Abstract
Background
Aspirin desensitization followed by high-dose aspirin therapy is routinely performed for patients with aspirin exacerbated respiratory disease (AERD). Little is known about the contributions of mediators other than cysteinyl leukotrienes to aspirin reactions and to the therapeutic benefit of high-dose aspirin therapy.
Objective
We investigated differences in urinary eicosanoid metabolites and blood eosinophils in subjects with AERD who tolerate and those who fail aspirin desensitization, and also in AERD subjects who were successfully treated with high-dose aspirin therapy.
Methods
29 subjects with AERD were stratified into those who tolerated aspirin desensitization (Group I) and those who did not (Group II). Urine was analyzed for eicosanoid metabolites at baseline, during aspirin reactions, and on high-dose aspirin therapy. Blood was analyzed for cell differentials at baseline and on aspirin therapy.
Results
Basal prostaglandin D2 metabolite (PGD-M) (13.6±2.7 vs. 7.0±0.8 pmol/mg Cr; P<.05), and thromboxane metabolite (TX-M) levels (1.4±0.3 vs. 0.9±0.1 pmol/mg Cr; P<.01) were higher in Group II than in Group I. During aspirin reactions, PGD-M remained unchanged while TX-M (0.7±0.1 pmol/mg Cr; P=.07) tended to decrease in Group I. In contrast, PGD-M rose dramatically in Group II (61.3±19.9 pmol/mg Cr; P<.05), while TX-M did not change. The decrease in FEV1 inversely correlated with basal urinary levels of both LTE4 and PGD-M. Blood eosinophil and basophil levels rose while urinary PGD-M (2.2±0.8 pmol/mg Cr, P<.001) decreased on two months of high-dose aspirin therapy in Group I.
Conclusion
Failure to tolerate aspirin desensitization in a subset of subjects with AERD is associated with PGD2 overproduction. The increase in blood eosinophils and basophils while on high-dose aspirin therapy may reflect the functional consequences of decreased PGD2 release and the therapeutic benefit of aspirin.
Keywords: Aspirin exacerbated respiratory disease, Samter's triad, Nasal polyps, Asthma, Prostaglandin D2, Thromboxane, Aspirin desensitization, Cysteinyl leukotrienes, Urinary eicosanoids, Eosinophils
INTRODUCTION
Aspirin exacerbated respiratory disease (AERD) is characterized by asthma, eosinophilic nasal polyposis, and respiratory reactions upon ingestion of cyclooxygenase (COX)-1 inhibitors. The pathogenesis of AERD involves dysregulation of arachidonic acid (AA) metabolism, as indicated by excessive basal generation of the cysteinyl leukotrienes (cysLTs), LTC4, LTD4 and LTE4, which is further increased by COX-1 inhibitors. A long-standing hypothesis for the etiology of the reactions to COX-1 inhibitors in AERD has been that COX-1 inhibition dysregulates 5-lipoxygenase (5-LO), resulting in overproduction of cysLTs. Reactions typically occur at a threshold aspirin dose of 40-160 mg (sufficient to block COX-1 but not COX-2), with symptoms generally confined to the respiratory tract. During reactions, both eosinophils and basophils are rapidly recruited to the respiratory tissue, accompanied by a decline in blood eosinophil levels 1,2. The reaction is usually followed by a desensitized state, during which time patients can benefit from high-dose daily aspirin which improves disease control3,4. Neither the mechanism responsible for the recruitment of effector cells nor the basis of the therapeutic benefit of high-dose aspirin are known.
AA is metabolized intracellularly to LTC4 through the actions of 5-LO and LTC4 synthase5-7. LTC4 is released and converted to LTD4, which in turn is rapidly metabolized to the stable end-metabolite LTE4 and is excreted in the urine8. At least three receptors (CysLT1, CysLT2, and GPR99)9-12 mediate the actions of cysLTs in vivo. Urinary LTE4 measurements reflect systemic cysLT production, and basal levels of LTE4 in the urine of subjects with AERD are higher than those in aspirin-tolerant asthmatic (ATA) controls11. Subjects with AERD who experience large (>30%) decreases in forced expiratory volume in 1 second (FEV1) during aspirin reactions have both higher basal levels of urinary LTE4 and greater aspirin-induced increases in LTE4 than subjects who experience lesser reductions in airflow13. Drugs that inhibit CysLT1 receptors or 5-LO attenuate the severity of respiratory symptoms and blunt the reductions in FEV1 that occur during aspirin reactions, validating the role of cysLTs14. Although the efficacy of these drugs justifies their use as prophylaxis for desensitization to aspirin, therapeutic responses to them are not uniform among subjects with AERD, and some experience severe aspirin reactions despite prophylaxis. Therefore it is likely that mediators other than cysLTs contribute to the disease in general and to the reactions to COX-1 inhibitors in particular. However, little is known about these other mediators.
COX-1 and COX-2 convert AA to prostaglandin (PG)H2, which is converted to PGE2, PGD2, PGF2, thromboxane (TXA2) and prostacyclin (PGI2) by cell type-restricted synthases. PGE2 can induce bronchodilation15, suppress the activation of mast cells and eosinophils, and block 5-LO activation16,17. While PGE2 is thought to play a protective role in AERD, less is known about the roles of other COX products. PGD2, the major COX product of mast cells, and its metabolite 9α,11β-PGF2, induce bronchoconstriction in asthmatic subjects18, and asthmatic subjects are hyperresponsive to PGD2 compared with healthy controls19. PGD2 is also a vasodilator20, and is chemotactic for eosinophils, basophils, Th2 cells, and ILC2 cells by acting at chemokine receptor homologous molecule expressed on Th2 lymphocytes (CRTH2), also known as the D prostanoid 2 (DP2) receptor21,22. The plasma levels of 9α,11β-PGF223,4 in AERD exceed those in ATA and healthy controls, and increase modestly within minutes of aspirin challenge24. Bronchoalveolar lavage (BAL) fluid levels of PGD2 remain unaffected by endobronchial challenge of subjects with AERD using lysine aspirin, whereas metabolites of other PGs decrease25. These studies suggest that COX function and production of PGD2 in AERD may be at least partly resistant to aspirin. Although the bronchoconstriction, vasodilation, and inflammation-promoting actions of PGD2 fit with a role in AERD, its functions in the disease remain largely unexplored.
We identified a subset of patients with AERD who did not tolerate desensitization to oral aspirin. Despite prophylaxis with the CysLT1 receptor antagonist montelukast, these patients failed to advance beyond a threshold dose of aspirin or had difficulty tolerating escalating doses of aspirin due to ongoing cutaneous and/or gastrointestinal symptoms. We hypothesized that differences in eicosanoid generation may account for the differences in reaction severity and the development of systemic extrapulmonary symptoms in this subgroup. Here we demonstrate that subjects with AERD who are unable to tolerate desensitization display markedly dysregulated PG production, particularly PGD2, which increases dramatically during their reactions. The production of PGD2 correlates with the severity of airflow obstruction during clinical reactions. We also show that subjects who are successfully desensitized and then treated with high-dose aspirin exhibit sharply reduced systemic production of PGD2, but not of cysLTs, and demonstrate that reduced tissue flux of classical effector cells (eosinophils and basophils) is potentially a beneficial consequence of the reduced PGD2 generation.
METHODS
Patient selection and stratification
Subjects with AERD who underwent aspirin desensitization at Brigham and Women's Hospital (Boston, MA) between 2009 and 2014 and signed informed consent to have urine and/or blood samples collected were included. All subjects had a history of asthma, nasal polyposis and characteristic respiratory reactions upon ingestion of COX-1 inhibitors. All were offered desensitization due to refractory rhinosinusitis and/or nasal polyposis, as specified in the 2007 practice parameter26. Aspirin desensitizations were performed while patients were not receiving the 5-LO inhibitor zileuton so that the production of cysLTs could be monitored. Patients took their regularly prescribed inhaled corticosteroids (ICS) with or without long-acting beta agonists the morning of desensitization as applicable. ICS use at the time of desensitization was recorded as low, medium, and high dose27. All subjects except two received montelukast (10mg) the evening prior to and the morning of aspirin desensitization to attenuate the severity of respiratory symptoms during the reaction14. Patients were assigned to either Group I (those who tolerated desensitization, n = 23) or Group II (those who failed to tolerate the procedure due to intractable abdominal pain, rash, or unresolved lower respiratory tract symptoms, n=6). Demographic and clinical data was extracted from the medical record at the time of desensitization.
Control subjects with ATA had a history of physician-diagnosed asthma and had tolerated a COX-1 inhibitor in the past 6 months. All subjects were non-smokers.
Aspirin desensitization protocol
Two subjects from Group I had a history of a previous reaction to non-steroidal anti-inflammatory drug (NSAID) ingestion that required epinephrine administration and underwent aspirin desensitization in our medical ICU; all other subjects underwent desensitization in our outpatient clinic. Oral aspirin desensitizations started with 40 mg of aspirin followed by dose increases (81 mg, 162 mg, 325mg) every 90 minutes28. Patients were observed for respiratory symptoms, ocular injection, flushing, rash and abdominal pain. The aspirin dose that caused upper and/or lower respiratory symptoms was recorded as the provocative dose. FEV1 for each patient in the outpatient clinic was recorded at baseline, prior to each dose, and at the time of reaction.
Urinary eicosanoid measurements
Basal (pre-aspirin administration) urine samples were collected from all subjects. Urine was also collected for AERD subjects 180 minutes after the onset of aspirin-induced reactions. 14 subjects with AERD also provided urine samples after at least 8 weeks of aspirin 1300 mg daily. ATA subjects provided basal urine samples which were collected off of all NSAIDs for more than 1 week. A subset of ATA subjects (n=5) also had urine collected 3 hours after ingestion of 325 mg of aspirin. All urine samples were stored at −80°C and analyzed via gas chromatography-mass spectrometry (GC-MS) at Vanderbilt University. As described previously, concentrations of LTE429, the major urinary thromboxane metabolite 11-dehydrothromboxane B2 (TXB-M)30, the major prostaglandin D2 metabolite 9α,11β-dihydroxy-15-oxo-2,3,18,19-tetranorprost-5-ene-1,20-dioic acid (PGD-M)31, the prostaglandin E metabolite 9,15-dioxo-11α-hydroxy-13,14-dihydro-2,3,4,5-tetranor-prostan-1,20-dioic acid (tetranor-PGE-M), and the prostacyclin metabolite 2,3-dinor-6-keto-PGF1α (PGI-M), were measured and reported as pmol/mg creatinine.
Peripheral blood leukocyte counts
Basal and on high-dose aspirin blood was collected for complete blood counts (LabCorp, Burlington, NC) in a subset of Group I subjects (n=11).
Statistics
All data presented are the mean + standard error of the mean (SEM) and were normally distributed. Baseline clinical characteristics and signs of the reaction to aspirin were compared between AERD Group I and Group II subjects using a two-tailed t-test or for categorical data, a Fisher's test. Basal and post-aspirin ingestion eicosanoids from ATA, Group I and Group II subjects were compared using a one-way ANOVA followed by unpaired, two-tailed t-tests if the ANOVA P-value was less than .05. P-values reported represent t-test results. Basal and reaction eicosanoids for each subject were compared using a paired two-tailed t-test. Pearson's correlation coefficient was used for comparisons between urinary eicosanoid levels and fall in FEV1 or change in blood eosinophils. For all analyses, a P <.05 was considered statistically significant. All analyses were performed using GraphPad Prism version 6.03 for Windows, GraphPad Software, La Jolla, CA, www.graphpad.com.
The Brigham and Women's Hospital human subjects Institutional Review Board approved the study and all subjects provided written consent.
RESULTS
Clinical characteristics of patients and reactions
Between 2009 and 2014, 111 subjects with AERD underwent aspirin desensitizations at our institution and agreed to participate in clinical research. Of these, 29 subjects provided urine for eicosanoid analysis, and 11 provided blood for complete blood count before desensitization and after 2 months of aspirin treatment. 23 (Group I) completed the desensitization and successfully initiated treatment with high-dose aspirin, and 6 (Group II) were unable to tolerate the desensitization due to marked extrapulmonary symptoms (n=5) or failure of lower respiratory tract symptoms to resolve (n=1). Age, race, baseline FEV1 (% predicted), FEV1/FVC ratio, ICS use, total IgE and peripheral blood eosinophil counts were not statistically different between the groups (Table I). Use of ICS or oral steroids remained the same throughout the study.
Table I.
Baseline clinical characteristics according to group stratification.
| Clinical Characteristics | Aspirin-Tolerant Asthmatics (n=10) | Group I AERD (n=23) | Group II AERD (n=6) | P Value (Group I vs. Group II) | |||
|---|---|---|---|---|---|---|---|
| mean | SEM | mean | SEM | mean | SEM | ||
| Age (years) | 36.3 | ±3.3 | 47.3 | ±2.3 | 47.3 | ±1.7 | .99 |
| Male | 40% | 39% | 50% | .67 | |||
| Baseline FEV1 (% predicted) | 91% | ±6 | 84% | ±3 | 86% | ±4 | .63 |
| FEV1/FVC | 80% | ±3 | 73% | ±2 | 71% | ±2 | .87 |
| Total IgE (IU/mL) | 168.0 | ±35 | 344.4 | ±85 | .22 | ||
| Peripheral Blood Eosinophils (k/μL) | 0.66 | ±0.11 | 0.40 | ±0.20 | .16 | ||
| ICS + LABA | 90% | 91% | 100% | .33 | |||
| High | 0% | 9% | 17% | ||||
| Medium | 10% | 57% | 50% | ||||
| Oral Prednisone | 0% | 13% | 17% | 1.0 | |||
| Montelukast Use | 40% | 91% | 100% | 1.0 | |||
Data are displayed as mean ± standard error of the mean (SEM). FEV1 – forced expiratory volume; ICS - inhaled corticosteroid; LABA – long-acting beta agonist.
Subjects in both AERD Group I and Group II experienced reductions in their FEV1 (15 ± 3% for Group I, 30 ± 10% for Group II, P < .05, Table II) during reactions to aspirin. The provocative aspirin dose (log2) that triggered the reaction was not significantly different between the two groups (Table II) and did not correlate with PG metabolite levels. Abdominal pain during reaction was more common (P<.05) and more severe in Group II with 4 subjects reporting sharp stabbing pain, 3 reporting nausea, and 1 subject experiencing watery diarrhea. A rash appeared during the reactions in all subjects in Group II, while none of the subjects in Group I developed rash (P<.001). The rash consisted of an erythematous and pruritic macular eruption that began on the distal upper and lower extremities bilaterally. It often involved the palmar and plantar surfaces (Fig 1), spread proximally and was not associated with urticaria or angioedema. Five of the 6 subjects in Group II were unable to tolerate aspirin therapy with dose escalation due to the persistence of gastrointestinal symptoms and/or rash. The one subject in Group II who eventually was able to tolerate aspirin therapy required a slower desensitization spaced over 2 days but continued to have rash and lower respiratory reactions with each successive dose of aspirin.
Table II.
Clinical characteristics of AERD subjects stratified by group following the provocative dose of aspirin.
| Clinical Characteristics During Aspirin Desensitization | |||||
|---|---|---|---|---|---|
| Group I AERD (n=23) | Group II AERD (n=6) | P Value | |||
| mean | SEM | mean | SEM | ||
| Maximum Change in FEV1 | −15% | ±3 | −30% | ±10 | <.05 |
| Abdominal Symptoms | 26% | 83% | <.05 | ||
| Rash | 0% | 100% | <0.001 | ||
| Provocative Aspirin Dose (mg) | 113 | ±16 | 68 | ±30 | 0.18 |
Data are displayed as mean ± standard error of the mean (SEM). FEV1 – forced expiratory volume.
Figure 1.
Rash observed following provocative dose of aspirin in 3 Group II AERD patients.
Urinary eicosanoid measurements and relationship to clinical outcomes
Basal levels of urinary LTE4 were higher in AERD Group II (2.9±1.0 pmol/mg Cr) than in the ATA control group (0.2±0.09 pmol/mg Cr, P<.01, Fig 2). Compared to their respective pre-reaction basal levels, the urinary levels of LTE4 in both AERD groups increased during the reaction to aspirin (Group I 10.5±1.8, Group II 16.5±4.6 pmol/mg Cr, P<.05 for both). Peak LTE4 levels were not significantly different.
Figure 2. Basal and post-aspirin (ASA) urinary LTE4 levels.
Basal and aspirin-induced urinary LTE4 levels analyzed by GC-MS from ATA controls (basal n=10, post-aspirin n=5), Group I AERD subjects (n=23) and Group II AERD subjects (n=6) are shown. Data are expressed as mean +SEM (٭P<.05, ٭٭P<.01).
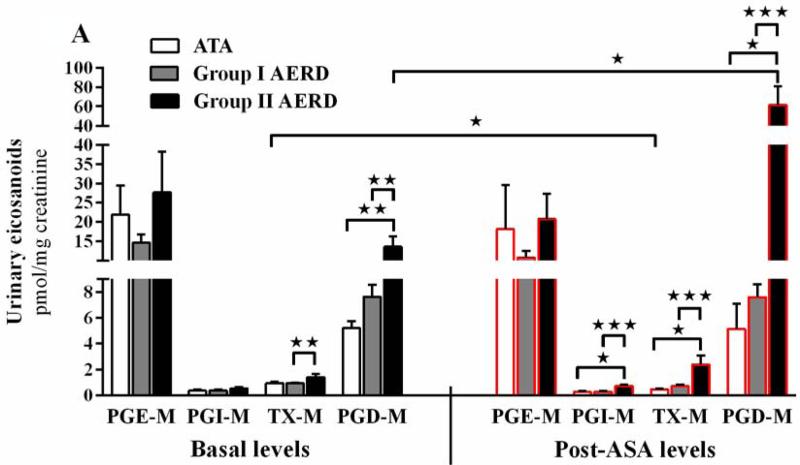
PGE-M was the most abundant prostanoid metabolite detected at baseline in all three groups (Fig 3,A, left panel). The basal levels of PGD-M (P<.05) and TX-M (P<.01) were higher in the Group II (13.6±2.7 and 1.4±0.3 pmol/mg Cr, respectively) than the Group I (7.0±0.8 and 0.9±0.1 pmol/mg Cr, respectively). Total basal urinary PG metabolites in Group II subjects exceeded that in Group I subjects by ~2-fold (43±2 vs. 23±2 pmol/mg Cr, P<.01, not shown).
Figure 3. Basal and post-aspirin (ASA) PG levels.
(A) Basal (left panel) and post-aspirin administration (right panel) urinary PG levels in subjects with ATA (basal n=10, post-aspirin n=5), Group I (n=23) and Group II (n=6) are shown. (B) Log2 of change from basal levels in urinary PGs induced by aspirin administration is shown for the three patient groups. Data are expressed as mean +SEM (٭P<.05, ٭٭P<.01, ٭٭٭P<.001).
Following the provocative dose, urinary PGD-M increased in 8, decreased in 12, and remained unchanged in 3 of the 23 subjects in Group I, but increased in all 6 subjects in Group II. As a result, the level of PGD-M during the reaction was ~7-fold higher in the urine of Group II subjects than Group I subjects (61.3±19.9 vs. 9.6±1.8 pmol/mg Cr, respectively, Fig 3,A, right panel, P <.001). Those 8 subjects in Group I who showed increased PGD-M during reaction had a basal level of 5.6±0.6 which increased to 18.2±3.2 pmol/mg Cr. The levels of PGI-M (0.7±0.2 vs 0.3±0.05 pmol/mg Cr; P <.001) and TX-M (2.4±0.7 vs. 0.7±0.1 pmol/mg Cr; P <.001) during the reaction were significantly higher in the post-aspirin urine of Group II than Group I (Fig 3,A).
Total PG metabolites in the urine of Group II subjects were significantly greater than total metabolites in the urine of the Group I subjects during the reaction (85±22 vs. 21±3 pmol/mg Cr, P <.001, not shown). All PG metabolites decreased in the urine of the ATA controls (n=5, data not shown) after the ingestion of 325 mg of aspirin with the exception of one subject who had a rise in PGD2. Figure 3B shows the log2 of the fold change (post-aspirin compared to basal level) of each metabolite in all three subject groups.
Basal and reaction eicosanoids were assessed for correlation with fall in FEV1 during aspirin reaction. Both basal urinary LTE4 (r=−.461, P<.05) and PGD-M (r=−.438, P<.02) inversely correlate with maximum fall in FEV1 during the reaction (Fig 4, AB). Reaction PGD-M (log2) also correlated with fall in FEV1 during reaction (r=−.41, P<.05, not shown).
Figure 4. Basal urinary eicosanoid levels correlate with change in FEV1 during aspirin desensitization.
Basal urinary (A) LTE4 and (B) PGD-M levels plotted against the corresponding percent change in FEV1 during aspirin reaction for each subject with AERD. Effect size, determined with Pearson correlation coefficient, is denoted as an r value.
To determine the effect of high-dose aspirin therapy on the production of eicosanoids in subjects with AERD, we compared basal levels of urinary PG metabolites and LTE4 to levels after 8 weeks of high-dose aspirin therapy in Group I subjects (n=14). Aspirin therapy resulted in significant decreases in PGE-M (8.7±2.1 pmol/mg Cr, P<.01), PGI-M (0.14±0.03 pmol/mg Cr, P <.01), TX-M (0.2±.0.1 pmol/mg Cr, P<.001) and PGD-M (2.2±0.8 pmol/mg Cr, P<.001) from basal levels, but there was no change in LTE4 levels (2.7±1.2 pmol/mg Cr, P=.24, Fig 5). The one subject in Group II who went on to tolerate high-dose aspirin also demonstrated a fall in PGD-M from baseline after 8 weeks.
Figure 5. Effect of high-dose aspirin therapy on urinary eicosanoid levels in AERD.
Urinary eicosanoid levels of Group I AERD subjects (n=14) are shown at baseline pre-aspirin and after 650mg twice-daily aspirin therapy for 8 weeks. Data are expressed as mean +SEM (٭٭P<.01, ٭٭٭P<.001).
Peripheral blood leukocyte counts and relationship to urinary PGD-M
Absolute peripheral blood eosinophil, basophil, and neutrophil counts were assessed in Group I AERD subjects (n=11) at baseline and again on high-dose aspirin. While on high-dose aspirin therapy, eosinophil counts rose in all subjects (0.31±0.06 to 0.93±0.19 K/μL, P<.01), and basophil counts rose or stayed the same (0.06±0.02 to 0.09±0.02 K/μL, P=.08). Neutrophil counts did not change (Fig 6). The percent increase in eosinophils on high-dose aspirin therapy trended to inversely correlate to the percent fall in urinary PGD-M from basal levels on high-dose aspirin (r =−0.51, P=.16; data not shown). The one subject in Group II who went on to tolerate high-dose aspirin also demonstrated an increase in absolute peripheral blood eosinophils at 8 weeks.
Figure 6. Peripheral blood eosinophils increase on high-dose aspirin therapy.

Blood eosinophil, basophil, and neutrophil counts (K/μL) for Group I AERD subjects (n=11) at baseline and on high-dose aspirin. Data are expressed as mean +SEM (٭٭P<.01).
Discussion
AERD involves complex dysregulation of proinflammatory (cysLTs, PGD2) and anti-inflammatory eicosanoid mediators (PGE2). Clinical reactions to drugs that block COX-1 are characterized by increases in urinary LTE4 and have been thought to be largely driven by the effector functions of cysLTs32. To our knowledge, ours is the first comprehensive examination of PG generation in subjects with AERD undergoing a therapeutic desensitization procedure. Unexpectedly, subjects unable to tolerate the procedure due to cutaneous and gastrointestinal symptoms (despite the use of a CysLT1 receptor antagonist) not only generate higher levels of cysLTs than those who tolerate the procedure, but also fail to suppress several prostanoids at a threshold dose of aspirin. In particular, individuals unable to tolerate the procedure generate markedly more PGD2 during reactions than do those who tolerate desensitization. These findings imply a potential role for “aspirin resistant” PGD2 as a mediator of AERD. Additionally, the suppression of PGD2 that results from high-dose aspirin therapy may prevent the recruitment of PGD2-responsive effector cells to the target tissue. This could account for why therapy with high-dose aspirin improves disease control without altering the high baseline urinary LTE4 levels typical of this disease24,33, 34, while increasing the blood levels of eosinophils and basophils.
The demographic and physiologic characteristics of the 23 subjects who tolerated the desensitization procedure (Group I) were comparable to those who did not tolerate the procedure (Group II) (Table 1). Every subject in Group I developed respiratory symptoms during the procedure, but these resolved and all subjects successfully continued on to higher aspirin doses without additional symptoms. The subjects in Group II also developed greater bronchoconstriction (Table 2), but were unable to complete the procedure due to ongoing abdominal pain and/or an atypical rash (Fig 1). The development of a rash during desensitization signaled a more challenging desensitization for subjects. The gastrointestinal manifestations could well reflect the actions of mediators released from gut mast cells during the reaction to aspirin35. The dose of aspirin that provoked symptoms was not statistically different between the groups (Table 2) and did not correlate with reaction PGD-M, consistent with previous studies reporting that neither the severity of reactions nor the changes in urinary LTE4 depend on the dose of aspirin ingested36,13. We cannot exclude the possibility that the subjects in Group II might tolerate an alternative protocol using intranasal ketorolac, which may be better tolerated than the standard oral aspirin desensitization protocol used in our study37.
The correlation between basal LTE4 and the fall in FEV1 (Fig 4,A) is consistent with the observations of Daffern et al, who reported that baseline urinary LTE4 levels predict the severity of airflow obstruction during aspirin challenges13. Unlike the Daffern study, however, all but two subjects in our cohort were pretreated with a CysLT1 receptor antagonist. Thus, the fact that basal urinary levels of LTE4 correlated with the fall in FEV1 in our subjects suggests that the cysLT-dependent component of airflow obstruction involves receptors other than CysLT1 receptors. Since we found that PGD-M levels at baseline and during reaction also correlated with the decrease in FEV1 (Fig 4,B), the bronchoconstricting effects of PGD2 could account for the portion of the bronchial response to aspirin that resists CysLT1 receptor blockade.
Although previous studies have monitored the production of PGs during aspirin challenges in AERD, none had simultaneously monitored all PG metabolites in subjects with vastly different clinical responses to aspirin. PGE2 suppresses 5-LO activity38. PGD2 and TXA2 are potent bronchoconstrictors39, and PGI2 is a vasodilator that decreases airflow when inhaled40. Thus all PGs measured in this study are potentially relevant to AERD. The increase in total PG metabolites observed in Group II during reactions to aspirin (from 43.2±9.4 to 85.2±22.1 pmol/mg Cr) contrast with the aspirin-induced changes in PGs observed in Group I and in the ATA controls. While much of the increase in total PGs in Group II reflects PGD-M, TX-M and PGI-M levels tended to increase in response to aspirin challenge in this group, whereas both tended to decrease in Group I (Fig 3, A,B). Although PGE2 is bronchoprotective in AERD due to inhibition of mast cell activation and 5-LO, PGE-M levels did not change significantly in either group during reactions to aspirin. It is possible that the high levels of PGE2 generated by the kidney mask changes that reflect aspirin-induced suppression of its production in the respiratory tissue41,42 at the low doses that elicit reactions. The slight reduction in PGE-M observed in Group I after 8 weeks of treatment may reflect the ability of high dose aspirin to interfere with COX-2 (from which most renal PGE2 is derived) as well as COX-1. The fact that both LTE4 levels and PG levels rose simultaneously suggests that aspirin provokes the release of large quantities of AA in Group II subjects by a to-be-determined mechanism. Additionally, the comparative resistance of their PG metabolites to suppression by aspirin at doses that block COX-1 (but not COX-2) suggests that COX-2 activity may mediate PG production in Group II subjects43. COX-2 protein expression by mast cells is increased in bronchial biopsies from patients with AERD compared to ATA controls44. COX-2 selective antagonists are almost universally well-tolerated by patients with AERD45, but whether COX-2 antagonists might actually be therapeutic in Group II AERD subjects remains to be determined.
As the effects of high-dose aspirin therapy on PG metabolites had not previously been studied comprehensively in AERD, we sought to determine the effect of high dose-aspirin therapy on urinary eicosanoids in Group I subjects. The sharply reduced levels of PGI-M, TX-M and PGD-M in the urine of Group I subjects while on aspirin (Fig 5) suggest that suppression of these bronchoconstrictive PGs may contribute to the clinical benefit of high-dose aspirin therapy.
PGD2 is potently chemotactic for eosinophils and basophils, both of which express CRTH2/DP221. Aspirin challenges induce a reduction in blood eosinophil counts in subjects with AERD 2, potentially reflecting (in retrospect) their recruitment to the tissue. Both eosinophils and basophils increase in nasal lavage fluids following aspirin challenge1, without an accompanying increase in the concentrations of eosinophil-active chemokines. Remarkably, the numbers of eosinophils rose, but not neutrophils (which lack CRTH2/DP2) (Fig 6), in all successfully desensitized subjects in our study, and tended to correlate with the reduction in PGD-M. We suspect that PGD2, through CRTH2/DP2, may facilitate the persistent eosinophilic inflammation of the respiratory tract that characterizes AERD. The suppression of PGD2 generation by high-dose aspirin therapy may increase the circulating pool of CRTH2/DP2+ effector cells by removing the chemotactic gradient that supports their recruitment to the tissue. Suppression of effector cell recruitment could contribute to the therapeutic benefit of high-dose aspirin in AERD, which is clearly not due to changes in the production of cysLTs46.
Previous studies had reported that levels of PGD2 metabolites in urine, plasma, or BAL fluid either remain unchanged24,33,47,4, or increase modestly with aspirin challenges in subjects with AERD48. The differences between these and our data likely reflect differences in sampling, the heterogeneous nature of AERD49, and the variable proportions of subjects with the phenotype defined by Group II. The pharmacologic properties of PGD2 and its metabolites make it likely that they play a causal role in the severe extrapulmonary manifestations of reactions to aspirin in Group II. The significant correlation between basal urinary PGD-M levels and the fall in FEV1 during aspirin reaction suggest that they also contribute to the respiratory response to aspirin (Fig 4). PGD2-mediated bronchoconstriction is sensitive to blockade with antagonists of the thromboxane receptor48,50. PGD2 also causes cutaneous vasodilation by acting at DP1 receptors21, and it seems plausible that the rash experienced by subjects in Group II (Fig 1) could reflect the direct effects of PGD2 in the skin microvasculature. Finally, the selective changes in blood eosinophils and basophils resulting from high-dose aspirin therapy in our study (Fig 6) may well reflect a prominent function for PGD2 in recruiting CRTH2/DP2+ effector cells to the respiratory tissues. Drugs are under development that block thromboxane, DP1, and DP2/CRTH2 receptors50,51. We speculate that these drugs, alone or in combination, could permit safe desensitization to aspirin in the PGD2-overproducing subgroup of patients with AERD, and could replicate some of the therapeutic benefits of high-dose aspirin by preventing the chemotaxis of CRTH2/DP2+ cells into the tissues. Our study is a necessary prerequisite to such trials.
Key Messages.
Cutaneous and gastrointestinal symptoms during aspirin desensitization and failure to tolerate desensitization in subjects with AERD are associated with marked increases in PGD2 generation.
High-dose aspirin therapy in subjects with AERD suppresses PGD2 production and raises peripheral blood eosinophil counts.
Capsule summary.
Increased PGD2 generation is associated with severe extrapulmonary reactions during aspirin desensitization in subjects with AERD. High-dose aspirin therapy suppresses PGD2 production, which may prevent the recruitment of effector cells into the respiratory tissue.
Acknowledgements
This work was supported by NIH grants AI007306-27, U19 AI095219-01, 5U19AI070412-06 Opportunity Fund Subaward No 153556/153044, K23HL111113-02 and by generous contributions from the Vinik Family and the Kaye Family. We thank Jing Cui, M.D., Ph.D. for her statistical assistance during manuscript preparation.
Abbreviations
- AERD
Aspirin exacerbated respiratory disease
- ASA
Aspirin
- COX-1
cyclooxygenase-1
- COX-2
cyclooxygenase-2
- CysLT1
type 1 receptor for the CysLTs
- DP
D prostanoid
- PG
prostaglandin
- PGD2
Prostaglandin D2
- PGE2
Prostaglandin E2
- LTE4
Leukotriene E4
- CysLT
Cysteinyl leukotrienes
- TXA2
Thromboxane
- PGI2
Prostacyclin
- PGI-M
Prostacyclin metabolites
- PGD-M
Prostaglandin D metabolites
- PGE-M
Prostaglandin E metabolites
- TX-M
Thromboxane metabolites
- 5-LO
5-lipoxygenase
- ATA
aspirin-tolerant asthmatics
- AA
Arachidonic acid
- BAL
bronchoalveolar lavage
- DP2/CRTH2
D prostanoid receptor 2/chemokine receptor homologous molecule expressed on Th2 lymphocytes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kupczyk M, Kurmanowska Z, Kuprys-Lipinska I, Bochenska-Marciniak M, Kuna P. Mediators of inflammation in nasal lavage from aspirin intolerant patients after aspirin challenge. Respiratory Medicine. 2010;104:1404–09. doi: 10.1016/j.rmed.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Sladek K, Szczeklik A. Cysteinyl leukotrienes overproduction and mast cell activation in aspirin-provoked bronchospasm in asthma. Eur Respir J. 1993;6:391–99. [PubMed] [Google Scholar]
- 3.Stevenson DD, Simon RA, Mathison DA. Aspirin-sensitive asthma: tolerance to aspirin after positive oral aspirin challenges. J Allergy Clin Immunol. 1980;66(1):82–8. doi: 10.1016/0091-6749(80)90143-8. [DOI] [PubMed] [Google Scholar]
- 4.Swierczynska-Krepa M, Sanak M, Bochenek Grazyna, Strek P, Cmiel A, Gielicz A, Plutecka H, Szczeklik, Nizankowska-Mogilnicka E. Aspirin desensitization in patients with aspirin-induced and aspirin-tolerant asthma: A double-blind study. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.02.041. epub April 25, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson DW, Ali A, Vaillancourt JP, Calaycay JR, Mumford RA, Zamboni RJ, Ford-Hutchinson AW. Purification to homogeneity and the N-terminal sequence of human leukotriene C4 synthase: a homodimeric glutathione S-transferase composed of 18-kDa subunits. Proc Natl Acad Sci U S A. 1993;90:2015–9. doi: 10.1073/pnas.90.5.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam BK, Penrose JF, Freedman GJ, Austen KF. Expression cloning of a cDNA for human leukotriene C4 synthase, a novel integral membrane protein conjugating reduced glutathione to leukotriene A4. Proc Natl Acad Sci U S A. 1994;91:7663–7. doi: 10.1073/pnas.91.16.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsch DJ, Creely DP, Hauser SD, Mathis KJ, Krivi GG, Isakson PC. Molecular cloning and expression of human leukotriene C4 synthase. Proc Natl Acad Sci U S A. 1994;91:9745–9. doi: 10.1073/pnas.91.21.9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumlin M, Stensvad F, Larsson L, Dahlén B, Dahlén SE. Validation and application of a new simple strategy for measurements of urinary leukotriene E4 in humans. Clin Exp All. 1995;25(5):467–79. doi: 10.1111/j.1365-2222.1995.tb01079.x. [DOI] [PubMed] [Google Scholar]
- 9.Kanaoka Y, Maekawa A, Austen KF. Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand. J Biol Chem. 2013;288(16):10967–72. doi: 10.1074/jbc.C113.453704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch KR, O'Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, Coulombe N, et al. Characterization of the human cysteinyl leukotriene cysLT1 receptor. Nature. 1999;399:789–93. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 11.Sarau HM, Ames RS, Chambers J, Ellis C, Elshourbagy N, Foley JJ, et al. Identification, molecular cloning, expression, and characterization of a cysteinyl leukotriene receptor. Mol Pharmacol. 1999;56:657–63. doi: 10.1124/mol.56.3.657. [DOI] [PubMed] [Google Scholar]
- 12.Heise CE, O'Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, et al. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem. 2000;275:30531–6. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- 13.Daffern PJ, Muilenburg D, Hugli TE, Stevenson DD. Association of urinary leukotriene E4 excretion during aspirin challenges with severity of respiratory responses. J Allergy Clin Immunol. 1999;104:559–64. doi: 10.1016/s0091-6749(99)70324-6. [DOI] [PubMed] [Google Scholar]
- 14.White A, Ludington E, Mehra P, Stevenson DD, Simon RA. Effect of leukotriene modifier drugs on the safety of oral aspirin challenges. Ann Allergy Asthma Immunol. 2006;97(5):688–93. doi: 10.1016/S1081-1206(10)61101-5. [DOI] [PubMed] [Google Scholar]
- 15.Benyahia C, Gomez I, Kanyinda L, Boukais K, Danel C, Leséche G, et al. PGE(2) receptor (EP(4)) agonists: potent dilators of human bronchi and future asthma therapy? Pulm Pharmacol Ther. 2012;25(1):115–8. doi: 10.1016/j.pupt.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Feng C, Beller EM, Bagga S, Boyce JA. Human mast cells express multiple EP receptors for prostaglandin E2 that differentially modulate activation responses. Blood. 2006;107(8):3243–3250. doi: 10.1182/blood-2005-07-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo M, Jones SM, Phare SM, Coffey MJ, Peters-Golden M, Brock TG. Protein kinase A inhibits leukotriene synthesis by phosphorylation of 5-lipoxygenase on serine 523. J Biol Chem. 2004;279(40):41512–41520. doi: 10.1074/jbc.M312568200. [DOI] [PubMed] [Google Scholar]
- 18.Beasley RC, Featherstone RL, Church MK, Faggerty P, Varley JG, Harris A, et al. Effect of a thromboxane receptor antagonist on PGD2- and allergen-induced bronchoconstriction. J Appl Physiol. 1989;66(4):1685–93. doi: 10.1152/jappl.1989.66.4.1685. [DOI] [PubMed] [Google Scholar]
- 19.Hardy CC, Robinson C, Tattersfield AE, Holgate ST. The bronchoconstrictor effect of inhaled prostaglandin D2 in normal and asthmatic men. N Engl J Med. 1984;311(4):209–13. doi: 10.1056/NEJM198407263110401. [DOI] [PubMed] [Google Scholar]
- 20.Cheng K, Wu TJ, Wu KK, Sturino C, Metters K, Gottesdiener K, et al. Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proc Natl Acad Sci U S A. 2006;103(17):6682–7. doi: 10.1073/pnas.0601574103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193(2):255–61. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue L, Salimi M, Panse I, Mjosberg JM, McKenzie AN, Spits H, et al. Prostaglandin D activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on T2 cells. J Allergy Clin Immunol. 2014;133(4):1184–94. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higashi N, Taniguchi M, Mita H, Yamaguchi H, Ono E, Akiyama K. Aspirin-intolerant asthma assessment using the urinary biomarkers, leukotriene E4 and Prostaglandin D2 metabolites. Allergology International. 2012;61:393–403. doi: 10.2332/allergolint.11-RA-0403. [DOI] [PubMed] [Google Scholar]
- 24.Bochenek G, Nagraba K, Nizankowska E, Szczeklik A. A controlled study of 9α,11β-PGF2 (a prostaglandin D2 metabolite) in plasma and urine of patients with bronchial asthma and healthy controls after aspirin challenge. J Allergy Clin Immunol. 2003;111:743–9. doi: 10.1067/mai.2003.1387. [DOI] [PubMed] [Google Scholar]
- 25.Szczeklik A, Sladek K, Dworski R, Bizankowska E, Soja J, Sheller J, Oates J. Am J Respir Crit Care Med. 1996;Bronchial aspirin challenge causes specific eicosanoid response in aspirin-sensitive asthmatics.154:1608–14. doi: 10.1164/ajrccm.154.6.8970343. [DOI] [PubMed] [Google Scholar]
- 26.Macy E, Bernstein JA, Castells MC, Gawchik SM, Lee TH, Settipane RA, Simon RA, Wald J, Woessner KM. Aspirin challenge and desensitization for aspirin-exacerbated respiratory disease: a practice paper. Ann Allergy Asthma Immunol. 2007;98:172–174. doi: 10.1016/S1081-1206(10)60692-8. [DOI] [PubMed] [Google Scholar]
- 27.National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Heart, Lung, and Blood Institute (US); Bethesda (MD): Aug, 2007. Available from: http://www.ncbi.nlm.nih.gov/books/NBK7232/ [Google Scholar]
- 28.Macy E, Bernstein JA, Castells MC, Gawchik SM, Lee TH, Settipane RA, et al. Aspirin challenge and desensitization for aspirin exacerbated respiratory disease: a practice paper. Ann Allergy Asthma Immunol. 2007;98:172–174. doi: 10.1016/S1081-1206(10)60692-8. [DOI] [PubMed] [Google Scholar]
- 29.Morrow JD, Minton TA. Improved assay for the quantification of 11-dehydrothromboxane B2 by gas chromatography-mass spectrometry. J Chromatogr. 1993;612(2):179–85. doi: 10.1016/0378-4347(93)80161-v. [DOI] [PubMed] [Google Scholar]
- 30.Awad JA, Morrow JD, Takahashi K, Roberts LJ., 2nd Identification of noncyclooxygenase-derived prostanoid (F2-isoprostane) metabolites in human urine and plasma. J Biol Chem. 1993;268(6):4161–9. [PubMed] [Google Scholar]
- 31.Morrow JD, Guzzo C, Lazarus G, Oates JA, Roberts LJ., 2nd Improved diagnosis of mastocytosis by measurement of the major urinary metabolite of prostaglandin D2. J Invest Dermatol. 1995;104(6):937–40. doi: 10.1111/1523-1747.ep12606209. [DOI] [PubMed] [Google Scholar]
- 32.Christie PE, Tagari P, Ford-Hutchinson AW, Charlesson S, Chee P, Arm JP, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143(5 Pt 1):1025–9. doi: 10.1164/ajrccm/143.5_Pt_1.1025. [DOI] [PubMed] [Google Scholar]
- 33.Szczeklik A, Sladek K, Dworski R, Nizankowska E, Soja J, Sheller J, et al. Bronchial aspirin challenge causes specific eicosanoid response in aspirinsensitive asthmatics. Am J Respir Crit Care Med. 1996;154:1608–14. doi: 10.1164/ajrccm.154.6.8970343. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida S, Amayasu H, Sakamoto H, Onuma K, Shoji T, Nakagawa H, Tajima T. Cromolyn sodium prevents bronchoconstriction and urinary LTE4 excretion in aspirin-induced asthma. Ann Allergy Asthma Immunol. 1998;80:171–6. doi: 10.1016/S1081-1206(10)62951-1. [DOI] [PubMed] [Google Scholar]
- 35.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112(11):1666–77. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mastalerz L, Sanak M, Gawlewicz-Mroczka A, Gielicz A, Szczeklik A. Prostaglandin E2 systemic production in patients with asthma with and without aspirin hypersensitivity. Thorax. 2008;63:27–34. doi: 10.1136/thx.2007.080903. [DOI] [PubMed] [Google Scholar]
- 37.Lee RU, White AA, Ding D, Dursun AB, Woessner KM, Simon RA, Stevenson DD. Use of intranasal ketorolac and modified oral aspirin challenge for desensitization of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2010;105(2):130–5. doi: 10.1016/j.anai.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Liu T, Laidlaw TM, Katz HR, Boyce JA. Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proc Natl Acad Sci U S A. 2013;110(42):16987–92. doi: 10.1073/pnas.1313185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song WL, Stubbe J, Ricciotti E, Alamuddin N, Ibrahim S, Crichton I, et al. Niacin and biosynthesis of PGD(2)by platelet COX-1 in mice and humans. J Clin Invest. 2012;122(4):1459–68. doi: 10.1172/JCI59262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardy CC, Bradding P, Robinson C, Holgate ST. Bronchoconstrictor and antibronchoconstrictor properties of inhaled prostacyclin in asthma. J Appl Physiol. 1988;64(4):1567–74. doi: 10.1152/jappl.1988.64.4.1567. [DOI] [PubMed] [Google Scholar]
- 41.Yoshimura T, Yoshikawa M, Otori N, Haruna S, Moriyama H. Correlation between the prostaglandin D(2)/E(2) ratio in nasal polyps and the recalcitrant pathophysiology of chronic rhinosinusitis associated with bronchial asthma. Allergology Int. 2008;57(4):429–36. doi: 10.2332/allergolint.o-08-545. [DOI] [PubMed] [Google Scholar]
- 42.Szczeklik A, Gryglewski RJ, Olszewski E, Dembinska-Kiec A, Czerniawska-Mysik G. Aspirin-sensitive asthma: the effect of aspirin on the release of prostaglandins from nasal polyps. Pharamacol Res Commun. 1977;9(5):415–25. doi: 10.1016/s0031-6989(77)80027-1. [DOI] [PubMed] [Google Scholar]
- 43.Smith WL, Meade EA, Dewitt DL. Interaction of PGH synthase isozymes-1 and -2 with nonsteroidal anti-inflammatory drugs. Adv Exp Med Biol. 1997;400A:189–96. doi: 10.1007/978-1-4615-5325-0_28. [DOI] [PubMed] [Google Scholar]
- 44.Sousa A, Pfister R, Christie PE, Lane SJ, Nasser SM, Schmitz-Schumann M, et al. Enhanced expression of cyclo-oxygenase isoenzyme 2 (COX-2) in asthmatic airways and its cellular distribution in aspirin-sensitive asthma. Thorax. 1997;52(11):940–5. doi: 10.1136/thx.52.11.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woessner KM, Simon RA, Stevenson DD. The safety of celecoxib in patients with aspirin-sensitive asthma. Arthritis Rheum. 2002;46(8):2201–6. doi: 10.1002/art.10426. [DOI] [PubMed] [Google Scholar]
- 46.Nasser SM, Patel M, Bell GS, Lee TH. The effect of aspirin desensitization on urinary leukotriene E4 concentrations in aspirin-sensitive asthma. Am J Respir Crit Care Med. 1995;151(5):1326–30. doi: 10.1164/ajrccm.151.5.7735581. [DOI] [PubMed] [Google Scholar]
- 47.Szczeklik A, Dworski R, Mastalerz L, Prokop A, Sheller J, Nizankowska E, et al. Salmeterol prevents aspirin-induced attacks of asthma and interferes with eicosanoid metabolism. Am J Respir Crit Care Med. 1998;158:1168–72. doi: 10.1164/ajrccm.158.4.9710043. [DOI] [PubMed] [Google Scholar]
- 48.Larsson AK, Hagfjard A, Dahlen SE, Adner M. Prostaglandin D(2) induces contractions through activation of TP receptors in peripheral lung tissue from the guinea pig. Eur J Pharmacol. 2011;669(1-3):136–42. doi: 10.1016/j.ejphar.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 49.Bochenek G, Kuschill-Dziurda J, Szanfraniec K, Plutecka H, Szczeklik A, Nizankowska-Mogilnicka E. Certain subphenotypes of aspirin-exacerbated respiratory disease distinguished by latent class analysis. J Clin Allergy Immunol. 2014;133:98–103. doi: 10.1016/j.jaci.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Johnston SL, Bardin PG, Harrison J, Ritter W, Joubert JR, Holgate ST. The effects of an oral thromboxane TP receptor antagonist BAY u 3405, on prostaglandin D2- and histamine-induced bronchoconstriction in asthma, and relationship to plasma drug concentrations. Br J Clin Pharmacol. 1992;34(5):402–8. doi: 10.1111/j.1365-2125.1992.tb05649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Busse WW, Wenzel SE, Meltzer EO, Kerwin EM, Liu MC, Zhang N, et al. Safety and efficacy of the prostaglandin D2 receptor antagonist AMG 853 in asthmatic patients. J Allergy Clin Immunol. 2013;131(2):339–45. doi: 10.1016/j.jaci.2012.10.013. [DOI] [PubMed] [Google Scholar]