Abstract
Single dose administration of dietary inorganic nitrate acutely reduces blood pressure in normotensive healthy volunteers, via bioconversion to the vasodilator nitric oxide. We assessed whether dietary nitrate might provide sustained blood pressure lowering in hypertensive patients.We randomly assigned 68 hypertensive patients in a double-blind, placebo-controlled clinical trial to receive daily dietary supplementation for 4-weeks with either dietary nitrate (250mL daily, as beetroot juice) or a placebo (250mL daily, as nitrate-free beetroot juice) following a 2-week run-in period and followed by a 2-week wash-out. We performed stratified randomization of drug-naïve (n=34) and treated (n=34) hypertensive patients aged 18-85 years. The primary end-point was change in clinic, ambulatory and home blood pressure compared to placebo. Daily supplementation with dietary nitrate was associated with reduction in blood pressure measured by 3 different methods. Mean (95% CI) reduction in clinic blood pressure was 7.7/2.4mmHg (3.6-11-8/0.0-4.9,p<0.001 and p=0.050). 24h ambulatory blood pressure was reduced by 7.7/5.2mmHg (4.1-11.2/2.7-7.7,p<0.001 for both). Home blood pressure was reduced by 8.1/3.8mmHg (3.8-12.4/0.7-6.9,p<0.001 and p<0.01) with no evidence of tachyphylaxis over the 4-week intervention period. Endothelial function improved by ~20% (p<0.001) and arterial stiffness was reduced by 0.59m/s (0.24-0.93;p<0.01) after dietary nitrate consumption with no change after placebo. The intervention was well tolerated. This is the first evidence of durable blood pressure reduction with dietary nitrate supplementation in a relevant patient group. These findings suggest a role for dietary nitrate as an affordable, readily-available, adjunctive treatment in the management of hypertensive patients.(Funded by The British Heart Foundation, Clinicaltrials.gov: NCT01405898).
Keywords: Blood pressure, Nitric Oxide, Nitrites, Nitrates, Diet
Introduction
Systemic hypertension remains the largest attributable risk factor for mortality worldwide.1, 2 Worryingly, the scale of the problem is increasing, with the proportion of adults with hypertension predicted to increase to almost 1-in-3 (1.57 billion) by 2025.3 Despite more than 60 years of innovation in the pharmacotherapy of hypertension,4 only ~half of hypertensives are treated for their blood pressure (BP) and of those only ~half are controlled to guideline-driven targets.5, 6 Thus, novel therapeutic strategies including dietary approaches are of great interest.
An approach that has been explored in the treatment of hypertension is the delivery of the potent vasodilator nitric oxide (NO). Endothelial NO generation, achieved through the conventional L-arginine/NO synthase pathway, plays a critical role in sustaining vascular health. However, in most cardiovascular diseases (CVDs), including hypertension, the levels of endothelial NO are diminished.7, 8 Accordingly, therapeutic strategies restoring NO levels in hypertension have been explored. However, supplementation with substrate (L-arginine) and other essential co-factors required for healthy NO generation from NO synthase have yielded equivocal results.9 In addition, NO donors (organic nitrates), such as nitroglycerin, have also been tested but suffer from problems of induced endothelial dysfunction10 and tachphylaxis11 that have limited their clinical utility.12
However, NO production from the chemical reduction of inorganic nitrite (NO2−), a phenomenon previously thought to occur only in extreme acidosis 13-15 has emerged as a potential pathway that might be exploited as a method for delivery of NO to the blood vessel. Evidence in healthy volunteers suggests nitrite reduction occurs readily within the circulation following elevation of plasma nitrite levels, by provision of dietary or oral inorganic nitrate salts.16, 17 It is now accepted that a significant proportion of orally ingested inorganic nitrate once absorbed across the upper intestine is extracted from the blood via the salivary glands and secreted into the oral cavity where it comes into contact with symbiotic bacteria that reduce inorganic nitrate (NO3−) to nitrite.18 Upon swallowing the saliva the nitrite then enters the circulation16, 17, 19 where it meets mammalian nitrite reductases that convert it to NO resulting in vasodilation20, 21 and significant BP-lowering.16, 17, 19 Indeed, exploitation of this NO3−-NO2−-NO (alternative) pathway by supplementation with dietary sources of NO3− (e.g. beetroot juice) is associated with elevations in both plasma nitrite and cyclic guanosine monophosphate (cGMP, an exquisitely sensitive marker of bioactive NO production22) and significant BP reductions over 24-hours in healthy volunteers and drug-naïve stage 1 hypertensive patients.17, 23 We explored in this phase 2 clinical study whether a once daily dietary nitrate supplementation for 4 weeks would confer sustained BP reduction in both drug-naïve and treated hypertensive patients.
Materials and Methods
Study design
This study was a prospective single-centre, double-blind, randomized, placebo-controlled trial. Eligible patients recruited were those that satisfied a range of inclusion/exclusion criteria (see online supplement) including aged between 18-85 years old, an estimated glomerular filtration rate (eGFR) >50 mL/min, no manifest CVD and uncontrolled BP on ambulatory BP (ABP) monitoring (day-time BP> 130/85 mmHg).24
Ethics approval was granted by the East London Research Ethics Committee and the trial registered on clinicaltrials.gov (unique identifier: NCT01405898). Patients were recruited between July 2011 and February 2013. Patients gave written informed consent, and the study conforms to the principles of the Declaration of Helsinki.
Study procedures
All tests were performed at the William Harvey Clinical Research Centre. 34 drug-naïve and 34 treated patients with hypertension were randomized to receive either 4-weeks daily supplementation with dietary nitrate (250mL beetroot juice, James White Drinks Ltd., Ipswich, UK) or placebo (250mL nitrate-depleted beetroot juice25, James White Drinks Ltd., Ipswich, UK). Patients were asked not to alter their usual diet and to keep anti-hypertensive medication(s) constant (in treated hypertensive patients) over the course of the study. Patients recorded once-daily home BP (using a validated oscillometric BP device:705IT, Omron Corp., Tokyo, Japan) over a 2-week run-in and then had a pre-intervention 24-hour ABP monitor (90207, Spacelabs Healthcare Ltd, Issaquah, USA) performed. Following this, patients attended in the morning after an overnight fast for clinic BP, vascular function testing and collection of saliva, urine and blood samples (Visit: Pre). Patients were then instructed to consume one bottle of juice at the same time in the morning daily and continue to record home BP daily during the ensuing 4-weeks. One day prior to the end of this period, patients returned for ABP monitoring, and then returned the following day after an overnight fast for clinic BP, vascular function testing and collection of saliva, urine and blood samples (Visit: Post). Following this visit, patients continued to measure BP at home for a 2-week wash-out period, at the end of which they returned for ABP monitoring, and then returned the following day after an overnight fast for clinic BP, vascular function testing and collection of saliva, urine and blood samples (Visit: W/O) (Figure S1). (For full details of BP measurements, vascular function tests and biological sample collection see online supplement). Transcutaneous arterial methaemoglobin concentrations were determined prior to venepuncture in a subset of patients (placebo n=12; dietary nitrate n=7) at all visits using a validated co-oximeter (Masimo Rad-57, Masimo Inc., Irvine, USA).
Statistical analyses
The data in the manuscript and in the figures are presented as mean±standard deviation or 95% confidence intervals (CIs) for comparisons between treatment allocations, unless otherwise specified. All statistical analyses were performed using Graphpad Prism™ software v6. For full statistical methods, including power analyses, pre-specified end-points and sub-groups, see online supplement.
Results
Of the 151 patients screened for the study 68 patients were enrolled, of whom 64 completed the study protocol and had complete data. 4 patients withdrew after randomization but before the first study visit and returned no analysable data (Figure S2). Both dietary nitrate and placebo interventions were well tolerated. No serious adverse events were reported. Common, expected findings were beeturia and faecal discolouration. All patients completed the dietary interventions for the duration of the study. The nitrate content of the active treatment juice was 25.7±5·3 mmol/L, giving ~6·4mmol nitrate in a 250mL daily dose. The nitrate content of the placebo juice was 0.028±0.008mmol/L, giving ~0.007mmol nitrate daily. The nitrite content was below the limits of detection in both interventions i.e. <50nmol/L. It has been shown previously that there are no significant differences in major cationic components (i.e. sodium, potassium) of the active and placebo juices.25 Demographics and baseline screening characteristics were similar in both treatment allocation groups (Table 1). All patients were confirmed to have significant hypertension by ABP monitoring at trial inception 24.
Table 1.
Baseline characteristics stratified by treatment allocation. Data are presented as mean±SD. Significance shown in the last column for unpaired Student t test, except for analysis of sex for which Fisher’s exact test was performed. (ABP=ambulatory blood pressure; ACE-i=angiotensin converting enzyme inhibitor; ARB=angiotensin receptor blocker; CCB=calcium channel blocker; DBP=diastolic blood pressure; eGFR=estimated glomerular filtration rate; HDL-C=high-density lipoprotein cholesterol; HR=heart rate; SBP=systolic blood pressure).
| Treatment Allocation | Placebo | Dietary Nitrate | Significance |
|---|---|---|---|
| Demographics | |||
| n (female) | 32 (22) | 32 (16) | 0.14 |
| age (y) | 56.3±16.4 | 57.6±13.9 | 0.73 |
| BMI (kg/m2) | 26.5±4.0 | 26.8±5.0 | 0.74 |
| Medications | |||
| hypertension drugs | 1.0±1.2 | 1.0±1.2 | 0.84 |
| patients on (n): | |||
| ACE-i/ARB | 10 | 11 | |
| β-blocker | 3 | 3 | |
| CCB | 14 | 10 | |
| diuretic | 5 | 4 | |
| α-blocker | 2 | 4 | |
| aldosterone antagonist | 1 | 1 | |
| statins | 3 | 4 | |
| antiplatelet drugs | 0 | 0 | |
| Screening ABP (mmHg) | |||
| SBP | 148.2±10.0 | 149.0±11.0 | 0.73 |
| DBP | 88.2±8.0 | 88.9±9.8 | 0.75 |
| HR | 70.6±8.3 | 72.9±10.7 | 0.32 |
| Biochemistry | |||
| eGFR (mL/min) | 79.1±16.3 | 85.0±16.6 | 0.17 |
| Total Cholesterol:HDL-C ratio | 3.4±1.3 | 3.1±0.7 | 0.37 |
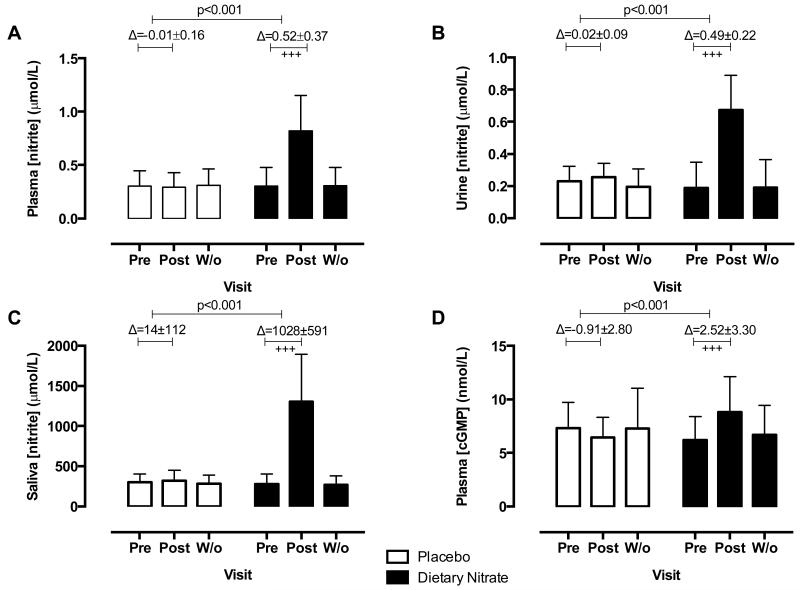
Dietary nitrate ingestion was associated with elevations in both nitrate and nitrite concentrations in all biological compartments assessed. These levels returned to baseline following a two-week washout period (Figure 1,S3). Importantly, plasma nitrite concentrations were also elevated from baseline by ~2.7-fold with a mean Δ0.52 μmol/L (95%CI 0.39-0.65) after consumption of dietary nitrate with no change in the placebo limb (p<0.001,Figure 2A). The change in circulating NOx levels in the dietary nitrate limb was also associated with an ~1.4 fold increase in plasma cGMP concentrations with no change in the placebo limb (p<0.001,Figure 1D). There were no changes in transcutaneous arterial methaemoglobin concentrations after either intervention (Figure S3D) and no changes from baseline or between groups in glycaemic, serum biochemical or haematological indices (Table S1).
Figure 1.
Dietary nitrate consumption elevates nitrite concentration in biological compartments in hypertensive patients. The effects of 4 weeks dietary nitrate consumption (nitrate-rich juice 250 mL daily) or placebo (nitrate-depleted juice 250 mL daily) on nitrite concentrations in (A) plasma, (B) urine (C) saliva and (D) plasma cGMP concentrations. Data are expressed as mean±SD. Significance shown for comparisons between treatment allocations of the change between Pre and Post for unpaired Student t test; and as +++p<0.001 for Dunnett’s post hoc test comparison to baseline (Pre) following 2 way ANOVA for changes within each treatment allocation cohort. (cGMP=cyclic guanosine monophosphate; Pre=1st visit pre-intervention; Post=2nd visit post-intervention; W/o=3rd visit washout).
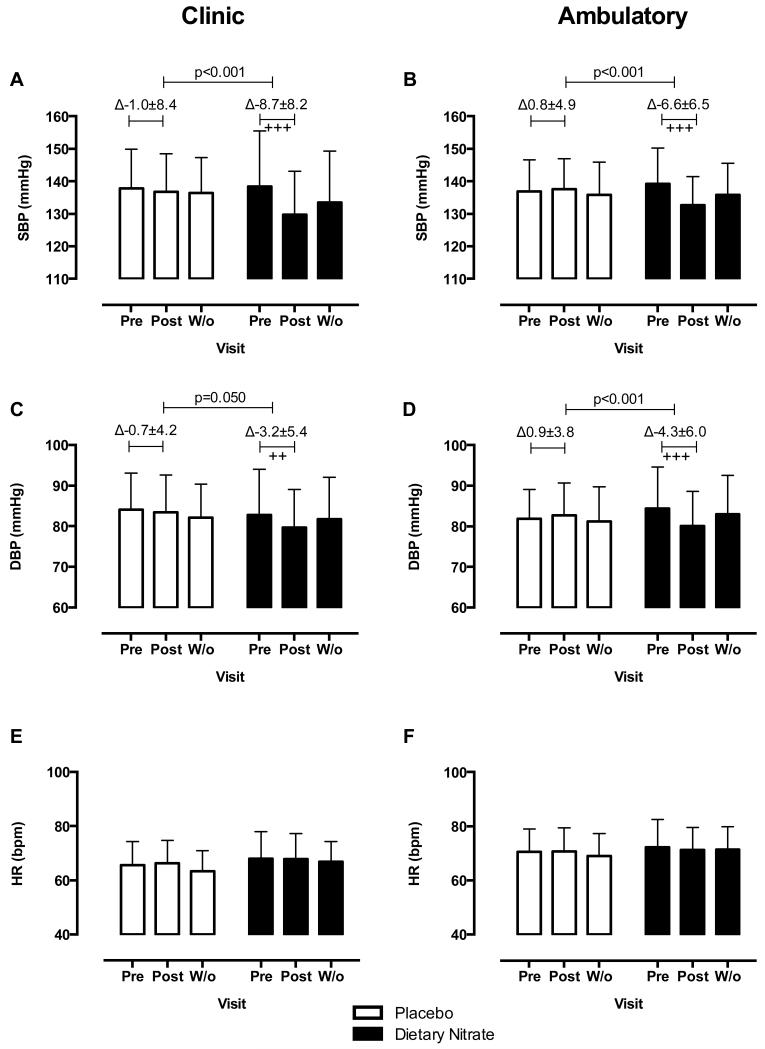
Figure 2.
Dietary nitrate consumption reduces clinic and 24h ABP in hypertensive patients. The effects of 4 weeks dietary nitrate consumption (beetroot juice 250 mL daily) or placebo (nitrate-depleted beetroot juice 250 mL daily) on clinic measures of (A) SBP and (C) DBP and (E) HR; and on 24h ABP measures of (B) SBP and (D) DBP and (F) HR. Data are expressed as mean±SD. Significance shown for comparisons between treatment allocations of the change between Pre and Post for unpaired Student t test; and as ++p<0.01 and +++p<0.001 for Dunnett’s post hoc test comparison to baseline (Pre) following 2 way ANOVA for changes within each treatment allocation cohort. (ABP=ambulatory blood pressure; BP=blood pressure; DBP=diastolic blood pressure; HR=heart rate; SBP=systolic blood pressure; Pre=1st visit pre-intervention; Post=2nd visit post-intervention; W/o=3rd visit washout).
The 2 treatment allocation groups were well matched for baseline (pre-intervention) BP and HR measured by the methods utilized in this study (Table S2). Consumption of dietary nitrate was associated with decreases in clinic BP (Figure 2). Clinic SBP and DBP decreased compared to baseline by 7.7mmHg (95%CI 3.5-11.8,p<0.001) and 2.4 mmHg (95%CI 0.0-4.9,p=0.050) respectively; changes not evident in the placebo group (Figure 2A,2C). Similarly, 24h ABP measurements exhibited a similar pattern with a mean decrease in SBP and DBP compared to baseline of 7.7mmHg (95%CI 4.1-11.2,p<0.001) and 5.2mmHg (95%CI 2.7-7.7,p<0.001) respectively (Figure 2B,2D). Observation of the hourly profile of the change in 24h ABP post intervention revealed that consumption of dietary nitrate was associated with reduction in BP over the entire 24h period for both SBP and DBP (Figure S4) compared to placebo. Splitting 24h ABP into day-time (0700-2300) and night-time (2300-0700) periods, dietary nitrate consumption was associated with decreases in BP in both time periods (Figure S5).
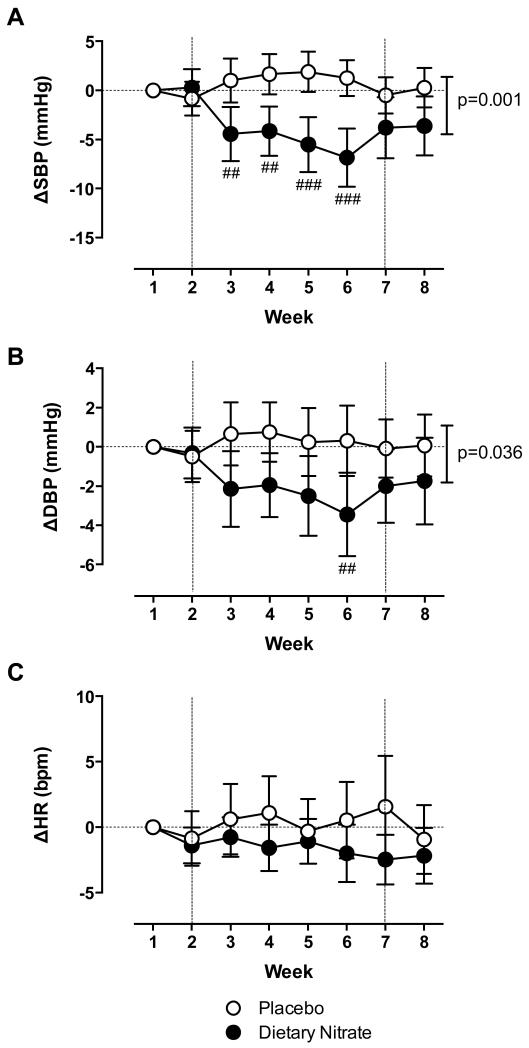
Home BP was reduced within 1 week of consumption of dietary nitrate, but not placebo, for both SBP and DBP and reduced over the entire 4-week intervention period (Figure 3A-B). Peak decreases in BP occurred at week 6 (i.e. last week of dietary nitrate intervention), with decreases in SBP compared to placebo of 8.1 mmHg (95%CI 3.8-12.4,p<0.001) and in DBP of 3.8mmHg (95%CI 0.7-6.8,p<0.01) (Figure 3A-B). Following washout both SBP and DBP started to return to baseline (Figure 3). There were no changes relative to baseline or compared to placebo in HR after dietary nitrate intervention by any method used (Figure 2E,2F,3C and S5E-F). Change in plasma nitrite from baseline in the intervention arm correlated inversely with change in SBP measured in clinic and at home, but not with ABP (Figure S6A-C).
Figure 3.
Dietary nitrate consumption reduces home BP over entire 4 week intervention period in hypertensive patients. The effects of 4 weeks dietary nitrate consumption (beetroot juice 250 mL daily) or placebo (nitrate-depleted beetroot juice 250 mL daily) on change in weekly (A) SBP and (B) DBP and (C) HR from baseline (Week 1) measured at home. Data are expressed as mean±SD. Significance shown for comparisons between treatment allocations for 2-way ANOVA; followed by ##p<0.01 and ###p<0.001 for Bonferroni post hoc test. (BP=blood pressure; DBP=diastolic blood pressure; HR=heart rate; SBP=systolic blood pressure). The vertical dotted lines at 2 and 7 weeks signify the end of the 2-week run-in and the beginning of the washout periods.
Dietary nitrate consumption was also associated with improvements in vascular function (Figure 4). PWV was reduced after dietary nitrate consumption by 0.59 m/s (95%CI 0.24-0.93;p<0.01) compared to baseline and 0.58m/s (95% CI 0.05-1.10,p<0.05) compared to placebo. AIx was also reduced after dietary nitrate consumption by 5.2% (95%CI 2.9-7.5;p<0.001) compared to baseline and 6.1% (95%CI 3.0-9.1,p<0.01) compared to placebo. Baseline brachial artery diameter and time to peak dilatation after reactive hyperaemia were similar across all treatment groups and visits (Table S3). However, dietary nitrate consumption was associated with an increase in peak FMD of 1.0% (95%CI 0.3-1.5;p<0.001) not evident in the placebo limb.
Figure 4.
Dietary nitrate consumption improves arterial function in hypertensive patients. The effects of 4 weeks dietary nitrate consumption (beetroot juice 250 mL daily) or placebo (nitrate-depleted beetroot juice 250 mL daily) on clinic measures of (A) PWV, (B) AIx and (C) % and (D) absolute increase in vessel diameter with FMD. Data are expressed as mean±SD. Significance shown for comparisons between treatment allocations of the change between Pre and Post for unpaired Student t test; and as ++p<0.01 and +++p<0.001 for Dunnett’s post hoc test comparison to baseline (Pre) following 2 way ANOVA for changes within each treatment allocation cohort. (AIx=augmentation index; FMD=flow mediated dilatation; PWV=pulse wave velocity; Pre=1st visit pre-intervention; Post=2nd visit post-intervention; W/o=3rd visit washout).
A priori sub-group analyses were conducted by stratification for baseline hypertension treatment status (drug-naïve or treated). We recruited n=34 into each sub-group. 4 patients dropped out prior to receiving intervention: all 4 were in the treated hypertension arm, split equally between intervention and placebo arms. There were no differences in baseline demographics, characteristics and BPs of the sub-groups (Table S4) and between the placebo and dietary nitrate sub-groups.
Dietary nitrate consumption produced similar rises in plasma, urine, saliva nitrite and nitrate, and plasma cGMP in both stratified limbs (Table S5). Importantly, BP was reduced compared to placebo by all methods used in both sub-groups (Figure S7-8). PWV velocity was not altered in drug-naïve patients after dietary nitrate consumption although endothelial function trended to improvement (p=0.06) though this failed to reach conventional levels of statistical significance (Figure S7C-D). In the treated hypertensives, PWV velocity was reduced after dietary nitrate (0.82±0.87 m/s, p<0.01) and endothelial function was improved (change in FMD compared to placebo: 1.1% (95%CI 0.1-2.0,p=0.04; Figure S8C-D).
Discussion
Previously the oxidation of endogenously generated NO to nitrite and nitrate was viewed as a one-way, linear termination of NO activity. However the discovery of the NO3−-NO2−-NO reductive pathway, dependent upon the enterosalivary circuit, has led to a radical revision of our understanding of the pathways that govern endogenous NO generation and NO metabolism.26 This novel paradigm reveals the nitrogen oxides to be in an NO cycle that can be augmented through the provision of inorganic nitrate, given either by dietary or inorganic supplementary route. By capitalizing on this cycle, herein we show that a once-daily dietary nitrate intervention augments NO generation through this pathway in hypertensive patients to lower BP. We have demonstrated that the intervention is well-tolerated, safe and is associated with robust BP reductions measured in and out of clinic.
Dietary nitrate supplementation providing ~6 mmol nitrate daily for 4 weeks, caused substantial increases in plasma nitrate concentrations (~5.5-fold) and is similar to the peak fold increases in previous studies with similar doses.23 Plasma nitrite concentration was elevated by ~2.7-fold from baseline; the reduced fold increase compared to plasma nitrate concentrations reflecting utilization of the entero-salivary circulation in the bio-conversion of ingested nitrate to nitrite that is critically dependent upon the symbiotic relationship between oral bacteria and host.27 In this study, dietary nitrate consumption elevated both saliva nitrate and nitrite concentrations with a ratio of saliva nitrate:nitrite of ~3. This is similar to the ratios identified in healthy volunteers following single dose nitrate supplementation28, 29 suggesting that the entero-salivary circulation is intact and functioning normally in hypertensive patients. In these processes, dietary (or inorganic) nitrate can be thought of as a pro-drug for the generation of plasma nitrite. The half-life of nitrite in the circulation following a single oral dosing has been estimated to be ~30min.30 In contrast nitrate has a half-life of ~6h after oral dosing of inorganic nitrate31. The recirculation of nitrate, however, also extends the apparent half-life of nitrate-derived nitrite, with plasma nitrite peaking at ~3h post inorganic nitrate ingestion and remaining elevated for at least a further 3h in both healthy and hypertensive subjects.17, 19, 23
In healthy volunteers it has been shown that at least some of the nitrite in the swallowed saliva enters the circulation where it is chemically reduced by the action of one or more mammalian nitrite reductases to generate vasodilator NO.32 That this occurs in hypertensive patients also is demonstrated biochemically in this study by increases in the downstream secondary messenger cGMP, that is elevated between 3-24h post dietary (or inorganic) nitrate ingestion.19, 23 confirming production of bioactive NO. Peak BP reductions coincide with these peak plasma nitrite elevation and plasma cGMP elevation, at ~3h following dietary (or inorganic) nitrate ingestion,17, 19, 23 and in this study, the increases in plasma nitrite concentration following dietary nitrate were associated with BP reductions measured both in- and out-of-clinic, providing further evidence that nitrite reduction to NO underlies the BP-lowering effects seen with dietary nitrate consumption.
Out-of-clinic BP measurements (i.e. home and ABP) are recognized to be more predictive of target organ damage and mortality in population cohort studies33, 34 and underlies the use of all three methods in this study. Interestingly, the magnitude of BP reduction following dietary nitrate consumption was similar across all 3 methods of measurement with clinic BP reduced by 7.7/2.4 mmHg, 24h ambulatory BP by 7.7/5.2mmHg and home BP by 8.1/3.8mmHg. Irrespective of the method of measurement, the magnitude of BP reduction is of clinical significance since it resembles the average BP reduction achieved with a single anti-hypertensive medication at standard dose (9.1/5.5mmHg).35 Most pharmacological treatments for raised BP give larger maximal BP reductions dependent on higher baseline BP values,35 including following acute, single inorganic and dietary nitrate dosing in healthy and drug-naïve stage 1 hypertensive patients.17, 19, 23 Thus, one may postulate that the BP-lowering effects seen in these patients with mild hypertensive phenotypes may be greater in patients with more severe hypertension. However, this has not been tested in our study and is not known at this time and there is the possibility that in patients on multiple anti-hypertensive agents with established vascular damage from long-standing uncontrolled hypertension, the effects could be attenuated rather than amplified.
It is noteworthy that the home BP measurements over the 4 weeks demonstrate, if anything, an increasing magnitude of the effect with time with a reversal occurring only upon washout. These findings are in agreement with primate studies demonstrating no tachyphylaxis to repeated and continuous systemic nitrite administration over 2 weeks,36 confirming an absence of the development of tolerance: a characteristic that has profoundly limited the clinical utility of the organic nitrates in CVD.37
Dietary nitrate consumption also improved indices of vascular function including aPWV, AIx and FMD. PWV is the gold standard measure of arterial stiffness and is recognized as a powerful predictor of cardiovascular events.38 The exact underlying mechanism for these changes in arterial stiffness is uncertain but pre-clinical studies of age-induced arterial stiffening in mice suggest that systemic nitrite therapy reduces oxidative stress and advanced glycation end-products that are associated with arterial stiffening.39 However, it is also possible that the improvement in endothelial function measured by FMD plays a role in the improved PWV. A recent report in healthy, elderly subjects supplemented with 0.1mmol/kg inorganic nitrate (~6-8mmol/day) for 4 weeks demonstrated a modest increase in FMD of 0.5%, compared to no change in the placebo group.40 We have previously demonstrated in young healthy individuals that whilst a single acute dose of 8mmol of nitrate does not alter FMD (in healthy individuals the FMD response ranges from between 7-14%) 28, that single acute doses ranging between 6-24mmol do protect against transient endothelial dysfunction induced by forearm ischaemia-reperfusion injury.17, 19
Finally, in all of the analyses, there were no significant changes in any functional parameter measured after consumption of the nitrate-free placebo intervention. This substantiates the proposal that inorganic nitrate is responsible for the beneficial effects seen with beetroot juice. The average intake of nitrate from regular food sources (predominantly vegetables) is 1.5-2mmol daily41 and the acceptable daily intake is set by the WHO at 3.7mg/kg/day42 (~4.2mmol daily for a 70kg person) due to concerns over methaemoglobinaemia and carcinogenesis. In this study, no suggestion of significant methaemoglobinaemia was evident and previous studies that were associated with micromolar plasma nitrite concentration have not demonstrated clinically significant methaemoglobinaemia.20, 43 Although there are well established links between pre-formed nitrosoamines and carcinogenesis,44 recent US National Toxicology Program reports on 2-year rodent feeding studies with nitrite45 and a comprehensive WHO report summarizing epidemiological cohort studies in humans evaluating the risk of cancer with nitrate intake have largely assuaged concerns regarding carinogenesis.42 Importantly, vegetable intake is associated with small reductions in cancer incidence, rather than increases.46 Patients predisposed to oxalate renal stones may need to avoid certain high-nitrate vegetables that also contain oxalate, such as spinach and beetroot. Although study participants reported no adverse effects apart from expected discolouration of urine (beeturia) and faeces from the purple betacyanin pigments in beetroot, we cannot be certain that prolonged intake of beetroot juice is reliably acceptable as a therapeutic source of dietary nitrate. There is batch-to-batch variation in nitrate content of vegetables and their juices and clinical studies using beetroot juice with varying concentrations delivering between ~4-24mmol. This natural variation could be controlled by use of anion-exchange resin to provide fixed nitrate concentrations25. Our previous studies have suggested that 4mmol is a threshold dose for BP lowering healthy volunteers though it is not clear from our data whether this is also true for hypertensives or what dose of dietary nitrate might provide maximal BP lowering is not known.
Overall, our results presented in this study demonstrate that dietary nitrate provision to hypertensive patients provides robust BP lowering that is dependent on the conversion of nitrate to nitrite and thence NO, with no suggestion of tachyphylaxis over a 4-week period.
Perspectives
The potential importance of our findings is substantial when one considers that each 2 mmHg increase in SBP increases mortality due to ischaemic heart disease and stroke by 7% and 10% respectively.47 Although the time-frame of the study is too short to make any supported claims to long-term BP control or to be able to extrapolate with any confidence to target organ damage reduction or CVD events, these appropriately powered data are the first to demonstrate robust, sustained BP-lowering with dietary nitrate in hypertensive patients that require BP control (rather than healthy subjects) and as such are very encouraging and should spur large-scale, long-term outcome studies to explore the utility of a dietary nitrate-based therapeutics approach to hypertension and CVD risk mitigation. Moreover, dietary nitrate provides a viable option to finally exploit the NO pathway, which has been implicated at multiple steps in the genetic architecture of BP and is a therapeutic modality targeted at gene products directly implicated in raised BP.48 With large populations of inadequately treated hypertensive patients at higher risk of CVD5, 6 an additional strategy, based on intake of nitrate-rich vegetables, may prove to be both cost-effective, affordable and favourable for a public health approach to hypertension.
Supplementary Material
Novelty and significance.
- What is new?
- Once a day dietary nitrate intervention for 4 weeks provides sustained blood pressure lowering in patients with hypertension
- This dietary intervention also results in improved function of the blood vessels.
- Prolonged dietary nitrate use is not associated with any tachyphylaxis of the beneficial effects on the cardiovascular system.
- A once a day dietary nitrate intervention results in further blood pressure lowering in patients taking 1-4 other blood pressure medications.
- What is relevant
- Dietary nitrate exerts potent and long-lasting blood pressure decrease in hypertension that is sustained with once a day dosing for 4 weeks.
- Daily dietary nitrate ingestion provides additional blood pressure lowering beyond conventional pharmacotherapy.
- Summary
- A once a day dietary nitrate regimen offers a strategy to lower blood pressure in hypertension either as monotherapy or in conjunction with conventional pharmacotherapy.
Acknowledgments
Funding
This work was funded by the British Heart Foundation. The study was conducted within The National Institute of Health Research (NIHR) Cardiovascular Biomedical Research Unit at Barts.
Footnotes
Conflicts of Interests
AA is a director of Heartbeet Ltd. All other authors declare that they have no conflict of interest.
References
- 1.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Global health risks: Mortality and burden of disease attributable to selected major risks. WHO Press; Geneva: 2009. [Google Scholar]
- 3.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 4.Laurent S, Schlaich M, Esler M. New drugs, procedures, and devices for hypertension. Lancet. 2012;380:591–600. doi: 10.1016/S0140-6736(12)60825-3. [DOI] [PubMed] [Google Scholar]
- 5.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 6.Falaschetti E, Mindell J, Knott C, Poulter N. Hypertension management in England: A serial cross-sectional study from 1994 to 2011. Lancet. 2014;383:1912–1919. doi: 10.1016/S0140-6736(14)60688-7. [DOI] [PubMed] [Google Scholar]
- 7.Forte P, Copland M, Smith LM, Milne E, Sutherland J, Benjamin N. Basal nitric oxide synthesis in essential hypertension. Lancet. 1997;349:837–842. doi: 10.1016/S0140-6736(96)07631-3. [DOI] [PubMed] [Google Scholar]
- 8.Linder L, Kiowski W, Buhler FR, Luscher TF. Indirect evidence for release of endothelium-derived relaxing factor in human forearm circulation in vivo. Blunted response in essential hypertension. Circulation. 1990;81:1762–1767. doi: 10.1161/01.cir.81.6.1762. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Janssens SP, Wingler K, Schmidt HHHW, Moens AL. Modulating endothelial nitric oxide synthase: A new cardiovascular therapeutic strategy. Am J Physiol Heart Circ Physiol. 2011;301:H634–H646. doi: 10.1152/ajpheart.01315.2010. [DOI] [PubMed] [Google Scholar]
- 10.Gori T, Mak SS, Kelly S, Parker JD. Evidence supporting abnormalities in nitric oxide synthase function induced by nitroglycerin in humans. J Am Coll Cardiol. 2001;38:1096–1101. doi: 10.1016/s0735-1097(01)01510-8. [DOI] [PubMed] [Google Scholar]
- 11.Elkayam U, Kulick D, McIntosh N, Roth A, Hsueh W, Rahimtoola SH. Incidence of early tolerance to hemodynamic effects of continuous infusion of nitroglycerin in patients with coronary artery disease and heart failure. Circulation. 1987;76:577–584. doi: 10.1161/01.cir.76.3.577. [DOI] [PubMed] [Google Scholar]
- 12.ISIS-4 Collaborative Group ISIS-4: A randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. Lancet. 1995;345:669–685. [PubMed] [Google Scholar]
- 13.Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 14.Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: Measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 16.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 17.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tannenbaum SR, Weisman M, Fett D. The effect of nitrate intake on nitrite formation in human saliva. Food Cosmet Toxicol. 1976;14:549–552. doi: 10.1016/s0015-6264(76)80006-5. [DOI] [PubMed] [Google Scholar]
- 19.Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, Macallister R, Hobbs AJ, Webb AJ, Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: Role for nitrite-derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 20.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 21.Modin A, Bjorne H, Herulf M, Alving K, Weitzberg E, Lundberg JO. Nitrite-derived nitric oxide: A possible mediator of ‘acidic-metabolic’ vasodilation. Acta Physiol Scand. 2001;171:9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 22.Batchelor AM, Bartus K, Reynell C, Constantinou S, Halvey EJ, Held KF, Dostmann WR, Vernon J, Garthwaite J. Exquisite sensitivity to subsecond, picomolar nitric oxide transients conferred on cells by guanylyl cyclase-coupled receptors. Proc Natl Acad Sci USA. 2010;107:22060–22065. doi: 10.1073/pnas.1013147107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh SM, Kapil V, Fuentes-Calvo I, Bubb KJ, Pearl V, Milsom AB, Khambata R, Maleki-Toyserkani S, Yousuf M, Benjamin N, Webb AJ, Caulfield MJ, Hobbs AJ, Ahluwalia A. Enhanced vasodilator activity of nitrite in hypertension: Critical role for erythrocytic xanthine oxidoreductase and translational potential. Hypertension. 2013;61:1091–1102. doi: 10.1161/HYPERTENSIONAHA.111.00933. [DOI] [PubMed] [Google Scholar]
- 24.Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, Sever PS, Thom SM. British hypertension society guidelines for hypertension management 2004 (BHS-IV): Summary. Br Med J. 2004;328:634–640. doi: 10.1136/bmj.328.7440.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilchrist M, Winyard PG, Fulford J, Anning C, Shore AC, Benjamin N. Dietary nitrate supplementation improves reaction time in type 2 diabetes: Development and application of a novel nitrate-depleted beetroot juice placebo. Nitric Oxide. 2014;40:67–74. doi: 10.1016/j.niox.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 27.Kapil V, Haydar SM, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Rad Biol Med. 2013;55:93–100. doi: 10.1016/j.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahra M, Kapil V, Pearl V, Ghosh S, Ahluwalia A. Inorganic nitrate ingestion improves vascular compliance but does not alter flow-mediated dilatation in healthy volunteers. Nitric Oxide. 2012;26:197–202. doi: 10.1016/j.niox.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velmurugan S, Kapil V, Ghosh SM, Davies S, McKnight A, Aboud Z, Khambata RS, Webb AJ, Poole A, Ahluwalia A. Antiplatelet effects of dietary nitrate in healthy volunteers: Involvement of cGMP and influence of sex. Free Rad Biol Med. 2013;65:1521–1532. doi: 10.1016/j.freeradbiomed.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunault CC, van Velzen AG, Sips AJ, Schothorst RC, Meulenbelt J. Bioavailability of sodium nitrite from an aqueous solution in healthy adults. Toxicol Lett. 2009;190:48–53. doi: 10.1016/j.toxlet.2009.06.865. [DOI] [PubMed] [Google Scholar]
- 31.van Velzen AG, Sips AJ, Schothorst RC, Lambers AC, Meulenbelt J. The oral bioavailability of nitrate from nitrate-rich vegetables in humans. Toxicol Lett. 2008;181:177–181. doi: 10.1016/j.toxlet.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Lundberg JO, Gladwin MT, Ahluwalia A, et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asayama K, Thijs L, Brguljan-Hitij J, et al. Risk stratification by self-measured home blood pressure across categories of conventional blood pressure: A participant-level meta-analysis. PLoS Med. 2014;11:e1001591. doi: 10.1371/journal.pmed.1001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: Follow-up results from the pressioni arteriose monitorate e loro associazioni (PAMELA) study. Circulation. 2005;111:1777–1783. doi: 10.1161/01.CIR.0000160923.04524.5B. [DOI] [PubMed] [Google Scholar]
- 35.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. Br Med J. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO, 3rd, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates: Endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 37.Munzel T, Daiber A, Gori T. More answers to the still unresolved question of nitrate tolerance. Eur Heart J. 2013;34:2666–2673. doi: 10.1093/eurheartj/eht249. [DOI] [PubMed] [Google Scholar]
- 38.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleenor BS, Sindler AL, Eng JS, Nair DP, Dodson RB, Seals DR. Sodium nitrite de-stiffening of large elastic arteries with aging: Role of normalization of advanced glycation end-products. Exp Gerontol. 2012;47:588–594. doi: 10.1016/j.exger.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rammos C, Hendgen-Cotta UB, Sobierajski J, Bernard A, Kelm M, Rassaf T. Dietary nitrate reverses vascular dysfunction in older adults with moderately increased cardiovascular risk. J Am Coll Cardiol. 2014;63:1584–1585. doi: 10.1016/j.jacc.2013.08.691. [DOI] [PubMed] [Google Scholar]
- 41.European Food Standards Agency Nitrate in vegetables: Scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008;689:1–79. [Google Scholar]
- 42.Food and Agriculture Organization of the United Nations. World Health Organization . Nitrate (and potential endogenous formation of n-nitroso compounds) WHO Press; Geneva: 2003. [Google Scholar]
- 43.Pluta RM, Oldfield EH, Bakhtian KD, Fathi AR, Smith RK, Devroom HL, Nahavandi M, Woo S, Figg WD, Lonser RR. Safety and feasibility of long-term intravenous sodium nitrite infusion in healthy volunteers. PLoS One. 2011;6:e14504. doi: 10.1371/journal.pone.0014504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bogovski P, Bogovski S. Animal species in which n-nitroso compounds induce cancer. Int J Cancer. 1981;27:471–474. doi: 10.1002/ijc.2910270408. [DOI] [PubMed] [Google Scholar]
- 45.National Toxicology Program . NTP technical report on toxicology and carcinogenesis studies of sodium nitrite in f344/n rats and b6c3f1 mice (drinking water studies) National Institutes of Health; Durham: 2001. [PubMed] [Google Scholar]
- 46.Boffetta P, Couto E, Wichmann J, et al. Fruit and vegetable intake and overall cancer risk in the european prospective investigation into cancer and nutrition (EPIC) J Natl Cancer Inst. 2010;102:529–537. doi: 10.1093/jnci/djq072. [DOI] [PubMed] [Google Scholar]
- 47.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 48.International Consortium for Blood Pressure Genome-Wide Association Studies Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.