibitory glycosaminoglycan chains in lesioned spinal cord. J Control Release. 2010;147:24–29. doi: 10.1016/j.jconrel.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Iaci JF, Vecchione AM, Zimber MP, et al. Chondroitin sulfate proteoglycans in spinal cord contusion injury and the effects of chondroitinase treatment. J Neurotrauma. 2007;24:1743–1759. doi: 10.1089/neu.2007.0366. [DOI] [PubMed] [Google Scholar]
- Inman DM, Steward O. Physical size does not determine the unique histopathological response seen in the injured mouse spinal cord. J Neurotrauma. 2003;20:33–42. doi: 10.1089/08977150360517164. [DOI] [PubMed] [Google Scholar]
- Iseda T, Okuda T, Kane-Goldsmith N, et al. Single, high-dose intraspinal injection of chondroitinase reduces glycosaminoglycans in injured spinal cord and promotes corticospinal axonal regrowth after hemisection but not contusion. J Neurotrauma. 2008;25:334–349. doi: 10.1089/neu.2007.0289. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Sakaguchi M, Kuroiwa T, et al. Human neural stem/progenitor cells, expanded in long-term neuro-sphere culture, promote functional recovery after focal ischemia in Mongolian gerbils. J Neurosci Res. 2004;78:215–223. doi: 10.1002/jnr.20246. [DOI] [PubMed] [Google Scholar]
- Iwanami A, Yamane J, Katoh H, et al. Establishment of graded spinal cord injury model in a nonhuman primate: the common marmoset. J Neurosci Res. 2005;80:172–181. doi: 10.1002/jnr.20435. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Guan Z, Wei P, et al. Traumatic spinal cord injury produced by controlled contusion in mouse. J Neurotrauma. 2000;17:299–319. doi: 10.1089/neu.2000.17.299. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Hoschouer EL, Basso DM. Injured mice at the gym: review, results and considerations for combining chondroitinase and locomotor exercise to enhance recovery after spinal cord injury. Brain Res Bull. 2011;84:317–326. doi: 10.1016/j.brainresbull.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res Brain Res Rev. 2008;57:46–55. doi: 10.1016/j.brainresrev.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson SC, Tester NJ, Rose M, et al. Cough following low thoracic hemisection in the cat. Exp Neurol. 2010;222:165–170. doi: 10.1016/j.expneurol.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery ND, Smith PM, Lakatos A, et al. Clinical canine spinal cord injury provides an opportunity to examine the issues in translating laboratory techniques into practical therapy. Spinal Cord. 2006;44:584–593. doi: 10.1038/sj.sc.3101912. [DOI] [PubMed] [Google Scholar]
- Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182:399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- Kakulas BA. The applied neuropathology of human spinal cord injury. Spinal Cord. 1999;37:79–88. doi: 10.1038/sj.sc.3100807. [DOI] [PubMed] [Google Scholar]
- Kakulas BA. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord. 2004;42:549–563. doi: 10.1038/sj.sc.3101670. [DOI] [PubMed] [Google Scholar]
- Kalia M, DiPalma JR. Ganglioside-induced acceleration of axonal transport following nerve crush injury in the rat. Neurosci Lett. 1982;34:1–5. doi: 10.1016/0304-3940(82)90083-0. [DOI] [PubMed] [Google Scholar]
- Kaneiwa T, Mizumoto S, Sugahara K, et al. Identification of human hyaluronidase-4 as a novel chondroitin sulfate hydrolase that preferentially cleaves the galactosaminidic linkage in the trisulfated tetrasaccharide sequence. Glycobiology. 2010;20:300–309. doi: 10.1093/glycob/cwp174. [DOI] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, et al. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657–1676. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner A, Gauthier P. Are rodents an appropriate pre-clinical model for treating spinal cord injury? Examples from the respiratory system. Exp Neurol. 2008;213:249–256. doi: 10.1016/j.expneurol.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Kazanis I. CNS injury research; reviewing the last decade: methodological errors and a proposal for a new strategy. Brain Res Brain Res Rev. 2005;50:377–386. doi: 10.1016/j.brainresrev.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, McGaughy VM, Popovich PG. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J Comp Neurol. 2006;494:578–594. doi: 10.1002/cne.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Li S, GrandPre T, et al. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Kleitman N. Keeping promises: translating basic research into new spinal cord injury therapies. J Spinal Cord Med. 2004;27:311–318. doi: 10.1080/10790268.2004.11753768. [DOI] [PubMed] [Google Scholar]
- Ko HY, Park JH, Shin YB, et al. Gross quantitative measurements of spinal cord segments in human. Spinal Cord. 2004;42:35–40. doi: 10.1038/sj.sc.3101538. [DOI] [PubMed] [Google Scholar]
- Kocsis JD. Neuroprotection and immunomodulation by cell transplantation are becoming central themes in potential therapeutic approaches for cell-based therapies. Neurosci Lett. 2009;456:99. doi: 10.1016/j.neulet.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Akiyama Y, Radtke C. Neural precursors as a cell source to repair the demyelinated spinal cord. J Neurotrauma. 2004;21:441–449. doi: 10.1089/089771504323004584. [DOI] [PubMed] [Google Scholar]
- Konya D, Liao WL, Choi H, et al. Functional recovery in T13–L1 hemisected rats resulting from peripheral nerve rerouting: role of central neuroplasticity. Regen Med. 2008;3:309–327. doi: 10.2217/17460751.3.3.309. [DOI] [PubMed] [Google Scholar]
- Kuluz J, Samdani A, Benglis D, et al. Pediatric spinal cord injury in infant piglets: description of a new large animal model and review of the literature. J Spinal Cord Med. 2010;33:43–57. doi: 10.1080/10790268.2010.11689673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BK, Oxland TR, Tetzlaff W. Animal models used in spinal cord regeneration research. Spine. 2002a;27:1504–1510. doi: 10.1097/00007632-200207150-00005. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Oxland TR, Tetzlaff W. Animal models used in spinal cord regeneration research. Spine (Phila Pa 1976) 2002b;27:1504–1510. doi: 10.1097/00007632-200207150-00005. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Fisher CG, Dvorak MF, et al. Strategies to promote neural repair and regeneration after spinal cord injury. Spine (Phila Pa 1976) 2005;30:S3–S13. doi: 10.1097/01.brs.0000175186.17923.87. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Hillyer J, Tetzlaff W. Translational research in spinal cord injury: a survey of opinion from the SCI community. J Neurotrauma. 2010a;27:21–33. doi: 10.1089/neu.2009.1048. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Stammers AM, Belanger LM, et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J Neurotrauma. 2010b;27:669–682. doi: 10.1089/neu.2009.1080. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Okon EB, Plunet W, et al. A systematic review of directly applied biologic therapies for acute spinal cord injury. J Neurotrauma. 2011a;28:1589–1610. doi: 10.1089/neu.2009.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BK, Okon EB, Tsai E, et al. A grading system to evaluate objectively the strength of pre-clinical data of acute neuroprotective therapies for clinical translation in spinal cord injury. J Neurotrauma. 2011b;28:1525–1543. doi: 10.1089/neu.2010.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BK, Okon E, Hillyer J, et al. A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J Neurotrauma. 2011c;28:1545–1588. doi: 10.1089/neu.2009.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammertse DP. Update on pharmaceutical trials in acute spinal cord injury. J Spinal Cord Med. 2004;27:319–325. doi: 10.1080/10790268.2004.11753769. [DOI] [PubMed] [Google Scholar]
- Lane MA. Spinal respiratory motoneurons and interneurons. Respir Physiol Neurobiol. 2011;179:3–13. doi: 10.1016/j.resp.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Lane MA, Fuller DD, White TE, et al. Respiratory neuroplasticity and cervical spinal cord injury: translational perspectives. Trends Neurosci. 2008a;31:538–547. doi: 10.1016/j.tins.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, et al. Cervical pre-phrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol. 2008b;511:692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Fuller DD, et al. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol. 2009;169:123–132. doi: 10.1016/j.resp.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty PH, Leskovar A, Breur GJ, et al. A preliminary study of intravenous surfactants in paraplegic dogs: polymer therapy in canine clinical SCI. J Neurotrauma. 2004;21:1767–1777. doi: 10.1089/neu.2004.21.1767. [DOI] [PubMed] [Google Scholar]
- Lee H, McKeon RJ, Bellamkonda RV. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2010a;107:3340–3345. doi: 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Tigchelaar S, Liu J, et al. Lack of neuroprotective effects of simvastatin and minocycline in a model of cervical spinal cord injury. Exp Neurol. 2010b;225:219–230. doi: 10.1016/j.expneurol.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Lee JK, Geoffroy CG, Chan AF, et al. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010c;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemons ML, Howland DR, Anderson DK. Chondroitin sulfate proteoglycan immunoreactivity increases following spinal cord injury and transplantation. Exp Neurol. 1999;160:51–65. doi: 10.1006/exnr.1999.7184. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Glendinning DS, Wilfong T, et al. Alterations of natural hand movements after interruption of fasciculus cuneatus in the macaque. Somatosens Mot Res. 1992;9:75–89. doi: 10.3109/08990229209144764. [DOI] [PubMed] [Google Scholar]
- Liebscher T, Schnell L, Schnell D, et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- Lim JH, Piedrahita JA, Jackson L, et al. Development of a model of sacrocaudal spinal cord injury in cloned Yucatan minipigs for cellular transplantation research. Cell Reprogramming. 2010;12:689–697. doi: 10.1089/cell.2010.0039. formerly “Cloning and Stem Cells”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Lu Y, Lee JK, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longbrake EE, Lai W, Ankeny DP, et al. Characterization and modeling of monocyte-derived macrophages after spinal cord injury. J Neurochem. 2007;102:1083–1094. doi: 10.1111/j.1471-4159.2007.04617.x. [DOI] [PubMed] [Google Scholar]
- Loy DN, Magnuson DS, Zhang YP, et al. Functional redundancy of ventral spinal locomotor pathways. J Neurosci. 2002a;22:315–323. doi: 10.1523/JNEUROSCI.22-01-00315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy DN, Talbott JF, Onifer SM, et al. Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp Neurol. 2002b;177:575–580. doi: 10.1006/exnr.2002.7959. [DOI] [PubMed] [Google Scholar]
- Ma M, Basso DM, Walters P, et al. Behavioral and histological outcomes following graded spinal cord contusion injury in the C57B1/6 mouse. Exp Neurol. 2001;169:239–254. doi: 10.1006/exnr.2001.7679. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Trinder TC, Zhang YP, et al. Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp Neurol. 1999;156:191–204. doi: 10.1006/exnr.1999.7016. [DOI] [PubMed] [Google Scholar]
- Mann CM, Lee JH, Hillyer J, et al. Lack of robust neurologic benefits with simvastatin or atorvastatin treatment after acute thoracic spinal cord contusion injury. Exp Neurol. 2010;221:285–295. doi: 10.1016/j.expneurol.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Margulies S, Hicks R. Combination therapies for traumatic brain injury: prospective considerations. J Neurotrauma. 2009;26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey JM, Amps J, Viapiano MS, et al. Increased chondroitin sulfate proteoglycan expression in denervated brainstem targets following spinal cord injury creates a barrier to axonal regeneration overcome by chondroitinase ABC and neurotrophin-3. Exp Neurol. 2008;209:426–445. doi: 10.1016/j.expneurol.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JE, Whishaw IQ. Complete compensation in skilled reaching success with associated impairments in limb synergies, after dorsal column lesion in the rat. J Neurosci. 1999;19:1885–1894. doi: 10.1523/JNEUROSCI.19-05-01885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JE, Prusky GT, Whishaw IQ. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. J Comp Neurol. 2000;419:286–296. doi: 10.1002/(sici)1096-9861(20000410)419:3<286::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Schreiber RC, Rudge JS, et al. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, Higuchi H. Targeting Rho to stimulate repair after spinal cord injury. J Neurotrauma. 2006;23:309–317. doi: 10.1089/neu.2006.23.309. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- Means ED, Anderson DK, Waters TR, et al. Effect of methylprednisolone in compression trauma to the feline spinal cord. J Neurosurg. 1981;55:200–208. doi: 10.3171/jns.1981.55.2.0200. [DOI] [PubMed] [Google Scholar]
- Merkler D, Metz GA, Raineteau O, et al. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J Neurosci. 2001;21:3665–3673. doi: 10.1523/JNEUROSCI.21-10-03665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir EM, Fyfe I, Gardiner S, et al. Modification of N-glycosylation sites allows secretion of bacterial chondroitinase ABC from mammalian cells. J Biotechnol. 2010;145:103–110. doi: 10.1016/j.jbiotec.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasonkin I, Mahairaki V, Xu L, et al. Long-term, stable differentiation of human embryonic stem cell-derived neural precursors grafted into the adult mammalian neostriatum. Stem Cells. 2009;27:2414–2426. doi: 10.1002/stem.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro R, Juhas S, Keshavarzi S, et al. Chronic spinal compression model in minipigs: a systematic behavioral, qualitative, and quantitative neuropathological study. J Neurotrauma. 2012;29:499–513. doi: 10.1089/neu.2011.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble LJ, Wrathall JR. Spinal cord contusion in the rat: morphometric analyses of alterations in the spinal cord. Exp Neurol. 1985;88:135–149. doi: 10.1016/0014-4886(85)90119-0. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Wrathall JR. Correlative analyses of lesion development and functional status after graded spinal cord contusive injuries in the rat. Exp Neurol. 1989;103:34–40. doi: 10.1016/0014-4886(89)90182-9. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Donnan GA, et al. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- Pettersson LG, Alstermark B, Blagovechtchenski E, et al. Skilled digit movements in feline and primate – recovery after selective spinal cord lesions. Acta Physiol (Oxf) 2007;189:141–154. doi: 10.1111/j.1748-1716.2006.01650.x. [DOI] [PubMed] [Google Scholar]
- Piao MS, Lee JK, Jang JW, et al. A mouse model of photochemically induced spinal cord injury. J Korean Neurosurg Soc. 2009;46:479–483. doi: 10.3340/jkns.2009.46.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzon A, Marcillo A, Pabon D, et al. A re-assessment of erythropoietin as a neuroprotective agent following rat spinal cord compression or contusion injury. Exp Neurol. 2008a;213:129–136. doi: 10.1016/j.expneurol.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzon A, Marcillo A, Quintana A, et al. A re-assessment of minocycline as a neuroprotective agent in a rat spinal cord contusion model. Brain Res. 2008b;1243:146–151. doi: 10.1016/j.brainres.2008.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado R, Dietrich WD, Watson BD, et al. Photochemically induced graded spinal cord infarction. Behavioral, electrophysiological, and morphological correlates. J Neurosurg. 1987;67:745–753. doi: 10.3171/jns.1987.67.5.0745. [DOI] [PubMed] [Google Scholar]
- Pritchard CD, Slotkin JR, Yu D, et al. Establishing a model spinal cord injury in the African green monkey for the preclinical evaluation of biodegradable polymer scaffolds seeded with human neural stem cells. J Neurosci Methods. 2010;188:258–269. doi: 10.1016/j.jneumeth.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu K, Lane MA, Lee KZ, et al. The phrenic motor nucleus in the adult mouse. Exp Neurol. 2010;226:254–258. doi: 10.1016/j.expneurol.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineteau O, Fouad K, Bareyre FM, et al. Reorganization of descending motor tracts in the rat spinal cord. Eur J Neurosci. 2002;16:1761–1771. doi: 10.1046/j.1460-9568.2002.02243.x. [DOI] [PubMed] [Google Scholar]
- Redmond DE, Jr, Freeman T. The American Society for Neural Transplantation and Repair Considerations and guidelines for studies of human subjects. The practice committee of the society. Approved by council. Cell Transplant. 2001;10:661–664. [PubMed] [Google Scholar]
- Reier PJ. Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx. 2004;1:424–451. doi: 10.1602/neurorx.1.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reier PJ, Anderson DK, Young W, et al. Workshop on intraspinal transplantation and clinical application. J Neurotrauma. 1994;11:369–377. doi: 10.1089/neu.1994.11.369. [DOI] [PubMed] [Google Scholar]
- Reier PJ, Golder FJ, Bolser DC, et al. Gray matter repair in the cervical spinal cord. Prog Brain Res. 2002;137:49–70. doi: 10.1016/s0079-6123(02)37007-9. [DOI] [PubMed] [Google Scholar]
- Rivlin AS, Tator CH. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg Neurol. 1978;10:38–43. [PubMed] [Google Scholar]
- Rosenzweig ES, McDonald JW. Rodent models for treatment of spinal cord injury: research trends and progress toward useful repair. Curr Opin Neurol. 2004;17:121–131. doi: 10.1097/00019052-200404000-00007. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Brock JH, Culbertson MD, et al. Extensive spinal decussation and bilateral termination of cervical corticospinal projections in rhesus monkeys. J Comp Neurol. 2009;513:151–163. doi: 10.1002/cne.21940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, Courtine G, Jindrich DL, et al. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci. 2010;13:1505–1510. doi: 10.1038/nn.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Schwab M, Schwartz M, et al. Spinal cord injury: time to move? J Neurosci. 2007;27:11782–11792. doi: 10.1523/JNEUROSCI.3444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Barriere G, Alluin O, et al. Re-expression of locomotor function after partial spinal cord injury. Physiology (Bethesda) 2009;24:127–139. doi: 10.1152/physiol.00042.2008. [DOI] [PubMed] [Google Scholar]
- Saino O, Taguchi A, Nakagomi T, et al. Immunodeficiency reduces neural stem/progenitor cell apoptosis and enhances neurogenesis in the cerebral cortex after stroke. J Neurosci Res. 2010;88:2385–2397. doi: 10.1002/jnr.22410. [DOI] [PubMed] [Google Scholar]
- Salazar DL, Uchida K, Hamers FPT, et al. Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PLoS One. 2010;5:e12272. doi: 10.1371/journal.pone.0012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Radtke C, Tan AM, et al. BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J Neurosci. 2009;29:14932–14941. doi: 10.1523/JNEUROSCI.2769-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Rabchevsky AG, Fugaccia I, et al. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- Schilsky RL, Gordon G, Gilmer TM, et al. The Translational Research Working Group developmental pathway for anticancer agents (drugs or biologics) Clin Cancer Res. 2008;14:5685–5691. doi: 10.1158/1078-0432.CCR-08-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Sprouting and regeneration of lesioned corticospinal tract fibres in the adult rat spinal cord. Eur J Neurosci. 1993;5:1156–1171. doi: 10.1111/j.1460-9568.1993.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Schrimsher GW, Reier PJ. Forelimb motor performance following dorsal column, dorsolateral funiculi, or ventrolateral funiculi lesions of the cervical spinal cord in the rat. Exp Neurol. 1993;120:264–276. doi: 10.1006/exnr.1993.1060. [DOI] [PubMed] [Google Scholar]
- Sharp J, Keirstead HS. Stem cell-based cell replacement strategies for the central nervous system. Neurosci Lett. 2009;456:107–111. doi: 10.1016/j.neulet.2008.04.106. [DOI] [PubMed] [Google Scholar]
- Shapiro S, Borgens R, Pascuzzi R, et al. Oscillating field stimulation for complete spinal cord injury in humans: a phase 1 trial. J Neurosurg Spine. 2005;2:3–10. doi: 10.3171/spi.2005.2.1.0003. [DOI] [PubMed] [Google Scholar]
- Shaw G, Yang C, Ellis R, et al. Hyperphosphorylated neurofilament NF-H is a serum biomarker of axonal injury. Biochem Biophys Res Commun. 2005;336:1268–1277. doi: 10.1016/j.bbrc.2005.08.252. [DOI] [PubMed] [Google Scholar]
- Simard JM, Gerzanich V. When replication teaches more than the original experiment–the saga of the unknown. Exp Neurol. 2012;233:623–624. doi: 10.1016/j.expneurol.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonen M, Pedersen V, Weinmann O, et al. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron. 2003;38:201–211. doi: 10.1016/s0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- Sipski ML. From the bench to the body: key issues associated with research aimed at a cure for SCI. J Rehabil Res Dev. 2003;40:1–7. doi: 10.1682/jrrd.2003.08.0001. [DOI] [PubMed] [Google Scholar]
- Smith PM, Jeffery ND. Histological and ultrastructural analysis of white matter damage after naturally-occurring spinal cord injury. Brain Pathol. 2006;16:99–109. doi: 10.1111/j.1750-3639.2006.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EY, Teng YD. Stem cells and spinal cord repair. N Engl J Med. 2012;366:1940–1942. doi: 10.1056/NEJMcibr1200138. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Gray JW, Reid BJ, et al. Translational Research Working Group developmental pathway for biospecimen-based assessment modalities. Clin Cancer Res. 2008;14:5672–5677. doi: 10.1158/1078-0432.CCR-08-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroga JM, Jones TB, Kigerl KA, et al. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 2003;462:223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- Steencken AC, Siebert JR, Stelzner DJ. Lack of axonal sprouting of spared propriospinal fibers caudal to spinal contusion injury is attributed to chronic axonopathy. J Neurotrauma. 2009;26:2279–2297. doi: 10.1089/neu.2009.0934. [DOI] [PubMed] [Google Scholar]
- Steeves J, Fawcett J, Tuszynski M. Report of international clinical trials workshop on spinal cord injury February 20–21, 2004, Vancouver, Canada. Spinal Cord. 2004;42:591–597. doi: 10.1038/sj.sc.3101669. [DOI] [PubMed] [Google Scholar]
- Steward O, Schauwecker PE, Guth L, et al. Genetic approaches to neurotrauma research: opportunities and potential pitfalls of murine models. Exp Neurol. 1999;157:19–42. doi: 10.1006/exnr.1999.7040. [DOI] [PubMed] [Google Scholar]
- Steward O, Sharp K, Yee KM, et al. A re-assessment of the effects of a Nogo-66 receptor antagonist on regenerative growth of axons and locomotor recovery after spinal cord injury in mice. Exp Neurol. 2008;209:446–468. doi: 10.1016/j.expneurol.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Sharp K, Yee KM. A re-assessment of the effects of intracortical delivery of inosine on transmidline growth of corticospinal tract axons after unilateral lesions of the medullary pyramid. Exp Neurol. 2012;233:662–673. doi: 10.1016/j.expneurol.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes BT, Noyes DH, Behrmann DL. An electromechanical spinal injury technique with dynamic sensitivity. J Neurotrauma. 1992;9:187–195. doi: 10.1089/neu.1992.9.187. [DOI] [PubMed] [Google Scholar]
- Stokols S, Tuszynski MH. The fabrication and characterization of linearly oriented nerve guidance scaffolds for spinal cord injury. Biomaterials. 2004;25:5839–5846. doi: 10.1016/j.biomaterials.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Straley KS, Foo CW, Heilshorn SC. Biomaterial design strategies for the treatment of spinal cord injuries. J Neurotrauma. 2010;27:1–19. doi: 10.1089/neu.2009.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JK, Miao L, Calvert JW, et al. A possible role of RhoA/Rho-kinase in experimental spinal cord injury in rat. Brain Res. 2003;959:29–38. doi: 10.1016/s0006-8993(02)03717-4. [DOI] [PubMed] [Google Scholar]
- Tang X, Davies JE, Davies SJ. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J Neurosci Res. 2003;71:427–444. doi: 10.1002/jnr.10523. [DOI] [PubMed] [Google Scholar]
- Tator CH. Strategies for recovery and regeneration after brain and spinal cord injury. Inj Prev. 2002;8:IV33–36. doi: 10.1136/ip.8.suppl_4.iv33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tator CH. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 2006;59:957–982. doi: 10.1227/01.NEU.0000245591.16087.89. discussion 982–987. [DOI] [PubMed] [Google Scholar]
- Teng YD, Lavik EB, Qu X, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A. 2002;99:3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Liao WL, Choi H, et al. Physical activity-mediated functional recovery after spinal cord injury: potential roles of neural stem cells. Regen Med. 2006;1:763–776. doi: 10.2217/17460751.1.6.763. [DOI] [PubMed] [Google Scholar]
- Tester NJ, Howland DR. Chondroitinase ABC improves basic and skilled locomotion in spinal cord injured cats. Exp Neurol. 2008;209:483–496. doi: 10.1016/j.expneurol.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester NJ, Plaas AH, Howland DR. Effect of body temperature on chondroitinase ABC's ability to cleave chondroitin sulfate glycosaminoglycans. J Neurosci Res. 2007;85:1110–1118. doi: 10.1002/jnr.21199. [DOI] [PubMed] [Google Scholar]
- Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28:1611–1682. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toffano G, Savoini G, Aldinio C, et al. Effects of gangliosides on the functional recovery of damaged brain. Adv Exp Med Biol. 1984;174:475–488. doi: 10.1007/978-1-4684-1200-0_40. [DOI] [PubMed] [Google Scholar]
- Tolias CM, Bullock MR. Critical appraisal of neuroprotection trials in head injury: what have we learned? NeuroRx. 2004;1:71–79. doi: 10.1602/neurorx.1.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Houle JD. Intraspinal microinjection of chondroitinase ABC following injury promotes axonal regeneration out of a peripheral nerve graft bridge. Exp Neurol. 2008;211:315–319. doi: 10.1016/j.expneurol.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Kadakia R, Santi L, et al. Administration of chondroitinase ABC rostral or caudal to a spinal cord injury site promotes anatomical but not functional plasticity. J Neurotrauma. 2009;26:2323–2333. doi: 10.1089/neu.2009.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai EC, Tator CH. Neuroprotection and regeneration strategies for spinal cord repair. Curr Pharm Des. 2005;11:1211–1222. doi: 10.2174/1381612053507404. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol. 2007;28:219–226. doi: 10.1016/j.it.2007.03.001. [DOI] [PubMed] [Google Scholar]
- van den Brand R, Heutschi J, Barraud Q, et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–1185. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Howells DW, Sena ES, et al. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7:e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velardo MJ, Burger C, Williams PR, et al. Patterns of gene expression reveal a temporally orchestrated wound healing response in the injured spinal cord. J Neurosci. 2004;24:8562–8576. doi: 10.1523/JNEUROSCI.3316-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierck CJ, Jr, Siddall P, Yezierski RP. Pain following spinal cord injury: animal models and mechanistic studies. Pain. 2000;89:1–5. doi: 10.1016/S0304-3959(00)00463-2. [DOI] [PubMed] [Google Scholar]
- Walmsley AR, Mir AK. Targeting the Nogo-A signalling pathway to promote recovery following acute CNS injury. Curr Pharm Des. 2007;13:2470–2484. doi: 10.2174/138161207781368611. [DOI] [PubMed] [Google Scholar]
- Watson C, Paxinos G, Kayalioglu G, editors. The Spinal Cord: A Christopher and Dana Reeve Foundation Text and Atlas. Elsevier AP; Amsterdam: 2009. [Google Scholar]
- Weidner N, Tuszynski MH. Spontaneous plasticity in the injured spinal cord–implications for repair strategies. Mol Psychiatry. 2002;7:9–11. doi: 10.1038/sj.mp.4000983. [DOI] [PubMed] [Google Scholar]
- Wennersten A, Holmin S, Al Nimer F, et al. Sustained survival of xenografted human neural stem/progenitor cells in experimental brain trauma despite discontinuation of immunosuppression. Exp Neurol. 2006;199:339–347. doi: 10.1016/j.expneurol.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Wewetzer K, Radtke C, Kocsis J, et al. Species-specific control of cellular proliferation and the impact of large animal models for the use of olfactory ensheathing cells and Schwann cells in spinal cord repair. Exp Neurol. 2011;229:80–87. doi: 10.1016/j.expneurol.2010.08.029. [DOI] [PubMed] [Google Scholar]
- White TE, Lane MA, Sandhu MS, et al. Neuronal progenitor transplantation and respiratory outcomes following upper cervical spinal cord injury in adult rats. Exp Neurol. 2010;225:231–236. doi: 10.1016/j.expneurol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, Tennissen AM. Activity-dependent spinal cord plasticity in health and disease. Annu Rev Neurosci. 2001;24:807–843. doi: 10.1146/annurev.neuro.24.1.807. [DOI] [PubMed] [Google Scholar]
- Yan J, Xu L, Welsh AM, et al. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007;4:e39. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JW, Jeong SM, Seo KM, et al. Effects of corticosteroid and electroacupuncture on experimental spinal cord injury in dogs. J Vet Sci. 2003;4:97–101. [PubMed] [Google Scholar]
- Yang H, Lu P, McKay HM, et al. Endogenous neurogenesis replaces oligodendrocytes and astrocytes after primate spinal cord injury. J Neurosci. 2006;26:2157–2166. doi: 10.1523/JNEUROSCI.4070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W. Spinal cord injury pathophysiology and therapy. In: Narayan RK, Wilberger JJE, Povlishock JT, editors. Neurotrauma. McGraw-Hill; New York: 1996. [Google Scholar]
- Young W, DeCrescito V, Flamm ES, et al. Pharmacological therapy of acute spinal cord injury: studies of high dose methylprednisolone and naloxone. Clin Neurosurg. 1988;34:675–697. [PubMed] [Google Scholar]
- Yu D, Neeley WL, Pritchard CD, et al. Blockade of peroxynitrite-induced neural stem cell death in the acutely injured spinal cord by drug-releasing polymer. Stem Cells. 2009;27:1212–1222. doi: 10.1002/stem.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Ho C, Li S, et al. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]
- Zorner B, Schwab ME. Anti-Nogo on the go: from animal models to a clinical trial. Ann N Y Acad Sci. 2010;1198:E22–E34. doi: 10.1111/j.1749-6632.2010.05566.x. [DOI] [PubMed] [Google Scholar]