Abstract
Background
With advances in multimodality therapy, colorectal cancer survivors are living longer. However, little is known about the quality of their long-term survival. We investigated the functional outcomes and symptoms among long-term survivors.
Methods
A cross-sectional study of 1,215 long-term (>5 years) colorectal cancer survivors was conducted using a validated disease-specific questionnaire. Younger-onset survivors (18–50 years) were matched 1:2 to later-onset survivors (> 50 years). Standardized mean scores were compared using one-way ANOVA. Key patient and treatment factors that impact function and symptoms were assessed by multivariate linear regression.
Results
830 survivors responded at an interval of 10.8±3 years from diagnosis (68% response rate). Younger-onset survivors underwent more surgery (97.9% vs 93.6%, P<0.001) and received more chemotherapy (86.1% vs. 77.7%, P=0.004). Anxiety, body image, sexual dysfunction, embarrassment by bowel movements, micturition problems, and impotence were significant concerns. Younger-onset survivors reported worse anxiety, body image, and embarrassment with bowel movements, whereas later-onset survivors highlighted sexual dysfunction, micturition problems, and impotence. Age-at-diagnosis was a key independent determinant of long-term function and symptoms.
Conclusion
Long-term survivors of CRC face ongoing functional deficits and symptoms, and their survivorship experience differs by age. Age-at-diagnosis should serve as a basis for tailored, personalized survivorship care plans.
Keywords: colorectal adenocarcinoma, quality of life, survivorship
INTRODUCTION
Colorectal cancer (CRC) is the third most commonly diagnosed and third leading cause of cancer death in men and women in the United States1. Both the incidence and mortality rates of CRC have decreased owing largely to population-based screening2–4. However, while the overall incidence of CRC decreased 3.0% per year in men and 2.4% per year in women between 1998 and 20063, the incidence of CRC in young adults (<50 years of age) has persistently increased1,5–7. Indeed, it is estimated that by 2030, 1 in 10 new cases of colon cancer and 1 in 4 new cases of rectal cancer will be diagnosed in these young adults7.
Multimodality therapy for CRC often includes extensive abdomino-pelvic surgery, radiation therapy, and chemotherapy. Surgical treatment can alter bowel function and may lead to sphincter loss and permanent ostomies. Additional concerns related to chemoradiation may include urinary and sexual dysfunction, perianal disorders, and stricture formation. These symptoms and functional concerns can lead to alterations in diet, clothing, work, travel, and social relationships, and thus impact the patients’ quality of life (QoL)8–10.
Several studies have documented significant sequelae in physical and functional symptoms at 1, 2 and 4 years after CRC treatment11–14. However, these studies only focused on shorter-term survivors within the immediate years after cancer treatment. Moreover, the majority of patients examined in these studies were older than 50 years of age. It is thus unknown if the previously reported findings would also be observed among long-term (>5 years) survivors or among the growing numbers of young adults diagnosed with CRC. Indeed, because young adults are more likely to present with more advanced disease, and more likely to receive more extensive multimodality therapy compared to patients diagnosed later in life, they may face unique issues during their cancer survivorship6,15. Therefore, we aimed to fill these gaps in knowledge by investigating the functional outcomes and symptoms reported by long-term CRC survivors. We specifically examined for differences by age-at-disease diagnosis, as well as other patient and treatment-related factors that may be key independent determinants of long-term functional outcomes.
METHODS
Study Design and Population
The University of Texas MD Anderson Cancer Center CRC registry was queried to identify all CRC survivors who were alive at least 5 years from their initial diagnosis. Patients with no active address or who declined future contact for research were excluded. To enrich for long-term survivors who had been diagnosed during young adulthood (YS, age 18–50 years), they were matched at a 1:2 ratio to survivors diagnosed during later adulthood (LS, age >50 years) for tumor site (colon vs. rectum). Medical records were retrospectively reviewed for patient demographics, as well as disease and treatment details, including surgery, chemotherapy, and radiation therapy. This study was approved by the Institutional Review Board.
Survey Instrument and Procedure
All eligible patients were mailed a generic cancer survivor survey16 and a disease-specific survey17. Both were self-administered, validated instruments, and we herein report findings from the disease-specific survey. To maximize survey response rates, initial non-responders were contacted by up to two subsequent mailings as well as a remainder telephone call.
The European Organization for Research and Treatment of Cancer CRC module (EORTC CR 29) is a validated disease-specific instrument specifically developed to measure health-related QoL in patients with CRC17. The EORTC CRC 29 consists of 29 single items which are divided into 4 function domains (anxiety, body image, male sexual function, and female sexual function) and 13 symptom domains (micturition problems, abdominal and pelvic pain, defecation problems, fecal incontinence, bloated feeling, dry mouth, hair loss, trouble with taste, sore skin, embarrassment with bowel movements, stoma-related problems, impotence, and dyspareunia). Patients were asked to report how frequently they experienced each concern over the past four weeks. Each single item is scored on a 4-point scale ranging from 1 (‘not at all’) to 4 (‘very much’).
Statistical Analysis
Patient, disease, and treatment factors for all survivors were described using summary statistics. They were compared between YS and LS by Student’s t-test for continuous variables and chi-square test for categorical variables.
A domain score was calculated for the 4 functional and 13 symptom domains from single item scores according to the EORTC CR 29 Scoring Manual. Each domain score was standardized by linear transformation and ranged from 0 to 100. A higher score in the functioning domains indicates better function, but a higher score in the symptom domains indicates more severe symptomatology. The standardized domain scores were compared between YS and LS using one-way ANOVA. For single item score analysis, we defined a priori that an item score of 3 (‘quite a bit’) or 4 (‘very much) indicated significant impact on long-term survivorship. The proportion of YS versus LS respondents indicating significant impact was compared using chi-square test.
Multivariate linear regression analysis was used to identify independent determinants of each domain score, while controlling for the potential confounding effect of patient demographics, tumor factors, and treatment-related factors including age at diagnosis, gender, race, site of disease, extent of disease at presentation, surgery, chemotherapy, radiation therapy, ostomy, and current active cancer. All reported P-values are two-sided and considered significant at the 0.05 level. We used STATA version 12 (Stata Corp, College Station, TX, USA) for statistical analyses.
Results
A total of 1,216 patients (415 YS and 801 LS) met inclusion criteria. Eight hundred thirty patients (282 YS and 548 LS) completed and returned their survey. The response rate was 68% overall and was the same for YS and LS. The mean time between CRC diagnosis and completion of the survey was 10.8 years for the entire cohort, 10.6 for YS, and 10.9 years for LS. Non-responders were more likely to be female (50.5% vs. 43.7%, P=0.027) and non-White (27.5% vs. 16.3%, P<0.001) and less likely to undergo surgical resection (91.5% vs. 95.0%, P=0.0143), receive chemotherapy (58.5% vs. 80.6%, P<0.001), and receive radiation therapy (29.8% vs. 53.6%, P<0.001) compared to responders. There was no difference in the age-at-diagnosis between non-responders and responders.
Patient Characteristics
Respondents represent a wide spectrum of demographic characteristics, disease stage at diagnosis, as well as treatments received (Table 1). When YS and LS were compared, they differed in mean age at diagnosis of CRC by design (YS 43.4 years vs. LS 62.6 years, P<0.001). YS were more likely to be female and non-White. Tumor site was well matched between the two groups. YS were more likely to present with regional and metastatic disease (54.9% vs. 33.4%, P<0.001) and receive chemotherapy (86.2% vs. 77.7%, P=0.004) compared to LS. There was no difference in receipt of radiation therapy, presence of a permanent ostomy, or presence of current active cancer between YS and LS.
Table 1.
Baseline patient, disease, and treatment characteristics.
| Characteristics | All Responders (N=830) |
Young-Onset Survivors (N=282) |
Late-Onset Survivors (N=548) |
P-value |
|---|---|---|---|---|
| Mean Age at Diagnosis | 56.1 ± 11.5 | 43.4 ± 6.1 | 62.6 ± 7.5 | <0.001 |
| Gender | ||||
| Male | 467 (56.3) | 143 (50.7) | 328 (59.9) | 0.004 |
| Female | 363 (43.7) | 139 (49.3) | 220 (40.1) | |
| Race | ||||
| White | 695 (83.7) | 226 (80.1) | 469 (85.6) | 0.008 |
| Hispanic | 57 (6.9) | 31 (11.0) | 26 (4.7) | |
| Black | 57 (6.9) | 17 (6.0) | 40 (7.3) | |
| Other | 21 (2.5) | 8 (2.8) | 13 (2.4) | |
| Tumor Site | ||||
| Colon | 385 (46.4) | 137 (48.6) | 248 (45.3) | 0.592 |
| Rectosigmoid/Rectum | 433 (52.2) | 142 (50.4) | 291 (53.1) | |
| Multiple Sites or Not Specified | 12 (1.4) | 3 (1.1) | 9 (1.6) | |
| Extent of Disease | ||||
| Localized | 415 (50) | 112 (39.7) | 303 (55.3) | <0.001 |
| Regional | 258 (31.1) | 114 (40.4) | 144 (26.3) | |
| Metastatic | 80 (9.6) | 41 (14.5) | 39 (7.1) | |
| Unknown | 77 (9.3) | 15 (5.3) | 62 (11.3) | |
| Surgery | ||||
| No | 32 (3.9) | 6 (2.1) | 35 (6.4) | <0.001 |
| Yes | 798 (96.1) | 276 (97.9) | 513 (93.6) | |
| Chemotherapy | ||||
| No | 161 (19.4) | 39 (13.8) | 122 (22.3) | 0.004 |
| Yes | 669 (80.6) | 243 (86.2) | 426 (77.7) | |
| Radiation | ||||
| No | 385 (46.4) | 135 (47.9) | 250 (45.6) | 0.538 |
| Yes | 445 (53.6) | 147 (52.1) | 298 (54.4) | |
| Permanent Ostomy | ||||
| No | 696 (83.9) | 236 (83.7) | 460 (83.9) | 0.607 |
| Yes | 124 (14.9) | 44 (15.6) | 80 (14.6) | |
| Unknown | 10 (1.2) | 2 (0.7) | 8 (1.5) | |
| Current Active Cancer | ||||
| No | 748 (90.1) | 258 (91.5) | 490 (89.4) | 0.343 |
| Yes | 82 (9.9) | 24 (8.5) | 58 (10.6) |
Functional Outcomes and Symptoms among Long-term CRC Survivors
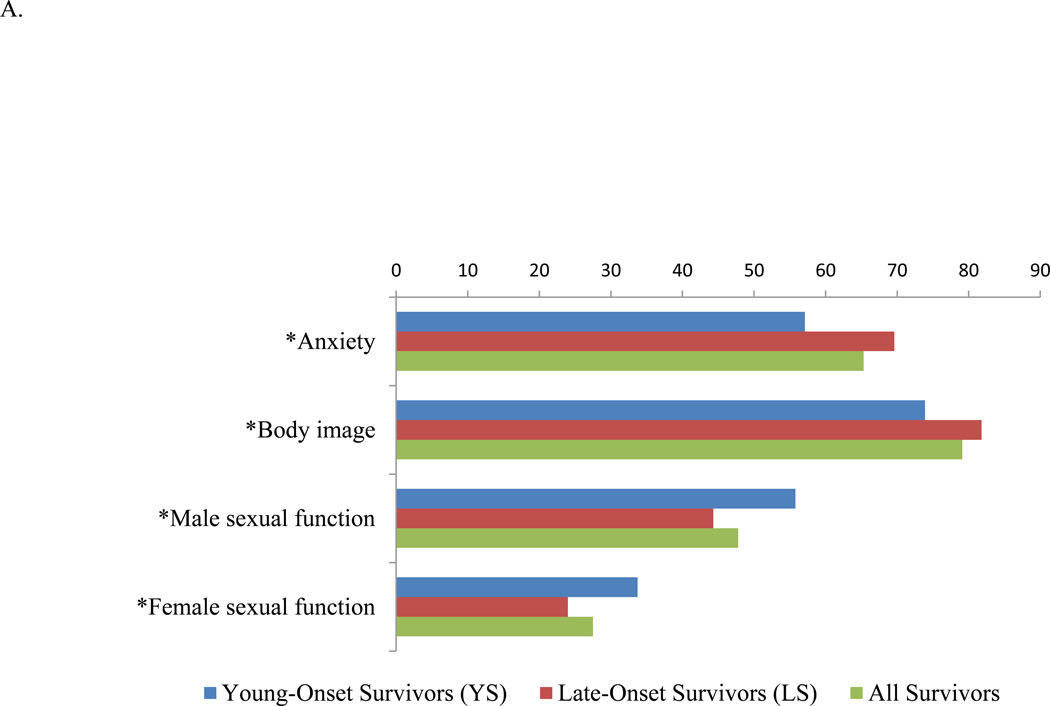
The standardized mean scores for the four functional domains demonstrated lowest scores for all survivors in sexual function (correlating with sexual dysfunction), followed by anxiety and body image (Figure 1A). Young adults were more troubled by worse anxiety (57.1 vs. 69.6, P<0.001) and poor body image perception (73.9 vs. 81.8, P<0.001) compared to LS. Both male and female sexual dysfunction was worse among LS compared to YS (male: 44.3 vs. 55.8, P=0.002; female: 24.0 vs. 33.7, P=0.014).
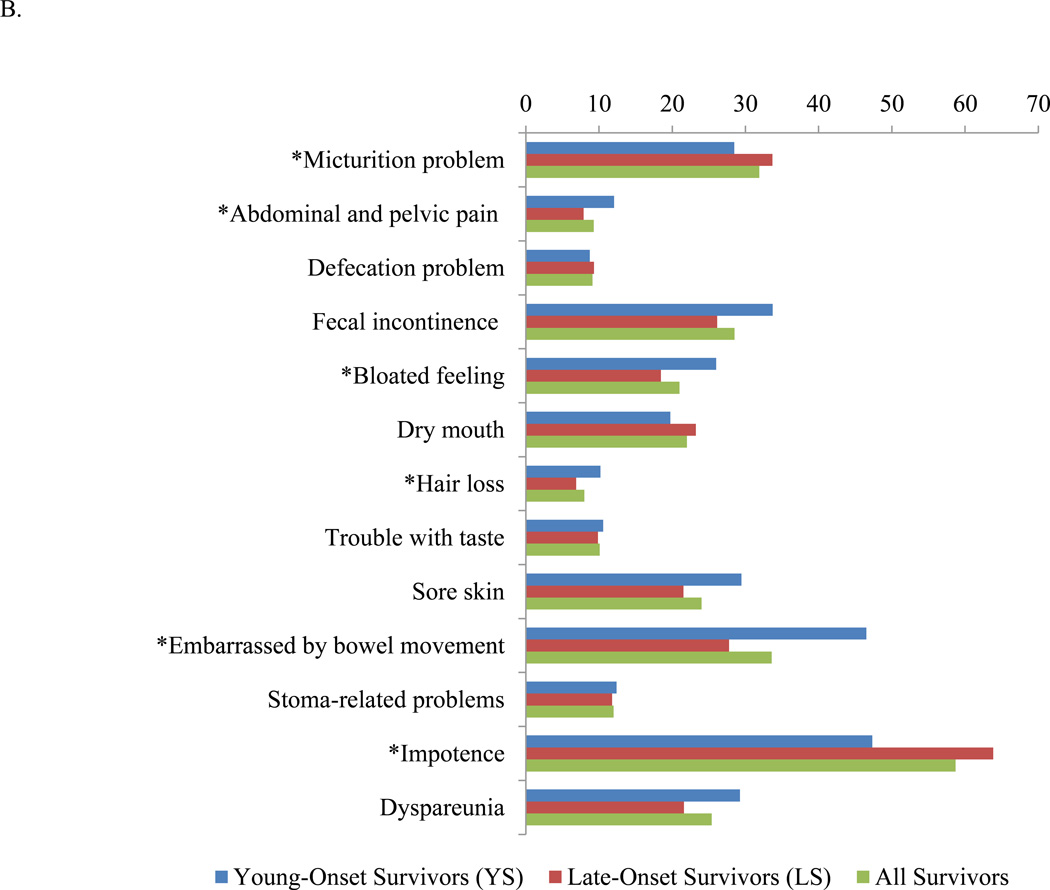
Figure 1.
Results of EORTC CR29. A. Functional domains. A higher functional scale score indicates better functioning. B. Symptom domains. A higher symptoms scale score indicates a higher level of symptomatology. EORTC CR29 = European Organization for Research and Treatment of Cancer Colorectal Cancer module. *P<0.05.
The standardized mean scores for the 13 symptom domains revealed that impotence, embarrassment by bowel movements, micturition problems, fecal incontinence, and dyspareunia are the five most common symptoms reported by all survivors (Figure 1B). Among the 13 symptoms assessed, YS differed from LS in that YS reported more abdominal and pelvic pain (12.1 vs. 7.9, P<0.001), bloated feeling (26.0 vs. 18.4, P=0.0002), hair loss (10.2 vs. 6.9, P=0.030), and embarrassment with bowel movements (46.5 vs. 27.8, P=0.002) compared to LS. LS reported more problems with micturition (33.7 vs. 28.5, P=0.002) and impotence (63.8 vs. 47.3, P<0.001) compared to YS. The scores for defecation problems, fecal incontinence, dry mouth, trouble with taste, sore skin, stoma-related problems, and dyspareunia did not significantly differ by age.
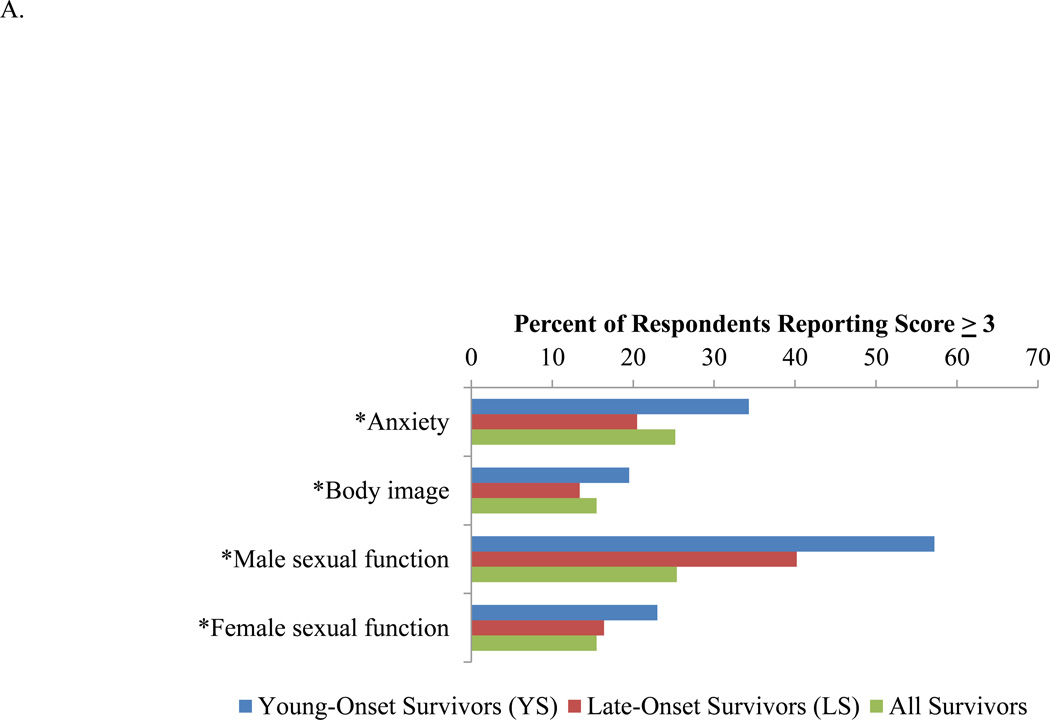
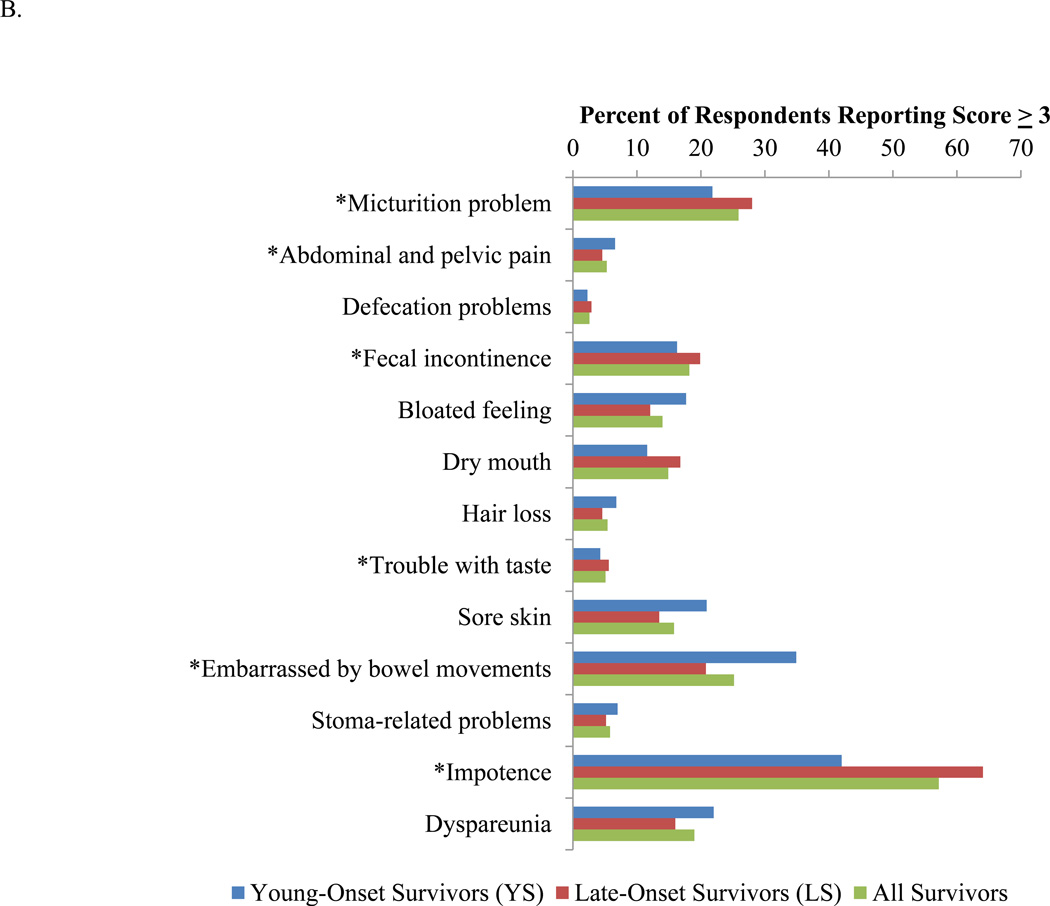
The percentages of patients who were significantly impacted (defined by reporting a score of 3 ‘quite a bit’ or 4 ‘very much’ for the single items) by functional deficits and symptoms are illustrated in Figure 2. Among the functional domains, the greatest proportion of survivors reported significantly impacted sexual function. Of all survivors, 15.5% and 25.2% were significantly affected by anxiety and body image concerns respectively (Figure 2A). These latter issues disproportionately affected YS. Among the symptom domains, at least 15% of all survivors reported that they were significantly impacted by micturition problems, fecal incontinence, sore skin, embarrassment with bowel movements, impotence and dyspareunia (Figure 2B). Greater proportions of YS complained of sore skin, embarrassment with bowel movements, and dyspareunia, whereas greater proportions of LS complained of problems with micturition, fecal incontinence, and impotence.
Figure 2.
Percentage of patients who recorded a score of 3 or higher for the single items in each domain. A. Functional domains. B. Symptom domains. *P<0.05.
Independent Determinants of Long-term Functional Outcomes and Symptoms
Among those functional deficits and symptoms noted to be significantly impactful, key independent determinants of the functional and symptom domain scores differed by the specific domain examined (Table 2). However, age-at-disease onset was an independent determinant in all 4 function and 4 of the 6 significantly impacted symptom domains. Specifically, younger age-at-disease onset was independently associated with more anxiety, worse body image perception, sore skin, and embarrassment with bowel movements among long-term CRC survivors. On the other hand, later age was independently associated with male and female sexual dysfunction, micturition problems, and impotence.
Table 2.
Independent significant factors influencing significantly impacted functional and symptom domain scores among long-term colorectal cancer survivors.
| Domain | Factors | Coefficient | 95% CI | |
|---|---|---|---|---|
| Functional Domainsa | ||||
| Anxiety | Age < 50 | −10.14 | −14.05 | −6.23 |
| Female gender | −5.52 | −14.11 | −6.70 | |
| Active cancer | −2.05 | −12.51 | −0.28 | |
| Metastatic disease | −6.77 | −13.49 | −0.04 | |
| Body Image | Age < 50 | −7.60 | −11.29 | −3.92 |
| Female gender | −5.15 | −8.65 | −1.67 | |
| Ostomy | −14.04 | −19.15 | −8.91 | |
| Active cancer | −8.59 | −14.41 | −2.77 | |
| Male Sexual Function | Age > 50 | −11.03 | −17.11 | −4.94 |
| Female Sexual Function | Age > 50 | −9.53 | −16.22 | −2.83 |
| Metastatic disease | −14.33 | −25.19 | −3.48 | |
| Symptom Domainsb | ||||
| Micturition problem | Age > 50 | 6.20 | 2.77 | 9.63 |
| Male gender | −6.30 | −9.56 | −3.03 | |
| Ostomy | 8.50 | 3.70 | 13.31 | |
| Fecal incontinence | Regional disease | −16.36 | −30.85 | −1.86 |
| Sore skin | Age < 50 | 10.23 | 0.03 | 20.43 |
| Hispanic | −22.53 | −41.82 | −3.24 | |
| Radiation therapy | −21.95 | −43.94 | 0.04 | |
| Embarrassed by bowel movements | Age < 50 | 18.47 | 5.89 | 31.01 |
| Other race | 38.97 | 0.67 | 77.56 | |
| Impotence | Age > 50 | 17.47 | 9.64 | 25.29 |
| Radiation therapy | 12.93 | 2.63 | 23.22 | |
| Active cancer | 12.64 | 1.20 | 24.08 | |
| Dyspareunia | Black race | −18.45 | −31.61 | −5.29 |
| Ostomy | 15.85 | 1.30 | 30.39 | |
| Regional disease | 6.91 | 1.03 | 22.56 | |
Abbreviations: CI, confidence interval
A higher functional domain score indicates better function. A negative coefficient indicates worse function, whereas a positive coefficient indicates better function.
A higher symptom domain score indicates worse symptomatology. A negative coefficient indicates fewer symptoms, whereas a positive coefficient indicates more symptoms.
Discussion
Multimodality therapy for CRC has improved the length of survival among CRC patients, however, it is often associated with adverse effects. As the number of cancer survivors continues to increase, improving the quality of cancer survivorship has emerged as a new priority in cancer care18,19. Survivors of CRC represent approximately 10% of all cancer survivors, but the residual functional outcomes and symptoms among long-term survivors have not been documented18. We measured these outcomes in 830 patients who had survived an average of 10.6 years after their initial CRC diagnosis and treatment using a validated disease-specific instrument and demonstrated that ongoing deficits in function and symptoms among long-term CRC survivors differed by age-at-diagnosis. YS reported more anxiety and worse body image perception, while LS had more concerns about sexual dysfunction. YS also experienced more abdominal and pelvic pain, bloating, hair loss, and embarrassment with bowel movements, while LS reported more problems with micturition and impotence. Because the initial age at cancer diagnosis was a key determinant for long-term CRC survivor experience, it should be strongly considered as a basis for designing tailored and individualized survivorship care programs.
Functional outcomes and symptoms experienced daily by long-term cancer survivors contribute to the quality of their survivorship experience. Increasing attention is being paid to the care of the growing number of survivors. Indeed, “Principles of Survivorship Care” has been a part of the National Comprehensive Cancer Network Clinical Practice Guidelines for Colon and Rectal Cancer since 200920. Investigating functional outcomes and symptoms among long-term CRC survivors allows for the identification of chronic and late effects of cancers and their treatments. Prior studies primarily focused on patients with rectal cancer and patients alive within 5 years of diagnosis12,21,22. Importantly, we herein have investigated patients with both colon and rectal cancer alive within at least 10 years of diagnosis. In previous reports, long-term CRC survivors experienced bowel problems (i.e. diarrhea and constipation), depression, poor body image perception, pain, and sexual dysfunction22–25. In our study, we not only corroborated these findings but further highlight that significant concerns for poor body image perception, pain, and sexual dysfunction do persist in the long-term, even 10 years after treatment. These findings indicate that surgeons and other physicians should be cognizant of the long-term impact of oncologic diagnosis and treatments to patients. Such an understanding can help shape counseling discussions even at the time of initial diagnosis and can facilitate management of patient expectations. The deficits and concerns documented herein should also motivate proactive interventions and supportive care for cancer survivors. For example, for patients experiencing sexual dysfunction and impotence, urologic, gynecologic and/or psychiatric referral may be appropriate for counseling and considerations for medical/hormonal therapy and assistive devices; urodynamic studies may be appropriate for patients with micturition problems; behavioral intervention and cognitive therapy may be appropriate for patients with body image concerns; and early referral to a pain clinic may be appropriate for patients with significant abdominal or pelvic pain. Currently, the American Society of Clinical Oncology recommends that all cancer survivors be periodically evaluated for symptoms of depression and anxiety using validated measures. Depending on the level of symptomatology, patients can be referred for support care services or consultation to a psychologist or psychiatrist for further treatment26.
Indeed, two important epidemiologic trends have recently emerged: first, CRC is being increasingly diagnosed among young adults between the ages of 18 to 50, prior to the age to commence average-risk screening for CRC in the United States5–7; secondly, nearly one third of all cancer survivors are adults between the ages of 18 and 5019. Unfortunately, to date, no study has specifically investigated the long-term functional deficits and symptoms experienced by young adults treated for CRC. Using EORTC CRC 29, a validated instrument specifically designed to evaluate functional outcomes in CRC patients17, we were able to document for the first time specific concerns of young adults. We found that the patterns of functional deficits and symptoms differed significantly by age. For example, YS were more likely to experience poor body image perception and complain of more abdominal and pelvic pain compared to LS, whereas LS reported worse sexual function compared to YS. We did not detect a difference in bowel problems (i.e. fecal incontinence and defecation problems) in our study and this may reflect that both YS and LS had largely adapted to new bowel patterns by 10 years post-treatment. Taken together, our findings indicate that increasing attention must be paid to young adults with CRC. Age should be considered while developing individualized survivorship care plans, and a tailored approach should be used to address age-specific concerns. Indeed, as patients survive into the long-term, patient age, rather than details related to their treatment, have arisen as key determinants of daily function and symptoms.
Our study includes the largest cohort of long-term CRC survivors stratified by age specifically evaluating functional and symptoms outcomes, but it remains limited in several aspects. First, as a first look at patients with long-term CRC survivorship, it was a cross-sectional study by design. Changes in patient-reported outcomes that can occur over time were not evaluated and are being investigated in an ongoing prospective study. Second, we recognize that some of the age-related disparities in our study appear to follow general trends that may be expected in the general population. Specifically, our later-onset survivors reported worse sexual function and more problems with micturition and impotence. Nonetheless, beyond relative trends, we highlight the high absolute proportions of patients significantly impacted by these symptoms. For example, in our study, 42% (almost 1 in 2) of young adult men and 63.2% (almost 2 in 3) of older adult men complained of impotence. These numbers are significantly higher than previously reported rates of approximately 5% of men ≤40 years of age and 15–25% of men ≥65 years of age experiencing impotence in the general population27. The lack of a normal population control in our survey specifically designed to assess the needs of long-term cancer survivors preclude a formal comparison with the population norm. Third, the aim of the current study is limited to reporting prevalence of symptoms to identify target areas for potential intervention to improve the current survivor experience. The EORTC CRC 29 questionnaire queries symptoms over the past 4 weeks but does not query symptoms that might have been present prior to cancer diagnosis. Therefore, the current study is limited in its lack of ability to assess the temporal trends of reported functional deficits. Fourth, our findings may be impacted by several confounders related to disease site and type of surgery. Indeed, our subgroup analysis found that patients with rectal cancer were troubled by poorer body image perception compared to patients with colon cancer; they also reported more problems with micturition, abdominal and pelvic pain, impotence, and dyspareunia. However, it is important to note that neither disease site nor type of surgery exerted an independent impact on functional outcomes in the long-term (>10 years) as demonstrated in our multivariate analysis. Indeed, patient-related factors such as age of diagnosis was a key independent determinant of our findings. Lastly, although we were able to achieve a remarkable 68% response rate among patients who were several years away from their initial treatments through meticulous survey procedures, demographic characteristics differed between responders and non-responders. Non-responders were more likely to be Hispanic and female, whereas responders were more likely to undergo surgical resection, receive chemotherapy, and receive radiation therapy. We were unable to measure the magnitude of the impact of potential responder bias.
In conclusion, long-term CRC survivors face ongoing deficits in function and symptoms. The initial age at CRC diagnosis significantly impacted patient-reported experiences and may be associated with long-term coping. It is important to be aware of the difference in functional outcomes and symptoms experienced by both young and old CRC survivors in order to tailor survivorship care. Providing quality survivorship care is becoming increasingly important with improvements in CRC treatment and increasing number of CRC survivors. Designing tailored and age-appropriate survivorship care plan can improve long-term cancer survivorship experience. The potential efficacy of age-tailored interventions as components of personalized survivorship care need to be investigated in future studies.
Acknowledgments
Funding/Support: This work was supported in part by National Institutes of Health/National Cancer Institute Grant T32CA009599 (C.E.B.), the University of Texas MD Anderson Cancer Center Institutional Grant (Y.N.Y), G.S. Hogan Gastrointestinal Cancer Research Grant (Y.N.Y.), and University of Texas MD Anderson Cancer Center Core Support Grant P30 CA016672.
Footnotes
This work was presented at a plenary session of the 55th Annual Meeting of the Society for Surgery of the Alimentary Tract, May 4th, 2014, Chicago, IL.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA: a cancer journal for clinicians. 2014 Mar;64(2):104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013 Jan;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010 Feb 1;116(3):544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Annals of internal medicine. 2002 Jul 16;137(2):132–141. doi: 10.7326/0003-4819-137-2-200207160-00015. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009 Jun;18(6):1695–1698. doi: 10.1158/1055-9965.EPI-09-0186. [DOI] [PubMed] [Google Scholar]
- 6.You YN, Xing Y, Feig BW, Chang GJ, Cormier JN. Young-onset colorectal cancer: is it time to pay attention? Archives of internal medicine. 2012 Feb 13;172(3):287–289. doi: 10.1001/archinternmed.2011.602. [DOI] [PubMed] [Google Scholar]
- 7.Bailey CEHC, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, Cantor SB, Chang GJ. Increasing Disparities in Age-Related Incidence of Colon and Rectal Cancers in the United States, 1975–2010. Manuscript submitted for publication. 2014 doi: 10.1001/jamasurg.2014.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caffo O, Amichetti M, Romano M, Maluta S, Tomio L, Galligioni E. Evaluation of toxicity and quality of life using a diary card during postoperative radiotherapy for rectal cancer. Diseases of the colon and rectum. 2002 Apr;45(4):459–465. doi: 10.1007/s10350-004-6220-2. discussion 465-457. [DOI] [PubMed] [Google Scholar]
- 9.Hendren SK, O'Connor BI, Liu M, et al. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Annals of surgery. 2005 Aug;242(2):212–223. doi: 10.1097/01.sla.0000171299.43954.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peeters ST, Heemsbergen WD, van Putten WL, et al. Acute and late complications after radiotherapy for prostate cancer: results of a multicenter randomized trial comparing 68 Gy to 78 Gy. International journal of radiation oncology, biology, physics. 2005 Mar 15;61(4):1019–1034. doi: 10.1016/j.ijrobp.2004.07.715. [DOI] [PubMed] [Google Scholar]
- 11.Camilleri-Brennan J, Steele RJ. Prospective analysis of quality of life and survival following mesorectal excision for rectal cancer. The British journal of surgery. 2001 Dec;88(12):1617–1622. doi: 10.1046/j.0007-1323.2001.01933.x. [DOI] [PubMed] [Google Scholar]
- 12.Engel J, Kerr J, Schlesinger-Raab A, Eckel R, Sauer H, Holzel D. Quality of life in rectal cancer patients: a four-year prospective study. Annals of surgery. 2003 Aug;238(2):203–213. doi: 10.1097/01.sla.0000080823.38569.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rauch P, Miny J, Conroy T, Neyton L, Guillemin F. Quality of life among disease-free survivors of rectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004 Jan 15;22(2):354–360. doi: 10.1200/JCO.2004.03.137. [DOI] [PubMed] [Google Scholar]
- 14.Schneider EC, Malin JL, Kahn KL, Ko CY, Adams J, Epstein AM. Surviving colorectal cancer : patient-reported symptoms 4 years after diagnosis. Cancer. 2007 Nov 1;110(9):2075–2082. doi: 10.1002/cncr.23021. [DOI] [PubMed] [Google Scholar]
- 15.You YN, Dozois EJ, Boardman LA, Aakre J, Huebner M, Larson DW. Young-onset rectal cancer: presentation, pattern of care and long-term oncologic outcomes compared to a matched older-onset cohort. Annals of surgical oncology. 2011 Sep;18(9):2469–2476. doi: 10.1245/s10434-011-1674-7. [DOI] [PubMed] [Google Scholar]
- 16.Avis NE, Smith KW, McGraw S, Smith RG, Petronis VM, Carver CS. Assessing quality of life in adult cancer survivors (QLACS) Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2005 May;14(4):1007–1023. doi: 10.1007/s11136-004-2147-2. [DOI] [PubMed] [Google Scholar]
- 17.Whistance RN, Conroy T, Chie W, et al. Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. European journal of cancer. 2009 Nov;45(17):3017–3026. doi: 10.1016/j.ejca.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA: a cancer journal for clinicians. 2012 Jul-Aug;62(4):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 19.Hewitt MGS, Stovall EE. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 20.Engstrom PF, Arnoletti JP, Benson AB, 3rd, et al. NCCN Clinical Practice Guidelines in Oncology: colon cancer. Journal of the National Comprehensive Cancer Network : JNCCN. 2009 Sep;7(8):778–831. doi: 10.6004/jnccn.2009.0056. [DOI] [PubMed] [Google Scholar]
- 21.Camilleri-Brennan J, Steele RJ. Objective assessment of morbidity and quality of life after surgery for low rectal cancer. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2002 Jan;4(1):61–66. doi: 10.1046/j.1463-1318.2002.00300.x. [DOI] [PubMed] [Google Scholar]
- 22.Fucini C, Gattai R, Urena C, Bandettini L, Elbetti C. Quality of life among five-year survivors after treatment for very low rectal cancer with or without a permanent abdominal stoma. Annals of surgical oncology. 2008 Apr;15(4):1099–1106. doi: 10.1245/s10434-007-9748-2. [DOI] [PubMed] [Google Scholar]
- 23.Ramsey SD, Berry K, Moinpour C, Giedzinska A, Andersen MR. Quality of life in long term survivors of colorectal cancer. The American journal of gastroenterology. 2002 May;97(5):1228–1234. doi: 10.1111/j.1572-0241.2002.05694.x. [DOI] [PubMed] [Google Scholar]
- 24.Krouse RS, Herrinton LJ, Grant M, et al. Health-related quality of life among long-term rectal cancer survivors with an ostomy: manifestations by sex. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Oct 1;27(28):4664–4670. doi: 10.1200/JCO.2008.20.9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phipps E, Braitman LE, Stites S, Leighton JC. Quality of life and symptom attribution in long-term colon cancer survivors. Journal of evaluation in clinical practice. 2008 Apr;14(2):254–258. doi: 10.1111/j.1365-2753.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- 26.Andersen BL, Derubeis RJ, Berman BS, et al. Screening, Assessment, and Care of Anxiety and Depressive Symptoms in Adults With Cancer: An American Society of Clinical Oncology Guideline Adaptation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Apr 14; doi: 10.1200/JCO.2013.52.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA: the journal of the American Medical Association. 1993 Jul 7;270(1):83–90. [PubMed] [Google Scholar]