Abstract
We analysed the responses of 11 ecosystem models to elevated atmospheric [CO2] (eCO2) at two temperate forest ecosystems (Duke and Oak Ridge National Laboratory (ORNL) Free-Air CO2 Enrichment (FACE) experiments) to test alternative representations of carbon (C)–nitrogen (N) cycle processes.
We decomposed the model responses into component processes affecting the response to eCO2 and confronted these with observations from the FACE experiments.
Most of the models reproduced the observed initial enhancement of net primary production (NPP) at both sites, but none was able to simulate both the sustained 10-yr enhancement at Duke and the declining response at ORNL: models generally showed signs of progressive N limitation as a result of lower than observed plant N uptake. Nonetheless, many models showed qualitative agreement with observed component processes. The results suggest that improved representation of above-ground–below-ground interactions and better constraints on plant stoichiometry are important for a predictive understanding of eCO2 effects. Improved accuracy of soil organic matter inventories is pivotal to reduce uncertainty in the observed C–N budgets.
The two FACE experiments are insufficient to fully constrain terrestrial responses to eCO2, given the complexity of factors leading to the observed diverging trends, and the consequential inability of the models to explain these trends. Nevertheless, the ecosystem models were able to capture important features of the experiments, lending some support to their projections.
Keywords: carbon (C) storage, CO2 fertilization, ecosystem modelling, elevated CO2, Free-Air CO2 Enrichment (FACE), model evaluation, nitrogen (N) limitation, plant physiology
Introduction
Rising atmospheric [CO2] from anthropogenic fossil fuel emissions fertilizes plants (Liebig, 1843; Arrhenius, 1896; Ainsworth & Long, 2005). Biosphere models integrating the effects of [CO2] on plant photosynthesis into projections of the global terrestrial carbon (C) balance suggest that elevated atmospheric [CO2] (eCO2) has caused a large fraction of the land C sequestration during recent decades (Cramer et al., 2001; Sitch et al., 2013). These models also project that the CO2-induced land C sequestration will continue in the future and thereby significantly reduce the accumulation rate of anthropogenic CO2 in the atmosphere (Arora et al., 2013). However, most of these models do not account for the limited availability of nitrogen (N) for plant uptake and growth in many terrestrial ecosystems (Vitousek & Howarth, 1991), which could attenuate ecosystem C storage in response to eCO2: increased C sequestration as a result of eCO2 is thought to bind N into less easily available forms of N within a few years after the onset of CO2 fertilization, a process referred to as progressive N limitation (PNL; Comins & McMurtrie, 1993; Luo et al., 2004). Terrestrial biosphere models that explicitly consider the C–N cycle interaction show that future land C sequestration could be reduced by 50% or more because of N cycle processes (Sokolov et al., 2008; Thornton et al., 2009; Zaehle et al., 2010). However, estimates of the magnitude of this N effect differ strongly among these projections as a result of uncertainty in the representation of key processes determining the strength of the N constraint on land C storage (Zaehle & Dalmonech, 2011).
Free-Air CO2 Enrichment (FACE) experiments in N-limited temperate forest ecosystems provide a unique source of empirical evidence for the ecosystem-scale response of the interacting C and N cycle processes to eCO2 (Oren et al., 2001; Norby et al., 2005; Palmroth et al., 2006; Finzi et al., 2007; Iversen et al., 2012). Specific site conditions (young, fast-growing forests established on abandoned soils previously used for agriculture or grazing) and the artificial nature of these experiments (step increase in [CO2]) limit the direct application of the measurements to estimate the N constraint on future global net primary production (NPP) and land C uptake. Nonetheless, the fact that the NPP enhancement resulting from experimentally elevated CO2 at several temperate forest FACE experiments converged towards a common response size (Norby et al., 2005) has led modellers to attempt benchmarking exercises, to evaluate the capacity of terrestrial ecosystem models to simulate average multi-year effects of CO2 fertilization (Sitch et al., 2008; Piao et al., 2013). However, this consistency of response to CO2 seen during the initial years has not been maintained as the length of the experiments increased, showing that a single number does not capture the complexities of ecosystem responses to eCO2: for instance, the NPP response strongly declined at Oak Ridge National Laboratory (ORNL) FACE towards the end of the experiment, whereas the Duke FACE site showed a sustained eCO2 response (McCarthy et al., 2010; Norby et al., 2010).
In this article, we use 11 ecosystem models to investigate the effects of N availability on the eCO2 response of forest productivity and C storage at two forest sites with fairly similar temperate climate (Köppen Cfa), comparable levels of N deposition, but contrasting vegetation: the evergreen, needle-leaved Duke Forest (McCarthy et al., 2010) and the deciduous, broad-leaved ORNL Forest (Norby et al., 2010) FACE experiments. As the observed ambient forest productivity and N requirement at the beginning of the experiment were comparable at the two sites (see Results), our hypothesis was that the ecosystem models should be able to explain the diverging long-term trends based on the different processes and time scales associated with the different vegetation types.
Our study forms part of a model intercomparison (A. P. Walker et al., unpublished) looking at the effect of eCO2 on water (De Kauwe et al., 2013), C (M. G. De Kauwe et al., unpublished) and N cycling. Each of the participating models incorporates the major processes by which the N cycle affects the ecosystem's response to eCO2, such as plant N uptake, net N mineralization and the ecosystem N balance, as well as emergent ecosystem properties, such as the N-use efficiency (NUE) of plant production (Fig.1). The representation of these processes varies greatly among models (Table1), illustrating a lack of consensus on the nature of the mechanisms driving these processes. Our objectives in this study were as follows:
Figure 1.
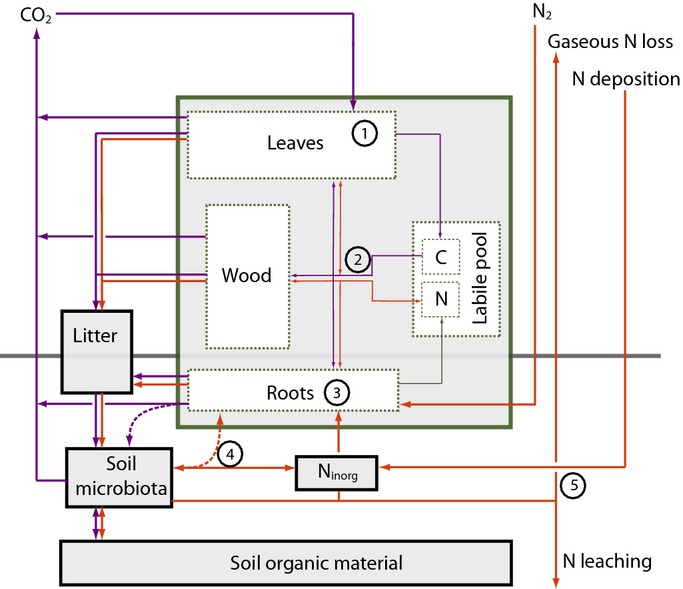
Conceptual diagram of the major nitrogen (N) and carbon (C) flows and stores in a terrestrial ecosystem. Blue arrows denote C fluxes and red arrows N fluxes between major plant compartments (green) and soil pools (black). Numbers 1–5 mark important C–N cycle linkages as described in the Evaluation framework section: 1, N-based gross primary production (GPPN): the return of C assimilates per unit canopy N Eqn 1; 2, whole-plant nitrogen-use efficiency (NUE): the total amount of foliar, root and woody production per unit of N taken up by plants; this process depends on the allocation of growth between different plant compartments (e.g. leaves, fine roots and wood) and the C : N stoichiometry of each compartment Eqn 2; 3, plant N uptake (fNup): the capacity of the plants to take up N from the soil Eqn 4. The plant-available soil N is determined by two factors: 4, net N mineralization (fNmin): the amount of N liberated from organic material through decomposition, which varies with microbial activity and litter quality Eqn 6; and 5, the net ecosystem nitrogen exchange (NNE): based on N inputs from biological N fixation (fNfix) and atmospheric deposition (fNdep) and N losses from the ecosystem as a result of leaching to groundwater (fNleach) and gaseous emission (fNgas) Eqn 5. As an emergent property, the net amount of C that can be stored in an ecosystem following an increase in CO2 depends on the elevated atmospheric [CO2] (eCO2) effect on the ecosystem's N balance and the whole-ecosystem stoichiometry, which, in turn, depends on the change in the C : N stoichiometry of vegetation and soil, as well as the partitioning of N between vegetation and soil (Rastetter et al., 1992).
to understand the eCO2 responses predicted by each model for the two sites in terms of their assumptions and representations of C–N cycle processes, and
to use experimental observations to constrain these model projections, where possible identifying the mechanisms that are supported vs those that are not.
Given the number and complexity of the C–N processes that determine the observed eCO2 responses (Fig.1), and the impracticality of measurement of every relevant C and N flux (e.g. N losses to leaching and gaseous emission) and stock (e.g. changes in organic soil N) with sufficient accuracy, we aimed to identify those process representations that lead to responses qualitatively in agreement with the available C and N cycle observations, rather than identifying the model best fitting the observed NPP responses.
Materials and Methods
Experimental sites
The Duke Forest FACE site was located in a loblolly pine (Pinus taeda L.) plantation (35.97°N, 79.08°W) established in 1983 in an open woodland partially covered with grass harvested as fodder (McCarthy et al., 2007). The soil is relatively nutrient poor, with forest production showing a substantial response to N fertilization (Oren et al., 2001; Crous et al., 2008; Maier et al., 2008), as evidenced from separate N fertilizer experiments in subplots, which were not analysed in the present study. At the start of the Duke FACE experiment in August 1996, trees were 15 yr old and c. 14 m tall, with a mean summer leaf area index (LAI) of 3–4 m2 m−2 (for the dominant pine species). The experiment consisted of three sets of paired plots (pairs of ambient and elevated [CO2], each 30 m in diameter) with different levels of tree productivity related to natural variations in soil N availability, affecting ambient NPP, LAI and the C allocation to above- vs below-ground compartments (Finzi et al., 2002; Palmroth et al., 2006; McCarthy et al., 2007). One of each set of plots received continuous enhanced [CO2] tracking ambient conditions + 200 μmol mol−1.
The ORNL FACE site was located in a sweetgum (Liquidambar styraciflua L.) plantation (35.9°N, 84.33°W) established in 1988 on a grassland. The soil at the site had a silty clay–loam texture, and was moderately well drained and slightly acidic (Norby et al., 2001; Warren et al., 2011). At the start of the experiment, the c. 90 trees per 25-m treatment plot were c. 12 m tall and in a linear growth phase. The LAI was 5.5 m2 m−2, and the canopy was no longer expanding (Norby et al., 2002). Five treatments plots were established at the site, in two of which exposure to eCO2 commenced in April 1998, and continued during the daylight hours of each growing season (April–November). The average daytime [CO2] from 1998 to 2008 growing seasons was 547 μmol mol−1 in the two CO2-enriched plots and 395 μmol mol−1 in the three ambient plots.
Evaluation framework
Our approach to analysing the N cycle dependence of the NPP response to eCO2 was to break NPP down into its component processes, thus benefitting from the suite of supplementary observations on these processes provided at each experiment. We investigated how each model represented these individual processes (Table1) and compared model outputs against relevant observations. The key C–N cycle processes controlling the ecosystem response to eCO2 (Fig.1) can be grouped into two major categories: Processes affecting NUE (see below), which has both photosynthetic and whole-plant components, and processes affecting N uptake (fNup), which include the rate of net N mineralization (fNmin), the competitive strength of plant vs soil microorganisms for N assimilation, and the ecosystem's balance of N inputs and losses (net ecosystem N exchange, NNE). All variables used in the following are listed in Table2.
N-use efficiency
The change in gross primary production (GPP) with eCO2 can be decomposed into the changed C return per unit of N investment into foliage, expressed as GPP per unit leaf N (N-based GPP; GPPN) and the change in the amount of leaf N. As the models only reported canopy-integrated values of GPP and foliar N (Ncan), and GPP and autotrophic respiration (Ra) could not be measured directly, we analysed the eCO2 effect on the relationship between NPP and Ncan at the whole-ecosystem level, by analysing the N-based NPP (NPPN) as:
| Eqn1 |
where CUE is the whole-plant C-use efficiency.
NPP is related to the amount of N available for growth by the N requirements set by the relative proportion of biomass growth of the different plant components and their C : N stoichiometry. We decomposed the whole-plant NUE into changes in tissue stoichiometry, changes in tissue allocation and retranslocation as follows:
| Eqn2 |
where a is the fraction of NPP allocated to foliage (f), fine roots (r) and woody (w) biomass, n is the respective tissue N concentration and 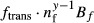 is the amount of N resorbed from the canopy in the previous year. Each of these terms is available from observations, including the amount of N retranslocated, which is calculated from the difference in N concentration between green foliage and leaf litter. Observed fNup at ORNL FACE also included an estimate of foliar N uptake from atmospheric N deposition, a process not included in the models, at the rate of 0.6 g N m−2 yr−1 for both ambient and elevated plots (Norby & Iversen, 2006).
is the amount of N resorbed from the canopy in the previous year. Each of these terms is available from observations, including the amount of N retranslocated, which is calculated from the difference in N concentration between green foliage and leaf litter. Observed fNup at ORNL FACE also included an estimate of foliar N uptake from atmospheric N deposition, a process not included in the models, at the rate of 0.6 g N m−2 yr−1 for both ambient and elevated plots (Norby & Iversen, 2006).
Net changes in vegetation C : N may differ from changes in NUE because N becomes allocated to tissues with different lifetimes. The effect of such changes is reflected in changes in the mean residence time of N in vegetation:
| Eqn3 |
where Nveg is the total N in vegetation.
Plant N uptake
The plant N uptake (fNup) can be expressed as the sum of three factors: the rate of net N mineralization into the inorganic N pool from litter and soil organic matter (SOM) decomposition (fNmin), the depletion of the soil inorganic N pool (ΔNinorg) and any changes in NNE:
| Eqn4a |
Changes in NNE depend on inputs from biological fixation (fNfix) and atmospheric deposition (fNdep) and losses caused by leaching (fNleach) and gaseous emission (fNgas):
| Eqn4b |
The rate of net N mineralization (fNmin) can also be separated into two factors: the effect of accumulating soil N during the course of the experiment and changes in the ratio of microbial N immobilization to gross N mineralization as follows:
| Eqn4c |
where NSOM is the size of the decomposing SOM pool, here including the litter layer, and 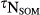 is its apparent turnover time.
is its apparent turnover time. 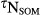 is constant, as long as the ratio of gross N mineralization to immobilization and the allocation of N to SOM pools with different lifetimes do not change. Increasing immobilization as a result of reduced litter quality will increase
is constant, as long as the ratio of gross N mineralization to immobilization and the allocation of N to SOM pools with different lifetimes do not change. Increasing immobilization as a result of reduced litter quality will increase 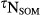 , whereas increased gross mineralization from increased microbial N uptake and release will decrease
, whereas increased gross mineralization from increased microbial N uptake and release will decrease 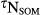 . Insufficient observations were available to constrain the change in fNup component processes during the course of the experiment (Iversen et al., 2011).
. Insufficient observations were available to constrain the change in fNup component processes during the course of the experiment (Iversen et al., 2011).
Ecosystem stoichiometry
The total ecosystem C stored in a forest relates to the total ecosystem N as follows (Rastetter et al., 1992):
| Eqn5 |
where N and C are the N and C pools, respectively, for vegetation (veg), soil (soil) or total organic (org), and fveg is the fraction of ecosystem N in vegetation. For the sake of simplicity, litter pools were subsumed to the soil pools.
Observations
Observed annual changes in C and N cycle parameters were taken from the FACE Data Management System web repository (http://public.ornl.gov/face), as well as published literature, where indicated below. N cycle observations from Duke FACE were only available from 1996 to 2005, and so most of the analyses in this article are focused on this period, although NPP and meteorological forcing data for each treatment plot were available until 2007. The ORNL FACE experiment ran from 1998 to 2009, and data through 2008 were available for this study.
For Duke FACE, standing biomass and biomass production in each plot for three plant compartments (foliage, fine roots and woody biomass, including branches and coarse roots) were taken from McCarthy et al. (2010), using the C and N concentration data for each plant compartment reported by Finzi et al. (2007) to estimate C and N stocks and fluxes. Plant N requirements and uptake were calculated from these data following Finzi et al. (2007). Forest floor and SOM C and N concentrations were obtained from Lichter et al. (2008).
For ORNL FACE, standing biomass, annual biomass production, their respective C and N concentrations, as well as inferred N requirements and plant N uptake by plot and plant compartment (foliage, fine roots and woody biomass, including branches and coarse roots), were obtained from Norby et al. (2010). Initial and final SOM stocks and their C and N concentrations were obtained from Johnson et al. (2004), Jastrow et al. (2005) and Iversen et al. (2012). Differences in sampling design and soil bulk density measurements prevent an accurate calculation of the change in soil C and N during the course of the experiment (Iversen et al., 2012). Comparing the % C and N data in Johnson et al. (2004) and Iversen et al. (2012), we estimated that 10 ± 21% of the greater C and N stocks in the elevated plots at the end of the experiment (Iversen et al., 2012) were a result of eCO2, whilst the rest were a result of initial differences among the plots. Combined with the standard errors of the measurements, eCO2 led to an increase in SOM to a depth of 90 cm of 160 ± 188 g C m−2 and 11.6 ± 24.6 g N m−2 between the beginning and end of the experiment.
The data analyses outlined in the Evaluation framework section were made using data by plot and year. For Duke FACE, responses were calculated per plot pair, and reported as the mean and standard error across the three pairs. For ORNL FACE, the analyses were performed with the mean and standard error across the average of the two eCO2 plots compared with the average of the three ambient CO2 plots.
Ecosystem models
In this study, we used the same set of 11 process-based ecosystem models as described by A. P. Walker et al. (unpublished), encompassing stand (GDAY, DAYCENT, TECO), age/size gap (ED2.1), land surface (CABLE, CLM4, EALCO, ISAM, OCN) and dynamic global vegetation (LPJ-GUESS, SDGVM) models. A detailed account of the major N cycle processes represented in each model is given in Table1. The model simulations covered the time periods representative of the FACE experiments. Meteorological and [CO2] data, as well as site history and stand characteristics, were provided in a standardized manner (http://public.ornl.gov/face).
All models (except CABLE and ED2.1) followed a similar protocol to derive the initial soil C and N pools of the sites, which considered the past land use, as well as the historic evolution of atmospheric CO2 concentration and N deposition, and site-specific meteorological driver data from during the FACE experiments were used throughout the spin-up. The forest vegetation of the plots was initialized such that the forests had the correct age and structure, as far as considered by the model, at the beginning of the eCO2 treatment. Details of the spin-up phase varied among models because of differences in model structure (A. P. Walker et al., unpublished). Inherently different assumptions of the models regarding soil C residence times and ecosystem N loss rates, as well as pre-FACE grassland productivity and N fixation, led to a notable spread in the initial amounts of modelled C and N pools, net N mineralization rates and thus NPP, despite the common initialization protocol.
Model outputs were provided at hourly or daily time steps, as appropriate. These outputs contained estimates of the various C, N and water fluxes and pools.
Results
Overall response to eCO2
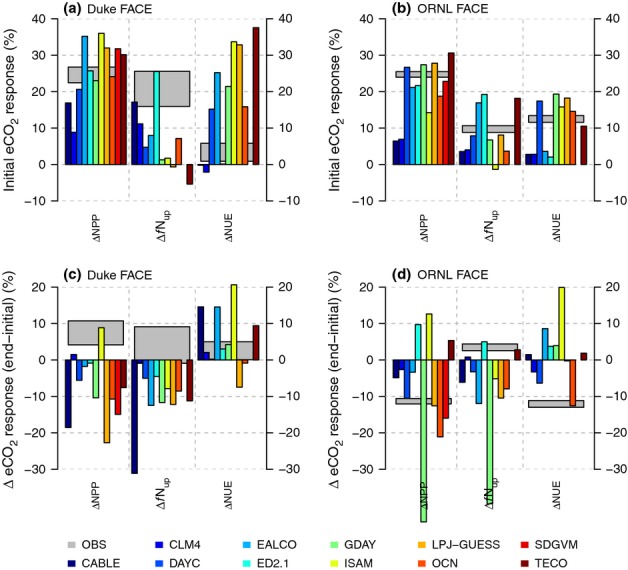
Observed ambient NPP and inferred fNup at Duke FACE were both slightly larger than at ORNL FACE (Figs3a,b), implying that the whole-plant NUE was similar between the sites (Fig.4) at 121 ± 2 g C g−1 N in the ambient plots (1997–2005 mean) for Duke FACE and 129 ± 13 g C g−1 N at ORNL. This similarity between sites is in contrast with an earlier study (Finzi et al., 2007), because the corrections in biomass estimates by McCarthy et al. (2010) resulted in a downward adjustment in the estimate of NUE at Duke Forest.
Figure 3.
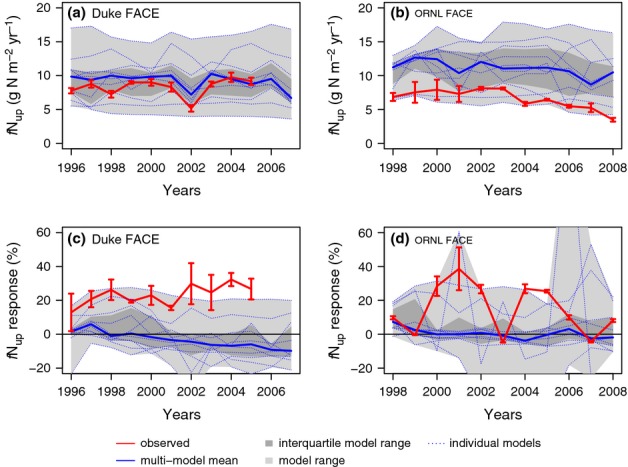
Ambient plant nitrogen (N) uptake (fNup; a, b) and its response to elevated CO2 (c, d) at the Duke (a, c) and Oak Ridge National Laboratory (ORNL) (b, d) Free-Air CO2 Enrichment (FACE) experiments. The observations are across-plot averages, and the error bars denote ± 1SE.
Figure 4.
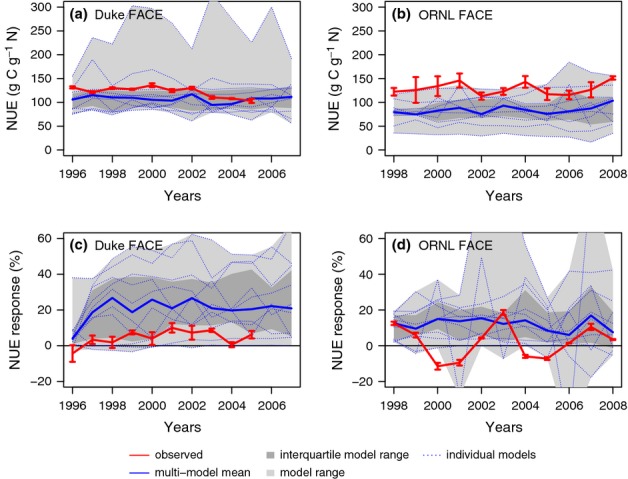
Ambient whole-plant nitrogen-use efficiency (NUE; a, b) and its response to elevated CO2 (c, d) at the Duke (a, c) and Oak Ridge National Laboratory (ORNL) (b, d) Free-Air CO2 Enrichment (FACE) experiments. The observations are across-plot averages, and the error bars denote ± 1SE.
The interquartile range of the model ensemble included the observed ambient NPP at both sites. However, there was significant spread across the models, resulting to a large extent from different model spin-ups, which led to different levels of N constraints on plant production. Only a few of the models (GDAY, OCN) captured the decline in NPP in the ORNL ambient plots related to declining soil N availability over the course of the experiment (Norby et al., 2010; Garten et al., 2011). Although the models, on average, matched the inferred, observation-based fNup at Duke Forest, they overestimated fNup at ORNL (Fig.3). On average, the models slightly underestimated NUE at Duke and more strongly at ORNL FACE (Fig.4). The primary cause for the underestimation was a high bias in the simulation of the fractional (C) allocation to fine roots at both sites (M. G. De Kauwe et al., unpublished). At ORNL FACE, this difference was accentuated by higher modelled than observed N concentration of the fine roots (average 1.4% modelled vs 0.7% observed).
Elevated CO2 increased NPP in the initial (first) year of the experiments by 25 ± 9% and 25 ± 1% at Duke and ORNL FACE, respectively, according to the measurements (Figs2c,d, 5a,b). Most models simulated an initial (first year) increase in NPP as a result of eCO2 that was close to the observations. Notable exceptions were CABLE and CLM4, which systematically underestimated the initial response at both sites, as well as EALCO and ISAM, which overestimated the response for Duke FACE (Fig.5a,b). Nonetheless, no model simulated the underlying changes in fNup and NUE correctly for both sites. At Duke Forest, according to the measurements, the increase in NPP was associated with a strong increase in fNup. The models generally underestimated the observed increase in fNup and overestimated the increase in NUE. At ORNL, according to the measurements, the initial increase in NPP was associated with nearly equal increases of fNup and NUE (Fig.5). Some models simulated a change in NUE in agreement with the observations (DAYCENT, GDAY, ISAM, LPJ-GUESS, OCN, TECO), but most models had a tendency to underestimate the increase in fNup.
Figure 2.
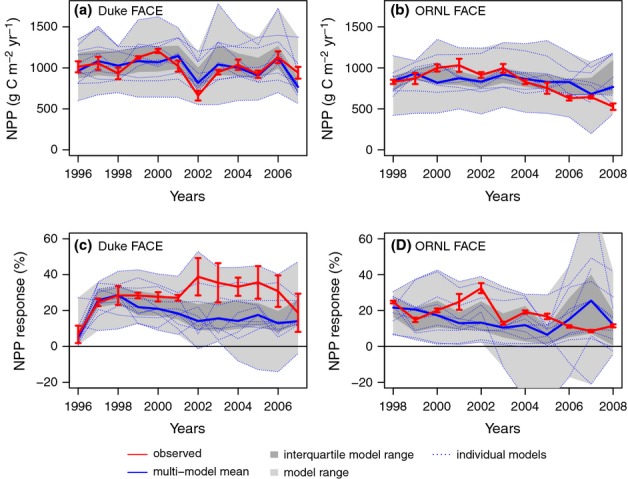
Ambient net primary production (NPP; a, b) and its response to elevated CO2 (c, d) at the Duke (a, c) and Oak Ridge National Laboratory (ORNL) (b, d) Free-Air CO2 Enrichment (FACE) experiments. The observations are across-plot averages, and the error bars denote ± 1SE.
Figure 5.
First year response of net primary production (NPP) to elevated atmospheric [CO2] (eCO2) (a, b) and the change between the first year and the final 5 yr of the experiment (c, d) at the Duke and Oak Ridge National Laboratory (ORNL) Free-Air CO2 Enrichment (FACE) sites, respectively, as well as the response of plant nitrogen (N) uptake (fNup) and whole-plant N-use efficiency (NUE). The grey boxes denote the mean observed eCO2 response ± 1SE.
The observed responses at the end of the experiment differed strongly between the two experiments (Fig.5c,d): the CO2 response of NPP at Duke Forest was maintained throughout the experiment, because the initial increase in fNup was sustained with little change in whole-plant NUE. At ORNL, the CO2 response of NPP declined over time, because the initial increase in NUE declined as a result of higher allocation to N-rich fine roots. At the end of the experiment, NUE and fNup were similar between ambient and elevated plots.
Most models showed signs of PNL (i.e. a progressively smaller enhancement in NPP as a result of N limitation) towards the end of the experiment at both sites (Fig.5c,d), but with varying strength and timing, causing an increasing spread among the models with the duration of the experiment. At Duke FACE, the models largely failed to capture the sustained NPP response to 11 yr of eCO2. The decline occurred despite increasing whole-plant NUE, because the models were not able to maintain an increased fNup as observed (with the exception of ED2.1). At ORNL FACE, three of the 11 models correctly simulated the 10% decline in the initial response towards the end of the experiment (DAYCENT, LPJ-GUESS, SDGVM), and two models (GDAY, OCN) showed an even stronger decline, related to an early simulated onset of N limitation in the ambient treatment. Two models (ED2.1 and TECO) predicted an increase in the NPP response over time, fuelled by increases in plant N uptake, which were supported by a large pool of easily degradable SOM and inorganic N prescribed as initial conditions. Contrary to the observations, NUE and vegetation C : N strongly increased at ORNL in most models by the end of the experiment.
Processes affecting NUE
N-based GPP and NPP
Models differed strongly in their initial NPPN response to eCO2 (Fig.6), generally overestimating the observed initial 11 ± 8% increase in NPPN at Duke FACE and underestimating the observed 35 ± 4% increase at ORNL FACE. Although N limitation did not strongly affect GPPN in the first year in most models, there were substantial differences in the first year's response among the models, in particular at ORNL FACE. Two models (CABLE and CLM4) showed an exceptionally low initial response of NPP at both sites (Fig.5). This low response was related to a near-zero response of GPPN (Fig.6a,b). In CLM4, this response resulted from the assumption that plants down-regulate GPP directly when N limited: CO2 fertilization of GPP is calculated in the absence of N limitation, and then reduced using N-limitation scalars if fNup is insufficient to support this amount of productivity. This low response did not happen in other models that followed a similar approach (DAYCENT and ED2.1), because of sufficient initial N supply. Another class of models simulated photosynthesis based on foliar N content (CABLE, GDAY, LPJ-GUESS, OCN, SDGVM, TECO). In these models, N limitation on GPP acts via foliar N concentrations: limited N availability reduces foliage N, which feeds back to limit GPP. This limitation takes time to develop, such that it was absent or weak in the initial response, but with a strong component of down-regulation in the longer term (Fig.6c,d).
Figure 6.
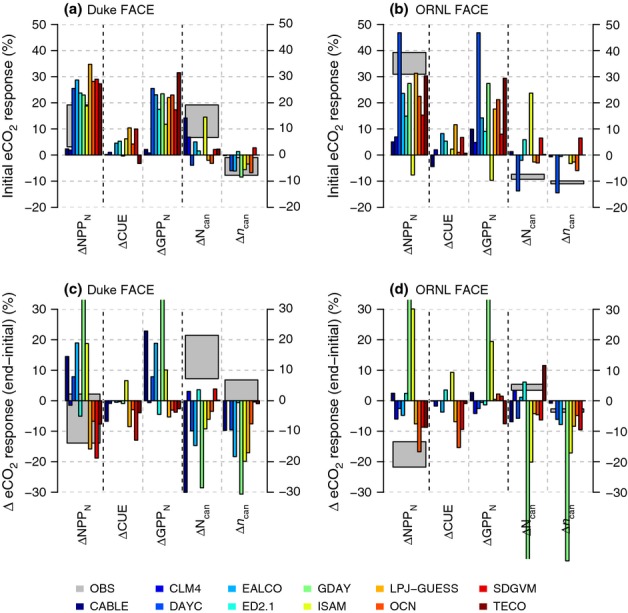
First year response of nitrogen (N)-based net primary production (NPPN) to elevated atmospheric [CO2] (eCO2) (a, b) and the change between the first year and the final 5 yr of the experiment (c, d) at the Duke and Oak Ridge National Laboratory (ORNL) Free-Air CO2 Enrichment (FACE) sites, respectively, as well as the response of plant carbon (C)-use efficiency (CUE), N-based gross primary production (GPPN) and canopy N, expressed as total canopy N (Ncan) and foliar N concentration (ncan). The grey boxes denote the mean observed eCO2 response ± 1SE, where observations corresponding to model output are available.
Model predictions of the eCO2 effect on the other component of NPPN, CUE 1, can be readily categorized into three groups as follows:
models that assume that NPP is a fixed proportion of GPP (GDAY and DAYCENT) showed no change in CUE;
models that estimate Ra directly from biomass and temperature (CABLE, CLM4, EALCO, ED2.1, ISAM, LPJ-GUESS, SDGVM, OCN and TECO) predicted a transient increase in CUE, because the increase in respiration as a result of increased biomass lagged behind the immediate eCO2 effect on GPP. These models generally showed that CUE returned to its original value within the time course of the experiment (10 yr). In addition to these processes;
some models (CABLE, OCN) increased Ra under nutrient stress, when stoichiometric constraints prevented allocation of the assimilated C to growth.
For example, at ORNL FACE, CUE in OCN fell noticeably during the last years of the experiment (Fig.6d). This change was driven by a growing N limitation, which resulted in a build-up of labile C. Increased respiration was used as a mechanism to remove this excess accumulated C.
Whole-plant NUE
With eCO2, observed NUE at Duke Forest increased by 5 ± 2%, mainly because of a shift of allocation towards lower C : N tissue (wood), whereas the 4 ± 3% decline in foliar N had little effect on NUE (Fig.7). Despite the initially observed increase in NUE at ORNL FACE, NUE did not change over the course of the experiment (+2 ± 5%), as the effects of increased tissue C : N were compensated by increased allocation towards N-rich roots.
Figure 7.
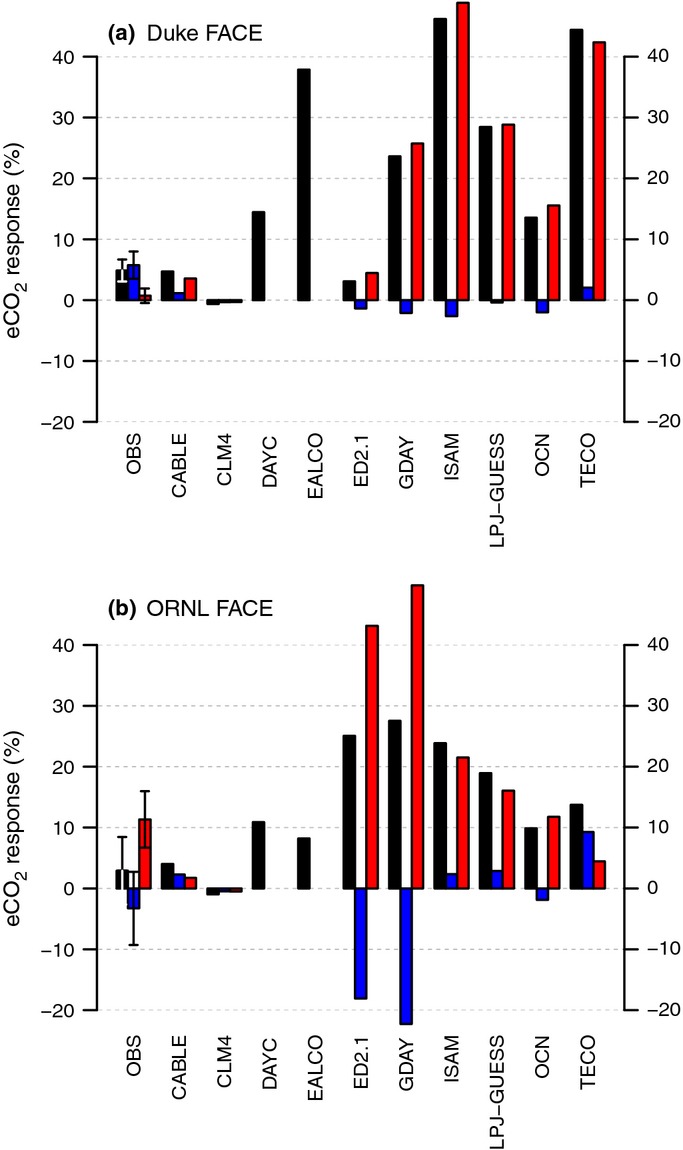
Change in nitrogen (N)-use efficiency of biomass production (NUE) at Duke (a) and Oak Ridge National Laboratory (ORNL) (b) Free-Air CO2 Enrichment (FACE) sites, integrated over the entire length of the experiment (1997–2005 and 1998–2008 for Duke and ORNL FACE, respectively). ΔNUEalloc denotes the change in NUE attributed to changes in allocation to leaves, fine roots and wood, whereas ΔNUEstoch denotes the change in NUE as a result of altered tissue C : N. The error bars denote ± 1SE. Black bars, ΔNUE; blue bars, ΔNUEalloc; red bars, ΔNUEstoch.
In the observations, the fraction of foliar N retranslocated before leaf shedding did not change significantly with eCO2 (−1.1 ± 0.4% at Duke Forest, 0.0 ± 14.3% at ORNL FACE), such that the retranslocation flux scaled with changes in total canopy N (see Fig.6). In most models (except EALCO), the retranslocation fraction did not vary with foliar N (or root N) content (Table1), such that, in agreement with observations, the retranslocation flux scaled with the total foliage (and root) N change. The effect of eCO2 on NUE can therefore be simply separated into its effects on stoichiometry and allocation (Fig.7) for those models that produced all of the variables required to perform these calculations. The model ensemble includes four alternative hypothesis combinations as to how whole-plant NUE changes with eCO2, namely:
assuming allocation and tissue stoichiometry to be constant (CLM4, TECO);
assuming flexible C : N ratios, but N-insensitive partitioning fractions (CABLE, GDAY, EALCO, SDGVM);
assuming constant tissue C : N ratios, but increasing root allocation with N stress (ED2.1); and
assuming the stoichiometry to be flexible and root allocation to increase with N stress (DAYCENT, ISAM, LPJ-GUESS, OCN).
Although the modelled NUE responses differed in magnitude among models, each model individually simulated similar trends at both sites, such that none of the models was able to simulate the observed difference in the NUE response between the sites, in particular, the observation-based interannual variability of the response at ORNL (Figs5). CABLE, which allows for the acclimation of tissue C : N only within narrow bounds, showed hardly any change in NUE, similar to CLM4, which simulates fixed tissue stoichiometry and allocation fractions (Fig.7). By contrast, models with a large flexibility in tissue stoichiometry (GDAY, LPJ-GUESS, OCN) consistently showed a stronger change in NUE as a result of increases in tissue C : N ratios rather than changes in allocation at both sites. The flexible C : N models showed a strong decline of foliar N at both sites, leading to a larger than observed decline in some models (Duke: CABLE, GDAY, LPJ-GUESS, OCN; ORNL: GDAY), which contributed to the excessive NUE response to eCO2 of these models.
The combined effect of the changes in allocation and stoichiometry in most models was that 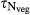 first declined, as a result of a greater growth of fast-overturning tissues (i.e. increased foliar growth as a result of increased NPP), but increased later in the experiment as tissue N concentration dropped and more N became incorporated into woody tissue. This model outcome is consistent with the observed response at Duke, but not ORNL FACE, where the strong increase in fine root growth resulted in a stronger decline in
first declined, as a result of a greater growth of fast-overturning tissues (i.e. increased foliar growth as a result of increased NPP), but increased later in the experiment as tissue N concentration dropped and more N became incorporated into woody tissue. This model outcome is consistent with the observed response at Duke, but not ORNL FACE, where the strong increase in fine root growth resulted in a stronger decline in 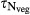 than suggested by the models.
than suggested by the models.
In summary, models that include representations of flexible tissue stoichiometry, photosynthesis calculations based on prognostic foliar N and increasing fine root allocation under nutrient stress were generally more consistent with the observed trends of the component processes. However, because of difficulties in capturing the timing and magnitude of the response of stoichiometry and allocation (as well as the diverging predictions of plant N uptake; see next section on Processes affecting plant N uptake), these models did not appear to be generally superior to the other models considered here in terms of predicting the CO2 response of NPP.
Processes affecting plant N uptake
As outlined in Materials and Methods a, changes in modelled fNup can be attributed to: changes in the rate of net N mineralization (fNmin), which depends on the total amount of SOM N (NSOM) and its turnover time (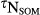 ); changes in the rate of depletion of the soil inorganic matter pool (ΔNinorg); and changes in NNE.
); changes in the rate of depletion of the soil inorganic matter pool (ΔNinorg); and changes in NNE.
In SDGVM, fNup was driven with observations and therefore this model is not considered further in this section. Among the other models, there are two alternative implementations of the processes that allow for a preferential increase in fNup compared with microbial N immobilization under eCO2, leading to contrasting predictions (Fig.8a,b).
Figure 8.
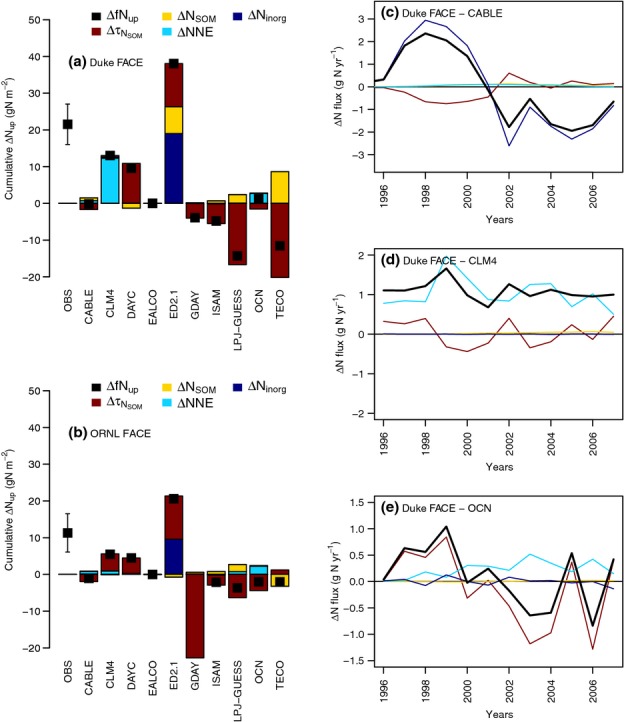
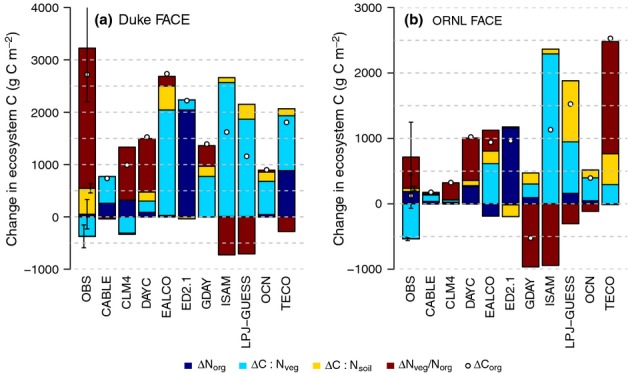
Cumulative plant nitrogen (N) uptake as a result of elevated atmospheric [CO2] (eCO2) over the length of the experiment, and its assignment to different mechanisms according to Eqns 4 and 5 at the Duke (a) and Oak Ridge National Laboratory (ORNL) (b) Free-Air CO2 Enrichment (FACE) sites. Positive values indicate an increase in plant N uptake, and negative values a decline. (c–e) Exemplary time courses of the net N balance for Duke forest, as predicted by CABLE (c), CLM4 (d) and OCN (e). ΔfNup, plant nitrogen uptake; 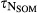 , change in net N mineralization caused by a change in the soil organic N turnover time relative to the soil organic C turnover time; ΔNSOM, change in net N mineralization caused by a change in the organic N pool; ΔNNE, change in the ecosystem N balance (sum of N increases from biological N fixation and atmospheric N deposition and N losses to leaching and gaseous emissions); ΔNinorg, changes in the inorganic N pool. The error bars on the observations denote ± 1SE.
, change in net N mineralization caused by a change in the soil organic N turnover time relative to the soil organic C turnover time; ΔNSOM, change in net N mineralization caused by a change in the organic N pool; ΔNNE, change in the ecosystem N balance (sum of N increases from biological N fixation and atmospheric N deposition and N losses to leaching and gaseous emissions); ΔNinorg, changes in the inorganic N pool. The error bars on the observations denote ± 1SE.
The first, employed by CLM4, is to increase the relative competitiveness of plants vs microbes for N. The plant's N demand is a function of potential GPP, which increases with eCO2. Conversely, the microbial N demand does not change strongly with eCO2, because CLM4 assumes fixed tissue C : N and therefore simulates no change in litter quality with eCO2, which would increase the N requirement of microbes and therefore immobilization. As a result, CLM4 showed a sustained increase in fNup at Duke FACE, because less N was immobilized than under ambient conditions (Fig.8d).
The second mechanism is an emergent property of the CENTURY model (used by CABLE, DAYCENT, GDAY, LPJ-GUESS and OCN): initial increases in fNup as a result of enhanced NPP lower soil inorganic N availability, which increases the C : N ratio of the newly formed SOM according to an empirical relationship. This reduces N immobilization during litter decomposition, as less N needs to be sequestered for the same amount of litter C transfer, increasing the availability of inorganic N for fNup (Fig.8e). In most of these models, the increase was dampened or reversed within a few months or years because the models also apply a flexible tissue C : N. Increased N stress increased tissue (and therefore also litter) C : N ratios, leading to higher microbial N immobilization and therefore a reduction in the net N mineralization (fNmin) to ambient or even below ambient rates, reflected as an increase in 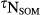 , and therefore a decrease in the availability of inorganic N (Fig.8e).
, and therefore a decrease in the availability of inorganic N (Fig.8e).
A second factor affecting the eCO2 response of fNup is the initial size of the inorganic N pool. Some models simulated an initial excess of inorganic N relative to plant N demand because of the site history (or the spin-up procedure; ED2.1, CABLE at Duke FACE and TECO at ORNL). An example is CABLE at Duke Forest (Fig.8c), in which the initial increase in fNup was supported by the initially available inorganic N pool. This pool became exhausted after a few years of the experiment, leading to lower fNup relative to the ambient plots in the later years of the experiment. The TECO model at ORNL had a much larger SOM pool, and with it gross N mineralization, than required by the forest's productivity, leading to a constant excess supply of N, which supported fNup under eCO2.
The third factor is the ecosystem N balance (NNE), which depends on the rates of input via deposition and fixation, and the rates of loss via leaching and volatilization. A few models in the ensemble (CABLE, CLM4) simulated biological N fixation explicitly, but none suggested that eCO2 would alter fixation such that it would affect the net N balance. For the other models, the principal difference affecting total ecosystem N balance was whether the N losses were assumed to be proportional to the amount of N mineralized (CABLE, CLM4, GDAY, TECO) or whether they were a function of the simulated inorganic N concentration (CABLE, CLM4, EALCO, ISAM, LPJ-GUESS, OCN). In some of the models (CABLE, CLM4, DAYCENT, GDAY, LPJ-GUESS, OCN), ecosystem N losses were reduced, but the causal mechanism differed between the models: for example, GDAY, in which fNup is assumed to be independent of plant N demand, and therefore eCO2, fNmin declined as a consequence of the higher microbial immobilization (higher litter C : N), which decreased directly the gaseous N losses in addition to reducing N leaching, because of lower soil inorganic N. In OCN, higher fNup and increased N immobilization led to lower inorganic N, causing both lower gaseous and leaching losses.
In most models, the change in NNE was of the order of 1 g N m−2 over 10 yr. This reduction in N loss was not sufficient to prevent the onset of PNL in forests that take up 8.3 ± 0.4 g N m−2 yr−1, on average. The only exception to this pattern was the simulation of CLM4 at Duke FACE, where larger increases in fNup substantially reduced gaseous N losses during autumn and winter, leading to a cumulative increase in fNup of 12 g N m−2 (Fig.8a). Although this sustained increase avoided the progressive decline of fNup in CLM4, it was not sufficient to explain the observed increase in vegetation N at Duke FACE.
Time-integrated effect of eCO2 on ecosystem C and N
At Duke, c. 80% of the observed increase in cumulated NPP (3.1 ± 0.6 kg C m−2; 1997–2005) was sequestered in vegetation (2.5 ± 0.5 kg C m−2) and forest floor C (0.3 ± 0.1 kg C m−2), whereas soil C declined by c. 0.2 ± 0.1 kg C m−2 (Supporting Information Fig. S1). These changes were associated with increased vegetation N (12.2 ± 2.9 g N m−2), litter N (6.8 ± 2.6 g N m−2) and decreased soil N (25.0 ± 7.0 g N m−2). At ORNL, the observed enhancement of NPP (1.7 ±0.4 kg C m−2; 1998–2008) did not result in a significant change in biomass (0.0 ± 0.7 kg C m−2 and 1.2 ± 1.7 g N m−2, respectively), but soil C and N pools were increased slightly (0.2 ± 0.2 kg C m−2 and 11.5 ± 12.3 g N m−2, respectively).
Most of the models suggested that a large fraction of the NPP enhancement remained in vegetation C (Fig. S1), in agreement with the observed trends at Duke FACE, but in disagreement with those observed at ORNL FACE. Nevertheless, most models underestimated vegetation C sequestration at Duke FACE, because they underestimated the NPP enhancement and failed to predict the decline in SOM. Most models overestimated vegetation C sequestration in ORNL FACE, mostly related to failure in capturing accurately the allocation pattern and response (M. G. De Kauwe et al., unpublished; Fig. S1).
The large observed increase in vegetation biomass at Duke Forest was supported mostly by a redistribution of N from soil to vegetation, as soil N stocks in the upper soil layers have probably declined over the course of the experiment (Fig.9a). However, there were significant differences in the magnitude of the transfer and vegetation C : N changes among the plots, causing large uncertainty in the attribution of the observed vegetation C increase. Although fNup also increased in ORNL FACE, there was not a sustained increase in biomass N and C, because the rapid turnover of leaves and roots did not lead to a sustained increase in biomass N and C, which instead caused C and N sequestration in SOM (within the detection limit; Fig.9b). At both sites, bulk vegetation C : N decreased slightly with eCO2, despite the larger C : N in foliage, because of the larger contribution of foliage and root biomass to total biomass.
Figure 9.
Total change in ecosystem carbon (ΔCorg) as a result of elevated atmospheric [CO2] (eCO2) at the Duke (a) and Oak Ridge National Laboratory (ORNL) (b) Free-Air CO2 Enrichment (FACE) sites resulting from changes in the total organic ecosystem nitrogen (N) store (ΔNorg), and vegetation and soil C : N ratios (ΔC : Nveg and ΔC : Nsoil), as well as changes in the fractionation of total ecosystem N between vegetation and soil, measured as the fraction of total ecosystem N in vegetation (fveg = Nveg/Norg). The error bars denote ± 1SE.
Consistent with the observations, increased organic ecosystem N (Norg) played a minor role in most models (Fig.9). The exceptions of ED2.1 and TECO at Duke Forest were related to the assumed initial conditions (see section on Processes affecting plant N uptake). Changes in the ecosystem N balance, that is reduction in N losses, led to < 500 g C m−2 additional C sequestration (CLM4 and CABLE at Duke Forest; DAYC and LPJ-GUESS at ORNL FACE). Contrary to the observations, models that assume a flexible tissue C : N ratio (CABLE, EALCO, GDAY, LPJ-GUESS, OCN) predicted that a large fraction of the increase in ecosystem C storage at both sites as a result of eCO2 resulted from the increase in vegetation C : N ratios (see section on Processes affecting NUE). Only CLM4, which assumes fixed tissue stoichiometry, correctly predicted the decline in total vegetation C : N ratio at Duke Forest and the ensuing reduction in vegetation C storage capacity; this response resulted from the increase in foliar and root biomass. Changes in litter and soil C : N were generally of lesser importance in absolute terms, and roughly agreed with the observations. An exception to this was the projected large increase in litter C : N by LPJ-GUESS at ORNL FACE, associated with large litter fall of the deciduous trees and a strong decline in leaf N concentrations.
At Duke Forest, most models suggested that there was a net transfer of N to the vegetation (as a result of the increased fNup), which supported C accumulation in vegetation. However, the predicted increase was always less than half that observed. In LPJ-GUESS, the cumulative effect was a net transfer of N to the soil, probably related to the large fraction of C (and thus N) allocated to fast-overturning tissues (M. G. De Kauwe et al., unpublished). A net N transfer to vegetation initially also occurred in most models at ORNL FACE. However, in GDAY, LPJ-GUESS and OCN, the larger litter fall and the decreased litter C : N ratio at the deciduous site led to increased immobilization of N during decomposition. This provided a mechanism by which plant-available N became trapped in the SOM pool, effectively reducing the fraction of ecosystem N stored in vegetation, consistent with the PNL hypothesis.
Discussion
The analyses presented here have separated the eCO2 response into time-dependent, observable components of the C and N cycle responses, which can be used to evaluate individual model processes and to identify key model weaknesses, as well as to identify the need for more observational constraints. The climate and N inputs, as well as the initial ambient levels of production, N uptake and NUE, were similar between the two sites, leading to the expectation that the different long-term trends in the eCO2 response of NPP and N uptake at Duke and ORNL FACE could be explained by processes associated with the different vegetation types encoded in the models. Despite the success of the models to simulate the initial eCO2 response of NPP at both sites, the models did not encode the relevant processes to explain the observed differences. Rather, most models followed the ORNL trajectory (progressively increasing N limitation) at both sites. In the following, we discuss the process representation of the most important C–N cycle linkages that contribute to the site and model–data differences.
Model responses and underlying processes
Plant N uptake and net N mineralization
The increase in fNup at Duke FACE was twice as large as that seen at ORNL FACE, in absolute terms and when integrated over the time of the experiment. This is a key factor in the observed, divergent NPP response at the two sites. The ensemble of models generally failed to simulate the magnitude of the observed increase in fNup and the large difference between the sites, although some of the models possess mechanisms to increase root growth, and the specific Ninorg uptake capacity of roots or whole plants, under N stress. In most models, fNup was tightly constrained by fNmin, but only few ecosystem-scale observations are available for this quantity (Iversen et al., 2011). At ORNL FACE, the increased fNup was probably related to the presence of plant-available N below the rooting zone of trees at the beginning of the experiment, resulting from past land use. Increased tree rooting depth and, probably, stimulation of SOM decomposition in these layers have added plant-accessible N (Iversen et al., 2008, 2011). The consideration of SOM depth profiles is missing in most ecosystem models, but this is likely to be relevant only under site conditions in which past land use determines the depth distribution of SOM. Increased microbial and fungal SOM decomposition following increased rhizodeposition (so called ‘priming’) is probably the cause of the large N transfer from soils to plants at Duke FACE (Drake et al., 2011); this is a further process not represented by the model ensemble. It is an open question whether this finding implies that models that do not incorporate such a mechanism must also have a low NPP response to gradually increasing atmospheric [CO2]. Under these conditions, the more gradual increase in plant N demand (Luo & Reynolds, 1999) might be satisfied by other mechanisms, such as the tightening of the ecosystem N balance or increased N fixation. Moreover, CENTURY-based models (DAYCENT, GDAY, OCN, LPJ-GUESS, TECO), which mimic the net transfer of N from soils to vegetation under increasing N stress, showed that the net N transfer based on N mining was limited. The pool of easily degradable N-rich material declined as a result of the increased N mining and declining litter quality, suggesting that ‘priming’ might be a temporary process relieving N stress.
NUE and ecosystem stoichiometry
The observed initial increase in whole-plant NUE was stronger at ORNL than at Duke Forest, and can largely be explained by the different magnitude of decline in foliar N concentrations and the diverging trends of total canopy N (Fig.6). The NUE enhancement decayed at ORNL FACE with increasing root allocation during the experiment, such that there was no strong change in NUE with eCO2 at both sites. The inclusion of flexible C : N stoichiometry, alongside increased below-ground allocation in response to eCO2 and increased plant N demand (M. G. De Kauwe et al., unpublished), appeared to be an important feature allowing the NUE response to CO2 to be captured because of the significant changes in foliar N concentrations. However, models that simulate flexible stoichiometry tended to overestimate the whole-plant NUE increase with eCO2. The probable reason for this overestimation is that the predicted changes in tissue C : N are not based on a hypothesis-driven prediction of C : N changes, but rather the emergent model outcome, as flexible stoichiometry in these models is the means to regulate C assimilation given plant-available N. Although the marginal change in photosynthetic capacity can be larger than the marginal change in foliar N (Friend et al., 1997), this does not seem to be sufficient to keep tissue C : N within the observed bounds, as shown by an exaggerated decline in foliar N concentrations at both sites. Other regulatory mechanisms, such as the acclimation of CUE under N stress, as implemented in the OCN model, can limit the reduction in tissue C : N ratios to variations within predefined bounds, but it is unclear whether such a mechanism exists in reality. Modelling approaches that maximize leaf photosynthetic gain given N and C availabilities may provide a more reliable framework to predict stoichiometric flexibility (Medlyn, 1996a; McMurtrie et al., 2008; Xu et al., 2012; McMurtrie & Dewar, 2013).
At both sites, the eCO2 effect on NPPN according to the measurements initially increased (more so at ORNL than Duke FACE), but then declined to very low values of enhancement. In deciduous trees at both sites, this decline was not associated with a change in the relationship of photosynthetic biochemistry (Vcmax, the maximum rate of carboxylation; Vjmax, the maximum rate of electron transport at saturating irradiance) with leaf N (Norby et al., 2010; Ellsworth et al., 2011), whereas, at Duke Forest, older pine needles showed a reduced Vcmax per unit leaf N (Ellsworth et al., 2011). A number of models implement a leaf N dependence of photosynthetic biochemistry (Table1), and a few captured the overall trend in foliar N and GPPN. However, there was a large spread in the simulated eCO2 response of GPPN, both initially and in the longer term, despite the fact that (with the exception of DAYCENT) all models inherit the CO2 sensitivity of photosynthesis from the Farquhar model (Farquhar et al., 1980). As the effect of eCO2 on GPPN is immediate, the uncertainty in the modelled initial GPPN response is independent of the representation of N cycle feedbacks, and therefore not affected by the step increase in CO2. The differences among models were maintained when analysing daily data with a restricted range of meteorological parameters, instead of annually integrated values, a finding that excludes any difference caused by phenological biases (A. P. Walker et al., unpublished) which could also affect GPPN. The probable cause of these differences is alternative assumptions about the fraction of the canopy that is limited by light availability vs carboxylation rate, related to the canopy scaling of N and the depths of the canopy (Medlyn, 1996b). Varying stomatal responses to eCO2 may also have played a role (De Kauwe et al., 2013). Reducing this uncertainty requires a better representation of the changes in foliar N and the slope of the Vjmax : Vcmax relationship within the canopy and across different ecosystems (Maire et al., 2012). At the ecosystem level, alternative data sources, light response curves of net ecosystem exchange or GPP, derived from eddy covariance measurements, could facilitate the evaluation of the canopy-level light response across ecosystem types (Lasslop et al., 2010; Bonan et al., 2012).
Ecosystem N balance
Uncertainties in the observed changes in soil N stocks prevent any statistically meaningful assessment of whether eCO2 increased N capital as a result of changes in N inputs or outputs. Some models simulated increased plant N availability through reduced N losses from the ecosystem. Although these mechanisms added up to 12 g N m−2 (accumulated over the length of the experiment) in the most extreme case, they did not contribute strongly to the simulated C sequestration. Changes in the N balance may be an important factor in modelled eCO2 responses (Rastetter et al., 1997), but the effect was not very pronounced in the ensemble used in this study. None of these N loss reduction mechanisms was sufficient to explain the observations at Duke FACE. In agreement with previous observationally based studies (Drake et al., 2011), we conclude that a mechanism that increases plant N availability under plant N stress based on the enhanced mineralization of organic N is required for models to explain the observed trends at Duke.
Limits of the observational constraints
The process inferences above rely on uncertain observations and implicit assumptions that require careful interpretation. The estimates of plant N uptake were inferred from the biomass production of plant tissues, their N concentrations and foliar N recovery on leaf shedding. Estimates of NPP and fNup are therefore not independent, and so the estimated whole-plant NUE should be considered with caution. Increases in NPP without statistically significant changes in tissue N concentrations imply an increase in fNup, irrespective of whether the rhizospheric N uptake has indeed increased, or whether changes in foliar N retention (or perhaps labile amino acid reserves not accounted for in the observed tissue N concentration changes) have affected the N balance of the plants. This situation leads to uncertainty in the fNup estimates for an individual year, and therefore the eCO2 response in the initial year of the experiment. However, the error associated with unaccounted for reserves diminishes when the estimates are integrated over time, and, on average, the translocation fractions did not change with time in the observations, further reducing the longer term error.
Uncertainty also results from the difficulties in measuring below-ground biomass and production, which is a fairly small contribution to total NPP at Duke Forest, but up to 40% of total NPP at ORNL under eCO2 (Iversen, 2009; McCarthy et al., 2010). Observations of fine root biomass should give suitably constrained estimates of the relative increase in root allocation under eCO2. However, uncertainty in the absolute below-ground C flux and, specifically, C flux to mycorrhizas propagates to uncertainty in annual NPP – and thus in the inferred N requirements to sustain the eCO2 response.
There is also substantial uncertainty in the observation-based estimates of net SOM changes with eCO2, resulting from a small signal-to-noise ratio and uncertainties in the sampling and analyses of the soil data (Jastrow et al., 2005). This uncertainty is primarily a result of the spatial variability of SOM, particularly for N (Iversen et al., 2012). The uncertainty in these measurements is sufficiently large to preclude reliable quantification of the net eCO2 effect on total soil and ecosystem C and N over the 10 yr of the experiment (Figs9, S1), as the expected change in SOM caused by CO2 is rather small. Therefore, the observations from Duke and ORNL Forests do not provide a robust constraint on the model N balance. Nonetheless, independent studies suggest that increased microbial decomposition may have resulted in a net transfer of N to vegetation at Duke FACE (Drake et al., 2011, 2013; Hofmockel et al., 2011), whereas increases in microbial activity with eCO2 may have been insufficient to compensate for the increased accumulation of N in SOM at ORNL FACE (Iversen et al., 2012).
Year-to-year variations in meteorological parameters influence both the ambient C and N cycling at the sites and the response to eCO2. These influences range from the direct effect of temperature on the CO2 sensitivity of photosynthesis (Hickler et al., 2008) to indirect effects resulting from interannual variations in the levels of drought stress (and thus eCO2–water-use efficiency interactions; De Kauwe et al., 2013) or N availability, following the sensitivity of SOM decomposition to soil temperature and moisture (Melillo et al., 2011). Assuming that the variability in the eCO2 response of NPP during the first 3 yr of the experiments was predominantly influenced by meteorological conditions, and not N availability (as suggested by most of the models), the weather-related standard error at Duke (1.3%) is lower than the across-ring variations (3%), whereas it is higher at ORNL (2.9% and 0.1%, respectively). These weather-related variations add uncertainty to our estimates of the initial response of NPP to eCO2, whereas they appear to be sufficiently small to allow us to decipher the long-term trend, which we assessed as a 5-yr mean towards the end of the experiment. We cannot rule out, however, that extreme events, such as the ice storm at Duke in December 2002 (McCarthy et al., 2007), have strongly altered the forest's C–N dynamics and thereby obscured the expected trajectory of NPP enhancement. Although the models' meteorological forcing contained these extreme events, none of the models incorporated the damage processes associated with, for instance, ice-break or wind damage.
A further complicating factor in the model–data analyses is that the magnitude of the N limitation of the CO2 response depends on various boundary conditions of the experiment, including the magnitude of the CO2 perturbation and the pool of plant-available N at the beginning of the experiment. The step increase in CO2 is much faster than projected future transient increases in atmospheric CO2. Thus, the experiment produces a suddenly increasing plant N demand (Luo & Reynolds, 1999) which could: (1) lead to an overestimate of the importance of nutrient constraints; and (2) trigger ecosystem processes that would not have occurred otherwise. The initial pool of easily plant-accessible N, either in the form of mineral N or readily decomposable dead organic material, is influenced by the land use history of the plots. It is difficult to estimate from bulk soil SOM measurements, as the net N mineralization depends on the partitioning of SOM into pools with different turnover times. In the absence of suitable initialization data, most models generated their initial condition based on site history, which caused uncertainty in the amount of net N mineralization, and thus N availability for plants, at the start of the experiment. Whether or not a model simulates PNL, and at what time scale, therefore depends not only on the model structure, but also on the initialization protocol. In particular, the ED2.1 model did not show signs of N limitation, because it did not simulate N inputs or losses; thus, the prescribed initial SOM pool provided ample inorganic N to support the growth of the trees throughout the simulation period. To minimize the effect of initial conditions, the models were evaluated in terms of the compatibility of their component processes with observations, rather than in terms of the average modelled productivity and N uptake response to CO2.
Concluding remarks and recommendations for future experiments
The two FACE experiments initially showed a similar productivity response to eCO2, relative to a comparable baseline, in terms of forest productivity and forest N use, as well as climate and atmospheric N inputs. The long-term responses diverged strongly: the cumulated NPP response to eCO2 at the deciduous site was about half that of the evergreen site. The primary reason for this difference was that altered SOM dynamics increased plant N availability at Duke Forest at a rate that allowed the vegetation to maintain elevated levels of N uptake, whereas this did not happen at a sufficient rate at ORNL FACE. Furthermore, a corollary of the different allocation responses to eCO2 was that almost the entire NPP enhancement remained in vegetation biomass in Duke, whereas eCO2 did not alter vegetation biomass at ORNL FACE.
Many models in the ensemble were capable of reproducing the observed initial increase in NPP with eCO2. However, in the majority of cases, this response resulted from compensating errors in the underlying process responses, as the models did not correctly simulate the magnitude of the observed initial increase in plant N uptake at both sites, and wrongly attributed a large share of the increased NPP to enhanced NUE. This result cautions against a too simplistic model–data comparison and underlines the necessity of the detailed process-level evaluation. Comparing the process responses of ecosystem models against the observations provided essential information on model validity: we were able to identify component processes within particular models that were operating well (qualitatively and quantitatively), although the overall observed ecosystem eCO2 response was not accurately reproduced.
Models with flexible stoichiometry and allocation patterns that respond to N stress captured the qualitative responses observed at both sites. Ecosystem models with flexible tissue stoichiometry predicted a larger CO2 response of the NPP response despite a lower than observed CO2 response of fNup, and generally overestimated the observed increase in vegetation C : N ratio. Despite the conceptually increased accuracy of the results, this clearly shows that a more explicitly process-based approach to the modelling of stoichiometric flexibility is important for capturing the eCO2 response at these sites.
Despite the diversity of the modelling approaches employed here, all 11 combinations of C–N cycle processes include mechanisms consistent with the PNL hypothesis (Comins & McMurtrie, 1993; Luo et al., 2004), although the extent to which PNL was simulated varied depending on the assumed tightness of the stoichiometric constraint and the openness of the N cycle. Although this generally agrees with the observed trends at ORNL FACE, most models failed to simulate the sustained NPP enhancement at the Duke FACE site, because the mechanisms to increase N availability for plant growth included in these models are insufficient to explain the observed increases. This tendency to underestimate the net transfer of N from soils to vegetation under elevated CO2 at Duke calls for a better representation of below-ground processes, in particular root allocation and microbial responses to enhanced rhizodeposition.
Large uncertainty as to whether the observed changes in above-ground N stocks are caused by a redistribution of N from soils or to newly acquired N stems from the low signal-to-noise ratio in soil N inventories. Precise inventories well below the active rooting depth at the beginning of the experiment (as it may increase as the experiment progresses) would help, as would additional regular measurements of N balance components (N leaching and gaseous emission). Additional experiments using open-top chambers may further help to reduce uncertainty with respect to the below-ground mass balance and the net transfer of nutrients from soil to plants. Replicated factorial manipulation of nutrient availability and atmospheric [CO2] treatments could help to elucidate process interactions regarding allocation and stoichiometric responses to altered C and N availability. The strong increase in atmospheric CO2 might have triggered processes that would not have occurred if CO2 had increased at a more gradual pace. It would be of interest to investigate nutrient responses in ecosystem-level experiments, where CO2 is elevated more gradually to the maximum level in instalments, allowing the ecosystem to adjust at least partially to the new conditions. To reduce the dependence of the experimental results on the initial state of the ecosystem, it would also be desirable to conduct future elevated CO2 experiments with replication of different soil fertilities. This model comparison exercise has also underlined the increasingly recognized need for datasets from large-scale experiments to be collated into a central, versioned data repository that is readily accessible to modellers, if we are to fully capitalize on the potential for such experiments to inform models.
The different responses of several key processes at the two experimental sites, which cannot be explained by any of the models, imply that we should be sceptical of overarching statements concerning the responses of ecosystems to increasing levels of atmospheric CO2. There is currently insufficient knowledge to fully constrain the eCO2 response of global terrestrial ecosystem models, despite the existing body of experimental evidence. Nevertheless, the ecosystem models were able to capture important features of the experiments, lending some support to their projections (e.g. Thornton et al., 2009; Zaehle et al., 2010; Zhang et al., 2011).
Acknowledgments
This study was conducted as part of the ‘Benchmarking ecosystem response models with experimental data from long-term CO2 enrichment experiments' Working Group supported by the National Center for Ecological Analysis and Synthesis, a Center funded by the National Science Foundation (NSF) (Grant #EF-0553768), the University of California, Santa Barbara and the State of California. The ORNL and Duke FACE sites and additional synthesis activities were supported by the US Department of Energy Office of Science, Biological and Environmental Research Program. In particular, Duke FACE research was supported under grant number FACE, DE-FG02-95ER62083). S.Z. was supported by the FP7 people programme through grant nos PERG02-GA-2007-224775 and 238366. M.G.D.K. and B.E-M. were supported by ARC Discovery Grant DP1094791, and T.H. by the research funding programme ‘LOEWE-Landesoffensive zur Entwicklung wissenschaftlich-ökonomischer Exzellenz’ of Hesse's Ministry of Higher Education.
Appendices
Table A1.
Overview of the models used and the representation of key processes in the carbon–nitrogen cycle. C, carbon; N, nitrogen; P, phosphorus; PFT, plant functional type; T, temperature; f(x), function of x.
| CABLE | CLM4 | DAYCENT | EALCO | ||
|---|---|---|---|---|---|
| Key reference | Wang et al. (2010, 2011) | Thornton & Zimmermann (2007), Thornton et al. (2007) | Parton et al. (2010) | Wang et al. (2001) | |
| Time step | 30 min | 30 min | 1 d | 30 min | |
| Plant C acquisition | Assimilation (GPP) | Farquhar et al. (1980) | Collatz et al. (1991) | 2 × NPPact | Farquhar et al. (1980) |
| N dependence of gross photosynthesis | f(leaf N) | NPPact/NPPpot | None | f(leaf N) | |
| Autotrophic respiration | f(tissue N, T) + f(growth rate) | f(tissue C, T) + f(growth rate) | 0.5 × GPP | f(tissue C, T) + i(growth rate) | |
| N dependence of whole-plant growth (if not GPP – Ra) | None | Potential growth (NPPpot) limited by stoichiometric N requirement for new tissue growth | Potential growth (f(PAR, T, moisture, CO2)) limited by stoichiometric N requirement for new tissue growth | None | |
| Plant N acquisition | Nitrogen fixation | Prescribed based on Wang & Houlton (2009) | f(NPP) | Plant-associated N fixation: f(N : P, plant N demand); soil N fixation: f(AET) | None |
| Nitrogen uptake | f(plant N demand, soil N availability) | f(relative strength of plant and microbial N demand, inorganic N pool size) | f(root biomass, plant demand, soil N availability) | Competition of soil mineral N between plant and microbial | |
| Plant growth | Allocation principle1 | Fixed allocation fractions, which vary according to phenological state | Fixed allocation fractions, derived from observations at the sites | Hierarchical allocation factors, in which fine roots have priority over leaves and over wood, with prescribed maximum pool sizes | Fixed allocation fractions, which vary according to phenological state |
| Maximum leaf area1 | Prescribed (LAI = 8; excess C is allocated to wood and roots) | Predicted | Predicted | Prescribed from observations at the site | |
| N effect on allocation1 | None | None | Nitrogen stress increases root allocation | None | |
| Plant tissue C : N stoichiometry | Flexible within 10% of the prescribed mean C : N | Fixed | Flexible within prescribed bounds | Flexible within prescribed bounds | |
| Plant N turnover | N effect on turnover/mortality | None | Indirect via changes in NPP | Leaf turnover increases linearly with leaf N concentration | None |
| N retention on leaf and root shedding | 50% of leaf N, 10% of root N | Litter has a fixed C : N (PFT specific) | 50% of leaf N | Retaining ratio depends on current tissue C : N ratio | |
| Soil N turnover | SOM decay (other than dependent on soil T and moisture) | 3 litter pools (metabolic, structural, coarse woody debris), 3 SOM pools with different turnover times, 1st order decay | 3 litter pools, 4 SOM pools, all with different turnover times, 1st order decay | 3 litter pools (above and below ground combined), 4 SOM pools, all with different turnover times, 1st order decay | 3 litter pools; 4 SOM pools with different turnover rates, 1st order decay |
| N effect on decomposition | Lignin : N ratio affects microbial efficiency and decomposition rate. Available soil mineral N constrains immobilization | Litter decomposition constrained by available soil N | Lignin : N ratio affects microbial efficiency and decomposition rate. Available soil mineral N constrains immobilization | Litter decomposition constrained by available soil N | |
| Soil C : N stoichiometry | Fixed for each pool | Fixed for each pool | f(mineral N concentration, within bounds) | f(mineral N concentration, within bounds) | |
| Ecosystem N losses | N leaching | Proportional to mineral N pool | f(soil water N concentration, drainage) | DON + N leaching = f(precipitation, NO3 pool size) | f(mineral N concentration, drainage and surface runoff) |
| gaseous N loss | Proportional to net N mineralization rate | Proportional to gross N mineralization + 10% of mineral N remaining in the soil | NOx, N2O, N2 fluxes, as a function of soil N pool size, temperature, water | None |
| ED2.1 | GDAY | ISAM | LPJ-GUESS | ||
|---|---|---|---|---|---|
| Key reference | Medvigy et al. (2009) | Comins & McMurtrie (1993) | Yang et al. (2009) | Smith et al. (2001, 2013) | |
| Time step | 15 min | 1 d | 30 min | 1 d | |
| Plant C acquisition | Assimilation | Farquhar et al. (1980) | Sands (1995, 1996) | Farquhar et al. (1980) | Collatz et al. (1991),Haxeltine & Prentice (1996) |
| N dependence of gross photosynthesis | None | f(leaf N) | Stoichiometric downregulation of Vcmax | f(leaf N) | |
| Autotrophic respiration | f(tissue C, T) + f(GPP) | 0.5 × GPP | f(tissue N, T) | f(tissue N, T) + f(GPP) | |
| N dependence of whole-plant growth (if not GPP – Ra) | Potential growth limited by stoichiometric N requirement for new tissue growth | None | None | None | |
| Plant N acquisition | Nitrogen fixation | None | Prescribed | Predicted | Prescribed |
| Nitrogen uptake | f(root biomass, plant N demand, soil N availability) | Fixed proportion of the inorganic N pool size | Michaelis–Menten kinetics, increases with increased plant N demand | f(plant N demand, soil T) | |
| Plant growth | Allocation principle1 | Functional relationships amongst leaf and sapwood (pipe-model), and sapwood and fine root biomass | Fixed allocation fractions, derived from observations at the sites | Dynamic allocation fractions, based on light, water and phenology | Functional relationships amongst leaf and sapwood (pipe-model), and leaf and fine root biomass |
| Maximum leaf area1 | Predicted | Predicted | Predicted | Predicted | |
| N effect on allocation1 | Nitrogen stress decreases leaf : root ratio | None | None | Nitrogen stress decreases leaf : root ratio | |
| Plant C : N stoichiometry | Fixed | Flexible | Flexible within prescribed bounds | Flexible within prescribed bounds | |
| Plant N turnover | N effect on turnover/mortality | Indirect via changes in NPP | None | Indirect via changes in NPP | Indirect via changes in NPP |
| N retention on leaf and root shedding | 50% of N is retained with leaf fall, but 0% with root turnover | 50% of N is retained with leaf fall, but 0% with root turnover | Biome dependent | 50% of N is retained | |
| Soil N turnover | SOM decay (other than dependent on soil T and moisture) | Three SOM pools with varying turnover rates | 4 litter pools (above/below metabolic and structural litter) and 3 SOM pools with varying turnover rates | 4 litter/SOM above-ground pools, 4 litter/SOM below-ground pools and one inert organic matter pool with different turnover rates | 5 litter pools (above/below metabolic and structural litter, plus an above CWD litter pool) and 5 SOM pools with varying turnover rates |
| N effect on decomposition | Litter decomposition constrained by available soil N | Lignin : N ratio affects microbial efficiency and decomposition rate. Available soil mineral N constrains immobilization | Litter decomposition constrained by available soil N | Litter decomposition constrained by available soil N | |
| Soil C : N stoichiometry | Fast pool: function of mineral N. Slow and structural pool: fixed C : N | f(mineral N concentration, within bounds) | Fixed | f(mineral N concentration, within bounds) | |
| Ecosystem N losses | N leaching | None | Fixed proportion of the inorganic N pool size | f(N pool size, drainage) | f(mineral N concentration, drainage) |
| gaseous N loss | None | None | NH3 volatilization and denitrification losses | None |
| OCN | SDGVM | TECO | ||
|---|---|---|---|---|
| Key reference | Zaehle & Friend (2010) | Woodward et al. (1995) | Weng & Luo (2009); updated | |
| Time step | 30 min | 1 d | 30 min | |
| Plant C acquisition | Assimilation | Farquhar et al. (1980),Kull & Kruijt (1998) | Farquhar et al. (1980),Harley et al. (1992) | Farquhar et al. (1980) |
| N dependence of gross photosynthesis | f(leaf N) | f(leaf N) | f(leaf N) | |
| Autotrophic respiration | f(tissue N) + f(growth rate) + excess respiration if labile C exceeds storage capacity, in the limits of the labile C pool size | f(tissue N, T) | f(leaf area, root and sapwood C) | |
| N dependence of whole-plant growth (if not GPP – Ra) | f(labile C pool size, stoichiometric N requirement for new tissue growth) | None | Surplus C under N stress is allocated to woody biomass | |
| Plant N acquisition | Nitrogen fixation | Prescribed | None | Prescribed |
| Nitrogen uptake | Michaelis–Menten kinetics, proportional to root biomass, increases with increased plant N demand | f(soil organic C and N) | f(root C, plant N demand) | |
| Plant growth | Allocation principle1 | Functional relationships amongst leaf and sapwood (pipe-model), and leaf and fine root biomass | Leaf allocation determined as C balance of lowest LAI layer of the previous year. Root and wood allocation fixed fraction if GPP > 0 | Resource limitations approach, prioritizing leaf over root and wood allocation |
| Maximum leaf area1 | Predicted | Predicted | Prescribed per plant functional type | |
| N effect on allocation1 | Increased plant N demand increases root : leaf ratio | None | N limitation increases allocation to woody biomass | |
| Plant C : N stoichiometry | Flexible within prescribed bounds | Foliar N is prescribed from observations | Flexible within prescribed narrow bounds | |
| Plant N turnover | N effect on turnover/mortality | Indirect via changes in NPP | None | None |
| N retention on leaf and root shedding | 50% of N is retained | None | 50% of N is retained | |
| Soil N turnover | SOM decay (other than dependent on soil T and moisture) | 3 litter pools; 4 SOM pools with different turnover times, 1st order decay | 4 litter pools, 4 SOM pools, with different turnover times, 1st order decay | 5 SOM pools (metabolic litter, structural litter, fast SOM, slow SOM, and passive SOM) with different turnover rates, 1st order decay |
| N effect on decomposition | Lignin : N ratio affects microbial efficiency and decomposition rate. Available soil mineral N constrains immobilization | n.a. | ||
| Soil C : N stoichiometry | f(mineral N concentration, within bounds) | Fixed | Flexible soil C : N ratios | |
| Ecosystem N losses | N leaching | f(mineral N concentration, drainage) | None | f(mineralized N, runoff) |
| gaseous N loss | f(mineral N concentration, soil T, moisture and respiration) | None | Fixed proportion of mineral N, regulated by soil T |
See M. G. De Kauwe et al. (unpublished) for details.
A2.
List of variable names used, as well as their description and unit. Tissue types considered are foliage (f), fine roots (r) and woody (w) biomass. C, carbon; N, nitrogen; DW, dry weight.
| Variable | Description | Unit |
|---|---|---|
| ai | Fractional allocation to tissue type i | – |
| AET | actual evapotranspiration | mm yr−1 |
| Bi | Biomass of tissue type i | g DW m−2 |
| Corg | Ecosystem organic carbon | g C m−2 |
| CSOM | Soil organic matter carbon (including the litter layer) | g C m−2 |
| Cveg | Vegetation carbon | g C m−2 |
| CUE | Carbon-use efficiency (NPP/GPP) | – (g C g−1 C) |
| CWD | coarse woody debris | g C m−2 |
| DON | dissolved organic nitrogen | g N m−2 yr−1 |
| GPP | Area-based gross primary production | g C m−2 yr−1 |
| GPPN | N-based gross primary production | g C g−1 Ncan yr−1 |
| fNdep | Atmospheric nitrogen deposition | g N m−2 yr−1 |
| fNfix | Biological nitrogen fixation | g N m−2 yr−1 |
| fNgas | Ecosystem loss of nitrogen through gaseous emission | g N m−2 yr−1 |
| fNleach | Ecosystem loss of nitrogen through leaching | g N m−2 yr−1 |
| fNmin | Net nitrogen mineralization | g N m−2 yr−1 |
| fNup | Plant nitrogen uptake | g N m−2 yr−1 |
| ftrans | Fraction of tissue N translocated before abscission | – |
| fveg | Fraction of organic ecosystem nitrogen in vegetation | – |
| LAI | leaf area index | m2 m−2 |
| ni | Nitrogen concentration of tissue type i | g N g−1 DW |
| Ncan | Canopy nitrogen | g N m−2 |
| Norg | Ecosystem organic nitrogen | g N m−2 |
| Ninorg | Inorganic nitrogen in the ecosystem | g N m−2 |
| nsom | Soil organic matter nitrogen (including the litter layer) | g N m−2 |
| Nveg | Vegetation nitrogen | g N m−2 |
| NNE | Net ecosystem nitrogen exchange | g N m−2 yr−1 |
| NPP | Area-based net primary production | g C m−2 yr−1 |
| NPPN | N-based net primary production | g C g−1 Ncan yr−1 |
| NUE | Nitrogen-use efficiency (NPP/fNup) | g C g−1 N |
| PAR | photosynthetically active radiation | μmol m−2 s−1 |
| Ra | Autotrophic respiration | g C m−2 yr−1 |
| ρi | tissue carbon density | g C g−1 DW |
| SOM | soil organic matter | g C|N m−2 |
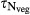 |
Turnover time of nitrogen in vegetation | yr−1 |
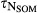 |
Turnover time of nitrogen in soil organic matter (including the litter layer) | yr−1 |
Supporting Information
Please note: Wiley Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 Cumulative effect of elevated atmospheric [CO2] (eCO2) on carbon (C) and nitrogen (N) storage in the Duke and Oak Ridge National Laboratory (ORNL) Free-Air CO2 Enrichment (FACE) sites.
References
- Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist. 2005;165:351–372. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- Arora VK, Boer GJ, Freidlingstein P, Eby M, Jones CD, Christian JR, Bonan G, Bopp L, Brovkin V, Cadule P. Carbon–concentration and carbon–climate feedbacks in CMIP5 Earth system models. Journal of Climate. 2013;26:5289–5314. [Google Scholar]
- Arrhenius S. On the influence of carbonic acid in the air upon the temperature of the ground. Philosophical Magazine and Journal of Science. 1896;41:237–276. [Google Scholar]
- Bonan GB, Oleson KW, Fisher RA, Lasslop G, Reichstein M. Reconciling leaf physiological traits and canopy flux data: use of the TRY and FLUXNET databases in the Community Land Model version 4. Journal of Geophysical Research. 2012;117:G02026. . doi: 10.1029-2011JG001913. [Google Scholar]
- Collatz GJ, Ball JT, Grivet C, Berry JA. Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration – a model that includes a laminar boundary layer. Agricultural and Forest Meteorology. 1991;54:107–136. [Google Scholar]
- Comins H, McMurtrie RE. Long-term biotic response of nutrient-limited forest ecosystems to CO2-enrichment: equilibrium behaviour of integrated plant–soil models. Ecological Applications. 1993;3:666–681. doi: 10.2307/1942099. [DOI] [PubMed] [Google Scholar]
- Cramer W, Bondeau A, Woodward FI, Prentice IC, Betts RA, Brovkin V, Cox PM, Fisher V, Foley JA, Friend AD. Global response of terrestrial ecosystem structure and function to CO2 and climate change: results from six dynamic global vegetation models. Global Change Biology. 2001;7:357–373. [Google Scholar]
- Crous KY, Walters MB, Ellsworth DS. Elevated CO2 concentration affects leaf photosynthesis–nitrogen relationships in Pinus taeda over nine years in FACE. Tree Physiology. 2008;28:607–614. doi: 10.1093/treephys/28.4.607. [DOI] [PubMed] [Google Scholar]
- De Kauwe M, Medlyn BE, Zaehle S, Walker AP, Dietze M, Thomas H, Jain AK, Luo Y, Parton WJ, Prentice IC. Forest water use and water use efficiency at elevated CO2: a model–data intercomparison at two contrasting temperate forest FACE sites. Global Change Biology. 2013;19:1759–1779. doi: 10.1111/gcb.12164. [DOI] [PubMed] [Google Scholar]
- Drake JE, Darby BA, Giasson MA, Kramer MA, Phillips RP, Finzi AC. Stoichiometry constrains microbial response to root exudation – insights from a model and a field experiment in a temperate forest. Biogeosciences. 2013;10:821–838. [Google Scholar]
- Drake JE, Gallet-Budynek A, Hofmockel KS, Bernhardt ES, Billings SA, Jackson RB, Johnsen KS, Lichter J, McCarthy HR, McCormack ML. Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecology Letters. 2011;14:349–357. doi: 10.1111/j.1461-0248.2011.01593.x. [DOI] [PubMed] [Google Scholar]
- Ellsworth DS, Thomas R, Crous KY, Palmroth S, Ward E, Maier C, DeLucia E, Oren R. Elevated CO2 affects photosynthetic responses in canopy pine and subcanopy deciduous trees over 10 years: a synthesis from Duke FACE. Global Change Biology. 2011;18:223–242. [Google Scholar]
- Farquhar GD, Caemmerer SV, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149:78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- Finzi AC, DeLucia E, Hamilton J, Schlesinger W, Richter D. The nitrogen budget of a pine forest under free air CO2 enrichment. Oecologia. 2002;132:567–578. doi: 10.1007/s00442-002-0996-3. [DOI] [PubMed] [Google Scholar]
- Finzi AC, Norby RJ, Calfapietra C, Gallet-Budynek A, Gielen B, Holmes WE, Hoosbeek MR, Iversen CM, Jackson RB, Kubiske ME. Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2. Proceedings of the National Academy of Sciences, USA. 2007;104:14014–14019. doi: 10.1073/pnas.0706518104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend AD, Stevens AK, Knox RG, Cannell MGR. A process-based, terrestrial biosphere model of ecosystem dynamics (Hybrid v3.0) Ecological Modelling. 1997;95:249–287. [Google Scholar]
- Garten CT, Jr, Iversen CM, Norby RJ. Litterfall 15N abundance indicated declining soil nitrogen availability in a free-air CO2 enrichment experiment. Ecology. 2011;92:133–139. doi: 10.1890/10-0293.1. [DOI] [PubMed] [Google Scholar]
- Harley PC, Loreto F, Marco GD, Sharkey TD. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analyses of the response of photosynthesis to CO2. Plant Physiology. 1992;98:1429–1436. doi: 10.1104/pp.98.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxeltine A, Prentice IC. A general model for the light-use efficiency of primary production. Functional Ecology. 1996;10:551–561. [Google Scholar]
- Hickler T, Smith B, Prentice IC, Mjofors K, Miller P, Arneth A, Sykes MT. CO2 fertilization in temperate FACE experiments not representative of boreal and tropical forests. Global Change Biology. 2008;14:1531–1542. [Google Scholar]
- Hofmockel KS, Gallet-Budynek A, McCarthy HR, Currie WS, Jackson RB, Finzi AC. Sources of increased N uptake in forest trees growing under elevated CO2: results of a large-scale 15N study. Global Change Biology. 2011;17:3338–3350. [Google Scholar]
- Iversen CM. Digging deeper: fine-root responses to rising atmospheric CO2 concentration in forested ecosystems. New Phytologist. 2009;186:346–357. doi: 10.1111/j.1469-8137.2009.03122.x. [DOI] [PubMed] [Google Scholar]
- Iversen CM, Hooker TD, Classen AT, Norby RJ. Net mineralization of N at deeper soil depths as a potential mechanism for sustained forest production under elevated [CO2] Global Change Biology. 2011;17:1130–1139. [Google Scholar]
- Iversen CM, Keller JK, Garten CT, Jr, Norby RJ. Soil carbon and nitrogen cycling and storage throughout the soil profile in a sweetgum plantation after 11 years of CO2-enrichment. Global Change Biology. 2012;18:1684–1697. [Google Scholar]
- Iversen CM, Ledford J, Norby RJ. CO2 enrichment increases carbon and nitrogen input from fine roots in a deciduous forest. New Phytologist. 2008;179:837–847. doi: 10.1111/j.1469-8137.2008.02516.x. [DOI] [PubMed] [Google Scholar]
- Jastrow JD, Miller MR, Matamala R, Norby RJ, Boutton TW, Rice CW, Owensby CE. Elevated atmospheric carbon dioxide increases soil carbon. Global Change Biology. 2005;11:2057–2064. doi: 10.1111/j.1365-2486.2005.01077.x. [DOI] [PubMed] [Google Scholar]
- Johnson DW, Cheng W, Joslin JD, Norby RJ, Edwards NT, Todd DE. Effects of elevated CO2 on nutrient cycling in a sweetgum plantation. Biogeochemistry. 2004;69:379–403. [Google Scholar]
- Kull O, Kruijt B. Leaf photosynthetic light response: a mechanistic model for scaling photosynthesis to leaves and canopies. Functional Ecology. 1998;12:767–777. [Google Scholar]
- Lasslop G, Reichstein M, Papale D, Richardson AD, Arneth A, Barr A, Stoy PC, Wohlfahrt G. Separation of net ecosystem exchange into assimilation and respiration using a light response curve approach: critical issues and global evaluation. Global Change Biology. 2010;16:187–208. [Google Scholar]
- Lichter J, Billings SA, Ziegler SE, Gaindh D, Ryals R, Finzi AC, Jackson RB, Stemmler EA, Schlesinger WH. Soil carbon sequestration in a pine forest after 9 years of atmospheric CO2 enrichment. Global Change Biology. 2008;14:2910–2922. [Google Scholar]
- Liebig JV. Die Chemie in ihrer Anwendung auf Agricultur und Physiologie. Braunschweig, Germany: Verlag Vieweg; 1843. [Google Scholar]
- Luo Y, Reynolds JF. Validity of extrapolating field CO2 experiments to predict carbon sequestration in natural ecosystems. Ecology. 1999;80:1568–1583. [Google Scholar]
- Luo Y, Su B, Currie WS, Dukes JS, Finzi AC, Hartwig U, Hungate BA, McMurtrie RE, Oren R, Parton WJ. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. BioScience. 2004;54:731–739. [Google Scholar]
- Maier CA, Palmroth S, Ward E. Short-term effects of fertilization on photosynthesis and leaf morphology of field-grown loblolly pine following long-term exposure to elevated CO2 concentration. Tree Physiology. 2008;28:597–606. doi: 10.1093/treephys/28.4.597. [DOI] [PubMed] [Google Scholar]
- Maire V, Martre P, Kattge J, Gastal F, Esser G, Fontaine S, Soussana J-F. The coordination of leaf photosynthesis links C and N fluxes in C3 plant species. PLoS ONE. 2012;7:e38345. doi: 10.1371/journal.pone.0038345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy HR, Oren R, Finzi AC, Ellsworth DS, Kim H-S, Johnsen KH, Millar B. Temporal dynamics and spatial variability in the enhancement of canopy leaf area under elevated atmospheric CO2. Global Change Biology. 2007;13:2479–2497. [Google Scholar]
- McCarthy HR, Oren R, Johnsen KH, Gallet-Budynek A, Pritchard SG, Cook CW, LaDeau SL, Jackson RB, Finzi AC. Re-assessment of plant carbon dynamics at the Duke free-air CO2 enrichment site: interactions of atmospheric [CO2] with nitrogen and water availability over stand development. New Phytologist. 2010;185:514–528. doi: 10.1111/j.1469-8137.2009.03078.x. [DOI] [PubMed] [Google Scholar]
- McMurtrie RE, Dewar RC. New insights into carbon allocation by trees from the hypothesis that annual wood production is maximized. New Phytologist. 2013;199:981–990. doi: 10.1111/nph.12344. [DOI] [PubMed] [Google Scholar]
- McMurtrie RE, Norby RJ, Medlyn BE, Dewar RC, Pepper DA, Reich PB, Barton CVM. Why is plant-growth response to elevated CO2 amplified when water is limiting, but reduced when nitrogen is limiting? A growth-optimisation hypothesis. Functional Plant Biology. 2008;35:521–534. doi: 10.1071/FP08128. [DOI] [PubMed] [Google Scholar]
- Medlyn BE. The optimal allocation of nitrogen within the C3 photosynthetic system at elevated CO2. Australian Journal of Plant Physiology. 1996a;23:593–603. [Google Scholar]
- Medlyn BE. Interactive effects of atmospheric carbon dioxide and leaf nitrogen concentration on canopy light use efficiency: a modeling analysis. Tree Physiology. 1996b;16:201–209. doi: 10.1093/treephys/16.1-2.201. [DOI] [PubMed] [Google Scholar]
- Medvigy D, Wofsy SC, Munger JW, Hollinger DY, Moorcroft PR. Mechanistic scaling of ecosystem function and dynamics in space and time: Ecosystem Demography Model version 2. Journal of Geophysical Research. 2009;114:G01002. [Google Scholar]
- Melillo JM, Butler S, Johnson J, Mohan J, Steudler P, Lux H, Burrows E, Bowles F, Smith R, Scott L. Soil warming, carbon–nitrogen interactions, and forest carbon budgets. Proceedings of the National Academy of Sciences, USA. 2011;108:9508–9512. doi: 10.1073/pnas.1018189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norby R, Iversen CM. Nitrogen uptake, distribution, turnover, and efficiency of use in a CO2 enriched sweetgum forest. Ecology. 2006;87:5–14. doi: 10.1890/04-1950. [DOI] [PubMed] [Google Scholar]
- Norby RJ, DeLucia EH, Gielen B, Calfapietra C, Giardina CP, King JS, Ledford J, McCarthy HR, Moore DJP, Ceulemans R. Forest response to elevated CO2 is conserved across a broad range of productivity. Proceedings of the National Academy of Sciences, USA. 2005;102:18052–18056. doi: 10.1073/pnas.0509478102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norby RJ, Hanson PJ, O'Neill EG, Tschaplinski TJ, Hansen RA, Cheng W, Wullschleger SD, Gunderson CA, Edwards NT, Johnson DW. Net primary productivity of a CO2-enriched deciduous forest and the implications for carbon storage. Ecological Applications. 2002;12:1261–1266. [Google Scholar]
- Norby RJ, Todd DE, Fults J, Johnson DW. Allometric determination of tree growth in a CO2-enriched sweetgum stand. New Phytologist. 2001;150:477–487. [Google Scholar]
- Norby RJ, Warren JM, Iversen CM, Medlyn BE, McMurtrie RE. CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proceedings of the National Academy of Sciences, USA. 2010;107:19368–19373. doi: 10.1073/pnas.1006463107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren R, Ellsworth DS, Johnsen KH, Phillips N, Ewers BE, Maier C, Schafer KVR, McCarthy H, Hendrey G, McNulty SG. Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature. 2001;411:469–472. doi: 10.1038/35078064. [DOI] [PubMed] [Google Scholar]
- Palmroth S, Oren R, McCarthy HR, Johnsen KH, Finzi AC, Butnor JR, Ryan MG, Schlese U. Aboveground sink strength in forests controls the allocation of carbon below ground and its [CO2]-induced enhancement. Proceedings of the National Academy of Sciences, USA. 2006;103:19362–19367. doi: 10.1073/pnas.0609492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton WJ, Hanson PJ, Swanston C, Torn M, Trumbore SE, Riley W, Kelly R. ForCent model development and testing using the Enriched Background Isotope Study experiment. Journal of Geophysical Research. 2010;115:G04001. [Google Scholar]
- Piao SL, Sitch SA, Ciais P, Friedlingstein P, Peylin P, Wang X, Ahlström A, Anav A, Canadell JG, Cong N. Evaluation of terrestrial carbon cycle models for their response to climate variability and to CO2 trends. Global Change Biology. 2013;19:2117–2132. doi: 10.1111/gcb.12187. [DOI] [PubMed] [Google Scholar]
- Rastetter EB, Agren GI, Shaver GR. Responses of N-limited ecosystems to increased CO2: a balanced-nutrition, coupled-element-cycles model. Ecological Applications. 1997;7:444–460. [Google Scholar]
- Rastetter EB, McKane RB, Shaver GR, Melillo JM. Changes in C storage by terrestrial ecosystems: how C–N interactions restrict responses to CO2 and temperature. Water, Air, and Soil Pollution. 1992;64:327–344. [Google Scholar]
- Sands PJ. Modelling canopy production I. Optimal distribution of photosynthetic resources. Australian Journal of Plant Physiology. 1995;22:593–601. [Google Scholar]
- Sands PJ. Modelling canopy production III. Canopy light-utilisation efficiency and its sensitivity to physiological and environmental variables. Australian Journal of Plant Physiology. 1996;23:103–114. [Google Scholar]
- Sitch SA, Friedlingstein P, Gruber N, Jones S, Murray-Tortarolo G, Ahlström A, Doney SC, Graven H, Heinze C, Huntingford C. Trends and drivers of regional sources and sinks of carbon dioxide over the past two decades. Biogeoscience Discussions. 2013;10:20113–20177. [Google Scholar]
- Sitch SA, Huntingford C, Gedney N, Levy PE, Lomas M, Piao SL, Betts R, Ciais P, Cox PM, Friedlingstein P. Evaluation of the terrestrial carbon cycle, future plant geography and climate–carbon cycle feedbacks using five Dynamic Global Vegetation Models (DGVMs) Global Change Biology. 2008;14:2015–2039. [Google Scholar]
- Smith B, Prentice IC, Sykes MT. Representation of vegetation dynamics in the modelling of terrestrial ecosystems: comparing two contrasting approaches within European climate space. Global Ecology and Biogeography. 2001;10:621–637. [Google Scholar]
- Smith B, Warlind D, Arneth A, Thomas H, Leadly P, Siltberg J, Zaehle S. Implications of incorporating N cycling and N limitations on primary production in an individual-based dynamic vegetation model. Biogeosciences Discussions. 2013;10:18563–18611. [Google Scholar]
- Sokolov AP, Kicklighter DW, Melillo JM, Felzer BS, Schlosser CA, Cronin TW. Consequences of considering carbon–nitrogen interactions on the feedbacks between climate and the terrestrial carbon cycle. Journal of Climate. 2008;21:3776–3796. [Google Scholar]
- Thornton PE, Doney SC, Lindsay K, Moore JK, Mahowald N, Randerson JT, Fung I, Lamarque JF, Feddema JJ, Lee YH. Carbon–nitrogen interactions regulate climate–carbon cycle feedbacks: results from an atmosphere–ocean general circulation model. Biogeosciences. 2009;6:2099–2120. [Google Scholar]
- Thornton PE, Lamarque J-F, Rosenbloom NA, Mahowald NM. Influence of carbon–nitrogen cycle coupling on land model response to CO2 fertilization and climate variability. Global Biogeochemical Cycles. 2007;21:GB4018. [Google Scholar]
- Thornton PE, Zimmermann NE. An improved canopy integration scheme for a land surface model with prognostic canopy structure. Journal of Climate. 2007;20:3902–3923. [Google Scholar]
- Vitousek PM, Howarth RW. Nitrogen limitation on land and in the sea – how it can occur. Biogeochemistry. 1991;13:87–115. [Google Scholar]
- Wang S, Grant RF, Verseghy DL, Black TA. Modelling plant carbon and nitrogen dynamics of a boreal aspen forest in CLASS – the Canadian Land Surface Scheme. Ecological Modelling. 2001;142:135–154. [Google Scholar]
- Wang Y-P, Houlton BZ. Nitrogen constraints on terrestrial carbon uptake: Implications for the global carbon-climate feedback. Geophysical Research Letters. 2009;36:L24403. . doi: 10.1029/2009GL041009. [Google Scholar]
- Wang Y-P, Kowalczyk E, Leuning R, Abramowitz G, Raupach MR, Pak B, van Gorsel E, Luhar A. Diagnosing errors in a land surface model (CABLE) in the time and frequency domains. Journal of Geophysical Research. 2011;116:G01034. [Google Scholar]
- Wang YP, Law RM, Pak B. A global model of carbon, nitrogen and phosphorus cycles for the terrestrial biosphere. Biogeosciences. 2010;7:2261–2282. [Google Scholar]
- Warren JM, Pötzelsberger E, Wullschleger SD, Thornton PE, Hasenauer H, Norby RJ. Ecohydrologic impact of reduced stomatal conductance in forests exposed to elevated CO2. Ecohydrology. 2011;4:196–210. [Google Scholar]
- Weng E, Luo Y. Soil hydrological properties regulate grassland ecosystem responses to multifactor global change: a modeling analysis. Journal of Geophysical Research. 2008;113:G03003. [Google Scholar]
- Woodward FI, Smith TM, Emanuel WR. A global land primary productivity and phytogeography model. Global Biogeochemical Cycles. 1995;9:471–490. [Google Scholar]
- Xu C, Fisher R, Wullschleger SD, Wilson CJ, Cai M, McDowell NG. Toward a mechanistic modeling of nitrogen limitation on vegetation dynamics. PLoS ONE. 2012;7:e37914. doi: 10.1371/journal.pone.0037914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wittig V, Jain AK, Post W. The integration of nitrogen cycle dynamics into the Integrated Science Assessment Model (ISAM) for the study of terrestrial ecosystem responses to global change. Global Biogeochemical Cycles. 2009;23:GB4029. [Google Scholar]
- Zaehle S, Ciais P, Friend AD, Prieur V. Carbon benefits of anthropogenic reactive nitrogen offset by nitrous oxide emissions. Nature Geoscience. 2011;4:1–5. [Google Scholar]
- Zaehle S, Dalmonech D. Carbon–nitrogen interactions on land at global scales: current understanding in modelling climate biosphere feedbacks. Current Opinion in Environmental Sustainability. 2011;3:311–320. [Google Scholar]
- Zaehle S, Friedlingstein P, Friend AD. Terrestrial nitrogen feedbacks may accelerate future climate change. Geophysical Research Letters. 2010;37:L01401. [Google Scholar]
- Zaehle S, Friend AD. Carbon and nitrogen cycle dynamics in the O-CN land surface model: 1. Model description, site-scale evaluation, and sensitivity to parameter estimates. Global Biogeochemical Cycles. 2010;24:GB1005. [Google Scholar]
- Zhang Q, Wang YP, Pitman AJ, Dai YJ. Limitations of nitrogen and phosphorous on the terrestrial carbon uptake in the 20th century. Geophysical Research Letters. 2011;38:GL049244. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Cumulative effect of elevated atmospheric [CO2] (eCO2) on carbon (C) and nitrogen (N) storage in the Duke and Oak Ridge National Laboratory (ORNL) Free-Air CO2 Enrichment (FACE) sites.


