Abstract
There remain no clear guidelines for the optimal management of patients with metastatic breast cancer. To better understand its natural history, we undertook a detailed examination of 197 autopsies performed on women who died of breast cancer. We reviewed clinical, treatment and pathological aspects of all cases and, additionally, pathological features and biomarker expression (ER, PgR, HER2, EGFR, p53, Ki67, c-Kit, CK AE1/AE3) were assessed in detail for the primary tumour and matched metastases for 55 of the cases. Genomes of the primary tumour and multiple metastases were analysed by array-based comparative genomic hybridization for six cases##. 945 metastatic deposits were identified, with a median of four/patient. The most common organs involved were lung/pleura (80%), bone (74%), liver (71%) and non-axillary lymph nodes (55%). Major findings included: (a) patients with CNS metastases were more likely to have bone metastases (p < 0.013); (b) younger age was associated with metastasis to the liver (≤ 49 years; p < 0.001) and to gynaecological organs (≤ 49 years; p = 0.001); (c) surgical excision of the primary tumour was associated with metastasis to the liver (p = 0.002); and (d) ER and PgR showed down-regulation during progression in a non-random manner, particularly in lung/pleura (ER; p < 0.001), liver and bone metastases. Genomic analysis revealed DNA copy number variation between the primary tumour and metastases (e.g. amplification of 2q11.2–q12.1 and 10q22.2–q22.3) but little variation between metastases from the same patient. In summary, the association of CNS and bone metastases, liver and gynaecological metastases in young women and the risk of liver metastases following surgery have important implications for the management of patients with breast cancer. Clonal heterogeneity of the primary tumour is important in developing metastatic propensity and the change in tumour phenotype during progression/colonization highlights the importance of sampling metastatic disease for optimal treatment strategies. © 2013 The Authors. Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Keywords: autopsy, breast cancer, metastasis, pathology, treatment, intratumour heterogeneity
Introduction
Breast cancer is one of the most common cancers to affect women and is a leading cause of cancer-related deaths worldwide. Approximately 90% of these deaths are due to metastatic dissemination of the disease. While implementation of breast cancer screening programmes and improved treatment of the primary tumour have contributed significantly to reducing overall mortality rates, clinical management of patients with metastatic progression is much less comprehensively structured.
To provide optimal treatment for patients with metastatic breast cancer we need to be able to predict both the presence and the distribution of metastases, together with their likely behaviour at metastatic sites. The considerable intertumoural heterogeneity, as exemplified by the 25 histological subtypes of breast cancer recently defined by the World Health Organization (WHO) 1, and the increasingly recognized intratumoural heterogeneity as genetic and phenotypic evolution occurs during disease progression 2–9 highlights the substantial challenges faced by physicians when deciding on treatment for individual patients. These complexities are only compounded when issues of metastatic disease are considered. Metastatic tumour deposits are not exact replicas of the primary tumour from which they arose in either a morphological or a molecular sense and, indeed, metastatic tumours at different sites within an individual may display widely disparate features 7,8. Basing treatment decisions solely on the morphological features of the primary tumour potentially ignores many key biological attributes of the metastases that may significantly affect disease outcome 10,11.
Here we present clinical, pathological and biological data derived from autopsies performed on a series of 197 women who died with metastatic breast cancer over a 50 year period at one institution. This gives novel insights into the metastatic progression of breast cancer and provides a rationally scientific basis for key treatment decisions.
Materials and methods
Patients
The cohort consisted of 197 female patients who died with metastatic breast cancer and on whom a complete autopsy was performed at the Royal Brisbane and Women's Hospital, Australia, between 1957 and 2007. Cases were included if paraffin-embedded tissue blocks were available. Clinical data, obtained from autopsy records and pathology reports, included the date of the autopsy, the age at diagnosis and at death, the time from diagnosis to death, the time from death to autopsy and treatment details. The study was approved by the Human Research Ethics Committee of the Royal Brisbane and Women's Hospital, Approval No. 2005/022, originally granted 4 April 2005, and by that of the University of Queensland, Approval No. 2005000785, originally granted 1 December 2005.
Pathological and immunohistochemical analysis
All autopsy and surgical blocks (for those patients who had prior breast surgery) were recut and stained with haematoxylin and eosin (H&E) and reviewed by two pathologists (MCC and SRL) to confirm the distribution of metastatic disease as described in the autopsy reports and to perform histological typing and grading of each primary tumour. Also, the preservation of each tumour was assessed. Whole sections of the primary cancers and the corresponding metastases from 55 cases were stained with antibodies to the following proteins: oestrogen receptor (ER), progesterone receptor (PgR), HER2, Ki67, c-kit, cytokeratin AE1/AE3, EGFR and p53. Fluorescence in situ hybridization (FISH) for HER2 was undertaken on cases where there was an equivocal score (2+) with immunohistochemistry using the HercepTest™ scoring system (see supplementary material).
Statistical analysis
Clinical and pathological data were summarized by frequencies and percentages for categorical outcomes, and by means and standard deviations (SDs) for continuous outcomes. Comparisons between groups for categorical outcomes were analysed using Pearson's χ2 test, while one-way ANOVA was used for continuous outcomes. Concordance of antibody positivity between primary and secondary tumours was analysed using McNemar's test. All statistical analyses were performed using SPSS v. 19 and a significance level of 5% was considered statistically significant (see supplementary material).
Array-based comparative genomic hybridization (aCGH)
Thirty-one tumours from six patients, including the primary breast tumour and several metastases from each patient, were analysed for DNA copy number alterations using SurePrint G3 Human CGH Microarrays (Agilent Technologies; for experimental details, see supplementary material). Validation of amplifications was performed by FISH and meta-analysis of publicly available data 12–15 was performed to investigate the role of alterations in driving tumour behaviour (see supplementary material).
Results
Clinical details
Full clinical details relating to the 197 patients are presented in the supplementary material, including Tables S2–S6. Briefly, 72% of the autopsies were performed in the 1960s and 1970s; the median age of the patients at clinical diagnosis and at death was 52 and 56 years, respectively; the median and interquartile range (IQR) time from diagnosis to death was 24 (range 8–51.5) months; and the median (IQR) time from death to autopsy was 20 (range 9–24) h.
Distribution of metastases
A total of 945 metastatic deposits were identified, involving 15 sites (see supplementary material, Table S7). The most common sites involved were lung or pleura (80.7%), bone (74.1%), liver (71.6%) and non-axillary lymph nodes (54.8%). Fifty-eight (29.4%) patients had three or less metastatic sites involved, 76 (38.6%) had four or five sites, while 63 (32.0%) had six to 13 sites involved, with the median number (IQR) per patient being four (range three to six) (see supplementary material, Figure S1).
We investigated whether the site of involvement correlated with the extent of disease. Patients with lung or pleural metastases were significantly more likely to have metastases at five or more other sites compared with those without lung or pleural metastases (37.1% versus 18.4%, p = 0.018). More widespread disease was also seen in those with liver (39.7% versus 21.4%, p = 0.021), adrenal (57.8% versus 21.5%, p < 0.001) and CNS metastases (63.6% versus 39.9%, p = 0.020) but not in those with metastases affecting bone, non-axillary lymph nodes or gynaecological organs (Table1).
Table 1.
Number of other metastatic sites involved according to the site of disease
| Site of metastasis | Number of other metastatic sites | p | ||
|---|---|---|---|---|
| 0–2 | 3–4 | 5+ | ||
| Lung or pleura | ||||
| No | 16 (42.1%) | 15 (39.5%) | 7 (18.4%) | |
| Yes | 35 (22.0%) | 65 (40.9%) | 59 (37.1%) | 0.018 |
| Bone | ||||
| No | 13 (25.5%) | 21 (41.2%) | 17 (33.3%) | |
| Yes | 34 (23.3%) | 55 (37.7%) | 57 (39.0%) | 0.77 |
| Liver | ||||
| No | 19 (33.9%) | 25 (44.6%) | 12 (21.4%) | |
| Yes | 27 (19.1%) | 58 (41.1%) | 56 (39.7%) | 0.021 |
| Non-axillary nodes | ||||
| No | 24 (27.0%) | 30 (33.7%) | 35 (39.3%) | |
| Yes | 19 (17.6%) | 49 (45.4%) | 40 (37.0%) | 0.16 |
| Adrenal glands | ||||
| No | 29 (27.1%) | 55 (51.4%) | 23 (21.5%) | |
| Yes | 3 (3.3%) | 35 (38.9%) | 52 (57.8%) | < 0.001 |
| CNS | ||||
| No | 27 (17.6%) | 65 (42.5%) | 61 (39.9%) | |
| Yes | 5 (11.4%) | 11 (25.0%) | 28 (63.6%) | 0.020 |
| Gynae organs | ||||
| No | 29 (17.9%) | 64 (39.5%) | 69 (42.6%) | |
| Yes | 2 (5.7%) | 12 (34.3%) | 21 (60.0%) | 0.089 |
The association between sites of involvement was also explored (see supplementary material, Table S8). Metastases in the CNS were present in 26.7% of women with bone metastases compared with 9.8% of women with no bone metastases (p = 0.013) (see supplementary material, Table S9). Metastases in the lung or pleura (p = 0.002), bone (p = 0.04), liver (p = 0.037), CNS (p = 0.001) and gynaecological organs (p = 0.009) were present more often in women who had adrenal metastases compared with those without adrenal metastases (see supplementary material, Table S10).
Effect of age and treatment on the distribution of metastases
Patterns of metastatic dissemination were analysed to determine if there were any statistically significant associations between specific clinical parameters. Patients diagnosed at a younger age (≤ 49 years) were significantly more likely to have metastases involving the liver (p < 0.001), adrenal glands (p = 0.019) and gynaecological organs (p = 0.001) and were more likely to have a larger number of metastatic sites involved (p = 0.002) compared with patients diagnosed at a later age (≥ 50 years) (Table2).
Table 2.
Sites of metastatic disease according to age at time of clinical diagnosis
| Site of metastasis | Age (years) at clinical diagnosis (n = 180) | p | ||
|---|---|---|---|---|
| 49 or younger | 50–64 | 65 or older | ||
| (n = 77) | (n = 71) | (n = 32) | ||
| Lung or pleura | ||||
| No | 14 (18.2%) | 14 (19.7%) | 10 (31.3%) | |
| Yes | 63 (81.8%) | 57 (80.3%) | 22 (68.8%) | 0.29 |
| Bone | ||||
| No | 15 (19.5%) | 26 (36.6%) | 5 (15.6%) | |
| Yes | 62 (80.5%) | 45 (63.4%) | 27 (84.4%) | 0.021 |
| Liver | ||||
| No | 13 (16.9%) | 20 (28.2%) | 18 (56.3%) | |
| Yes | 64 (83.1%) | 51 (71.8%) | 14 (43.8%) | <0.001 |
| Non-axillary nodes | ||||
| No | 37 (48.1%) | 26 (36.6%) | 18 (56.3%) | |
| Yes | 40 (51.9%) | 45 (63.4%) | 14 (43.8%) | 0.14 |
| Adrenal glands | ||||
| No | 35 (45.5%) | 39 (54.9%) | 24 (75.0%) | |
| Yes | 42 (54.5%) | 32 (45.1%) | 8 (25.0%) | 0.019 |
| CNS | ||||
| No | 54 (70.1%) | 59 (83.1%) | 26 (81.3%) | |
| Yes | 23 (29.9%) | 12 (16.9%) | 6 (18.8%) | 0.14 |
| Gynae organs | ||||
| No | 54 (70.1%) | 65 (91.5%) | 30 (93.8%) | |
| Yes | 23 (29.9%) | 6 (8.5%) | 2 (6.3%) | 0.001 |
| Number of metastatic sites | ||||
| 0–3 | 12 (15.6%) | 24 (33.8%) | 17 (53.1%) | |
| 4–5 | 33 (42.8%) | 26 (36.6%) | 8 (25.0%) | |
| 6–13 | 32 (41.6%) | 21 (29.6%) | 7 (21.9%) | 0.002 |
We investigated whether the distribution of metastases was affected by the particular treatment of the breast carcinoma and found that patients who had the primary tumour removed surgically were significantly more likely to develop liver metastases compared with those who did not have surgery (p = 0.002) (Table3). No differences were seen for women treated or not treated with either radiotherapy or chemotherapy and there were no significant differences for involvement of other metastatic sites or for the number of metastatic sites involved.
Table 3.
Distribution of metastases according to surgical treatment of the primary tumour
| Site of metastasis | Surgery | p | |
|---|---|---|---|
| No | Yes | ||
| (n = 46) | (n = 150) | ||
| Lung or pleura | |||
| No | 9 (19.6%) | 29 (19.3%) | |
| Yes | 37 (80.4%) | 121 (80.7%) | 0.97 |
| Bone | |||
| No | 12 (26.1%) | 39 (26.0%) | |
| Yes | 34 (73.9%) | 111 (74.0%) | 0.99 |
| Liver | |||
| No | 21 (45.7%) | 34 (22.7%) | |
| Yes | 25 (54.3%) | 116 (77.3%) | 0.002 |
| Non-axillary nodes | |||
| No | 19 (41.3%) | 69 (46.0%) | |
| Yes | 27 (58.7%) | 81 (54.0%) | 0.58 |
| Adrenal glands | |||
| No | 29 (63.0%) | 78 (52.0%) | |
| Yes | 17 (37.0%) | 72 (48.0%) | 0.19 |
| CNS | |||
| No | 36 (78.3%) | 117 (78.0%) | |
| Yes | 10 (21.7%) | 33 (22.0%) | 0.97 |
| Gynae organs | |||
| No | 39 (84.8%) | 122 (81.3%) | |
| Yes | 7 (15.2%) | 28 (18.7%) | 0.59 |
| Number of metastatic sites | |||
| 0–3 | 16 (34.8%) | 42 (28.0%) | |
| 4–5 | 17 (37.0%) | 58 (38.7%) | |
| 6–13 | 13 (28.3%) | 50 (33.3%) | 0.65 |
Features of the primary tumour correlating with survival and metastatic patterns
A subset of 55 patients had the primary tumour available for analysis, including 24 in whom the primary tumour was present at autopsy and 31 who had previous breast surgery. Overall, there were 261 metastases available from these patients. No association was found between the histological type, grade or receptor status of the primary tumour and spread to individual sites, or with the number of sites involved, although this approached significance for HER2, with no patients with HER2-positive primary tumours showing involvement of gynaecological organs (p = 0.052) (see supplementary material).
Patients with hormone receptor-negative primary disease had a shorter survival time compared with those with hormone receptor-positive disease: 25% of 28 patients who died within 2 years of a clinical diagnosis of breast cancer had ER-positive tumours, compared with 75% of 24 patients who died 2 or more years after their tumours were diagnosed (p < 0.001) (see supplementary material, Table S12). Similarly, 39.3% of those dying within 2 years of diagnosis were PgR-positive, compared with 75% who died 2 or more years later (p = 0.01). The primary tumours were classified into four Perou-type molecular subcategories: luminal A, luminal B, HER2 and triple-negative, using the hormone receptor HER2 and Ki67 status 16. There was no statistical association between the molecular subcategory of the primary tumour and metastatic sites involved, with the number of sites involved or with the age of the patient, either at diagnosis or at autopsy.
Concordance of biomarker expression at different metastatic sites
We investigated whether biomarker expression was consistent between the primary tumour and the corresponding metastases within individuals and, if not, whether spread to specific sites was associated with any change in expression (see supplementary material). Expression of HER2, CK AE1/AE3, EGFR and c-kit was concordant between the primary tumour and all metastases within an individual in more than 89% of cases, while concordant expression of all metastases for ER, PgR, Ki67 and p53 ranged between 56.4% for ER and 72.7% for p53 (see supplementary material, Table S14). For HER2, Ki67, p53, CK AE1/AE3, EGFR and c-kit there was no consistent alteration in biomarker expression according to the site of metastasis, while for ER there was significant loss of expression in metastases in any or all of the lung or pleura, bone, liver and non-axillary lymph nodes. For PgR there was significant loss of expression in the bone and liver (Table4).
Table 4.
Changes in oestrogen and progesterone receptor status according to site of metastatic disease
| Metastatic site (n) | Oestrogen receptor (ER) | Progesterone receptor (PgR) | ||
|---|---|---|---|---|
| Primary versus metastatic status | Significance | Primary versus metastatic status | Significance | |
| Lung or pleura (47) | 13 pos to neg | < 0.001 | 10 pos to neg | 0.09 |
| 0 neg to pos | 3 neg to pos | |||
| 24 neg to neg | 15 neg to neg | |||
| 10 pos to pos | 18 pos to pos | |||
| Bone (38) | 11 pos to neg | 0.022 | 8 pos to neg | 0.039 |
| 2 neg to pos | 1 neg to pos | |||
| 15 neg to neg | 13 neg to neg | |||
| 10 pos to pos | 16 pos to pos | |||
| Liver (32) | 10 pos to neg | 0.002 | 8 pos to neg | 0.039 |
| 0 neg to pos | 1 neg to pos | |||
| 18 neg to neg | 14 neg to neg | |||
| 4 pos to pos | 9 pos to pos | |||
| Non-axillary nodes (24) | 8 pos to neg | 0.008 | 3 pos to neg | 0.25 |
| 0 neg to pos | 0 neg to pos | |||
| 14 neg to neg | 13 neg to neg | |||
| 2 pos to pos | 8 pos to pos | |||
McNemar test.
Genomic nature of metastatic progression
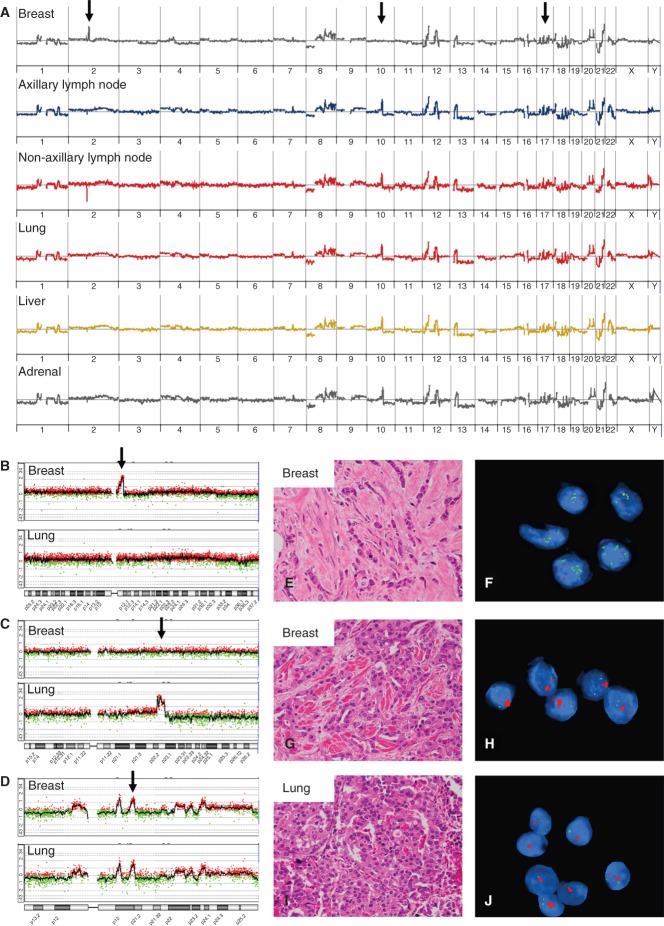
Most copy number alterations (CNAs; deletions, gains and amplifications) were shared between the primary tumour and its metastases, reflecting the close clonal relationship of all tumour foci within a case (Figure 1; see also supplementary material, Figures S3–S7). The majority of CNAs therefore occurred as early events in the major clonal expansion of the primary tumour. However, evidence of clonal diversity was observed between the primary tumour and its corresponding metastases in four cases. For example, in case 7, focal amplification of 2q11.2–q12.1 was detected in the primary tumour but not in the metastases, and amplification of 10q22.2–q22.3 was identified in the metastases but not in the primary tumour. FISH analysis demonstrated that this clonal heterogeneity occurred within the primary tumour (Figure 1; see also supplementary material, Figure S2) and that the clone harbouring the 10q22 amplification had spread. Similarly, in other cases, additional CNAs were detected in all the metastases compared with the corresponding primary tumour (see supplementary material, Figures S5–S7), suggesting (as for case 7) that the clonal diversity most likely occurred prior to dissemination. Of these six cases, only one patient received adjuvant chemotherapy, suggesting that the genomic heterogeneity observed was not treatment-induced.
Figure 1.
Clonal nature of metastatic progression. The primary breast tumour and metastases from lymph nodes (axilla and non-axillary), lung, liver and adrenal gland from autopsy case 7 were studied by aCGH. (A) Whole-genome copy number profiles were strikingly similar, indicating a close clonal relationship between tumours during progression. Individual plots for chromosomes 2 (B), 10 (C) and 17 (D) are shown for the primary breast tumour and the lung metastases, which are representative of all metastases, and DNA copy number alterations along chromosome 17 emphasize this clonal relatedness of tumours; arrow in (D) marks the amplification of HER2/ERBB2 and all tumour deposits were HER2 3+ positive (not shown). Array CGH also detected an amplification of 2q11.2–q12.1 in the primary tumour only [arrow in (B)] and an amplification of 10q22.2–q22.3 in all metastases, but not in the primary tumour [arrow in (C)]. FISH analysis indicated that this clonal diversity occurred in the primary tumour: the 2q amplification (green) was restricted to the primary tumour (E, F), but the 10q22 amplification (red) was found in a different subclone (in a different tissue block) of the primary tumour (G, H) and was identified in all metastases (I, J) (see also supplementary material, Figure S2).
Discussion
Analysis of clinical, pathological and biological data from this large and unique autopsy series provides new insight into metastatic progression that may alter the management of women with breast cancer. Key findings include statistically significant associations between: (a) CNS and bone involvement; (b) young patient age and metastatic spread to liver, gynaecological organs and adrenal glands, together with more widespread sites of involvement; (c) surgical excision of the primary breast tumour and the subsequent development of liver metastases; and (d) changing tumour phenotype particularly affecting ER and PgR expression in metastases in the lung or pleura, liver, non-axillary nodes and bone.
The link between metastases in bone and in the CNS is an important finding. Previous studies have associated lung as the first site of metastasis, with later CNS disease 17. While Altundag et al 18 described lung as the first site of disease in 20% of 420 patients with brain metastases, a further 18% of patients had bone as the initial site, with liver disease at 6%. Our study compared sites of involvement overall, not just the first metastatic sites, which may partly explain this significant bone and CNS association.
That ER-positive breast cancers metastasize to bone and ER-negative breast cancers metastasize to lung and liver was first described in 1976 by Singhakowinta et al 19. That study primarily looked at initial sites of metastatic disease: 47% of the primary tumours in our study were ER-positive but, at the time of death, 74.1% of the patients had bone metastases; conversely, 53% of the primary tumours were ER-negative, while at death 80.7% of the patients had lung or pleural disease and 71.6% had liver disease. The apparent lack of association between ER status and metastatic sites most probably reflects late-stage disease.
Women who had surgical removal of their primary breast tumour were significantly more likely to develop liver metastases compared with those who did not have surgery. Such critical information could only be derived from an archival study such as this, as it would obviously not be possible to conduct a prospective study in which a cohort of women were denied surgery. That surgical excision of the primary tumour may enhance the growth of micrometastases is supported by the work of Demicheli et al 20, who compared the time to death for 1173 patients who underwent mastectomy with 250 untreated breast cancer patients. The treated group showed two peaks in mortality hazard function curves, compared with a single later peak in the untreated group, surgical removal of the primary tumour being held to alter the growth kinetics of metastatic foci. Excision of the primary tumour and the specific development of visceral metastases were documented by Barista et al 21 in 1996, who described a group of 370 women with metastatic breast cancer; for the 53.8% treated with radical or modified radical mastectomy, the first metastases were predominantly in visceral sites.
There is experimental support for the role of surgery in activating dormant metastases. Mice in which Lewis lung carcinoma cells were implanted developed pulmonary micrometastases which remained dormant while the primary tumour was intact, but which grew rapidly after resection of the primary tumour 22, possibly via activating angiogenesis 23,24.
An increased frequency of liver metastases occurring in those aged < 50 years was also shown by Viadana et al 25 in an autopsy study of 374 patients who died of metastatic breast cancer; liver metastases were more frequent in those whose primary breast cancers were diagnosed at age < 50 years (80% versus 56%, p < 0.01) but only in those who survived < 5 years. The possible dual effects of surgery and younger age on increasing distant metastases was described by Retsky et al 26, where in the first 10 months after surgery, for women with node-positive disease there was a surge of distant metastases for premenopausal but not for postmenopausal women.
In addition to demonstrating liver involvement occurring in younger patients, we showed that younger women were significantly more likely to have disease in gynaecological organs and adrenal glands, as well as having a greater number of metastatic sites involved. de la Monte et al 27, in an autopsy study of 187 patients with metastatic breast cancer, showed less frequent involvement of both the adrenal glands and the ovaries with increasing age. In the current study, metastases to the adrenal glands may reflect widespread disease but there was no association between the presence of gynaecological metastases and involvement of a large number of metastatic sites. The oestrogen-rich environment of the premenopausal ovary may partly explain the influence of younger age on the initial establishment of breast cancer metastases at this site.
We found that the majority of metastases retained the molecular features (immunophenotype and genomic profile) of the primary tumour. However, evidence of intratumoural heterogeneity was detected between the primary tumour and its metastases in some cases. With respect to genomic CNAs, this clonal diversity most likely occurred within the primary tumour (e.g. amplification of 10q22) rather than during dissemination, and in these cases was independent of treatment. Furthermore, a meta-analysis of genes affected by these CNAs indicated that adverse expression of some (e.g. VDAC2, TGFß1, ITGBL1) is significantly associated with metastasis-free survival. We hypothesize, therefore, that the acquisition of these CNAs may confer a more aggressive nature to the primary tumour, potentially through enhancing the metastatic capability to the tumour cells (see supplementary material, Figures S8–S10). During progression and colonization of distant organs, however, the tumour genome remained relatively stable, with little variation observed between metastases within a case. Analysis of a larger cohort would give a clearer indication of how CNA heterogeneity during progression varies with respect to the molecular subtype of the primary tumour, the site of metastasis or treatment. In the current series expression of ER and PgR showed a more dynamic modulation 4,8,28 with significant down-regulation in metastases in lung or pleura, bone, liver and non-axillary nodes. This was also heterogeneous, such that expression could vary from one metastasis to another within an individual. Further studies are required to determine whether different subclones within a heterogeneous primary tumour preferentially spread to different sites 3,5,9, giving rise to this phenotypic variability, or whether biomarker expression changes occur as a consequence of adjuvant treatment or in response to local factors at particular metastatic sites. Additionally, the effects of specific targeted therapeutic agents, such as Herceptin, on potentially altering the distribution of metastases will need to be explored.
By studying the clinical and pathological features of 197 women who died of metastatic breast cancer and on whom an autopsy was performed, we provide new information about disease progression in breast cancer, for which there are significant treatment implications. Patients with bone metastases are at an increased risk of developing CNS metastases and should be monitored for their occurrence. Women aged < 50 years have an increased likelihood of developing metastases in the liver or in gynaecological organs and clinicians need to be aware of this when screening for metastatic disease. Surgical excision of the primary tumour was associated with an increased risk of liver metastases. The mechanisms by which this occurs need to be thoroughly delineated, so that appropriate and timely therapeutic intervention can be instituted. Biomarker discordance between primary and secondary sites occurs, especially for ER and PgR, with down-regulation in lung or pleura, bone and liver, and metastases at these sites should be biopsied if possible to determine the most appropriate treatment.
Acknowledgments
The authors would like to thank Helen Speirs from the Ramaciotti Centre for Gene Function Analysis (University of NSW, Australia) and Dan Belluoccio (Agilent Technologies Australia) for assistance with aCGH analysis of autopsy samples. The work was funded through NHMRC Australia (Programme Grant No. 1017028). Peter Simpson is supported by a Fellowship from the National Breast Cancer Foundation, Australia. We also acknowledge the help of many staff members of Pathology Queensland, who over many years contributed to the detailed autopsy investigations reported here.
Author contributions
MC, SL and PS designed the study; MC collected and collated clinical data; LR prepared histological sections and did immunohistochemical staining; MC and SL assessed pathological material; LM and PO'R performed statistical analysis; JJ, JK, AMcC, PS and SS performed genomic analysis; and AM and JS performed FISH. All authors were involved in writing the paper and had final approval of the submitted and published versions.
SUPPLEMENTARY MATERIAL ON THE INTERNET
The following supplementary material may be found in the online version of this article:
Histological assessment.
Immunohistochemistry.
Table S1. Details of antibodies used and immunohistochemical methods.
FISH testing for HER2 status.
Array-based comparative genomic hybridization.
FISH testing for 2q and 10q amplification.
Clinical details of autopsy series.
Table S2. Dates of autopsies.
Table S3. Age at time of clinical diagnosis and at death.
Table S4. Time from clinical diagnosis to death.
Table S5. Time between death and autopsy.
Table S6. Treatment details.
Distribution of metastases.
Table S7. Distribution of metastases in 197 patients at autopsy.
Figure S1. Primary tumour and metastases at multiple sites in one patient.
Table S8. Statistical association between sites of metastatic involvement, giving p values.
Table S9. Associations between metastatic involvement of bone and other sites.
Table S10. Associations between metastatic involvement of adrenal glands and other sites.
Clinical and pathological features related to the primary tumour.
Table S11. The histological type of the primary breast tumour.
Biomarker expression, survival and metastatic progression.
Table S12. Hormone receptor status and time to death.
Table S13. Biomarker expression and histological grade.
Concordance of biomarker expression at metastatic sites.
Table S14. Proportion of patients with concordant staining of all metastases with that of the corresponding primary tumour.
Table S15. The proportion of the metastases in each case with oestrogen and progesterone receptor status concordant with that of the corresponding primary tumour.
Genomic analysis of autopsy cases.
Table S16. Overview of samples used for genomic analysis by array-based Comparative Genomic Hybridization.
Figure S2. FISH analysis of amplifications 2q11–q12 and 10q22 in multiple metastases.
Figure S3. aCGH analysis of autopsy case 1 (primary tumour surgery 1978, autopsy 1978.).
Figure S4. aCGH analysis of autopsy case 11 (2007).
Figure S5. aCGH analysis of autopsy case 13 (primary surgery 1961, autopsy 1964).
Figure S6. aCGH analysis of autopsy case 15 (1967).
Figure S7. aCGH analysis of autopsy case 33 (primary surgery 1966, autopsy 1969).
Impact of intratumoural heterogeneity on metastatic progression.
Figure S8. Meta-analysis of the 10q22 amplicon.
Figure S9. Meta-analysis of the 5q31.1–q31.3 amplicon.
Figure S10. Meta-analysis of the 13q32–q33 deletion.
Supporting Information
Primary tumour and metastases at multiple sites in one patient
FISH analysis of amplifications 2q11–q12 and 10q22 in multiple metastases
aCGH analysis of autopsy case 1 (primary tumour surgery 1978, autopsy 1978.)
aCGH analysis of autopsy case 11 (2007)
aCGH analysis of autopsy case 13 (primary surgery 1961, autopsy 1964)
aCGH analysis of autopsy case 15 (1967)
aCGH analysis of autopsy case 33 (primary surgery 1966, autopsy 1969)
Meta-analysis of the 10q22 amplicon
Meta-analysis of the 5q31.1–q31.3 amplicon
Meta-analysis of the 13q32–q33 deletion
Appendix S1.
Details of antibodies used and immunohistochemical methods
Dates of autopsies
Age at time of clinical diagnosis and at death
Time from clinical diagnosis to death
Time between death and autopsy
Treatment details
Distribution of metastases in 197 patients at autopsy
Statistical association between sites of metastatic involvement, giving p values
Associations between metastatic involvement of bone and other sites
Associations between metastatic involvement of adrenal glands and other sites
The histological type of the primary breast tumour
Hormone receptor status and time to death
Biomarker expression and histological grade
Proportion of patients with concordant staining of all metastases with that of the corresponding primary tumour
The proportion of the metastases in each case with oestrogen and progesterone receptor status concordant with that of the corresponding primary tumour
Overview of samples used for genomic analysis by array-based Comparative genomic hybridization
References
- Lakhani SR, Ellis IO, Schnitt SJ. WHO Classification of Tumours of the Breast. 4th edn. Lyon: International Agency for Research on Cancer (IARC); 2012. [Google Scholar]
- Ding L, Ellis MJ, Li S. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlinger M, Rowan AJ, Horswell S. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idirisinghe PK, Thike AA, Cheok PY. Hormone receptor and c-ERBB2 status in distant metastatic and locally recurrent breast cancer. Pathologic correlations and clinical significance. Am J Clin Pathol. 2010;133:416–429. doi: 10.1309/AJCPJ57FLLJRXKPV. [DOI] [PubMed] [Google Scholar]
- Navin N, Kendall J, Troge J. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472:90–94. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SP, Morin RD, Khattra J. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009;461:809–813. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
- Singhi AD, Cimino-Mathews A, Jenkins RB. MYC gene amplification is often acquired in lethal distant breast cancer metastases of unamplified primary tumors. Mod Pathol. 2012;25:378–387. doi: 10.1038/modpathol.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JM, Fackler MJ, Halushka MK. Heterogeneity of breast cancer metastases: comparison of therapeutic target expression and promoter methylation between primary tumors and their multifocal metastases. Clin Cancer Res. 2008;14:1938–1946. doi: 10.1158/1078-0432.CCR-07-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachida S, Jones S, Bozic I. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzer B, Klein CA. Metastasis awakening: the challenges of targeting minimal residual cancer. Nat Med. 2013;19:274–275. doi: 10.1038/nm.3121. [DOI] [PubMed] [Google Scholar]
- Stoecklein NH, Klein CA. Genetic disparity between primary tumours, disseminated tumour cells, and manifest metastasis. Int J Cancer. 2010;126:589–598. doi: 10.1002/ijc.24916. [DOI] [PubMed] [Google Scholar]
- Curtis C, Shah SP, Chin SF. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell JC, Prat A, Parker JS. Genomic analysis identifies unique signatures predictive of brain, lung, and liver relapse. Breast Cancer Res Treat. 2012;132:523–535. doi: 10.1007/s10549-011-1619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TCGA. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Vijver MJ, He YD, van't Veer LJ. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- Perou CM, Sørlie T, Eisen MB. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Slimane K, Andre F, Delaloge S. Risk factors for brain relapse in patients with metastatic breast cancer. Ann Oncol. 2004;15:1640–1644. doi: 10.1093/annonc/mdh432. [DOI] [PubMed] [Google Scholar]
- Altundag K, Bondy ML, Mirza NQ. Clinicopathologic characteristics and prognostic factors in 420 metastatic breast cancer patients with central nervous system metastasis. Cancer. 2007;110:2640–2647. doi: 10.1002/cncr.23088. [DOI] [PubMed] [Google Scholar]
- Singhakowinta A, Potter HG, Buroker TR. Estrogen receptor and natural course of breast cancer. Ann Surg. 1976;183:84–88. doi: 10.1097/00000658-197601000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demicheli R, Valagussa P, Bonadonna G. Does surgery modify growth kinetics of breast cancer micrometastases? Br J Cancer. 2001;85:490–492. doi: 10.1054/bjoc.2001.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barista I, Baltali E, Gullu IH. Factors influencing the distribution of metastases and survival in metastatic breast carcinoma. Am J Clin Oncol. 1996;19:569–573. doi: 10.1097/00000421-199612000-00007. [DOI] [PubMed] [Google Scholar]
- O'Reilly MS, Holmgren L, Shing Y. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Viadana E, Cotter R, Pickren JW. An autopsy study of metastatic sites of breast cancer. Cancer Res. 1973;33:179–181. [PubMed] [Google Scholar]
- Retsky M, Demicheli R, Hrushesky W. Premenopausal status accelerates relapse in node positive breast cancer: hypothesis links angiogenesis, screening controversy. Breast Cancer Res Treat. 2001;65:217–224. doi: 10.1023/a:1010626302152. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Hutchins GM, Moore GW. Influence of age on the metastatic behavior of breast carcinoma. Hum Pathol. 1988;19:529–534. doi: 10.1016/s0046-8177(88)80199-0. [DOI] [PubMed] [Google Scholar]
- Amir E, Clemons M, Purdie CA. Tissue confirmation of disease recurrence in breast cancer patients: pooled analysis of multi-centre, multi-disciplinary prospective studies. Cancer Treat Rev. 2012;38:708–714. doi: 10.1016/j.ctrv.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. . I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. [DOI] [PubMed] [Google Scholar]
- Lipson D, Aumann Y, Ben-Dor A. Efficient calculation of interval scores for DNA copy number data analysis. J Comput Biol. 2006;13:215–228. doi: 10.1089/cmb.2006.13.215. [DOI] [PubMed] [Google Scholar]
- Ruiz C, Lenkiewicz E, Evers L. Advancing a clinically relevant perspective of the clonal nature of cancer. Proc Natl Acad Sci USA. 2011;108:12054–12059. doi: 10.1073/pnas.1104009108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop HM, Blamey RW, Elston CW. Relationship of oestrogen-receptor status to survival in breast cancer. Lancet. 1979;2:283–284. doi: 10.1016/s0140-6736(79)90295-2. [DOI] [PubMed] [Google Scholar]
- Blamey RW, Bishop HM, Blake JR. Relationship between primary breast tumor receptor status and patient survival. Cancer. 1980;46:2765–2769. doi: 10.1002/1097-0142(19801215)46:12+<2765::aid-cncr2820461404>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Maynard PV, Davies CJ, Blamey RW. Relationship between oestrogen-receptor content and histological grade in human primary breast tumours. Br J Cancer. 1978;38:745–748. doi: 10.1038/bjc.1978.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primary tumour and metastases at multiple sites in one patient
FISH analysis of amplifications 2q11–q12 and 10q22 in multiple metastases
aCGH analysis of autopsy case 1 (primary tumour surgery 1978, autopsy 1978.)
aCGH analysis of autopsy case 11 (2007)
aCGH analysis of autopsy case 13 (primary surgery 1961, autopsy 1964)
aCGH analysis of autopsy case 15 (1967)
aCGH analysis of autopsy case 33 (primary surgery 1966, autopsy 1969)
Meta-analysis of the 10q22 amplicon
Meta-analysis of the 5q31.1–q31.3 amplicon
Meta-analysis of the 13q32–q33 deletion
Appendix S1.
Details of antibodies used and immunohistochemical methods
Dates of autopsies
Age at time of clinical diagnosis and at death
Time from clinical diagnosis to death
Time between death and autopsy
Treatment details
Distribution of metastases in 197 patients at autopsy
Statistical association between sites of metastatic involvement, giving p values
Associations between metastatic involvement of bone and other sites
Associations between metastatic involvement of adrenal glands and other sites
The histological type of the primary breast tumour
Hormone receptor status and time to death
Biomarker expression and histological grade
Proportion of patients with concordant staining of all metastases with that of the corresponding primary tumour
The proportion of the metastases in each case with oestrogen and progesterone receptor status concordant with that of the corresponding primary tumour
Overview of samples used for genomic analysis by array-based Comparative genomic hybridization