Abstract
Nitroxyl (HNO), the reduced and protonated form of nitric oxide (NO.), confers unique physiological effects including vasorelaxation and enhanced cardiac contractility. These features have spawned current pharmaceutical development of HNO donors as heart failure therapeutics. HNO interacts with selective redox sensitive cysteines to effect signaling, but is also proposed to activate soluble guanylate cyclase (sGC) in vitro to induce vasodilation and potentially enhance contractility. Here we tested whether sGC stimulation is required for these HNO effects in vivo and if HNO also modifies a redox-sensitive cysteine (C42) in protein kinase G-1α (PKG1α) to control vasorelaxation. Intact mice and isolated arteries lacking the sGC-β subunit (sGCKO, results in full sGC deficiency) or expressing solely a redox-dead C42S mutant PKG1α were exposed to the pure HNO donor, CXL-1020. CXL-1020 induced dose-dependent systemic vasodilation while increasing contractility in controls; however, vasodilator effects were absent in sGCKO mice whereas contractility response remained. The CXL-1020 dose reversing 50% of pre-constricted force in aortic rings was ~400-fold greater in sGCKO than controls. Cyclic-GMP and cAMP levels were unaltered in myocardium exposed to CXL-1020 despite its inotropicvasodilator activity. In PKG1αC42S mice, CXL-1020 induced identical vasorelaxation in vivo and in isolated aortic and mesenteric vessels as in littermate controls. In both groups, dilation was near fully blocked by pharmacologically inhibiting sGC. Thus, sGC and cGMP-dependent signaling are necessary and sufficient for HNO-induced vasodilation in vivo, but are not required for positive inotropic action. Redox modulation of PKG1α is not a mechanism for HNO-mediated vasodilation.
Keywords: cardiovascular physiology, vasodilation, pharmacology, contractility
Introduction
Nitroxyl (HNO) is the protonated, one electron-reduced form of the signaling molecule nitric oxide (NO.)1, 2. Like NO., HNO has prominent vascular effects inducing vasodilation in conduit and resistance arteries3-7. However, HNO differs from NO. in that tolerance does not develop with repeated exposure8, and its effects are not suppressed by oxidative stress, but rather by administration of reducing agents such as L-cysteine9 Exogenously administered HNO donors exhibit prominent pharmacological activity on the cardiovascular system10-12, combining venous and arterial dilation with an augmentation of cardiac contractility and relaxation11-17. This net constellation of effects has triggered interest in HNO as a heart failure therapy17, with clinical trials now underway (Clinicaltrials.gov/NCT02157506).
Several mechanisms for HNO-mediated vasodilation have been proposed, including NO.-like activity on soluble guanylate cyclase (sGC) triggering cGMP-dependent signaling, activation of voltage and calcium-dependent potassium hyperpolarizing channels3, 6, and the stimulation of calcitonin gene related peptide (CGRP)18. HNO-vasodilation is blocked in vitro by the sGC antagonist [1H-[1,2,4]oxadiazolo-[4, 3-a]quinoxalin-1-one] (ODQ) 4-6, 19, 20, which has been interpreted as supporting an sGC dependent mechanism. However, whether HNO directly interacts with sGC heme has been questioned by molecular model analysis21 and data showing this requires HNO conversion to NO. by superoxide dismutase22. Inhibition by ODQ does not guarantee sGC is solely involved. ODQ oxidizes the heme in sGC to block NO-responsiveness, but it can also modify other heme-containing proteins including hemoglobin, nitric oxide synthase, and cytochrome p-450 enzymes that impact vasodilation23, 24. Lastly, alternative mechanisms including CGRP and cysteine-42 oxidation in protein-kinase G-1α (PKG1α) that mediates cGMP-independent H2O -induced vasodilation, could play a role25,26.
There are also controversies surrounding the role of sGC in mediating HNO cardiac contractility. Low levels of cGMP stimulate myocyte contractility by impairing cAMP hydrolysis by phosphodiesterase type 3 (PDE3)27, 28, whereas higher levels blunt contractility by PKG1α-dependent mechanisms29 and cGMP activation of PDE2 resulting in cAMP hydrolysis30. Though ODQ has no effect on HNO-stimulated inotropy in isolated ventricular myocytes12, 17, it reportedly blunts HNO-inotropy in isolated rat hearts31. Yet, unlike NO, HNO donors do not inhibit β-adrenergic stimulated contractility11, 12. This has suggested different mechanisms, most notably HNO modification of selective cysteines to form reversible S-S bonds or sulfinamides (RS(O)NH2)1. In the heart, this chemistry alters phospholamban (PLN) 13, 32, sarcoplasmic reticular (SR) ATPase33, the ryanodine receptor12, myosin light chain, and tropomyosin10, resulting in enhanced Ca2+ cycling and myofilament sensitivity.
Critically, no prior work has tested whether sGC is required for HNO effects in vivo as the compounds to inhibit sGC or quench NO. cannot be administered in the intact animal, and genetic deletion studies have not been performed. Furthermore, prior HNO studies have mostly employed Angeli's salt (AS) that degrades into HNO but also nitrite which is itself a vasodilator34. Some have used IPA/NO, which is a pH dependent HNO donor35, while others acyloxy-nitroso compounds that are limited to in vitro use36, 37. Here, we performed studies in mice genetically lacking the sGCβ1 subunit38 that results in loss of the entire sGC protein complex. The role of PKG1α oxidation at C42 was also tested using mice with a knock-in mutation (C42S) generating a PKG1α redox-dead protein26. Lastly, we employed the pure HNO donor CLX-1020 that is stable at room temperature and can be administered both in vitro and in vivo17. We find both vasodilator and inotropic effects of HNO are observed in control mice, but vasodilation is absent while positive inotropy persists in sGCKO mice. By contrast, HNO-induced dilation is unaltered by expressing solely the C42S-mutant PKG1α.
Methods
Mouse Models
Studies were performed in C57Bl6/J adult mice (Jackson Laboratories, Bar Harbor, ME), and in sGCKO and PKGC42S mice and their respective littermate controls. Both genetic models have been previously described26, 38, and develop hypertension (+ ~30 mmHg systolic pressure vs. controls). sGCKO mice38 develop gastrointestinal dysmotility and consequent early lethality, but can survive by using a fiber-free diet. These mice were maintained and studied at the University of Würzburg to avoid trauma from shipping. PKGC42S mice (provided by Phil Eaton, Kings College, London, UK) display no gastro-intestinal dysfunction and live a normal lifespan. Isolated and in vivo resistance vessels in these mice dilate in response to cGMP stimuli but show reduced responsiveness to hydrogen peroxide26. Mice aged 2-4 months were used in the study. The protocols were approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions, or University of Würzburg.
Pharmaceuticals
CXL-1020 (2-Methylsulfonyl benzene N-hydroxy sulfonamide, Cardioxyl Pharmaceuticals Inc., NC), which chemically decomposes into HNO and an organic byproduct (CXL-1051)17, was dissolved in 15% beta-cyclodextrin (Captisol®) in sterile water at pH 4.0 to generate a stock solution (60mM). For in vivo intravenous administration, CXL-1020 was diluted in 0.9% NaCl and infused at incremental doses of 200, 300, or 500 μg/kg/min (with 2-10 μL/min infusion rate). For the vascular ring studies, CXL-1020 was administered at doses ranging 1nM-1mM, prepared from a 100mM stock solution.
In vivo Hemodynamics
Cardiac function and arterial loading were assessed by pressure-volume (PV) analyses, using a miniature micromanometer/conductance catheter (Millar, Inc.) as described previously39. Briefly, mice [C57Bl/6 controls (n=10), sGCKO and littermate controls, (n=5 each group), and PKG1αC42S (n=4)] were anesthetized using an established protocol (1-2% isoflurane, followed by i.p. 750-1000 mg/kg urethane, 5-10 mg/kg etomidate, and 1-2 mg/kg morphine) 39. Following tracheostomy, they were ventilated using 6-7 μl/g tidal volume at 130 breaths/min, and administered 12.5% human albumin (50–100 μl over 5 min) to provide modest intravascular volume expansion. The LV apex was then exposed and a 1.4-Fr PV catheter (SPR 839; Millar Instruments Inc.) was advanced through the apex to lie along the longitudinal axis. Data were measured at steady state with each dose of CXL-1020, allowing sufficient time to establish steady state responses. The volume signal was calibrated using ultrasound-aortic flow (Transonics, NY) and the hypertonic saline method39. Total ventricular afterload was indexed by effective arterial elastance (Ea = ventricular end-systolic pressure/stroke volume) and by total systemic arterial resistance. Ventricular contractility was determined by peak rate of pressure rise normalized to instantaneous developed pressure (dP/dtmax/IP), and relaxation by a logistic-model time-constant40. Analysis used custom software (WinPVAN) developed in our laboratory.
Isolated vascular rings
Direct vasodilator effects of CXL-1020 were tested in aortic rings or mesenteric vessels using tissue bath force-transducer systems. Animals were euthanized with an overdose of isoflurane, and thoracic aorta or mesenteric vessels excised, cleaned from connective tissue, and placed in Krebs-Henseleit solution (118 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 7.5 mM Glucose, pH 7.4) gassed with 95% O2 and 5% CO2. Aortic rings (2-3 mm) were mounted in a myograph 700 (Danish Myo Technology, Aarhus, Denmark) set to a rest tension of 5 mN. Mesenteric 3rd order vessels of similar length were attached to a micromanipulator and force transducer under microscopic visualization, and passively stretched in 60 mM KCl, washed with Krebs buffer41. After equilibration (at least 60 minutes at 37 °C) in the presence of diclofenac or indomethacin (3 μM) and N-nitro-L-arginine methyl ester (L-NAME) (200 μM), rings were pre-contracted with phenylephrine (1μM; Sigma). Each experiment was conducted in parallel with aortic rings or mesenteric vessels derived from littermates of sGCKO or PKGC42S mice.
PKG1α-non-reducing gel electrophoresis
Snap frozen mesentaric vessels from control and PKGC42S mice were lysed in buffer containing N-ethyl maleimide (100 mM, Sigma) to prevent further thiol oxidation during protein isolation, and run in non-reducing conditions as previously described.42 Protein concentration was determined by BCA assay (Pierce) and samples probed with primary antibodies against PKG1α (gift of Robert Blanton, Tufts University) and alpha-tubulin (loading control; Cell Signaling Technology). Antibody binding was imaged and analyzed using an infrared system (Odyssey, Licor).
Cyclic nucleotide assay
Ventricular myocardium was homogenized in 6% trichloroacetic acid, centrifuged and extracted with water-saturated ether. The aqueous phase was then transferred and vacuum dried, and the pellet resuspended in sodium acetate buffer for cGMP or cAMP immunoassay (Amersham Pharmacia Biotech) following manufacturers instruction.
Statistical Analysis
Dose-dependent effects were tested by multiple regression analysis, with a dummy variable encoding each mouse. For single-dose analysis testing genotype-drug treatment interaction, a 2-way analysis of variance was used. Analysis was performed using Systat 11.0 software.
Results
CXL-1020 induces combined inotropy and vasodilation in the mouse heart in vivo
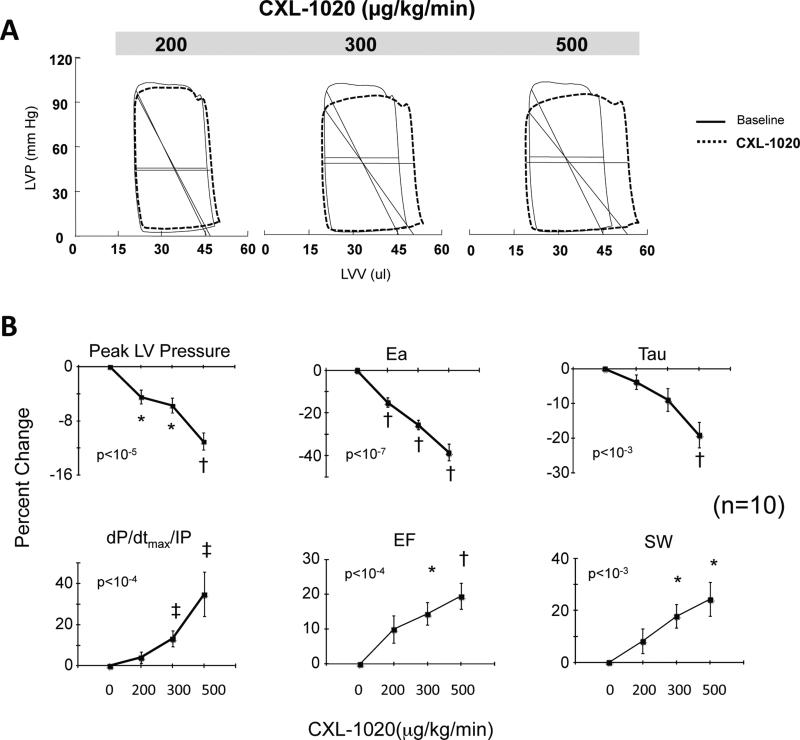
Since in vivo cardiovascular effects of any HNO donor have not been previously reported in mice, we first established this by a dose-response to CXL-1020. The HNO donor displayed both dose-dependent arterial vasodilation and positive cardiac contractility and enhanced relaxation (Figure 1). Example pressure-volume loops before and after CXL-1020 (Fig 1A) reveal dose-dependent reductions in total ventricular afterload (Ea, slope of diagonal line in each loop) consistent with a decline in systemic resistance. Summary data for the various loop-derived parameters are provided in Fig 1B and in Supplemental Table S1. The doses of CXL-1020 required to generate cardiovascular changes in intact mice were about 10-fold greater than in dog17, but the effects (e.g. peak LV pressure: −10%; total afterload-Ea: −40%; and dP/dtmx/IP: +35%) were similar (p<0.001 for each). Isovolumic relaxation time constant shortened by nearly 20%. The net effect of these changes was a near 20% rise in ejection fraction and stroke work.
Figure 1.
A) Example of pressure-volume loops in control mice exposed to incremental doses of the pure HNO donor, CXL-1020. There is a gradual decline in ventricular afterload indexed by Ea (diagonal line). B) Summary data (n=10) for dose-dependent changes in ventricular endsystolic pressure (LV-ESP), effective arterial elastance (Ea), LV contractility (dP/dtmax/IP) and relaxation time constant (Tau-l), and integrated function (ejection fraction, EF, and stroke work, SW). Data are shown as percent change from baseline. P-values in figure are for multiple regression analysis of variable vs. dose that also included a dummy variable for each mouse. * p≤0.01, †≤0.001, ‡p≤0.03 vs. baseline (Bonferroni corrected for multiple comparisons).
CXL-1020 induces inotropy but not vasodilation in sGCKO mice
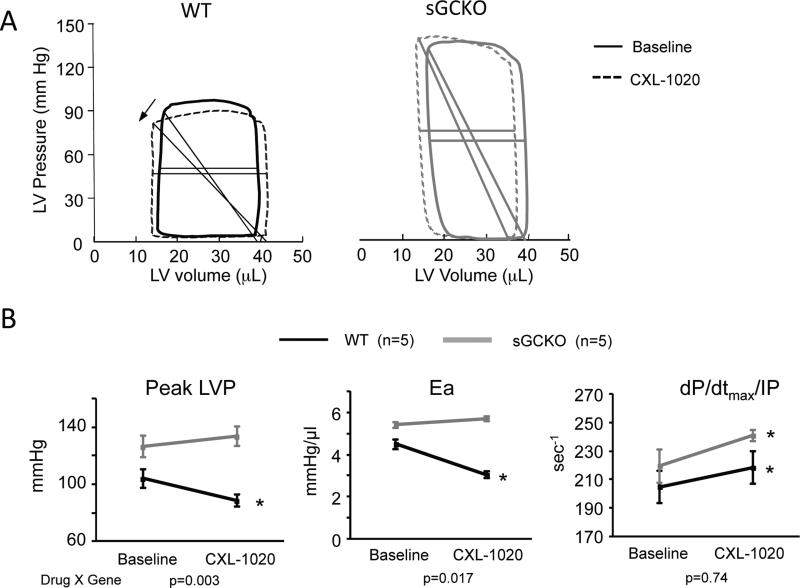
We next tested if sGC activation is required for HNO induced arterial vasodilation, cardiac inotropy, or both. sGCKO mice and their littermate controls were studied using the same in vivo pressure-volume analysis, employing a single 500 μg/kg/min dose of CXL-1020 based on the dose-response data. CLX-1020 lowered systolic blood pressure and Ea in littermate controls, but both changes were absent in sGCKO mice (Fig 2A, 2B). By contrast, contractility rose similarly in both groups (Fig 2B, Supplemental Table S2).
Figure 2.
A) Example PV loops from WT-littermate control and GC-KO mice, before and after exposure to 500 μg/kg/min CXL-1020. The HNO donor reduced ventricular afterload in controls (arrow notes decline in Ea), but not in mice lacking sGC. B) Summary data for these studies (n=5 per group). SBP – systolic blood pressure; Ea – effective arterial elastance. P-values below are for interaction term of two-way ANOVA, with dose and genotype as the two groups. * p≤0.05 vs. baseline.
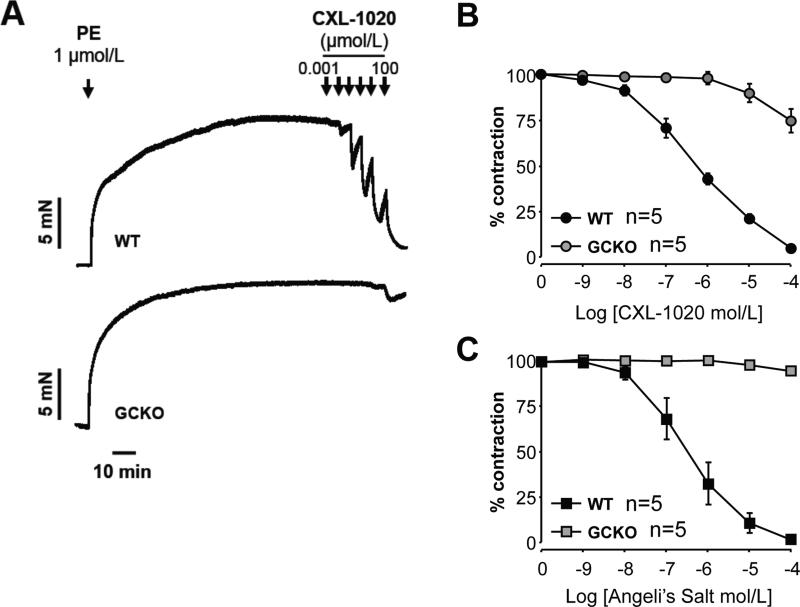
To more directly test the requirement of sGC for CXL-1020 vasodilation, aortic rings were pre-constricted with phenylephrine in the presence of the pan-NOS inhibitor L-NAME and the COX-2 inhibitor diclofenac, and exposed to varying doses of CXL-1020. CXL-1020 induced dose-dependent vasodilation, with 50% reduction in pre-constriction force (IC50) achieved with ~0.5 μM in control rings (Fig 3A,B). By contrast, rings from sGCKO mice showed marked insensitivity to CXL-1020, with a ~400-fold higher IC50. By way of comparison, we also tested the dilator response to the prototypic HNO donor, Angeli's salt, and found a similar IC50 for reversing PE-constriction in control rings, and negligible effect in sGCKO rings (Figure 3C).
Figure 3.
A) Dose-dependent vasorelaxation of isolated aortic rings from control and GCKO mice. Reversal of phenylephrine (PE) induced constriction was produced by CXL-1020 at more than 100-fold lower concentrations in controls than in the GCKO animals. B) Summary results for CXL-1020 dose-response relations (n=5 for each group). C) Summary results for Angeli's salt dose-response relations (n=5 for each group).
CXL-1020 vasodilation is not mediated by PKG1α C42-oxidation
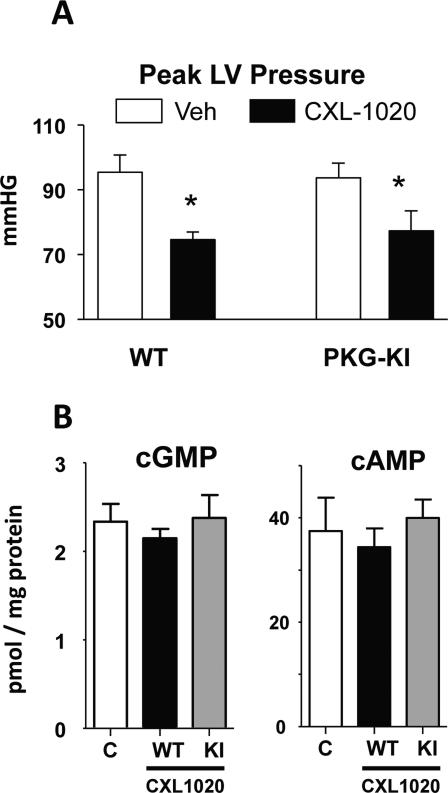
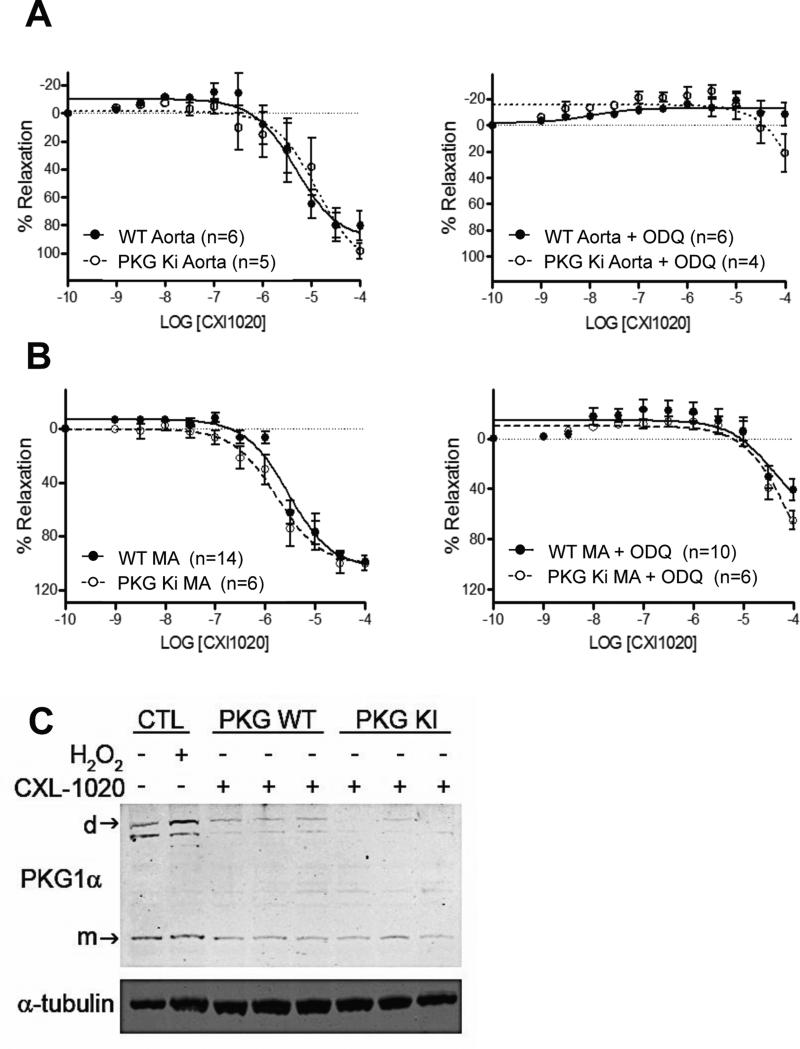
The data from sGCKO mice indicated sGC was required for HNO-vasodilation; however, as some mild dilation was observed at the highest CXL-1020 doses in vascular rings, we further tested if HNO might directly alter PKG1α at its redox sensitive C42 residue to induce cGMP-independent vasorelaxation. Mice expressing solely wild-type PKG1α or the C42S mutation were administered CXL-1020 at 500 μg/kg/min (x10 min), and an identical decline in blood pressure was observed in both groups (Fig 4A). CXL-1020 exposure had no impact on myocardial cGMP or cAMP levels in the hearts of either genotype (Fig 4B). Consistent with these in vivo data, aortic rings (Fig 5A) and mesenteric vessels (Fig 5B) from PKG1αC42S or controls showed near identical CXL-1020 dose responses, with an IC50 between 1-10 μM. As a control, we applied ODQ (0.3 μM) to block sGC activity, and found this markedly blocked CXL-1020 stimulated relaxation similarly in both groups. Lastly, we directly tested if HNO stimulates formation of PKG1α dimer in mesenteric vessels. As shown in Fig 5C, whereas H2O2 (10 μM) enhanced PKG1α dimer, exposure to CXL-1020 showed no change over non-stimulated control. In vessels expressing PKG1αC42S the dimer signal was negligible. Collectively, these data show that HNO does not modify PKG1α-C42 to induce vasorelaxation.
Figure 4.
A) Peak left ventricular pressure declines similarly in mice with a knock-in redox-dead PKG1αC42S mutation as in littermate controls (n=4 for each group); *p<0.05. B) Myocardial cGMP and cAMP levels measured in control myocardium, and after 10 minute exposure to HNO (500 μg/kg/min CLX-1020). Neither cyclic nucleotide was altered by CXL-1020 exposure.
Figure 5.
Dose-dependent reversal of PE-induced vasoconstriction in mice with WT vs. redox-dead (C42S) PKG1α (n=4-16/group). The absence of PKG1α redox sensitivity did not alter CXL-1020 vasodilation dose-sensitivity in both A) conduit (aorta) and B) resistance (B) (mesenteric) arteries. In both cases, application of ODQ suppressed CXL-1020 vasodilation. C) Non-reducing gel electrophoresis for PKG1α monomer and dimer in response to H2O2 (positive control) versus CXL-1020 (the latter tested in both wild type (WT) and PKG1αC42S mutant mesenteric arteries. CXL-1020 did not stimulate dimer formation.
Discussion
The first question tested by the present study was whether sGC signaling is required for HNO mediated vasodilation in vivo. Despite prior work conducted in vitro, this was far from a forgone conclusion given the potential for ODQ off-target effects23, 24 and concerns as to whether HNO can indeed interact with the heme in sGC as NO does21, 22. Additionally, our prior conscious animal data had found arterial and venous blood cGMP failed to rise after AS infusion whereas equal-vasodilating doses of an NO. donor augmented levels16. There were alternatives such as stimulation of hyperpolarizing potassium channels3, 6, 19, though this too has been ODQ suppressible, and release of CGRP acting via by a cAMP-dependent mechanism16. The latter has been recently proposed to arise by HNO generated from the interaction of NO and hydrogen sulfide (H2S), leading to stimulation of the chemoreceptor channel TRPA1 to trigger CGRP release.18. Lastly, vasodilation from the oxidation of PKG1α at C4225, 26 raised a possibility that HNO could also target this residue and reduce vascular tone while bypassing sGC.
By using a gene targeting approach, we isolated the role of sGC and found it is indeed required and sufficient to explain HNO-vasodilation in vivo and in vitro. Use of CXL-1020 avoided ambiguities of AS which co-generates the vasodilator nitrite, or IPA/NO which donates HNO or NO depending on acid/base conditions35. CXL-1020 is also the only HNO donor to have been thus far administered to humans17 where arterial vasodilation and improved LV function were observed, conferring clinical relevance to the current findings. The negative results obtained in the PKG1αC42S mutant mice indicate that this redox modification does not contribute to HNO vasodilation. The control data in Figure 5 shows that CXL-1020 counters vasoconstriction with similar potency in both aorta and mesenteric arteries, both responses being largely blocked by ODQ. Though slight residual dilation was seen at the highest dose (as in the sGSKO aorta), this most likely reflected non-specific effects at this concentration. We did not study mesenteric arteries from sGCKO mice, but the failure of CXL-1020 to vasodilate in vivo indicated that resistance-vessel targeting was central.
The second major question we addressed was whether sGC is required for acute positive inotropic effects of HNO, as prior studies had also led to some conflicting results. Low levels sGC-derived cGMP can induce a positive inotropic response 27, 43 due to competitive inhibition of phosphodiesterase type 3 for cAMP hydrolysis28. However, at higher concentrations, cGMP stimulates PKG to phosphorylate troponin I and desensitize the myofilaments to Ca2+ 44, 45, reducing contractility. Cyclic GMP also activates PDE2 to increase cAMP hydrolysis30. PKG1α stimulation has not been linked to positive cardiac inotropy.
Given that sGC was required for HNO-stimulated vasodilation, some modulation of contraction seemed possible and indeed was recently reported in a study performed in isolated rat hearts perfused with constant-pressure crystalloid buffer31. The investigators found that in addition to vasodilation (reflected the rise in coronary flow), AS enhanced LV systolic pressure and dP/dtmx, both being suppressed by ODQ. However, in isolated crystalloid-perfused hearts, increasing coronary flow can itself result in enhanced ventricular function46, 47. By contrast, using a constant-flow isolated heart preparation, we found hearts lacking PLN displayed identical vasodilation to AS as in controls, yet no inotropic response13. This is consistent with direct evidence for HNO-mediated thiol modification of PLB32 and consequent dis-inhibition of the SR-ATPase13. Additional evidence against cGMP-mediated contractility from HNO is the failure of ODQ to suppress it in isolated myocytes12, 17, and finding that HNO-inotropy is additive to β-adrenergic stimulation in vivo and in vitro, whereas the latter is blunted by NO donors 11, 12. The present finding of similar in vivo HNO inotropy in the absence or presence of sGC indicates these alternative mechanisms are central, confirming a dichotomy for sGC dependent (vessels) and independent (heart) effects.
Our study has several limitations. One is that both inotropic and lusitropic responses to HNO were modest in the in vivo mouse heart, making it more difficult to assess cardiac responses. Given the small changes in inotropy, we relied on dP/dtmax/IP as the index that could be most reliably measured from multiple steady-state cycles. The difference between mouse and canine or human dose responses may stem from very high basal Ca2+ cycling in mice, with 90% or more of the Ca2+ recycled via the SR48 that leaves little room for HNO-derived contractile enhancement. Isolated murine myocytes display greater effects17 but this outcome could result from the lower temperatures used that slow basal Ca2+ cycling kinetics, and removal from intrinsic adrenergic stimulation. Species differences in circulating or intracellular thiols may also impact the HNO response and concentration needed for physiological effects. Lastly, we did not specifically examine the role of α1/β1 versus α2/β1 forms of sGC, as the sGCKO mouse deletes both. This contrasts to mice lacking solely α1 or α2 subunits, where compensatory effects from the other isoform are observed49.
In conclusion, we show that sGC activation is required and sufficient to explain arterial vasodilation from a pure HNO donor, but is not necessary for HNO positive inotropy. Redox targeting of HNO to C42 in PKG1α does not play a significant role to its vasomotor regulation. The importance of sGC to vascular modulation raises questions regarding its targeting in chronic disease conditions where the cyclase may become oxidized. While this can blunt NO-responsiveness, HNO appears to remain effective in such settings50. Lastly, the chronic influence of HNO-sGC interactions on cardiovascular disease remains unsettled. ODQ-suppressible anti-hypertrophic effects in myocytes exposed to the short-acting HNO donor IPA/NO have been reported51. These findings need to be confirmed using a stable and much longer-acting pure HNO donor combined with sGC gene-deletion, and then further tested in vivo to identify their translational potential.
Perspectives
The physiological biochemistry of HNO (nitroxyl) is attracting growing interest given its promising combination of vasodilator and positive inotropic effects that may benefit the failing heart. Unlike NO. , HNO-stimulated vasodilation does not induce tolerance. Furthermore, its vasorelaxant and inotropic properties are preserved in heart failure11 and diabetes52, both diseases that involve increased oxidative stress. The latter can suppress NO.- but not HNO-dependent signaling. The present study resolves controversies regarding mechanisms for arterial dilation and contractility enhancement by HNO by using genetic KO and KI mouse models to provide unambiguous support for sGC, but not PKG1α dependence for vasomotor responses. Their lack of influence on contractility modulation means that varying cGMP/PKG signaling as occurs with co-treatment by natriuretic peptides or PDE5 inhibitors is unlikely to impact HNO-mediated inotropy. In addition, reduced sGC functionality that might accompany cardiac disease is unlikely to impact the positive contractility efficacy from HNO donors.
Supplementary Material
Novelty and Significance.
1) What is New?
This study demonstrates that HNO requires soluble guanylate cyclase to effect systemic vasodilation in vivo. It also shows potential HNO targeting to PKG1α itself does not play a role in this vasomotor modulation. By contrast, positive inotropic and lusitropic actions exerted by HNO donors in vivo do not require the presence of sGC, and thus are fully cGMP independent.
2) What is relevant?
The mechanisms by which HNO impacts cardiac inotropy have been well studied at the molecular level, but data have remained limited with respect to its modulation of arterial tone; particularly in vivo. The current data provide definite evidence that sGC is the essential and required transducer of HNO-mediated vasorelaxation both in isolated vascular tissue and in the intact circulation.
3) Summary
Systemic arterial vasodilation by HNO requires sGC-dependent signaling, whereas cardiac contractility enhancement does not. HNO does not modulate vascular tone by forming an intermolecular disulfide in PKG1α at C42 to activate the kinase.
Acknowledgements
We thank Philip Eaton for generously providing the PKG C42S KI mice, and Cardioxyl Pharmaceuticals Inc. for providing CXL-1020.
Sources of Funding:
This study was supported by grants from the National Institute of Health (HL-119012, and HL093432), Deutsche Forschungsgemeinschaft (Fr 1725/1-5), a research grant from Cardioxyl Inc., and by the Japan Heart Foundation / Bayer Yakuhin Research Grant Abroad (DH).
Footnotes
Disclosures:
Drs. Paolocci and Kass are founders and scientific advisors to Cardioxyl, Inc.
Conflict of Interest:
The authors report no conflict of interest.
References
- 1.Flores-Santana W, Salmon DJ, Donzelli S, Switzer CH, Basudhar D, Ridnour L, Cheng R, Glynn SA, Paolocci N, Fukuto JM, Miranda KM, Wink DA. The specificity of nitroxyl chemistry is unique among nitrogen oxides in biological systems. Antioxid Redox Signal. 2011;14:1659–1674. doi: 10.1089/ars.2010.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukuto JM, Cisneros CJ, Kinkade RL. A comparison of the chemistry associated with the biological signaling and actions of nitroxyl (HNO) and nitric oxide (NO). J Inorg Biochem. 2013;118:201–208. doi: 10.1016/j.jinorgbio.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Andrews KL, Irvine JC, Tare M, Apostolopoulos J, Favaloro JL, Triggle CR, Kemp-Harper BK. A role for nitroxyl (HNO) as an endothelium-derived relaxing and hyperpolarizing factor in resistance arteries. Br J Pharmacol. 2009;157:540–550. doi: 10.1111/j.1476-5381.2009.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botden IP, Batenburg WW, de Vries R, Langendonk JG, Sijbrands EJ, Danser AH. Nitrite- and nitroxyl-induced relaxation in porcine coronary (micro-) arteries: Underlying mechanisms and role as endothelium-derived hyperpolarizing factor(s). Pharmacol Res. 2012;66:409–418. doi: 10.1016/j.phrs.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Favaloro JL, Kemp-Harper BK. The nitroxyl anion (HNO) is a potent dilator of rat coronary vasculature. Cardiovasc Res. 2007;73:587–596. doi: 10.1016/j.cardiores.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Yuill KH, Yarova P, Kemp-Harper BK, Garland CJ, Dora KA. A novel role for HNO in local and spreading vasodilatation in rat mesenteric resistance arteries. Antioxid Redox Signal. 2011;14:1625–1635. doi: 10.1089/ars.2010.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuto JM, Chiang K, Hszieh R, Wong P, Chaudhuri G. The pharmacological activity of nitroxyl: A potent vasodilator with activity similar to nitric oxide and/or endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1992;263:546–551. [PubMed] [Google Scholar]
- 8.Irvine JC, Favaloro JL, Widdop RE, Kemp-Harper BK. Nitroxyl anion donor, angeli's salt, does not develop tolerance in rat isolated aortae. Hypertension. 2007;49:885–892. doi: 10.1161/01.HYP.0000259328.04159.90. [DOI] [PubMed] [Google Scholar]
- 9.Pino RZ, Feelisch M. Bioassay discrimination between nitric oxide (NO.) and nitroxyl (NO-) using l-cysteine. Biochem Biophys Res Commun. 1994;201:54–62. doi: 10.1006/bbrc.1994.1668. [DOI] [PubMed] [Google Scholar]
- 10.Gao WD, Murray CI, Tian Y, Zhong X, DuMond JF, Shen X, Stanley BA, Foster DB, Wink DA, King SB, Van Eyk JE, Paolocci N. Nitroxyl-mediated disulfide bond formation between cardiac myofilament cysteines enhances contractile function. Circ Res. 2012;111:1002–1011. doi: 10.1161/CIRCRESAHA.112.270827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paolocci N, Katori T, Champion HC, St John ME, Miranda KM, Fukuto JM, Wink DA, Kass DA. Positive inotropic and lusitropic effects of HNO/NO- in failing hearts: Independence from beta-adrenergic signaling. Proc Natl Acad Sci USA. 2003;100:5537–5542. doi: 10.1073/pnas.0937302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tocchetti CG, Wang W, Froehlich JP, et al. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ Res. 2007;100:96–104. doi: 10.1161/01.RES.0000253904.53601.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sivakumaran V, Stanley BA, Tocchetti CG, et al. HNO enhances SERCA2A activity and cardiomyocyte function by promoting redox-dependent phospholamban oligomerization. Antioxid Redox Signal. 2013;19:1185–1197. doi: 10.1089/ars.2012.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Armouche A, Wahab A, Wittkopper K, Schulze T, Bottcher F, Pohlmann L, King SB, DuMond JF, Gerloff C, Boger RH, Eschenhagen T, Carrier L, Donzelli S. The new HNO donor, 1-nitrosocyclohexyl acetate, increases contractile force in normal and beta-adrenergically desensitized ventricular myocytes. Biochem Biophys Res Commun. 2010;402:340–344. doi: 10.1016/j.bbrc.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Dai T, Tian Y, Tocchetti CG, Katori T, Murphy AM, Kass DA, Paolocci N, Gao WD. Nitroxyl increases force development in rat cardiac muscle. J Physiol. 2007;580:951–960. doi: 10.1113/jphysiol.2007.129254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paolocci N, Saavedra WF, Miranda KM, Martignani C, Isoda T, Hare JM, Espey MG, Fukuto JM, Feelisch M, Wink DA, Kass DA. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc Natl Acad Sci USA. 2001;98:10463–10468. doi: 10.1073/pnas.181191198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabbah HN, Tocchetti CG, Wang M, Daya S, Gupta RC, Tunin RS, Mazhari R, Takimoto E, Paolocci N, Cowart D, Colucci WS, Kass DA. Nitroxyl (HNO): A novel approach for the acute treatment of heart failure. Circ Heart Fail. 2013;6:1250–1258. doi: 10.1161/CIRCHEARTFAILURE.113.000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eberhardt M, Dux M, Namer B, et al. H2s and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat Commun. 2014;5:4381. doi: 10.1038/ncomms5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irvine JC, Favaloro JL, Kemp-Harper BK. NO-activates soluble guanylate cyclase and Kv channels to vasodilate resistance arteries. Hypertension. 2003;41:1301–1307. doi: 10.1161/01.HYP.0000072010.54901.DE. [DOI] [PubMed] [Google Scholar]
- 20.Wanstall JC, Jeffery TK, Gambino A, Lovren F, Triggle CR. Vascular smooth muscle relaxation mediated by nitric oxide donors: A comparison with acetylcholine, nitric oxide and nitroxyl ion. Br J Pharmacol. 2001;134:463–472. doi: 10.1038/sj.bjp.0704269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodrich LE, Lehnert N. The trans effect of nitroxyl (HNO) in ferrous heme systems: Implications for soluble guanylate cyclase activation by HNO. J Inorg Biochem. 2013;118:179–186. doi: 10.1016/j.jinorgbio.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Zeller A, Wenzl MV, Beretta M, Stessel H, Russwurm M, Koesling D, Schmidt K, Mayer B. Mechanisms underlying activation of soluble guanylate cyclase by the nitroxyl donor Angeli's salt. Mol Pharmacol. 2009;76:1115–1122. doi: 10.1124/mol.109.059915. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Brandish PE, Di Valentin M, Schelvis JP, Babcock GT, Marletta MA. Inhibition of soluble guanylate cyclase by ODQ. Biochemistry. 2000;39:10848–10854. doi: 10.1021/bi9929296. [DOI] [PubMed] [Google Scholar]
- 24.Feelisch M, Kotsonis P, Siebe J, Clement B, Schmidt HH. The soluble guanylyl cyclase inhibitor 1h-[1,2,4]oxadiazolo[4,3,-a] quinoxalin-1-one is a nonselective heme protein inhibitor of nitric oxide synthase and other cytochrome p-450 enzymes involved in nitric oxide donor bioactivation. Mol Pharmacol. 1999;56:243–253. doi: 10.1124/mol.56.2.243. [DOI] [PubMed] [Google Scholar]
- 25.Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schroder E, Browning DD, Eaton P. Cysteine redox sensor in PKG1α enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 26.Prysyazhna O, Rudyk O, Eaton P. Single atom substitution in mouse protein kinase G eliminates oxidant sensing to cause hypertension. Nat Med. 2012;18:286–290. doi: 10.1038/nm.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vila-Petroff MG, Younes A, Egan J, Lakatta EG, Sollott SJ. Activation of distinct cAMPdependent and cGMP-dependent pathways by nitric oxide in cardiac myocytes. Circ Res. 1999;84:1020–1031. doi: 10.1161/01.res.84.9.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stangherlin A, Gesellchen F, Zoccarato A, Terrin A, Fields LA, Berrera M, Surdo NC, Craig MA, Smith G, Hamilton G, Zaccolo M. cGMP signals modulate cAMP levels in a compartment-specific manner to regulate catecholamine-dependent signaling in cardiac myocytes. Circ Res. 2011;108:929–939. doi: 10.1161/CIRCRESAHA.110.230698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66:12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 30.Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD, Zaccolo M. Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res. 2006;98:226–234. doi: 10.1161/01.RES.0000200178.34179.93. [DOI] [PubMed] [Google Scholar]
- 31.Chin KY, Qin C, Cao N, Kemp-Harper BK, Woodman OL, Ritchie RH. The concomitant coronary vasodilator and positive inotropic actions of the nitroxyl donor Angeli's salt in the intact rat heart: Contribution of soluble guanylyl cyclase-dependent and - independent mechanisms. Br J Pharmacol. 2014;171:1722–1734. doi: 10.1111/bph.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Froehlich JP, Mahaney JE, Keceli G, Pavlos CM, Goldstein R, Redwood AJ, Sumbilla C, Lee DI, Tocchetti CG, Kass DA, Paolocci N, Toscano JP. Phospholamban thiols play a central role in activation of the cardiac muscle sarcoplasmic reticulum calcium pump by nitroxyl. Biochemistry. 2008;47:13150–13152. doi: 10.1021/bi801925p. [DOI] [PubMed] [Google Scholar]
- 33.Lancel S, Zhang J, Evangelista A, Trucillo MP, Tong X, Siwik DA, Cohen RA, Colucci WS. Nitroxyl activates SERCA in cardiac myocytes via glutathiolation of cysteine 674. Circ Res. 2009;104:720–723. doi: 10.1161/CIRCRESAHA.108.188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO, 3rd, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates: Endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 35.Salmon DJ, Torres de Holding CL, Thomas L, Peterson KV, Goodman GP, Saavedra JE, Srinivasan A, Davies KM, Keefer LK, Miranda KM. HNO and NO release from a primary amine-based diazeniumdiolate as a function of pH. Inorganic Chemistry. 2011;50:3262–3270. doi: 10.1021/ic101736e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DuMond JF, King SB. The chemistry of nitroxyl-releasing compounds. Antioxid Redox Signal. 2011;14:1637–1648. doi: 10.1089/ars.2010.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miranda KM, Katori T, Torres de Holding CL, et al. Comparison of the NO and HNO donating properties of diazeniumdiolates: Primary amine adducts release HNO in vivo. Journal of Medicinal Chemistry. 2005;48:8220–8228. doi: 10.1021/jm050151i. [DOI] [PubMed] [Google Scholar]
- 38.Friebe A, Mergia E, Dangel O, Lange A, Koesling D. Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc Natl Acad Sci U S A. 2007;104:7699–7704. doi: 10.1073/pnas.0609778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cingolani OH, Kass DA. Pressure-volume relation analysis of mouse ventricular function. Am J Physiol Heart Circ Physiol. 2011;301:H2198–2206. doi: 10.1152/ajpheart.00781.2011. [DOI] [PubMed] [Google Scholar]
- 40.Senzaki H, Fetics B, Chen CH, Kass DA. Comparison of ventricular pressure relaxation assessments in human heart failure: Quantitative influence on load and drug sensitivity analysis. J Am Coll Cardiol. 1999;34:1529–1536. doi: 10.1016/s0735-1097(99)00362-9. [DOI] [PubMed] [Google Scholar]
- 41.Winters B, Mo Z, Brooks-Asplund E, Kim S, Shoukas A, Li D, Nyhan D, Berkowitz DE. Reduction of obesity, as induced by leptin, reverses endothelial dysfunction in obese (Lep(ob)) mice. J Appl Physiol. 2000;89:2382–2390. doi: 10.1152/jappl.2000.89.6.2382. [DOI] [PubMed] [Google Scholar]
- 42.Burgoyne JR, Eaton P. Detecting disulfide-bound complexes and the oxidative regulation of cyclic nucleotide-dependent protein kinases by H2O2. Methods Enzymol. 2013;528:111–128. doi: 10.1016/B978-0-12-405881-1.00007-0. [DOI] [PubMed] [Google Scholar]
- 43.Paolocci N, Ekelund UE, Isoda T, Ozaki M, Vandegaer K, Georgakopoulos D, Harrison RW, Kass DA, Hare JM. cGMP-independent inotropic effects of nitric oxide and peroxynitrite donors: Potential role for nitrosylation. Am J Physiol Heart Circ Physiol. 2000;279:H1982–H1988. doi: 10.1152/ajpheart.2000.279.4.H1982. [DOI] [PubMed] [Google Scholar]
- 44.Layland J, Li JM, Shah AM. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J Physiol. 2002;540:457–467. doi: 10.1113/jphysiol.2001.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee DI, Vahebi S, Tocchetti CG, Barouch LA, Solaro RJ, Takimoto E, Kass DA. PDE5A suppression of acute beta-adrenergic activation requires modulation of myocyte beta-3 signaling coupled to PKG-mediated troponin I phosphorylation. Basic Res Cardiol. 2010;105:337–347. doi: 10.1007/s00395-010-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westerhof N, Boer C, Lamberts RR, Sipkema P. Cross-talk between cardiac muscle and coronary vasculature. Physiol Rev. 2006;86:1263–1308. doi: 10.1152/physrev.00029.2005. [DOI] [PubMed] [Google Scholar]
- 47.Sutherland FJ, Shattock MJ, Baker KE, Hearse DJ. Mouse isolated perfused heart: Characteristics and cautions. Clin Exp Pharmacol Physiol. 2003;30:867–878. doi: 10.1046/j.1440-1681.2003.03925.x. [DOI] [PubMed] [Google Scholar]
- 48.Gao WD, Perez NG, Marban E. Calcium cycling and contractile activation in intact mouse cardiac muscle. J Physiol. 1998;507(Pt 1):175–184. doi: 10.1111/j.1469-7793.1998.175bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mergia E, Friebe A, Dangel O, Russwurm M, Koesling D. Spare guanylyl cyclase NO receptors ensure high NO sensitivity in the vascular system. J Clin Invest. 2006;116:1731–1737. doi: 10.1172/JCI27657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bullen ML, Miller AA, Dharmarajah J, Drummond GR, Sobey CG, Kemp-Harper BK. Vasorelaxant and antiaggregatory actions of the nitroxyl donor isopropylamine nonoate are maintained in hypercholesterolemia. Am J Physiol Heart Circ Physiol. 2011;301:H1405–1414. doi: 10.1152/ajpheart.00489.2011. [DOI] [PubMed] [Google Scholar]
- 51.Irvine JC, Cao N, Gossain S, Alexander AE, Love JE, Qin C, Horowitz JD, Kemp-Harper BK, Ritchie RH. HNO/CGMP-dependent antihypertrophic actions of isopropylaminenonoate in neonatal rat cardiomyocytes: Potential therapeutic advantages of HNO over NO. Am J Physiol Heart Circ Physiol. 2013;305:H365–377. doi: 10.1152/ajpheart.00495.2012. [DOI] [PubMed] [Google Scholar]
- 52.Leo CH, Joshi A, Hart JL, Woodman OL. Endothelium-dependent nitroxyl-mediated relaxation is resistant to superoxide anion scavenging and preserved in diabetic rat aorta. Pharmacol Res. 2012;66:383–391. doi: 10.1016/j.phrs.2012.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.