Abstract
Accumulating evidence has shown that the hypoxic microenvironment, which is critical during cancer development, plays a key role in regulating breast cancer progression and metastasis. The effects of hypoxia-inducible factor 1 (HIF-1), a master regulator of the hypoxic response, have been extensively studied during these processes. In this review, we focus on the roles of HIF-1 in regulating breast cancer cell metastasis, specifically its effects on multiple key steps of metastasis, such as epithelial-mesenchymal transition (EMT), invasion, extravasation, and metastatic niche formation. We also discuss the roles of HIF-1-regulated non-coding RNAs in breast cancer metastasis, and therapeutic opportunities for breast cancer through targeting the HIF-1 pathway.
Keywords: Breast cancer, Hypoxia-inducible factor 1 (HIF-1), Metastasis
1. Hypoxic microenvironment and breast cancer
Hypoxia has been extensively studied for decades within the tumor microenvironment, which influences malignant cells in various ways (Hu and Polyak, 2008). While the O2 supply matches the need of cell growth in normal tissues, the diffusion of O2 from a blood capillary in a tumor mass that reaches a size of 1–2 mm3 is limiting because of structural and functional abnormalities of the newly formed tumor vessels, thus leading to the development of hypoxic areas in the tumor mass (Vaupel et al., 2004).
Breast cancer is the most frequently diagnosed cancer and a major cause of death in women worldwide (Jemal et al., 2011; Lech and Przemyslaw, 2011). About 25%–40% of invasive breast cancers exhibit hypoxic regions (Lundgren et al., 2007). Studies using polarographic electrodes have shown that the median partial pressure of oxygen (pO2) in normal human breast tissue is 65 mmHg (1 mmHg=133.3 Pa). In contrast, human breast cancers have a median pO2 of 10 mmHg. In addition, more than half of all breast cancers studied had a pO2 of less than 2.5 mmHg (Vaupel et al., 1991; 2007; Hohenberger et al., 1998). Studies have shown that intra-tumoral hypoxia has negative implications for survival of breast cancer patients, independent of prognostic parameters, such as the clinical tumor stage, histological grade, and lymph node status. Hypoxic tumors are associated with a more aggressive phenotype, increased risk of metastasis, increased resistance to radiotherapy and chemotherapy, and induced cancer immune suppression (Brizel et al., 1996; Mees et al., 2009).
2. Basic biology of hypoxia-inducible factor 1 (HIF-1)
In most human solid tumors, adaptation to decreased O2 availability is achieved primarily through the activity of two hypoxia-inducible factors (HIF-1 and HIF-2), which regulate the expression of over 1000 target genes (Semenza, 2012). Overexpression of HIF-1 has been confirmed in many solid tumors, including ovarian, bladder, uterus, breast, colon, brain, pancreatic, renal, and prostate (Zhong et al., 1999; Talks et al., 2000). HIF-1 is a hetero-dimeric transcription factor consisting of a constitutively expressed HIF-1β subunit (also known as the aryl hydrocarbon receptor nuclear translocator [ARNT]) and a tightly regulated HIF-1α subunit (Wang et al., 1995). Both HIF-1α and HIF-1β are members of the bHLH-PAS superfamily of proteins containing basic-helix-loop-helix (bHLH) and PER-ARNT-SIM (PAS) domains involved in DNA binding and dimerization, respectively (Wang et al., 1995). HIF-1α also contains an oxygen-dependent degradation (ODD) domain and two transactivation domains (N-TAD and C-TAD).
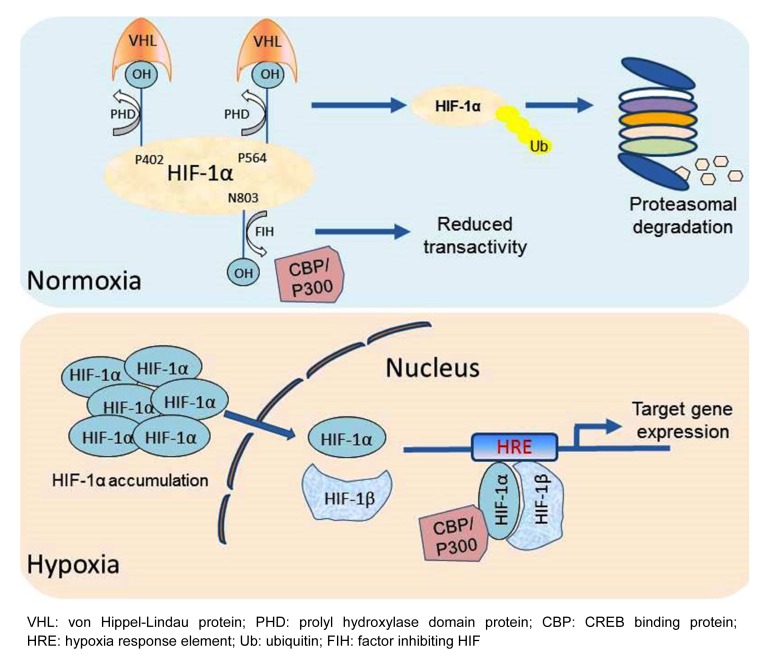
O2 regulates HIF-1α stability and activity through hydroxylation of proline and asparagine residues. Under normoxic conditions, two proline residues (P402 and P564) in the human HIF-1α ODD domain are hydroxylated by prolyl hydroxylase domain proteins (PHDs). Hydroxylation is required for binding of the HIF-1α protein to the von Hippel-Lindau protein (VHL). VHL is the recognition subunit of an E3 ubiquitin ligase that adds a poly-ubiquitin chain to HIF-1α, which targets the protein for degradation in the proteasome. Morever, O2-dependent hydroxylation of asparagine residue 803 (N803) in HIF-1α by factor inhibiting HIF-1 (FIH-1) suppresses the interaction of HIF-1α with the coactivators p300 and CBP (CREB binding protein), and therefore inhibits the HIF-1α transactivation function under normoxic conditions (Mahon et al., 2001; Lando et al., 2002). Under hypoxic conditions, both the prolyl and asparaginyl hydroxylation reactions are inhibited, leading to the stabilization of HIF-1α protein and increased interaction with its co-activators. Thus, in hypoxic cells, HIF-1α protein accumulates, hetero-dimerizes with HIF-1, binds to the consensus DNA sequence 5'-RCGTG-3' within hypoxia response elements (HREs) located in target genes, and activates their transcription (Fig. 1).
Fig. 1.
Hypoxia-inducible factor 1 (HIF-1) regulated by oxygen tension
It is not only that the HIF-1 pathway is regulated by oxygen tension, but also that hypoxia-independent growth factor stimulation, loss of tumor suppressor function, and oncogenic activation have been reported to regulate HIF-1α activity (Semenza, 2003). Growth factor signals affecting the HIF-1α pathway include human insulin-like growth factor, human epidermal growth factor (EGF), and prostaglandin E2 (Feldser et al., 1999; Laughner et al., 2001; Fukuda R. et al., 2002; Liu et al., 2002; Fukuda N. et al., 2003; Grimshaw, 2007). Function loss of tumor suppressor proteins, such as VHL, phosphatase, and phosphatase and tension homolog deleted on chromosome 10 (PTEN) and p53 in cancer cells, impairs the degradation of HIF-1α protein, or increases the synthesis of HIF-1 (Krieg et al., 2000; Zhong et al., 2000; Zundel et al., 2000; Ravi et al., 2001), thus leading to HIF-1α protein accumulation. Activation of the oncogenic PI3K/Akt signaling pathway stimulates HIF-1α expression through mTOR-dependent translation (Zhong et al., 2000; Laughner et al., 2001). In addition, cellular reactive oxygen species and nitric oxide have also been shown to stabilize HIF-1α, causing protein accumulation (Kasuno et al., 2004; Quintero et al., 2006; Gao et al., 2007).
3. HIF-1 and breast cancer metastasis
Over 90% of all breast cancer deaths are the result of metastasis, primarily to the bone, lung, liver, brain and lymph nodes. Tumor metastasis is the dissemination of cancer cells from the initial site of primary tumor growth to distant organs where they survive, proliferate, and form secondary tumors. Metastasis is a complex process containing a series of discrete steps: (1) epithelial-mesenchymal transition (EMT), during which cancer cells lose cell-to-cell contact and gain motility; (2) local tissue invasion, which is facilitated by the degradation of extracellular matrix (ECM); (3) intravasation, during which cancer cells penetrate the wall of a blood vessel and enter the circulation; (4) homing, during which cancer cells must survive within the circulation; (5) extravasation, during which cancer cells pass through the vascular wall and exit the blood stream at distant organs; and, (6) metastatic niche formation at the metastatic site to create a milieu that is favorable for cancer cell growth.
A high level of HIF-1α at diagnosis is predictive of early relapse and metastasis, and correlates with poor clinical outcomes in human breast cancer (Bos et al., 2003; Gruber et al., 2004; Generali et al., 2006). It has been reported that expression of HIF-1 target genes is increased in the triple-negative breast cancer subgroup (Zhong et al., 1999; Cancer Genome Atlas Network, 2012). HIF-1 plays key roles in many crucial aspects of breast cancer biology, including angiogenesis, stem cell maintenance, metabolic reprogramming, EMT, invasion, metastasis, and resistance to radiation therapy and chemotherapy (Semenza, 2012). Inhibition of HIF-1 activity in mice after orthotopic transplantation of triple-negative breast cancer cells has a dramatic effect on primary tumor growth as well as metastasis to lymph nodes and lungs (Zhang et al., 2012). Understanding the molecular and cellular mechanisms underlying metastasis is required for accurate prognoses and for developing new therapeutic strategies. Recent studies have shown that the HIF-1 pathway regulates breast cancer metastasis through multiple mechanisms. Next, we will focus on the regulatory roles of HIF-1 in a series of discrete steps of breast cancer metastatic progression.
3.1. HIF-1 mediates EMT
During metastasis, epithelial cells detach from the primary tumor, adhere to and invade the surrounding stroma, intravasate into the bloodstream, and disseminate to distant tissues where they extravasate out of blood vessels and form secondary tumors. The process of epithelial cells detaching from primary tumors closely resembles that of cells undergoing EMT, and EMT has been regarded as the possible first step in the complex process of metastasis (Chaffer and Weinberg, 2011; Valastyan and Weinberg, 2011). Epithelial cells that undergo EMT lose their epithelial phenotype and acquire mesenchymal cell-associated characteristics (Thiery, 2002; Kalluri and Weinberg, 2009; Creighton et al., 2010; Singh and Settleman, 2010; May et al., 2011). Thus, EMT is characterized by cellular and molecular changes that include loss of cell-to-cell adhesion, which involves E-cadherin in adherent junctions and claudins in tight junctions, upregulation of the mesenchymal protein vimentin, reorganization of the cytoskeleton to acquire a more spindle-like morphology, and increased motility that involves dynamic actin microfilament networks.
HIF-1 provokes EMT through up-regulating EMT-associated transcription factors or repressors, activating the EMT-associated signaling pathways, modulating EMT-associated inflammatory cytokines, as well as regulating other pathways like epigenetic regulators (Bao et al., 2012). For instance, HIF-1 induces EMT through the transcriptional control of E-cadherin, SNAIL (zinc finger protein snail), ZEB1 (zinc finger E-box-binding homeobox 1), TWIST, and TCF3 (transcription factor 3, also known as E47) (Krishnamachary et al., 2006; Moreno-Bueno et al., 2008). In breast cancer cells, EMT was activated by ZEB1-MYB-E-cadherin signaling under hypoxic stress, which induced higher expression of ZEB1 and lower expression of MYB (Hugo et al., 2013). Recently, it has been discovered that hypoxia-induced biomolecules, including Jagged2, cyclooxygenase-2 (COX-2), and urokinase receptor (uPAR) (Jo et al., 2009; Xing et al., 2011; Bocca et al., 2014), also contribute to EMT changes and invasive ability of breast cancer cells. The HIF-1 pathway may also regulate EMT via regulating long non-coding RNAs (lncRNAs; such as H19 lncRNA) and calcium signaling in breast cancer cells to promote cancer cell invasion in hypoxic microenvironments (Lock et al., 2013; Davis et al., 2014; Matouk et al., 2014).
3.2. HIF-1 mediates invasion and intravasation
To invade the surrounding tissues, cancer cells need to degrade the surrounding basement membrane (BM) and extracellular matrix barriers. Matrix metallo-proteinases (MMPs) are members of zinc-dependent endo-peptidases that degrade many of the components of the ECM, including the BM. Among MMPs, MMP-2 and MMP-9 degrade type IV collagen, which is a major component of the BM. MMP-2 and MMP-9 have been positively correlated with a higher incidence of metastases and with a poor prognosis in breast cancer (Duffy et al., 2000; Pritchard et al., 2001; Incorvaia et al., 2007). Cells at the invasive front of solid tumors are frequently found to have a high expression level of HIF-1α (Horrée et al., 2007). Many reports suggest that hypoxia and HIF-1 upregulate the expression levels and/or activities of MMP-2 and MMP-9 (Krishnamachary et al., 2006; Munoz-Najar et al., 2006).
Type I collagen is very important for cell migration during invasion. When tumor size increases, collagen fibers straighten and align, which facilitates cancer cell invasion (Maglione et al., 2006; Provenzano et al., 2006; Conklin et al., 2011). Recent studies showed that HIF-1α plays a critical role in collagen biogenesis in breast cancers by upregulating the expression levels of pro-collagen prolyl (P4HA1 and P4HA2) and lysyl (PLOD1 and PLOD2) hydroxylases in both cancer and stromal cells (Gilkes et al., 2013a; 2013b; 2013c). P4HA1 and P4HA2 are required for collagen deposition, while PLOD2 is important for collagen fiber crosslinking (Gilkes et al., 2013a). Expression of P4HA1, P4HA2, and PLOD2 is required for breast cancer metastasis.
3.3. HIF-1 and extravasation
Breast cancer cells may spread via either the lymphatic or vascular circulation (Weigelt et al., 2005). However, less than 0.1% of cancer cells that enter the vascular circulation establish a metastatic lesion (Fidler, 1970). This indicates that extravasation of cancer cells from the circulation into a tissue microenvironment where cancer cells can survive and proliferate is a limiting step in the metastatic process. To extravasate, a cancer cell must first adhere to endothelial cells (ECs) in the lung vasculature, a process known as margination, and then disrupt the tight interactions between ECs to penetrate out of the blood vessel.
Zhang et al. (2012) showed that HIF-1 promoted the extravasation of breast cancer cells in the lung. In addition, the invasion of naive breast cancer cells through EC monolayers increased when the cells were exposed to conditioned medium generated by hypoxic breast cancer cells. Biophysical measurement of EC-EC interactions using transendothelial electrical resistance showed decreased EC-EC interactions in the presence of conditioned medium generated by hypoxic breast cancer cells. These effects were not observed when conditioned medium from hypoxic breast cancer cells expressing shRNAs-targeting HIF-1 was used (Zhang et al., 2012). Further experiments identified angiopoietin-like 4 (ANGPTL4) as a factor secreted from cancer cells that was induced by hypoxia in an HIF-1-dependent manner. ANGPTL4 expression in cancer cells disrupts vascular EC-EC junctions, increases the permeability of lung capillaries, and facilitates the extravasation of cancer cells (Padua et al., 2008). In vivo studies also showed that ANGPTL4 induced vascular leakiness and facilitated lung metastasis in mice (Huang et al., 2011). ANGPTL4 expression in primary breast cancers is associated with lung metastasis (Padua et al., 2008).
Hypoxic breast cancer cells also showed increased adherence to EC monolayers in an HIF-1-dependent manner. Microarray analysis of metastasis-related genes identified a cell surface receptor, L1 cell adhesion molecule (L1CAM), which is a direct transcriptional target of HIF-1 induced by hypoxia in triple negative breast cancer cells (Zhang et al., 2012). L1CAM is a protein involved in mediating cell-cell adherence by homophilic and heterophilic interactions with integrins, neuropilin 1, or CD24 (Semenza, 2012). Overexpression of L1CAM in cancer cells promoted the adhesion of cancer cells to EC monolayers in vitro, and increased the number of extravasated cancer cells in lung tissue after tail vein injection (Zhang et al., 2012). HIF-regulated SDF-1/CXCR4 (stromal cell-derived factor-1/CXC chemokine receptor 4) signaling in ECs under hypoxic conditions may also promote the adhesion of breast cancer cells to ECs (Jin et al., 2012).
3.4. HIF-1 and metastatic niche formation
HIF-1 also regulates premetastatic niche formation at distant organs prior to cancer cell arrival (Erler et al., 2006; 2009; Wong et al., 2011; 2012). During metastasis, bone marrow-derived cells (BMDCs) are recruited to metastatic sites, where they form cell clusters that precede tumor cell arrival and are subsequently colonized by metastatic cancer cells (Kaplan et al., 2005; Xing et al., 2011). Hypoxic breast cancer cells produce multiple members of the lysyl oxidase (LOX) family, including LOX, LOXL2, and LOXL4, in an HIF-1-dependent manner (Erler et al., 2009; Wong et al., 2011; 2012). LOX family members are enzymes secreted from primary breast cancer cells that catalyze collagen crosslinking at hydroxylated lysine residues. LOX was initially found to remodel ECM in the primary tumor. Recent studies revealed that LOX can remodel ECM at sites distant from the primary tumor. Primary breast cancer cells secrete LOX family members into the circulation, leading to collagen crosslinking in metastatic tissues, which facilitates the recruitment of BMDCs and, subsequently, breast cancer premetastatic niche formation (Erler et al., 2006; 2009; Wong et al., 2011; 2012). In addition, CD11b+/Ly6Cmed/Ly6G+ myeloid cells and CD3−/NK1.1+ natural killer cells are two BMDC populations that promote metastasis. Indeed, it has been found that recruitment of CD11b+/Ly6Cmed/mLy6G+ cells was enhanced by hypoxic breast cancer cells (Sceneay et al., 2012).
Interestingly, LOX family members, LOX, LOXL2, and LOXL4 have variable expression in human breast cancers and breast cancer cell lines (Wong et al., 2011). A clinical study revealed that LOX expression was associated with intra-tumoral oxygen tension and metastases of breast cancer in patients (Erler et al., 2006). Since LOX family members are extracellular secreted proteins that play critical roles in metastasis, a monoclonal antibody or small molecule inhibitor directed against LOX might be useful for breast cancer therapy.
4. HIF-1-regulated non-coding RNA in breast cancer metastasis
HIF-1 plays important roles in breast cancer metastasis by mediating hypoxia-induced expression of mRNA-encoding genes. HIF-1 also regulates the expression of non-coding RNAs, which are critical regulators of migration, invasion, and metastasis. Various types of non-coding RNAs have been implicated in cancer progression, such as microRNAs (miRNAs), lncRNAs, endogenous small interfering RNAs (end-siRNAs), PIWI-interacting RNAs (piRNAs), circular RNAs (circRNAs), and long intergenic non-coding RNAs (lincRNAs) (Ling et al., 2013). Non-coding RNAs play pivotal roles in migration and invasion of breast carcinoma cells, and provide new insights into the molecular mechanisms that determine metastatic propensity. In this section, we summarize the published research reporting HIF-1-regulated non-coding RNAs in the pathogenesis of breast cancer metastasis, with an emphasis on miRNAs and lncRNAs.
HIF-1 regulates the expression of many miRNAs in breast cancer cells. In MCF-7 breast cancer cells, 41 miRNAs were significantly up-regulated and 28 miRNAs were down-regulated by the hypoxic treatment (Camps et al., 2014). Expression of miR-210, for instance, which is induced by HIF-1 in most cancer cells, promotes tumor proliferation and invasion, and is associated with a poor clinical outcome in breast cancer (Volinia et al., 2012). miR-10b is activated by HIF-1/TWIST1 (twist-related protein 1) signaling, and overexpression of miR-10b initiates robust invasion and metastasis in otherwise non-metastatic breast cancers (Ma et al., 2007; 2010; Haque et al., 2011). miR-20a and miR-20b, which are co-expressed with vascular endothelial growth factor (VEGF)-A and HIF-1α, also play important roles in breast cancer metastasis (Li et al., 2012) (Table 1).
Table 1.
Examples of the hypoxia/HIF-1α regulated ncRNAs in cancer metastasis
| ncRNA | Type (genomic location) | Regulation by hypoxia | Effect on metastasis | Target gene or effector | Reference |
| miR-10b | MicroRNA (chr 2) | ↑ | ↑ | HOXD10 | Ma et al., 2007; Haque et al., 2011 |
| miR-20a | MicroRNA (chr 13) | ↓ | ↓ | VEGF-A | Li et al., 2012 |
| miR-20b | MicroRNA (chr X) | ↓ | ↓ | VEGF-A | Cascio et al., 2010; Li et al., 2012 |
| miR-210 | MicroRNA (chr 11) | ↑ | ↑ | BRCA1, FANCD, FANCF, PARP1, E-cadherin, Rb1 | Camps et al., 2008; Rothé et al., 2011; Volinia et al., 2012 |
| miR-24 | MicroRNA (chr X) | ↑ | ↑ | PTPN9, PTPRF, Sprouty2 (SPRY2) | Kulshreshtha et al., 2007; Du et al., 2013; Li et al., 2013; Camps et al., 2014 |
| miR-27a | MicroRNA (chr 19) | ↑ | ↑ | Sprouty2 (SPRY2) | Kulshreshtha et al., 2007; Camps et al., 2014; Li et al., 2013 |
| miR-372/373 | MicroRNA (chr 19) | ↑ | ↑ | RECK, NF-κB, and TGF-β pathways | Loayza-Puch et al., 2010; Keklikoglou et al., 2012 |
| miR-495 | MicroRNA (chr 14) | ↑ | ↑ | E-cadherin, REDD1 | Hwang-Verslues et al., 2011 |
| miR-675 | MicroRNA (chr 11) | ↑ | ↑ | RECK, NF-κB, and TGF-β pathways | Loayza-Puch et al., 2010; Keklikoglou et al., 2012 |
| Oncofetal H19 RNA | lncRNA (chr 11) | ↑ | ↑ | Slug, E-cadherin, angiogenin, FGF18 | Matouk et al., 2007; 2010; 2014 |
| linc-RoR | lncRNA (chr 18) | ↑ | ↑ | mi-205, ZEB2 | Takahashi et al., 2013; Hou et al., 2014 |
| UCA1 | lncRNA (chr 19) | ↑ | ↑ | p27 (Kip1) | Huang et al., 2014; Xue et al., 2014 |
chr: chromosome; lncRNA: long non-coding RNA; HOXD10: homeobox D10; VEGF-A: vascular endothelial growth factor A; BRCA1: breast cancer 1; FANCD: Fanconi anemia, complementation group D; FANCF: Fanconi anemia, complementation group F; PARP1: poly(ADP-ribose) polymerase 1; RB1: retinoblastoma 1; PTPN9: protein tyrosine phosphatase, non-receptor type 9; PTPRF: protein tyrosine phosphatase, receptor type F; RECK: reversion-inducing-cysteine-rich protein with Kazal motifs; NF-κB: nuclear factor of kappa light polypeptide gene enhancer in B cells; TGF-β: transforming growth factor β; REDD1: DNA-damage-inducible transcript 4; FGF18: fibroblast growth factor 18; ZEB2: zinc finger E-box binding homeobox 2; p27: p27 protein; UCA1: urothelial cancer associated 1
lncRNAs are novel molecules that participate in a broad range of biological processes and complex diseases, especially in cancer progression. For example, the oncofetal H19 lncRNA is concomitantly induced by both transforming growth factor beta (TGF-β) and hypoxia in a mouse breast cancer model, and regulates E-cadherin expression and stimulates tumor metastasis through a positive feedback loop between Slug and H19/miR-675 (Matouk et al., 2014). Several other lncRNAs, such as linc-RoR (regulator of reprogramming) and UCA1 (urothelial cancer associated 1), have been proved to be regulated by hypoxia/HIF-1α, but their detailed mechanistic functions remain elusive (Takahashi et al., 2013; Hou et al., 2014; Huang et al., 2014; Xue et al., 2014) (Table 1).
5. HIF-1 as a therapeutic target
Solid tumors, including breast cancers, contain a hypoxic microenvironment due to poor vascularization in the rapidly growing tumor tissues and fast proliferation of cancer cells. HIF-1 plays a pivotal role in the adaptation of cancer cells to hypoxia by activating transcription of target genes that regulate angiogenesis, cell proliferation and survival, glucose metabolism, pH regulation, migration, invasion, and metastasis (Kim et al., 2006; Fukuda et al., 2007; Zhang et al., 2007; Semenza, 2011; 2012). Increased HIF-1 expression shows strong correlations with poor prognostic outcomes and low survival rates of patients with breast cancers (Rose et al., 2003). Therefore, targeting the HIF pathway provides an attractive strategy to treat hypoxic tumors. The HIF-1 pathway is activated in the basal molecular subtype of breast cancer (Cancer Genome Atlas Network, 2012), suggesting that HIF-1 inhibitors may be particularly effective in the treatment of triple negative breast cancers.
The current HIF-1 inhibitors are classified by their mechanism of action and include those that affect HIF-1α protein levels, HIF-1 dimerization, HIF-1 DNA binding, or HIF-1 transactivation of target genes (Table 2). Inhibitors that decrease HIF-1α protein levels can be further divided into two groups, inhibitors of HIF-1α translation and inducers of HIF-1α proteasomal degradation. HIF-1 inhibitors, such as digoxin and acriflavine, showed convincing potential therapeutic effects by decreasing primary tumor growth, vascularization, invasion and metastasis in animal models of breast cancer (Lee et al., 2008; Zhang et al., 2008; 2012; Wong et al., 2011; 2012; Schito et al., 2012).
Table 2.
Inhibitors of HIF-1 pathway
| Mechanism of action | Inhibitor |
| Inhibition of HIF-1α mRNA | Aminoflavone, GL331 |
| Inhibition of HIF-1α protein synthesis | Topoisomerase inhibitors (such as rapamycin, temsirolimus, and everolimus); cardiac glycosides (such as digoxin and digtoxin), microtubule targeting agents (such as 2-methoxyestradiol, epothilone B, and taxotere), topoisomerase inhibitors (such as camptothecin, topotecan, and NSC 644221), oligonucleotides, AF, PX-478 |
| Inhibition of HIF-1α protein stabilization | HSP90 inhibitors (such as 17-AAG, 17-DMAG, and apigenin), HDAC inhibitors (such as FK228 and LAQ824), antioxidants (such as ascorbic acid), oligonucleotides, selenium compound (such as berberine and MSC), PX-12, YC-1 |
| Inhibition of HIF-1α:HIF-1β dimerization | Acriflavine |
| Inhibitions of HIF-1:DNA binding | Anthracyclines, echinomycin |
| Inhibition of HIF-1 transactivity | Bortezomib |
HSP90: heat-shock protein 90; 17-AAG: 17-allylamino-demethoxygeldanamycin; HDAC: histone deacetylase; MSC: methylselenocysteine; 17-DMAG: 17-dimethylaminoethylamino-17-demethoxygeldanamycin
The combination of HIF inhibitors with existing treatments may prove to be useful clinically. Currently, for breast cancer therapy, the selective estrogen receptor (ER) modulator tamoxifen is widely used. Combination chemotherapy using trastuzumab targeting human epidermal growth factor receptor 2 (HER2) and bevacizumab targeting VEGF is another widely used therapeutic strategy. However, triple negative breast cancers respond poorly to currently available therapies. Since the HIF-1 pathway is highly activated in triple negative breast cancers, HIF-1 inhibitors could be effective in treatment of such cancers. Clinical trials are warranted to determine if they may increase the survival of breast cancer patients alone or in combination with current therapeutic regimens.
6. Concluding remarks and future directions
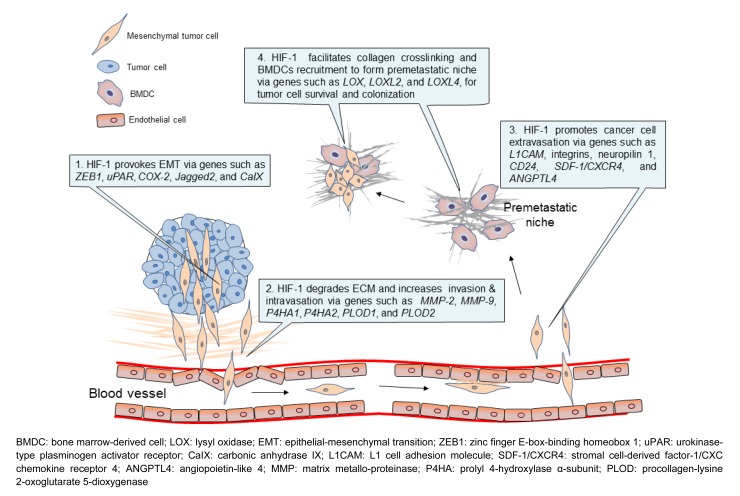
HIF-1, as a master regulator of cell response to hypoxic stress, plays important roles in breast cancer metastasis. As summarized in Fig. 2, HIF-1 is involved in almost every key step of the metastatic process, such as EMT, invasion, intravasation, extravasation, and metastatic niche formation, as discussed in detail in this review.
Fig. 2.
Cancer metastasis regulated by HIF-1
While most current studies have focused on HIF-1-regulated proteins, such as transcription factors, repressors and cytokines, recently, there has been growing appreciation of the role of ncRNAs, especially miRNAs and lncRNAs connected with HIF-1 controlled breast cancer metastasis. More comprehensive studies in this regard will shed light on the mechanisms underlying the regulation of hypoxia in breast cancer metastasis. A new concept has also been proposed that involves cancer stem cells in the connections between hypoxia and cancer metastasis (Hill et al., 2009). The metastasis-promoting effects of HIF-1 help to maintain an expanding/renewing population of cancer stem cells ready to be distributed much like seeds or pollen blowing in the wind (Philip et al., 2013). A few studies have supported this point of view by demonstrating that hypoxia/HIF-1 induces cancer stem-like cells by Jagged2 or mediates paracrine signaling between breast cancer cells and mesenchymal stem cells to promote metastasis (Xing et al., 2011; Chaturvedi et al., 2013). Further studies along these lines will be potentially valuable for dissecting the mechanisms of breast cancer metastasis.
Thus, while intensive mechanistic studies are under way, targeting HIF-1 has emerged as a new therapeutic approach for treating breast cancer patients. Novel strategies targeting HIF-1 are likely to be useful in combination with current therapeutic regimens. Future work is warranted to identify more selective HIF-1 inhibitors, to study their mechanism of action, and to incorporate them in clinical trials.
Footnotes
Project supported partially by the National Basic Research Program (973) of China (Nos. 2014CB910604 and 2012CB910104), the National Natural Science Foundation of China (Nos. 31171358 and 31371429), the Research Fund for the Doctoral Program of Higher Education of China (No. 20133402110020), the Fundamental Research Funds for the Central Universities in China, and the ‘1000 Youth Talent Program’ by the Chinese Government for Hua-feng ZHANG
Compliance with ethics guidelines: Zhao-ji LIU, Gregg L. SEMENZA, and Hua-feng ZHANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Bao B, Azmi AS, Ali S, et al. The biological kinship of hypoxia with CSC and EMT and their relationship with deregulated expression of miRNAs and tumor aggressiveness. BBA Rev Cancer. 2012;1826(2):272–296. doi: 10.1016/j.bbcan.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bocca C, Ievolella M, Autelli R, et al. Expression of Cox-2 in human breast cancer cells as a critical determinant of epithelial-to-mesenchymal transition and invasiveness. Exp Opin Therap Targets. 2014;18(2):121–135. doi: 10.1517/14728222.2014.860447. [DOI] [PubMed] [Google Scholar]
- 3.Bos R, van der Groep P, Greijer AE, et al. Levels of hypoxia-inducible factor-1α independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003;97(6):1573–1581. doi: 10.1002/cncr.11246. [DOI] [PubMed] [Google Scholar]
- 4.Brizel DM, Scully SP, Harrelson JM, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56(5):941–943. [PubMed] [Google Scholar]
- 5.Camps C, Buffa FM, Colella S, et al. hsa-miR-210 is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008;14(5):1340–1348. doi: 10.1158/1078-0432.CCR-07-1755. [DOI] [PubMed] [Google Scholar]
- 6.Camps C, Saini HK, Mole DR, et al. Integrated analysis of microRNA and mRNA expression and association with HIF binding reveals the complexity of microRNA expression regulation under hypoxia. Mol Cancer. 2014;13:28. doi: 10.1186/1476-4598-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Network Cancer Genome Atlas. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cascio S, D'Andrea A, Ferla R, et al. miR-20b modulates VEGF expression by targeting HIF-1α and STAT3 in MCF-7 breast cancer cells. J Cell Physiol. 2010;224(1):242–249. doi: 10.1002/jcp.22126. [DOI] [PubMed] [Google Scholar]
- 9.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 10.Chaturvedi P, Gilkes DM, Wong CC, et al. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Invest. 2013;123(1):189–205. doi: 10.1172/JCI64993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conklin MW, Eickhoff JC, Riching KM, et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011;178(3):1221–1232. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creighton CJ, Chang JC, Rosen JM. Epithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J Mammary Gland Biol Neopl. 2010;15(2):253–260. doi: 10.1007/s10911-010-9173-1. [DOI] [PubMed] [Google Scholar]
- 13.Davis FM, Azimi I, Faville RA, et al. Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene. 2014;33(18):2307–2316. doi: 10.1038/onc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du WW, Fang L, Li M, et al. MicroRNA miR-24 enhances tumor invasion and metastasis by targeting PTPN9 and PTPRF to promote EGF signaling. J Cell Sci. 2013;126(6):1440–1453. doi: 10.1242/jcs.118299. [DOI] [PubMed] [Google Scholar]
- 15.Duffy MJ, Maguire TM, Hill A, et al. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000;2(4):252–257. doi: 10.1186/bcr65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erler JT, Bennewith KL, Nicolau M, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440(7088):1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 17.Erler JT, Bennewith KL, Cox TR, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15(1):35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldser D, Agani F, Iyer NV, et al. Reciprocal positive regulation of hypoxia-inducible factor 1α and insulin-like growth factor 2. Cancer Res. 1999;59(16):3915–3918. [PubMed] [Google Scholar]
- 19.Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1970;45(4):773–782. [PubMed] [Google Scholar]
- 20.Fukuda N, Nakayama M, Jian T, et al. Leukocyte angiotensin II levels in patients with essential hypertension: relation to insulin resistance. Am J Hypertens. 2003;16(2):129–134. doi: 10.1016/S0895-7061(02)03145-X. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda R, Hirota K, Fan F, et al. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 2002;277(41):38205–38211. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda R, Zhang HF, Kim JW, et al. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129(1):111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 23.Gao P, Zhang HF, Dinavahi R, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo . Cancer Cell. 2007;12(3):230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Generali D, Berruti A, Brizzi MP, et al. Hypoxia-inducible factor-1α-expression predicts a poor response to primary chemoendocrine therapy and disease-free survival in primary human breast cancer. Clin Cancer Res. 2006;12(15):4562–4568. doi: 10.1158/1078-0432.CCR-05-2690. [DOI] [PubMed] [Google Scholar]
- 25.Gilkes DM, Bajpai S, Chaturvedi P, et al. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J Biol Chem. 2013;288(15):10819–10829. doi: 10.1074/jbc.M112.442939. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Gilkes DM, Bajpai S, Wong CC, et al. Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Mol Cancer Res. 2013;11(5):456–466. doi: 10.1158/1541-7786.MCR-12-0629. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Gilkes DM, Chaturvedi P, Bajpai S, et al. Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer Res. 2013;73(11):3285–3296. doi: 10.1158/0008-5472.CAN-12-3963. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Grimshaw MJ. Endothelins and hypoxia-inducible factor in cancer. Endocr Rel Cancer. 2007;14(2):233–244. doi: 10.1677/ERC-07-0057. [DOI] [PubMed] [Google Scholar]
- 29.Gruber G, Greiner RH, Hlushchuk R, et al. Hypoxia-inducible factor 1α in high-risk breast cancer: an independent prognostic parameter? Breast Cancer Res. 2004;6(3):R191–R198. doi: 10.1186/bcr775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haque I, Banerjee S, Mehta S, et al. Cysteine-rich 61-connective tissue growth factor-nephroblastoma-overexpressed 5 (CCN5)/Wnt-1-induced signaling protein-2 (WISP-2) regulates microRNA-10b via hypoxia-inducible factor-1α-TWIST signaling networks in human breast cancer cells. J Biol Chem. 2011;286(50):43475–43485. doi: 10.1074/jbc.M111.284158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill RP, Marie-Egyptienne DT, Hedley DW. Cancer stem cells, hypoxia and metastasis. Semin Radiat Oncol. 2009;19(2):106–111. doi: 10.1016/j.semradonc.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Hohenberger P, Felgner C, Haensch W, et al. Tumor oxygenation correlates with molecular growth determinants in breast cancer. Breast Cancer Res Treat. 1998;48(2):97–106. doi: 10.1023/A:1005921513083. [DOI] [PubMed] [Google Scholar]
- 33.Horrée N, van Diest PJ, Daisy MDSG, et al. The invasive front in endometrial carcinoma: higher proliferation and associated derailment of cell cycle regulators. Human Pathol. 2007;38(8):1232–1238. doi: 10.1016/j.humpath.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Hou P, Zhao Y, Li Z, et al. lincRNA-RoR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death Dis. 2014;5(6):e1287. doi: 10.1038/cddis.2014.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu M, Polyak K. Microenvironmental regulation of cancer development. Curr Opin Genet Dev. 2008;18(1):27–34. doi: 10.1016/j.gde.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang J, Zhou N, Watabe K, et al. Long non-coding RNA UCA1 promotes breast tumor growth by suppression of p27 (Kip1) Cell Death Dis. 2014;5(1):e1008. doi: 10.1038/cddis.2013.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang RL, Teo ZQ, Chong HC, et al. ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE-cadherin and claudin-5 clusters. Blood. 2011;118(14):3990–4002. doi: 10.1182/blood-2011-01-328716. [DOI] [PubMed] [Google Scholar]
- 38.Hugo HJ, Pereira L, Suryadinata R, et al. Direct repression of MYB by ZEB1 suppresses proliferation and epithelial gene expression during epithelial-to-mesenchymal transition of breast cancer cells. Breast Cancer Res. 2013;15(6):R113. doi: 10.1186/bcr3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hwang-Verslues WW, Chang PH, Wei PC, et al. miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via downregulation of E-cadherin and REDD1. Oncogene. 2011;30(21):2463–2474. doi: 10.1038/onc.2010.618. [DOI] [PubMed] [Google Scholar]
- 40.Incorvaia L, Badalamenti G, Rini G, et al. MMP-2, MMP-9 and activin A blood levels in patients with breast cancer or prostate cancer metastatic to the bone. Anticancer Res. 2007;27(3B):1519–1525. [PubMed] [Google Scholar]
- 41.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 42.Jin FY, Brockmeier U, Otterbach F, et al. New insight into the SDF-1/CXCR4 axis in a breast carcinoma model: hypoxia-induced endothelial SDF-1 and tumor cell CXCR4 are required for tumor cell intravasation. Mol Cancer Res. 2012;10(8):1021–1031. doi: 10.1158/1541-7786.MCR-11-0498. [DOI] [PubMed] [Google Scholar]
- 43.Jo M, Lester RD, Montel V, et al. Reversibility of epithelial-mesenchymal transition (EMT) induced in breast cancer cells by activation of urokinase receptor-dependent cell signaling. J Biol Chem. 2009;284(34):22825–22833. doi: 10.1074/jbc.M109.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaplan HG, Malmgren JA, Atwood M. Tumor size, age and stage in patient detected breast cancer. J Clin Oncol. 2005;23(16):716. [Google Scholar]
- 46.Kasuno K, Takabuchi S, Fukuda K, et al. Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling. J Biol Chem. 2004;279(4):2550–2558. doi: 10.1074/jbc.M308197200. [DOI] [PubMed] [Google Scholar]
- 47.Keklikoglou I, Koerner C, Schmidt C, et al. MicroRNA-520/373 family functions as a tumor suppressor in estrogen receptor negative breast cancer by targeting NF-κB and TGF-β signaling pathways. Oncogene. 2012;31(37):4150–4163. doi: 10.1038/onc.2011.571. [DOI] [PubMed] [Google Scholar]
- 48.Kim JW, Tchernyshyov I, Semenza GL, et al. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3(3):177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Krieg M, Haas R, Brauch H, et al. Up-regulation of hypoxia-inducible factors HIF-1α and HIF-2α under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene. 2000;19(48):5435–5443. doi: 10.1038/sj.onc.1203938. [DOI] [PubMed] [Google Scholar]
- 50.Krishnamachary B, Zagzag D, Nagasawa H, et al. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;66(5):2725–2731. doi: 10.1158/0008-5472.CAN-05-3719. [DOI] [PubMed] [Google Scholar]
- 51.Kulshreshtha R, Ferracin M, Wojcik SE, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27(5):1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lando D, Peet DJ, Whelan DA, et al. Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science. 2002;295(5556):858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 53.Laughner E, Taghavi P, Chiles K, et al. Her2 (neu) signaling increases the rate of hypoxia-inducible factor 1 alpha (HIF-1α) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21(12):3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lech R, Przemyslaw O. Epidemiological models for breast cancer risk estimation. Ginekol Pol. 2011;82(6):451–454. [PubMed] [Google Scholar]
- 55.Lee HS, Seo EY, Kang NE, et al. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J Nutr Biochem. 2008;19(5):313–319. doi: 10.1016/j.jnutbio.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 56.Li JY, Zhang Y, Zhang WH, et al. Differential distribution of miR-20a and miR-20b may underly metastatic heterogeneity of breast cancers. Asian Pac J Cancer Prev. 2012;13(5):1901–1906. doi: 10.7314/APJCP.2012.13.5.1901. [DOI] [PubMed] [Google Scholar]
- 57.Li X, Liu X, Xu W, et al. c-MYC-regulated miR-23a/24-2/27a cluster promotes mammary carcinoma cell invasion and hepatic metastasis by targeting Sprouty2 . J Biol Chem. 2013;288(25):18121–18133. doi: 10.1074/jbc.M113.478560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Disc. 2013;12(11):847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu XH, Kirschenbaum A, Lu M, et al. Prostaglandin E2 induces hypoxia-inducible factor-1α stabilization and nuclear localization in a human prostate cancer cell line. J Biol Chem. 2002;277(51):50081–50086. doi: 10.1074/jbc.M201095200. [DOI] [PubMed] [Google Scholar]
- 60.Loayza-Puch F, Yoshida Y, Matsuzaki T, et al. Hypoxia and RAS-signaling pathways converge on, and cooperatively downregulate, the RECK tumor-suppressor protein through microRNAs. Oncogene. 2010;29(18):2638–2648. doi: 10.1038/onc.2010.23. [DOI] [PubMed] [Google Scholar]
- 61.Lock FE, Mcdonald PC, Lou Y, et al. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene. 2013;32(44):5210–5219. doi: 10.1038/onc.2012.550. [DOI] [PubMed] [Google Scholar]
- 62.Lundgren K, Holm C, Landberg G. Hypoxia and breast cancer: prognostic and therapeutic implications. Cell Mol Life Sci. 2007;64(24):3233–3247. doi: 10.1007/s00018-007-7390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 64.Ma L, Reinhardt F, Pan E, et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010;28(4):341–347. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maglione E, Ferreira LS, Cattapan G. Asymptotic properties of bound states in coupled quantum wave guides. J Phys A Math General. 2006;39(5):1207–1228. doi: 10.1088/0305-4470/39/5/013. [DOI] [Google Scholar]
- 66.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Devel. 2001;15(20):2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matouk IJ, Degroot N, Mezan S, et al. The H19 non-coding RNA is essential for human tumor growth. PLoS ONE. 2007;2(9):e845. doi: 10.1371/journal.pone.0000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matouk IJ, Mezan S, Mizrahi A, et al. The oncofetal H19 RNA connection: hypoxia, p53 and cancer. BBA Mol Cell Res. 2010;1803(4):443–451. doi: 10.1016/j.bbamcr.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 69.Matouk IJ, Raveh E, Abu-Lail R, et al. Oncofetal H19 RNA promotes tumor metastasis. BBA Mol Cell Res. 2014;1843(7):1414–1426. doi: 10.1016/j.bbamcr.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 70.May CD, Sphyris N, Evans KW, et al. Epithelial-mesenchymal transition and cancer stem cells: a dangerously dynamic duo in breast cancer progression. Breast Cancer Res. 2011;13(1):202. doi: 10.1186/bcr2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mees G, Dierckx R, Vangestel C, et al. Molecular imaging of hypoxia with radiolabelled agents. Eur J Nucl Med Mol Imag. 2009;36(10):1674–1686. doi: 10.1007/s00259-009-1195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27(55):6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 73.Munoz-Najar UM, Neurath KM, Vumbaca F, et al. Hypoxia stimulates breast carcinoma cell invasion through MT1-MMP and MMP-2 activation. Oncogene. 2006;25(16):2379–2392. doi: 10.1038/sj.onc.1209273. [DOI] [PubMed] [Google Scholar]
- 74.Padua D, Zhang XHF, Wang QQ, et al. TGF-β primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133(1):66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Philip B, Ito K, Moreno-Sanchez R, et al. HIF expression and the role of hypoxic microenvironments within primary tumours as protective sites driving cancer stem cell renewal and metastatic progression. Carcinogenesis. 2013;34(8):1699–1707. doi: 10.1093/carcin/bgt209. [DOI] [PubMed] [Google Scholar]
- 76.Pritchard SC, Nicolson MC, Lloret C, et al. Expression of matrix metalloproteinases 1, 2, 9 and their tissue inhibitors in stage II non-small cell lung cancer: implications for MMP inhibition therapy. Oncol Rep. 2001;8(2):421–424. [PubMed] [Google Scholar]
- 77.Provenzano PP, Eliceiri KW, Campbell JM, et al. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4(1):38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quintero M, Brennan PA, Thomas GJ, et al. Nitric oxide is a factor in the stabilization of hypoxia-inducible factor-1α in cancer: role of free radical formation. Cancer Res. 2006;66(2):770–774. doi: 10.1158/0008-5472.CAN-05-0333. [DOI] [PubMed] [Google Scholar]
- 79.Ravi D, Ramadas K, Mathew BS, et al. Apoptosis, angiogenesis and proliferation: trifunctional measure of tumour response to radiotherapy for oral cancer. Oral Oncol. 2001;37(2):164–171. doi: 10.1016/S1368-8375(00)00082-8. [DOI] [PubMed] [Google Scholar]
- 80.Rose C, Vtoraya O, Pluzanska A, et al. An open randomised trial of second-line endocrine therapy in advanced breast cancer. Comparison of the aromatase inhibitors letrozole and anastrozole. Eur J Cancer. 2003;39(16):2318–2327. doi: 10.1016/S0959-8049(03)00630-0. [DOI] [PubMed] [Google Scholar]
- 81.Rothé F, Ignatiadis M, Chaboteaux C, et al. Global microRNA expression profiling identifies miR-210 associated with tumor proliferation, invasion and poor clinical outcome in breast cancer. PLoS ONE. 2011;6(6):e20980. doi: 10.1371/journal.pone.0020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sceneay J, Chow MT, Chen A, et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72(16):3906–3911. doi: 10.1158/0008-5472.CAN-11-3873. [DOI] [PubMed] [Google Scholar]
- 83.Schito L, Rey S, Tafani M, et al. Hypoxia-inducible factor 1-dependent expression of platelet-derived growth factor b promotes lymphatic metastasis of hypoxic breast cancer cells. PNAS. 2012;109(40):E2707–E2716. doi: 10.1073/pnas.1214019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 85.Semenza GL. Oxygen sensing, homeostasis, and disease reply. N Engl J Med. 2011;365(19):537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 86.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33(4):207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takahashi K, Yan IK, Haga H, et al. The hypoxia-induced long non-coding RNA linc-RoR modulates tumor cell resistance to hypoxia by an extra-cellular vesicle mediated regulation of hypoxia-signaling pathways in hepatocellular cancer. Hepatology. 2013;58:1067A. [Google Scholar]
- 89.Talks KL, Turley H, Gatter KC, et al. The expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157(2):411–421. doi: 10.1016/S0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 91.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vaupel P, Schlenger K, Knoop C, et al. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991;51(12):3316–3322. [PubMed] [Google Scholar]
- 93.Vaupel P, Mayer A, Hockel M. Tumor hypoxia and malignant progression. Meth Enzymol. 2004;381:335–354. doi: 10.1016/S0076-6879(04)81023-1. [DOI] [PubMed] [Google Scholar]
- 94.Vaupel P, Hockel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal. 2007;9(8):1221–1235. doi: 10.1089/ars.2007.1628. [DOI] [PubMed] [Google Scholar]
- 95.Volinia S, Galasso M, Sana ME, et al. Breast cancer signatures for invasiveness and prognosis defined by deep sequencing of microRNA. PNAS. 2012;109(8):3024–3029. doi: 10.1073/pnas.1200010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang GL, Jiang BH, Rue EA, et al. Hypoxia-inducible factor-1 is a basic-helix-loop-helix-pas heterodimer regulated by cellular O2 tension. PNAS. 1995;92(12):5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5(8):591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 98.Wong CCL, Gilkes DM, Zhang HF, et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. PNAS. 2011;108(39):16369–16374. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wong CCL, Zhang H, Gilkes DM, et al. Inhibitors of hypoxia-inducible factor 1 block breast cancer metastatic niche formation and lung metastasis. J Mol Med. 2012;90(7):803–815. doi: 10.1007/s00109-011-0855-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xing F, Okuda H, Watabe M, et al. Hypoxia-induced Jagged2 promotes breast cancer metastasis and self-renewal of cancer stem-like cells. Oncogene. 2011;30(39):4075–4086. doi: 10.1038/onc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xue M, Li X, Li Z, et al. Urothelial carcinoma associated 1 is a hypoxia-inducible factor-1α-targeted long noncoding RNA that enhances hypoxic bladder cancer cell proliferation, migration, and invasion. Tumour Biol. 2014;35(7):6901–6912. doi: 10.1007/s13277-014-1925-x. [DOI] [PubMed] [Google Scholar]
- 102.Zhang H, Gao P, Fukuda R, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11(5):407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 103.Zhang H, Qian DZ, Tan YS, et al. Digoxin and other cardiac glycosides inhibit HIF-1α synthesis and block tumor growth. PNAS. 2008;105(50):19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang H, Wong CCL, Wei H, et al. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2012;31(14):1757–1770. doi: 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Zhong H, de Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res. 1999;59(22):5830–5835. [PubMed] [Google Scholar]
- 106.Zhong H, Chiles K, Feldser D, et al. Modulation of hypoxia-inducible factor 1α expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60(6):1541–1545. [PubMed] [Google Scholar]
- 107.Zundel W, Schindler C, Haas-Kogan D, et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Gene Devel. 2000;14(4):391–396. [PMC free article] [PubMed] [Google Scholar]