Abstract
In utero or early-life vitamin D deficiency is associated with skeletal problems, type 1 diabetes, and schizophrenia, but the prevalence of vitamin D deficiency in U.S. pregnant women is unexplored. We sought to assess vitamin D status of pregnant women and their neonates residing in Pittsburgh by race and season. Serum 25-hydroxyvitamin D (25(OH)D) was measured at 4–21 wk gestation and predelivery in 200 white and 200 black pregnant women and in cord blood of their neonates. Over 90% of women used prenatal vitamins. Women and neonates were classified as vitamin D deficient [25(OH)D <37.5 nmol/L], insufficient [25(OH)D 37.5–80 nmol/L], or sufficient [25(OH)D >80 nmol/L]. At delivery, vitamin D deficiency and insufficiency occurred in 29.2% and 54.1% of black women and 45.6% and 46.8% black neonates, respectively. Five percent and 42.1% of white women and 9.7% and 56.4% of white neonates were vitamin D deficient and insufficient, respectively. Results were similar at <22 wk gestation. After adjustment for prepregnancy BMI and periconceptional multivitamin use, black women had a smaller mean increase in maternal 25(OH)D compared with white women from winter to summer (16.0 ± 3.3 nmol/L vs. 23.2 ± 3.7 nmol/L) and from spring to summer (13.2 ± 3.0 nmol/L vs. 27.6 ± 4.7 nmol/L) (P < 0.01). These results suggest that black and white pregnant women and neonates residing in the northern US are at high risk of vitamin D insufficiency, even when mothers are compliant with prenatal vitamins. Higher-dose supplementation is needed to improve maternal and neonatal vitamin D nutriture.
Introduction
Rickets, once thought to have been nearly eradicated in the United States in the 1930s (1), has again become a major public health problem. Several reports have been published describing recent cases of rickets in infants, most of whom were black and exclusively breastfed (2–5). The reemergence of rickets is thought to be due to an epidemic of vitamin D deficiency in mothers and children (6). A newborn’s vitamin D stores are completely reliant on vitamin D from the mother (7). Not surprisingly, poor maternal vitamin D status during pregnancy is a major risk factor for infant rickets (8–10).
In addition to causing poor global mineralization of the skeleton, vitamin D deficiency has implications for numerous other nonskeletal health outcomes. In utero or early life vitamin D deficiency has been linked to an increased risk of type 1 diabetes (11), asthma (12), and schizophrenia (13,14). Fascinating new data also show that vitamin D regulates placental development and function (15), which suggests that maternal vitamin D status may be associated with adverse outcomes of pregnancy, such as miscarriage, preeclampsia, and preterm birth.
The most important source of vitamin D is the skin’s synthesis of the vitamin from UV B solar radiation (16). Any process that reduces UV B photons from entering the epidermis will diminish cholecalciferol (vitamin D-3) production. The skin pigment melanin absorbs UV B photons and can reduce vitamin D-3 synthesis by >90% (17). Consequently, African Americans are at high risk of vitamin D deficiency. The most recent data from the National Health and Nutrition Examination Survey (1988–1994) indicated that vitamin D deficiency [25-hydroxy vitamin D[25(OH)D]≤37.5 nmol/L] was prevalent in42%of black childbearing-aged women and only 4% of white childbearing-aged women residing throughout the United States (18). Vitamin D status is also worsened in winter months (November through March), when, at latitudes above 37°, less UV B radiation reaches the earth and little or no vitamin D can be synthesized in the skin (16,19). Indeed, vitamin D deficiency in U.S. childbearing-aged women was more than 3 times as common in winter than summer in both blacks and whites (18).
Despite the striking racial disparity in vitamin D deficiency and the strong influence of season, there are few recent investigations into the vitamin D status of U.S. black and white pregnant women and their neonates throughout the year. Given the public health importance of adequate vitamin D in this group and the racial disparity in a number of relevant disease outcomes, we sought to assess the vitamin D status of a cohort of nulliparous pregnant women residing in Pittsburgh, Pennsylvania (latitude 40°N) and their neonates by race and season.
Subjects and Methods
Data came from the Pregnancy Exposures and Preeclampsia Prevention Study (20). Women carrying singleton fetuses were enrolled at <16 wk gestation from outpatient clinics at Magee-Women’s Hospital in Pittsburgh, Pennsylvania and affiliated private practices from 1997 to 2001. The response rate was 72%. After providing informed, written consent, all subjects completed an interviewer-administered questionnaire at enrollment to collect data on sociodemographic factors, medical history, and health behaviors. Nonfasting blood samples were collected at times of usual blood draws for clinical indications (initial visit, 16–18 wk, 26–29 wk, and predelivery) and banked. Medical records were abstracted to ascertain blood pressures and urinary protein measurements throughout gestation, delivery data, and neonatal outcomes. Venous cord serum samples were also collected and banked. The University of Pittsburgh and Magee-Women’s Hospital Institutional Review Boards approved the study.
A total of 2211 women enrolled in the study and had complete data on pregnancy outcomes. For the current analysis, we used SAS random number generator to randomly select 200 white women and 200 black women who were nulliparous (i.e. had no previous pregnancies lasting >20 wk), had no preexisting medical conditions, and had an uncomplicated index pregnancy delivered at term (37–42 wk) with an appropriately grown infant. From each of the 400 women, we sought to select 1 serum sample at <22 wk gestation, 1 predelivery serum sample, and a cord serum sample. If a woman had more than 1 sample collected at <22 wk gestation, we randomly selected 1 sample using SAS random number generator. Of the 200 white women selected, 198 had an available banked serum sample collected at <22 wk, 199 had an available banked serum sample collected predelivery, and 195 had an available banked cord serum sample. These numbers were 194, 185, and 171 for the 200 black women, respectively.
Quantitation of serum 25(OH)D
Maternal and cord serum samples were stored in aliquots at −80°C until they were analyzed for 25(OH)D. Quantitation of serum 25(OH)D was performed using a commercial ELISA from Immunodiagnostic Systems Limited and validated against an HPLC method. The ELISA was performed according to the manufacturer’s written protocol and all samples were assayed in duplicate. The ELISA could detect 25(OH)D in the range of 5–300 nmol/L. No sample in our analysis fell outside this detectable range. The interassay CV for the ELISA was 10.3%. The ELISA recognized 100% of 25(OH) D3 and 75% of 25(OH) ergocalciferol (vitamin D-2) but did not distinguish between these 2 forms. In our initial HPLC validation, we observed that only 3 of 32 samples (<10%) had any measurable 25(OH) D2, and within these samples, 25(OH)D2 accounted for only 10% of the total measurable 25(OH)D.
The HPLC method for the quantification of 25(OH)D was modified from those published by Horst et al. (21) and Alvarez and De Mazancourt (22). Briefly, serum samples were spiked with an internal standard (1 α-hydroxy vitamin D-3 purchased from Sigma) to a final concentration of 50 nmol/L. Samples were processed twice by solid phase extraction, first using C18 Sep-pak cartridges and then using Silica Sep-pak cartridges. After extraction, samples were dried completely under a nitrogen stream at 37°C and reconstituted in 100% acetonitrile. Fifty microliters of the extracted samples was injected onto a prepared HPLC system that consisted of a Waters 600E pump, a Waters 717 plus autosampler, and a Waters 996 photodiode array. We separated samples on a C18 column (150 × 4.8 mm, 5µm, Supelco) with a 20- × 4.8-mm guard column in line. The HPLC method could separate 1 α-hydroxy vitamin D-3, 25(OH)D2, and 25(OH)D3 using isocratic conditions with a mobile phase that consisted of 87% acetonitrile with all components being detected at a wavelength of 265 nm. For most serum samples, only 25(OH)D3 was detectable. However, a few samples had small but measurable amounts of 25(OH)D2. The interassay CV for 25(OH)D3 using the HPLC method was 5.8%. The sensitivity of the HPLC method was <10 nmol/L and had a linear range to 1000 nmol/L. The relation between serum 25(OH)D concentrations obtained from the ELISA compared with HPLC was as follows: slope = 1.14, intercept = 22, r = 0.88. Because the ELISA overestimated concentrations of 25(OH)D by ~25%, we adjusted the values obtained by ELISA so they would be in better agreement with data obtained by HPLC.
For our analysis, we classified women and neonates into 1 of 3 groups that defined vitamin D status: vitamin D deficiency [25(OH)D <37.5 nmol/L], vitamin D insufficiency [25(OH)D37.5–80nmol/L], and vitamin D sufficiency [25(OH)D >80 nmol/L] (6,23). The same cutoffs were used for both women and neonates, because experts contend that there is no reason to think the definition of vitamin D sufficiency varies by age (6).
Race/ethnicity was self-reported as non-Hispanic white or non- Hispanic black. Season of sample collection was defined as winter (December, January, February), spring (March, April, May), summer (June, July, August), and fall (September, October, November). Prepregnancy BMI [weight (kg)/height (m)2] was based on measured height and maternal self-report of prepregnancy weight at the initial visit. Self-reported data were available on gravidity (i.e. number of times a woman has been pregnant: 1, 2, ≥3), marital status (married, unmarried), maternal education (<12, 12, >12 y), and smoking status (smoker, nonsmoker). At enrollment, women self-reported their regular use of multivitamins or prenatal vitamins in the periconceptional period (defined as the 3 mo before and 3 mo after conception), and at delivery, women reported their regular use in the last 3 mo of pregnancy.
Statistical analysis
Nonparametric data were log-transformed before statistical tests were performed. Student’s t tests and chi-square tests were used to compare mean 25(OH)D concentrations and proportion of vitamin D deficiency and insufficiency by race/ethnicity. A P-value of 0.05 defined significance. Spearman rank correlation coefficient was used to test for correlations between maternal and cord serum 25(OH)D concentrations. Multivariable regression models were built to assess the independent effect of season on vitamin D status of mothers and newborns. First, we fitted 2 multivariable linear regression models with either maternal or neonatal 25(OH)D concentration as the dependent variable and a series of indicator variables for season as the primary independent variables. Second, we fitted 2 multivariable log-binomial regression models with either maternal or neonatal vitamin D insufficiency (binary) as the dependent variable and season as the primary independent variable. We used generalized estimating equations for both maternal linear and log-binomial models to account for the nonindependence of repeated serum measurements among women (24).
We fit parsimonious regression models by specifying full models with potential effect modifiers and confounding variables (season, race/ethnicity, maternal age, education, marital status, gravidity, maternal prepregnancy BMI, smoking status, periconceptional multivitamin use, multivitamin use in the last 3 mo of pregnancy, and sample gestational age). Effect modification by race and blood sample gestational age were tested separately using Wald P-values (α = 0.10). Potential confounders were considered to not be influential and were removed from the model if their inclusion did not satisfy our a priori change-in-estimate criterion (a change in the coefficient of >8%). Sample gestational age, maternal prepregnancy BMI, and periconceptional multivitamin use met our definition of confounding and were included in the final models.
Values in the text are means ± SEM or mean [95% CI]. Stata software version 8.0 was used for all data analysis.
Results
Black women were more likely than white women to be <20 y old, unmarried, less educated, nonsmokers, obese, and nonusers of periconceptional multivitamins (Table 1). The maternal serum samples were drawn at similar gestational ages in black and white women.
TABLE 1.
Characteristics of white and black pregnant nulliparous women1
| White women, n = 200 |
Black women, n = 200 |
P-value | |
|---|---|---|---|
| Maternal age, % | |||
| <20 y | 23.5 | 54.0 | <0.01 |
| 20–29 y | 56.5 | 41.0 | |
| ≥30 y | 41.0 | 5.0 | |
| Marital status, % | |||
| Married | 38.0 | 3.0 | <0.01 |
| Unmarried | 62.0 | 97.0 | |
| Maternal education, % | |||
| <12 y | 15.5 | 31.0 | <0.01 |
| 12 y | 25.0 | 33.0 | |
| >12 y | 59.5 | 36.0 | |
| Gravidity,2 % | |||
| 1 | 72.5 | 69.5 | 0.31 |
| 2 | 19.5 | 24.0 | |
| ≥3 | 8.0 | 6.5 | |
| Smoking status, % | |||
| Smokers | 52.5 | 41.5 | <0.05 |
| Nonsmokers | 47.5 | 58.5 | |
| Prepregnancy BMI, % | |||
| <18.5 kg/m2 | 8.0 | 4.0 | <0.01 |
| 18.5–24.9 kg/m2 | 58.0 | 48.5 | |
| 25.0–29.9 kg/m2 | 21.0 | 23.0 | |
| ≥30.0 kg/m2 | 13.0 | 24.5 | |
| Regular3 periconceptional4 multivitamin use, % | |||
| Yes | 66.5 | 33.5 | <0.01 |
| No | 44.5 | 55.5 | |
| Regular3 multivitamin use in the last 3 mo of pregnancy, % | |||
| Yes | 94.3 | 91.9 | 0.36 |
| No | 5.7 | 8.1 | |
| Median (range) gestational age of blood sample <22 wk | 10.2 (4.4–20.9) | 11.7 (4.7–20.4) | 0.06 |
| Median (range) gestational age of blood sample at delivery, wk | 40.0 (37–42.3) | 39.9 (37–42) | 0.89 |
Data are presented as percent or median (range).
Defined as the number of times a woman has been pregnant.
Defined as self-reported use of multivitamins or prenatal vitamins at least once per week.
Defined as the 3 mo before conception and the 3 mo after conception.
The unadjusted mean maternal serum 25(OH)D concentrations at 4–21 wk gestation and at term were significantly higher among white women than black women (Table 2). At 4–21 wk gestation, the vast majority of black women were either vitamin D deficient or insufficient. This is in sharp contrast to white women, almost none of whom were vitamin D deficient at 4–21 wk gestation. However, white women had a high likelihood of vitamin D insufficiency. Results were similar for black and white women in samples collected before delivery (Table 2). Notably, we observed differences in mean 25(OH)D at 4–21 wk by periconceptional multivitamin use. Regular users of periconceptional multivitamins had higher serum 25(OH)D than nonusers in both white [76.7 (72.0, 81.7) nmol/L vs. 68.8 (63.2, 74.9) nmol/L, P < 0.05] and black women [46.0 (42.0, 50.5) nmol/L vs. 37.7 (35.0, 40.6) nmol/L, P < 0.01].
TABLE 2.
Vitamin D status of white and black pregnant women and their neonates1
| White women, n = 200 |
Black women, n = 200 |
|
|---|---|---|
| 4–21 wk gestation | ||
| Serum 25(OH)D,2 nmol/L | 73.1 (69.4, 76.9) | 40.2 (37.9, 42.7)* |
| Vitamin D status, % | ||
| Deficient: 25(OH)D <37.5 nmol/L | 2.0 | 44.9** |
| Insufficient: 25(OH)D 37.5–80 nmol/L | 60.3 | 51.0 |
| Sufficient: 25(OH)D >80 nmol/L | 37.3 | 4.1 |
| 37–42 wk gestation | ||
| Serum 25(OH)D, nmol/L | 80.4 (76.0, 85.1) | 49.4 (46.1, 52.9)* |
| Vitamin D status, % | ||
| Deficient: 25(OH)D <37.5 nmol/L | 5.0 | 29.2** |
| Insufficient: 25(OH)D 37.5–80 nmol/L | 41.2 | 54.1 |
| Sufficient: 25(OH)D >80 nmol/L | 53.8 | 16.7 |
| Cord blood | ||
| Serum 25(OH)D, nmol/L | 67.4 (63.8, 71.3) | 39.0 (36.3, 41.8)* |
| Vitamin D status, % | ||
| Deficient: 25(OH)D <37.5 nmol/L | 9.7 | 45.6** |
| Insufficient: 25(OH)D 37.5–80 nmol/L | 56.4 | 46.8 |
| Sufficient: 25(OH)D >80 nmol/L | 33.9 | 7.6 |
Values are geometric means [95%CI] or %.
Different from white women, P < 0.001 (student’s t test);
different from white women, P < 0.001 (chi-square test).
Log-transformed to ensure normality.
The unadjusted mean cord serum 25(OH)D concentrations were significantly higher in offspring of white mothers than offspring of black mothers (Table 2). Among white neonates, about two-thirds had 25(OH)D ≤80 nmol/L. Furthermore, unadjusted mean cord serum 25(OH)D was lower in mothers who had a predelivery serum 25(OH)D ≤80 nmol/L than mothers with 25(OH)D >80 nmol/L [mean [95%CI]: black mothers: 34.2 (32.0, 36.7) nmol/L vs. 76.0 (68.6, 84.1) nmol/L, P < 0.001; white mothers: 51.2 (47.6, 55.2) nmol/L vs. 86.2 (82.4, 90.1) nmol/L, P < 0.001]. Cord serum 25(OH)D concentrations had a moderate positive correlation with maternal serum 25(OH)D at 4–21 wk gestation (r = 0.58, P < 0.001) and a strong positive correlation with maternal serum 25(OH)D before delivery (r = 0.89, P < 0.001).
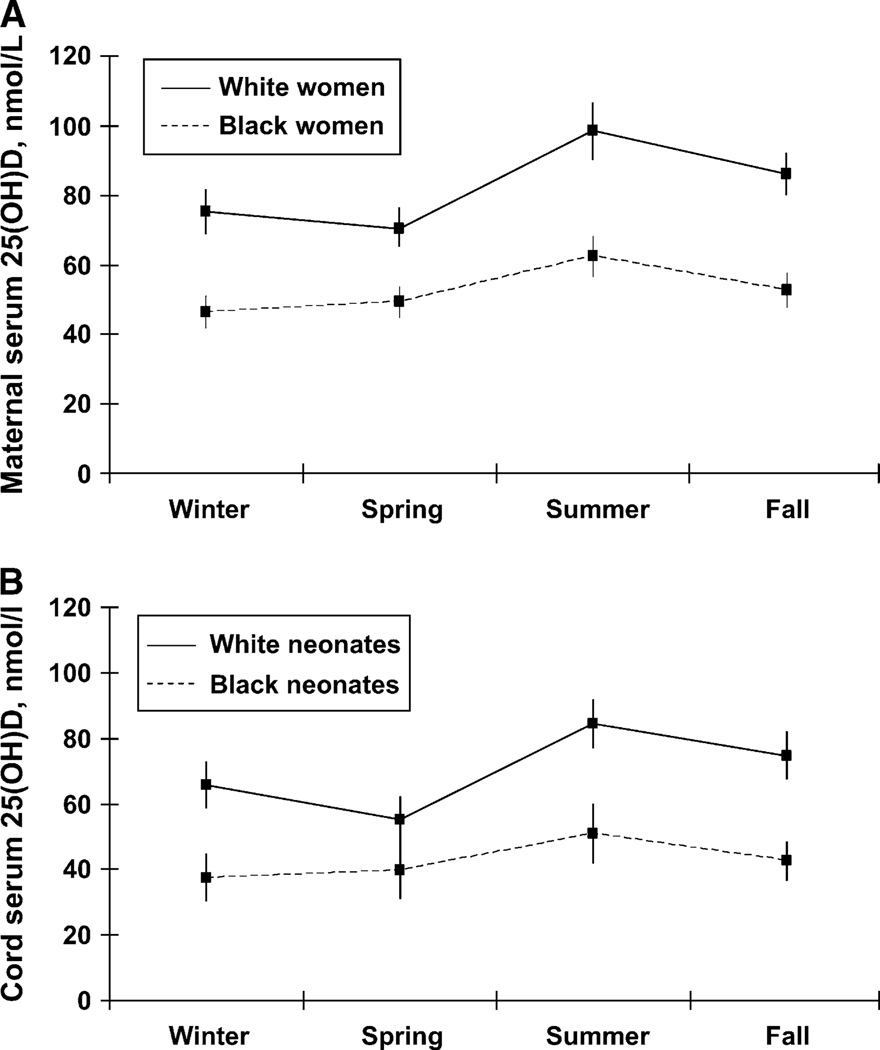
In both racial/ethnic groups, maternal (Fig. 1A) and cord serum (Fig. 1B) 25(OH)D concentrations were highest in summer and lowest in winter and spring (Fig. 1A,B). However, the amplitude of the difference from winter to summer and spring to summer varied by race/ethnicity. After adjustment for sample gestational age, prepregnancy BMI, and multivitamin use, black women had a smaller mean difference in adjusted maternal 25(OH)D compared with white women from winter to summer (16.0 ± 3.3 nmol/L vs. 23.2 ± 3.7 nmol/L) and from spring to summer (13.2 ± 3.0 nmol/L vs. 27.6 ± 4.7 nmol/L). This race-by-season interaction was important (P < 0.01). Similarly, black newborns had a smaller mean adjusted cord blood 25(OH)D increase than white newborns from spring to summer (11.3 ± 6.1 nmol/L vs. 29.3 ± 4.8 nmol/L, P < 0.05). There were no significant differences in other seasonal contrasts among black and white neonates.
Figure 1.
Maternal serum 25(OH)D concentrations by season in 200 white and 200 black pregnant women (A) and cord serum 25(OH)D concentrations of their neonates (B). All values were adjusted for prepregnancy BMI, prenatal vitamin use, and gestational age.
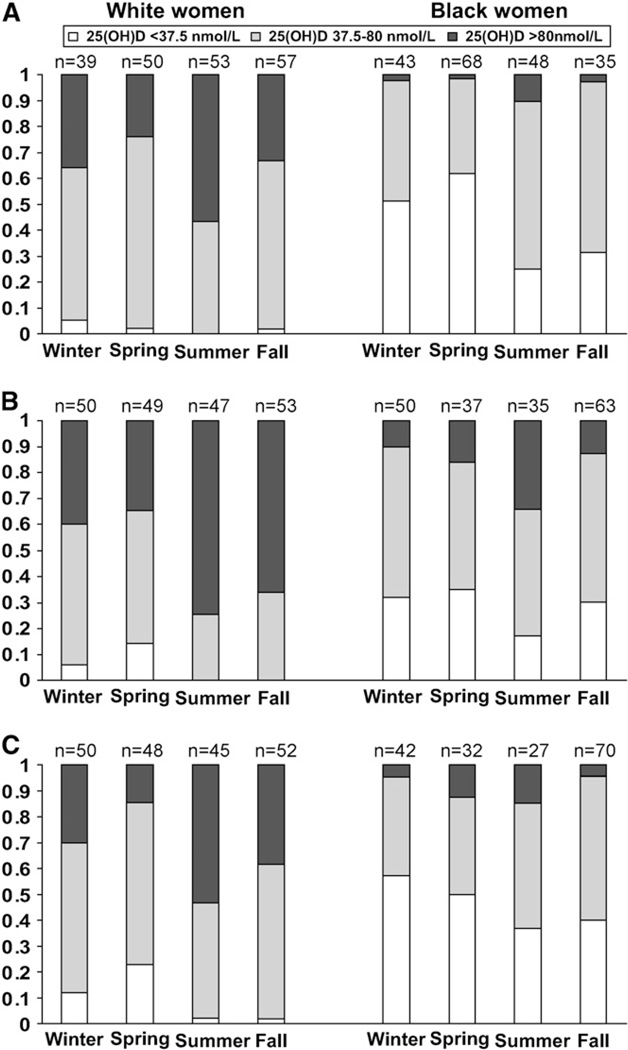
Vitamin D insufficiency in mothers and their neonates was most common in spring among whites and in winter among blacks (Fig. 2A–C). After adjustment for gestational age, prepregnancy BMI, and multivitamin use, we observed an interaction between race and season on the likelihood of vitamin D insufficiency [defined here as 25(OH)D ≤80 nmol/L] in maternal samples (P < 0.001). Compared with summer, the adjusted prevalence of vitamin D insufficiency was 1.9-fold, 2.1-fold, and 1.5-fold in winter, spring, and fall, respectively, among white mothers (Table 3). White neonates born in spring had a 76% increased prevalence of vitamin D insufficiency compared with white neonates born in summer. Among black women and newborns, the adjusted prevalence ratios were significantly attenuated compared with those seen in whites and did not show great seasonal variability. Indeed, compared with summer, there was only a 16–21% increase in the likelihood of vitamin D insufficiency in winter, spring, and fall among black women and no significant seasonal effect in black neonates (Table 3).
Figure 2.
Prevalence of vitamin D deficiency [25(OH)D <37.5 nmol/L], insufficiency [25(OH)D 37.5–80 nmol/L], and sufficiency [25(OH)D >80 nmol/L] among 200 white and 200 black women at 4–21 wk gestation (A), at term (B), and in their neonates (C).
TABLE 3.
Prevalence ratios (PR) for maternal vitamin D insufficiency [serum 25(OH)D ≤80 nmol/L] among white and black pregnant women and their offspring1
| White women, n = 200 | Black women, n = 200 | |
|---|---|---|
| Maternal serum2 | ||
| Winter | 1.90 (1.44, 2.49) | 1.21 (1.07, 1.35) |
| Spring | 2.08 (1.60, 2.70) | 1.16 (1.04, 1.30) |
| Summer | 1.00 (ref) | 1.00 (ref) |
| Fall | 1.50 (1.13, 2.00) | 1.19 (1.05, 1.36) |
| Cord serum3 | ||
| Winter | 1.55 (0.90, 2.66) | 1.12 (0.67, 1.88) |
| Spring | 1.76 (1.03, 3.00) | 1.01 (0.57, 1.79) |
| Summer | 1.0 (ref) | 1.0 (ref) |
| Fall | 1.30 (0.75, 2.27) | 1.11 (0.69, 1.79) |
Values are adjusted PR (95% CI). Adjusted for gestational age of the sample, prepregnancy BMI, and multivitamin use.
Results are based on a multivariable log-binomial regression model using generalized estimating equations to account for the nonindependence of repeated 25(OH)D measures in mothers across pregnancy.
Results are based on a multivariable log-binomial regression model.
Discussion
In this cohort of black and white nulliparous pregnant women with a normal pregnancy outcome who resided in Pittsburgh (latitude 40°N), we found a remarkably high proportion of vitamin D deficiency and insufficiency among mothers throughout gestation and among their infants at birth. Although black women and newborns carried the burden of vitamin D insufficiency, a striking number of white women and their neonates were also affected. Notably, we observed low concentrations of 25(OH)D despite >90% of women reporting regular multivitamin use in the last trimester of pregnancy and 45% reporting regular multivitamin use in the periconceptional period.
Studies reporting a high prevalence of vitamin D deficiency among pregnant women and their neonates are abundant, particularly among Middle Eastern women (25–30), veiled or deeply pigmented Australians (31), non-Westerners in the Netherlands (32), Athenians (33), and South Asians living in Asia (34,35), the United Kingdom (36,37), and Europe (38,39). Yet, we are aware of only 2 studies that assessed 25(OH)D concentrations in black and white pregnant women in the United States, both of which were conducted over 20 y ago with small sample sizes. In a cross-sectional study of 10 black and 12 white pregnant women residing in Cleveland (latitude 41°N) in February and March, investigators reported results consistent with ours: plasma 25(OH)D at term was lower in black mothers (45.5 ± 30.3 nmol/L) than white mothers (68.5 ± 21.8 nmol/L, P < 0.05) and in black neonates (23.8 ± 16.8 nmol/L) than white neonates (37.3 ± 13.5 nmol/L, P < 0.05) (7). In contrast, another study in St. Louis (latitude 38°N) of 56 women in February and 61 women in August found that the mean 3rd-trimester serum 25(OH)D was significantly lower in February (38.5 ± 14.8 nmol/L) than August (105 ± 34.8 nmol/L) but that there were no differences between black and white women at either time (40). In a postpartum study of 40 mostly black mother-infant pairs, investigators reported that 50% of mothers had 25(OH)D <30 nmol/L at 24–48 h postpartum despite a majority of women using a multivitamin during pregnancy (41).
To our knowledge, ours is the first pregnancy study that used the 80-nmol/L cut-point to define vitamin D insufficiency. This cutoff is recommended because it correlates with a number of nutritional biomarkers that are impaired by inadequate vitamin D status (23). Previous investigators used more conservative cut-points, which underestimate the magnitude of the problem (23). If we had only reported 25(OH)D concentrations <37.5 nmol/L, nearly all of the white women identified as insufficient with the 80 nmol/L cutoff would have been missed.
Similar to other investigators (7,34,35,42), we found a strong correlation between maternal and cord blood 25(OH)D. Indeed, the high incidence of vitamin D insufficiency in mothers is additionally relevant, because newborns will also have inadequate vitamin D stores to draw on in early life. Although we lacked data on other functional indicators of vitamin D status, such as intact parathyroid hormone, we can assume that when 25(OH)D concentrations are ≤80 nmol/L, bone mineral density is compromised, and below 37.5 nmol/L, individuals are at risk of osteomalacia or rickets (6).
The seasonal changes that we observed in 25(OH)D concentrations are well recognized (19,43,44), but few investigators have examined how these seasonal patterns differ between blacks and whites. Our finding of an attenuated increase in mean 25(OH)D and the likelihood of vitamin D insufficiency from winter and spring to summer among black women and black neonates compared with their white counterparts is consistent with 2 studies conducted in U.S. childbearing-aged women (18,45) and 1 in New Zealand nonpregnant adults (46). These data support the observation that with typical sunlight exposure, black individuals synthesize less cutaneous vitamin D than do whites (47), leading to an inability of their vitamin D status to recover to adequate levels when sunlight increases in summer-time.
Our study was limited by a lack of data on sunlight exposure, dietary vitamin D intake, and skin type/skin pigmentation. Such data would have allowed us to examine the extent to which important determinants of vitamin D status contribute to deficiency in pregnant women and newborns and how they vary by race and season. We also lacked data on the prenatal vitamin brand and dose used by our subjects. Such information would have allowed us to determine whether women were supplemented with vitamin D-2 or vitamin D-3 and the amount they received daily. As mentioned previously, we did not measure functional indicators of maternal or neonatal vitamin D status, such as bone density measurements. Nevertheless, our biracial cohort of 400 mostly low-income women residing at 40°N latitude allowed us to study patterns of 25(OH)D concentrations across seasons. Importantly, our use of the 80-nmol/L cut-point to define vitamin D insufficiency provided the most accurate description of vitamin D nutriture in pregnant women and neonates. Another major strength was the validation of our method of quantifying serum 25(OH)D against HPLC, the gold standard (48).
Our study suggests that black and white pregnant women residing in northern United States and their neonates are at high risk of vitamin D insufficiency, even when they regularly use a prenatal vitamin or multivitamin. Our data add to the growing body of research supporting assertions that the current vitamin D dietary intake recommendations are inadequate to meet the increased demands of pregnancy (16,23,49). Research into the true vitamin D requirements of pregnancy is greatly needed. With the mounting evidence that vitamin D insufficiency increases the risk of skeletal problems, autoimmune diseases, cancers, type 1 diabetes, heart disease, and schizophrenia (14,16), and the striking racial disparities in many of these health outcomes, improving maternal vitamin D status in pregnancy has a tremendous capacity to benefit public health.
Footnotes
Supported by NIH grants PPG 2PO1 HD30367 and 5MO1 RR00056. Dr. Bodnar was supported by NIH grant K01 MH074092. Dr. Simhan was supported by NIH grants R01 HD041663 and R01 HD052732.
The authors do not declare any conflicts of interest.
Literature Cited
- 1.Welch TR, Bergstrom WH, Tsang RC. Vitamin D-deficient rickets: the reemergence of a once-conquered disease. J Pediatr. 2000;137:143–145. doi: 10.1067/mpd.2000.109008. [DOI] [PubMed] [Google Scholar]
- 2.Kreiter SR, Schwartz RP, Kirkman HN, Jr, Charlton PA, Calikoglu AS, Davenport ML. Nutritional rickets in African American breast-fed infants. J Pediatr. 2000;137:153–157. doi: 10.1067/mpd.2000.109009. [DOI] [PubMed] [Google Scholar]
- 3.Pugliese MT, Blumberg DL, Hludzinski J, Kay S. Nutritional rickets in suburbia. J Am Coll Nutr. 1998;17:637–641. doi: 10.1080/07315724.1998.10718814. [DOI] [PubMed] [Google Scholar]
- 4.Sills IN, Skuza KA, Horlick MN, Schwartz MS, Rapaport R. Vitamin D deficiency rickets. Reports of its demise are exaggerated. Clin Pediatr (Phila) 1994;33:491–493. doi: 10.1177/000992289403300808. [DOI] [PubMed] [Google Scholar]
- 5.Tomashek KM, Nesby S, Scanlon KS, Cogswell ME, Powell KE, Parashar UD, Mellinger-Birdsong A, Grummer-Strawn LM, Dietz WH. Nutritional rickets in Georgia. Pediatrics. 2001;107:E45. doi: 10.1542/peds.107.4.e45. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollis BW, Pittard WB., III Evaluation of the total fetomaternal vitamin D relationships at term: evidence for racial differences. J Clin Endocrinol Metab. 1984;59:652–657. doi: 10.1210/jcem-59-4-652. [DOI] [PubMed] [Google Scholar]
- 8.American Academy of Pediatrics. Work Group on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 1997;100:1035–1039. doi: 10.1542/peds.100.6.1035. [DOI] [PubMed] [Google Scholar]
- 9.Specker BL. Do North American women need supplemental vitamin D during pregnancy or lactation? Am J Clin Nutr. 1994;59:S484–S490. doi: 10.1093/ajcn/59.2.484S. [DOI] [PubMed] [Google Scholar]
- 10.Zeghoud F, Vervel C, Guillozo H, Walrant-Debray O, Boutignon H, Garabedian M. Subclinical vitamin D deficiency in neonates: definition and response to vitamin D supplements. Am J Clin Nutr. 1997;65:771–778. doi: 10.1093/ajcn/65.3.771. [DOI] [PubMed] [Google Scholar]
- 11.Stene LC, Joner G. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. Am J Clin Nutr. 2003;78:1128–1234. doi: 10.1093/ajcn/78.6.1128. [DOI] [PubMed] [Google Scholar]
- 12.Camargo CA, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Gillman MW. Prospective study of maternal intake of vitamin D during pregnancy and risk of wheezing illness in children at age 2 years [abstract] J Allergy Clin Immunol. 117:721–722. [Google Scholar]
- 13.McGrath J. Hypothesis: is low prenatal vitamin D a risk-modifying factor for schizophrenia? Schizophr Res. 1999;40:173–177. doi: 10.1016/s0920-9964(99)00052-3. [DOI] [PubMed] [Google Scholar]
- 14.McGrath J, Saari K, Hakko H, Jokelainen J, Jones P, Jarvelin MR, Chant D, Isohanni M. Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophr Res. 2004;67:237–245. doi: 10.1016/j.schres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Evans KN, Bulmer JN, Kilby MD, Hewison M. Vitamin D and placental-decidual function. J Soc Gynecol Investig. 2004;11:263–271. doi: 10.1016/j.jsgi.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 17.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 18.Nesby-O’Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: Third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 19.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 20.Ness RB, Markovic N, Bass D, Harger G, Roberts JM. Family history of hypertension, heart disease, and stroke among women who develop hypertension in pregnancy. Obstet Gynecol. 2003;102:1366–1371. doi: 10.1016/j.obstetgynecol.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Horst RL, Reinhardt TA, Hollis BW. Improved methodology for the analysis of plasma vitamin D metabolites. Kidney Int Suppl. 1990;29:S28–S35. [PubMed] [Google Scholar]
- 22.Alvarez JC, De Mazancourt P. Rapid and sensitive high-performance liquid chromatographic method for simultaneous determination of retinol, alpha-tocopherol, 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human plasma with photodiode-array ultraviolet detection. J Chromatogr B Biomed Sci Appl. 2001;755:129–135. doi: 10.1016/s0378-4347(01)00047-0. [DOI] [PubMed] [Google Scholar]
- 23.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 24.StataCorp. Stata Statistical Software: Release 8.0. College Station (TX): Stata Corporation; 2003. [Google Scholar]
- 25.Bassir M, Laborie S, Lapillonne A, Claris O, Chappuis MC, Salle BL. Vitamin D deficiency in Iranian mothers and their neonates: a pilot study. Acta Paediatr. 2001;90:577–579. [PubMed] [Google Scholar]
- 26.Pehlivan I, Hatun S, Aydogan M, Babaoglu K, Gokalp AS. Maternal vitamin D deficiency and vitamin D supplementation in healthy infants. Turk J Pediatr. 2003;45:315–320. [PubMed] [Google Scholar]
- 27.Taha SA, Dost SM, Sedrani SH. 25-Hydroxyvitamin D and total calcium: extraordinarily low plasma concentrations in Saudi mothers and their neonates. Pediatr Res. 1984;18:739–741. doi: 10.1203/00006450-198408000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Nehama H, Wientroub S, Eisenberg Z, Birger A, Milbauer B, Weisman Y. Seasonal variation in paired maternal-newborn serum 25-hydroxy vitamin D and 24,25-dihydroxyvitamin D concentrations in Israel. Isr J Med Sci. 1987;23:274–277. [PubMed] [Google Scholar]
- 29.Serenius F, Elidrissy AT, Dandona P. Vitamin D nutrition in pregnant women at term and in newly born babies in Saudi Arabia. J Clin Pathol. 1984;37:444–447. doi: 10.1136/jcp.37.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markestad T, Elzouki A, Legnain M, Ulstein M, Aksnes L. Serum concentrations of vitamin D metabolites in maternal and umbilical cord blood of Libyan and Norwegian women. Hum Nutr Clin Nutr. 1984;38:55–62. [PubMed] [Google Scholar]
- 31.Grover SR, Morley R. Vitamin D deficiency in veiled or dark-skinned pregnant women. Med J Aust. 2001;175:251–252. doi: 10.5694/j.1326-5377.2001.tb143558.x. [DOI] [PubMed] [Google Scholar]
- 32.van der Meer IM, Karamali NS, Boeke AJP, Lips P, Middelkoop BJC, Verhoeven I, Wuister JD. High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am J Clin Nutr. 2006;84:350–353. doi: 10.1093/ajcn/84.1.350. [DOI] [PubMed] [Google Scholar]
- 33.Nicolaidou P, Hatzistamatiou Z, Papadopoulou A, Kaleyias J, Floropoulou E, Lagona E, Tsagris V, Costalos C, Antsaklis A. Low vitamin D status in mother-newborn pairs in Greece. Calcif Tissue Int. 2006;78:337–342. doi: 10.1007/s00223-006-0007-5. [DOI] [PubMed] [Google Scholar]
- 34.Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr. 2005;81:1060–1064. doi: 10.1093/ajcn/81.5.1060. [DOI] [PubMed] [Google Scholar]
- 35.Goswami R, Gupta N, Goswami D, Marwaha RK, Tandon N, Kochupillai N. Prevalence and significance of low 25-hydroxyvitamin D concentrations in healthy subjects in Delhi. Am J Clin Nutr. 2000;72:472–475. doi: 10.1093/ajcn/72.2.472. [DOI] [PubMed] [Google Scholar]
- 36.Alfaham M, Woodhead S, Pask G, Davies D. Vitamin D deficiency: a concern in pregnant Asian women. Br J Nutr. 1995;73:881–887. doi: 10.1079/bjn19950093. [DOI] [PubMed] [Google Scholar]
- 37.Brooke OG, Brown IR, Cleeve HJ, Sood A. Observations on the vitamin D state of pregnant Asian women in London. Br J Obstet Gynaecol. 1981;88:18–26. doi: 10.1111/j.1471-0528.1981.tb00931.x. [DOI] [PubMed] [Google Scholar]
- 38.Brunvand L, Haug E. Vitamin D deficiency amongst Pakistani women in Oslo. Acta Obstet Gynecol Scand. 1993;72:264–268. doi: 10.3109/00016349309068035. [DOI] [PubMed] [Google Scholar]
- 39.Henriksen C, Brunvand L, Stoltenberg C, Trygg K, Haug E, Pedersen JI. Diet and vitamin D status among pregnant Pakistani women in Oslo. Eur J Clin Nutr. 1995;49:211–218. [PubMed] [Google Scholar]
- 40.Hillman LS, Haddad JG. Perinatal vitamin D metabolism. III. Factors influencing late gestational human serum 25-hydroxyvitamin D. Am J Obstet Gynecol. 1976;125:196–200. [PubMed] [Google Scholar]
- 41.Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr. 2007 doi: 10.1177/0009922806289311. in press. [DOI] [PubMed] [Google Scholar]
- 42.Hillman LS, Haddad JG. Human perinatal vitamin D metabolism. I. 25-Hydroxyvitamin D in maternal and cord blood. J Pediatr. 1974;84:742–749. doi: 10.1016/s0022-3476(74)80024-7. [DOI] [PubMed] [Google Scholar]
- 43.Brot C, Vestergaard P, Kolthoff N, Gram J, Hermann AP, Sorensen OH. Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. Br J Nutr. 2001;86(Suppl 1):S97–S103. doi: 10.1079/bjn2001345. [DOI] [PubMed] [Google Scholar]
- 44.McGrath JJ, Kimlin MG, Saha S, Eyles DW, Parisi AV. Vitamin D insufficiency in south-east Queensland. Med J Aust. 2001;174:150–151. doi: 10.5694/j.1326-5377.2001.tb143195.x. [DOI] [PubMed] [Google Scholar]
- 45.Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr. 1998;67:1232–1236. doi: 10.1093/ajcn/67.6.1232. [DOI] [PubMed] [Google Scholar]
- 46.Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, Dryson E. Serum 25-hydroxyvitamin D3 is related to physical activity and ethnicity but not obesity in a multicultural workforce. Aust N Z J Med. 1995;25:218–223. doi: 10.1111/j.1445-5994.1995.tb01526.x. [DOI] [PubMed] [Google Scholar]
- 47.Loomis WF. Skin-pigment regulation of vitamin-D biosynthesis in man. Science. 1967;157:501–506. doi: 10.1126/science.157.3788.501. [DOI] [PubMed] [Google Scholar]
- 48.Zerwekh JE. The measurement of vitamin D: analytical aspects. Ann Clin Biochem. 2004;41:272–281. doi: 10.1258/0004563041201464. [DOI] [PubMed] [Google Scholar]
- 49.Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79:717–726. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]