Abstract
Environmental exposure of parents or early in life may affect disease development in adults. We found that hypertension and renal injury induced by a high-salt diet were substantially attenuated in Dahl SS/JrHsdMcwiCrl (SS/Crl) rats that had been maintained for many generations on the grain-based 5L2F diet compared to SS/JrHsdMcwi rats (SS/Mcw) maintained on the casein-based AIN-76A diet (mean arterial pressure 116±9 vs. 154±25 mmHg; urinary albumin excretion 23±12 vs. 170±80 mg/day). RNA-seq analysis of the renal outer medulla identified 129 and 82 genes responding to a high-salt diet uniquely in SS/Mcw and SS/Crl rats, respectively, along with minor genetic differences between the SS substrains. The 129 genes responding to salt in the SS/Mcw strain included numerous genes with homologs associated with hypertension, cardiovascular disease, or renal disease in human. To narrow the critical window of exposure, we performed embryo transfer experiments in which single-cell embryos from one colony (SS/Mcw or SS/Crl) were transferred to surrogate mothers from the other colony, with parents and surrogate mothers maintained on their respective original diet. All offspring were fed the AIN-76A diet after weaning. Salt-induced hypertension and renal injury were substantially exacerbated in rats developed from SS/Crl embryos transferred to SS/Mcw surrogate mothers. Conversely, salt-induced hypertension and renal injury were significantly attenuated in rats developed from SS/Mcw embryos transferred to SS/Crl surrogate mothers. Together, the data suggests that maternal diet during the gestational-lactational period has substantial effects on the development of salt-induced hypertension and renal injury in adult SS rats.
Keywords: Salt-sensitivity, Dahl salt-sensitive, rats, hypertension, casein, blood pressure, renal injury
INTRODUCTION
Clinical and experimental results indicate that diet can have large effects on arterial blood pressure. The effects of sodium chloride intake in salt-sensitive hypertension are well described1–3; less well-appreciated are the effects of other dietary components. In humans, increased cholesterol, fat, and carbohydrate consumption has been associated with increased blood pressure; and increased protein intake has been linked to decreased arterial pressure4–7. Studies in animal models of hypertension have also demonstrated altered disease phenotypes depending on dietary protein8, carbohydrate9–12, and fat12, 13. Experiments in our laboratory have demonstrated that the development of hypertension and renal damage in SS (Dahl salt-sensitive) rats is also dependent upon components of the diet other than NaCl levels. Specifically, our studies have demonstrated that grain-based diets attenuate the degree of hypertension and renal damage compared to that observed in Dahl SS rats fed a casein-based diet14–16. Since the phenotypic characteristics of salt-sensitive disease in the Dahl SS rat are similar to those observed in humans1, 2 an understanding of these differences in this animal model of disease may provide important insight into the role of diet in humans. The mechanisms responsible for these sodium-independent dietary effects in the Dahl SS rat are unclear, however, particularly the timing of casein-based diet exposure which increases disease susceptibility.
Previous studies by others have shown that hypertension and renal damage in the SS model can be attenuated or delayed by transfer of embryos between genetically unrelated strains17, 18. Those studies demonstrated that SS rat embryos transferred into the SR (Dahl salt-resistant) strain had a marked decrease in blood pressure at 7 weeks of age18 and concluded that the gestational environment, but not the lactational environment, of SS rat embryos played a critical role in determining the hypertensive phenotypes of the resulting offspring. Similarly, transfer of SHR (Spontaneous Hypertensive Rat) embryos into the WKY (Wistar-Kyoto) strain resulted in a significant attenuation in blood pressure19 and it was shown by similar experiments that the SHR maternal in utero (gestational) and suckling (lactational) periods both contributed to the rate of blood pressure increase of the SHR, but not the WKY strain20. These embryo transfers between genetically distinct strains provide insight into the sensitivity of the SS and SHR rat strain offspring to genetically encoded differences between their developmental environments, however the exposure to dietary factors was tightly controlled in those studies. In contrast, building upon our earlier work, we hypothesized that salt-induced blood pressure changes and renal damage in the SS rat may be influenced most directly by the dietary exposure of SS rat embryos during early development.
To distinguish potential diet-induced altered mechanisms sensitizing casein-fed animals to salt-induced disease phenotypes, experiments were initially performed to phenotype SS rats derived from parents maintained on a 0.4% NaCl casein-based (C0.4) or 0.75% NaCl grain-based (G0.75) diet for many generations. The experimental rats were fed these diets identical to their parents from weaning to 6 weeks of age, after which the rats were then fed an identical high salt 4.0% NaCl casein-based (C4.0) diet for three weeks and the development of hypertension and renal damage was assessed. A significant effect of the initial casein-based diet was observed in the SS rats, leading to increased blood pressure change and renal injury after C4.0 intake. To assess potential mechanisms of this NaCl-independent effect on salt-induced hypertension, an RNA-seq analysis of transcripts from the renal outer medullary tissue of the rats fed the grain- or casein-based diets before and after high salt intake was performed. The outer medulla was chosen based upon the alterations in renal medullary function that have been observed in Dahl SS hypertension. Finally, reciprocal embryo transfer experiments were used to demonstrate and narrow the phase of life that confers significant sensitivity of the SS rat to casein-based diet exposure to the gestational/lactational period prior to weaning.
METHODS
Experimental Animals
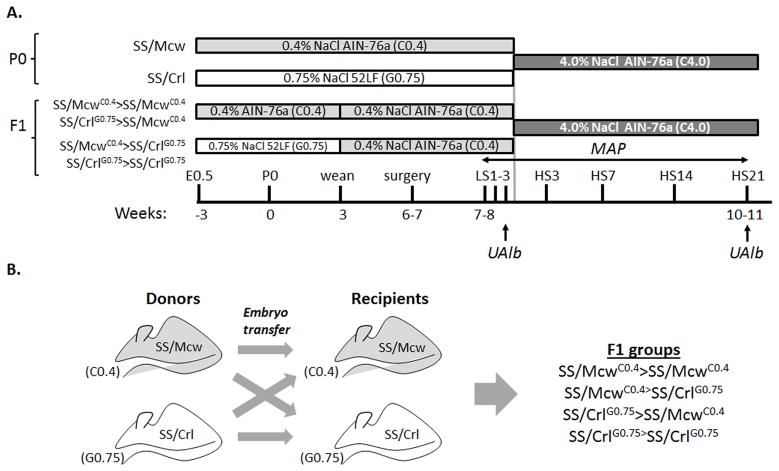
Experiments were performed on inbred Dahl SS rats obtained from two colonies that are maintained on different diets. Inbred SS/JrHsdMcwi rats (herein after called SS/Mcw) have been maintained as a closed colony at the Medical College of Wisconsin since 1991 and have been fed the casein-based 0.4% NaCl (C0.4) purified diet AIN-76A (Dyets, Inc., Bethlehem, PA) for many generations (http://www.hmgc.mcw.edu/ServiceCenters/RRSC.htm). Breeding pairs from this colony were sent to Charles River (SS/JrHsdMcwiCrl) in 2001 (herein after called SS/Crl), where they are maintained on a 0.75% NaCl milled grain (G0.75) diet 5L2F (Charles River Laboratories). The C0.4 AIN-76A and G0.75 5L2F diets are composed of approximately the same percentage of protein (18% for both), carbohydrates (60% to 65%), fat (5%), and fiber (4% to 5%). The diet feeding and phenotyping protocols and embryo transfer are shown in Figure 1.
FIGURE 1.
Phenotyping and embryo-transfer procedures. (A) Mean arterial pressure (MAP) and urinary albumin excretion (UAlb) were measured in SS/Mcw, SS/Crl and F1 offspring beginning at 7–8 weeks of age on C0.4 or G0.75 diets, respectively, for three days before switching animals to the C4.0 diet for 21 days. (B) An embryo transfer strategy to test the role of maternal in utero environmental exposure to the casein-based diet on blood pressure and renal injury. Embryos from SS/McwC0.4 mate pairs were transferred into either SS/McwC0.4 or SS/CrlG0.75 recipients (SS/McwC0.4>SS/McwC0.4, SS/McwC0.4>SS/CrlG0.75). Similarly, embryos from SS/CrlG0.75 mate pairs were transferred into those same recipients (SS/CrlG0.75>SS/McwC0.4, SS/CrlG0.75>SS/CrlG0.75).
For the parental (P0) strain studies, SS/Mcw and SS/Crl pups were maintained on their ‘parental’ C0.4 and G0.75 diets (SS/McwC0.4 and SS/CrlG0.75), respectively, from weaning though 6 weeks of age. At 7 weeks of age, SS/McwC0.4 and SS/CrlG0.75 rats were switched to the high-salt, casein-based AIN-76A diet containing 4.0% NaCl (C4.0) for three weeks. First generation (F1) offspring resulting from embryo transfers within and between the two strains were kept on the same diet as the recipient mother (C0.4 or G0.75), but were then weaned and fed the C0.4 diet from 3–6 weeks of age, before switching to the high salt C4.0 diet at 7 weeks of age. The numbers of studied animals and litter numbers for all groups are shown in Supplemental Table S1. The MCW Institutional Animal Care and Use Committee approved all experimental protocols.
Blood Pressure and Renal Disease Phenotyping
At approximately 6 weeks of age, the rats were deeply anesthetized with a mixture of ketamine (75 mg/kg i.p.), xylazine (10 mg/kg i.p.) and acepromazine (2.5 mg/kg i.p.) with supplemental anesthesia administered as needed. Using aseptic technique, a telemetry transmitter (Data Sciences International) for measuring arterial blood pressure was implanted in the carotid artery; the body of the transmitter implanted subcutaneously in the back of the animal. Animals were maintained on warming trays during and following surgery. Analgesics and antibiotics were administered after surgery to control pain and infection.
Following 5 days of recovery from surgery, daily blood pressure measurements were obtained (from 9:00am–12:00pm daily) for three days while the rats were maintained on the appropriate parental diet and are expressed as mean arterial pressure (MAP) throughout the manuscript. An overnight urine collection was obtained from the rats for the assessment of urinary excretion of albumin, protein, creatinine, and electrolytes. Daily blood pressure measurements were then obtained after 1, 2, 3, 7, 10, 14, and 21 days of the C4.0 diet. An overnight urine collection was also obtained on day 21 of high salt. At the conclusion of the experiment, the animals were deeply anesthetized with sodium pentobarbital, the kidneys were flushed with saline to remove red blood cells, and the kidneys were placed in formalin for histological analysis.
Urine electrolytes were measured by flame photometry (IL-943, Instrumentation Laboratories, Lexington, MA). Urine creatinine and protein values were measured with an autoanalyzer (ACE, Alfa Wasserman, Fairfield, NJ). Urine albumin was quantified with a fluorescent assay utilizing Albumin Blue 580 dye (Molecular Probes, Eugene, OR) and a fluorescent plate reader (FL600, Bio-Tek, Winooski, VT).
Histological Analysis of Kidney Tissues
The grading of glomerular and medullary damage was performed in a blinded manner. Formalin-fixed kidneys were paraffin-embedded in an automatic tissue processor (Microm HMP 300), cut in 3 μm sections (Microm HM355S), mounted on silanized/charged slides, and stained with Gomori’s One-Step Trichrome and the samples randomized. Slides were photographed using a Nikon E-400 fitted with a Spot Insight camera; digital micrographs were taken at different magnifications. Individual glomeruli (>40 per rat) were evaluated using a semiquantitative index method21, glomeruli lesions were scored from 0 (best) to 4 (worst) on the basis of glomerulosclerosis and mesangial expansion, where a lesion score of 1 represents involvement of 25% of the glomerulus while a lesion score of 4 indicates that 100% of the glomerulus is involved, as we previously described15. Thus, the most severely damaged glomeruli have a score of 4. The percentage of the outer medullary tissue containing blocked tubules filled with protein was quantified by determining the proportion of red-stained structures in this region using Metamorph Image Analysis software (version 4.6, Universal Imaging Systems Corp.).
RNA-seq
Transcriptomes in the renal outer medulla were analyzed in the SS/McwC0.4 and SS/CrlG0.75, as well as SS/Mcw and SS/Crl groups of male rats fed C4.0 chow for 14 days starting at 6 weeks of age. Each group included 4 rats that were age matched and were 8 weeks old at the time of tissue collection. Transcriptome analysis was done in each individual rat using RNA-seq as described previously22, 23. Briefly, Total RNA was extracted from isolated renal outer medulla using TRIzol (Invitrogen). Double stranded cDNA libraries were prepared from 4 μg of total RNA using TruSeq RNA Sample Preparation Kit (Illumina). Libraries were quantified by quantitative PCR. The libraries underwent cluster generation using TruSeq PE Cluster Kit v3-cBot-HS and 100 cycles of paired-end sequencing using TruSeq SBS Kit v3-HS (Illumina) and an Illumina HiSeq 2000 sequencer. Eight libraries were multiplexed on one lane of a 300 Gb flow cell.
The adapter sequences were removed from the output reads by the tool cutadapt (http://code.google.com/p/cutadapt/). Sequences with low quality (base quality < 13) at both ends of reads were further trimmed, and trimmed reads with less than 25 bp were removed using SolexaQA (http://solexaqa.sourceforge.net/). The remaining reads were aligned with genome (rn4) and exon junctions using Bowtie (http://bowtie-bio.sourceforge.net/index.shtml) and Tophat (http://tophat.cbcb.umd.edu/). A parsimonious set of transcripts was constructed from alignments using Cufflinks (http://cufflinks.cbcb.umd.edu/). Transcript abundance was expressed as Fragments Per Kilobase of exon per Million fragments mapped (FPKM). Finally, we tested differential expression of these identified transcripts using cuffdiff, implemented in the package Cufflinks. Pathway enrichment in differentially expressed genes was assessed by the DAVID pathway and gene ontology analysis tool (http://david.abcc.ncifcrf.gov/).
Statistical Analysis
All data are presented as the mean ± standard error of the mean (SEM). A one-way analysis of variance was utilized to determine the differences in parameters between the rats maintained on the different diets. A Tukey post-hoc test was used when appropriate. For RNAseq, the Benjamini-Hochberg method was used to control for false discovery rates (FDR). FDR<0.05 was considered significant. The 95% confidence interval was considered significant.
RESULTS
Salt-induced hypertension and renal injury were attenuated in SS/Crl rats compared to SS/Mcw rats
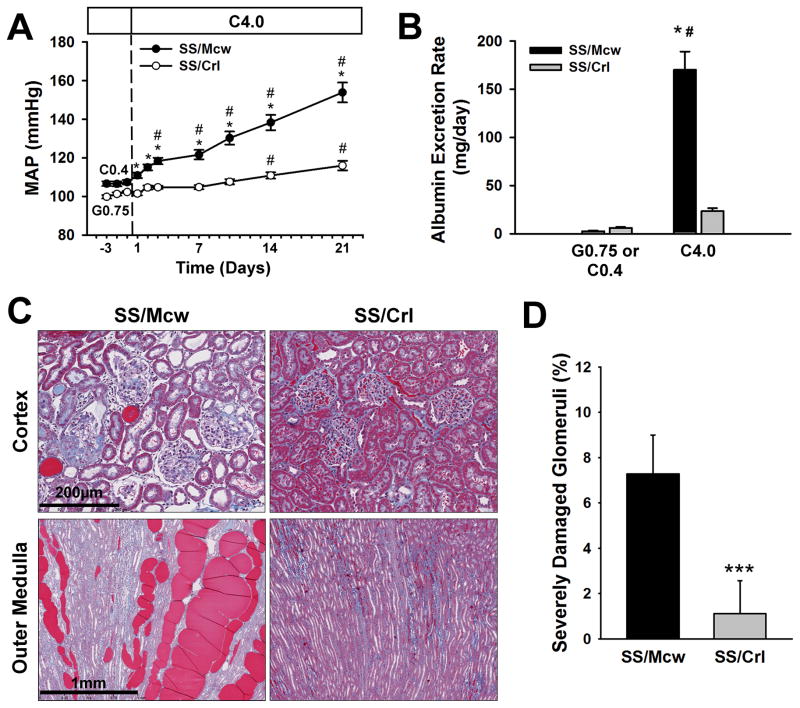
SS (Dahl salt-sensitive) rats fed two different diets were characterized for their response to a uniform high salt diet (Figure 2). The purified, casein-based AIN-76A diet (Dyets, Inc.) comes in two sodium chloride concentrations, 0.4% and 4.0%, while the grain-based 5L2F diet (Charles River Laboratories) contains 0.75% sodium chloride. We will refer to these diets as the C0.4, C4.0, and G0.75 diets, respectively, and refer to the C4.0 diet as a ‘high-salt’ diet (see Figure 1). Mean arterial blood pressure (MAP) and renal damage were assessed in the highly related (see Supplemental Table S2) SS/Mcw and SS/Crl substrains of Dahl salt-sensitive rats fed the C0.4 and G0.75 diets (SS/McwC0.4 and SS/CrlG0.75), respectively, for many generations. At six weeks of age, we measured baseline MAP and urinary albumin excretion (UAE) in male rats for three days, before switching them to the C4.0 high salt diet for 21 days. SS/McwC0.4 male rats were smaller than SS/CrlG0.75 males (178±4.8 g vs. 201±4.8g, p<0.005) at the beginning of the study, but ended at the same weight (357±12 vs. 353±5.0, respectively). No significant differences in mean arterial blood pressure (MAP) were detected between the SS/McwC0.4 and SS/CrlG0.75 rats during the three days of baseline recording. However, after switching both strains to the C4.0 high-salt diet, MAP was significantly greater than base line in SS/Mcw rats by the third day of the high salt intake (Figure 2a). The level of MAP continued to increase during the high salt period reaching a peak of 154±5 mmHg after three weeks. In contrast, the increase in MAP developed at a far slower rate in SS/Crl rats fed the same high salt diet, reaching a peak of 116±2 mmHg after 21 days of high salt intake.
FIGURE 2.
Assessment of hypertensive phenotypes in SS/Mcw and SS/Crl rats. (A) Mean arterial blood pressure (MAP) and (B) albumin excretion rate were measured in SS/Mcw (n=23–26) and SS/Crl (n=13–14) rats fed a C0.4 or G0.75 diet, respectively, followed by the C4.0 high-salt diet for 21 days. After 21 days of the C4.0 diet, kidneys were extracted, sectioned, and trichrome stained. (C) Cortex and outer medulla of the kidney of SS/MCW rats was far more damaged than SS/Crl rats. (D) Quantification of glomerular injury indicates that SS/MCW glomeruli are more damaged than SS/Crl glomeruli. # P<0.05 vs values obtained on the final day of C0.4 or G0.75 diet; * P<0.05 vs SS/Crl on the same day; *** P<0.001 vs SS/Mcw. Error bars represent SEM.
Similar to MAP, no differences in albumin excretion rate, as an index of renal injury, were measured between the SS/McwC0.4 and SS/CrlG0.75 rats (Figure 2b). Following three weeks of the C4.0 chow, both groups demonstrated an increase in albumin excretion rate, but the daily albumin (UAlb) excretion rate was significantly greater in SS/Mcw rats (170±18 mg/day) compared to SS/Crl (23±3 mg/day). Representative histological images of the renal cortex and outer medulla of the rats following three weeks of high salt are presented in Figure 2c. SS/Mcw kidneys after high-salt intake tended to be larger, but were not significantly different from SS/Crl. Consistent with previous reports in the Dahl SS/Mcw rat15, severe glomerular damage (blue fibrotic tissue and collapsed capillary structure) and blocked tubules in the outer medulla (red protein deposition casts) are readily apparent in the SS/Mcw rats. Less glomerular and tubular injury is evident in the kidneys of the SS/Crl rats. The overall glomerular injury index (n=5/group) was not significantly different between the groups (2.78±0.06% vs. 2.64±0.17%), however a significantly higher percentage of severely damaged glomeruli (glomerular lesion score of 4) were observed in the SS/Mcw males after high salt intake (7.3±0.8% vs. 1.1±0.7%, p<0.0003; Figure 2d). Similarly, the degree of tubular damage, as assessed by the area of the outer medulla stained positive for protein casts in dilated tubules, was significantly greater in the outer medulla of the SS/Mcw rats (17.0±0.7%) compared to SS/Crl (3.5±0.8%). Cumulatively, the data support our earlier findings of a susceptibility of the SS rat to the AIN-76A casein-based diet; where exposure to the casein-based C0.4 diet prior to 6 weeks of age sensitizes SS animals to a rapid increase in blood pressure and organ damage after switching to the high salt diet, while the grain-based G0.75 diet attenuates these effects.
Gene expression profiles and genes responsive to high salt were substantially different between SS/Mcw and SS/Crl
We reasoned that the altered sensitivity to high salt between SS/Mcw and SS/Crl could potentially be the result of gene expression differences induced in early development by dietary exposure. We performed RNA-seq analysis in the renal outer medulla in groups of naïve 6–7 week old SS/McwC0.4 and SS/CrlG0.75 male rats and similar groups after 14 days of C4.0 high-salt diet exposure (n=4 per group). The renal outer medulla has been demonstrated to play an especially important role in the development of hypertension and related renal injury24, 25. The yield of the RNA-seq analysis, quality of the sequencing reads, and the mapping rates are summarized in Supplementary Table S2. Transcript abundance data obtained from biological replicates were highly correlated (Supplementary Figure S1), supporting the reproducibility of the analysis. A comparison of expressed sequences confirmed a high degree of genetic identity between the two SS substrains. RNA-seq yielded 8× coverage for 172,301,707 nucleotides, or 5–6% of the SS/Mcw and SS/Crl rat genomes for comparison. Of these nucleotides, only 102 (or 0.00001%) simple nucleotide variants (SNVs) were different between the two colonies (Supplementary Tables S3). The 102 SNVs were not evenly distributed across the genome; 43 and 16 variants between the strains, respectively, were clustered in two regions on chromosomes 16 and X.
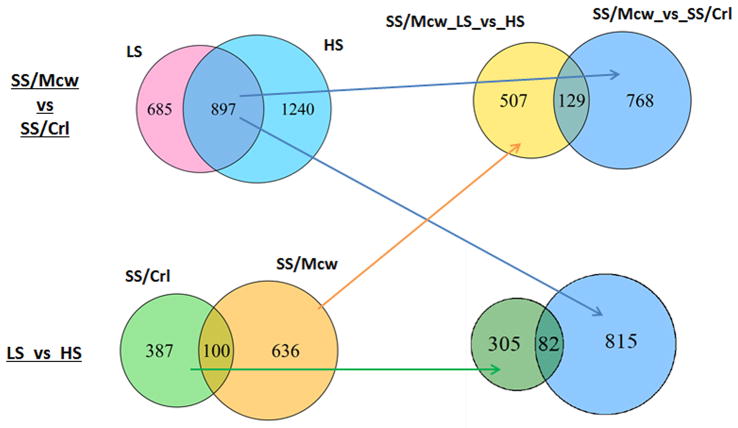
Substantial differences in the transcriptomes in the renal outer medulla existed between SS/Mcw and SS/Crl colonies. As shown in Figure 3, 1,582 genes were differentially expressed (FDR<0.05) between SS/McwC0.4 and SS/CrlG0.75 rats prior to the high-salt exposure, which was 2–3 fold greater than the number of genes responding to two weeks of high salt exposure in either colony of rats. Of the 1,582 genes, 897 (or 57%) remained differentially expressed following the high-salt exposure. This was a highly significant degree of overlap (Chi-square test P=0), consistent with a high degree of data reproducibility. Another 1,240 genes were differentially expressed between the two colonies of rats on high salt. Cluster analysis confirmed substantial differences in the transcriptomes existed between SS/Mcw and SS/Crl (Supplementary Figure S2). Interestingly, mostly overlapping subsets of 21 of the identified 102 SNVs were located within or nearby genes that are differentially expressed in each group between the parental and high salt diets (Supplemental Figure S3), suggesting that a small fraction of gene expression differences might arise from genetic differences between the strains.
FIGURE 3.
Numbers and overlaps of genes differentially expressed in the renal outer medulla. LS: C0.4 chow for SS/Mcw and G0.75 chow for SS/Crl; HS: C4.0 for two weeks.
The responses of the transcriptome to the high-salt exposure were largely different between the two colonies of rats, while some overlaps also existed. Of 487 genes responding to the high-salt diet in SS/Crl, 100 (or 21%) also responded to the high-salt diet in SS/Mcw (Figure 3). The degree of overlap was smaller than the overlap between prior to and after high-salt exposure, but was still highly significant (chi-square test P= 5.4×10−57). 636 genes responded to high salt only in SS/Mcw and 387 responded only in SS/Crl. KEGG pathways over-represented in the differentially expressed genes among the comparisons are shown in Table 1. Seven pathways were over-represented in genes responding to the two weeks of high salt exposure in SS/Mcw. Remarkably, five of the seven pathways (glutathione metabolism, tryptophan metabolism, drug metabolism, PPAR signaling, and histidine metabolism) overlapped with our previous study of SS/Mcw rats exposed to seven days of high salt diet which identified a total of nine pathways23. The degree of overlap was highly significant (Chi-square test P= 1.8×10−18), again demonstrating the high level of reproducibility of our transcriptome analysis. The pathways identified at one time point but not the other might contribute to the effects of the high salt diet in either early or established phases of the disease progression. Only one pathway (circadian rhythm) was over-represented in genes responding to the high salt diet in SS/Crl rats (Table 1). The Genes differentially expressed between SS/Mcw and SS/Crl rats prior to the high salt exposure were enriched for genes involved in immune function, hematopoietic cell lineage, and complement and coagulation cascades. As we emphasized previously26, the name of a canonical pathway may not always be informative. For example, the complement and coagulation cascades pathway includes the kallikrein-kinin system known to influence blood pressure.
Table 1.
Pathways enriched in gene expression comparisons between groups and diet
| KEGG Pathway | Count | Fold Enrichment | FDR |
|---|---|---|---|
| SS/Mcw, C0.4, vs. SS/Crl, G0.75 | |||
| rno04640:Hematopoietic cell lineage | 26 | 4.0 | 1.88E-06 |
| rno04610:Complement and coagulation cascades | 17 | 2.9 | 1.90E-01 |
| rno04662:B cell receptor signaling pathway | 16 | 2.5 | 1.36E+00 |
| rno04650:Natural killer cell mediated cytotoxicity | 19 | 2.3 | 1.65E+00 |
| rno05340:Primary immunodeficiency | 10 | 3.5 | 1.75E+00 |
| rno04666:Fc gamma R-mediated phagocytosis | 17 | 2.3 | 2.68E+00 |
| rno04060:Cytokine-cytokine receptor interaction | 29 | 1.8 | 4.12E+00 |
| rno04660:T cell receptor signaling pathway | 19 | 2.1 | 4.38E+00 |
| SS/Mcw, C4.0, vs. SS/Crl, C4.0 | |||
| rno00280:Valine, leucine and isoleucine degradation | 21 | 4.8 | 1.54E-06 |
| rno04670:Leukocyte transendothelial migration | 29 | 2.7 | 2.22E-03 |
| rno00071:Fatty acid metabolism | 15 | 3.8 | 2.07E-02 |
| rno04710:Circadian rhythm | 8 | 6.5 | 8.28E-02 |
| rno04610:Complement and coagulation cascades | 17 | 2.6 | 7.88E-01 |
| rno00640:Propanoate metabolism | 11 | 3.5 | 8.03E-01 |
| rno03320:PPAR signaling pathway | 17 | 2.5 | 9.30E-01 |
| rno04920:Adipocytokine signaling pathway | 16 | 2.5 | 1.45E+00 |
| rno05210:Colorectal cancer | 18 | 2.3 | 1.51E+00 |
| rno04514:Cell adhesion molecules (CAMs) | 27 | 1.9 | 1.59E+00 |
| rno04110:Cell cycle | 24 | 2.0 | 1.76E+00 |
| rno04640:Hematopoietic cell lineage | 17 | 2.3 | 2.66E+00 |
| rno04510:Focal adhesion | 32 | 1.7 | 3.04E+00 |
| rno04666:Fc gamma R-mediated phagocytosis | 18 | 2.2 | 3.81E+00 |
| SS/Mcw, C0.4 vs. C4.0 | |||
| rno00480:Glutathione metabolism | 14 | 6.6 | 1.02E-04 |
| rno00380:Tryptophan metabolism | 13 | 7.1 | 1.38E-04 |
| rno00982:Drug metabolism | 15 | 4.9 | 1.55E-03 |
| rno00280:Valine, leucine and isoleucine degradation | 9 | 4.6 | 6.89E-01 |
| rno00350:Tyrosine metabolism | 7 | 4.9 | 3.10E+00 |
| rno03320:PPAR signaling pathway | 10 | 3.3 | 3.24E+00 |
| rno00340:Histidine metabolism | 6 | 5.9 | 3.31E+00 |
| rno00980:Metabolism of xenobiotics by cytochrome P450 | 9 | 3.5 | 4.00E+00 |
| SS/Crl, G0.75 vs. C4.0 | |||
| rno04710:Circadian rhythm | 6 | 21.3 | 5.35E-03 |
| rno00980:Metabolism of xenobiotics by cytochrome P450 | 7 | 5.4 | 1.89E+00 |
| rno04115:p53 signaling pathway | 7 | 4.9 | 3.08E+00 |
| rno00480:Glutathione metabolism | 6 | 5.5 | 4.57E+00 |
Of the 897 genes differentially expressed between SS/Mcw and SS/Crl both prior to and after the high salt exposure, a set of 129 genes responded to high salt only in SS/Mcw and 82 responded only in SS/Crl (Figure 3). The 129 genes, which had a high likelihood of contributing to the disease progression in SS/Mcw, included 33 genes with homologs that have been genetically associated with human disease (Table 2). The 129 genes were enriched for Gene Ontology terms including “regulation of blood pressure” and, prominently, terms related to extracellular matrix that is central to the development of renal interstitial fibrosis (Supplementary Table S4). In contrast, the 82 genes uniquely responsive to high salt in the SS/Crl substrain had no known genetic association with hypertension, cardiovascular, or renal disease and were associated with just a few Gene Ontology terms, including oxygen transport. KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways were not significantly over-represented in either enriched gene set, likely due to the small number of genes.
Table 2.
Genes responding to high-salt in SS/Mcw animals which have been genetically linked to human disease
| Human trait | Genes |
|---|---|
| Hypertension | CASR, FGB, HMOX1, SLC9A3, ELN, SCN7A, ATP1A2 |
| Cardiovascular disease | CASR, OLR1, CORIN, SLC9A3, COL3A1, ELN, GJA1, MGP, ATP1A2, GCGR, TGFB2, FGG, FGB, HMOX1, FCGR2A, SCN7A, LOX, CD14 |
| Renal disease | TSC22D1, CASR, FGB, HMOX1, MGP, FCGR2A, MYC, CD14 |
Dietary effects on blood pressure and renal damage can be reversed by embryo transfer
Our previous studies demonstrated that grain-based diets attenuate the degree of hypertension and renal damage in SS/Mcw rats compared to those observed fed a casein-based diet14–16, and support the difference between the SS/McwC0.4 and SS/CrlG0.75 rats respectively, shown above. Subsequent RNAseq on these rats has now revealed a small number of SNVs correlating with gene expression differences that could potentially contribute to the altered phenotypes of these strains to high salt intake. We reasoned that reciprocal embryo transfers between the two strains would reveal whether the environmental (diet exposure) or genetic differences is the primary driver of the differences in between the SS/Mcw and SS/Crl to high salt. Shown in Figure 1 is the embryo transfer design and phenotyping strategy for the resulting F1 offspring, where animals were exposed to either the C0.4 or G0.75 diets in the gestational-lactational environment before all pups were weaned at 21 days of age to the same C0.4 diet. Animals were again measured for urine excretion parameters and MAP by telemetry for three days starting at 6–7 weeks of age, followed by 21 days of C4.0 high-salt feeding. Control embryo transfers within the same strain were used to control for confounding factors of the embryo transfer procedure on salt-induced phenotypes.
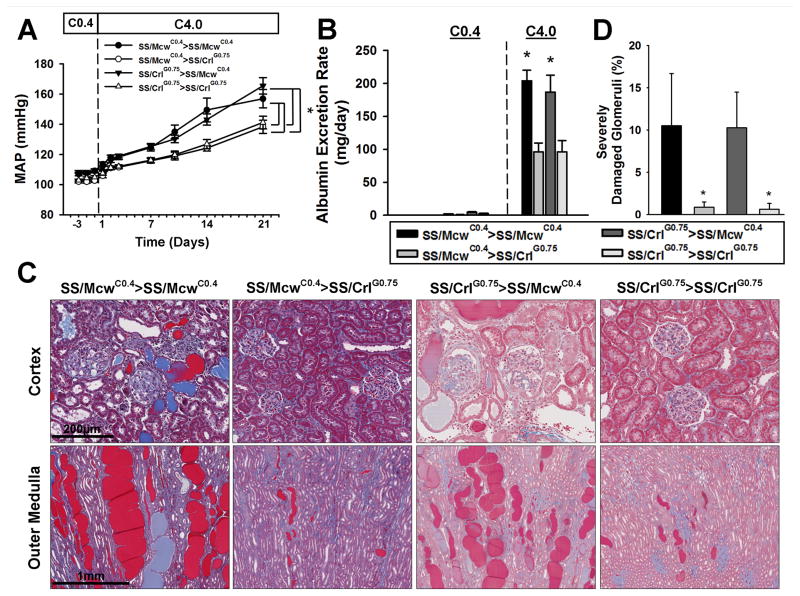
Similar to the parental substrains, F1 offspring were sensitized to high salt-induced changes in blood pressure and renal damage by exposure to the casein-based C0.4 diet. Male offspring of either strain born from embryos surgically transferred to SS/McwC0.4 recipients displayed significantly increased sensitivity to high-salt intake compared to F1 offspring resulting embryos of either strain surgically transferred to SS/CrlG0.75 recipients (Figure 4). MAP of offspring from SS/CrlG0.75 or control SS/McwC0.4 donors transferred into SS/McwC0.4 recipients (SS/CrlG0.75>SS/McwC0.4 and SS/McwC0.4>SS/McwC0.4, respectively) was significantly increased after high-salt intake compared to those resulting from transfer into SS/CrlG0.75 recipients (SS/CrlG0.75>SS/CrlG0.75 and SS/McwC0.4>SS/CrlG0.75; Figure 4a). Renal damage followed the same pattern, with SS/CrlG0.75>SS/McwC0.4 and SS/McwC0.4>SS/McwC0.4 offspring having larger and more damaged kidneys with higher UAlb, increased tubular casting, and a significantly higher percentage of severely damaged glomeruli compared to SS/CrlG0.75>SS/CrlG0.75 and SS/McwC0.4>SS/CrlG0.75 animals (Figure 4b-d). Similar differences in blood pressure and renal damage were seen in F1 females (Supplementary Figure S4), suggesting that the sensitizing effects of the C0.4 diet are not gender specific.
FIGURE 4.
Assessment of hypertensive phenotypes in embryo transferred F1 male rats. Following embryo transfer, offspring were phenotyped for (A) Mean arterial blood pressure (MAP) and (B) albumin excretion rate in response to being fed the C0.4 diet from weaning, followed by 21 days of the C4.0 diet. (C) Kidneys were extracted, sectioned, and trichrome stained to determine the level of renal damage between the groups. The SS/McwC0.4>SS/McwC0.4 and the SS/CrlG0.75>SS/McwC0.4 F1 kidneys were far more damaged compared to the SS/McwC0.4>SS/CrlG0.75 and the SS/CrlG0.75>SS/CrlG0.75 kidneys. (D) Consistent with these observations, SS/McwC0.4>SS/McwC0.4 and the SS/CrlG0.75>SS/McwC0.4 kidneys had significantly more severely-damaged glomeruli compared to the SS/McwC0.4>SS/CrlG0.75 and the SS/CrlG0.75>SS/CrlG0.75 glomeruli. *P<0.05 vs SS/McwC0.4>SS/CrlG0.75 and the SS/CrlG0.75>SS/CrlG0.75. Error bars represent SEM.
Table 3 provides a comparison of the parental SS/McwC0.4 and SS/CrlG0.75 rats to the F1 groups after C4.0 high salt intake. For all parameters with the exception of urinary sodium excretion (UNaE), F1 groups where embryos were transferred into the SS/McwC0.4 recipients demonstrated significantly worse disease than the groups from transfers into the SS/CrlG0.75 recipients. MAP and UAE in SS/CrlG0.75>SS/McwC0.4 (and control SS/McwC0.4>SS/McwC0.4) F1 offspring was comparable to the parental SS/Mcw group, demonstrating that the attenuated disease phenotypes of the SS/CrlG0.75 rats can be reversed by exposing embryos to the C0.4 diet during gestation and lactation. Because the SS/CrlG0.75>SS/McwC0.4 and SS/McwC0.4>SS/McwC0.4 phenotypes were indistinguishable, this also demonstrates that the observed genetic differences between the donor embryos of these SS substrains do not play a significant role in the observed sensitivity of blood pressure and renal damage in response to high salt intake. Notably, both MAP and renal damage in the F1 animals transferred into recipients and fed the G0.75 diet until weaning at 3-weeks of age (SS/CrlG0.75>SS/CrlG0.75 and SS/McwC0.4>SS/CrlG0.75) were not attenuated to the same levels as the SS/Crl animals fed the G0.75 diet for the 6-weeks prior to high salt intake (Table 3), suggesting that there is also a sensitizing effect of the C0.4 diet between 3 and 6 weeks of age.
Table 3.
Comparison of parental and F1 phenotypes after 21 days of high salt intake
| Group | n= | MAP (mmHg) | UAlb (mg/day) | %Glom score of 4 | Casting (%) | UNaE (mEq/day) | BW at surgery(g) | Final BW (g) | Kidney/ BW (g/g*1000) |
|---|---|---|---|---|---|---|---|---|---|
| SS/McwC0.4 | 23 | 154±5* | 170±18* | 7.3±0.8* | 17.0±0.7* | 11.0±0.7 | 178±4.8 | 357±12 | 4.45±0.40 |
| SS/McwC0.4 >SS/McwC0.4 | 8 | 157 ±6† | 204±16† | 10.5±2.8† | 21.7± 3.3† | 11.6± 0.4 | 145±3.9 | 309±6.8 | 5.70±0.18† |
| SS/CrlG0.75 >SS/McwC0.4 | 21 | 166 ±5‡ | 187±26‡ | 10.3±1.9‡ | 18.9± 1.7‡ | 10.2± 0.8 | 168±4.1 | 318±6.3 | 5.71±0.25‡ |
| SS/CrlG0.75 | 13 | 116 ±2 | 23±3 | 1.1±0.7 | 3.5±0.8 | 13.3± 0.7 | 201±4.8 | 353±5.0 | 3.93±0.07 |
| SS/McwC0.4 >SS/CrlG0.75 | 14 | 138 ±4 | 92±13 | 0.9±0.3 | 4.9±0.6 | 12.5± 0.4 | 157±2.6 | 331±3.7 | 4.56±0.11 |
| SS/CrlG0.75 >SS/CrlG0.75 | 14 | 141 ±4 | 96±17 | 1.4±0.8 | 7.2±2.5 | 12.0± 0.6 | 159±4.7 | 326±5.6 | 4.86±0.15 |
| SS/CrlC0.4 | 15 | 153 ±7 | 143±23 | ND | ND | 11.3± 0.9 | 173±4.8 | 353±17 | 5.13±0.39 |
significantly increased compared to SS/Crl, p<0.05;
significantly increased compared to SS/McwC0.4>SS/CrlG0.75, p<0.05;
significantly increased compared to SS/CrlG0.75>SS/CrlG0.75, p<0.05.
note animals in the SS/Crl parental group were maintained on the G0.75 diet until 6–7 weeks of age, whereas the SS/Mcw and all F1 group animals were all weaned to the C0.4 diet (see Figure 1). BW: body weight. BW at surgery was measured at ~6 weeks of age, before the 21 days of high salt intake.
While the embryo transfer experiments suggest that the genetic differences of the embryo do not impact disease progression, it did not rule out that the genetics of the recipient gestational environment could play a role. To address this, we intercrossed F1 SS/CrlG0.75>SS/CrlG0.75 animals that had been switched to the C0.4 diet at wean to generate a second generation of SS/Crl rats which had been exposed to the C0.4 diet for a full generation. As shown in Table 3, after 21 days of C4.0 chow, these SS/CrlC0.4 rats were indistinguishable from the parental SS/Mcw rats fed the C0.4 diet for many generations. We conclude that neither the genetic differences between the donor embryos nor the recipient females can account for the differences in salt sensitivity between SS/Mcw and SS/Crl rats. Cumulatively, the data suggest that exposure to the 0.4% NaCl casein-based diet during gestation, lactation, and up to 6 weeks of age sensitizes SS rats to high salt-induced hypertension and renal damage, while increasing exposure to the grain-based diet progressively attenuates salt sensitivity.
DISCUSSION
Previous studies from our laboratory have demonstrated that, compared to a grain-based diet, the 0.4% NaCl casein-based AIN-76A (C0.4) diet exacerbates the degree of hypertension and renal damage in SS/Mcw rats after high salt intake14–16. These observations were confirmed in SS/Mcw and SS/Crl animals fed the C0.4 diet and G0.75 diet for many generations, respectively. In parental SS/Mcw rats, F1 animals from embryo transfer into SS/McwC0.4 recipients, and SS/Crl rats fed the C0.4 diet for a whole generation, MAP and renal injury were markedly increased compared to those exposed to the G0.75 diet during gestation and early life. While both of these parental diets contain more salt than rats would normally consume27, likely contributing to cardiovascular disease in both SS/Mcw and SS/Crl rats, the present data indicate that the gestational-lactational environmental exposure to the C0.4 diet is sufficient and necessary to markedly sensitize SS animals to high-salt diet. The mean glomerular injury index scores were not different between SS/Mcw and SS/Crl animals, likely owing to the subjective nature of this scoring method, however, the numbers of severely damaged glomeruli (100% fibrosed) were clearly increased in C0.4 exposed SS/Mcw rats at the end of the study. Due to the fact that the sensitizing C0.4 diet contains less sodium than the G0.75 diet and that the attenuated phenotypes of the SS/Crl rats could be reversed by the C0.4 diet, we conclude that salt-independent components of the diet, and not genetic differences between the strains, is an important driver of salt-sensitivity in SS rats. These findings are significant for researchers using SS rats from colonies fed grain-based diets, such as SS/Crl.
Gene expression analysis identified more than 1500 genes differentially expressed between the SS/Mcw and SS/Crl strains and confirmed the high degree of genetic identity between the strains, though some observed genetic differences did map to genes which are differentially expressed between them. Unique subsets of 129 and 82 genes responded to high-salt intake in the SS/Mcw and SS/Crl. Among the 129 salt-induced genes in the SS/Mcw rats were several which have been genetically associated with hypertension, cardiovascular disease, or renal disease in humans and with pathways and gene ontologies associated with the control of blood pressure, further demonstrating significant parallels in disease mechanisms between the SS rat model and human hypertension. Further studies using this model and embryo transfer strategy can now be used to ascertain mechanisms by which diet exposure leads to initial gene expression differences and alter subsequent response to salt. For instance, the large number of genes differentially expressed in the outer medulla might suggest that the casein-based diet exposure alters kidney development and that the resulting gene expression changes potentially reflect altered cell composition. Alternatively, dietary exposure during development could result in altered patterns of epigenetic marks, such as CpG methylation or histone modifications, which lead to altered gene expression and salt sensitivity. Such epigenetic effects have been demonstrated to be heritable for other model systems28, 29 and have been postulated to play a significant role in human hypertension and other diseases30, 31. The SS rat model, with its sensitivity to diet and maternal environment and embryo transfer strategy, now provide a unique opportunity to dissect these potential mechanisms using modern high-throughput genomic techniques23, 32.
The embryo transfer strategy between these two strains again verifies the importance of the maternal environment, both gestational and lactational, in the susceptibility or resistance of SS rats to disease later in life. The important difference between this study and prior findings17–19 is the importance of the role of maternal diet as a driver of hypertension and renal damage, although we cannot completely rule out whether the observed genetic differences between the SS/Mcw and SS/Crl play a role in other phenotypes. The earlier studies of transfers between the SS and SR (Dahl salt-resistant)17, 18 demonstrated that the gestational environment was most important in delaying the progression of hypertension upon high salt intake. More studies involving cross-fostering between casein- and grain-fed SS rats are now needed to further narrow down whether the gestational or lactational exposure to the casein-based diet is most critical. However, an interesting new study by Tran, et al.33, points to potential confounding limitations of embryo studies in differentiating gestational environment and germ line effects. In that model, offspring of embryo transfers into uteroplacental insufficiency growth restricted embryo recipients gave rise underwent significantly accelerated growth in the peripubertal phase (8–12 weeks) and had altered cardiorenal function compared to naturally gestated restricted offspring. As growth trajectory may influence cardiovascular disease risk34, the authors cautioned the interpretation of cardiovascular phenotypes from these types of embryo transfer studies. In our model, comparing the growth of the embryo transferred SS/McwC0.4>SS/McwC0.4 F1 animals to the parental group of naturally mated SS/McwC0.4 rats (Table 3), we observe lower in body weight at surgery (~6 weeks of age, 18.5% reduction) and after 21 days of high salt intake (~10–11 weeks, 13.4% reduction), suggesting that we did not observe accelerated growth of the embryo transferred animals during this period. Indeed, all F1 groups had similar growth rates and were consistently smaller than naturally mated animals.
PERSPECTIVES
The present studies validate and demonstrate the significant parallels between the mechanisms of hypertension and renal damage between Dahl salt-sensitive rats and humans and indicate the importance of dietary protocol when using these rats. Exposure to the 0.4% NaCl casein-based AIN-76A diet during gestational-lactational period sensitizes the SS model to hypertension and renal damage after high salt intake, while the 0.75% NaCl grain-based 5L2F diet exposure during these same periods has strong attenuating effects. These diet exposures alter gene expression patterns in young animals by unknown mechanisms, which then alter the numbers and types of genes that respond to high salt, resulting in differences in their susceptibility to disease. We postulate that dietary exposure early in life of SS rats alters developmental programs, possibly through epigenetic mechanisms controlling gene expression, leading to altered gene expression patterns and possibly organ development to subsequently modulate disease susceptibility in adult animals.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
Dahl salt-sensitive (SS) rats fed a grain-based diet for many generations demonstrate significant attenuation of changes in mean arterial pressure and renal injury after high salt intake, which can be reversed by exposure to the casein-based diet in the gestational-lactational period.
Groups of highly genetically similar SS rats fed casein- or grain-based diets for many generations demonstrate significant differences in global gene expression and in the subsets of genes that respond to high salt intake.
What is relevant?
The present studies demonstrate that exposure to sodium-independent components of the diet during the gestational-lactational period can have a profound influence on disease phenotypes in the offspring as adults. The present studies were performed on inbred rats fed defined diets; further studies to explore similar effects in humans are warranted.
While many studies demonstrate therapeutic effects of casein in hypertensive humans and rats, exposure to dietary casein during the gestational-lactational period has significant deleterious effects on the adult SS rat model after high-salt intake, suggesting that exposure during early development could also lead to disease susceptibility later in life in humans.
Exposure to a casein-based diet significantly exacerbates disease-relevant phenotypes in widely studied Dahl SS rats, especially the SS/JrHsdMcwi and SS/JrHsdMcwiCrl available from Charles Rivers Laboratories. This is currently the only available commercial source of these animals.
Summary
Hypertension and renal injury induced by a high-salt diet are attenuated in Dahl SS/JrHsdMcwiCrl rats maintained for many generations on a grain-based diet compared to SS/JrHsdMcwi rats maintained on a casein-based diet. RNA-seq analysis identified unique subsets of genes responding to a high salt diet in each strain where genes responding to salt in the casein-fed SS/JrHsdMcwi strain included numerous genes with homologs associated with hypertension, cardiovascular disease, or renal disease in humans. To test the hypothesis that in utero exposure to casein was critical to induce salt-sensitivity in this model, we performed embryo transfer experiments between SS rats fed the casein or grain-based diets and found that hypertension and renal injury were substantially exacerbated in rats exposed to the casein diet in utero. Conversely, salt-induced hypertension and renal injury were significantly attenuated in rats exposed to the grain-based diet in utero. Together, the data suggests that maternal diet during the gestational-lactational period has substantial effects on the development of salt-induced hypertension and renal injury in adult SS rats.
Acknowledgments
We thank Hayley Lund, Louise Evans, Ph.D., Kristie Usa, and Robert P. Ryan for their excellent technical assistance in preparing animals and collecting tissues.
SOURCES OF FUNDING
These studies were supported by National Institutes of Health grants OD-8396, HL-116264, and HL-82798.
Footnotes
DISCLOSURES/CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare
References
- 1.Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension. 1994;23:531–550. doi: 10.1161/01.hyp.23.4.531. [DOI] [PubMed] [Google Scholar]
- 2.Grim CE, Wilson TW, Nicholson GD, Hassell TA, Fraser HS, Grim CM, Wilson DM. Blood pressure in blacks. Twin studies in barbados. Hypertension. 1990;15:803–809. doi: 10.1161/01.hyp.15.6.803. [DOI] [PubMed] [Google Scholar]
- 3.Kotchen TA, Cowley AW, Jr, Frohlich ED. Salt in health and disease--a delicate balance. The New England journal of medicine. 2013;368:2531–2532. doi: 10.1056/NEJMc1305326. [DOI] [PubMed] [Google Scholar]
- 4.Hajjar I, Kotchen T. Regional variations of blood pressure in the united states are associated with regional variations in dietary intakes: The nhanes-iii data. The Journal of nutrition. 2003;133:211–214. doi: 10.1093/jn/133.1.211. [DOI] [PubMed] [Google Scholar]
- 5.Hajjar IM, Grim CE, George V, Kotchen TA. Impact of diet on blood pressure and age-related changes in blood pressure in the us population: Analysis of nhanes iii. Archives of internal medicine. 2001;161:589–593. doi: 10.1001/archinte.161.4.589. [DOI] [PubMed] [Google Scholar]
- 6.Kesteloot H, Joossens JV. Relationship of serum sodium, potassium, calcium, and phosphorus with blood pressure. Belgian interuniversity research on nutrition and health. Hypertension. 1988;12:589–593. doi: 10.1161/01.hyp.12.6.589. [DOI] [PubMed] [Google Scholar]
- 7.Stamler J, Caggiula A, Grandits GA, Kjelsberg M, Cutler JA. Relationship to blood pressure of combinations of dietary macronutrients. Findings of the multiple risk factor intervention trial (mrfit) Circulation. 1996;94:2417–2423. doi: 10.1161/01.cir.94.10.2417. [DOI] [PubMed] [Google Scholar]
- 8.Nevala R, Vaskonen T, Vehniainen J, Korpela R, Vapaatalo H. Soy based diet attenuates the development of hypertension when compared to casein based diet in spontaneously hypertensive rat. Life sciences. 2000;66:115–124. doi: 10.1016/s0024-3205(99)00569-x. [DOI] [PubMed] [Google Scholar]
- 9.Mori Y, Murakawa Y, Yokoyama J, Tajima N, Ikeda Y, Nobukata H, Ishikawa T, Shibutani Y. Effect of highly purified eicosapentaenoic acid ethyl ester on insulin resistance and hypertension in dahl salt-sensitive rats. Metabolism: clinical and experimental. 1999;48:1089–1095. doi: 10.1016/s0026-0495(99)90120-8. [DOI] [PubMed] [Google Scholar]
- 10.Preuss HG, Knapka JJ, MacArthy P, Yousufi AK, Sabnis SG, Antonovych TT. High sucrose diets increase blood pressure of both salt-sensitive and salt-resistant rats. American journal of hypertension. 1992;5:585–591. doi: 10.1093/ajh/5.9.585. [DOI] [PubMed] [Google Scholar]
- 11.Young JB, Landsberg L. Effect of oral sucrose on blood pressure in the spontaneously hypertensive rat. Metabolism: clinical and experimental. 1981;30:421–424. doi: 10.1016/0026-0495(81)90173-6. [DOI] [PubMed] [Google Scholar]
- 12.Zhang HY, Reddy S, Kotchen TA. A high sucrose, high linoleic acid diet potentiates hypertension in the dahl salt sensitive rat. American journal of hypertension. 1999;12:183–187. doi: 10.1016/s0895-7061(98)00238-6. [DOI] [PubMed] [Google Scholar]
- 13.Shimokawa T, Moriuchi A, Hori T, Saito M, Naito Y, Kabasawa H, Nagae Y, Matsubara M, Okuyama H. Effect of dietary alpha-linolenate/linoleate balance on mean survival time, incidence of stroke and blood pressure of spontaneously hypertensive rats. Life sciences. 1988;43:2067–2075. doi: 10.1016/0024-3205(88)90356-6. [DOI] [PubMed] [Google Scholar]
- 14.De Miguel C, Lund H, Mattson DL. High dietary protein exacerbates hypertension and renal damage in dahl ss rats by increasing infiltrating immune cells in the kidney. Hypertension. 2011;57:269–274. doi: 10.1161/HYPERTENSIONAHA.110.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, Jacob HJ, Cowley AW., Jr Influence of diet and genetics on hypertension and renal disease in dahl salt-sensitive rats. Physiol Genomics. 2004;16:194–203. doi: 10.1152/physiolgenomics.00151.2003. [DOI] [PubMed] [Google Scholar]
- 16.Mattson DL, Meister CJ, Marcelle ML. Dietary protein source determines the degree of hypertension and renal disease in the dahl salt-sensitive rat. Hypertension. 2005;45:736–741. doi: 10.1161/01.HYP.0000153318.74544.cc. [DOI] [PubMed] [Google Scholar]
- 17.Kubisch HM, Gomez-Sanchez EP. Embryo transfer in the rat as a tool to determine genetic components of the gestational environment. Lab Anim Sci. 1999;49:90–94. [PubMed] [Google Scholar]
- 18.Kubisch HM, Mathialagan S, Gomez-Sanchez EP. Modulation of blood pressure in the dahl ss/jr rat by embryo transfer. Hypertension. 1998;31:540–545. doi: 10.1161/01.hyp.31.1.540. [DOI] [PubMed] [Google Scholar]
- 19.Lee JY, Azar SH. Wistar-kyoto and spontaneously hypertensive rat blood pressure after embryo transfer into different wombs and cross-suckling. Experimental biology and medicine (Maywood, NJ) 2010;235:1375–1384. doi: 10.1258/ebm.2010.010081. [DOI] [PubMed] [Google Scholar]
- 20.Di Nicolantonio R, Koutsis K, Westcott KT, Wlodek ME. Relative contribution of the prenatal versus postnatal period on development of hypertension and growth rate of the spontaneously hypertensive rat. Clin Exp Pharmacol Physiol. 2006;33:9–16. doi: 10.1111/j.1440-1681.2006.04317.x. [DOI] [PubMed] [Google Scholar]
- 21.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in dahl rats. Kidney Int. 1984;26:137–143. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- 22.Kriegel AJ, Liu Y, Liu P, Baker MA, Hodges MR, Hua X, Liang M. Characteristics of micrornas enriched in specific cell types and primary tissue types in solid organs. Physiol Genomics. 2013;45:1144–1156. doi: 10.1152/physiolgenomics.00090.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Liu P, Yang C, Cowley AW, Jr, Liang M. Base-resolution maps of 5-methylcytosine and 5-hydroxymethylcytosine in dahl s rats: Effect of salt and genomic sequence. Hypertension. 2014;63:827–838. doi: 10.1161/HYPERTENSIONAHA.113.02637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowley AW., Jr Long-term control of arterial blood pressure. Physiological reviews. 1992;72:231–300. doi: 10.1152/physrev.1992.72.1.231. [DOI] [PubMed] [Google Scholar]
- 25.Cowley AW, Jr, Mattson DL, Lu S, Roman RJ. The renal medulla and hypertension. Hypertension. 1995;25:663–673. doi: 10.1161/01.hyp.25.4.663. [DOI] [PubMed] [Google Scholar]
- 26.Yang C, Stingo FC, Ahn KW, et al. Increased proliferative cells in the medullary thick ascending limb of the loop of henle in the dahl salt-sensitive rat. Hypertension. 2013;61:208–215. doi: 10.1161/HYPERTENSIONAHA.112.199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martus W, Kim D, Garvin JL, Beierwaltes WH. Commercial rodent diets contain more sodium than rats need. Am J Physiol Renal Physiol. 2005;288:F428–431. doi: 10.1152/ajprenal.00310.2004. [DOI] [PubMed] [Google Scholar]
- 28.Nadeau JH. Transgenerational genetic effects on phenotypic variation and disease risk. Human molecular genetics. 2009;18:R202–210. doi: 10.1093/hmg/ddp366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson VR, Nadeau JH. Transgenerational genetic effects. Epigenomics. 2010;2:797–806. doi: 10.2217/epi.10.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cowley AW, Nadeau JH, Baccarelli A, et al. Report of the national heart, lung, and blood institute working group on epigenetics and hypertension. Hypertension. 2012;59:899–905. doi: 10.1161/HYPERTENSIONAHA.111.190116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation: Linking early developmental environment to adult disease. Reproductive Toxicology. 2007;23:297–307. doi: 10.1016/j.reprotox.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Liang M, Cowley AW, Jr, Mattson DL, Kotchen TA, Liu Y. Epigenomics of hypertension. Seminars in nephrology. 2013;33:392–399. doi: 10.1016/j.semnephrol.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran M, Gallo LA, Hanvey AN, Jefferies AJ, Westcott KT, Cullen-McEwen LA, Gardner DK, Moritz KM, Wlodek ME. Embryo transfer cannot delineate between the maternal pregnancy environment and germ line effects in the transgenerational transmission of disease in rats. Am J Physiol Regul Integr Comp Physiol. 2014;306:R607–618. doi: 10.1152/ajpregu.00523.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early growth and coronary heart disease in later life: Longitudinal study. BMJ (Clinical research ed) 2001;322:949–953. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.