Abstract
Objectives
A better dosing strategy can improve clinical outcomes for patients. We sought to compare the extended or continuous infusion with conventional intermittent infusion of piperacillin/tazobactam, investigating which approach is better and worthy of recommendation for clinical use.
Methods
Articles were gathered from PubMed, Web of Science, ProQuest, Science Direct, Cochrane, two Chinese literature databases (CNKI, Wan Fang Data) and related ICAAC and ACCP conferences. Randomized controlled and observational studies that compared extended or continuous infusion with conventional intermittent infusion of piperacillin/tazobactam were identified from the databases above and analyzed. Two reviewers independently extracted and investigated the data. A meta-analysis was performed using Revman 5.2 software. The quality of each study was assessed. Sensitivity analysis and publication bias were evaluated.
Results
Five randomized controlled trials and nine observational studies were included in this study. All included studies had high quality and no publication bias was found. Compared to the conventional intermittent infusion approach, the extended or continuous infusion group had a significantly higher clinical cure rate (OR 1.88, 95% CI 1.29-2.73, P = 0.0009) and a lower mortality rate (OR 0.67, 95% CI 0.50-0.89, P = 0.005). No statistical difference was observed for bacteriologic cure (OR 1.40, 95% CI 0.82-2.37, P = 0.22) between the two dosing regimens. The sensitivity analysis showed the results were stable.
Conclusions
Our systematic review and meta-analysis suggested that the extended or continuous infusion strategy of piperacillin/tazobactam should be recommended for clinical use considering its higher clinical cure rate and lower mortality rate in comparison with conventional intermittent strategy. Data from this study could be extrapolated for other β-lactam antimicrobials. Therefore, this dosing strategy could be considered in clinical practice.
Introduction
Piperacillin/tazobactam is an extended-spectrum β-lactamase inhibitor combination antibiotic. Because of its broad coverage, piperacillin/tazobactam is commonly recommended as a first-line therapy for severe bacterial infections including intra-abdominal infection, hospital-acquired pneumonia, febrile neutropenia, and skin or soft-tissue infection [1], [2]. As a time-dependent antibiotic, the bactericidal activity of piperacillin/tazobactam is optimized when drug concentrations exceed the fractional time above the minimum inhibitory concentration (fT>MIC) for at least 30% to 50% [3–5].
As more is understood about antimicrobial agents through research, it is evidenced that proper use of antimicrobials can improve clinical outcomes and reduce resistance, while maintaining antimicrobial sensitivity in general population [6–10]. Conventional dosing of piperacillin/tazobactam is an intermittent 30-minute infusion, potentially resulting in serum concentrations below minimum inhibitory concentration (MIC) for a prolonged period of time [11]. Numerous studies have investigated alternative dosing strategies that increase the drug’s fT>MIC, of which the extended or continuous infusion strategy (>3 hours) was an option [12–14], but no consistent conclusions were obtained. Therefore, a definitive recommendation is necessary because of its significant meaning for clinical practice. Though a meta-analysis comparing the two dosing strategies of piperacillin/tazobactam was done, the study had some limitations. Only two databases, PubMed and Scopus, were searched, articles published after January 2012 were not analyzed, and only one randomized controlled trial (RCT) was included in the meta-analysis [15]. Many new studies with better study design have been published since January 2012. Therefore, it is important and necessary to systematically investigate the clinical outcome differences between the two dosing strategies of piperacillin/tazobactam from those clinical trials in order to produce an evidence-based recommendation for clinical practice.
Methods
Literature search
Published articles were systematically searched (until April 30, 2014) from PubMed, Web of Science, ProQuest, Science Direct, Cochrane, two Chinese literature databases: [China National Knowledge Infrastructure (CNKI), Wan Fang Data] and related Interscience Conference on Antimicrobial Agents & Chemotherapy (ICAAC) and American College of Clinical Pharmacology (ACCP) conferences databases. References of the retrieved articles were also searched for additional studies. The following research pattern was utilized: (piperacillin/tazobactam) AND (extended OR continuous OR prolonged OR intermittent OR discontinuous OR short OR traditional OR conventional OR intermittent) AND (duration OR infusion OR administration OR interval OR dosing). No language restriction was applied to the search.
Study selection
Articles reporting the comparative outcomes of patients treated with the two different dosing strategies of piperacillin/tazobactam were eligible for the meta-analysis, and the types of studies included were prospective study, retrospective study and RCT.
Data extraction
Two reviewers (H.Y and Q.Y. Z) independently extracted relevant information for the meta-analysis. The extracted data included the characteristics of each study (author, study design, years, country), patient population (numbers of patients, type and etiology of infection), drug regimens, and clinical outcomes (clinical cure, mortality, bacteriologic cure, days in hospital, adverse events, cost) of the two groups in each study. Adverse events were directly described instead of statistical analysis considering few sample sizes.
Quality assessment
Two authors (H.Y and Y.K.W) independently assessed the included studies for quality without blinding to journal or study authorship. Discrepancies were resolved by involvement of a third review author (C.Z) if required.
The quality of included RCT studies was assessed according to the criteria developed by the Cochrane risk of bias tool: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. The quality of observational studies was assessed using the Newcastle-Ottawa Scales (NOS) [16].
Statistical analysis
The meta-analysis was performed using Review Manager for Windows (version 5.2). Odds ratio (OR) and 95% confidence interval (CI) were calculated for each outcome. Statistical heterogeneity among studies was assessed by χ2 test (P <0.10 was defined to indicate significant heterogeneity) and I2 test. Mantel-Haenszel fixed effects model (FEM) was used when there was no significant heterogeneity between studies; otherwise, a random effects model was chosen. Adverse events were directly described instead of statistical analysis considering few sample sizes included. In order to evaluate the stability of results without estimation bias from individual study, especially considering relative small size studies being included [17–19], sensitivity analysis was performed by exclusion of each study one by one. This process of excluding one study at a time allowed for identification of any single article that may have a large influence on the final results. Publication bias was evaluated using the funnel plot method, of which funnel plot asymmetry was assessed by Egger’s linear regression test [20].
Results
Literature search
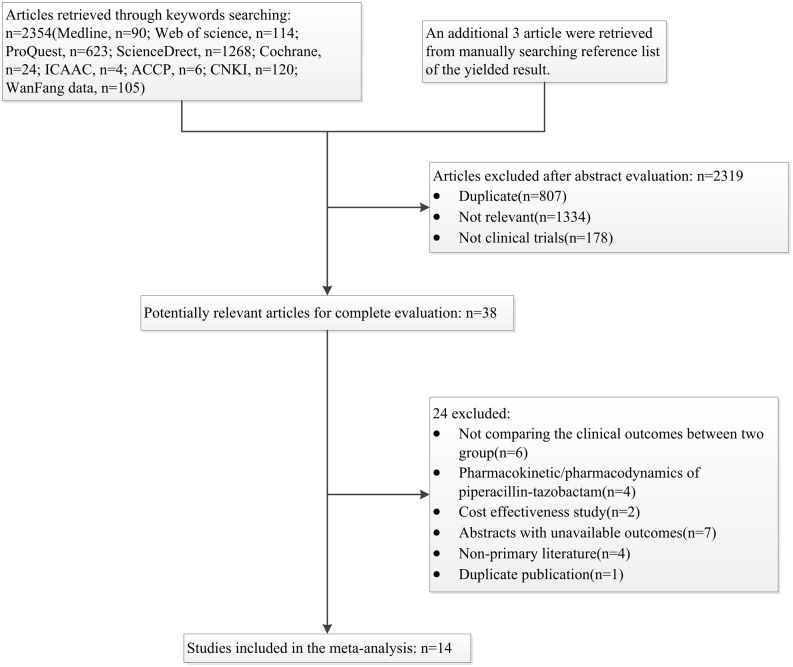
The search strategy yielded 2354 titles and abstracts. In addition, 3 articles were retrieved manually by searching from references lists. A total of 2319 articles were excluded after the review of abstracts, and 38 articles remained for full-text analysis. 24 articles were excluded after full-text review, leaving a total of 14 articles to be included in the analysis. The whole literature search process is summarized in Fig. 1.
Figure 1. Flow chart depicting the selection process of studies included in the meta-analysis.
Study description
Characteristics of the eligible studies are presented in Table 1. This meta-analysis included fourteen studies, among which were two prospective studies [18], [21], seven retrospective studies [17], [24–26], [29–31] and five RCTs [19], [22], [27], [28], [32]. The patients of five of the included studies were persons who were admitted to the Intensive Care Unit (ICU) with severe infection, and the other nine studies included only non-ICU patients with moderate or severe infection. The infections of patients included pneumonia, sepsis, complicated intra-abdominal infection and ICU infections. The severity of underlying illness of the patients was evaluated using APACHE II, SAPS II or SOFA score and there was no statistically significant difference for their average values between two dosing groups in each study. In total, 1786 patients were included in the analysis and the sample sizes ranged from 16 to 359 in the identified studies. In the included studies, conventional intermittent infusion regimens were 2.25–4.5g over 20 or 30min three or four times daily. The extended infusion regimens lasted greater than 3 hours and the continuous infusion regimens lasted 24 hours with the doses ranging from 6.75 to 13.5g daily.
Table 1. The characteristics of included studies.
| Author, year, reference | Study design; years, country | No. of patients, infections | bacteria | CI or EI | II | Clinical cure | Mortality | Bacteriologic cure | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CI, n/N (%) | II, n/N (%) | P value | CI, n/N (%) | II, n/N (%) | P value | CI, n/N (%) | II, n/N (%) | P valve | ||||||
| Grant,2002, [21] | Prospective, open-lable;1999–2000, USA | 98,NR | NR | 9g q24h for HAP(n = 24), 13.5g q24h for nosocomial infections(n = 23) | 3.375 q6h(n = 2),4.5g q8h(n = 49) | 44/47(94) | 42/51(82) | 0.081 | 1/47(2.1) | 5/51(9.8) | >0.5 | 25/28(89) | 23/32(73) | 0.092 |
| Buck,2005, [18] | Prospective, open-lable;NR, Germany | 24,CAP or HAP | NR | 9g q12h(n = 12) a | 4.5g q8h(n = 12) | 8/12(67) | 8/12(67) | >0.05 | NR | NR | NR | NR | NR | NR |
| Lau,2006, [22–23] | RCT, open-lable;2002–2004,USA | 167,cIAIs | Gram(-)/(+) bacteria | 13.5g q24h(n = 130) b | 3.375g over 30min q6h(n = 132) | 70/81(86) | 76/86(88) | 0.817 | 1/130(0.8) | 3/132(2.3) | >0.05 | 47/56(83.9) | 51/58(87.9) | 0.597 |
| Rafati,2006, [19] | RCT;2003–2004,Iran | 40,ICU septic | NR | 8g daily over 24h g(n = 20) c | 3g over 0.5h q6h(n = 20) | NR | NR | NR | 5/20(25) | 6/20(30) | 0.72 | NR | NR | NR |
| Lodise,2007, [24] | Retrospective cohort;2000–2004,USA | 194,P. aeruginosa Infection | P. aeruginosa | 3.375g over 4h,q8h(n = 102) d | 3.375 over 30min, q4h or q6h(n = 92) | NR | NR | NR | 5/41(12.2) | 12/38(31.6) | 0.04 | NR | NR | NR |
| Patel,2009, [25] | Retrospective cohort;2006–2007,USA | 129,Gram(-) infection | Gram(-) bacteria | 3.375g over 4h,q8h(n = 70) | 3.375 to 4.5g over 30min q6h or q8h(n = 59) | NR | NR | NR | 4/70(5.7) | 5/59(8.5) | 0.54 | NR | NR | NR |
| Lorente,2009, [26] | Retrospective cohort;2002–2007,Spain | 83,VAP | Gram(-) bacteria | 4.5g over 6h q6h(n = 37) e | 4.5g over 30min q6h(n = 46) | 33/37(89.2) | 26/46(56.2) | 0.001 | 8/37(21.6) | 14/46(30.4) | 0.46 | NR | NR | NR |
| Li,2010, [27] | RCT;2006–2008,China | 66,severe pneumonia | NR | 4.5g 2ml/h bid(n = 28) | 4.5g over 30min q8h(n = 31) | 24/32(75.0) | 17/34(50.0) | 0.001 | NR | NR | NR | 11/32(34.4) | 11/34(32.4) | <0.05 |
| Robort,2010, [17] | Retrospective;2005,Australia | 16,ICU | NR | 13.5 continuous(n = 8) | 4.5g over 20min q6h or q8h(n = 8) | 8/8(100) | 8/8(100) | NR | 0/8(0) | 0/8(0) | NR | NR | NR | NR |
| Ye, 2011, [28] | RCT;2009–2010,China | 66,ICU | Gram(-) bacteria | 4.5g over 3h q8h(n = 35) | 4.5g over 30min q8h(n = 31) | 24/35(68.6) | 13/31(41.9) | <0.05 | 8/35(22.9) | 8/31(25.8) | >0.05 | 15/35(42.8) | 10/31(32.2) | >0.05 |
| Yost,2011, [29] | Retrospective Cohort;2007–2010,USA | 359,Pseudomonas aeruginosa infections | Pseudomonas aeruginosa | 3.375g over 4h q8h(n = 186) | NR(n = 84) | NR | NR | NR | 18/186(9.7) | 17/84(20.2) | 0.03 | NR | NR | NR |
| Pereira,2012, [30] | Retrospective cohort;2006–2010,Portugal | 346,ICU | NR | NR(n = 173) | t = 30min,dose NR(n = 173) | NR | NR | NR | 49/173(28.3) | 49/173(28.3) | 1.0 | NR | NR | NR |
| Lee,2012, [31] | Retrospective;2009–2011,USA | 148,ICU | Gram(-) bacteria | 3.375g over 4h q8h(n = 68) | 2.25–4.5g over 30min q6h or q8h(n = 80) | NR | NR | NR | 13/68(19) | 30/80(38) | 0.01 | NR | NR | NR |
| Lv,2013, [32] | RCT;2012,China | 50,HAP | Pseudomonas aeruginosa, e. coli, klebsiella pneumoniae | 4.5g over 3h q6h(n = 25) | 4.5g over 30min q6h(n = 25) | 22/25(88) | 20/25(80) | NR | NR | >0.05 | NR | NR | NR | |
CI, Continuous infusion; EI, Extended infusion; II, Intermittent infusion; CAP, Community acquired pneumonia; HAP, Hospital acquired pneumonia; IAIs, Complicated intra-abdominal infection; ICU, Intensive care unit; VAP, Ventilator-associated pneumonia.
a 2.5g single loading dose before starting continuous infusion.
b A loading dose was administered before continuous infusion: 2.25g over 30min.
c Loading dose was administered before continuous infusion: 2g.
d Among patients with Acute Physiological and Chronic Health Evaluation-II score≥17.
e A loading dose was administered before continuous infusion: 4.5g over 30min. A loading dose was administered before continuous infusion.
Quality of included studies
Seven factors were used to evaluate the bias of the five included RCT studies according to the Cochrane risk of bias tool. Most factors for all studies showed low bias. However, the methods used to generate the allocation sequence in four studies were unclear. On the whole, the included RCTs in our study were of relatively high quality.
There were nine observational studies, including two prospective studies and seven retrospective studies. Eight factors were used to assess study quality according to NOS. The more factors the study met, the higher the quality of the study was. Except one study (Buck 2005) missed one indicator, the other seven studies were adequate in all criteria. The results showed that all observational studies were high quality.
Clinical cure
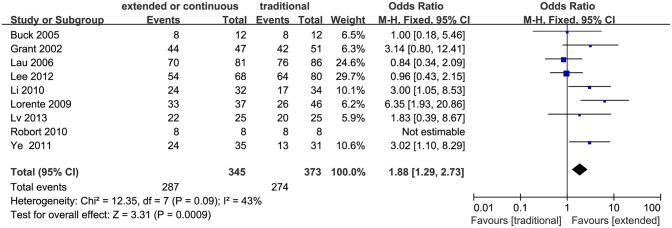
Clinical cure was defined as “cure” (the complete resolution of clinical signs and symptoms of infection, with no new signs or symptoms associated with the original infection) or “improvement” (the patient was not cured, but there was a resolution or a reduction of the majority of the clinical signs and symptoms of infection and no new or worsened signs associated with the original infection) in these studies. Nine studies including four RCTs, reported clinical cure rate [17], [18], [21], [22], [26–28], [31], [32]. Compared to the conventional intermittent infusion, the extended or continuous infusion had a significantly higher clinical cure rate (718 patients, OR 1.88, 95% CI 1.29–2.73, P = 0.0009; Fig. 2). No significant heterogeneity was found among all the studies (I2 = 43%, P = 0.09). The funnel plot did not show obvious asymmetry, and there was no publication bias presented by Egger’s test (P = 0.849).
Figure 2. Forest plot depicting the odds ratios of clinical cure of patients receiving extended or continuous versus conventional intermittent infusion of piperacillin/tazobactam.
Mortality
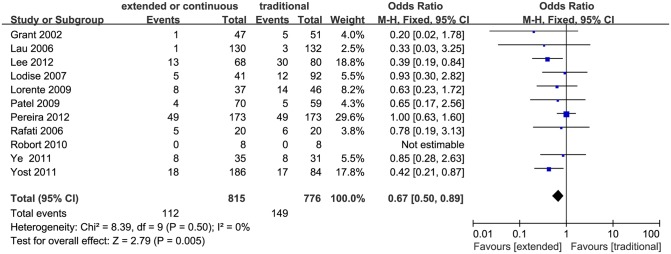
Eleven studies, including three RCTs, compared mortality rate between the extended or continuous infusion and the conventional intermittent infusion strategy [17], [19], [21], [22], [24–26], [28–31]. The extended or continuous infusion strategy was found having lower mortality rate compared to the conventional intermittent infusion (1591 patients, OR 0.67, 95% CI 0.50–0.89, P = 0.005; Fig. 3). No significant heterogeneity was found among the studies (I2 = 0%, P = 0.50). Obvious asymmetry was not found in the funnel plot. Egger’s test showed no publication bias, but the p value was 0.058, which indicated no statistically significant difference.
Figure 3. Forest plot depicting the odds ratios of mortality of patients receiving extended or continuous versus conventional intermittent infusion of piperacillin/tazobactam.
Bacteriologic success
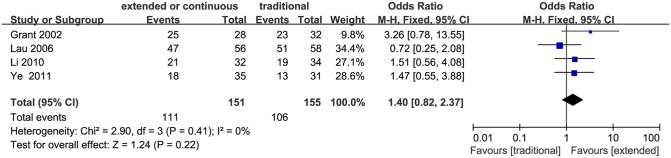
Bacteriological success was defined as success (“eradication” or “presumed eradication”) versus failure (“persistence” or “presumed persistence”). A total of four studies evaluated bacteriologic success, of which Li et al only reported overall success rates [27], and Grant et al [21], Lau et al [22], Ye et al [28] reported both overall and classified bacteriologic success rate for each type of bacteria. The average bacteriological success rate was 73.5% for the extended or continuous infusion group and 68.4% for the conventional intermittent infusion group, respectively. Bacteriological success rate showed no significant difference between the two infusion strategies (306 patients, OR 1.40, 95% CI 0.82–2.37, P = 0.22; Fig. 4).
Figure 4. Forest plot depicting the odds ratios of bacteriologic success of patients receiving extended or continuous versus conventional intermittent infusion of piperacillin/tazobactam.
Adverse events
Adverse event data were few since piperacillin/tazobactam is rather well tolerated, and therefore, statistical analysis was not applied due to limited available data. Four studies described adverse events [17], [21], [22], [27] but two of them did not find any [17], [21]. In the RCT study by Lau et al, 22 of 130 patients (16.9%) in the continuous infusion group experienced adverse events versus 18 of 132 (13.6%) in the conventional intermittent group [22]. Six serious adverse events were found in the continuous group, including Clostridium difficile colitis, renal failure, confusion, tachycardia, and a tonic/clonic seizure, but none led to death. The incidence of adverse events was not significantly different between the two dosing groups. Another RCT study by Li et al reported 2 of 32 patients in the extended or continuous infusion group and 2 of 34 patients in the conventional intermittent group experienced adverse effects [27]. No serious adverse event was found in either group.
Sensitivity analysis
The results of sensitivity analysis showed no substantial modification of the estimates after exclusion of individual study one by one.
Discussion
This meta-analysis was performed to compare extended or continuous infusion with the conventional intermittent infusion approach of piperacillin/tazobactam. Our meta-analysis, including fourteen studies (two prospective studies, seven retrospective studies, and five RCTs), showed that the extended or continuous infusion strategy was associated with a higher clinical cure rate and lower mortality than the conventional intermittent approach. The bacteriological success rates and adverse events were not found to be significantly different between the two dosing approaches. These results ignored differences in severity of infection among different studies, but the average level of severity of infection between two dosing groups for each study was not significantly different.
Higher clinical cure rate for the extended infusion approach was found in our study, which is an important result, indicating the merit of using the extended or continuous infusion instead of the conventional intermittent infusion approach in clinical practice. The reason for which the extended or continuous infusion leads to increased clinical cure rate may be related to the increase in the time that the drug concentrations exceed the MIC since piperacillin/tazobactam is a time-dependent antimicrobial [1], [12–14], [17], [19], [24], [30], [33], [34]. However, Falagas et al concluded no difference in clinical cure rate between extended and conventional intermittent infusion strategy after their meta-analysis [15]. Possible reason may be related to limited numbers and few high quality studies included in their analysis (i.e. only one RCT incorporated). High quality researches with good study design are important and can avoid much of the bias of estimation [35]. As is well known, RCT is a study design of high quality, the best way to evaluate the clinical outcome, and can reduce bias to the maximum extent [36]. Five RCT studies were included in our analysis, avoiding possible bias to a large extent. Moreover, heterogeneity of the study is another main factor that may influence the final conclusion. FEM was used in our analysis since there was no significant statistical heterogeneity present among included studies (Fig. 2). If significant heterogeneity did exist, a random effect model would have been used instead, similar to the work of Falagas et al. Compared to the random-effects model, the fixed-effects model is more sensitive and accurate [37], [38]. Sometimes, an opposite result could even be obtained using the random effect model compared to the fixed effect model [39]. Sensitivity analysis is used to measure the stability of results, especially when heterogeneity exists. It is a good way to find the source of heterogeneity and eliminate it. There was one small size study included in the analysis [18], but it did not modify the conclusion of the study when excluded during the sensitivity analyses.
Our meta-analysis suggested that extended or continuous infusion of piperacillin/tazobactam resulted in significantly lower mortality rate compared to the conventional intermittent infusion. No publication bias was discovered and the sensitivity analysis showed no substantial modification in this meta-analysis, but the Egger’s test result did not reveal obvious statistical significance.
The bacteriologic success rate was not found to be statistically different in this meta-analysis. The β-lactam antibiotics exhibit their bactericidal effects by inhibiting enzymes involved in cell wall synthesis [40]. However, bacteriologic success rate is not as good as other endpoints since it is easily influenced by many factors, of which some are not measured routinely, for example, the specimen storage conditions. In most instances, the end point is imputed (i.e. clinical success without resampling from the infection site equals microbiological success). There were four included studies that reported bacteriologic cure, of which two were from the United States [21], [22] and two were from China [27], [28]. The bacteriologic success rate was about 80% in the United States, which was relatively high, compared to about 50% in China. Low bacteriologic success rate may be attributed to high resistance rates due to overuse in some areas of China [41], [42]. No matter what reasons led to a lower bacteriologic success rate, a better dosing strategy is linked to increased bacteriologic success rate and more rational use of antimicrobials.
Drug-related adverse effects were mild and reported in similar numbers in both dosing strategy in all studies. This was powerful evidence against the traditional concept that an extended or continuous infusion could further induce toxicity reactions due to the high drug concentration lingering for a long-time within tissues [43]. However, more well-designed trials are needed to clarify this issue.
Eight different studies, including one RCT, provided inconsistent results towards the question that whether the extended or continuous infusion approach of piperacillin/tazobactam could reduce the length of hospital stay [21], [24–26], [29–32]. Grant et al reported that days of therapy were similar with both treatment groups (7.3 ± 4.8 days for continuous infusion versus 8.7 ± 7.1 days for conventional intermittent infusion, P = 0.26) [21]. This finding was also found by Patel et al [25], Lorente et al [26], Yost et al [29] and Pereira et al [30]. However, Lodise et al found that the median duration of hospital stay was significantly shorter in patients who received extended infusion therapy compared to conventional intermittent therapy (21 days versus 38 days, P = 0.02) [24]. Lee et al [31] and Lv et al [32] also found a similar result when comparing the mean duration of therapy.
MIC ranges and pathogens are very important factors for consideration of their influence on patient outcomes. Unfortunately, the studies included in the meta-analysis did not report data based on the source of infection. However, the evaluation indicators used in this study were clinical signs and symptoms, which were consistent with the types of pathogens and MIC ranges between two dosing groups, reducing the influence to the maximum extent.
Budget restrictions have put most institutions under pressure to curtail pharmacy costs. Except studies by Grant et al [21] and Heinrich et al [44], no economic advantage was observed for the extended or continuous infusion strategy in other studies. A lower total daily dose may be required for the extended infusion in order to achieve a similar drug concentration [45],which is the link to a decreased cost. Nevertheless, based on the current available evidence, we cannot make the conclusion that the extended or continuous infusion of piperacillin/tazobactam has any economic advantage.
All studies that we analyzed were of high quality, including RCTs and observational studies. Therefore, our conclusions were relatively reliable, but there were still multiple confounding factors. Small sample trials might bring bias. Additionally, disease status and drug doses were not the same in all studies, which could influence the clinical outcomes. Also, information regarding concurrent medications was not given in the studies analyzed. Therefore, drug-drug interactions were unknown and could not be considered during our evaluation.
In conclusion, evidences demonstrated that the extended or continuous infusion of piperacillin/tazobactam led to a higher clinical cure rate and a lower mortality rate than the conventional intermittent strategy. Therefore, this alternative infusion strategy could be recommended in clinical practice. Further data on the impact on adverse effects and economic budget should be generated for a better understanding of the extended or continuous infusion strategy.
Supporting Information
(DOC)
(DOC)
Acknowledgments
We thank all the original authors of the included studies for their wonderful work.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by Peking University Third Hospital research funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mah GT, Mabasa VH, Chow I, Ensom MH (2012) Evaluating outcomes associated with alternative dosing strategies for piperacillin/tazobactam: a qualitative systematic review. Ann Pharmacother 46: 265–75. 10.1345/aph.1Q378 [DOI] [PubMed] [Google Scholar]
- 2. Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, et al. (2010) Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 5: 133–64. 10.1086/649554 [DOI] [PubMed] [Google Scholar]
- 3. Javad A, Vahid P, Kazem G, Alireza S, Gholamali G, et al. (2006) Piperacillin/tazobactam in treatment of brain abscess. Scand J Infect Dis 38: 224–6. 10.1080/00365540500348978 [DOI] [PubMed] [Google Scholar]
- 4. Lodise TP, Lomaestro BM, Drusano GL (2006) Application of antimicrobial pharmacodynamic concepts into clinical practice: focus on b-lactam antibiotics. Pharmacotherapy 26: 1320–32. 10.1592/phco.26.9.1320 [DOI] [PubMed] [Google Scholar]
- 5. Roberts JA, Paratz J, Paratz E, Krueger WA, Lipman J (2007) Continuous infusion of beta-lactam antibiotics in severe infections: a review of its role. Int J Antimicrob Agents 30: 11–8. 10.1016/j.ijantimicag.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 6. Fehér C, Rovira M, Soriano A, Esteve J, Martínez JA, et al. (2014) Effect of meropenem administration in extended infusion on the clinical outcome of febrile neutropenia: a retrospective observational study. J Antimicrob Chemother: 69:2556–62. 10.1093/jac/dku150 [DOI] [PubMed] [Google Scholar]
- 7. Thalhammer F, Traunmüller F, El Menyawi I, Frass M, Hollenstein UM, et al. (1999) Continuous infusion versus intermittent administration of meropenem in critically ill patients. J Antimicrob Chemother 43:523–7. 10.1093/jac/43.4.523 [DOI] [PubMed] [Google Scholar]
- 8. Bauer KA, West JE, O'Brien JM, Goff DA (2013) Extended-infusion cefepime reduces mortality in patients with Pseudomonas aeruginosa infections. Antimicrob Agents Chemother 7: 2907–12. 10.1128/AAC.02365-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Papich MG (2014) Pharmacokinetic-pharmacodynamic (PK-PD) modeling and the rational selection of dosage regimes for the prudent use of antimicrobial drugs. Vet Microbiol 16: 480–6. 10.1016/j.vetmic.2013.12.021 [DOI] [PubMed] [Google Scholar]
- 10. Felton TW, Goodwin J, O'Connor L, Sharp A, Gregson L, et al. (2013) Impact of Bolus dosing versus continuous infusion of Piperacillin and Tazobactam on the development of antimicrobial resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 57: 5811–9. 10.1128/AAC.00867-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Waele JJ, Carrette S, Carlier M, Stove V, Boelens J, et al. (2014) Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: a randomised controlled trial. Intensive Care Med, 40:380–7. 10.1007/s00134-013-3187-2 [DOI] [PubMed] [Google Scholar]
- 12. Buck C, Bertram N, Ackermann T, Sauerbruch T, Derendorf H, et al. (2005) Pharmacokinetics of piperacillin-tazobactam: intermittent dosing versus continuous infusion. Int J Antimicrob Agents 25: 62–7. 10.1016/j.ijantimicag.2004.08.012 [DOI] [PubMed] [Google Scholar]
- 13. Drusano GL (2003) Prevention of resistance: a goal for dose selection for antimicrobial agents. Clin Infect Dis 36:S42–50. 10.1086/344653 [DOI] [PubMed] [Google Scholar]
- 14. Butterfield JM, Lodise TP, Beegle S (2014) Pharmacokinetics and pharmacodynamics of extended-infusion piperacillin/tazobactam in adult patients with cystic fibrosis-related acute pulmonary exacerbations. J Antimicrob Chemother 69: 176–9. 10.1093/jac/dkt300 [DOI] [PubMed] [Google Scholar]
- 15. Falagas ME, Tansarli GS, Ikawa K, Vardakas KZ (2013) Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: a systematic review and meta-analysis. Clin Infect Dis 56: 272–82. 10.1093/cid/cis857 [DOI] [PubMed] [Google Scholar]
- 16. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, et al. (2005) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed 10 May 2014.
- 17. Roberts JA, Kirkpatrick CM, Roberts MS, Dalley AJ, Lipman J (2010) First dose and steady-state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int J Antimicrob Agents 35: 156–63. 10.1016/j.ijantimicag.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 18. Roberts JA, Kirkpatrick CM, Roberts MS, Dalley AJ, Lipman J (2005) Pharmacokinetics of piperacillin-tazobactam: intermittent dosing versus continuous infusion. Int J Antimicrob Agents 25: 62–7. 10.1016/j.ijantimicag.2004.08.012 [DOI] [PubMed] [Google Scholar]
- 19. Rafati MR, Rouini MR, Mojtahedzadeh M, Najafi A, Tavakoli H, et al. (2006) Clinical efficacy of continuous infusion of piperacillin compared with intermittent dosing in septic critically ill patients. Int J Antimicrob Agents 28: 122–7. 10.1016/j.ijantimicag.2006.02.020 [DOI] [PubMed] [Google Scholar]
- 20. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grant EM, Kuti JL, Nicolau DP, Nightingale C, Quintiliani R (2002) Clinical efficacy and pharmacoeconomics of a continuous-infusion piperacillin-tazobactam program in a large community teaching hospital. Pharmacotherapy 22: 471–83. 10.1592/phco.22.7.471.33665 [DOI] [PubMed] [Google Scholar]
- 22. Lau WK, Mercer D, Itani KM, Nicolau DP, Kuti JL, et al. (2006) Randomized, open-label, comparative study of piperacillin-tazobactam administered by continuous infusion versus intermittent infusion for treatment of hospitalized patients with complicated intra-abdominal infection. Antimicrob Agents Chemother 50: 3556–61. 10.1128/AAC.00329-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DeRyke CA, Kuti JL, Mansfield D, Dana A, Nicolau DP (2006) Pharmacoeconomics of continuous versus intermittent infusion of piperacillin-tazobactam for the treatment of complicated intraabdominal infection. Am J Health Syst Pharm 63: 750–5. 10.2146/ajhp050323 [DOI] [PubMed] [Google Scholar]
- 24. Lodise TP, Lomaestro B, Drusano GL (2007) Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis 44: 357–63. 10.1086/510590 [DOI] [PubMed] [Google Scholar]
- 25. Patel GW, Patel N, Lat A, Trombley K, Enbawe S, et al. (2009) Outcomes of extended infusion piperacillin/tazobactam for documented gram-negative infections. Diagn Microbiol Infect Dis 64: 236–40. 10.1016/j.diagmicrobio.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 26. Lorente L, Jiménez A, Martín MM, Iribarren JL, Jiménez JJ, et al. (2009) Clinical cure of ventilator-associated pneumonia treated with piperacillin/tazobactam administered by continuous or intermittent infusion. Int J Antimicrob Agents 33: 464–8. 10.1016/j.ijantimicag.2008.10.025 [DOI] [PubMed] [Google Scholar]
- 27. Li ZQ, Zhang YG, Wang CY, Qiu F (2010) Clinical efficacy of continuous infusion of piperacillin/tazobactam in severe pneumonia patients: a randomized controlled clinical trial. Modern Preventive Medicine 3: 2949–51. [Google Scholar]
- 28. Ye LQ, Cai T, Jin YH (2011) Clinical study of extending administration time of piperacillin/tazobactam in treatment of Gram-negative bacteria infection. Chin J Nosocomiol 21: 3476–79. [Google Scholar]
- 29. Yost RJ, Cappelletty DM (2011) The retrospective cohort of infusion piperacillin/tazobactam (RECEIPT) study: a multicenter study. Pharmacotherapy 31: 767–75. 10.1592/phco.31.8.767 [DOI] [PubMed] [Google Scholar]
- 30. Gonçalves-Pereira J, Oliveira BS, Janeiro S, Estilita J, Monteiro C, et al. (2012) Continuous infusion of piperacillin/tazobactam in septic critically ill patients--a multicenter propensity matched analysis. PLoS One 7: e49845 10.1371/journal.pone.0049845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee GC, Liou H, Yee R, Quan CF, Neldner K (2012) Outcomes of extended-infusion piperacillin-tazobactam: a retrospective analysis of critically ill patients. Clin Ther 34: 2297–300. 10.1016/j.clinthera.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 32. Lü Y, Yan Z, Wang DH, Dong WL, Yang Y, et al. (2013) Treatment study of hospital acquired pneumonia by optimizing dosing regimen of piperaeillin/tazobactam:prolonged vs regular infusion. Chin Crit Care Med 25: 479–83. [DOI] [PubMed] [Google Scholar]
- 33. Kasiakou SK, Lawrence KR, Choulis N, Falagas ME (2005) Continuous versus intermittent intravenous administration of antibacterials with time-dependent action: a systematic review of pharmacokinetic and pharmacodynamics parameters. Drugs 65: 2499–511. 10.2165/00003495-200565170-00006 [DOI] [PubMed] [Google Scholar]
- 34. Li C, Kuti JL, Nightingale CH, Mansfield DL, Dana A, et al. (2005) Population pharmacokinetics and pharmacodynamics of piperacillin/tazobactam in patients with complicated intra-abdominal infection. J Antimicrob Chemother 56: 388–95. 10.1093/jac/dki243 [DOI] [PubMed] [Google Scholar]
- 35. Bandolier, Evidence And Diagnostics. Available: http://www.medicine.ox.ac.uk/bandolier/booth/diagnos/Diagessy.html Accessed 10 July 2014.
- 36. Nelson EA (1999) Critical appraisal. 5. Randomised controlled trials: questions for valid evidence. NT Learn Curve 3: 6–8. [PubMed] [Google Scholar]
- 37. Austin PC, Alter DA, Tu JV (2003) The use of fixed- and random-effects models for classifying hospitals as mortality outliers: a Monte Carlo assessment. Med Decis Making 23: 526–39. 10.1177/0272989X03258443 [DOI] [PubMed] [Google Scholar]
- 38. Chen TA (2012) Fixed or random testlet effects: a comparison of two multilevel testlet models. J Appl Meas 13: 231–47. [PubMed] [Google Scholar]
- 39. Villar J, Mackey ME, Carroli G, Donner A (2001) Meta-analyses in systematic reviews of randomized controlled trials in perinatal medicine: comparison of fixed and random effects models. Stat Med 20: 3635–47. 10.1002/sim.1096 [DOI] [PubMed] [Google Scholar]
- 40. Yoon YG1, Koob MD (2005) Transformation of isolated mammalian mitochondria by bacterial conjugation. Nucleic Acids Res 33: e139 10.1093/nar/gni140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang J, Luo YP, Zhang XJ, Yao YH (2003) Resistant Mechanisms of Staphylococcus aureus: Analysis of Clinical Isolates to 20 Antimicrobial Agents. Chin J Nosocomiol 13: 1107–9. [Google Scholar]
- 42. Li XZ, Ling BD (2007) Progress in studying mechanisms of antimicrobial resistance in bacteria in 2006. Chin J of Antibiotics 32: 193–200. [Google Scholar]
- 43. Persson M, Løye AF, Mow T, Hornberg JJ (2013) A high content screening assay to predict human drug-induced liver injury during drug discovery. J Pharmacol Toxicol Methods 68: 302–13. 10.1016/j.vascn.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 44. Heinrich LS, Tokumaru S, Clark NM, Garofalo J, Paek JL, et al. (2011) Development and implementation of a piperacillin-tazobactam extended infusion guideline. J Pharm Pract 24: 571–6. 10.1177/0897190011406984 [DOI] [PubMed] [Google Scholar]
- 45. Roberts JA, Paratz J, Paratz E, Krueger WA, Lipman J (2007) Continuous infusion of beta-lactam antibiotics in severe infections: a review of its role. Int J Antimicrob Agents 30: 11–8. 10.1016/j.ijantimicag.2007.02.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper.