Abstract
Purpose
To evaluate plan quality and delivery efficiency gains of Volumetric Modulated Arc Therapy (VMAT) versus a Multi-Criteria Optimization based IMRT (MCO-IMRT) for stereotactic radiosurgery of spinal metastases.
Methods and Materials
MCO-IMRT plans (RayStation V2.5, RaySearch Laboratories, Stockholm, Sweden) of ten spinal radiosurgery cases using 7–9 beams were developed for clinical delivery, and patients were replanned using VMAT with partial arcs. The prescribed dose was 18 Gy, and target coverage was maximized such that the maximum dose to the planning organ-at-risk volume (PRV) of the spinal cord was 10 or 12 Gy. DVH constraints from the clinically acceptable MCO-IMRT plans were utilized for VMAT optimization. Plan quality and delivery efficiency with and without collimator rotation for MCO-IMRT and VMAT were compared and analyzed based upon DVH, PTV coverage, homogeneity index, conformity number, cord PRV sparing, total MU and delivery time.
Results
VMAT plans were capable of matching most DVH constraints from the MCO-IMRT plans. The ranges of MUs were 4808–7193 for MCO-IMRT without collimator rotation, 3509–5907 for MCO-IMRT with collimator rotation, 4444–7309 for VMAT without collimator rotation and 3277–5643 for VMAT with collimator of 90 degrees. MU for the VMAT plans were similar to their corresponding MCO-IMRT plans, depending upon the complexity of the target and PRV geometries, but had a larger range. The delivery times of the MCO-IMRT and VMAT plans, both with collimator rotation, were 18.3 ± 2.5 minutes and 14.2 ± 2.0 minutes, respectively (p < 0.05).
Conclusion
MCO-IMRT and VMAT can create clinically acceptable plans for spinal radiosurgery. The MU for MCO-IMRT and VMAT can be reduced significantly by utilizing a collimator rotation following the orientation of the spinal cord. Plan quality for VMAT is similar to MCO-IMRT, with similar MU for both modalities. Delivery times can be reduced by nominally 25% with VMAT.
INTRODUCTION
Stereotactic radiosurgery (SRS) has gained increasing importance in the treatment of patients with spine metastases1–3. Many studies have been presented using intensity modulated radiation therapy (IMRT) for spine SRS and established this technique as a treatment option for rapid pain relief and safe and effective tumor control3–5. However, IMRT treatments for spine SRS often require 30–60 minutes including imaging verification time in our clinic, which can be challenging for patients with substantial pain from tumor involvement or recent spine surgery.
Volumetric modulated arc therapy (VMAT) is an alternative technique to IMRT with the beam modulated by variable gantry speed, dose rate and multileaf collimator (MLC) motion. Earlier studies have shown that VMAT provides comparable dose distributions and improved delivery efficiency when compared to IMRT for a variety of treatment sites6–11, but very few data exists on spine SRS cases.
All IMRT and VMAT SRS cases in our department are planned on the RayStation treatment planning system (Version 2.5, RaySearch Laboratories, Stockholm, Sweden). IMRT plans are generated using the Multicriteria Optimization (MCO) approach, which has been demonstrated to be beneficial for a variety of treatment sites12–13. The MCO approach is based on Pareto- surface techniques to generate a database of optimal plans that satisfy different objectives and criteria. The planner can navigate interactively through this database by exploring convex combinations of the database to achieve an optimal plan with the desired trade-off between different planning objectives14–15. MCO has only recently become clinically available for VMAT. We utilize an approach termed MCO-IMRT guided VMAT optimization, where output DVH point values of MCO-IMRT from a clinically acceptable MCO-IMRT plan are utilized as a starting point for VMAT optimization16.
The presented study compares plan quality and delivery efficiency between VMAT and MCO-IMRT plans for spine metastases treated with spine SRS. Furthermore, the influence of collimator rotation was also evaluated for both treatment methods, since rotating the collimator may provide benefits given the relative geometry of the spinal cord and target.
METHODS AND MATERIALS
Patients, volume definition and SRS dose prescription
Ten patients were randomly selected from a clinical set of spinal radiosurgery cases treated in our department. The treatment sites range from T1 to L4. Figure 1 shows the patient anatomy for an L3-4 spine case. All patients were immobilized in a vacuum cushion. For site T4 and above, a head and shoulder mask was also used. The target volume was defined per guidelines of RTOG 0631, with a clinical target volume (CTV) defined for radiation planning purposes. For gross disease limited to the vertebral body, the CTV included the vertebral body and both pedicles. For gross disease that extended into a pedicle, the CTV was extended to include the ipsilateral pedicle, lamina, and spinous process at the discretion of the radiation oncologist. For gross disease that involves the vertebral body and both pedicles, the CTV included entire vertebral elements. For gross disease limited only to the posterior elements, the CTV included the spinous process and bilateral laminae. The planning target volume (PTV) was then defined by adding a uniform margin of 3 mm to the CTV. In most cases, the spinal cord or cauda equina was defined using both MRI and myelogram at the time of CT simulation, when clinically appropriate. The planning OAR volume (PRV) of the spinal cord was a 1–2 mm expansion of the structure. The prescription for all plans was 18 Gy in a single fraction. Dose constraint for cord PRV was Dmax < 12 Gy in radiation-naïve patients and < 10 Gy in the setting of retreatment. If at the level of the cauda equina PRV, the Dmax was < 14 Gy and < 12 Gy, respectively. In general, esophageal PRV constraints were Dmax < 15 Gy and V12–15 Gy < 5 cc. In patients with normal renal function, combined kidneys were limited to V8 < 40%.
Figure 1.
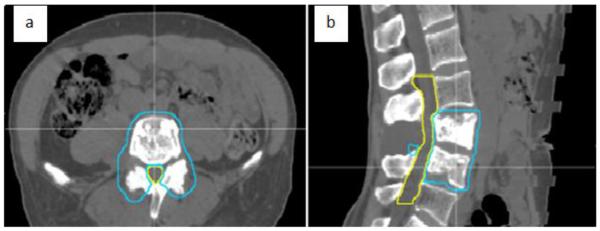
Patient anatomy on (a) transversal CT slice and (b) sagittal CT slice. Blue contour: PTV; yellow contour: spinal cord PRV.
Pre-treatment Quality Assurance (QA), imaging procedures, and Linac isocenter accuracy
Patient-specific dosimetry QA is performed prior to each treatment with the ArcCheck device (Sun Nuclear Corp., Melbourne, FL), which consists of 1386 diodes arranged in a helical grid. Dose is calculated on the ArcCheck virtual phantom with the treatment couch in place.
Imaging QA is also performed prior to every treatment. An anthropomorphic plastic phantom containing a 6 mm tungsten ball is positioned at an offset position from isocenter. A cone beam computed tomography (CBCT) scan is acquired and then registered to the reference CT. Shifts are calculated and applied using the robotic couch. Another CBCT is acquired to verify the accuracy of the shifts made to the phantom. After confirmation of isocentricity, a Winston-Lutz test17 is performed with the mega-volutage (MV) treatment beam, analyzing the position of the phantom/tungsten ball and radiation isocenter.
Treatment is delivered on an Elekta Synergy S linac. We have determined the gantry isocenter accuracy to be within 0.3 mm with a sag of ± 1mm (+1 mm from the AP direction and −1 mm from the PA direction) for a total drift of almost 2 mm during the rotation of the gantry. The collimator rotation iscoenter accuracy is within 0.5 mm with a total walkout of ± 0.5 mm. During commissioning and subsequent routine maintenance, the gantry and collimator rotation isocentricities are measured and the average position is calculated for the radiation isocenter. The imaging system is aligned to the average radiation isocenter.
Patient setup is 7–15 minutes with 2–3 CBCT acquisitions depending upon the amount of initial setup error with 3 acquisitions required for greater initial error, specifically for patients with greater than 1 degree of rotational error. IMRT beam delivery requires approximately 20 minutes with a mid-treatment CBCT for position verification. The treatment session concludes with a final CBCT acquisition to measure any residual positioning errors. Intra-fraction motion is typically less than 1 mm. Pre-treatment QA requires about 30–40 minutes for dosimetric measurements and 15 minutes for isocentricity, patient positioning and imaging system checks.
MCO guided IMRT and VMAT planning
All plans were designed and optimized on a RayStation treatment planning system (TPS) for a 6MV Elekta Synergy S linac equipped with a “Beam Modulator” multileaf collimator (MLC) with 40 leaf pairs each with a 4 mm leaf width at isocenter. The slice thickness of the CT scans was 1.25 mm. The final dose calculation was done using the collapsed cone calculation algorithm with a calculation dose grid of 2 mm × 2 mm × 2 mm. For each patient, two MCO-IMRT plans with 7–9 beams were performed with collimator setting of 0 degrees (colli: 0) and collimator rotation (colli: Rot). For “colli: Rot”, the collimator angle was chosen to allow the leaf travel direction to best match the orientation of the spinal cord for each beam entry18. Couch rotations were not used. The goal of MCO-IMRT planning in this study was to achieve maximum dose coverage of PTV while fulfilling the OAR constraints. The MCO-IMRT approach for other sites is described in greater detail by Craft et al19–20.
All ten clinically accepted MCO-IMRT plans were then replanned using the VMAT modality. The two-step VMAT optimization for RayStation is described by Bzdusek et al21. Using an MCO-guided VMAT optimization approach, the DVH values for the PTV and relevant PRVs from the clinically acceptable MCO-IMRT plans were used as initial values for the VMAT optimization. VMAT plans were generated with collimator setting of 0 degrees (colli: 0) and 90 degrees (colli: 90). The system configuration for VMAT delivery includes a variable dose rate ranging from 39 to 450 MU/minute, a maximum leaf speed of 2.5 cm/s and a maximum gantry speed of 5.5 degree/s. Partial arcs were utilized for VMAT plans with the same start and end gantry angle of the corresponding MCO-IMRT plan. Dual arcs with a gantry angle spacing of 2 degrees, and a maximum delivery time of 20 minutes were also used for VMAT optimization. If the initial iteration of VMAT optimization was inferior to the MCO-IMRT plan, the weights of the objectives and/or constraints were further adjusted to best match the plan quality of MCO-IMRT.
Plan quality, delivery efficiency evaluation, and statistical analysis
Plan quality with and without collimator rotation for both MCO-IMRT and VMAT were compared and analyzed based upon DVH curves in terms of conformity number (CN), homogeneity index (HI), and coverage (D80) for PTV, and D0.5 for spinal PRV, where DN is the dose to the hottest N% of the volume. CN was defined as (TVPI)2/(TV * VPI), as proposed by van't Riet et al22, which accounts for overlap of the prescription isodose line with the PTV as well as the dose to the normal tissue. VPI is the total volume of the prescription isodose, TV is the PTV volume and TVPI is the total PTV volume covered by the prescription isodose. CN=1 implies ideal conformity. Target HI was defined as (D0.5-D80)/D80. Spine SRS plans are evaluated primarily based on the spinal PRV dose, but in order to facilitate IMRT vs VMAT plan quality comparisons, the PTV CN, HI, and D80 were also determined. Delivery efficiency was evaluated by comparing MU and delivery time of MCO-IMRT and VMAT. MUs were compared among MCO-IMRT (colli: 0), MCO-IMRT (colli: Rot), VMAT (colli: 0) and VMAT (colli: 90). Timing measurements were performed for the MCO-IMRT (colli: Rot) plans, delivered with auto field sequencing. VMAT delivery times were calculated by the TPS, based on MU, gantry speed, dose rate and other relevant delivery parameters. Our clinical experience has confirmed that these calculated delivery times are accurate. Furthermore, paired-sample t-tests were used to perform statistical analysis among the four groups, in terms of PTV CN, HI, and D80, spinal PRV sparing, MU, and delivery time.
RESULTS
Dose distribution and DVH comparison
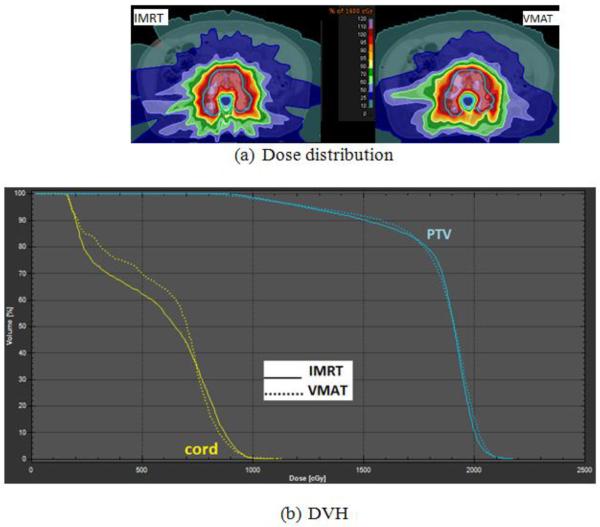
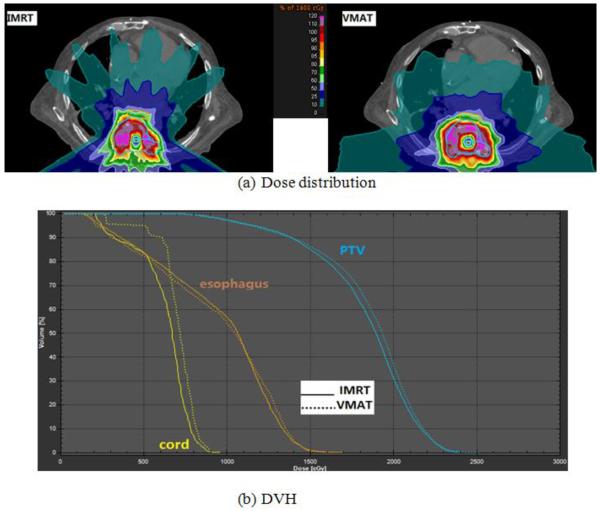
For all MCO-IMRT plans with or without collimator rotation, plan quality was similar, in terms of PTV coverage, homogeneity and OAR sparing. Plan quality of VMAT also matches that of MCO-IMRT. Figure 2a shows the dose distribution comparison between MCO-IMRT (colli: Rot) and VMAT (colli: 90) plans of an L3-4 spine SRS patient. No visible differences in the high dose region are observed, and the 100% isodose line (18 Gy) covered similar region of the PTV for both treatment methods. Figure 2b shows the DVH comparison between MCO-IMRT and VMAT. A dose distribution and DVH comparison between MCO-IMRT and VMAT of a T8 spine case is displayed in Figure 3. In this study, PTV coverage ranged between 60–90% at 18 Gy for all plans, depending upon the geometric complexity of the target/PRVs and the maximum dose limit of the spinal cord. A summary of plan quality metrics is included in Table 1. A statistically significant difference was only observed for PTV CN between MCO-IMRT and VMAT for plans without a collimator rotation.
Figure 2.
(a) Dose distribution comparison between MCO-IMRT and VMAT and (b) DVH comparison for an L3-4 spine SRS patient (blue: PTV; yellow: spinal cord PRV).
Figure 3.
(a) Dose distribution comparison between MCO guided MCO-IMRT and VMAT and (b) DVH comparison for a T8 spine SRS patient (blue: PTV; yellow: spinal cord PRV; orange: esophagus PRV).
Table 1.
Summary of dosimetric analysis for PTV and cord PRV among MCO-IMRT and VMAT plans with or without collimator rotation.
| Colli: 0 |
Colli: Rot |
||||
|---|---|---|---|---|---|
| MCO-IMRT | VMAT | MCO-IMRT | VMAT | ||
| PTV | D80 (Gy) | 17.28 ± 1.64 | 17.64 ± 1.43 | 17.31 ± 1.70 | 17.56 ± 1.43 |
| CN | 0.52 ± 0.05* | 0.54 ± 0.08* | 0.52 ± 0.09 | 0.55 ± 0.06 | |
| HI | 0.30 ± 0.15 | 0.28 ± 0.08 | 0.32 ± 0.14 | 0.29 ± 0.09 | |
| Cord PRV | D0.5 (Gy) | 11.22 ± 1.54 | 11.59 ± 1.71 | 11.31 ± 1.20 | 11.23 ± 1.38 |
Statistically significant differences (p < 0.05) were only observed for CN between MCO-IMRT and VMAT planning without collimator rotation.
Delivery efficiency
Though plan quality for all four groups, MCO-IMRT and VMAT with or without collimator rotation, are comparable, the number of MUs was influenced by the collimator setting. On average, for either MCO-IMRT or VMAT, MUs decreased by about 25% when using a collimator rotation. For individual cases, the MU reduction was as large as 37%. No statistically significant differences were observed for MU when comparing MCO-IMRT and VMAT for the same collimator setting. Introducing a collimator rotation for either MCO-IMRT or VMAT leads to a statistically significant reduction in MU. With collimator rotation, the 18 Gy prescription dose can be delivered in about 4500 MU. MU data are summarized in Table 2. The MUs ranged from 4808–7193 for MCO-IMRT (colli: 0), 3509–5907 for MCO-IMRT (colli: Rot), 4444–7309 for VMAT (colli: 0) and 3277–5643 for VMAT (colli: 90).
Table 2.
MU and delivery time (average ± standard deviation) comparison for MCO-IMRT and VMAT with or without collimator rotation.
| Colli: 0 |
Colli: Rot |
|||
|---|---|---|---|---|
| MCO-IMRT | VMAT | MCO-IMRT | VMAT | |
| MU | 6216 ± 756a | 5861 ± 896b | 4681 ± 726a | 4360 ± 722b |
| Delivery time (min) | - | - | 18.3 ± 2.5c | 14.2 ± 2.0c |
Statistically significant differences (p < 0.05) for MU were observed between (a) MCO-IMRT (Colli:0) vs. MCO-IMRT (Colli: Rot) and (b)VMAT (Colli:0) vs. VMAT (Coll: 90). No statistically significant differences for MU were observed between IMRT and VMAT for the same collimator setting.
A statistically significant difference (p < 0.05) was also observed between delivery time for IMRT and VMAT.
In our clinical practice, MCO-IMRT plans with collimator rotation are used for treatment. Therefore, the delivery time of MCO-IMRT (colli: Rot) and VMAT (colli: 90) were compared. Mean delivery times for both MCO-IMRT and VMAT are shown in Table 2. For MCO-IMRT (colli: Rot), the mean delivery time is 18.3 minutes; while the mean delivery time for VMAT (colli: 90) is 14.2 minutes, or a reduction of 25% (p < 0.05).
In summary, the two techniques of IMRT and VMAT for radiosurgery of spinal metastases present comparable dosimetry, and VMAT with collimator rotation has significant less delivery time than that of IMRT for spine SRS.
DISCUSSION
This study presents the first comparison of VMAT and IMRT using Multi-Criteria Optimization for spine metastases SRS. VMAT is a rotational IMRT modality that has the potential advantage of reduced delivery time by means of one or more arcs with continuously varying beam apertures, gantry speed, and dose rate. The aim of this work was to compare the plan quality and delivery efficiency of MCO guided IMRT and VMAT plans. Planning for spine metastases SRS is challenging due to the concave-shaped PTV and adjacent cord PRV. A highly conformal dose distribution with strict dose constraints for the spinal cord is required. The MCO-IMRT approach provides an optimal dose distribution but the delivery time (not including patient setup and imaging) for IMRT-based spine SRS is around 18 minutes in our clinic. Shorter delivery times may improve patient comfort, particularly for patients with substantial pain from tumor involvement or recent spine surgery. Reduced delivery times may reduce the need for multiple CBCT verification, and may minimize patient motion during treatment, although the amount of intra-fraction motion is currently small. Our comparisons show little difference between dosimetric metrics for MCO-IMRT and VMAT delivery, as summarized in Table 1. No differences were observed for the cord PRV (D0.5), or PTV HI and D80. Statistically significant differences, although perhaps not clinically significant, were observed for PTV CN, for collimator 0 only. In our clinic, the most relevant dosimetric parameter is maximum dose (or D0.5) to the cord PRV, followed by PTV D80. A study by Wu et al23, reporting the feasibility of VMAT for 10 spine SRS cases on the Eclipse treatment planning system demonstrated that VMAT yielded comparable plan quality to IMRT with a 50% reduction in treatment time and a 26% reduction in MU. This study utilized a prescription dose of 16 Gy and a maximum dose rate of 1000 MU/minute, with a mean delivery time of 8 minutes and mean MU of 6317. Matuszak et al11 compared a single spine SRS patient, planned in Pinnacle, and showed that VMAT improved dose conformity and reduced treatment time by 37%, but had similar MU as IMRT. The results of a comparison between VMAT and IMRT may depend on the optimization approach of different treatment planning systems. Our DVH analysis shows comparable plan quality for VMAT and MCO-IMRT. This may be because MCO guided IMRT has determined the optimal trade-off between target coverage and OAR sparing.
Another parameter examined in this study is the collimator setting for spine SRS for both IMRT and VMAT methods. In spine metastases, the PTV often partially encircles the spinal cord and a steep dose gradient along the PTV–cord boundary is required. A collimator orientation parallel to the spinal cord may allow for more efficient beam modulation. In our study, a collimator rotation for either IMRT or VMAT can significantly reduce the required MU. With the collimator rotated to an appropriate angle (usually around 90), only a few leaf pairs are needed to block the cord and the remaining leaves are available to shape the dose distribution. In other words, optimal choice of collimator angle could simultaneously block the spine and allow delivery to both sides of the PTV, depending on the beam's eye view. The collimator angle can be set independently for each IMRT gantry angle, but current VMAT planning methods require a fixed collimator angle for VMAT, set to 90 degrees in our study. The MU reduction was approximately 25% with a collimator rotation for both IMRT and VMAT. Also, MU differences for IMRT and VMAT, for the same collimator setting, were not statistically significant in our study. The mean delivery time, however, is reduced by approximately 25% with VMAT, which may have clinical significance, particularly for spine SRS patients. The maximum dose rate used in this study is only 450 MU/minute, and treatment times for both IMRT and VMAT can be further reduced with flattening filter free high dose rate treatments.
The VMAT optimization and delivery parameter settings included dual partial arcs, gantry spacing of 2 degrees, collimator of 90 degrees, and a specified maximum delivery time of 20 minutes. These are recommended for spine SRS VMAT planning on current RayStation treatment planning system (V2.5), which allows plan quality to match that of MCO-IMRT. Further parameter sensitivity analysis of VMAT planning for spine SRS will be the subject of future work.
CONCLUSION
MCO-IMRT and VMAT can create clinically acceptable plans for spinal radiosurgery. The MU for IMRT and VMAT can be reduced significantly by utilizing a collimator rotation. For the same collimator setting, the required MU for VMAT and IMRT are similar. Plan quality for VMAT is similar to MCO-IMRT, and VMAT can reduce delivery time by about 25%. VMAT with collimator rotation is the most efficient way to perform spine SRS.
ACKNOWLEDGEMENTS
This work was supported by NCI Federal Share Proton Beam Program Income Grant.
Conflicts of Interest Notification Dr. Chen reports grants from NCI Federal Share Proton Beam Program Income Grant, during the conduct of the study. Dr. Winey reports grants from NCI Federal Share Proton Beam Program Income Grant for the Massachusetts General Hospital, during the conduct of the study. Dr. Oh reports grants from NCI Federal Share Proton Beam Program Income Grant for the Massachusetts General Hospital, during the conduct of the study. Dr. Gierga reports grants from NCI Federal Share Proton Beam Program Income Grant, during the conduct of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gerszten PC, Mendel E, Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: What are the options, indications, and outcomes? Spine (Phila Pa 1976) 2009;34(22 Suppl):S78–92. doi: 10.1097/BRS.0b013e3181b8b6f5. [DOI] [PubMed] [Google Scholar]
- 2.Schipani S, Wen W, Jin JY, et al. Spine radiosurgery: A dosimetric analysis in 124 patients who received 18 Gy. Int J Radiat Oncol Biol Phys. 2012;84(5):e571–6. doi: 10.1016/j.ijrobp.2012.06.049. [DOI] [PubMed] [Google Scholar]
- 3.Wen N, Walls N, Kim J, et al. Clinical use of dual image-guided localization system for spine radiosurgery. Technol Cancer Res Treat. 2012;11(2):123–131. doi: 10.7785/tcrt.2012.500241. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed KA, Stauder MC, Miller RC, et al. Stereotactic body radiation therapy in spinal metastases. Int J Radiat Oncol Biol Phys. 2012;82(5):e803–9. doi: 10.1016/j.ijrobp.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 5.Gerszten PC, Monaco EA, 3rd, Quader M, et al. Setup accuracy of spine radiosurgery using cone beam computed tomography image guidance in patients with spinal implants. J Neurosurg Spine. 2010;12(4):413–420. doi: 10.3171/2009.10.SPINE09249. [DOI] [PubMed] [Google Scholar]
- 6.Palma D, Vollans E, James K, et al. Volumetric modulated arc therapy for delivery of prostate radiotherapy: Comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):996–1001. doi: 10.1016/j.ijrobp.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Moret J, Pohl F, Koelbl O, et al. Evaluation of volumetric modulated arc therapy (VMAT) with oncentra MasterPlan(R) for the treatment of head and neck cancer. Radiat Oncol. 2010;5 doi: 10.1186/1748-717X-5-110. 110-717X-5-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quan EM, Li X, Li Y, et al. A comprehensive comparison of IMRT and VMAT plan quality for prostate cancer treatment. Int J Radiat Oncol Biol Phys. 2012;83(4):1169–1178. doi: 10.1016/j.ijrobp.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff D, Stieler F, Welzel G, et al. Volumetric modulated arc therapy (VMAT) vs. serial tomotherapy, step-and-shoot IMRT and 3D-conformal RT for treatment of prostate cancer. Radiother Oncol. 2009;93(2):226–233. doi: 10.1016/j.radonc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Diot Q, Kavanagh B, Timmerman R, et al. Biological-based optimization and volumetric modulated arc therapy delivery for stereotactic body radiation therapy. Med Phys. 2012;39(1):237–245. doi: 10.1118/1.3668059. [DOI] [PubMed] [Google Scholar]
- 11.Matuszak MM, Yan D, Grills I, et al. Clinical applications of volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys. 2010;77(2):608–616. doi: 10.1016/j.ijrobp.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 12.Wala J, Salari E, Chen W, et al. Optimal partial-arcs in VMAT treatment planning. Phys Med Biol. 2012;57(18):5861–5874. doi: 10.1088/0031-9155/57/18/5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craft DL, Hong TS, Shih HA, et al. Improved planning time and plan quality through multicriteria optimization for intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(1):e83–90. doi: 10.1016/j.ijrobp.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craft D, Halabi T, Shih HA, et al. An approach for practical multiobjective IMRT treatment planning. Int J Radiat Oncol Biol Phys. 2007;69(5):1600–1607. doi: 10.1016/j.ijrobp.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Craft DL, Halabi TF, Shih HA, et al. Approximating convex pareto surfaces in multiobjective radiotherapy planning. Med Phys. 2006;33(9):3399–3407. doi: 10.1118/1.2335486. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Craft DL, Gierga DP. Multicriteria optimization informed VMAT planning. Med Dosim. 2014;39:64–73. doi: 10.1016/j.meddos.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutz W, Winston KR, Maleki N. A system for stereotactic radiosurgery with a linear accelerator. Int J Radiat Oncol Biol Phys. 1988;14(2):373–381. doi: 10.1016/0360-3016(88)90446-4. [DOI] [PubMed] [Google Scholar]
- 18.Zhang P, Happersett L, Yang Y, et al. Optimization of collimator trajectory in volumetric modulated arc therapy: Development and evaluation for paraspinal SBRT. Int J Radiat Oncol Biol Phys. 2010;77(2):591–599. doi: 10.1016/j.ijrobp.2009.08.056. [DOI] [PubMed] [Google Scholar]
- 19.Craft D, Richter C. Deliverable navigation for multicriteria step and shoot IMRT treatment planning. Phys Med Biol. 2012;58(1):87–103. doi: 10.1088/0031-9155/58/1/87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craft D, Bortfeld T. How many plans are needed in an IMRT multi-objective plan database? Phys Med Biol. 2008;53(11):2785–2796. doi: 10.1088/0031-9155/53/11/002. [DOI] [PubMed] [Google Scholar]
- 21.Bzdusek K, Friberger H, Eriksson K, et al. Development and evaluation of an efficient approach to volumetric arc therapy planning. Med Phys. 2009;36(6):2328–2339. doi: 10.1118/1.3132234. [DOI] [PubMed] [Google Scholar]
- 22.van't Riet A, Mak AC, Moerland MA, et al. A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: Application to the prostate. Int J Radiat Oncol Biol Phys. 1997;37(3):731–736. doi: 10.1016/s0360-3016(96)00601-3. [DOI] [PubMed] [Google Scholar]
- 23.Wu QJ, Yoo S, Kirkpatrick JP, et al. Volumetric arc intensity-modulated therapy for spine body radiotherapy: Comparison with static intensity-modulated treatment. Int J Radiat Oncol Biol Phys. 2009;75(5):1596–1604. doi: 10.1016/j.ijrobp.2009.05.005. [DOI] [PubMed] [Google Scholar]