Abstract
Background
A number of heritable immune dysregulatory diseases result from defects affecting T regulatory (TR) cell development and/or function. They include Immune dysregulation, Polyendocrinopathy, Enteropathy, X-Linked (IPEX), due to mutations in FOXP3, and IPEX-like disorders caused by mutations in IL2RA, STAT5b and STAT1. However, the genetic defects underlying many cases of IPEX-like disorders remain unknown.
Objective
We sought to identify the genetic abnormalities in subjects with idiopathic IPEX-like disorders.
Methods
We performed whole exome and targeted gene sequencing, and phenotypic and functional analyses of TR cells.
Results
A child who presented with an IPEX-like syndrome and severe TR cell deficiency was found to harbor a nonsense mutation in the gene encoding LPS-responsive beige-like anchor (LRBA), previously implicated as cause of common variable immunodeficiency with autoimmunity. Analysis of subjects with LRBA deficiency revealed marked TR cell depletion, profoundly decreased expression of canonical TR cell markers, including FOXP3, CD25, Helios, and CTLA4 and impaired TR cell-mediated suppression. There was skewing in favor of memory T cells and intense autoantibody production with marked expansion of T follicular helper and contraction of T follicular regulatory cells. Whereas the frequency of recent thymic emigrants and the differentiation of induced TR cells were normal, LRBA-deficient T cells exhibited increased apoptosis and reduced activities of the metabolic sensors mammalian target of rapamycin 1 and 2 complexes.
Conclusion
LRBA deficiency is a novel cause of IPEX-like syndrome and TR cell deficiency associated with metabolic dysfunction and increased apoptosis of TR cells.
Keywords: Autoantibodies, Autoimmunity, FOXP3, IPEX, LRBA, mTOR, T Follicular Helper Cells, T Follicular Regulatory Cells, T Regulatory Cells
Introduction
A number of Mendelian disorders of immune dysregulation and autoimmunity result from defects affecting T regulatory (TR) cell development and function 1. The best characterized of these is Immune dysregulation, Polyendocrinopathy, Enteropathy, X-linked (IPEX), resulting from deleterious mutations affecting FOXP3 2–4. Subjects with IPEX suffer from a plethora of autoimmune manifestations, including enteropathy, endocrinopathies especially type 1 diabetes and thyroiditis, and cytopenias 5, 6. FOXP3 directs the differentiation of TR cells and is essential for their suppressive functions 7–9. It reinforces and stabilizes transcriptional networks characteristic of TR cells and maintains TR cell phenotypic markers 10, 11. In the absence of a functional FOXP3 protein, the expression of these networks and markers is attenuated and/or disrupted.
In cohorts of subjects with an IPEX-like phenotype, up to half of the patients fail to exhibit mutations in FOXP3 5, 12. A number of other gene defects that affect TR cell function give rise to an IPEX-like phenotype 1. These include loss of function mutations in genes encoding components of the interleukin 2 receptor pathway, including IL2RA and STAT5b, and gain of function mutations in STAT1 1, 13–16. Nevertheless, defects underlying the majority of cases with IPEX-like phenotype remain uncharacterized.
To identify novel genetic causes of IPEX-like disorders, we performed whole exome sequencing (WES) of DNA from candidate subjects. A boy with an IPEX-like disorder, profound TR cell deficiency, and normal FOXP3 gene sequence was found to have a homozygous nonsense mutation in the LPS-responsive beige-like anchor (LRBA) gene, previously implicated as a cause of common variable immunodeficiency (CVID) with autoimmunity 17–19. Analysis of other subjects with LRBA deficiency also revealed TR cell deficiency, profoundly decreased canonical TR cell markers, and impaired TR cell suppressive function. Patients with LRBA deficiency manifested elevated autoantibodies against autologous antigens in association with a dramatic increase in the frequency of circulating T follicular helper T (TFH) cells and depressed circulating T follicular regulatory (TFR) cells. Our findings implicate LRBA deficiency as a cause of IPEX-like syndrome and suggest that TR cell dysfunction is a major contributor of the autoimmune manifestations in affected subjects.
Materials and Methods
Patients
Affected individuals with LRBA deficiency from 3 families were analyzed. The index cases of families A and B are described herein for the first time. Those of family C have been previously reported 18. A patient with IPEX due to an A384T amino acid substitution in FOXP3 was identified by WES (M. Garcia-Lloret and T. A. Chatila; manuscript in preparation). Subjects with the diagnosis of SLE were identified at the Rheumatology clinic at the Boston children’s Hospital. Control subjects were age group-matched. All study participants were recruited using written informed consent approved by the local Institutional Review Boards. Studies at the Boston Children’s Hospital were conducted under approved protocol #04-09-113R.
Antibodies and flow cytometry
Information on the antibodies employed is provided in the Online Methods in the Repository. Whole blood was incubated with mAbs against surface markers for 30 min on ice. Intracellular staining with FOXP3, CTLA4, Helios, Bcl6 and CD40L mAbs was performed using eBioscience Fixation/Permeabilization according to the manufacturer’s instructions. Intracellular staining for pS6, p4E-BP1 and pAKT (S473) were performed on freshly isolated peripheral blood mononuclear cells (PBMCs) either left untreated or stimulated with anti-CD2/3/28 mAb-coated beads (Miltenyi) for 4 or 12 hours using eBioscience Fixation/Permeabilization buffer. Annexin V (Biolegend) staining was performed on PBMCs using Biolegend Annexin V binding buffer according to the manufacturer’s instructions. Data were collected with a Fortessa cytometer (BD Biosciences) and analyzed with FlowJo software (TreeStar).
Whole exome sequencing (WES)
WES was performed on genomic DNA of the probands of families A and B, their parents, and their healthy siblings through Axeq, Inc. The Agilent SureSelect Target enrichment kit was used for exon capture (Agilent). Paired-end sequencing was performed using an Illumina HiSeq2000 instrument, which generated 100 base pair reads. Average coverage of the exome was 183-208x. Analysis of WES data was performed with MolBioLib 20. Variants were identified using Genome Analysis Toolkit with standard filtering parameters according to Genome Analysis Toolkit Best Practices Recommendations 21. Variant calls with a read coverage ≤2x and a Phred-scaled SNP quality ≤20 were eliminated.
Immunoblotting
Protein lysates derived from fibroblasts (patient P1) or lymphocytes (patients P2, P3, P5 and P6) were resolved on SDS-PAGE gels and transferred to nitrocellulose filters as described 22. Immunoblots were carried out using polyclonal rabbit anti-LRBA antibodies (Sigma-Aldrich, St. Louis, MO). The blots were reprobed with mouse anti-β-actin mAb (Clone C4) (Millipore, Billerica, MA).
Regulatory T cell Suppression Assays
PBMCs were isolated from whole blood by centrifugation over Ficoll-Hypaque gradients. CD4+ T cells were separated by negative selection using magnetic beads (Miltenyi). TR cells were purified via cell sorting for CD4+CD25+CD127low T cells. The suppressor function of TR cells of both patient and control subjects was tested against CD4+CD25− T effector (Teff) cells of a common third party donor. Teff cells were isolated by depleting donor PBMCs of CD25+ T cells using anti-CD25 beads (Miltenyi Biotec). They were labeled with CellTrace Violet dye (Life Technologies) to monitor cell divisions by flow cytometry, and resuspended in complete media (RPMI-1640 with 10% fetal calf serum, 10 µM HEPES buffer, 1 mM pyruvate, non-essential amino acids, and penicillin/streptomycin). The cells placed in round bottom wells of 96 tissue culture plates at 0.5-1×104 cells/well alone or together with anti-CD2/CD3/CD28 mAb-coated beads. TR cells were added at 1:1 ratio to Teff cells, as indicated. On day 4, the Teff cells were evaluated by flow cytometry for cell divisions as revealed by dye dilution. The percentage of Teff cells that had more than one division was determined. The percent suppression was calculated by dividing the percentage of divided Teff cells from each suppressed culture by the percentage of divided Teff cells in the non-suppressed cultures and subtracting this fraction from 1.
In vitro generation of induced regulatory T cells
CD4+ T cells were isolated from PBMCs by negative selection using magnetic beads (Miltenyi). Naïve CD3+CD4+CD25− CD45RO−CD45RA+ cells were purified from CD4+ T cells by cell sorting on a FACS ARIA (purity > 98%). Naïve CD4 T cells were resuspended in complete media and seeded at a concentration of 5X104/well of a 96-well plate and stimulated with anti-CD2/CD3/CD28 mAb-coated beads (Miltenyi) and 5ng/mL of recombinant human TGF-β1 (R&D system). After 4 days, intracellular staining for FOXP3 was performed.
Cytokine and autoantibody assays
For IL-10 assays, cell-sorted TR cells were seeded in round bottom wells at 5×103 cells/well and either left untreated or treated with anti-CD2/CD3/CD28 mAb-coated beads or with PMA/ionomycin (20 ng/ml and 1 µM, respectively). The supernatant were harvested 4 days later and analyzed for IL-10 by ELISA (eBioscience). For autoantibody detection, plasma aliquots from patient and control subjects were analyzed using microarrays spotted with 84 autoantigens (University of Texas Southwestern Medical Center, Genomic and Microarray Core Facility), as described 23. Data was normalized to healthy controls. Anti-nuclear antigens (ANAs) and dsDNA (double stranded DNA) antibodies were measured by enzyme-linked immunosorbant assay (ELISA) (Genway Biotech and Alpha Diagnostics).
Statistical Analysis
Aggregate results are presented as means ± standard error of the means (S.E.M.). Comparison between groups was carried out using Student’s unpaired two tailed t test and 2-way ANOVA with Bonferroni post-test analysis, as indicated. Differences in mean values were considered significant at a p < 0.05.
Results
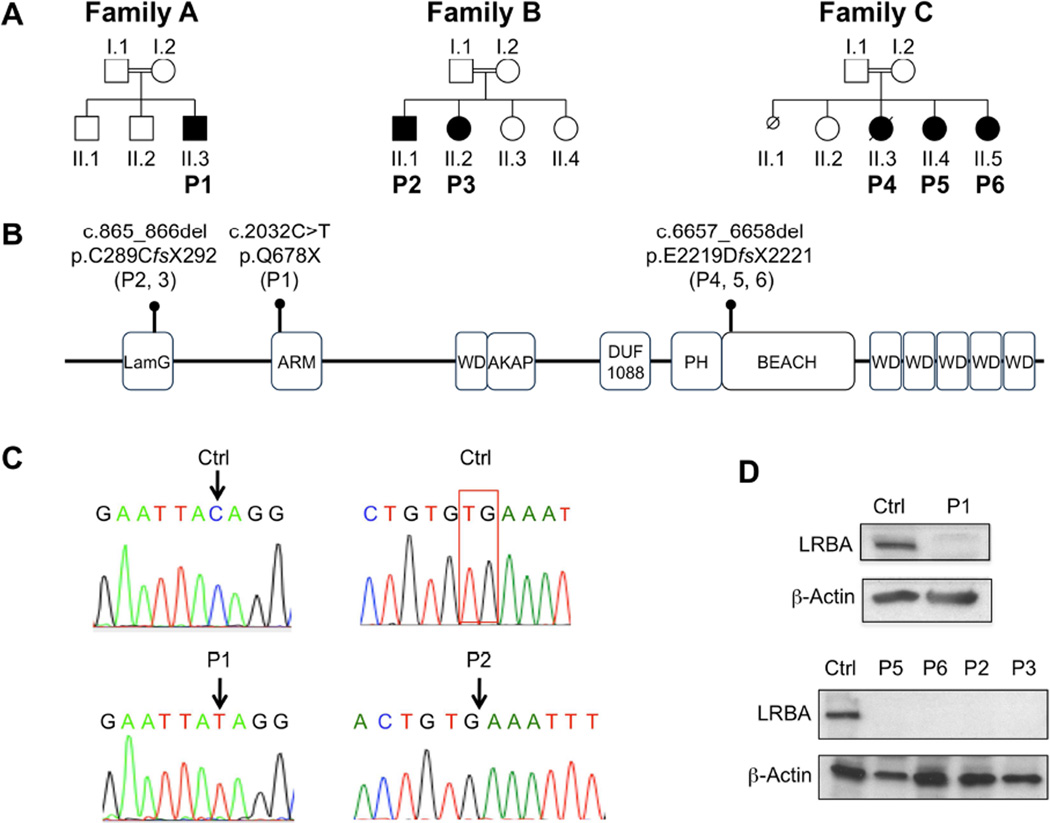
In family A (Figure 1 and Figure E1 in the Online Repository), the index case (P1) is a 2 year 10 month old Saudi Arabian boy who presented at the age of 3 months with protracted diarrhea following his first childhood vaccination. Evaluation was negative for celiac disease, and the pathology was consistent with an autoimmune enteropathy. Within a few weeks, he was diagnosed with type 1 diabetes with positive insulin antibodies and hypothyroidism with positive thyroid antibodies. He also developed a Coombs positive autoimmune hemolytic anemia. In addition, he suffered recurrent episodes of sepsis, including Pseudomonas aeruginosa septicemia and Streptococcus pneumoniae-related septic shock. Analysis of his immunological status revealed reduced T cell proliferative responses to phytohemagglutinin (~50% of normal), normal B cell numbers, and elevated immunoglobulin levels in the setting of low normal specific antibody responses (Table E1 in the Online Repository). Analysis of his FOXP3 gene sequence failed to reveal the presence of deleterious mutations. In view of his consanguineous heritage, we undertook WES to identify gene variants which were homozygous in the patient, heterozygous in his mother, and either heterozygous or absent in his healthy brother. This filtering approach identified 18 candidate variants which were non-synonymous, absent from dbSNP, and not present in the homozygous state in any of our 80 in-house Middle Eastern exomes (Table E2 in the Online Repository). While the majority of these variants scored benign by Polyphen and/or SIFT protein function prediction algorithms, the one variant that stood out in relation to its deleterious impact on the immune system involved LRBA 17–19. The patient suffered a homozygous c.2032 C>T substitution in exon 16 of LRBA, which encodes the third armadillo domain of LRBA, resulting in the replacement of amino acid glutamine 678 with a stop codon (Q678X) and in absent protein expression (Fig. 1A-D). The mutation was confirmed by Sanger sequencing, and both parents were heterozygous carriers of the mutation (Figure E1 in the Online Repository).
Figure 1.
A. Pedigrees of the LRBA-deficient families. Generations are designated by Roman numerals (I–II) and individuals by Arabic numerals. Double lines connecting parents indicate consanguinity. Probands in the respective families are indicated by P1-P6 labeling. Squares, males; circles, females; filled symbols, patients. B. Schematic representation of LRBA protein showing the respective domains and the location of patient mutations. C. Sanger sequencing flurograms showing genomic DNA mutations of patient P1 and P2 as compared to the equivalent DNA sequences of healthy controls (Ctrl). D. Western blot analysis of LRBA protein expression in fibroblasts of patient P1 and lymphocytes of patients P2, P3, P5, and P6 as compared to those of controls. The blots were reprobed for β-actin as a positive control.
LRBA deficiency was also found in a second newly described Lebanese family with two siblings (Family B; P2 and P3), whose clinical histories were notable for recurrent infections and immune dysregulation (Supplementary Text in the Online Repository) A homozygous exonic deletion in LRBA (c.865_866del) was the lead candidate variant identified by WES under the aforementioned filtering conditions (Table E3 in the Online Repository). It was confirmed by Sanger sequencing, and resulted in absent protein expression (Figure 1A-D). Both parents and the patients’ two clinically unaffected siblings (II.3, and II.4; Figure 1A) were heterozygous carriers of the mutation (Figure E2 in the Online Repository). We also studied three previously described Saudi Arabian siblings (P4, P5 and P6; Family C) with LRBA deficiency due to a homozygous deletion in the BEACH domain of LRBA that abolished protein expression (Figure 1A, B, D) 18. The clinical and laboratory findings of these patients are detailed in Table E1 in the Online Repository.
In view of their immunodysregulatory phenotypes, most notably the IPEX-like disease of patient P1, we examined our cohort of LRBA-deficient subjects for evidence of TR cell abnormalities. Flow cytometric analysis of peripheral blood TR cells of patient P1 demonstrated a markedly reduced number of CD4+FOXP3+ TR cells (Figure 2A). Analysis of the five other patients with LRBA deficiency revealed that they all share a profound decrease in TR cell frequency in the peripheral blood (controls: 7.54±0.64% vs. patients: 2.45±0.29%) (Figure 2B). Importantly, expression of several canonical TR cell markers, including FOXP3, CD25 (IL-2RA), CTLA-4, and Helios, was profoundly decreased in LRBA-deficient subjects relative to controls (Figure 2C). Thus LRBA deficiency was associated with decreased numbers and aberrant phenotype of TR cells.
Figure 2. LRBA deficiency leads to defect in TR cell frequency and phenotype.
A. Representative dot plot analysis of FOXP3 expression in patient P1 vs. a control subject. B. Percent CD4+FOXP3+ T cells in the peripheral blood of healthy controls (n=8; open circles) and LRBA deficient patients (n=6; closed circles). C. Representative flow cytometric histogram plots of FOXP3, CD25, CTLA4 and Helios expression in FOXP3+CD4+ T cells from controls (dotted line) and LRBA deficient patients (solid line). Gray area represents control isotype staining. D. Mean fluorescence intensity of the respective TR cell marker in patient and control subjects. ** p<0.01, *** p<0.001 by unpaired two-tailed Student's t-test.
We further analyzed the impact of LRBA deficiency on TR cell suppressive function using an in vitro suppression assay of T cell proliferation to mitogenic stimulation. TR cells were isolated by cell sorting of CD4+CD25+CD127low TR cells. They were confirmed by intracellular staining to be >90% positive for FOXP3, indicative of their TR cell lineage (data not shown). Equal numbers of patient and control TR cells, were added to an equal number of control CD4+CD25− Teff cells loaded with the proliferation dye CellTrace Violet and treated with a mitogenic combination of CD2/CD3/CD28 mAbs. LRBA-deficient TR cells manifested decreased suppression of T cell proliferation, measured by tracer dye dilution in target Teff cells, as compared to control TR cells, indicative of their impaired function (Figure 3A, B). Defective in vitro suppression by LRBA-deficient TR cells could not be ascribed to decreased IL-10 production, whose deficiency in TR cells is associated with colitis 24. IL-10 production was increased in patient as compared to control TR cells (Figure 3C).
Figure 3. LRBA-deficient TR cells have decreased in vitro suppressive ability.
A. Flow cytometric analysis of CellTrace Violet proliferation dye dilution in Teff (CD4+CD25−) cells stimulated with anti-CD2/CD3/CD28 mAb-coated bead in the absence or presence of TR cells (CD4+CD25+CD127low) from healthy controls or LRBA deficient patients. B. Cumulative Results of in vitro suppression assays in patient and control subjects. N=6 individuals per group, ****p<0.0001 by unpaired two-tailed Student's t-test. C. IL-10 production in patient and control TR cells at baseline and following stimulation with the aCD2/CD3/CD28 or PMA + Ionomycin (Iono) for 4 days. N.D.: Not detected. *P<0.05 by 2-way ANOVA with post-test analysis.
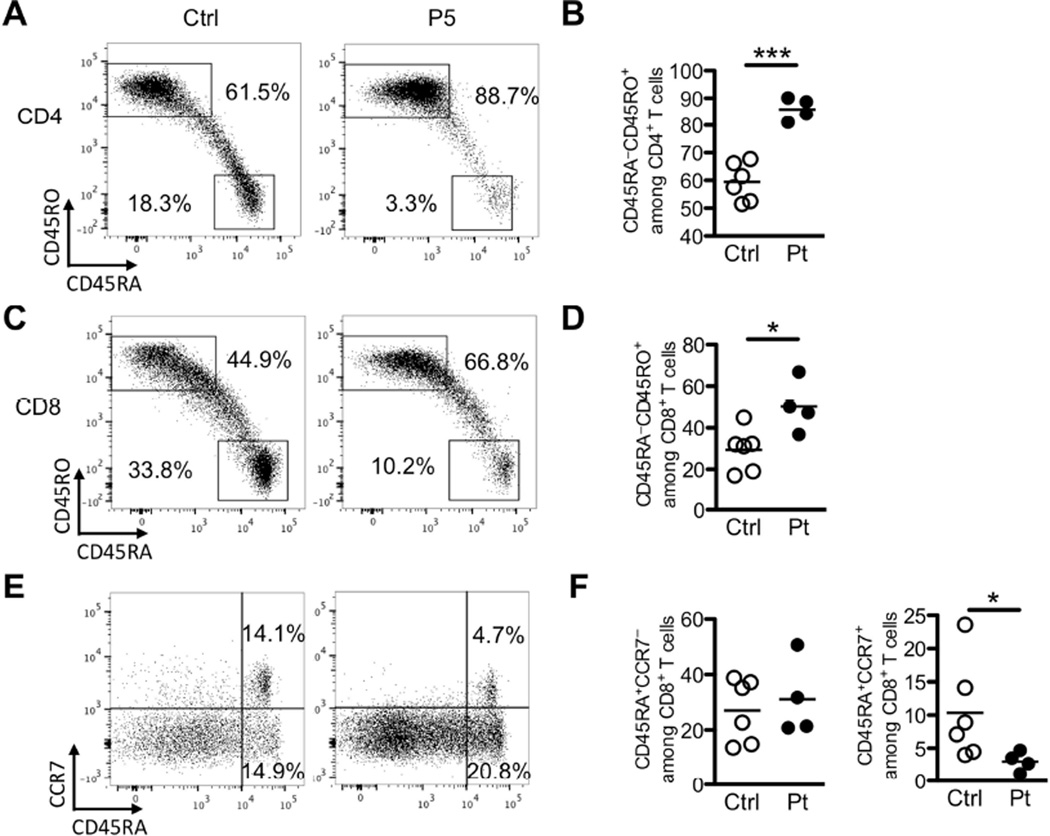
TR cell deficiency is associated with dysregulated T cell activation and skewing towards a memory phenotype. Consistent with immune dysregulation, LRBA deficiency resulted in skewing of peripheral CD4 and CD8 T cell towards a memory (CD45RA−CD45RO+) phenotype (control vs. LRBA deficient subjects: 59.5%±2.8 vs. 86%±2.1 for CD4 T cells, and 29.4%±4.2 vs. 50.3%±6.2 for CD8 T cells, respectively) (Figure 4A-D). The frequency of naïve CD45RA+CCR7+ CD8+T cells was decreased in LRBA-deficient subjects (control vs. LRBA deficient subjects: 10.4%±3 vs. 3%±0.7). However, there was no difference in the frequency of CD8+CD45RA+CCR7− [(CD45RA+ effector memory (TEMRA)] T cells as compared to control subjects (Figure 4E-F).
Figure 4. Increased frequency of memory T cells in LRBA-deficient subjects.
A. Flow cytometric analysis of circulating CD45RO−CD45RA+ and CD45RO+CD45RA− CD4+ T cells in an LRBA-deficient and a control subject. B. Frequency of activated CD45RO+CD45RA− within the peripheral blood CD4+ T cell pool of LRBA-deficient and control subjects. C. Flow cytometric analysis of circulating CD45RO−CD45RA+ and CD45RO+CD45RA− CD8 T cells in an LRBA-deficient and a control subject. D. Frequency of activated CD45RO+CD45RA− within the peripheral blood CD8+ T cell pool of LRBA-deficient and control subjects. E. Flow cytometric analysis of circulating TEMRA CD45RA+CCR7− and naïve CD45RA+CCR7+ CD8 T cells in an LRBA-deficient and a control subject. F. Frequency of circulating TEMRA CD45RA+CCR7− and naïve CD45RA+CCR7+ within the peripheral blood CD8+ T cell pool of LRBA-deficient and control subjects. *p<0.05, ***p<0.001 by Student’s unpaired two tailed t test.
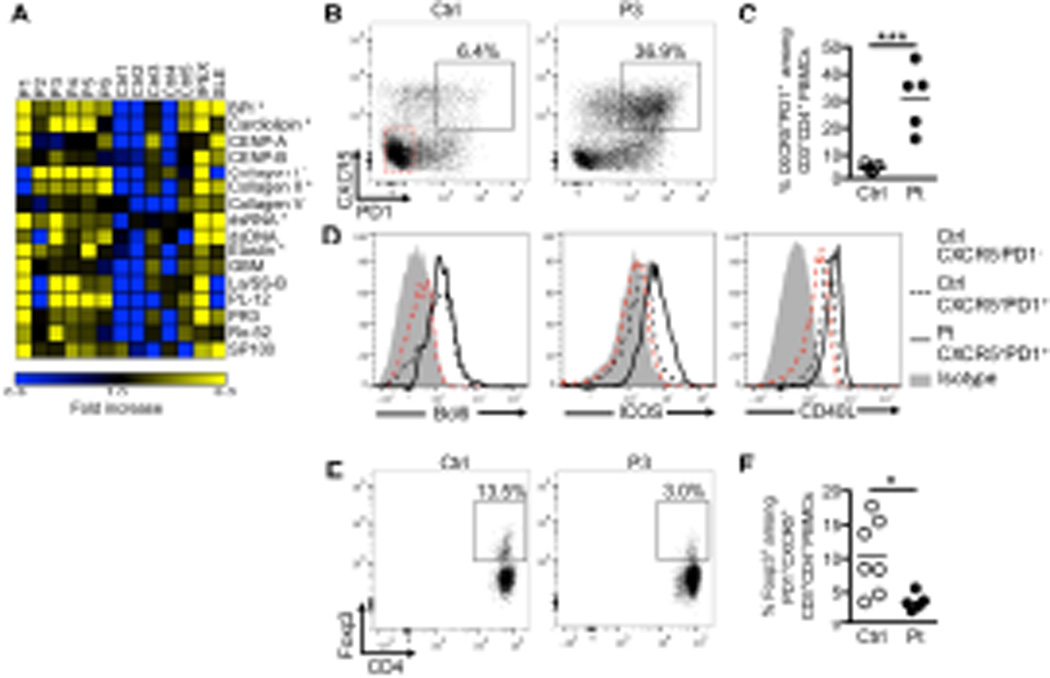
LRBA-deficient subjects suffer from diverse autoimmune phenomena, including autoimmune hemolytic anemia, thrombocytopenia, thyroiditis, and end organ inflammation including colitis and pneumonitis 17–19. Using an array of 84 autoantigens, we determined the spectrum of circulating autoantibodies in the 6 LRBA deficient patients as compared to an IPEX patient with a missense mutation in forkhead domain of FOXP3 (p.A384T), an adolescent patient with systemic lupus erythematosus (SLE), and five healthy control subjects. When normalized to the results of the control subjects, the LRBA-deficient patients had increased autoantibodies present in their plasma. This was especially true for patient P1, whose phenotype and autoantibody profile were similar to those of the subject with true IPEX disease (Figure 5A and data not shown). There was a predominance of cytoplasmic- and RNA-directed autoantibodies in LRBA deficient patients. Similar to IPEX patients and unlike SLE patients, they were negative for anti-nuclear and anti-dsDNA antibodies (Figure 5A; Figure E3 in the Online Repository and data not shown).
Figure 5. Increased autoantibodies and dysregulated TFH cell response in LRBA-deficient patients.
A. Heat map display of autoantibody reactivity against self-antigens in sera of LRBA-deficient and control subjects, a patient with IPEX and a patient with SLE. A value of 1 (black) is equal to the control average + 1 standard deviation (SD). B. Flow cytometric analysis of circulating TFH (CD4+CXCR5+PD-1hi) cells in patient and control subjects. C. Frequency of TFH cells within the peripheral blood CD4+ T cells of LRBA-deficient and control subjects. D. Flow cytometric overlays of the TFH markers BCL6, ICOS and CD40L within the peripheral blood CD4+CXCR5+PD-1+ cell pool of LRBA-deficient and control subjects. E. Flow cytometric analysis of circulating TFR cells. T cells within the CD4+CXCR5+PD-1hi gate were analyzed for Foxp3 expression. F. Frequencies of TFR cells within the peripheral blood CD4+CXCR5+PD-1+ cell pool. *p<0.05, ***p<0.001 by Student’s unpaired two tailed t test.
A specialized subset of TR cells, termed T follicular regulatory (TFR) cells, regulates the ability of T follicular helper (TFH) cells to provide support for cognate B cells in developing germinal centers and mounting T cell-dependent antibody responses 25–28. TFR deficiency, as seen in IPEX patients and in mice with FOXP3 deficiency, results in dysregulated expansion of TFH cells and germinal center B cells, leading to intense autoantibody formation 25, 29. Both TFH and TFR cells express the chemokine receptor CXCR5, and also have high expression of the inhibitory receptor Programmed Death-1 (PD-1) 25–28. Circulating TFH (CD4+FOXP3−CXCR5+PD-1hi) and TFR (CD4+FOXP3+CXCR5+PD-1hi) cells are found in the peripheral blood, and the former are detected in greater abundance in human diseases associated with intense autoantibody production such as SLE, myasthenia gravis, and juvenile dermatomyositis 30–32. The gating strategy for detecting circulating TFH and TFR cells and the specificity of the staining are illustrated in Figures E4 and E5 in the Online Repository, respectively. There was a striking increase in the frequency of TFH cells in the peripheral blood of LRBA-deficient patients (controls: 4.9%±0.6 vs. patients: 31.2%±5.3) (Figure 5B, C). The patient CXCR5+PD-1hi cells expressed high levels of B cell lymphoma 6 protein (BCL6), inducible co-stimulator protein (ICOS) and CD40 ligand (CD40L), consistent with their TFH phenotype (Figure 5D). Within the TFH cell population, the frequency of FOXP3+ TFR cells was significantly decreased in patients relative to controls (controls: 10.3%±2 vs. patients: 3.5%±0.6) (Figure 5E, F). These results are consistent with dysregulated TFH and compromised TFR cell compartments in LRBA-deficient subjects.
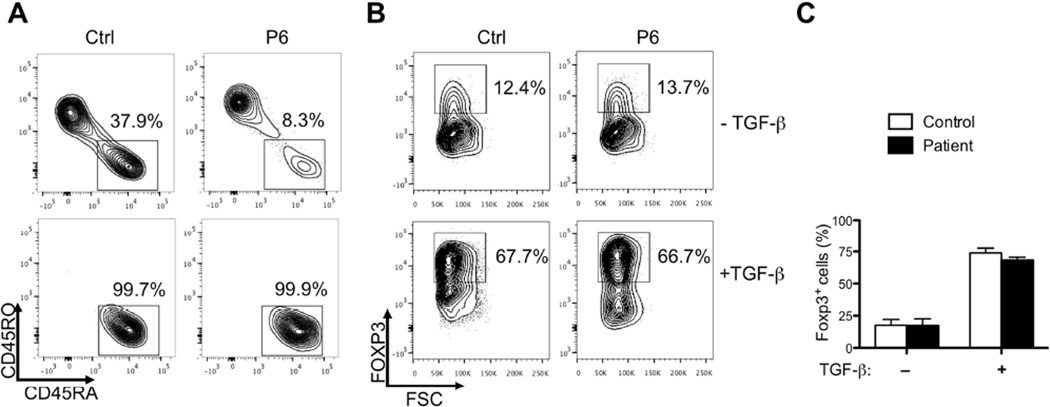
To determine if LRBA deficiency impaired the induction of FOXP3 expression, we analyzed the capacity of LRBA-sufficient and deficient naïve CD4+ T cells to differentiate into induced (i)TR cells upon stimulation with anti-CD2/CD3/CD28 mAbs in presence of TGF-beta. CD4+ T cells of patient and control subjects were enriched by magnetic separation, and naïve CD4+ T cells were purified >98% purity by cell sorting (Figure 6A and data not shown). LRBA sufficient and deficient T cells were found equivalent in their capacity to differentiate into iTR cells that equally expressed high levels of FOXP3 (Figure 6B, C).
Figure 6. LRBA deficiency does not affect the in vitro generation of FOXP3+ iTR cells.
A. Representative flow cytometric analyses of CD45RA/RO markers in purified CD4+ T cells (upper panel) and in cell sorted CD3+CD4+CD25−CD45RO−CD45RA+ T cells (lower panels) of a control and an LRBA-deficient subject. B. Representative flow cytometric analyses of Foxp3 expression in T cells sorted as in panel A and stimulated in culture with anti-CD2/3/28 mAbs in the absence or presence of TGF-β1. C. Collated frequencies of FOXP3+ iTR cells in control and LRBA-deficient subjects (N=4–5/group). Results are representative of 2 independent experiments.
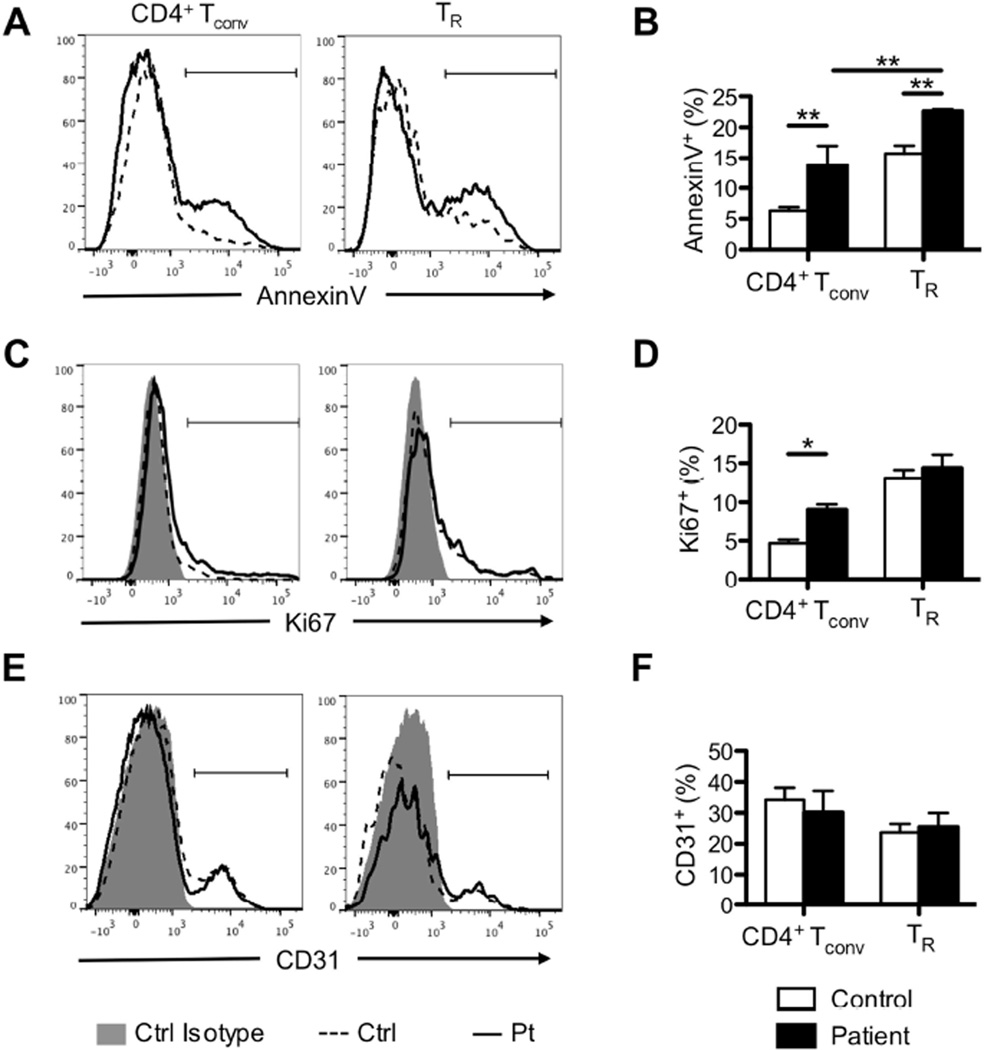
Given the normal differentiation of LRBA-deficient T cells into iTR cells, we examined the turnover of peripheral blood TR and CD4+ conventional T (Tconv) cells in patient vs. control subjects. Results revealed that both TR and Tconv cells of LRBA-deficient subjects exhibited increased AnnexinV staining as compared to those of controls, indicative of increased apoptosis (control vs. LRBA deficient subjects: 6.3%±0.6 vs. 13.7%±3.2 for CD4+ Tconv cells and 15.7%±1.3 vs. 22.6%±0.3 for TR cells, respectively) (Figure 7A, B). LRBA-deficient Tconv but not TR cells also exhibited increased Ki67 staining, indicative of increased proliferation (control vs. LRBA deficient subjects: 4.7%±0.5 vs. 9%±0.7 for CD4+ Tconv cells and 13%±1 vs. 14.4%±1.7 for TR cells, respectively) (Figure 7C, D). In contrast, the frequency of CD31+ T cells, a marker of recent thymic emigrant cells (RTEs), was similar in peripheral blood of patient and control subjects (Figure 7E, F). These results pointed to increased turnover (proliferation and apoptosis) of Tconv cells and increased attrition (apoptosis) of TR cells in the periphery of LRBA-deficient subjects in the face of apparently normal thymic efflux of the respective population.
Figure 7. Increased turnover of LRBA-deficient T cells.
A, C E. Flow cytometric analysis of AnnexinV (A), Ki67 (B) and CD31 (C) staining in ex-vivo CD4+ Tconv and TR cells from LRBA-deficient and control subjects. B, D, F. Frequencies of AnnexinV+ (B), Ki67+ (D) and CD31+ (F) cells within Tconv and TR cell populations of LRBA-deficient and control subjects. N=4–5 patients and 5–8 controls/group . *p<0.05, **p<0.01 by 2-way ANOVA with post-test analysis.
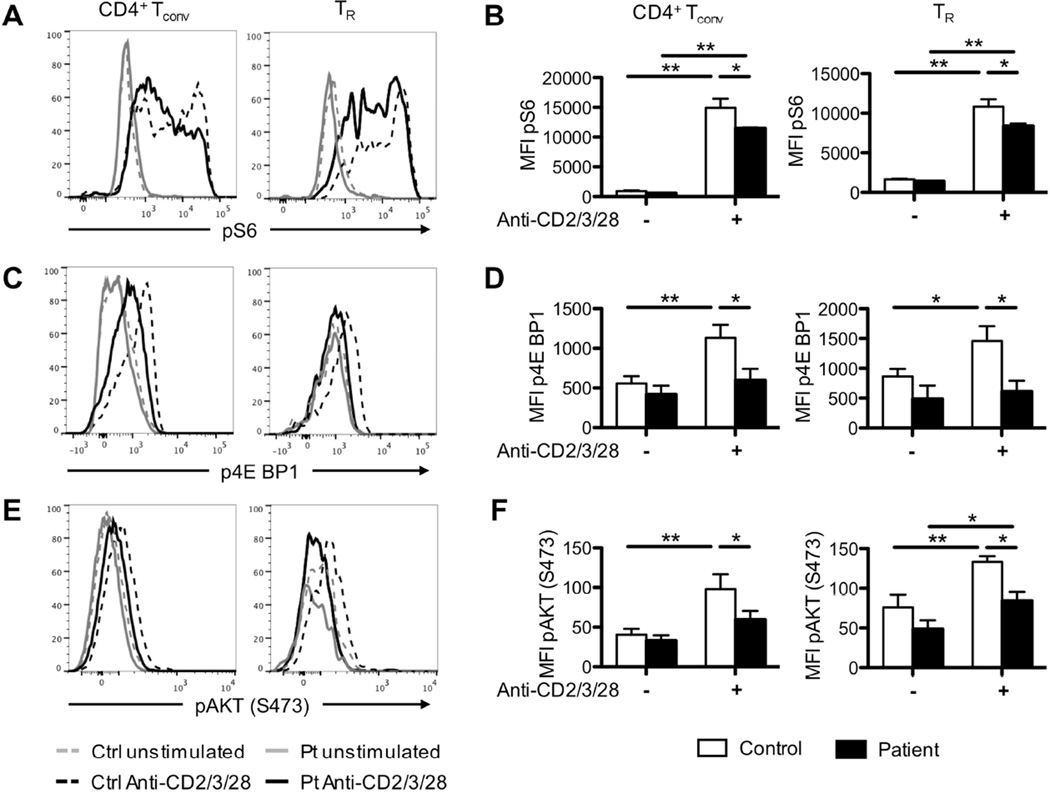
The increased apoptosis in peripheral LRBA-deficient T cells promoted us to examine the activity of the metabolic sensors mammalian target of rapamycin 1 and 2 complexes (mTORC1 and mTORC2, respectively). In particular, mTORC1 deficiency in TR cells precipitates an IPEX like phenotype in experimental mice, indicative of the essential role this pathway plays in regulating TR cell metabolism 33. Stimulation of PBMCs of LRBA-deficient and control subjects with anti-CD2/CD3/CD28 mAbs revealed that phosphorylation of S6 and eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1), downstream substrates of mTORC1, was decreased in patient vs. control TR and Tconv cells (Figure 8A-D) 33. Furthermore, phosphorylation of the kinase AKT at S473 residue (pAKTS473), a measure of mTORC2 kinase activity, was also decreased in patient vs. control TR and Tconv cells (Figure 8E-F) 33. These findings indicated that LRBA deficiency was associated with impaired activation of mTORC1 and mTORC2 complexes, with potentially deleterious impact on TR cell function.
Figure 8. Impaired mTORC1 and mTORC2 activities in LRBA-deficient Tconv and TR cells.
A. Flow cytometric analysis of pS6 of conventional (Tconv) and regulatory (TR) T cells from LRBA-deficient and control subjects before after anti-CD2/3/28 stimulation. B. Mean Fluorescence Intensity (MFI) of pS6 within Tconv and TR cells of LRBA-deficient and control subjects before and after anti-CD2/3/28 stimulation. C. Flow cytometric analysis of p4E-BP1 of Tconv and TR cells from LRBA-deficient and control subjects after anti-CD2/3/28 stimulation. D. MFI of p4E-BP1 within Tconv and TR cells of LRBA-deficient and control subjects before and after anti-CD2/3/28 stimulation. E. Flow cytometric analysis of p-AKT (S473) of Tconv and TR cells from LRBA-deficient and control subjects after anti-CD2/3/28 stimulation. F. MFI of p-AKT (S473) within Tconv and TR cells of LRBA-deficient and control subjects before and after anti-CD2/3/28 stimulation. *p<0.05, **p<0.01 by 2-way ANOVA with post-test analysis.
Discussion
We demonstrate that LRBA deficiency results in decreased frequency, aberrant phenotype, and depressed suppressive function of TR cells, leading in at least one case to an IPEX-like condition similar in severity to classical IPEX disease. A compromised TR cell compartment is thus a cardinal feature of LRBA deficiency and may play a critical role in the ubiquitous autoimmune manifestations of the disease.
LRBA is widely expressed multi-domain protein that shares a highly conserved beige and Chediak-Higashi syndrome (BEACH) domain with other BEACH family proteins including LYST, encoded by the Chediak-Higashi syndrome gene (CHS1) 34. It is highly conserved from Homo sapiens down to C. elegans, particularly in the functional domains, and has been implicated in regulating endosomal trafficking, cell proliferation and survival 35, 36. LRBA deficiency did not impact the in vitro differentiation of iTR cells nor did it affect the fraction of recent thymic immigrants amongst peripheral Tconv and TR cells. Instead, it was associated with increased apoptosis of TR and Tconv cells, with increased turnover of the latter but not the former cells. A disproportionately constrained TR cell population especially in the context of a lymphopenic environment has been invoked as a trigger for the development of autoimmune lymphoproliferation, related to a severely restricted TR cell T cell receptor repertoire 37.
TR cell depletion in LRBA deficiency is compounded by impaired TR cell phenotype and function, with decreased expression of key effector proteins involved in TR cell suppression such as CD25 and CTLA-4 38. LRBA localizes to cytosol at lysosomes, the trans-Golgi network, the ER, the perinuclear ER, and endocytic vacuoles 39. It is thus spatially segregated from FOXP3, whose expression is exclusively nuclear 7. In searching for a mechanism that accounts for adverse impact of LRBA deficiency on TR cells, the finding of impaired activation of mTOR complexes invoked deranged metabolic sensing in disease pathogenesis 40. In particular, defective mTORC1 cell activation may provide one mechanism by which LRBA deficiency impairs TR cell function. In mice, TR cell-specific mTORC1 deficiency, effected through deletion of the gene encoding the essential mTORC1 component Raptor, precipitates a Foxp3 deficiency-like disease, with runting, lymphoproliferation and autoimmunity 33. mTORC1 directs TR cell lipid metabolism, relevant to their energy generation and suppressor function 33. It impacts the expression of several TR cell markers, most notably CTLA-4, which may contribute to immune dysregulation upon mTORC1 deficiency 33.
Mechanisms by which LRBA deficiency impairs mTOR activation may involve defective autophagy, as originally described by Lopez-Herrera et al 17. Autophagy regulates energy metabolism in T cells, and its dysfunction may result in decreased activation of mTOR complexes and attenuated phosphorylation of downstream targets including S6 and 4E-BP1 41, 42. Alternatively, LRBA may act as a scaffold that enables the assembly and activation of mTOR complexes and/or recruitment of downstream intermediates such as AKT, as has been described for other scaffold proteins 43.
LRBA deficiency is associated with intense autoantibody responses even in the context of B cell lymphopenia and hypogammaglobulinemia. Our studies have identified increase TFH and decreased TFR cell frequencies in the peripheral blood of LRBA deficient subjects, consistent with ineffective regulation of antibody responses. TFR cell deficiency and its associated TFH cell dysregulation has been implicated in the development of autoantibodies in immune dysregulatory diseases, such as IPEX 25–29. We hypothesize that this abnormality is a key mechanism in the development of humoral autoimmunity in LRBA deficiency. While the precise cause of TFH dysregulation remains to be mapped, enhanced FOXO1/3a induced activation of BCL6 due to decreased negative regulation of the former by pAKTS473 may provide one putative mechanism 44. Further studies would be required to validate such a link.
Supplementary Material
Key messages.
-
–
LRBA deficiency results in profound T regulatory (TR) cell deficiency and impaired phenotype and function.
-
–
LRBA deficiency may present with Immune dysregulation, Polyendocrinopathy, Enteropathy, X-linked-like disease, reflecting their profound TR cell deficiency.
-
–
LRBA deficient subjects exhibit high frequency of memory T cells and a dysregulated T follicular helper cell compartment with increased autoantibody production.
Acknowledgements
The work was supported by the National Institutes of Health (NIH) grants R01AI085090 to T.A.C., 1R01AI100315 to R.S.G, Children's Health Research Centers Career Development Award 5K12HD052896 and 1K12HD052896-01A1 to E.J and J.C., respectively, by a Jeffrey Modell Foundation Translational Research Program Grant Award to J.C., by grants from the Dubai-Harvard Foundation for Medical Research and the Jeffrey Model Foundation to R.S.G. and by a Scientific and Technological Research Council of Turkey grant 059B191300622 to S.K.
Abbreviations
- AR
autosomal recessive
- CVID
Common variable immunodeficiency
- dsDNA
double stranded DNA
- ELISA
enzyme-linked immunosorbant assay
- FACS
fluorescence-activated cell sorting
- FOXP3
forkhead box P3
- IPEX
immune dysregulation, polyendocrinopathy, enteropathy, X-linked
- MFI
mean fluorescence intensity
- MHC
major histocompatibility complex
- mTORC1 and mTORC2
mammalian target of rapamycin 1 and 2 complexes
- PBMC
peripheral blood mononuclear cell
- RTE
recent thymic emigrants
- Teff
T effector
- TR
T regulatory
- SLE
systemic lupus erythematosus
- WES
Whole exome sequencing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Verbsky JW, Chatila TA. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases. Curr Opin Pediatr. 2013;25:708–714. doi: 10.1097/MOP.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 4.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 5.Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked: forkhead box protein 3 mutations and lack of regulatory T cells. J Allergy Clin Immunol. 2007;120:744–750. doi: 10.1016/j.jaci.2007.08.044. quiz 51-2. [DOI] [PubMed] [Google Scholar]
- 6.Barzaghi F, Passerini L, Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Front Immunol. 2012;3:211. doi: 10.3389/fimmu.2012.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, et al. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- 8.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 9.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, et al. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Chatila T. The regulatory T cell transcriptosome: E pluribus unum. Immunity. 2007;27:693–695. doi: 10.1016/j.immuni.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Barzaghi F, Passerini L, Gambineri E, Ciullini Mannurita S, Cornu T, Kang ES, et al. Demethylation analysis of the FOXP3 locus shows quantitative defects of regulatory T cells in IPEX-like syndrome. J Autoimmun. 2012;38:49–58. doi: 10.1016/j.jaut.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caudy AA, Reddy ST, Chatila T, Atkinson JP, Verbsky JW. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J Allergy Clin Immunol. 2007;119:482–487. doi: 10.1016/j.jaci.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Sharfe N, Dadi HK, Shahar M, Roifman CM. Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc Natl Acad Sci U S A. 1997;94:3168–3171. doi: 10.1073/pnas.94.7.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen AC, Nadeau KC, Tu W, Hwa V, Dionis K, Bezrodnik L, et al. Cutting edge: Decreased accumulation and regulatory function of CD4+ CD25(high) T cells in human STAT5b deficiency. J Immunol. 2006;177:2770–2774. doi: 10.4049/jimmunol.177.5.2770. [DOI] [PubMed] [Google Scholar]
- 16.Sampaio EP, Bax HI, Hsu AP, Kristosturyan E, Pechacek J, Chandrasekaran P, et al. A novel STAT1 mutation associated with disseminated mycobacterial disease. J Clin Immunol. 2012;32:681–689. doi: 10.1007/s10875-012-9659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Herrera G, Tampella G, Pan-Hammarstrom Q, Herholz P, Trujillo-Vargas CM, Phadwal K, et al. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am J Hum Genet. 2012;90:986–1001. doi: 10.1016/j.ajhg.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alangari A, Alsultan A, Adly N, Massaad MJ, Kiani IS, Aljebreen A, et al. LPS-responsive beige-like anchor (LRBA) gene mutation in a family with inflammatory bowel disease and combined immunodeficiency. J Allergy Clin Immunol. 2012;130:481–488 e2. doi: 10.1016/j.jaci.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burns SO, Zenner HL, Plagnol V, Curtis J, Mok K, Eisenhut M, et al. LRBA gene deletion in a patient presenting with autoimmunity without hypogammaglobulinemia. J Allergy Clin Immunol. 2012;130:1428–1432. doi: 10.1016/j.jaci.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohsumi TK, Borowsky ML. MolBioLib: a C++11 framework for rapid development and deployment of bioinformatics tasks. Bioinformatics. 2012;28:2412–2416. doi: 10.1093/bioinformatics/bts458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, del Angel G, Levy-Moonshine A, et al. From FastQ Data to High-Confidence Variant Calls: The Genome Analysis Toolkit Best Practices Pipeline. Current Protocols in Bioinformatics. 2013;43:11.0.1-.0.33. doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124:1289–1302. doi: 10.1016/j.jaci.2009.10.038. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li QZ, Zhou J, Wandstrat AE, Carr-Johnson F, Branch V, Karp DR, et al. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol. 2007;147:60–70. doi: 10.1111/j.1365-2249.2006.03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2013;14:152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wollenberg I, Agua-Doce A, Hernandez A, Almeida C, Oliveira VG, Faro J, et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol. 2011;187:4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- 29.Kinnunen T, Chamberlain N, Morbach H, Choi J, Kim S, Craft J, et al. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood. 2013;121:1595–1603. doi: 10.1182/blood-2012-09-457465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito R, Onodera H, Tago H, Suzuki Y, Shimizu M, Matsumura Y, et al. Altered expression of chemokine receptor CXCR5 on T cells of myasthenia gravis patients. J Neuroimmunol. 2005;170:172–178. doi: 10.1016/j.jneuroim.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 33.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499:485–490. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cullinane AR, Schaffer AA, Huizing M. The BEACH is hot: a LYST of emerging roles for BEACH-domain containing proteins in human disease. Traffic. 2013;14:749–766. doi: 10.1111/tra.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Souza N, Vallier LG, Fares H, Greenwald I. SEL-2, the C. elegans neurobeachin/LRBA homolog, is a negative regulator of lin-12/Notch activity and affects endosomal traffic in polarized epithelial cells. Development. 2007;134:691–702. doi: 10.1242/dev.02767. [DOI] [PubMed] [Google Scholar]
- 36.Wang JW, Gamsby JJ, Highfill SL, Mora LB, Bloom GC, Yeatman TJ, et al. Deregulated expression of LRBA facilitates cancer cell growth. Oncogene. 2004;23:4089–4097. doi: 10.1038/sj.onc.1207567. [DOI] [PubMed] [Google Scholar]
- 37.Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–122. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Wang JW, Howson J, Haller E, Kerr WG. Identification of a novel lipopolysaccharide-inducible gene with key features of both A kinase anchor proteins and chs1/beige proteins. J Immunol. 2001;166:4586–4595. doi: 10.4049/jimmunol.166.7.4586. [DOI] [PubMed] [Google Scholar]
- 40.Dunlop EA, Tee AR. mTOR and autophagy: A dynamic relationship governed by nutrients and energy. Semin Cell Dev Biol. 2014 doi: 10.1016/j.semcdb.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Hubbard VM, Valdor R, Patel B, Singh R, Cuervo AM, Macian F. Macroautophagy regulates energy metabolism during effector T cell activation. J Immunol. 2010;185:7349–7357. doi: 10.4049/jimmunol.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang HT, Lee KB, Kim SY, Choi HR, Park SC. Autophagy impairment induces premature senescence in primary human fibroblasts. PLoS One. 2011;6:e23367. doi: 10.1371/journal.pone.0023367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fritzius T, Moelling K. Akt- and Foxo1-interacting WD-repeat-FYVE protein promotes adipogenesis. EMBO J. 2008;27:1399–1410. doi: 10.1038/emboj.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hurtz C, Hatzi K, Cerchietti L, Braig M, Park E, Kim YM, et al. BCL6-mediated repression of p53 is critical for leukemia stem cell survival in chronic myeloid leukemia. J Exp Med. 2011;208:2163–2174. doi: 10.1084/jem.20110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.