Abstract
Background and aims: Ingestion of paraquat (PQ), a widely used herbicide, can cause severe toxicity in humans, leading to a poor survival rate and prognosis. One of the main causes of death by PQ is PQ-induced pulmonary fibrosis, for which there are no effective therapies. The aim of this study was to evaluate the effects of rapamycin (RAPA) on inhibiting PQ-induced pulmonary fibrosis in mice and to explore its possible mechanisms. Methods: Male C57BL/6J mice were exposed to either saline (control group) or PQ (10 mg/kg body weight, intraperitoneally; test group). The test group was divided into four subgroups: a PQ group (PQ-exposed, non-treated), a PQ+RAPA group (PQ-exposed, treated with RAPA at 1 mg/kg intragastrically), a PQ+MP group (PQ-exposed, treated with methylprednisolone (MP) at 30 mg/kg intraperitoneally), and a PQ+MP+RAPA group (PQ-exposed, treated with MP at 30 mg/kg intraperitoneally and with RAPA at 1 mg/kg intragastrically). The survival rate and body weight of all the mice were recorded every day. Three mice in each group were sacrificed at 14 d and the rest at 28 d after intoxication. Lung tissues were excised and stained with hematoxylin-eosin (H&E) and Masson’s trichrome stain for histopathological analysis. The hydroxyproline (HYP) content in lung tissues was detected using an enzyme-linked immunosorbent assay (ELISA) kit. The expression of transforming growth factor-β1 (TGF-β1) and α-smooth muscle actin (α-SMA) in lung tissues was detected by immunohistochemical staining and Western blotting. Results: A mice model of PQ-induced pulmonary fibrosis was established. Histological examination of lung tissues showed that RAPA treatment moderated the pathological changes of pulmonary fibrosis, including alveolar collapse and interstitial collagen deposition. HYP content in lung tissues increased soon after PQ intoxication but had decreased significantly by the 28th day after RAPA treatment. Immunohistochemical staining and Western blotting showed that RAPA treatment significantly down-regulated the enhanced levels of TGF-β1 and α-SMA in lung tissues caused by PQ exposure. However, RAPA treatment alone could not significantly ameliorate the lower survival rate and weight loss of treated mice. MP treatment enhanced the survival rate, but had no significant effects on attenuating PQ-induced pulmonary fibrosis or reducing the expression of TGF-β1 and α-SMA. Conclusions: This study demonstrates that RAPA treatment effectively suppresses PQ-induced alveolar collapse and collagen deposition in lung tissues through reducing the expression of TGF-β1 and α-SMA. Thus, RAPA has potential value in the treatment of PQ-induced pulmonary fibrosis.
Keywords: Paraquat, Pulmonary fibrosis, Rapamycin, Transforming growth factor-β1, α-Smooth muscle actin, Methylprednisolone
1. Introduction
Paraquat (N,N-dimethyl-4,4'-bipyridinium dichloride; PQ) is one of the most widely used herbicides in developing countries. However, it is also a highly toxic compound for both humans and animals, which accounts for a high mortality rate each year resulting from accidental or voluntary ingestion (Vale et al., 1987; Baltazar et al., 2013). Regular care of PQ-poisoned patients, including gastric lavage and haemoperfusion, does not reduce their mortality rate. This is mainly because PQ selectively accumulates in the lungs, leading to irreversible pulmonary fibrosis. Previous studies have revealed that a combination of glucocorticoid and cyclophosphamide can improve the survival rate of PQ-poisoned patients or animal models, and reduce the pathological changes in the lung tissues, such as inflammatory cell infiltration, in the early stages of acute lung injury (ALI) (Rocco et al., 2003; Li et al., 2014), but this therapy does not have a significant effect in preventing progressive pulmonary fibrosis. Some other potential agents, such as docosahexaenoic acid (DHA) (Chen J. et al., 2013) and naringin (Chen Y. et al., 2013; Blanco-Ayala et al., 2014), have been shown to ameliorate PQ-induced pulmonary fibrosis in animal models. However, these new therapies take a relatively long time to achieve a noticeable antifibrotic effect. Therefore, more effective therapies are urgently required.
Rapamycin (RAPA), also known as sirolimus, is a potent inhibitor of the mammalian target of rapamycin (mTOR), which has been increasingly used to prevent graft rejection in solid organ transplantation because of its immunosuppressive effects. RAPA is also attractive in clinical practice due to its potent antifibrotic property. The mTOR protein is a serine-threonine kinase known to be central to a complex intracellular signaling pathway and involved in the processes of cell growth and proliferation. Downstream of mTOR are two main effectors, p70S6 kinase and the translation inhibitor 4E binding protein 1, both of which control the translation of specific mRNAs and the synthesis of particular proteins. RAPA binds intracellularly to the FK506 (tacrolimus)-binding protein to form a complex, which subsequently binds to mTOR to inhibit its ability to signal adequately to its downstream effectors (Hartford and Ratain, 2007). It has been reported that in animal models RAPA can attenuate progressive chronic kidney diseases, such as tubulointerstitial fibrosis, diabetic nephropathy and compensatory renal hypertrophy (Bonegio et al., 2005; Chen J.K. et al., 2005; Lloberas et al., 2006). In rat models of liver fibrosis, low-dose RAPA treatment can reduce collagen deposition (Neef et al., 2006). More recently, several studies have revealed that RAPA can prevent the progression of bleomycin-or transforming growth factor-α (TGF-α)-induced pulmonary fibrosis (Korfhagen et al., 2009; Madala et al., 2011). As a result, we hypothesized that RAPA treatment may be an effective therapy to ameliorate PQ-induced pulmonary fibrosis.
Pulmonary fibrosis is characterized by fibroblast proliferation, myofibroblast differentiation, and deposition of extra-cellular matrix (ECM) in the lung parenchyma (Tulek et al., 2011). TGF-β1 is a key fibrogenic cytokine, which plays a critical role in the expression of α-smooth muscle actin (α-SMA) and induction of lung fibrosis (Oka et al., 2013). α-SMA is the hallmark of myofibroblasts, which are generally considered to be the key effector cells in the development of fibrosis (Wang et al., 2012). The aim of this study was to evaluate RAPA treatment as a potential novel therapy for attenuating PQ-induced pulmonary fibrosis by assessing its effects on the survival rate, body weight, histology, and hydroxyproline (HYP) content of PQ-treated mice. The possible mechanisms of RAPA treatment in this model were also studied.
2. Materials and methods
2.1. Animals
This study was performed on healthy male C57BL/6J mice, 6 to 8 weeks old, weighing between 20 and 22 g, and kept in the animal center of Zhejiang University, China. The animals were housed in a ventilated room at (22±2) °C with a 12-h dark/12-h light cycle and provided with free access to fresh water and standard rodent chow.
2.2. Experimental design
The mice were assigned to either a control group or test groups, each group containing six mice. All the animals were weighed before treatment and then daily throughout the experiment.
In the control group, saline (10 ml/kg body weight) was administered intraperitoneally and then dimethyl sulfoxide (DMSO; 10 ml/kg body weight) was given intragastrically daily. Mice in the test groups received PQ (dissolved in saline) at 10 mg/kg body weight intraperitoneally on Day 0. The mice in the test groups were divided according to the treatment received during the experiment as follows: (1) PQ group: mice in this group were treated daily with DMSO intragastrically 1 h after PQ administration; (2) PQ+RAPA group: mice in this group were treated daily with RAPA (1 mg/kg, intragastrically) dissolved in DMSO 1 h after PQ administration; (3) PQ+MP group: mice in this group were treated with a single dose injection of MP (30 mg/kg, intraperitoneally) 1 h after PQ administration and then daily with DMSO intragastrically; (4) PQ+MP+RAPA group: mice in this group were treated with a single dose injection of MP (30 mg/kg, intraperitoneally) 1 h after PQ administration and then daily with RAPA (1 mg/kg, intragastrically). Three mice in each group were sacrificed at 14 d and the rest at 28 d after PQ exposure.
2.3. Lung preparation
The animals were anesthetized with pentobarbital sodium (10 mg/kg body weight, intraperitoneally) and killed by exsanguination via the arteria angularis. After perfusion of the lungs with 10 ml of normal saline via the right ventricle, individual lung lobes were removed en bloc for further experimentation.
2.4. Morphological analysis
The left lower lung lobes were inflation-fixed overnight with 10% neutral buffered formalin. After fixation, lung tissues were embedded in paraffin and cut into 2-μm sections on a cryostat-microtome (Leica Microsystems Nussloch GmbH, RM2245). To visualize the overall tissue architecture and collagen deposition, representative sections were stained with hematoxylin-eosin (H&E) and Masson’s trichrome stain. Stained sections were viewed using a microscope (Leica Microsystems Wetzlar GmbH, DFC490) at a magnification of 400×, and the images were analyzed using LAS AF Lite V3.6 software.
2.5. HYP content assay
Lung tissues (30 mg) from each mouse were rinsed with 1× phosphate buffer saline (PBS), and then homogenized in 0.3 ml radioimmunoprecipitation assay buffer supplemented with protease and phosphatase inhibitors (Beyotime Institute of Biotechnology, st506-1) at 4 °C. The homogenate was centrifuged for 45 min at 15 000 r/min at 4 °C. The supernatant was collected immediately. The HYP content of the supernatant was then determined using an HYP enzyme-linked immunosorbent assay (ELISA) kit (MyBioSource, MBS703512) according to the manufacturer’s instructions.
2.6. Immunohistochemical staining of lung sections
Paraffin sections with 2-μm thickness were dewaxed in xylene and rehydrated in grade alcohol, and then subjected to heat-mediated antigen retrieval in a pressure cooker. To block endogenous peroxidase activity, sections were immersed in 0.3% H2O2 for 30 min, followed by a serum block with 5% non-fat dry milk for 30 min. The sections were then incubated with primary monoclonal mouse anti-TGF-β1 antibody (1:40 (v/v), R&D, MAB240) at 4 °C overnight and anti-α-SMA antibody (1:100 (v/v), Abcam, ab7817) at 37 °C for 1 h. After washing three times with PBS (pH 7.4) for 5 min each, the sections were incubated with horseradish peroxidase (HRP)-conjugated secondary anti-mouse antibody (Gene Tech, GK500705) for 30 min at room temperature and then developed by diaminobenzidine (DAB). The sections were restained with hematoxylin for 1 min and then dehydrated, cleared, and mounted. Stained sections were photographed using a Leica microscope (Leica Microsystems Wetzlar GmbH, DFC490), and the images were analyzed using LAS AF Lite V3.6 software.
2.7. Western blots
Lung tissues were minced and homogenized in 1 ml radioimmunoprecipitation assay buffer supplemented with protease and phosphatase inhibitors (Beyotime Institute of Biotechnology, st506-1) at 4 °C. Supernatants were collected after 45 min of centrifugation at 15 000 r/min. Protein concentrations were measured by BCA protein assay (Thermo Scientific, MK166714). Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was used to separate denatured proteins (100 °C, 5 min; 30 μg per lane). Proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad, 162-0177) by electroblotting (Bio-Rad, Power Pac Basic). Blots were blocked at room temperature for 2 h with Tris-buffered saline and Tween 20 (TBST) containing 5% (0.05 g/ml) fat-free milk powder, and were subsequently incubated overnight at 4 °C with primary monoclonal mouse anti-α-SMA antibody (1:500 (v/v), Abcam, ab7817) and anti-TGF-β1 antibody (1:1000 (v/v), R&D, MAB240). Monoclonal mouse anti-β-actin antibody at 1:2000 (v/v) dilution (Dawen Biotech, DW9562) was also added as an internal control. Blots were washed three times with TBST for 10 min each and then incubated for 30 min at room temperature with HRP-conjugated secondary anti-mouse antibodies (1:2000 (v/v), Abbkine, A21010-1). After rinsing, signals were detected using electrochemiluminescence (ECL; Biological Industries, 1236507) and scanned with a ChemiDoc MP System (Bio-Rad). The intensity of protein bands was analyzed using Image Lab 4.1 software (Bio-Rad).
2.8. Statistical analysis
Data are presented as mean±standard deviation (SD). All analyses were performed using GraphPad Prism 5.0 software (GraphPad Inc., San Diego, CA, USA). Statistical comparisons were performed using one-way analysis of variance (ANOVA). The significance of survival curves was estimated by the Kaplan-Meier method with a log-rank test. P value of <0.05 was considered statistically significant for all experiments.
3. Results
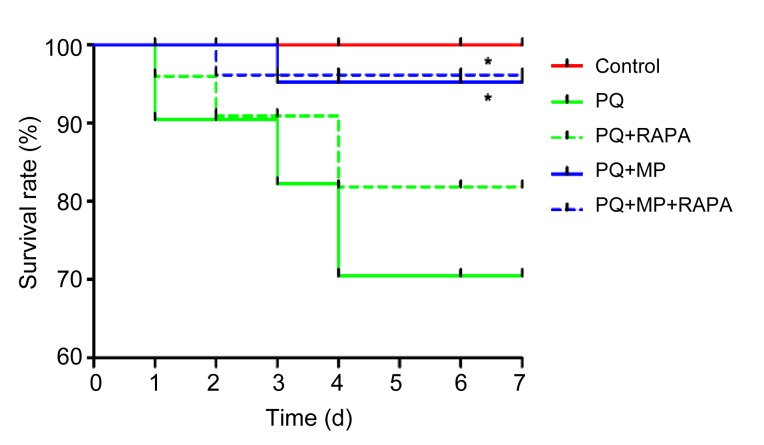
3.1. Survival rate of mice
At 7 d after PQ intoxication, MP treatment had significantly increased the survival rate, with 95% survival in the PQ+MP group (P<0.05) and 96% in the PQ+MP+RAPA group (P<0.05) compared with 70% in the PQ group. RAPA treatment improved the survival rate to 82% in the PQ+RAPA group compared with 70% in the PQ group, and to 96% in the PQ+MP+RAPA group compared with 95% in the PQ+MP group, but the improvements were not statistically significant (Fig. 1).
Fig. 1.
Survival rate analysis
MP treatment significantly increased the survival rate (n=6;* P<0.05, compared with PQ group). The survival rate was recorded up to 7 d after RAPA or DMSO treatment. The survival rates were estimated by the Kaplan-Meier method and compared by a log-rank test
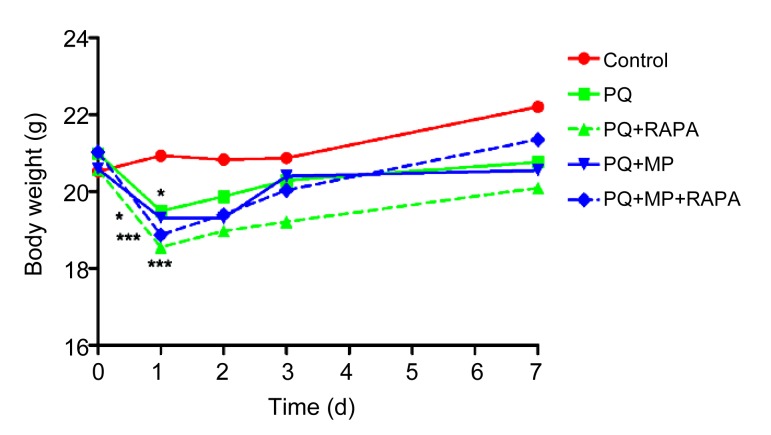
3.2. Body weight changes
There was a remarkable loss of weight 1 d after PQ intoxication (PQ group, PQ+MP group vs. control, P<0.05; PQ+RAPA group, PQ+MP+RAPA group vs. control, P<0.001; Fig. 2). Instead of attenuating the body weight loss, RAPA treatment was also associated with weight loss, but the reduction was not statistically significant. By the 7th day after PQ administration, the body weight of mice in all the test groups had recovered to baseline.
Fig. 2.
Body weight changes
PQ intoxication caused a marked weight loss after one day (* P<0.05, *** P<0.001, compared with control). There was also a body weight reduction after RAPA treatment, but it was not statistically significant
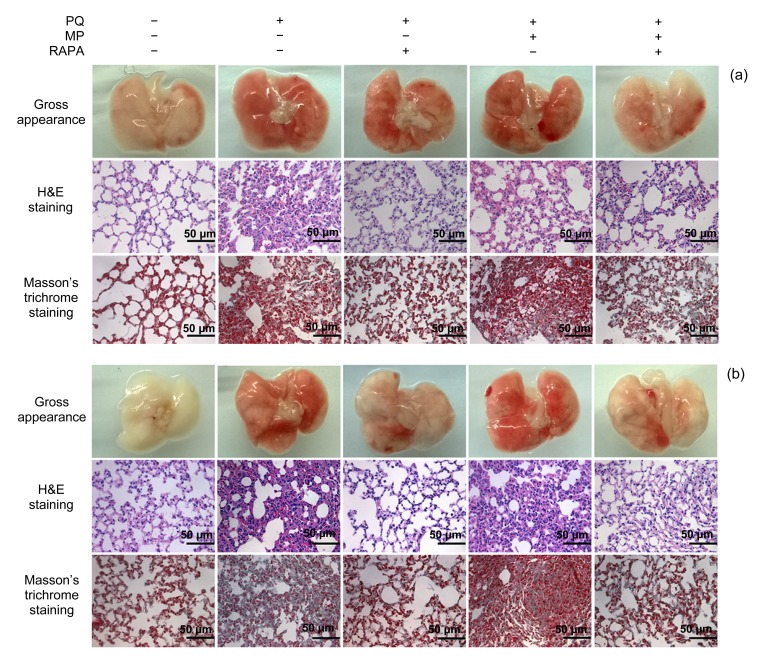
3.3. Morphopathological changes in lung tissues
Typical morphopathological changes in the lung tissues from the mice 14 and 28 d after pulmonary fibrosis induction are shown in Fig. 3. Compared with the lung tissues of the mice in the PQ and PQ+MP groups, those of the PQ+RAPA and PQ+MP+RAPA groups presented with significantly less pulmonary fibrosis. Gross examination of lungs from the PQ and PQ+MP groups 14 d after intoxication revealed dark red coloration with evident hemorrhagic foci on the surface, whereas lungs from RAPA-treated mice appeared nearly normal compared with those of mice in the control group (Fig. 3a). H&E staining revealed diffuse alveolar collapse and thickening in lung tissue sections of the PQ and PQ+MP groups. These changes were ameliorated by RAPA treatment. Masson’s trichrome staining also showed diffuse perialveolar, peribronchial, and interstitial fibrosis in lung tissue sections of the PQ and PQ+MP groups. In lung tissue sections of the PQ+RAPA and PQ+MP+RAPA groups, however, there was mild collagen deposition. Effects of MP treatment (PQ+MP and PQ+MP+RAPA groups) against pulmonary fibrosis were not evident when the MP groups were compared with the PQ group and the PQ+RAPA group (Fig. 3a). The PQ-induced pathological changes in gross appearance, H&E staining, and Masson’s trichrome staining in lung tissues were more markedly blockaded after RAPA treatment after 28 d (Fig. 3b).
Fig. 3.
Morphopathological changes in lung tissues
Mice were treated with a single dose injection of MP 1 h after PQ administration and then DMSO or RAPA once daily for 14 d (a) or 28 d (b). The gross appearance, H&E staining, and Masson’s trichrome staining of lung tissue sections showed less focal hemorrhage, alveolar collapse and thickening, and interstitial collagen deposition, indicating that RAPA treatment could prevent PQ-induced pulmonary fibrosis. The PQ-induced pathological changes after 28 d (b) were more markedly blockaded by RAPA treatment than those after 14 d (a). However, no effect of MP treatment against pulmonary fibrosis was observed
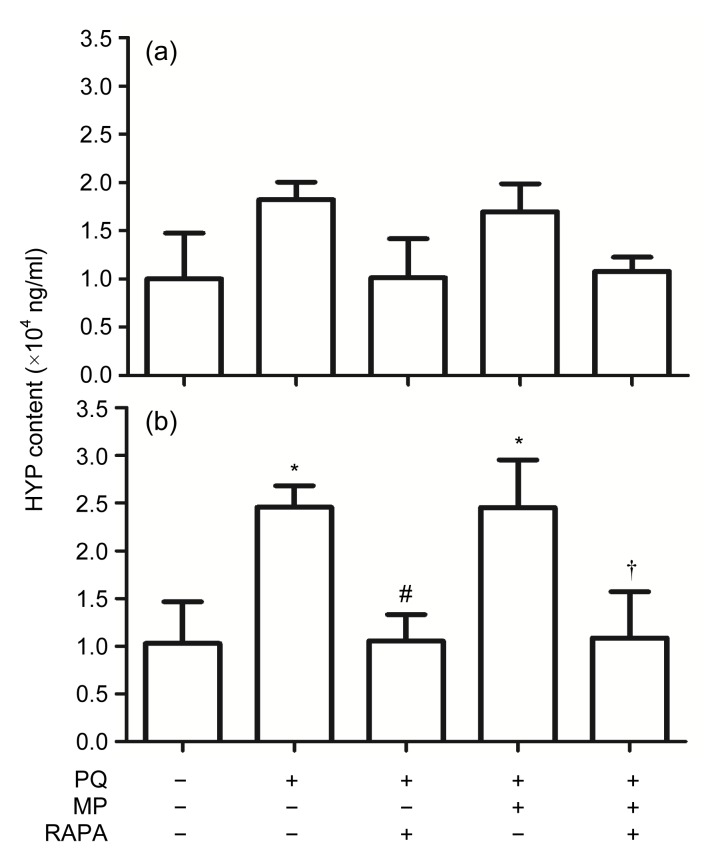
3.4. HYP content analysis of lung tissues
Pulmonary fibrosis is characterized by the accumulation of collagen. Because HYP is found uniquely in collagen (Helene et al., 1999), HYP content was determined to assess further the severity of the fibrosis presented in lung tissues (Fig. 4).
Fig. 4.
Changes of hydroxyproline (HYP) content in lung tissues
(a) HYP content decreased after RAPA treatment for 14 d compared with that of non-treated PQ-poisoned mice, but the decrease was not statistically significant. (b) HYP content had decreased significantly in lung tissues of the mice in the PQ+RAPA and PQ+MP+RAPA groups, compared with the PQ and PQ+MP groups, respectively, 28 d after RAPA treatment. Data are expressed as mean±SEM (n=6). * P<0.05, compared with control; # P<0.05, compared with PQ group; † P<0.05, compared with PQ+MP group
HYP content in lung tissues of the mice in the PQ group was significantly increased (P<0.05, compared with control). HYP content decreased after RAPA treatment for 14 d, but not significantly. HYP content also decreased significantly in the lung tissues of the mice in the PQ+RAPA and PQ+MP+RAPA groups, compared with the PQ and PQ+MP groups, respectively, 28 d after RAPA treatment (both P<0.05), indicating that PQ-induced increased collagen deposition was attenuated. MP treatment did not significantly reduce the HYP content.
3.5. Expression of TGF-β1 and α-SMA in lung tissues
The expression of TGF-β1 and α-SMA in lung tissues 14 and 28 d after PQ intoxication is summarized in Figs. 5 and 6.
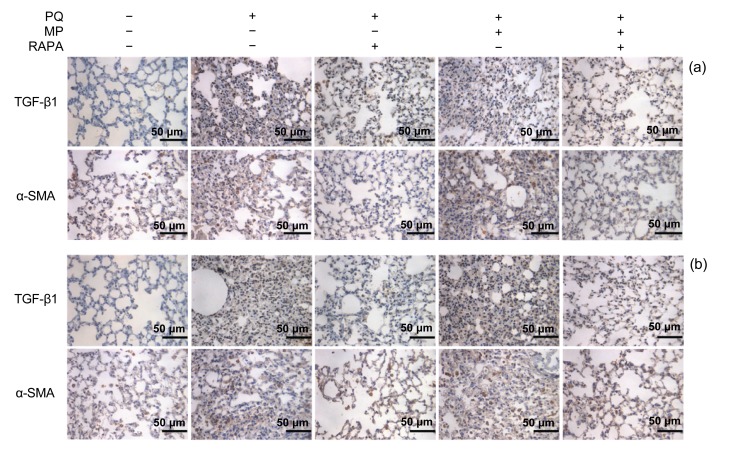
Fig. 5.
Immunohistochemical staining of TGF-β1 and α-SMA in lung tissues
The stainings of TGF-β1 and α-SMA (brown in the cytoplasm) were markedly down-regulated by RAPA treatment for 14 d (a) and 28 d (b) after PQ intoxication (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
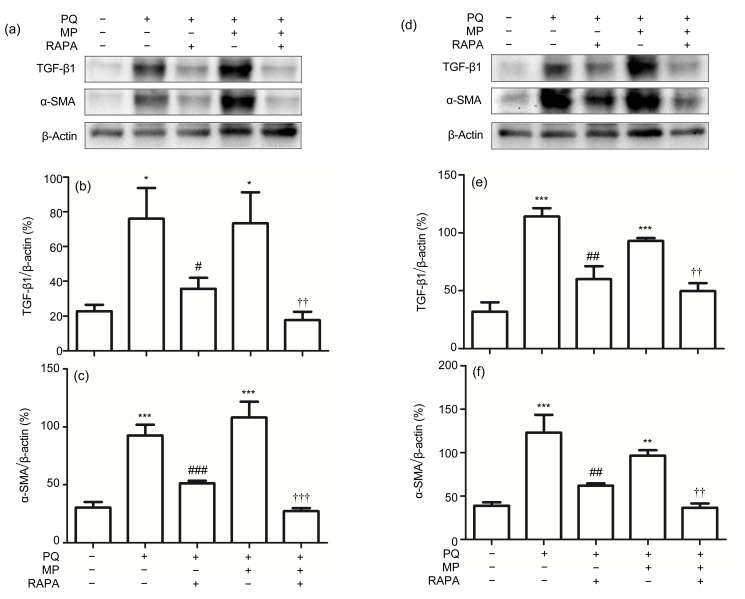
Fig. 6.
Protein levels of TGF-β1 and α-SMA in lung tissues
The protein levels of TGF-β1 and α-SMA were significantly reduced by RAPA treatment for 14 d (a–c) and 28 d (d–f), respectively. Data are expressed as mean±SEM (n=6). * P<0.05, ** P<0.01, *** P<0.001, compared with control; # P<0.05, ## P<0.01, ### P<0.001, compared with PQ group; †† P<0.01, ††† P<0.001, compared with PQ+MP group. The blots with anti-TGF-β1 antibody and anti-α-SMA antibody are shown in (a) and (d), respectively, and densitometric analysis normalized against β-actin is shown in (b), (c), (e), and (f)
Low levels of TGF-β1 and α-SMA were detected in lung tissues of the mice in the control group. However, there was increased deposition of TGF-β1 and α-SMA 14 d after poisoning in the PQ group and the PQ+MP group (Fig. 5a). The immunohistochemical staining of α-SMA and TGF-β1 was reduced in lung tissues from the mice treated with RAPA. MP treatment had no significant effects on the regulation of the expression of α-SMA and TGF-β1. The intense staining of TGF-β1 and α-SMA in lung tissues of PQ-poisoned mice was more significantly blockaded after RAPA treatment for 28 d (Fig. 5b).
PQ exposure for 14 d (Figs. 6a–6c) and 28 d (Figs. 6d–6f) resulted in a significant increase in the expression of protein levels of TGF-β1 (PQ group, PQ+MP group vs. control: P<0.05 or P<0.001; Figs. 6b and 6e) and α-SMA (PQ group vs. control: P<0.001; PQ+MP group vs. control: P<0.01 or P<0.001; Figs. 6c and 6f) in lung tissues from the mice of the PQ and PQ+MP groups. In addition, the protein levels of TGF-β1 (PQ+RAPA group vs. PQ group: P<0.05 or P<0.01; PQ+MP+RAPA group vs. PQ+MP group: P<0.01; Figs. 6b and 6e) and α-SMA (PQ+RAPA group vs. PQ group: P<0.01 or P<0.001; PQ+MP+RAPA group vs. PQ+MP group: P<0.01 or P<0.001; Figs. 6c and 6f) were markedly down-regulated by RAPA treatment for 14 and 28 d, respectively. MP treatment had no significant effects on regulating the protein levels of TGF-β1 and α-SMA.
4. Discussion
The purpose of this study was to examine the antifibrotic effects of RAPA, an increasingly used immunosuppressant, in a mice model of PQ-induced pulmonary fibrosis. Our results showed that RAPA treatment could attenuate pulmonary fibrosis progression (Figs. 3 and 4) through down-regulating the expression of TGF-β1 and α-SMA (Figs. 5 and 6). However, RAPA did not significantly improve the survival rate or reduce the loss of body weight in this model (Figs. 1 and 2).
The significant attenuation of alveolar collapse and collagen deposition in histological changes after RAPA treatment was comparable to the effects obtained previously with this model using other potential antifibrotic agents such as DHA and naringin (Chen J. et al., 2013; Chen Y. et al., 2013; Blanco-Ayala et al., 2014). Interestingly, the observation period of the test groups in our study (14 and 28 d after RAPA treatment) differed from that of other studies of novel antifibrotic agents mentioned above. Our results showed that the therapy of RAPA treatment for 14 d could ameliorate progressive pulmonary fibrosis, and the antifibrotic effects were more obvious in the lung tissues of the mice given RAPA for 28 d. The more rapid effect of RAPA compared with DHA (35 d) and naringin (21 d) suggests that RAPA treatment may have a more potent anti-fibrogenesis impact. The key effector cells in the development of pulmonary fibrosis are myofibroblasts (Wang et al., 2012), which are thought to arise from proliferation or differentiation of resident lung fibroblasts, epithelial-to-mesenchymal transition (EMT) and recruitment of circulating fibrocytes (Tulek et al., 2011; Kendall and Feghali-Bostwick, 2014). Various cytokines, growth factors, and signaling pathways are involved in these events. TGF-β1 is one of the most potent fibrogenic cytokines to drive EMT, fibroblast proliferation, and myofibroblast differentiation, and then induce ECM production (Cui et al., 2011; Chen et al., 2014). Previous studies have demonstrated that TGF-β1 contributes to the progress of PQ-induced lung fibrosis (Chen C.M. et al., 2005; Cutroneo et al., 2007). Moreover, inhibition of TGF-β1 activity or its signaling pathways can significantly attenuate PQ-induced pulmonary fibrosis (Chen Y. et al., 2013). On the other hand, the mTOR protein is involved in the growth and proliferation of fibrosis response cells (Hartford and Ratain, 2007; Xu et al., 2013). As an inhibitor of mTOR, RAPA is thought to affect the cell cycle through the FK506-RAPA complex, by increasing p27 levels in S phase and decreasing p21 levels in G1 phase, to diminish the number of interstitial fibroblasts and myofibroblasts (Biecker et al., 2005). Recent research also suggests that TGF-β signals via not only Smad-2 and Smad-3, but also the AKT/mTOR axis (Geissler and Schlitt, 2010). In the present study, RAPA treatment displayed inhibitory effects on the expression of TGF-β1 and α-SMA along with a reduction in HYP content. These results provide further evidence for the antifibrotic effects of RAPA treatment and support the potential role of TGF-β1 in PQ-induced pulmonary fibrosis.
Despite the promising antifibrotic effects of RAPA on pulmonary fibrosis shown in our study, the survival rate failed to improve significantly after RAPA treatment alone. However, the mice that received the combination of RAPA and MP treatments had a longer life expectancy. We suggest two possible reasons for this. RAPA has an insignificant effect on ameliorating the progression of ALI, which accounts for early mortality after PQ intoxication (Park et al., 2014). On the other hand, MP, a kind of synthetic corticosteroid drug, can improve the survival rate of PQ patients mainly because of its potent anti-inflammatory effects (Chen G.H. et al., 2002; Wu et al., 2014). In our study, MP treatment did not alleviate pulmonary fibrosis. This finding is in agreement with the conclusions of some recent studies which indicated that MP treatment could decrease inflammatory cell infiltration, but could not further improve lung histology (Chen C.M. et al., 2002). Our study also showed that RAPA treatment could not block the trend of body weight loss and may even worsen it. This result was inconsistent with some previous studies (Korfhagen et al., 2009; Madala et al., 2011). We speculate that this discrepancy may be caused mainly by the different animal models established: TGF-α- and bleomycin-induced pulmonary fibrosis might have less severe lung damage and a lower incidence of multiple organ dysfunction syndrome (MODS). The reduction in body weight after RAPA treatment indicated that RAPA had adverse effects on the PQ-poisoned mice, caused mainly by its immunosuppressive property (Pham et al., 2004; Lee and Gabardi, 2012).
In summary, our study showed for the first time that RAPA has significant inhibitory effects on progressive pulmonary fibrosis in the PQ intoxication mice model. These effects may be partly ascribed to the inhibition of TGF-β1. In some clinical practices, this agent has been used to rescue PQ-poisoned patients (Barrueto et al., 2008; Lorenzen et al., 2010), but the success rate is not adequate. Hence, further studies of RAPA are needed to confirm its suitability as a new antifibrotic agent in PQ-induced pulmonary fibrosis.
Footnotes
Project supported by the National Key Technology R&D Program of China (No. 2011BAI10B07), the National Basic Research Program (973) of China (No. 2012CB517603), and the National High-Tech R&D Program (863) of China (No. 2012AA02A512)
Compliance with ethics guidelines: Xue SHAO, Meng LI, Chong LUO, Ying-ying WANG, Ying-ying LU, Shi FENG, Heng LI, Xia-bing LANG, Yu-cheng WANG, Chuan LIN, Xiu-jin SHEN, Qin ZHOU, Hong JIANG, and Jiang-hua CHEN declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Baltazar T, Dinis-Oliveira RJ, Duarte JA, et al. Paraquat research: do recent advances in limiting its toxicity make its use safer? Br J Pharmacol. 2013;168(1):44–45. doi: 10.1111/j.1476-5381.2012.02017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrueto F, Lee C, Pajoumand M, et al. Use of sirolimus in a case of severe paraquat poisoning. Clin Toxicol. 2008;46(8):778–779. doi: 10.1080/15563650701546219. [DOI] [PubMed] [Google Scholar]
- 3.Biecker E, de Gottardi A, Neef M, et al. Long-term treatment of bile duct-ligated rats with rapamycin (sirolimus) significantly attenuates liver fibrosis: analysis of the underlying mechanisms. J Pharmacol Exp Ther. 2005;313(3):952–961. doi: 10.1124/jpet.104.079616. [DOI] [PubMed] [Google Scholar]
- 4.Blanco-Ayala T, Andérica-Romero AC, Pedraza-Chaverri J. New insights into antioxidant strategies against paraquat toxicity. Free Radic Res. 2014;48(6):623–640. doi: 10.3109/10715762.2014.899694. [DOI] [PubMed] [Google Scholar]
- 5.Bonegio RG, Fuhro R, Wang Z, et al. Rapamycin ameliorates proteinuria-associated tubulointerstitial inflammation and fibrosis in experimental membranous nephropathy. J Am Soc Nephrol. 2005;16(7):2063–2072. doi: 10.1681/ASN.2004030180. [DOI] [PubMed] [Google Scholar]
- 6.Chen CM, Su B, Hsu CC, et al. Methylprednisolone does not enhance the surfactant effects on oxygenation and histology in paraquat-induced rat lung injury. Intensive Care Med. 2002;28(8):1138–1144. doi: 10.1007/s00134-002-1350-2. [DOI] [PubMed] [Google Scholar]
- 7.Chen CM, Chou HC, Hsu HH, et al. Transforming growth factor-β1 upregulation is independent of angiotensin in paraquat-induced lung fibrosis. Toxicology. 2005;216(2-3):181–187. doi: 10.1016/j.tox.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Chen GH, Lin JL, Huang YK. Combined methyl-prednisolone and dexamethasone therapy for paraquat poisoning. Crit Care Med. 2002;30(11):2584–2587. doi: 10.1097/00003246-200211000-00030. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Zeng T, Zhao X, et al. Docosahexaenoic acid (DHA) ameliorates paraquat-induced pulmonary fibrosis in rats possibly through up-regulation of Smad 7 and SnoN. Food Chem Toxicol. 2013;57:330–337. doi: 10.1016/j.fct.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 10.Chen JK, Chen J, Neilson EG, et al. Role of mammalian target of rapamycin signaling in compensatory renal hypertrophy. J Am Soc Nephrol. 2005;16(5):1384–1391. doi: 10.1681/ASN.2004100894. [DOI] [PubMed] [Google Scholar]
- 11.Chen T, Nie H, Gao X, et al. Epithelial-mesenchymal transition involved in pulmonary fibrosis induced by multi-walled carbon nanotubes via TGF-β/Smad signaling pathway. Toxicol Lett. 2014;226(2):150–162. doi: 10.1016/j.toxlet.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Nie YC, Luo YL, et al. Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food Chem Toxicol. 2013;58:133–140. doi: 10.1016/j.fct.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Cui Y, Robertson J, Maharaj S, et al. Oxidative stress contributes to the induction and persistence of TGF-β1 induced pulmonary fibrosis. Int J Biochem Cell Biol. 2011;43(8):1122–1133. doi: 10.1016/j.biocel.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Cutroneo KR, White SL, Phan SH, et al. Therapies for bleomycin induced lung fibrosis through regulation of TGF-β1 induced collagen gene expression. J Cell Physiol. 2007;211(3):585–589. doi: 10.1002/jcp.20972. [DOI] [PubMed] [Google Scholar]
- 15.Geissler EK, Schlitt HJ. The potential benefits of rapamycin on renal function, tolerance, fibrosis, and malignancy following transplantation. Kidney Int. 2010;78(11):1075–1079. doi: 10.1038/ki.2010.324. [DOI] [PubMed] [Google Scholar]
- 16.Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clin Pharmacol Ther. 2007;82(4):381–388. doi: 10.1038/sj.clpt.6100317. [DOI] [PubMed] [Google Scholar]
- 17.Helene M, Lake-Bullock V, Zhu J, et al. T cell independence of bleomycin-induced pulmonary fibrosis. J Leukoc Biol. 1999;65(2):187–195. doi: 10.1002/jlb.65.2.187. [DOI] [PubMed] [Google Scholar]
- 18.Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korfhagen TR, Le Cras TD, Davidson CR, et al. Rapamycin prevents transforming growth factor-α-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2009;41(5):562–572. doi: 10.1165/rcmb.2008-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee RA, Gabardi S. Current trends in immune-suppressive therapies for renal transplant recipients. Am J Health Syst Pharm. 2012;69(22):1961–1975. doi: 10.2146/ajhp110624. [DOI] [PubMed] [Google Scholar]
- 21.Li LR, Sydenham E, Chaudhary B, et al. Glucocorticoid with cyclophosphamide for paraquatinduced lung fibrosis. Cochrane Database Syst Rev. 2014;8:CD008084. doi: 10.1002/14651858.CD008084.pub4. [DOI] [PubMed] [Google Scholar]
- 22.Lloberas N, Cruzado JM, Franquesa M, et al. Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol. 2006;17(5):1395–1404. doi: 10.1681/ASN.2005050549. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzen JM, Schonenberger E, Hafer C, et al. Failed rescue therapy with rapamycin after paraquat intoxication. Clin Toxicol. 2010;48(1):84–86. doi: 10.3109/15563650903376089. [DOI] [PubMed] [Google Scholar]
- 24.Madala SK, Maxfield MD, Davidson CR, et al. Rapamycin regulates bleomycin-induced lung damage in SP-C-deficient mice. Pulm Med. 2011;2011:653524. doi: 10.1155/2011/653524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neef M, Ledermann M, Saegesser H, et al. Low-dose oral rapamycin treatment reduces fibrogenesis, improves liver function, and prolongs survival in rats with established liver cirrhosis. J Hepatol. 2006;45(6):786–796. doi: 10.1016/j.jhep.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 26.Oka H, Ishii H, Iwata A, et al. Inhibitory effects of pitavastatin on fibrogenic mediator production by human lung fibroblasts. Life Sci. 2013;93(25-26):968–974. doi: 10.1016/j.lfs.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Park JS, Park KH, Kim H, et al. Effects of sivelestat treatment on acute lung injury in paraquat-intoxicated rats. Drug Chem Toxicol. 2014;37(1):114–120. doi: 10.3109/01480545.2013.834351. [DOI] [PubMed] [Google Scholar]
- 28.Pham PT, Pham PC, Danovitch GM, et al. Sirolimus-associated pulmonary toxicity. Transplantation. 2004;77(8):1215–1220. doi: 10.1097/01.TP.0000118413.92211.B6. [DOI] [PubMed] [Google Scholar]
- 29.Rocco PR, Souza AB, Faffe DS, et al. Effect of corticosteroid on lung parenchyma remodeling at an early phase of acute lung injury. Am J Respir Crit Care Med. 2003;168(6):677–684. doi: 10.1164/rccm.200302-256OC. [DOI] [PubMed] [Google Scholar]
- 30.Tulek B, Kiyan E, Toy H, et al. Anti-inflammatory and anti-fibrotic effects of sirolimus on bleomycin-induced pulmonary fibrosis in rats. Clin Invest Med. 2011;34(6):E341. doi: 10.25011/cim.v34i6.15894. [DOI] [PubMed] [Google Scholar]
- 31.Vale JA, Meredith TJ, Buckley BM. Paraquat poisoning: clinical features and immediate general management. Hum Toxicol. 1987;6(1):41–47. doi: 10.1177/096032718700600107. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Yu ZH, Zhou ZY, et al. Inhibition of α-SMA by the ectodomain of FGFR2c attenuates lung fibrosis. Mol Med. 2012;18:992–1002. doi: 10.2119/molmed.2011.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu WP, Lai MN, Lin CH, et al. Addition of immunosuppressive treatment to hemoperfusion is associated with improved survival after paraquat poisoning: a nationwide study. PLoS ONE. 2014;9(1):e87568. doi: 10.1371/journal.pone.0087568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu X, Wan X, Geng J, et al. Rapamycin regulates connective tissue growth factor expression of lung epithelial cells via phosphoinositide 3-kinase. Exp Biol Med. 2013;238(9):1082–1094. doi: 10.1177/1535370213498976. [DOI] [PubMed] [Google Scholar]