Abstract
Background
Nudix hydrolases form a protein family whose function is to hydrolyse intracellular nucleotides and so regulate their levels and eliminate potentially toxic derivatives. The genome of the radioresistant bacterium Deinococcus radiodurans encodes 25 nudix hydrolases, an unexpectedly large number. These may contribute to radioresistance by removing mutagenic oxidised and otherwise damaged nucleotides. Characterisation of these hydrolases is necessary to understand the reason for their presence. Here, we report the cloning and characterisation of the DR0975 gene product, a nudix hydrolase that appears to be unique to this organism.
Results
The DR0975 gene was cloned and expressed as a 20 kDa histidine-tagged recombinant product in Escherichia coli. Substrate analysis of the purified enzyme showed it to act primarily as a phosphatase with a marked preference for (deoxy)nucleoside 5'-diphosphates (dGDP > ADP > dADP > GDP > dTDP > UDP > dCDP > CDP). Km for dGDP was 110 μM and kcat was 0.18 s-1 under optimal assay conditions (pH 9.4, 7.5 mM Mg2+). 8-Hydroxy-2'-deoxyguanosine 5'-diphosphate (8-OH-dGDP) was also a substrate with a Km of 170 μM and kcat of 0.13 s-1. Thus, DR0975 showed no preference for 8-OH-dGDP over dGDP. Limited pyrophosphatase activity was also observed with NADH and some (di)adenosine polyphosphates but no other substrates. Expression of the DR0975 gene was undetectable in logarithmic phase cells but was induced at least 30-fold in stationary phase. Superoxide, but not peroxide, stress and slow, but not rapid, dehydration both caused a slight induction of the DR0975 gene.
Conclusion
Nucleotide substrates for nudix hydrolases conform to the structure NDP-X, where X can be one of several moieties. Thus, a preference for (d)NDPs themselves is most unusual. The lack of preference for 8-OH-dGDP over dGDP as a substrate combined with the induction in stationary phase, but not by peroxide or superoxide, suggests that the function of DR09075 may be to assist in the recycling of nucleotides under the very different metabolic requirements of stationary phase. Thus, if DR0975 does contribute to radiation resistance, this contribution may be indirect.
Background
The Nudix hydrolases are a family of nucleotide hydrolases found in virtually all organisms. They hydrolyse a wide range of substrates including (d)NTPs, pyridine nucleotides, coenzyme A, dinucleoside polyphosphates and nucleotide sugars, all of which conform to the general structure of a nucleoside diphosphate linked to another moiety X (NDP-X) [1-3]. More recently, non-nucleotide compounds that do not conform to the NDP-X structure, such as diphosphoinositol polyphosphates and phosphoribosyl pyrophosphate, have been added to the range of substrates degraded by some of these enzymes [4-7].
Catalytic activity resides in a distinctive motif, the Nudix (formerly MutT) motif, usually resulting in the cleavage of a pyrophosphate bond. Substrate specificity, which can be relatively broad or narrow, results from interactions with other regions of the protein structure, some of which have been defined. For example, the sequence LLTxR[SA]x3Rx3Gx3FPGG located immediately upstream of the Nudix motif, is found in hydrolases that prefer CoA and CoA esters as substrates [8-10], while the sequence SQPWPFPxS is found a short distance downstream of the Nudix motif in hydrolases characterized as NADH diphosphatases [11-14]. Other motifs may characterise ADP-ribose pyrophosphatases [2], diadenosine tetraphosphate hydrolases [2] and UTP pyrophosphohydrolases [15].
The proposed functions of this family are to eliminate potentially toxic nucleotide metabolites from the cell, e.g. oxidised, mutagenic purine nucleotides in the case of E. coli MutT [16-19] and human NUDT1 (MTH1) [20,21], and to regulate the concentrations of nucleotide cofactors and signalling molecules for optimal cell growth and survival in response to the cellular environment [1]. The number of genes encoding Nudix hydrolases varies widely, from zero in most Mycoplasma species to around 30 in streptomycetes. This variation correlates fairly closely with genome size and presumably reflects the metabolic capacity and growth or environmental adaptability of the different organisms. However, some bacteria have many more Nudix genes than would be expected from their DNA content. One such is the radiation- and desiccation-resistant Deinococcus radiodurans [22,23] which has roughly three times as many Nudix genes as would be expected from its genome size of 3.3 Mb [24,25], suggesting a particular selective pressure on this organism to maintain an expanded set of these genes. In order to understand how the possession of 25 Nudix hydrolases relates to the biology of D. radiodurans, we are studying the gene products, particularly those that have no obvious orthologues in other organisms. Here, we describe a new Nudix hydrolase that has a marked degree of specificity for ribonucleoside and deoxyribonucleoside 5'-diphosphates [(d)NDPs].
Results
Expression and purification of the DR0975 gene product
The DR0975 gene was cloned by PCR from genomic DNA into the pET15b expression vector to yield a 20 kDa N-terminally hexahistidine-tagged protein. This protein was purified by chromatography on a Ni-CAM HC affinity column (Sigma) and the resulting product was judged to be 95% pure (not shown).
Substrate analysis
The ability of the DR0975 gene product to hydrolyse a range of potential nucleotide substrates was determined under a standard set of conditions comprising 100 μM nucleotide, pH 8 and 5 mM Mg2+ ions. Assays involving (d)NTPs included inorganic pyrophosphatase to release Pi from any PPi produced initially, assays involving dinucleoside polyphosphates (e.g. Ap4A, NADH) included alkaline phosphatase to release Pi from primary products, while those involving (d)NDPs contained neither auxiliary enzyme. Under these conditions, high activity was obtained with the purine (deoxy)ribonucleoside 5'-diphosphates (d)GDP and (d)ADP with dGDP being the best substrate (Table 1). dTDP was also hydrolysed but UDP and (d)CDP appeared to be resistant to breakdown. Lower activity was observed with the long chain (di)nucleoside polyphosphates Ap5A, Ap6A, p4A and p5A and also with NADH, but not with other compounds of this general structure. Notably, all (d)NTPs, NDP-sugars and NDP-alcohols tested were inactive as substrates. These included (d)ATP, (d)GTP, (d)CTP, UTP, dTTP, ADP-glucose, ADP-mannose, ADP-ribose, IDP-ribose, UDP-glucose, UDP-galactose, GDP-glucose, GDP-mannose, GDP-α-fucose, CDP-choline, CDP-ethanolamine, CDP-glucose and CDP-glycerol.
Table 1.
Hydrolysis of nucleotide substrates by DR0975 protein. Nucleotides were screened for substrate activity as described in Materials and Methods.
| Nucleotide | nmol hydrolysed |
| dGDP | 9.3 |
| dADP | 2.8 |
| GDP | 2.7 |
| dTDP | 2.1 |
| ADP | 1.7 |
| (d)CDP, UDP | 0 |
| (d)NTPs | 0 |
| Ap3A, Ap4A | 0 |
| Ap5A | 1.8 |
| Ap6A | 0.8 |
| p4A | 0.4 |
| p5A | 0.7 |
| NADH | 0.35 |
| NAD, NADP(H), FAD, CoA | 0 |
| NDP-sugars | 0 |
Assay conditions were then optimised using dGDP as substrate and an HPLC assay. Maximum activity was obtained at pH 9.4 and 7.5 mM Mg2+. DTT was not required. Mn2+ at 0.5 mM supported 18% of the optimum activity with Mg2+. The (d)NDPs were then retested as substrates under these new conditions using a higher substrate concentration of 500 μM. The results in Table 2 show that purine (d)NDPs were still the preferred substrates, although activity was now evident with all the pyrimidine compounds as well.
Table 2.
Hydrolysis of (d)NDP substrates by DR0975 protein under optimised conditions. Assays containing 50 mM BisTrisPropane, pH 9.4, 7.5 mM Mg acetate, 3 μg/DR0975 protein and 500 μM substrate were incubated at 37°C for 15 min.
| Nucleotide | nmol hydrolysed |
| dGDP | 19.8 |
| ADP | 13.6 |
| dADP | 13.4 |
| GDP | 11.7 |
| dTDP | 9.3 |
| UDP | 3.4 |
| dCDP | 3.2 |
| CDP | 1.9 |
HPLC analysis of the products of dGDP hydrolysis showed them to be dGMP and Pi (results not shown). No further degradation of the dGMP was observed, therefore the enzyme is acting primarily as a nucleoside diphosphate phosphohydrolase (EC3.6.1.6). Since the low activity observed with Ap5A, Ap6A and NADH would require pyrophosphatase activity, degradation of these substrates was checked by colorimetric and HPLC assays and confirmed. In the case of Ap5A, for example, initial products were ADP and ATP, the ADP then being rapidly converted to AMP + Pi (results not shown).
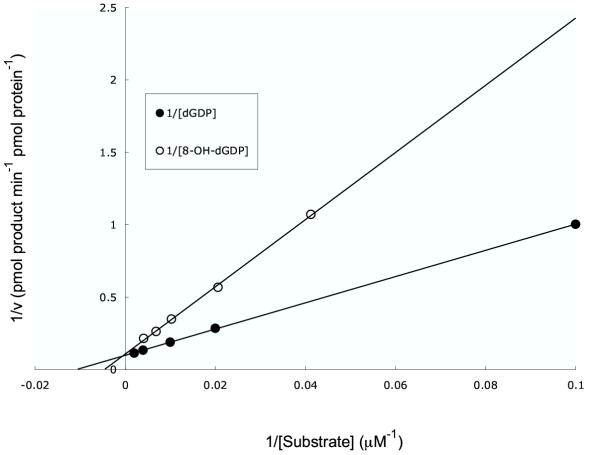
The preference for (d)NDPs was surprising. At the time of conducting these experiments, no other nudix hydrolase had been reported to have activity with (d)NDPs. Recently, however, the human NUDT5 hydrolase, previously characterized as an ADP-sugar pyrophosphatase [26,27] and the orthologue of the yeast YSA1 protein [2], was shown to have a preference for 8-OH-dGDP as substrate [28]. We therefore compared the kinetic constants for dGDP and 8-OH-dGDP hydrolysis by DR0975 (Fig. 1). Km values of 110 μM and 170 μM and kcat values of 0.18 s-1 and 0.13 s-1 were determined for dGDP and 8-OH-dGDP respectively. Km/kcat ratios were 1.68 × 103 s-1 M-1 for dGDP and 0.78 × 103 s-1 M-1 for 8-OH-dGDP. Thus, DR0975 exhibits a 2-fold preference for dGDP as a substrate. This, coupled with the relatively high Km of 170 μM for 8-OH-dGDP, a substrate that is likely to exist at very low concentrations, suggests that the primary function of DR0975 is not the elimination of 8-OH-dGDP. These results should be contrasted with the properties of the E. coli MutT 8-OH-dGTPase, which has similar kcat values for dGTP and 8-OH-dGTP hydrolysis but respective Km values of 1100 and 0.48 μM for these nucleotides [16] and with human MTH1, which has respective Km values of 870 and 12.5 μM for dGTP and 8-OH-dGTP, again with similar kcat values [29]. Both these enzymes display a marked preference for the oxidised dGTP derivative.
Figure 1.
Lineweaver-Burk plot of the hydrolysis of dGDP and 8-OH-dGDP by DR0975 protein. Assays were performed by HPLC as described in Materials and Methods.
Non-nudix (d)NDP phosphohydrolase activities have previously been described in mammalian tissues, and some of these enzymes are also active towards the structurally similar enzyme cofactor thiamine pyrophosphate [30-32]. Therefore, TPP was tested as a possible substrate for the DR0975 protein, but no activity was detected.
Expression analysis of the DR0975 gene
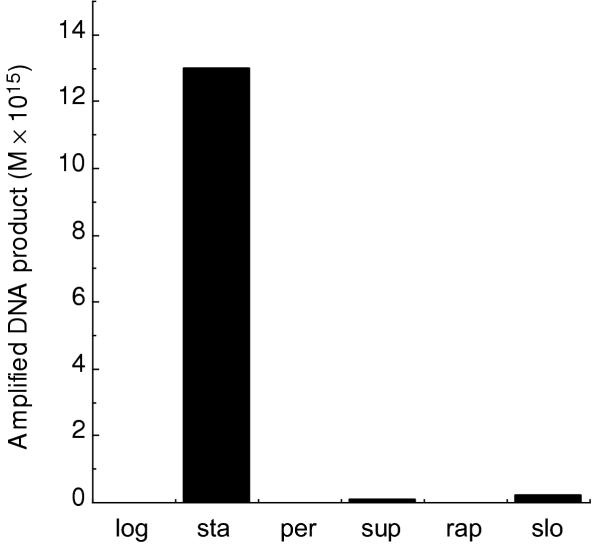
Expression of the DR0975 gene was measured by quantitative RT-PCR analysis of RNA isolated from logarithmic and stationary phase cells, from cells exposed to hydrogen peroxide or menadione (a superoxide generator) and from cells subjected to rapid desiccation by freeze drying or slow dehydration, followed by rehydration in each case. These conditions were chosen to reflect the known resistance of D. radiodurans to ionizing radiation and dehydration/rehydration, both of which result in DNA damage, particularly double-strand breaks and oxidised lesions [23,33,34]. The concentration of hydrogen peroxide used (10 mM for 1 h) is known to have little effect on the growth and survival of D. radiodurans [35] while 10 mM menadione leads to growth arrest (J. Cartwright, unpublished observation). The results show that DR0975 mRNA expression was below the limit of detection in logarithmically growing cells but was induced at least 30-fold in stationary phase cells, (Fig. 2). Superoxide, but not peroxide, and slow, but not rapid, dehydration both caused a slight induction of the DR0975 gene. It appears, therefore, that the requirement for (d)NDPase activity is confined to the stationary phase of the growth cycle and is not a response to oxidative stress.
Figure 2.
Expression analysis of the DR0975 gene in cultures exposed todifferent conditions. The level of DR0975 mRNA expressed in logarithmic (log) and stationary (sta) phase cells, in cells exposed to hydrogen peroxide (per) or menadione (sup) and from cells subjected to rapid desiccation by freeze drying (rap) or slow (slo) dehydration, followed by rehydration in each case, was quantified by RT-PCR as described in Materials and Methods. Each value represents the mean of triplicate amplifications of each of three independently prepared RNA samples for each condition. The amount of amplified product was determined by densitometry by comparison with the concentrations of the standards amplified from genomic DNA by gene-specific primers. The limit of detection was 4.23 × 10-16 M.
Discussion
The DR0975 protein is unusual in its preference for (d)NDPs, showing that X can be H in the commonly used NDP-X substrate designation. Such activity has only recently been observed in one other nudix hydrolase, the human NUDT5 ADP-sugar pyrophosphatase that, surprisingly, is actually more selective for 8-OH-dGDP [28]. However, DR0975 has no activity with any of the NDP-sugars tested and so seems unlikely to be a NUDT5 orthologue. Phosphatase activity among the nudix hydrolases has previously been observed with those active towards diphosphoinositol polyphosphates and phosphoribosyl pyrophosphate [4-7]. Interestingly, these enzymes also act as pyrophosphatases with the alternative diadenosine polyphosphate substrates. Similarly, DR0975 has low activity with some long chain (di)adenosine polyphosphates and with NADH. Determination of the structure of this enzyme should reveal how this is achieved.
The D. radiodurans genome contains 23 nudix genes, two of which encode proteins with two distinct nudix motifs (active sites). It has been suggested that this large number is related to the ability of this organism to withstand high doses of ionizing radiation [24,25], itself a probable consequence of the desiccation tolerance of the organism [34]. Both ionising radiation and dehydration/rehydration impose severe oxidative stress and might be expected to generate potentially toxic and mutagenic oxidised derivatives within the nucleotide pool. Nudix hydrolases with a degree of specificity for oxidised nucleotide such as 8-OH-dGTP, 2-OH-dATP, 8-OH-dATP, 5-OH-CTP and 8-OH-dGDP have been isolated from E. coli [16,17,36,37] and mammalian cells [20,28,38,39] and so it is possible that some of the additional nudix genes in the D. radiodurans genome encode enzymes active towards other oxidized nucleotides. However, the lack of preference for 8-OH-dGDP compared to dGDP suggests that the former nucleotide is unlikely to be an important substrate for this enzyme in vivo. It is possible, of course, that some other oxidized (d)NDP derivative or derivatives are physiologically relevant substrates and this highlights the problem with the study of new nudix hydrolases – that conclusions are often limited by the availability of suitable, novel substrates. In the case of DR0975, however, the dramatic induction of activity upon entry into stationary phase and the lack of significant induction by peroxide or superoxide suggest an alternative function.
The bacterial stationary phase is characterised by extensive changes in patterns of gene expression leading to physical and morphological adaptations that are designed to maintain viability during starvation [40,41]. DNA synthesis ceases and there is considerable RNA degradation to supply energy for maintenance metabolism. These changes will inevitably involve nucleotide pools as they are diverted to other activities, such as cell wall synthesis. Thus, the DR0975 protein may have the relatively non-specific function of recycling nucleic acid nucleotides. Alternative, more specific functions can also be imagined. For example, the nucleoside diphosphatase of the mammalian endoplasmic reticulum is believed to eliminate UDP, a product of UDP-glucose:glycoprotein glucosyltransferase [32]. UDP inhibits this enzyme and, if allowed to accumulate, would inhibit protein glucosylation. Thus, although not a nudix hydrolase, this enzyme seems to fulfil the housecleaning role proposed for nudix hydrolases [1]. The D. radiodurans DR0975 protein may serve a similar function in stationary phase bacteria in pathways leading to the synthesis of various NDP-sugars. Hence, DR0975 may be involved in the reprogramming of nucleotide pools to meet the requirements of stationary phase. Stationary phase D. radiodurans are more radiation resistant than logarithmic phase cells [42], so an indirect contribution of DR0975 to radiation tolerance is conceivable. Such a role may indeed be unique to this organism as a BLAST search reveals no sequences of close similarity to DR0975 among the nudix genes of other sequenced bacterial genomes (>100).
Conclusions
In the absence of evidence to the contrary, we would suggest that a likely role of the DR0975 nucleoside diphosphate phosphohydrolase is to recycle (deoxy)nucleoside diphosphates as part of the general reprogramming of metabolism that occurs during stationary phase. Whether this is true of others among the large number of nudix genes in this organism remains to be determined. Ultimately, an understanding of the roles of the nudix hydrolases of D. radiodurans will require systematic gene disruption and phenotypic analysis. Due to the overlapping substrate specificity of these enzymes, multiple deletions may be required in order to observe a phenotype. This will present an interesting but worthwhile challenge for future research.
Methods
Materials
8-Hydroxy-2'-deoxyguanosine 5'-diphosphate (8-OH-dGDP) was prepared as described previously [21]. All other nucleotides were from Sigma. Calf intestinal alkaline phosphatase and yeast inorganic pyrophosphatase were from Roche. NdeI, BamHI and the pET15b expression vector were from Novagen. Pfu DNA polymerase was from Stratagene and M-MLV reverse transcriptase (RNase H minus) was from Promega. Oligonucleotides were from MWG Biotech. TRIzol and DNAse I (Amplification Grade) were from Invitrogen.
Cloning of DR0975 from genomic DNA
The DR0975 coding region was amplified from genomic DNA by PCR using Pfu DNA polymerase, a 33-mer oligonucleotide forward primer d(CGAGGACCCATATGGGGCGGCGTGATCTGCTGG) and 32-mer reverse primer d(GCCCTGCCTGGATCCGCTAGCGGTCCTTGACC). These primers incorporated an NdeI restriction site at the start of the gene, and a BamHI site at the end. After amplification, the DNA was recovered by phenol-chlorofom extraction and digested with NdeI and BamHI. The gel-purified restriction fragment was ligated into the appropriate restriction sites of plasmid pET15b and the resulting construct, pET-0975 containing the DR0975 coding region with an upstream His tag sequence under the control of a T7 lac promoter, was used to transform E. coli XL1-Blue cells for propagation.
Protein expression in E. coli and purification
E. coli strain BL21(DE3) was transformed with pET-0975. A single colony was inoculated into 10 ml LB medium containing 60 μg/ml ampicillin and grown overnight at 37°C. The cells were transferred to 1 litre LB medium containing 60 μg/ml ampicillin and grown to an A600 of 0.6. Isopropyl-1-thio-β-D-galactopyranoside was added to 1 mM and the cells induced for 3 h. The cells (4.3 g) were harvested, washed and resuspended in 25 ml breakage buffer (50 mM Tris-HCl, pH 8.0, 300 mM NaCl). The cell suspension was sonicated and centrifuged at 10,000 g for 10 min. The supernatant was recovered and loaded in 10 ml aliquots at 1 ml/min on to a 7 × 50 mm Ni-CAM HC affinity resin column equilibrated with breakage buffer. After elution of unbound protein, a 20 min linear gradient of 0–50 mM histidine in breakage buffer was applied at 1 ml/min. Fractions of 1 ml were collected and analysed by SDS-PAGE. Fractions containing the 20.9 kDa His-tagged DR0975 protein were pooled and DTT was added to a final concentration of 1 mM.
Enzyme assay
Nucleotide substrates were assayed as previously described [26] using the following conditions. Assays (200 μl) contained 50 mM Tris-HCl, pH8.0, 5 mM Mg acetate, 1 mM DTT, 100 μM substrate, 0.5 μg inorganic pyrophosphatase or 1 μg/ml alkaline phosphatase as appropriate (except with (d)NDP substrates) and 5 μg DR0975 protein and were incubated at 37°C for 15 mins.
HPLC analysis of dGDP and 8-OH-dGDP hydrolysis
Assay samples (50 μl) were injected directly on to a 2.1 × 100 mm Brownlee AX300 column in buffer A (0.1 M potassium phosphate, pH 6.1) and eluted with a gradient of 0–5% buffer B (4.5 min), 5–40% B (7.3 min), 40% B (13.0 min), where B = 0.1 M potassium phosphate, pH 6.1, 50% (v/v) acetonitrile at a flow rate of 0.5 ml/min.
Expression analysis of the DR0975 gene
Expression of the DR0975 gene was determined in cells grown under different conditions using a reverse transcription-coupled PCR (RT-PCR) assay. D. radiodurans was grown in TGY medium (0.8% w/v tryptone, 0.1% w/v glucose and 0.4% w/v yeast extract) at 30°C, 200 rpm and samples removed hourly for turbidity analysis at 600 nm. Cultures (100 ml) were grown to early log phase (A600 = 0.1) and well into stationary phase (40 h after inoculation). Oxidised log phase cells were prepared by incubating early log phase cells with 10 mM H2O2 for 1 h. Superoxide-treated log phase cells were prepared by incubating early log phase cells with 10 mM menadione for 1 h. Rehydrated log phase cells were prepared by harvesting early log phase cells by centrifugation (2000 g for 10 min) and then lyophilising. Several days later the cells were resuspended in TGY medium and incubated at 30°C, 200 rpm for 2 h before processing for RT-PCR. Slowly dehydrated, rehydrated log phase cells were prepared by harvesting early log phase cells by centrifugation (including a wash with PBS) and then resuspending the pellets in 1 ml PBS. The resuspended pellets were placed into 35 mm petri-dishes and then sealed in a desiccator over silica-gel at 30°C for 58 days. Cells were resuspended in TGY medium and incubated at 30°C, 200 rpm for 2 hours before processing for RT-PCR. Cultures were prepared in triplicate for each set of conditions.
RNA was extracted from the cell pellets using TRIzol reagent according to the manufacturers instructions, dissolved in ddH2O and adjusted to 125 μg/ml (from A260). The RNA was then further treated with DNase I according to the manufacturers recommendations in order to remove traces of genomic DNA. Reverse transcription was performed in a 10 μl reaction containing 10 pmol specific-downstream primer (5' AACGCTAGCGGTCCTTGACCACCG 3'), 0.5 μg DNase-treated RNA, 500 μM of each dNTP and 100 U M-MLV reverse transcriptase. Reactions were incubated at 50°C for 1 h before being terminated by the addition of 40 μl TE buffer and storage at -20°C. Control RT reactions were also performed which contained all of the reaction components except the specific-downstream primer.
Polymerase Chain Reaction (PCR) was performed in 20 μl assays and contained 1.0 U Pfu polymerase, 200 μM of each dNTP, 10 pmol each of upstream primer (5' ACCAGCATGGGGCGGCGTGATCTG 3') and downstream primer (5' TCCCAGCCCTTGAAGGCATAGAAG 3'), 5 μl RT or control-RT reaction and 5% (v/v) DMSO. The PCR cycle was 45 sec at 95°C (dissociation), 45 sec at 60°C, and 75 sec at 72°C (extension) for 40 cycles. All conditions were analysed in a single PCR experiment which contained samples of the triplicate first-strand cDNAs for each of the six growth conditions, individual controls for each of these samples, and a range of empirically determined dilutions of a gene-specific template which demonstrated that amplification was within the exponential range. The gene-specific template was prepared from D. radiodurans genomic DNA by PCR using primers external to those used in the RT-PCR reactions and the product was quantified according to adsorption at 260 nm [43]. The identity of the RT-PCR product was confirmed by its co-migration with the product of the gene-specific template as determined by agarose gel electrophoresis. Data were obtained by densitometry (Syngene GeneGenius) of PCR products resolved using agarose gel electrophoresis and stained with ethidium bromide. Densitometric data for each sample were converted to the equivalent concentration of gene-specific template from a linear calibration plot of log [gene-specific template concentration] versus relative density using GeneTools software (Syngene) [44].
Authors' contributions
DIF performed the cloning, purification and substrate analysis and participated in the design of the study. JLC carried out the expression analysis and assisted with the design. HH and HK synthesised the 8-OH-dGDP and provided helpful advice. AGM conceived the study, participated in its design and coordination and drafted the manuscript.
Acknowledgments
Acknowledgements
We are grateful to the Biotechnology and Biological Sciences Research Council for financial support and for the award of a studentship to DIF.
Contributor Information
David I Fisher, Email: dfisher@liv.ac.uk.
Jared L Cartwright, Email: jc46@york.ac.uk.
Hideyoshi Harashima, Email: harasima@pharm.hokudai.ac.jp.
Hiroyuki Kamiya, Email: hirokam@pharm.hokudai.ac.jp.
Alexander G McLennan, Email: agmclen@liv.ac.uk.
References
- Bessman MJ, Frick DN, O'Handley SF. The MutT proteins or ''nudix'' hydrolases, a family of versatile, widely distributed, ''housecleaning'' enzymes. J Biol Chem. 1996;271:25059–25062. doi: 10.1074/jbc.271.41.25059. [DOI] [PubMed] [Google Scholar]
- Dunn CA, O'Handley SF, Frick DN, Bessman MJ. Studies on the ADP-ribose pyrophosphatase subfamily of the Nudix hydrolases and tentative identification of trgB, a gene associated with tellurite resistance. J Biol Chem. 1999;274:32318–32324. doi: 10.1074/jbc.274.45.32318. [DOI] [PubMed] [Google Scholar]
- McLennan AG. The MutT motif family of nucleotide phosphohydrolases in man and human pathogens. Int J Mol Med. 1999;4:79–89. doi: 10.3892/ijmm.4.1.79. [DOI] [PubMed] [Google Scholar]
- Caffrey JJ, Safrany ST, Yang XN, Shears SB. Discovery of molecular and catalytic diversity among human diphosphoinositol-polyphosphate phosphohydrolases - An expanding Nudt family. J Biol Chem. 2000;275:12730–12736. doi: 10.1074/jbc.275.17.12730. [DOI] [PubMed] [Google Scholar]
- Fisher DI, Safrany ST, Strike P, McLennan AG, Cartwright JL. Nudix hydrolases that degrade dinucleoside and diphosphoinositol polyphosphates also have 5-phosphoribosyl 1-pyrophosphate (PRPP) pyrophosphatase activity that generates the glycolytic activator ribose 1,5-bisphosphate. J Biol Chem. 2002;277:47313–47317. doi: 10.1074/jbc.M209795200. [DOI] [PubMed] [Google Scholar]
- Leslie NR, McLennan AG, Safrany ST. Cloning and characterisation of hAps1 and hAps2, human diadenosine polyphosphate-metabolising Nudix hydrolases. BMC Biochem. 2002;3:20. doi: 10.1186/1472-2091-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safrany ST, Ingram SW, Cartwright JL, Falck JR, McLennan AG, Barnes LD, Shears SB. The diadenosine hexaphosphate hydrolases from Schizosaccharomyces pombe and Saccharomyces cerevisiae are homologues of the human diphosphoinositol polyphosphate phosphohydrolase - Overlapping substrate specificities in a MutT-type protein. J Biol Chem. 1999;274:21735–21740. doi: 10.1074/jbc.274.31.21735. [DOI] [PubMed] [Google Scholar]
- Gasmi L, McLennan AG. The mouse Nudt7 gene encodes a peroxisomal nudix hydrolase specific for coenzyme A and its derivatives. Biochem J. 2001;357:33–38. doi: 10.1042/0264-6021:3570033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AbdelRaheim S, McLennan AG. The Caenorhabditis elegans Y87G2A.14 Nudix hydrolase is a peroxisomal coenzyme A diphosphatase. BMC Biochem. 2002;3:5. doi: 10.1186/1472-2091-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright JL, Gasmi L, Spiller DG, McLennan AG. The Saccharomyces cerevisiae PCD1 gene encodes a peroxisomal nudix hydrolase active towards coenzyme A and its derivatives. J Biol Chem. 2000;275:32925–32930. doi: 10.1074/jbc.M005015200. [DOI] [PubMed] [Google Scholar]
- AbdelRaheim SR, Spiller DG, McLennan AG. Mammalian NADH diphosphatases of the Nudix family. Cloning and characterization of the human peroxisomal NUDT12 protein. Biochem J. 2003;374:329–335. doi: 10.1042/BJ20030441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AbdelRaheim SR, Cartwright JL, Gasmi L, McLennan AG. The NADH diphosphatase encoded by the Saccharomyces cerevisiae NPY1 nudix hydrolase gene is located in peroxisomes. Arch Biochem Biophys. 2001;388:18–24. doi: 10.1006/abbi.2000.2268. [DOI] [PubMed] [Google Scholar]
- Frick DN, Bessman MJ. Cloning, purification, and properties of a novel NADH pyrophosphatase - Evidence for a nucleotide pyrophosphatase catalytic domain in MutT-like enzymes. J Biol Chem. 1995;270:1529–1534. doi: 10.1074/jbc.270.4.1529. [DOI] [PubMed] [Google Scholar]
- Xu WL, Dunn CA, Bessman MJ. Cloning and characterization of the NADH pyrophosphatases from Caenorhabditis elegans and Saccharomyces cerevisae, members of a Nudix hydrolase subfamily. Biochem Biophys Res Commun. 2000;273:753–758. doi: 10.1006/bbrc.2000.2999. [DOI] [PubMed] [Google Scholar]
- Xu WL, Shen JY, Dunn CA, Bessman MJ. A new subfamily of the nudix hydrolase superfamily active on 5- methyl-UTP (ribo-TTP) and UTP. J Biol Chem. 2003;278:37492–37496. doi: 10.1074/jbc.M307639200. [DOI] [PubMed] [Google Scholar]
- Maki H, Sekiguchi M. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature. 1992;355:273–275. doi: 10.1038/355273a0. [DOI] [PubMed] [Google Scholar]
- Bhatanagar SK, Bessman MJ. Studies on the mutator gene, mutT, of Escherichia coli: molecular cloning of the gene, purification of the gene product, and identification of a novel nucleoside triphosphatase. J Biol Chem. 1988;263:8953–8957. [PubMed] [Google Scholar]
- Taddei F, Hayakawa H, Bouton M-F, Cirinesi A-M, Matic I, Sekiguchi M, Radman M. Counteraction by MutT protein of transcriptional errors caused by oxidative damage. Science. 1997;278:128–130. doi: 10.1126/science.278.5335.128. [DOI] [PubMed] [Google Scholar]
- Tassotto ML, Mathews CK. Assessing the metabolic function of the MutT 8- oxodeoxyguanosine triphosphatase in Escherichia coli by nucleotide pool analysis. J Biol Chem. 2002;277:15807–15812. doi: 10.1074/jbc.M200965200. [DOI] [PubMed] [Google Scholar]
- Kakuma T, Nishida J, Tsuzuki T, Sekiguchi M. Mouse MTH1 protein with 8-oxo-7,8-dihydro-2'-deoxyguanosine 5'-triphosphatase activity that prevents transversion mutation - cDNA cloning and tissue distribution. J Biol Chem. 1995;270:25942–25948. doi: 10.1074/jbc.270.43.25942. [DOI] [PubMed] [Google Scholar]
- Fujikawa K, Kamiya H, Yakushiji H, Fujii Y, Nakabeppu Y, Kasai H. The oxidized forms of dATP are substrates for the human MutT homologue, the hMTH1 protein. J Biol Chem. 1999;274:18201–18205. doi: 10.1074/jbc.274.26.18201. [DOI] [PubMed] [Google Scholar]
- Minton KW. Repair of ionizing-radiation damage in the radiation resistant bacterium Deinococcus radiodurans. Mutat Res. 1996;363:1–7. doi: 10.1016/0921-8777(95)00014-3. [DOI] [PubMed] [Google Scholar]
- Battista JR. Against all odds: The survival strategies of Deinococcus radiodurans. Annu Rev Microbiol. 1997;51:203–224. doi: 10.1146/annurev.micro.51.1.203. [DOI] [PubMed] [Google Scholar]
- Xu WL, Shen JY, Dunn CA, Desai S, Bessman MJ. The nudix hydrolases of Deinococcus radiodurans. Mol Microbiol. 2001;39:286–290. doi: 10.1046/j.1365-2958.2001.02267.x. [DOI] [PubMed] [Google Scholar]
- Makarova KS, Aravind L, Wolf YI, Tatusov RL, Minton KW, Koonin EV, Daly MJ. Genome of the extremely radiation-resistant bacterium Deinococcus radiodurans viewed from the perspective of comparative genomics. Microbiol Mol Biol Rev. 2001;65:44–79. doi: 10.1128/MMBR.65.1.44-79.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasmi L, Cartwright JL, McLennan AG. Cloning, expression and characterization of YSA1H, a human adenosine 5'-diphosphosugar pyrophosphatase possessing a MutT motif. Biochem J. 1999;344:331–337. doi: 10.1042/0264-6021:3440331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HJ, Slupska MM, Wei YF, Tai JH, Luther WM, Xia YR, Shih DM, Chiang JH, Baikalov C, FitzGibbon S, Phan IT, Conrad A, Miller JH. Cloning and characterization of a new member of the nudix hydrolases from human and mouse. J Biol Chem. 2000;275:8844–8853. doi: 10.1074/jbc.275.12.8844. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Hayakawa H, Sekiguchi M. A novel mechanism for preventing mutations caused by oxidation of guanine nucleotides. EMBO Rep. 2003;4:479–483. doi: 10.1038/sj.embor.embor838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo J-Y, Maki H, Sekiguchi M. Hydrolytic elimination of a mutagenic nucleotide, 8-oxodGTP, by human 18-kilodalton protein: sanitization of nucleotide pool. Proc Natl Acad Sci USA. 1992;89:11021–11025. doi: 10.1073/pnas.89.22.11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano S, Matsuda Y, Nakagawa H. Type B nucleoside-diphosphatase of rat brain. Purification and properties of an enzyme with high thiamin pyrophosphatase activity. Eur J Biochem. 1988;171:231–236. doi: 10.1111/j.1432-1033.1988.tb13781.x. [DOI] [PubMed] [Google Scholar]
- Wang TF, Guidotti G. Golgi localization and functional expression of human uridine diphosphatase. J Biol Chem. 1998;273:11392–11399. doi: 10.1074/jbc.273.18.11392. [DOI] [PubMed] [Google Scholar]
- Trombetta ES, Helenius A. Glycoprotein reglucosylation and nucleotide sugar utilization in the secretory pathway: identification of a nucleoside diphosphatase in the endoplasmic reticulum. EMBO J. 1999;18:3282–3292. doi: 10.1093/emboj/18.12.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts M. Desiccation tolerance: a simple process? Trends Microbiol. 2001;9:553–559. doi: 10.1016/S0966-842X(01)02231-4. [DOI] [PubMed] [Google Scholar]
- Mattimore V, Battista JR. Radioresistance of Deinococcus radiodurans: Functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J Bacteriol. 1996;178:633–637. doi: 10.1128/jb.178.3.633-637.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Schellhorn HE. Induction of resistance to hydrogen peroxide and radiation in Deinococcus radiodurans. Can J Microbiol. 1995;41:170–176. doi: 10.1139/m95-023. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Murata-Kamiya N, Iida E, Harashima H. Hydrolysis of oxidized nucleotides by the Escherichia coli Orf135 protein. Biochem Biophys Res Commun. 2001;288:499–502. doi: 10.1006/bbrc.2001.5781. [DOI] [PubMed] [Google Scholar]
- Fujikawa K, Kasai H. The oxidized pyrimidine ribonucleotide, 5-hydroxy-CTP, is hydrolyzed efficiently by the Escherichia coli recombinant Orf135 protein. DNA Repair. 2002;1:571–576. doi: 10.1016/S1568-7864(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Fujikawa K, Kamiya H, Yakushiji H, Nakabeppu Y, Kasai H. Human MTH1 protein hydrolyzes the oxidized ribonucleotide, 2- hydroxy-ATP. Nucleic Acids Res. 2001;29:449–454. doi: 10.1093/nar/29.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai JP, Ishibashi T, Takagi Y, Hayakawa H, Sekiguchi M. Mouse MTH2 protein which prevents mutations caused by 8- oxoguanine nucleotides. Biochem Biophys Res Commun. 2003;305:1073–1077. doi: 10.1016/S0006-291X(03)00864-7. [DOI] [PubMed] [Google Scholar]
- Siegele DA, Kolter R. Life after log. J Bacteriol. 1992;174:345–348. doi: 10.1128/jb.174.2.345-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihama A. Adaptation of gene expression in stationary phase bacteria. Current Opin Gen Dev. 1997;7:582–588. doi: 10.1016/S0959-437X(97)80003-2. [DOI] [PubMed] [Google Scholar]
- Minton KW. DNA repair in the extremely radioresistant bacterium Deinococcus radiodurans. Mol Microbiol. 1994;13:9–15. doi: 10.1111/j.1365-2958.1994.tb00397.x. [DOI] [PubMed] [Google Scholar]
- Puntschart A, Vogt M. A stochastic PCR approach for RNA quantification in multiple samples. In: Kochanowski B and Reischl U, editor. Methods in Molecular Medicine: Quantitative PCR Protocols. Vol. 26. Totowa, NJ, Humana Press; 1999. pp. 277–287. [DOI] [PubMed] [Google Scholar]
- Wen X, Fuhrman S, Michaels GS, Carr DB, Smith S, Barker JL, Somogyi R. Large-scale temporal gene expression mapping of central nervous system development. Proc Natl Acad Sci USA. 1998;95:334–339. doi: 10.1073/pnas.95.1.334. [DOI] [PMC free article] [PubMed] [Google Scholar]