Abstract
Several agents used for treatment of colon and other cancers induce reactive oxygen species (ROS) and this plays an important role in their anticancer activities. In addition to the well-known proapoptotic effects of ROS inducers, these compounds also decrease expression of specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4 and several pro-oncogenic Spregulated genes important for cancer cell proliferation, survival and metastasis. The mechanism of these responses involve ROS-dependent downregulation of microRNA-27a (miR-27a) or miR-20a (and paralogs) and induction of two Sp-repressors, ZBTB10 and ZBTB4 respectively. This pathway significantly contributes to the anticancer activity of ROS inducers and should be considered in development of drug combinations for cancer chemotherapy.
Keywords: Colon cancer, reactive oxygen Species, antioxidants, specificity transcription factors, targeted therapy, mechanism based drugs, zinc finger DNA binding proteins, microRNA
Introduction
Colorectal cancer is the third most diagnosed cancer in men and second most frequently observed cancer in women worldwide [1-3] and over 1 million new cases of colon cancer were diagnosed in 2008 and it is estimated that the annual number of deaths due to colorectal cancer are more than half a million. This accounts for 8% of all cancer deaths, making it the fourth leading cause of mortality from cancer. Colon cancer incidence is higher in the more developed countries with 0.7 million cases compared to the less developed nations with 0.5 million cases in 2008 [1]. Although, the prevalence rates for colorectal cancer are higher in individuals from the developed and industrialized nations, western lifestyles in less developed countries are resulting in increasing incidence rates [4]. Individuals from under developed countries that migrate to an industrialized country with high a incidence of colon cancer eventually exhibit the higher incidence rates [5].
Risk factors for colon cancer include obesity, increased alcohol intake, smoking, increased consumption of red meat, processed meats, highly refined grains, starch and low intakes of fruits and vegetables. The risks for colon cancer are decreased with increasing consumption of white meat such as poultry and fish, plant-based food sources of protein, substitution of saturated with unsaturated fats and incorporating legumes, sugars and unrefined grains as the major source of carbohydrates [6, 7]. A recent large case-control study demonstrated a strong correlation between a diet rich in fruit and vegetables and reduced risk of colorectal cancers. According to the study the total antioxidant capacity from different food sources rather than levels of their individual antioxidants is considered to be critical for decreasing colorectal cancer risk [8]. Western diets which are comprised of large portions of red meat with high levels of iron stores are thought to be a cancer risk factor. Heme iron is present in significant quantities in red meat and promotes growth of transformed cells and increases DNA damage by acting as a pro-oxidant and generating oxidative stress in cells [9, 10].
Reactive Oxygen Species and Cancer
Reactive oxygen species (ROS) which includes hydrogen peroxide, hydroxyl radical, superoxide anion and peroxynitrites are chemically active pro-oxidant molecules that are generated by incomplete reduction of oxygen. ROS are involved in a variety of physiological and pathological processes in the cell [11, 12]. It has been established that cancer cells thrive under some levels of oxidative stress and compared to non-cancer tissue, ROS levels are elevated in breast, colon, pancreatic, prostate and other cancers [13-15]. In addition oxidative DNA damage by ROS contributes to carcinogenesis by causing mutations in the genetic material. ROS can cause single strand breaks and genetic instability and this is due to oxidation of purines and pyrimidines and generation of alkali labile sites. Oxidative damage that commonly leads to mutations are associated with modification of GC base pairs and these mutations are primarily due to base-pair substitution and less frequently deletions and insertions [16]. Furthermore ROS plays a crucial role in regulating expression of genes associated with cancer cell growth and survival, angiogenesis, invasion and metastasis by activating transcription factors including NF-kB, activator protein-1 (AP-1) and hypoxia inducible factor-1 (HIF-1α) [17].
In addition to facilitating tumor growth and survival, ROS also plays a role as an important inhibitor of cancer cell proliferation and in induction of apoptosis. Excessive production of ROS in cancer cells induces apoptosis or autophagy and in some cases necrosis by damaging cellular components such as DNA, protein and lipid membranes [17]. Disruption of the mitochondrial membrane integrity can result in release of cytochrome-c, which in turn activates the intrinsic apoptotic machinery [18]. Similarly, Fas ligand, an upstream activator of the extrinsic apoptotic pathway, mediates induction of ROS that is essential for initiating the apoptotic signaling cascade [19]. ROS also has an important function in autophagic cell death in many cancer types including colon, pancreatic, breast, glioma, glioblastoma and non-small cell lung cancer [20-24]. In addition to programmed cell death, necrotic cell death is observed when in cancer cells the levels of ROS exceed those necessary for inducing apoptosis and autophagy and this has been reported in multiple myeloma, prostate cancer, hepatoma and leukemia cells [25-28].
ROS and Cancer Therapy
The phenomenon of drug-induced ROS in mediating induction of apoptosis has been extensively exploited to design effective strategies for cancer chemotherapy. Several studies investigating the effects of both natural and synthetic anti-cancer agents in colorectal cancers have shown that many of these compounds induce ROS and although not always recognized this pathway may be the predominant mechanism of action for several drugs used in cancer chemotherapy. The effects of antioxidants in attenuating the responses of various chemotherapeutic drugs provide critical evidence for the role of ROS in mediating their activites.
Recent Reports of ROS Inducing Anticancer Agents (Table 1)
Table 1.
ROS inducing anticancer agents
| Compound | Cell lines | Mechanism of Action |
|---|---|---|
| Betulinic acid [40] | RKO | ROS and MMP, miR27a-ZBTB10 axis |
| Curcumin [41] | SW480, RKO | ROS, MMP, miR27a-ZBTB10/miR20a-miR- 17-5p-ZBTB4 |
| RL197 [41] | SW480, RKO | ROS, MMP, miR27a-ZBTB10/miR20a-miR- 17-5p-ZBTB4 |
| GT-094 [42] | SW480, RKO | ROS and loss of MMP, miR27a-ZBTB10 axis |
| Ascorbic Acid [43] | RKO, SW480 | ROS |
| Emilia sonchifolia extract | HCT116 | ROS, upregulated ATM, p53, Fas, caspase 3,8 and 9 |
| Emodin | LS1034 | ROS, Ca2+, caspase 9 and 3 activation |
| Quercetin, Quercetin-5′,8- disulphonate |
LoVo | ROS and cell cycle arrest (S) |
| Patulin | HCT116, Caco2, SW620 | ROS, cell cycle arrest (G1/S), caspase 3activation, ATF3 upregulation |
| Resveratrol | HT-29, COLO 201 | ROS, caspase 8 and 3 activation, increase in LC3-II |
| Capsazepine | HCT116 | ROS, TRAIL, JNK, CHOP, DR5 activation |
| Laminarin | LoVo | ROS, Ca2+, caspase 9 and 3 activation |
| LS-1 | HT-29 | ROS, cell cycle arrest (G1), activation of caspase 3,8 and 9 |
| (5Z)-7-oxozeaenol | HT-29 | ROS, inhibits NF-kB, caspase 3 and 7 activation |
| Pulasan extract | HT-29, HCT116 | ROS, increase in Bax, caspase 3,7 and 9 activation |
| Caffeic acid | HCT116 | ROS, cell cycle arrest (G1) |
| Luffa echinata extract | HT-29 | ROS, cell cycle arrest (G2/M), Bax, caspase 3 accumulation |
| Berberine | Immorto-Min cells | ROS, cathepsin B, PARP, AIF activation |
| Bortezomib | ROS, cell cycle arrest (G2/M), ATM activation | |
| Salinomycin | RKO, SW620 | ROS, caspase 3,7 (RKO), LC3 (SW620) |
| 7-piperazinethylchrysin | HCT116 | ROS, p53, Bax, caspase 3 and 9 activation |
| Di-spiropyrrolizidino oxindole derivatives |
HCT116 | ROS, cell cycle arrest (G1), Bax, p53, caspase 3 and 9 activation |
| WYCO2-9 | HCT116 | ROS, cell cycle arrest (G2/M), MAP2K3/6 MAPK14 activation, |
| Sanguinarine | HCT116 | ROS, caspase 3,8 and 9, Egr-1 activation |
The anti-carcinogenic properties of curcumin, the active ingredient of the Indian spice, turmeric has been extensively investigated and its role in ROS-dependent growth inhibition has been reported in several studies. A structural analogue of curcumin, dehydrozingerone extracted from ginger (Zingiber officinale) was recently shown to induce intracellular ROS and induce cell cycle arrest at G2/M phase in HT-29, HCT-116 and HCT-15 colon cancer cells. Cotreatment with ROS scavenger, N-acetyl-L-cysteine, repressed dehydrozingerone mediated cell cycle arrest at G2/M phase in HT-29 cells. However, other antioxidants did not reverse the effects of dehydrozingerone on cell cycle arrest. The authors indicate that further studies are required to confirm the involvement of ROS, since dehydrozingerone is structurally similar to curcumin and could conjugate with glutathione, the direct metabolite of N-acetyl-L-cysteine to reduce its potency in the cell [29]. A study on the anticancer effects of curcuminoids in both HT-29 and SW480 colon cancer cells and CCD-18Co colon fibroblasts reported inhibition of HT-29 and SW480 colon cancer cell growth and enhanced activity of the chemotherapeutic agent 5-fluorouracil. These effects were due to decrease in levels of the multi-drug resistant gene1 (MDR1) and ROS mediated repression of specificity protein (Sp) transcription factors which regulates MDR1 and this will be discussed in detail later in the review [30]. Another study showed that curcumin inhibited HCT-15 colon cancer cell proliferation and induced apoptosis and this was accompanied by generation of ROS. Furthermore treatment of HCT-15 colon cancer cells with curcumin in this study decreased levels of pre-mRNA processing factor 4B (Prp4B) and mutated forms of p53 [31].
The active ingredients of Grape seed extract (GSE) such as epigallocatechins and procyanidins also inhibit growth of tumors derived from various cancer types and are cytoprotective to normal human gastric mucosal cells. GSE induced apoptosis and increase in ROS and Ca2+ levels in Caco-2 colon cancer cells and this was accompanied by inactivation of extracellular signalregulated kinase (ERK). Cotreatment of Caco-2 cells with antioxidant, N-acetyl cysteine and calcium chelator ethylene glycol tetraacetic acid suppressed GSE-induced apoptosis and ERK inactivation indicating a critical role of ROS in the anticarcinogenic activity of GSE [32]. Another study demonstrated a synergistic effect between (−)-epigallocatechin-3-gallate, vitexin-2-O-xyloside and raphasatin (4-methylsulphanyl-3-butenyl isothiocyanates) on growth inhibition in LoVo and CaCo-2 colon cancer cells. These responses were accompanied by increased levels of ROS, G0/G1 phase cell cycle arrest and induction of apoptosis by modulation of Bax, Bcl2 and caspase-9 suggestive of ROS-dependent mitochondrial activation of apoptosis [33]. Similarly, hirsutanol A, a sesquiterpene compound extracted from fungus Chondrostereum sp. caused accumulation of ROS in SW620 colon cancer cells altering the mitochondrial membrane potential followed by release of cytochrome-c and induction of apoptosis and these responses were blocked by addition of the ROS scavenger N-acetyl-L-cysteine. In addition results from this study indicate that ROS generation by hirsutanol A was mitochondrial respiratory chain independent in SW620 colon cancer cells [34].
Fatty acid derivatives such as dietary conjugated linoleic acids (CLA) and 6 iodo-δ-lactone (IL-δ) (5-hydroxy-6 iodo-8,11,14-eicosatrienoic delta lactone) an iodinated arachidonic acid derivative induced apoptosis in SW480 and HCT116 and HT29 colon cancer cells respectively. The compounds t10 and c12 CLA activated ER stress and induced apoptosis in HCT116 colon cancer cells and this was accompanied by increased levels of ROS and these responses were blocked by cotreatment of cells with antioxidants N-acetyl-cysteine and vitamin E [35]. Similarly, the anticancer agent, 6-iodo-δ-lactone, inhibited HT-29 colon cancer cell growth and induced apoptosis by increasing caspase-3 activity and levels of the pro-apoptotic protein Bax-2 and cotreatment of HT-29 cells with the antioxidant trolox reversed IL-δ mediated induction of apoptosis [36].
In addition to phytochemicals and naturally-occurring anti-cancer agents several synthetic anticancer drugs induce ROS. Treatment of HCT-116 colon cancer cells with a novel Osmium-based organometallic compound (Os) induced autophagy and apoptosis, disrupted the electron transport chain, changed mitochondrial morphology leading to decreased mitochondrial membrane potential and ATP levels and increased ROS levels in these cells. Colon cancer cells that were sensitive to effects of Os such as HCT-116 and RKO induced mitochondria-dependent ROS levels whereas cells that were insensitive to Os treatment such as HT-29 and SW620 failed to show any increase in ROS levels indicating that the ROS-dependent effects Os was cell context dependent. In addition, cotreatment of HCT116 with the antioxidant N-acetyl-cysteine reversed the proapoptotic effects of Os on these colon cancer cells [37].
Similarly, a study investigating the anti-neoplastic effects of a stable mixed phosphine gold (I) complex called [Au(tBu3P)(dppet)Cl] (complex I) also demonstrated the role of ROS in mediating its anticarcinogenic effects in colorectal cancer cells. Complex I induced apoptosis, increased the percentage of cells in sub-G1 phase; oligonucleosomal fragmentation and cyctochrome-c release from mitochondria and caspase 3 activation was also observed in HCT-116 colon cancer cells. Induction of apoptosis was due to inhibition of the ROS scavenging enzyme thioredoxin reductase which is responsible for reducing levels of hydrogen peroxide that is released into the cell after mitochondrial damage. Treatment of HCT-116 cells with complex I inhibited both cytosolic and mitochondrial isoforms of the enzyme [38].
Chromate (Cr(VI)) containing compounds are also carcinogenic however their mechanisms of action are unclear. However, a recent study in DLD1 colon cancer cells showed that Cr(VI) inhibited cell cycle progression at the S phase and these effects were blocked by cotreatment with the antioxidant catalase indicating that the effects of Cr(VI) may be mediated by hydrogen peroxide. In addition, activation of p53 was observed when DLD1 cells were treated with CR(IV) and cotreatment of cells with small hairpin RNA for p53 blocked S phase cell cycle arrest and these responses were also dependent on induction of ROS [39].
Specificity Protein Transcription Factors as a Target of Drug-Induced ROS
Results from numerous clinical studies and in vitro experiments show that even targeted therapies for disrupting growth and survival of cancer cells are complex and not completely understood. Although some mechanism-based drugs show some promise their beneficial therapeutic efficacy has been limited due to the complex labyrinth-like signaling pathways and regulatory networks that allow cancer cells to grow and survive. Thus inhibition of individual growth/survival-promoting and angiogenic pathways are not effective since cancer cells can function by relying on alternate pathways. Hence there is a need for therapeutic targets that can simultaneously target multiple pathways in cancer cells and tumors. To this effect our laboratory has focused on specificity protein (Sp) transcription factors (TFs) since Sp1 and other Sp TFs regulate expression of multiple genes that are important for cancer cell growth and survival and Sp TFs can be effectively targeted by various anti-cancer agents.
Sp Transcription Factors
Members of the Sp/KLF family have a highly conserved DNA binding domain that consists of three contiguously placed C2H2-type zinc fingers that are located at the C-terminal region. Members of the family bind to GC boxes (GGGGCGGGG), GT/CACCC boxes (GGTGTGGGG) and basic transcription elements to regulate gene transcription [44-47]. Evidence from studies conducted in this laboratory and others indicate that Sp transcription factors are overexpressed in several cancer cell lines including colon, bladder, pancreatic, prostate, breast, thyroid and esophageal cancer cell lines and play a crucial role in tumor growth, development and metastasis [48-53]. RNA interference (RNAi) studies conducted in pancreatic cancer cells reveals that Sp1, Sp3 and Sp4 proteins are involved in VEGF, VEGFR1 and VEGFR2 expression and knockdown of Sp1, Sp3 and Sp4 by RNAi also affected pancreatic cancer cell growth and cell cycle progression with a decreased percentage of cells in G2/M and S and increased percentage of cells in G0/G1 phase. This was accompanied by increased expression of cyclin-dependent kinase inhibitor p27 with Sp3 knockdown [54-56]. Although Sp TFs are important for embryonic and postnatal growth and development, expression of Sp1 decreases with age and in adults there are large differences in expression of Sp-TFs in tumor (high) vs non-tumor tissue [53, 57-61].
Targeting Sp Transcription Factors
Research in this laboratory has focused on developing anticancer drugs that downregulate Sp1, Sp3 and Sp4 protein expression and thereby inhibit pathways required for cancer growth, proliferation, survival, angiogenesis and metastasis. Anti-cancer agents that decrease expression of Sp. TFs include compounds such as betulinic acid (BA), curcumin, arsenic trioxide, synthetic triterpenoids and NSAIDs and these agents decrease expression of key regulators of cell growth (EGFR, cyclin D1, c-MET), survival (bcl-2, survivin), inflammation (NKκB) and angiogenesis (VEGF, VEGFR1, VEGFR2) [50, 51, 54, 62]. Multiple mechanistic pathways are involved in drug mediated downregulation of Sp TFs and these mechanisms are dependent on the individual drug and cancer cell line. Several of these agents that downregulate Sp TFs act through a transcriptional repression pathway that is activated by ROS.
Role of ROS in Drug Mediated Dowregulation of Sp Transcription Factors
Studies in this laboratory have shown that several anti-cancer compounds mediate their effects via induction of oxidative stress and generation of ROS, which is necessary for decreased expression of Sp TFs.
Ethyl 2-((2,3 bis(nitrooxy)propyl)disulfanyl)benzoate (GT-094), a novel nitro-NSAID induced ROS and decreased mitochondrial membrane potential (MMP) in SW480 and RKO colon cancer cells and treatment with antioxidants GSH and DTT inhibited ROS generation, prevented the loss of MMP and reversed the effects on downregulation of Sp1, Sp3 and Sp4 proteins and Sp dependent genes [42]. Similarly ascorbic acid (vitamin C) which induced hydrogen peroxide decreased SW480 and RKO colon cancer cell proliferation and induced apoptosis and necrosis and this was accompanied by downregulation of Sp1, Sp3 and Sp4 proteins and Sp regulated gene products such as c-Met, EGFR, cyclin-D1, survivin, bcl-2, VEGF, VEGFR1 and VEGFR2. Moreover these responses and affected genes were inhibited in cells cotreated with the antioxidant GSH [43]. In another study curcumin and a synthetic analog of curcumin (3E,5E)-3,5-Bis(2,5-dimethoxybenzylidene)-1-tbutoxycarbonylpiperidin-4-one (RL197) all decreased Sp TFs in SW480 and RKO colon cancer cells via ROS activation and cotreatment with antioxidants inhibited downregulation of Sp TFs and other responses including cell proliferation, induction of ROS and loss of MMP [41].
Role of ZBTB10 and MiR27a in ROS Dependent Drug Induced Downregulation of Sp Proteins
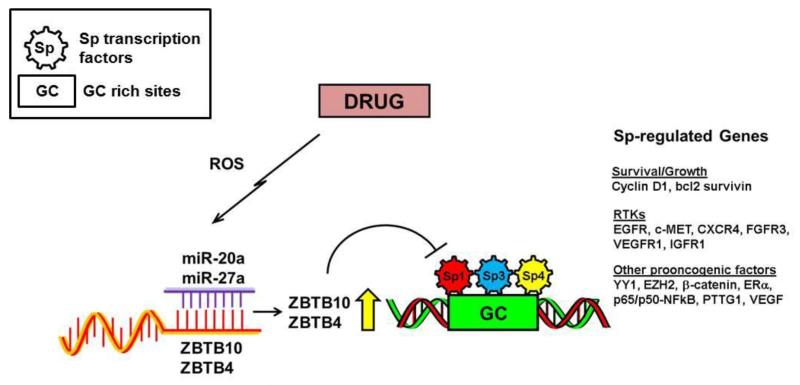
A study investigating the transcriptional regulation of gastrin, a gastrointestinal tract growth factor discovered that a novel zinc finger and BTB domain containing 10 (ZBTB-10/RIN ZF) protein that binds to the CACC cis-regulatory elements of the gastrin promoter and interferes with Sp1 binding and transactivation of the gastrin gene suggesting that ZBTB10 may function as an Sp1 repressor [63]. Subsequent studies showed that after transfection of the 2′-O-methyl RNA antisense miR-27a in SKBr3 breast cancer cells ZBTB10 mRNA was induced indicating that miR27a transcriptionally suppressed the expression of the Sp1 repressor, ZBTB10 [64]. Subsequent overexpression studies and experiments employing antisense miR27a in breast cancer cells established that downregulation of the oncogenic microRNA, miR-27a was necessary for induction of ZBTB10 expression which in turn acts as a transcriptional repressor for Sp TFs and Sp-regulated genes by competitively binding to GC-rich sites on their promoter sequences. Drugs such as curcumin and GT-094 induce ROS in colon cancer cells and this is accompanied by decreased miR-27a levels and induction of ZBTB10 which in turn results in downregulation of Sp proteins and Sp dependent genes. Cotreatment of these cells with antioxidants such as DTT and GSH blocks induction of ZBTB10 and suppression of miR27a levels in these cells indicating that these compounds mediate their effects on Sp proteins and Sp-regulated genes by inducing ROS (Figure 1.) [41, 42]. In addition, betulinic acid a naturally occurring pentacyclic triterpenoid also induces ROS levels in RKO colon cancer cells and ROS generation disrupts the miR27a-ZBTB10 axis leading to repression of Sp 1, Sp3 and Sp4 proteins and Sp dependent genes. However, this response is cell context dependent and betulinc acid does not induce ROS levels in SW480 colon cancer cells and decrease in expression of Sp TFs is ROS-independent [40].
Figure 1. ROS dependent downregulation of Sp proteins and other oncogenic factors.
Treatment of colon cancer cells with ROS inducing drugs disrupt miR-ZBTB interaction to induce Sp repressors ZBTB10 and or ZBTB4 that act as transcriptional repressors on GC-rich cis elements [41, 42].
Role of ZBTB4/MiR-20a/MiR-17-5-p Axis in ROS-mediated Transcriptional Repression of Sp Proteins
Recent studies investigating the role of human POK family members as transcriptional repressors showed that ZBTB4, another zinc finger and BTB domain containing protein also bind GC-rich sequences [65]. In addition, the Kaplan-Meier survival analysis of two publicly available breast cancer patient data sets showed that patients with high expression levels of ZBTB4 exhibited increased relapse free-survival compared patients with low ZBTB4 expression. Analysis of publicly available mRNA and miR data sets indicated that ZBTB4 can be negatively regulated by miR-17-5p and miR-20a which form part of the miR-17-92 cluster. Knocking down miR20a and overexpression of ZBTB4 in MCF-7 and MDA-MB-231 breast cancer cell lines downregulated expression of Sp proteins and Sp regulated gene products indicating that miR-20a and related paralogs enhance expression of Sp proteins by suppressing the Sp repressor ZBTB4 [66]. Studies carried out in this laboratory show that drugs including curcumin and RL197 target Sp1, Sp3 and Sp4 proteins and Sp dependent genes by inducing ROS which in turn disrupt the ZBTB4/miR-20a/miR-17-5-p axis in RKO and SW480 colon cancer cells and these effects are blocked by cotreating the cells with glutathione indicating that the anticarcinogenic effects of these drugs are ROS dependent (Figure 1.) [41]. Although the mechanisms of ROS-dependent downregulation of miR27a/miR20a (paralogs) are unknown it is possible that trans-acting factors are modulated through genome wide chromatin shifts as recently reported for hydrogen peroxide in SW480 colon cancer cells [67].
Conclusions
Anticancer agents that induce ROS have long been recognized as an important class of drugs that inhibit cancer cell proliferation and survival and this has been attributed, in part, to either direct or indirect effects on mitochondria. However, the discovery that ROS also disrupts miR-ZBTB interactions (Figure 1.) which leads to downregulation of Sp TFs and Sp regulated genes now defines an important new ROS-mediated pathway that explains the efficacy of ROS inducers and this will facilitate their future development for combination therapies with other anticancer agents.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Sandeep Sreevalsan and Stephen Safe declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Sandeep Sreevalsan, VMR 1197, Room 413, Texas A&M University, College Station, TX, 77843 979-845-9182 ssreevalsan@cvm.tamu.edu.
Stephen Safe, VMR 1197, Room 410, Texas A&M University, College Station, TX, 77843 979-845-5988
References
Papers of particular interest, published recently, have been highlighted as:
*Of importance
**Of major importance
- 1.Ferlay J, S.H., Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide. 2010 [cited 2012 July 17th]; Available from: http://globocan.iarc.fr.
- 2.Peter Boyle BL. World Cancer Report 2008. IARC; Lyon, France: 2008. p. 15. [Google Scholar]
- 3.Jemal A, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Curado MP, E.B., Shin HR. Cancer incidence in five continents. IX. IARC; Lyon, France: 2007. [Google Scholar]
- 5.Parkin DM, et al. Global cancer statistics. CA Cancer J Clin. 2002;2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 6.Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138(6):2029–2043 e10. doi: 10.1053/j.gastro.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1688–94. doi: 10.1158/1055-9965.EPI-09-0090. [DOI] [PubMed] [Google Scholar]
- 8.La Vecchia C, et al. Dietary total antioxidant capacity and colorectal cancer: A large case-control study in Italy. Int J Cancer. 2013;27(10):28133. doi: 10.1002/ijc.28133. [DOI] [PubMed] [Google Scholar]
- 9.Bird CL, et al. Plasma ferritin, iron intake, and the risk of colorectal polyps. Am J Epidemiol. 1996;144(1):34–41. doi: 10.1093/oxfordjournals.aje.a008852. [DOI] [PubMed] [Google Scholar]
- 10.Stevens RG, et al. Body iron stores and the risk of cancer. N Engl J Med. 1988;319(16):1047–52. doi: 10.1056/NEJM198810203191603. [DOI] [PubMed] [Google Scholar]
- 11*.Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. 2011;7(8):504–11. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hancock JT, Desikan R, Neill SJ. Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans. 2001;29(Pt 2):345–50. doi: 10.1042/0300-5127:0290345. [DOI] [PubMed] [Google Scholar]
- 13.Afanas’ev I. Reactive oxygen species signaling in cancer: comparison with aging. Aging Dis. 2011;2(3):219–30. [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar B, et al. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68(6):1777–85. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 15.Vaquero EC, et al. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem. 2004;279(33):34643–54. doi: 10.1074/jbc.M400078200. [DOI] [PubMed] [Google Scholar]
- 16.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta SC, et al. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal. 2012;16(11):1295–322. doi: 10.1089/ars.2011.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192(1):1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 19.Medan D, et al. Regulation of Fas (CD95)-induced apoptotic and necrotic cell death by reactive oxygen species in macrophages. J Cell Physiol. 2005;203(1):78–84. doi: 10.1002/jcp.20201. [DOI] [PubMed] [Google Scholar]
- 20.Shrivastava A, et al. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol Cancer Ther. 2011;10(7):1161–72. doi: 10.1158/1535-7163.MCT-10-1100. [DOI] [PubMed] [Google Scholar]
- 21.Coriat R, et al. The organotelluride catalyst LAB027 prevents colon cancer growth in the mice. Cell Death Dis. 2011;2:e191. doi: 10.1038/cddis.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donadelli M, et al. Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Dis. 2011;28(2):36. doi: 10.1038/cddis.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, et al. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15(1):171–82. doi: 10.1038/sj.cdd.4402233. [DOI] [PubMed] [Google Scholar]
- 24.Li J, et al. Tephrosin-induced autophagic cell death in A549 non-small cell lung cancer cells. J Asian Nat Prod Res. 2010;12(11):992–1000. doi: 10.1080/10286020.2010.513034. [DOI] [PubMed] [Google Scholar]
- 25.Garbarino JA, et al. Demalonyl thyrsiflorin A, a semisynthetic labdane-derived diterpenoid, induces apoptosis and necrosis in human epithelial cancer cells. Chem Biol Interact. 2007;169(3):198–206. doi: 10.1016/j.cbi.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 26.Kim JS, et al. Role of reactive oxygen species-mediated mitochondrial dysregulation in 3-bromopyruvate induced cell death in hepatoma cells: ROS-mediated cell death by 3-BrPA. J Bioenerg Biomembr. 2008;40(6):607–18. doi: 10.1007/s10863-008-9188-0. [DOI] [PubMed] [Google Scholar]
- 27.Nair RR, et al. HYD1-induced increase in reactive oxygen species leads to autophagy and necrotic cell death in multiple myeloma cells. Mol Cancer Ther. 2009;8(8):2441–51. doi: 10.1158/1535-7163.MCT-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naito M, et al. Caspase-independent necrotic cell death induced by a radiosensitizer, 8-nitrocaffeine. Cancer Sci. 2004;95(4):361–6. doi: 10.1111/j.1349-7006.2004.tb03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yogosawa S, et al. Dehydrozingerone, a structural analogue of curcumin, induces cellcycle arrest at the G2/M phase and accumulates intracellular ROS in HT-29 human colon cancer cells. J Nat Prod. 2012;75(12):2088–93. doi: 10.1021/np300465f. [DOI] [PubMed] [Google Scholar]
- 30.Noratto GD, et al. The drug resistance suppression induced by curcuminoids in colon cancer SW-480 cells is mediated by reactive oxygen species-induced disruption of the microRNA-27a-ZBTB10-Sp axis. Mol Nutr Food Res. 2013;8(10):201200609. doi: 10.1002/mnfr.201200609. [DOI] [PubMed] [Google Scholar]
- 31.Shehzad A, et al. Curcumin induces apoptosis in human colorectal carcinoma (HCT-15) cells by regulating expression of Prp4 and p53. Mol Cells. 2013;16:16. doi: 10.1007/s10059-013-0038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinicola S, et al. Grape seed extract triggers apoptosis in Caco-2 human colon cancer cells through reactive oxygen species and calcium increase: extracellular signal-regulated kinase involvement. Br J Nutr. 2013;25:1–13. doi: 10.1017/S0007114512006095. [DOI] [PubMed] [Google Scholar]
- 33.Papi A, et al. Vitexin-2-O-xyloside, raphasatin and (-)-epigallocatechin-3-gallate synergistically affect cell growth and apoptosis of colon cancer cells. Food Chem. 2013;138(2-3):1521–30. doi: 10.1016/j.foodchem.2012.11.112. [DOI] [PubMed] [Google Scholar]
- 34.Yang F, et al. Hirsutanol A, a novel sesquiterpene compound from fungus Chondrostereum sp., induces apoptosis and inhibits tumor growth through mitochondrial-independent ROS production: Hirsutanol A inhibits tumor growth through ROS production. J Transl Med. 2013;11(32):1479–5876. doi: 10.1186/1479-5876-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pierre AS, et al. Trans-10, cis-12 conjugated linoleic acid induced cell death in human colon cancer cells through reactive oxygen species-mediated ER stress. Biochim Biophys Acta. 2013;4:759–68. doi: 10.1016/j.bbalip.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Thomasz L, et al. 6 Iodo-delta-lactone: a derivative of arachidonic acid with antitumor effects in HT-29 colon cancer cells. Prostaglandins Leukot Essent Fatty Acids. 2013;88(4):273–80. doi: 10.1016/j.plefa.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Maillet A, et al. A novel Osmium-based compound targets the mitochondria and triggers ROS-dependent apoptosis in colon carcinoma. Cell Death Dis. 2013;6(4):185. doi: 10.1038/cddis.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lupidi G, et al. Synthesis, properties, and antitumor effects of a new mixed phosphine gold(I) compound in human colon cancer cells. J Inorg Biochem. 2013;124:78–87. doi: 10.1016/j.jinorgbio.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 39.Sun L, et al. Reactive oxygen species mediate Cr(VI)-induced S phase arrest through p53 in human colon cancer cells. J Environ Pathol Toxicol Oncol. 2012;31(2):95–107. doi: 10.1615/jenvironpatholtoxicoloncol.v31.i2.20. [DOI] [PubMed] [Google Scholar]
- 40.Chintharlapalli S, et al. Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome-dependent and -independent downregulation of specificity proteins (Sp) transcription factors. BMC Cancer. 2011;11:371. doi: 10.1186/1471-2407-11-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Gandhy SU, et al. Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs. BMC Cancer. 2012;12(564):1471–2407. doi: 10.1186/1471-2407-12-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Pathi SS, et al. GT-094, a NO-NSAID, inhibits colon cancer cell growth by activation of a reactive oxygen species-microRNA-27a: ZBTB10-specificity protein pathway. Mol Cancer Res. 2011;9(2):195–202. doi: 10.1158/1541-7786.MCR-10-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pathi SS, et al. Pharmacologic doses of ascorbic acid repress specificity protein (Sp) transcription factors and Sp-regulated genes in colon cancer cells. Nutr Cancer. 2011;63(7):1133–42. doi: 10.1080/01635581.2011.605984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suske G. The Sp-family of transcription factors. Gene. 1999;238(2):291–300. doi: 10.1016/s0378-1119(99)00357-1. [DOI] [PubMed] [Google Scholar]
- 45.Gidoni D, et al. Bidirectional SV40 transcription mediated by tandem Sp1 binding interactions. Science. 1985;230(4725):511–7. doi: 10.1126/science.2996137. [DOI] [PubMed] [Google Scholar]
- 46.Giglioni B, et al. The same nuclear proteins bind the proximal CACCC box of the human beta-globin promoter and a similar sequence in the enhancer. Biochem Biophys Res Commun. 1989;164(1):149–55. doi: 10.1016/0006-291x(89)91695-1. [DOI] [PubMed] [Google Scholar]
- 47.Imataka H, et al. Two regulatory proteins that bind to the basic transcription element (BTE), a GC box sequence in the promoter region of the rat P-4501A1 gene. Embo J. 1992;11(10):3663–71. doi: 10.1002/j.1460-2075.1992.tb05451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdelrahim M, Safe S. Cyclooxygenase-2 inhibitors decrease vascular endothelial growth factor expression in colon cancer cells by enhanced degradation of Sp1 and Sp4 proteins. Mol Pharmacol. 2005;68(2):317–29. doi: 10.1124/mol.105.011825. [DOI] [PubMed] [Google Scholar]
- 49.Abdelrahim M, et al. Tolfenamic acid and pancreatic cancer growth, angiogenesis, and Sp protein degradation. J Natl Cancer Inst. 2006;98(12):855–68. doi: 10.1093/jnci/djj232. [DOI] [PubMed] [Google Scholar]
- 50.Chintharlapalli S, et al. Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res. 2007;67(6):2816–23. doi: 10.1158/0008-5472.CAN-06-3735. [DOI] [PubMed] [Google Scholar]
- 51.Chadalapaka G, et al. Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res. 2008;68(13):5345–54. doi: 10.1158/0008-5472.CAN-07-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mertens-Talcott SU, et al. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67(22):11001–11. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- 53.Safe S, Abdelrahim M. Sp transcription factor family and its role in cancer. Eur J Cancer. 2005;41(16):2438–48. doi: 10.1016/j.ejca.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Abdelrahim M, et al. Regulation of vascular endothelial growth factor receptor-1 expression by specificity proteins 1, 3, and 4 in pancreatic cancer cells. Cancer Res. 2007;67(7):3286–94. doi: 10.1158/0008-5472.CAN-06-3831. [DOI] [PubMed] [Google Scholar]
- 55.Higgins KJ, et al. Regulation of vascular endothelial growth factor receptor-2 expression in pancreatic cancer cells by Sp proteins. Biochem Biophys Res Commun. 2006;345(1):292–301. doi: 10.1016/j.bbrc.2006.04.111. [DOI] [PubMed] [Google Scholar]
- 56.Abdelrahim M, et al. Role of Sp proteins in regulation of vascular endothelial growth factor expression and proliferation of pancreatic cancer cells. Cancer Res. 2004;64(18):6740–9. doi: 10.1158/0008-5472.CAN-04-0713. [DOI] [PubMed] [Google Scholar]
- 57.Bouwman P, Philipsen S. Regulation of the activity of Sp1-related transcription factors. Mol Cell Endocrinol. 2002;195(1-2):27–38. doi: 10.1016/s0303-7207(02)00221-6. [DOI] [PubMed] [Google Scholar]
- 58.Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188(2):143–60. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- 59.Ammendola R, et al. Sp1 DNA binding efficiency is highly reduced in nuclear extracts from aged rat tissues. J Biol Chem. 1992;267(25):17944–8. [PubMed] [Google Scholar]
- 60.Adrian GS, et al. YY1 and Sp1 transcription factors bind the human transferrin gene in an age-related manner. J Gerontol A Biol Sci Med Sci. 1996;51(1):B66–75. doi: 10.1093/gerona/51a.1.b66. [DOI] [PubMed] [Google Scholar]
- 61.Oh JE, Han JA, Hwang ES. Downregulation of transcription factor, Sp1, during cellular senescence. Biochem Biophys Res Commun. 2007;353(1):86–91. doi: 10.1016/j.bbrc.2006.11.118. [DOI] [PubMed] [Google Scholar]
- 62*.Jutooru I, et al. Arsenic trioxide downregulates specificity protein (Sp) transcription factors and inhibits bladder cancer cell and tumor growth. Exp Cell Res. 2010;316(13):2174–88. doi: 10.1016/j.yexcr.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tillotson LG. RIN ZF, a novel zinc finger gene, encodes proteins that bind to the CACC element of the gastrin promoter. J Biol Chem. 1999;274(12):8123–8. doi: 10.1074/jbc.274.12.8123. [DOI] [PubMed] [Google Scholar]
- 64.Scott GK, et al. Rapid alteration of microRNA levels by histone deacetylase inhibition. Cancer Res. 2006;66(3):1277–81. doi: 10.1158/0008-5472.CAN-05-3632. [DOI] [PubMed] [Google Scholar]
- 65.Weber A, et al. Zbtb4 represses transcription of P21CIP1 and controls the cellular response to p53 activation. Embo J. 2008;27(11):1563–74. doi: 10.1038/emboj.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66**.Kim K, et al. Identification of oncogenic microRNA-17-92/ZBTB4/specificity protein axis in breast cancer. Oncogene. 2012;31(8):1034–44. doi: 10.1038/onc.2011.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67**.O’Hagan HM, et al. Oxidative damage targets complexes containing DNA methyltransferases, SIRT1, and polycomb members to promoter CpG Islands. Cancer Cell. 2011;20(5):606–19. doi: 10.1016/j.ccr.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]