Abstract
Male factor infertility is a relatively common condition, affecting at least 6% of men of reproductive age. Typically men with unknown genetic abnormalities resort to using assisted reproductive technologies (ART) to achieve their reproductive goals. Infertile men who father biological children using ART could have a higher incidence of aneuploidy, which is a deviation from the normal haploid or diploid chromosomal state. Aneuploidy can be evaluated using fluorescent in situ hybridization (FISH), a cytogenetic assay that gives an estimate of the frequencies of chromosomal abnormalities. The chromosomes that are generally analyzed in FISH are associated with aneuploidies that are compatible with life, that is, chr.13, 18, 21, X, and Y. The technique is indicated for a variety of reasons, but most importantly in the following: 1) men who despite normal semen parameters suffer recurrent pregnancy loss and 2) men with normal semen parameters, undergoing IVF, but still experiencing recurrent implantation failure. It may be used as a screening tool to help in reproductive and genetic counseling of affected couples, or those who have previously experienced failure of ART. A qualitative analysis of FISH study results allows them to make informed reproductive choices. Given its increasing clinical use in various infertility diagnoses and the development of novel adjunct technologies, one can expect much progress in the areas of preimplantation genetic screening, diagnostics, and therapeutics.
Keywords: male infertility, genetics, aneuploidy, FISH
Introduction
By generally agreed definition, infertility is the inability to produce offspring despite actively attempting to do so for one year. This problem affects ~12% of the population of reproductive age, and in about 50% of cases, it could be due to a male factor (1). Many causes of male factor infertility (for instance, low sperm counts or compromised motility) can be overcome with the use of the assisted reproductive technologies (ART), such as in vitro fertilization (IVF). For more severe male factor infertility, intracytoplasmic sperm injection (ICSI) is used together with IVF to enhance the likelihood of fertilization. These technologies allow males previously considered to be infertile to now father children of their own. Errors arising during stem cell division can be due to various underlying mechanisms, such as extrinsic failure of proper segregation of chromosomes, or from the accrual of DNA damage that is independent of replication. Typically, both types of errors are recognized by the cell’s DNA repair mechanisms, but these (as will be discussed below) can often be faulty themselves, resulting in perpetuation of errors in the affected cell’s progeny. While not every single etiology of male factor infertility is clearly understood, its natural history is known to be multifactorial. With the advent of ARTs such as ICSI, many males affected by the aforementioned conditions are now able to produce offspring, but offspring produced by such means have an increased risk of aneuploidies, in particular of the sex chromosomes (2–4). Given that ICSI is a relatively new technology (less than 30 years old), the long-term effects of the procedure are still poorly understood. One of the major shortcomings of ICSI is the mechanism of selection of the spermatozoon (5). While close attention is paid to selecting from the patient’s sample a sperm that displays the best possible combination of sperm parameters, that is, motility and normal gross (but not strict) morphology (count obviously being irrelevant in this case as the technology is designed to overcome shortcomings in this very parameter), neither of these parameters will ensure the genetic integrity of the resultant embryo. Spermatozoa that are used to undergo a FISH analysis cannot be subsequently used for ART as the various steps of fixing, washing, incubation, but most importantly formamide denaturation prior to sperm head condensation, make the sperm non-viable, and thus not amenable to fertilization. For this reason, a thorough cytogenetic analysis of a particular spermatozoon prior to its fusion with an oocyte (through ICSI) is not possible. The protocol for human sperm FISH illustrates certain limitations with regards to sperm selection. A rather homogenous centrifuged cell mass is placed on a slide and a target region of the probe mixture is selected for fluorescent labeling. The process does not allow for the targeting and selection of individual cells, a potential shortcoming of the assay.
Importantly, men with normal semen parameters who are partners in a couple with recurrent pregnancy loss or recurrent IVF failure are one of the most commonly overlooked subpopulations as sperm aneuploidies in these men could represent a significant but clinically under-appreciated cause of infertility. Cytogenetic analysis of sperm FISH can help with evaluating possible causes of recurrent pregnancy loss or recurrent IVF failure.
What does FISH measure?
FISH is a cytogenetic assay that provides an estimate of the frequency of chromosomal abnormalities by measuring sperm aneuploidy, which is defined as any deviation from the normal haploid or diploid state. It is accepted that gametes of infertile individuals tend to display rates of chromosomal abnormalities higher than those seen in fertile men (6). Arguably, the vast majority of fetuses with any chromosomal aneuploidy are not viable. Therefore, ideally clinical diagnosis of sperm aneuploidy should measure all human autosomes and sex chromosomes. However, because of the high cost to run the assay, the aneuploidies that are of major clinical importance are those that can be non-lethal, and thus compatible with survival (7). They include the autosomal trisomies, such as Trisomy 13 (Patau syndrome), Trisomy 18 (Edwards syndrome), and Trisomy 21 (Down syndrome); and aneuploidies of the sex chromosomes, such as X monosomy (the only human monosomy compatible with life) and Klinefelter syndrome (XXY-XXXXY). Aneuploidy can result from one of three principal mechanisms (7): non-disjunction, anaphase lag, and ineffective checkpoint control. In non-disjunction, which is considered the main etiology of aneuploidy (8), chromosome pairs or sister chromatids fail to segregate properly during the process of cell division and thus result in chromosomal gains or losses. Losses or gains of whole chromosomes are the most significant type of oocyte aneuploidy, similar anomalies also can occur in sperm at an overall rate of 4–5% (9). It is important to note that with increasing paternal age, the chromosomal aberrations that occur in human sperm are more often structural changes, rather than numerical aberrations. Studies specifically show that in males there exists a very significant correlation between structural chromosomal abnormalities and age (10), whereas there is no evidence for increased risk of trisomy with paternal age. Thus, increased paternal age alone would not be an indication for a FISH analysis as aneuploidy rates, all other things being equal, are largely unchanged. It has been proposed that these structural aberrancies stem from underlying errors that arise during stem cell division of spermatogonia in the adult testis (11). Nonetheless, it has been demonstrated that increased gonosomal disomy (X-X-8 in particular) is observed in aged mice, suggesting that advanced paternal age is associated with meiotic defects (12).
Since most aneuploid embryos do not survive past the early stages of development, many instances of aneuploidy are effectively subclinical and cannot be detected by simple observation, that is, by antenatal radiographic diagnostic modalities, or simply at birth. Costly invasive measures such as array comparative genomic hybridization microarrays in combination with preimplantation genetic diagnosis (PGD) and ICSI are used today for identifying aneuploid embryos. However, for couples with high percentages of aneuploid gametes, identification of these anomalies before undertaking expensive ART procedures allows for couples to make educated informed decisions regarding their reproductive options. Sperm FISH analysis provides a simple but effective option to assess the male gamete.
The sperm must be studied directly. In the sperm FISH cytological assay of aneuploidy, the compacted chromatin in the sperm head firstly must undergo treatment to decondense the chromatin in order to enable probes to access the DNA of the spermatozoon. The analysis of these decondensed heads makes possible the study a large number of spermatozoa, (approximately 10^4 per individual probe) in a relatively short period of time. In addition to analyzing a large sample of sperm in order to estimate the frequency of abnormalities, multicolor FISH is employed, which is necessary for reliable results. In fact, two-color FISH is needed for analysis of autosomes, and three colors are used for sex chromosomes to distinguish diploidy from disomy (13). Currently, a major technological advantage of this assay is the use of sophisticated image analysis software that has remarkable sensitivity and is less operator-dependent, although methodology is always validated by direct analysis of selected samples by a trained cytogeneticist. Notable drawbacks of the assay are its limitations in surveying structural chromosomal aberrancies, and its detection of mere segments of chromosomes, rather than entire chromosomes. Despite these shortcomings, multicolor FISH remains the most effective and efficient tool available in the andrology laboratory to assess sperm aneuploidy.
The procedural details of sample preparation and analysis are beyond the scope of this discussion, but a detailed protocol is outlined by Sarrate & Anton (14); they describe the process as having five phases: 1) sample (can be ejaculated, epididymal, or testicular sperm) processing with cell fixation, 2) decondensation, 3) hybridization, 4) post-hybridization washes, and 5) visualization. Hybridized, multicolored, fluorescently labeled DNA probes specific for each chromosome are visualized by a special fluorescence microscope that is equipped with filter sets for the following commonly used colors: FITC, Texas Red, and DAPI/FITC/Texas Red (Figure 1). The number and features of observed signals form the basis for chromosome aneuploidy scoring. Strict scoring criteria ensure the absence of subjective variation. These criteria include: (1) overlapped spermatozoa or sperm heads without a well-defined boundary are not counted; (2) in cases of disomy (one or more chromosomes present in two copies (n+1)) or diploidy (having two sets of chromosomes, (2n)), all signals should have the same intensity and be separated from each other by a distance longer than the diameter of each signal; and, finally, (3) nullisomies (complete lack of one chromosomal pair (2n−2) due to loss of said pair) are not directly scored and are conservatively considered as equivalent to the incidence of disomies (15). Adhering to the procedural steps makes the assay one of the most powerful and accurate tools for the study aneuploidy in human sperm.
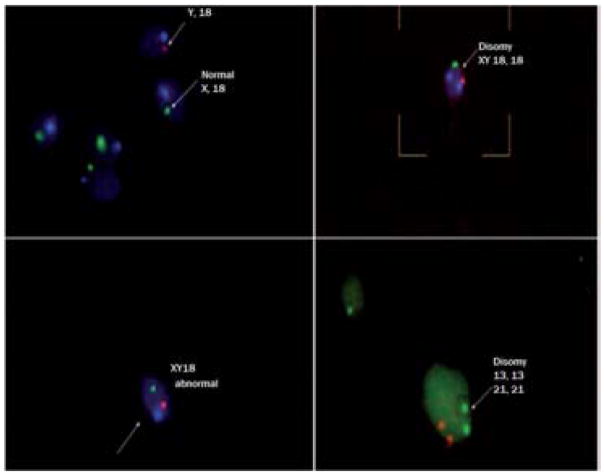
Figure 1.
Examples of sperm fluorescent in situ hybridization (FISH) visualized under a fluorescence microscope. Clockwise from top left: normal sperm, sperm with chromosome 18 and XY disomy, sperm with chromosome 13 and 18 disomy, and sperm with sex chromosome XY disomy.
What are the indications for FISH analysis?
The patients in whom a FISH analysis is clearly indicated fall into the following two categories: 1) Males with normal gross semen parameters whose partners suffer recurrent pregnancy loss and 2) females who have recurrent pregnancy loss after repeated IVF failure (even with a partner who has normal sperm parameters). In addition to genetic counseling, FISH analysis allows these patients to make informed reproductive choices by allowing them to gauge their relative risk of producing offspring with genetic defects. Some patients who experience recurrent pregnancy loss appear to have a significant increase of sperm chromosome aneuploidy, apoptosis, and abnormal sperm morphology. The percentage of aneuploidy sperm is correlated to the percentage of apoptotic sperm and higher frequency of abnormal sperm morphology(16). It is widely known that the gametes of infertile individuals display higher rates of chromosomal abnormalities than the general population (6). Indeed, most studies to date indicate that infertile oligozoospermic men generally have increased numbers of spermatozoa with aneuploidies (Table 1; adapted with permission from (17)). Sarrate and colleagues (18) have demonstrated that the relationship between sperm count and the overall increased rates of chromosome abnormalities in spermatozoa truly illustrates an inverse correlation between these two parameters. Indeed, extremely high levels of sperm aneuploidy were identified in testicular sperm extraction (TESE) samples of men with non-obstructive azoospermia (NOA) (19). A recent study demonstrated that testicular sperm exhibited significantly lower DNA damage in comparison with ejaculated spermatozoa; however, testicular sperm had higher aneuploidy rates in chromosomes 13, 18, 21, X, and Y (20). Whereas testicular spermatozoa seem most suitable for ICSI because of their lower rates of DNA damage, this potential benefit may actually be offset by increased aneuploidy rates in testicular spermatozoa.
Table 1.
Indications for sperm fluorescent in situ hybridization (FISH), (24)
| Indication | Study |
|---|---|
| Recurrent pregnancy loss | Bernardini et al. [2004] |
| Repeated in vitro fertilization failure | Petit et al. [2005] |
| Oligozoospermia | Martin [2007] |
| Asthenozoospermia | Collodel et al. [2007a] |
| Nonobstructive azoospermia (testicular sperm) | Huang et al. [1999] Palermo et al. [2002] |
| Obstructive azoospermia (epididymal sperm) | Sukcharoen et al. [2003] |
| Teratozoospermia | |
| Tail agenesis | Carrell et al. [2004] |
| Multiflagellar sperm/Macrocephalic sperm | Devillard et al. [2002] |
| Round head-only syndrome (globozoospermia) | Morel et al. [2004] |
| Double-head or multinucleated sperm | Moretti et al. [2007] |
| Other severe morphologic defects | Lewis-Jones et al. [2003] |
| Genetic abnormalities | |
| Robertsonian translocations | Sarrate et al. [2005] |
| Reciprocal translocations | Sarrate et al. [2005] |
| Chromosome inversions | Sarrate et al. [2005] |
| Ring chromosome | Arnedo et al. [2005] |
| Klinefelter syndrome (mosaic and nonmosaic) | Kruse et al. [1998] Lim et al. [1999] Rives et al. [2000] |
| Y-chromosome microdeletions | Minor et al. [2007] |
There are numerous checkpoints to ensure genomic integrity is maintained during spermatogenesis, which lead to arrest of replication of cells marked by any chromosomal abnormalities. Globally, this restriction on output leads to a reduced production of gametes, which could, in part, explain the lower sperm counts of individuals with higher frequencies of chromosomal abnormalities in their spermatozoa. Particularly in men with NOA, the incidence of aneuploidy was significantly greater among the diploid nuclei, which suggests that chromosome instability is a result of altered genetic control during mitotic cell division and proliferation that occurs during spermatogenesis (19).
A plausible mechanism for the increased rates of aforementioned abnormalities may be underlying deficiencies in the control mechanisms that make up the various selective checkpoints. Such deficiencies could be, for instance, errors in identification of cells with abnormalities, a malfunctioning of the cellular elimination process, or an abnormal cell count that overwhelms the functional capacity of the control systems (18). The clinical manifestations of these insufficiencies are quite heterogeneous, complicating the delineation of groups of patients who would have a clear indication for FISH analyses (Table 2; adapted with permission from (18). Typically, the semen parameters of those partners of couples with recurrent pregnancy loss are within the normal range for count, motility and morphology.
Table 2.
Patients with abnormal FISH results classified according to their seminal parameters (23)
| Seminal Parameters | Men with abnormal FISH (%) |
|---|---|
| Asthenoteratozoospermic (AT) | 6/71 (8.5) |
| Asthenozoospermic (A) | 4/67 (6.0) |
| Normozoospermic (N) | 4/34 (11.8) |
| Oligoasthenoteratozoospermic (OAT) | 13/62 (21) |
| Oligoasthenozoospermic (OA) | 17/51 (33) |
| Oligoteratozoospermic (OT) | 2/13 (15) |
| Oligozoospermic (O) | 2/4 (50) |
| Teratozoospermic (T) | 1/17 (6) |
| Total | 49/319 (15.3) |
How can we interpret FISH results?
The chromosomes that are generally analyzed in FISH are those aneuploidies that are compatible with life, that is, 13, 18, 21, X, and Y. While other studies (21) examined additional chromosomes, such as 4, 6, 7, 8, 9, 10, 11, 12, and 17, among others, these chromosomes are not included in a routine sperm FISH analysis, as their diagnostic yield is relatively low when compared to the five relevant chromosomes. All current studies agree that there is a definite relationship between aneuploidies and male factor infertility. There is also agreement that most chromosomal anomalies are not isolated events, but frequently involve the sex chromosomes in addition to one or more autosomes (18). One study suggests that the analysis of recombination events between sex chromosomes could be a useful indicator for identifying men with an increased risk of producing chromosomally abnormal spermatozoa (22).
Given the subject of the analysis, the question that arises is how FISH results are interpreted. Abnormal results can be evaluated using the qualitative and the quantitative approach (23). The quantitative approach enables interpretation of FISH as a numerical value (a score, essentially) that would indicate the patient’s degree of risk. Major disadvantages of this approach are the features intrinsic to this technique: An analysis of the entire karyotype (that is, all autosomes and the sex chromosomes of an individual) as opposed to the ones with demonstrated clinical relevance (13, 18, 21, X, and Y) is a rather difficult task. Furthermore, some chromosomal aberrancy, for instance, nullisomies, is not accounted for. An additional restriction is the strictness of inclusion criteria, which tends to underestimate the extent of anomaly rates, thus calling for the consideration of qualitative analysis, which is, in fact, the second perspective: here, significant escalations of anomalies are essentially seen as evidence of some insult to proper spermatogenesis, typically the various types of meiotic defects (mis-association of pairs, recombination errors, or mis-segregation). A qualitative way of analyzing defects substantiates the idea that the examination of a few chromosomes (21, X, and Y) is sufficient to pinpoint meiotic defects, thus identifying most at-risk patients. That such an approach should be favored over a quantitative one is also demonstrated by the fact that the latter fails to adequately explain the clinical sequelae of chromosomal defects in spermatozoa during the treatment process of ART (18). Proper qualitative analysis of results allows for the next logical step, the consideration of their clinical significance.
Can FISH results change therapy?
The advent of new reproductive technologies such as IVF and ICSI has helped previously infertile men with abnormal sperm density to father biological children. Unfortunately, men with abnormal semen parameters can have an increased proportion of chromosomal defects, and chromosomal defects potentially can be passed on to the offspring of couples taking advantage of ART. A routine semen analysis provides clinical information on gross seminal parameters, specifically valuable information regarding spermatogenesis, genital tract secretions and ejaculation, but unless the male is azoospermic the results are quite limited with regard to defining infertility, risk stratification and prognostication (24). Currently, sperm FISH is used mainly in the determination of aneuploidy in the sex chromosomes and autosomes of normospermic partners in couples with recurrent pregnancy loss and for men with infertility. However, depending upon the probes used for diagnosis, sperm FISH analysis can aid in the quantification of the probability of transmitting aneuploidies and complex chromosomal rearrangements, such as translocations and inversions (25) to offspring. Therefore, sperm FISH is increasingly utilized in infertility diagnostic protocols, because its diagnostic yield allows affected couples to make informed reproductive choices (17). For instance, if the likelihood of transmitting chromosomal defects to offspring is deemed too high, they may choose to pursue other options, such as PGD with ICSI to select seemingly normal embryos. FISH analysis of spermatozoa is an effective genetic screening tool for infertile patients. Nevertheless, the simple quantitative approach described above, (the numerical chromosomal copy value), may not be adequate when applied in isolation. However, the results become very powerful when used in the context of other tests, such as the semen analysis or the patient’s clinical history. Stochastic chromosomal abnormalities, different baselines with regards to mutation, and selection of adequate controls, among other things, are important considerations when assessing a patient’s risk for aneuploidy. Abnormal results would place a patient in the “at risk” category, and the next step in management at that time would include genetic counseling prior to undergoing ART with ICSI so couples can make an informed choice.
Does the test improve pregnancy/delivery rates?
Men affected by male factor infertility have higher rates of aneuploidy than healthy males. And despite the higher rates of disomy in the sex chromosomes of sperm in male partners of women with recurrent miscarriage, a cytogenetic survey of the products of conception (POC) of couples who suffered recurrent pregnancy loss (RPL) often does not reveal a higher rate of sex chromosome aneuploidy (26). This suggests that sperm with abnormal karyotypes may be selected against prior to potential fertilization, which is consistent with what has been suggested earlier, a mechanism that tries to prevent deleterious alleles from being passed on to offspring. Importantly, there is a subset of men whose partners experience unexplained pregnancy loss. Many of these men have relatively normal semen parameters, but display exceedingly high levels of sperm aneuploidy with the majority of chromosomes measured abnormal.
FISH is not routinely performed in this country because there are few laboratories skilled in this analysis, also because of its high cost, and lack of insurance coverage. Nonetheless, sperm FISH does identify meiotic defects and thus guides reproductive counseling and clinical management, and it allows the couple to make informed, albeit often difficult, reproductive decisions, including the pursuit of PGD, adoption, or donor sperm (17). The next step in the management of such a case would be genetic counseling. Given the nature of this cytogenetic test that is useful for screening and diagnostics, sperm FISH does not affect the quality of sperm or pregnancy outcomes directly, but because of its capability to detect aneuploidy with remarkable sensitivity and specificity, it does guide reproductive choices, which, in turn, may indirectly lead to a net increase of favorable pregnancy outcomes.
Appropriate study designs needed to determine the utility of sperm FISH
In order to assess the utility of sperm FISH, one would ideally conduct a randomized controlled trial (RCT) to evaluate the utility of FISH. In such a case, PICO method (Population, Intervention, Comparison, and Outcome) would lead to a study design that randomizes a study population of clinically infertile men into two groups, one that undergoes the diagnostic intervention, that is, analysis of spermatozoa with FISH, and another one that does not. Following the intervention or placebo (i.e. sample collection without diagnostic test), both groups would present for follow-up for comparison of results. In theory, this sounds rather simple; however, in reality, it is marked by numerous problems: most RCTs on preimplantation genetic screening (PGS, a test that screens for numerical chromosomal aberrancies in individuals with compromised fertility despite normal karyotypes) tests, of which FISH is one (27), demonstrate no favorable effects in the treatment groups (28). Because of the relative lack of significant positive findings to date, there appears to be a movement of discouraging physicians from enrolling patients in such trials, citing ethical reasons (29). On the other hand, it is argued by others that PGS RCTs are methodologically flawed (30). Procedural shortcomings can be addressed by implementing a stratification protocol that mandates sub-analysis for the various indications of PGS tests like FISH. Other types of studies, such as retrospective reviews, meta-analyses and systematic reviews can be conducted instead. Retrospective studies are limited because of paucity of data, and lack of usage of standardized protocols. Similar restrictions affect meta-analyses or systematic reviews, whose output is limited by the existence of much disagreement among the RCTs implemented to date. Furthermore, a lack of standardization of data interpretation further complicates the picture.
Conclusions
FISH is the quickest, most sophisticated, accurate, and reliable test to assess aneuploidy of autosomes and sex chromosomes in sperm nuclei of infertile men and couples with unexplained pregnancy loss. Clinically, it is a widely used screening tool that can be used in counseling couples affected by male factor infertility to make informed choices regarding their reproductive plans. This is particularly important considering the increased risk of aneuploidies affecting embryos conceived by means of ART and the increased likelihood of chromosomal defects associated with recurrent pregnancy loss. Further studies are obviously required to assess the value of routine use of multicolor sperm FISH in diagnostic medicine, but to this date, limitations such as high cost, limited accessibility, and lack of standardized risk assessment protocols indicate that despite being a highly sophisticated assay, its routine clinical application in evaluation of male infertility is something that remains to be seen.
Acknowledgments
Financial support: RR is a K12 scholar supported by a Male Reproductive Health Research (MRHR) Career Development Physician-Scientist Award (Grant # HD073917-01) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Program to DJL.
Footnotes
Conflict of Interest: The authors have no potential conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital and health statistics Series 23, Data from the National Survey of Family Growth. 2005:1–160. [PubMed] [Google Scholar]
- 2.Hansen M, Bower C, Milne E, de Klerk N, Kurinczuk JJ. Assisted reproductive technologies and the risk of birth defects--a systematic review. Human reproduction. 2005;20:328–38. doi: 10.1093/humrep/deh593. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig M. Risk during pregnancy and birth after assisted reproductive technologies: an integral view of the problem. Seminars in reproductive medicine. 2005;23:363–70. doi: 10.1055/s-2005-923394. [DOI] [PubMed] [Google Scholar]
- 4.Rimm AA, Katayama AC, Diaz M, Katayama KP. A meta-analysis of controlled studies comparing major malformation rates in IVF and ICSI infants with naturally conceived children. Journal of assisted reproduction and genetics. 2004;21:437–43. doi: 10.1007/s10815-004-8760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alter L, Boitrelle F, Sifer C. How can we nowadays select the best embryo to transfer? Gynecologie, obstetrique & fertilite. 2014;42:515–25. doi: 10.1016/j.gyobfe.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Egozcue J, Blanco J, Anton E, Egozcue S, Sarrate Z, Vidal F. Genetic Analysis of Sperm and Implications of Severe Male Infertility—A Review. Placenta. 2003;24:S62–S5. doi: 10.1016/s0143-4004(03)00186-3. [DOI] [PubMed] [Google Scholar]
- 7.Templado C, Uroz L, Estop A. New insights on the origin and relevance of aneuploidy in human spermatozoa. Molecular human reproduction. 2013;19:634–43. doi: 10.1093/molehr/gat039. [DOI] [PubMed] [Google Scholar]
- 8.Márquez C, Egozcue J, Martorell MR, Moreno V, Templado C. Colcemid increases the frequency of chromosome abnormalities in human sperm. Cytogenet Cell Genet. 1996;72:164–70. doi: 10.1159/000134177. [DOI] [PubMed] [Google Scholar]
- 9.Templado C, Vidal F, Estop A. Aneuploidy in human spermatozoa. Cytogenet Genome Res. 2011;133:91–9. doi: 10.1159/000323795. [DOI] [PubMed] [Google Scholar]
- 10.Martin RH, Rademaker AW. The effect of age on the frequency of sperm chromosomal abnormalities in normal men. Am J Hum Genet. 1987;41:484–92. [PMC free article] [PubMed] [Google Scholar]
- 11.Templado C, Donate A, Giraldo J, Bosch M, Estop A. Advanced age increases chromosome structural abnormalities in human spermatozoa. Eur J Hum Genet. 2011;19:145–51. doi: 10.1038/ejhg.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinkworth MH, Schmid TE. Effect of age on testicular germ cell apoptosis and sperm aneuploidy in MF-1 mice. Teratogenesis, carcinogenesis, and mutagenesis. 2003;(Suppl 2):103–9. doi: 10.1002/tcm.10085. [DOI] [PubMed] [Google Scholar]
- 13.Shi Q, Martin RH. Aneuploidy in human spermatozoa: FISH analysis in men with constitutional chromosomal abnormalities, and in infertile men. Reproduction. 2001;121:655–66. doi: 10.1530/rep.0.1210655. [DOI] [PubMed] [Google Scholar]
- 14.Sarrate Z, Anton E. Fluorescence in situ hybridization (FISH) protocol in human sperm. Journal of visualized experiments: JoVE. 2009 doi: 10.3791/1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanco J, Gabau E, Gomez D, Baena N, Guitart M, Egozcue J, et al. Chromosome 21 disomy in the spermatozoa of the fathers of children with trisomy 21, in a population with a high prevalence of Down syndrome: increased incidence in cases of paternal origin. Am J Hum Genet. 1998;63:1067–72. doi: 10.1086/302058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrell DT, Wilcox AL, Lowy L, Peterson CM, Jones KP, Erickson L, et al. Elevated sperm chromosome aneuploidy and apoptosis in patients with unexplained recurrent pregnancy loss. Obstetrics and gynecology. 2003;101:1229–35. doi: 10.1016/s0029-7844(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 17.Hwang K, Weedin JW, Lamb DJ. The use of fluorescent in situ hybridization in male infertility. Therapeutic advances in urology. 2010;2:157–69. doi: 10.1177/1756287210373758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarrate Z, Vidal F, Blanco J. Role of sperm fluorescent in situ hybridization studies in infertile patients: indications, study approach, and clinical relevance. Fertility and Sterility. 2010;93:1892–902. doi: 10.1016/j.fertnstert.2008.12.139. [DOI] [PubMed] [Google Scholar]
- 19.Huang WJ, Lamb DJ, Kim ED, de Lara J, Lin WW, Lipshultz LI, et al. Germ-cell nondisjunction in testes biopsies of men with idiopathic infertility. Am J Hum Genet. 1999;64:1638–45. doi: 10.1086/302402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moskovtsev SI, Alladin N, Lo KC, Jarvi K, Mullen JB, Librach CL. A comparison of ejaculated and testicular spermatozoa aneuploidy rates in patients with high sperm DNA damage. Systems biology in reproductive medicine. 2012;58:142–8. doi: 10.3109/19396368.2012.667504. [DOI] [PubMed] [Google Scholar]
- 21.Pang MG, Hoegerman SF, Cuticchia AJ, Moon SY, Doncel GF, Acosta AA, et al. Detection of aneuploidy for chromosomes 4, 6, 7, 8, 9, 10, 11, 12, 13, 17, 18, 21, X and Y by fluorescence in-situ hybridization in spermatozoa from nine patients with oligoasthenoteratozoospermia undergoing intracytoplasmic sperm injection. Human reproduction. 1999;14:1266–73. doi: 10.1093/humrep/14.5.1266. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson KA, Wong EC, Chow V, Nigro M, Ma S. Abnormal meiotic recombination in infertile men and its association with sperm aneuploidy. Hum Mol Genet. 2007;16:2870–9. doi: 10.1093/hmg/ddm246. [DOI] [PubMed] [Google Scholar]
- 23.Colls P, Templado C, Martinez-Pasarell O, Darroudi F, Natarajan AT. Sequential G-banding FISH on human sperm chromosomes. Chromosome research: an international journal on the molecular, supramolecular and evolutionary aspects of chromosome biology. 1997;5:457–61. doi: 10.1023/a:1018464929628. [DOI] [PubMed] [Google Scholar]
- 24.Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, et al. Sperm morphology, motility, and concentration in fertile and infertile men. The New England journal of medicine. 2001;345:1388–93. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 25.Egozcue J, Sarrate Z, Codina-Pascual M, Egozcue S, Oliver-Bonet M, Blanco J, et al. Meiotic abnormalities in infertile males. Cytogenet Genome Res. 2005;111:337–42. doi: 10.1159/000086907. [DOI] [PubMed] [Google Scholar]
- 26.Practice Committee of the American Society for Reproductive M. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103–11. doi: 10.1016/j.fertnstert.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 27.Scriven PN, Ogilvie CM. FISH for Pre-implantation Genetic Diagnosis. In: Bridger JM, Volpi EV, editors. Fluorescence in situ Hybridization (FISH) Humana Press; 2010. pp. 269–82. [DOI] [PubMed] [Google Scholar]
- 28.Rubio C, Bellver J, Rodrigo L, Bosch E, Mercader A, Vidal C, et al. Preimplantation genetic screening using fluorescence in situ hybridization in patients with repetitive implantation failure and advanced maternal age: two randomized trials. Fertil Steril. 2013;99:1400–7. doi: 10.1016/j.fertnstert.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 29.Hernández ER. What next for preimplantation genetic screening? Beyond aneuploidy Hum Reprod. 2009;24:1538–41. doi: 10.1093/humrep/dep078. [DOI] [PubMed] [Google Scholar]
- 30.Simpson JL. What next for preimplantation genetic screening? Randomized clinical trial in assessing PGS: necessary but not sufficient. Human reproduction. 2008;23:2179–81. doi: 10.1093/humrep/den250. [DOI] [PubMed] [Google Scholar]