Abstract
Hypertension and aging are both recognized to increase aortic stiffness, but their interactions are not completely understood. Most prior studies have attributed increased aortic stiffness to changes in extracellular matrix proteins that alter mechanical properties of the vascular wall. Alternatively, we hypothesized that a significant component of increased vascular stiffness in hypertension is due to changes in the mechanical and adhesive properties of vascular smooth muscle cells, and that aging would augment the contribution from vascular smooth muscle cells compared to the extracellular matrix. Accordingly, we studied aortic stiffness in young (16 wks) and old (64 wks) spontaneously hypertensive rats and Wistar-Kyoto wild-type controls. Systolic and pulse pressures were significantly increased in young spontaneously hypertensive rats, compared to young Wistar-Kyoto rats, and these continued to rise in old spontaneously hypertensive rats, compared to age-matched controls. Excised aortic ring segments exhibited significantly greater elastic moduli in both young and old spontaneously hypertensive rats vs. Wistar-Kyoto rats. Vascular smooth muscle cells were isolated from the thoracic aorta, and stiffness and adhesion to fibronectin were measured by atomic force microscopy. Hypertension increased both vascular smooth muscle cell stiffness and vascular smooth muscle cell adhesion, and these increases were both augmented with aging. By contrast, hypertension did not affect histological measures of aortic collagen and elastin, which were predominantly changed by aging. This supports the concept that stiffness and adhesive properties of vascular smooth muscle cells are novel mechanisms contributing to the increased aortic stiffness occurring with hypertension superimposed on aging.
Keywords: collagen, elastin, fibronectin, vascular stiffness, aorta, atomic force microscopy, focal adhesion
Introduction
In the United States, the residual lifetime risk of developing hypertension is greater than 90% in both men and women1. Long-term sustained hypertension increases morbidity and mortality from myocardial infarction, stroke, atherosclerosis, and renal failure. Large-artery stiffening is com or bid with hypertension2, but is also accelerated in patients with uncontrolled blood pressure3. However, increased aortic stiffness is also characteristic of the normal aging process4. Clinically, elderly hypertensive patients harbor stiffness levels above that of age-matched normotensive older patients5, suggesting an additive or synergistic effect of hypertension and aging. However, the pathogenesis of aortic stiffening in hypertension, especially as it progresses with age, is not completely understood.
Old spontaneously hypertensive rats (SHR) are the most commonly studied model of aging hypertension, with several reports of advancing aortic pressure6-8 and accelerated changes in either vascular stiffness or decreased vascular compliance in the aging SHR8-10. The most predominant mechanism believed to under lie changes vascular mechanical properties is an increase in collagen deposition and a breakdown of elastin. It is generally agreed that these changes are seen with vascular aging, and can contribute to increases in vascular stiffness11. However, these changes are not consistently increased with hypertension6,12-14, with several reports actually showing decreases in vascular collagen15,16. Furthermore, extracellular matrix (ECM) changes alone do not provide a mechanistic explanation for the acceleration, per se, of vascular stiffness seen hypertensive aging.
Despite these inconsistencies, few alternative structural and cellular mechanisms have been considered. Bezie and colleagues have previously speculated that the connections between vascular smooth muscle cells (VSMCs) and the extracellular matrix (ECM) may play a role in vascular stiffness in hypertension. They have reported an increased VSMC surface occupied by dense plaques17, and expression of fibronectin and α-5 integrin12 within the abdominal aortas of SHR, compared to Wistar-Kyoto(WKY) controls. This strongly suggests that an alteration in the ECM adhesive properties of VSMCs may also be involved, but this has yet to be investigated at the cellular level using biophysical approaches that can directly assess cellular changes in stiffness and adhesion.
Recently, we have described another important mechanism mediating the increased vascular stiffness with aging18,19 and with hypertension20, i.e., increased intrinsic VSMC stiffness accompanied by an increase in fibronectin adhesion (which binds to α5B1 integrin). Essentially nothing is known about whether these alterations interact when both hypertension and aging coexist, or if the result of this interaction accelerates the stiffening process.
Accordingly, a primary goal of the present investigation was to test two hypotheses: (1) hypertension superimposed on aging alters the course of VSMC stiffness changes in the aorta, and (2) if changes in VSMC adhesion are also associated with advancing aortic pressure or accelerated vascular stiffness in aging hypertension. To accomplish these goals we studied age-matched young (16 wks) and old (64 wks) SHR and their WKY littermates. We quantified changes in arterial pressure, overall aortic stiffness, aortic collagen and elastin content and densities, and measured intrinsic VSMC stiffness in cells isolated from the thoracic aorta. We also investigated if hypertension and aging act to alter the innate abilities of VSMCs to form adhesion attachments to fibronectin. The overall stiffness for aortic tissue was assessed by mechanically testing intact vessel rings; cellular stiffness and adhesion of isolated VSMCs were assessed by atomic force microscopy. These changes were compared against morpho metric content and density assessments of collagen and elastin within the aortic wall.
Methods
Animal Model
In this study, adult male SHR and WKY rats were studied at 16 weeks of age and at 64 weeks of age. Throughout the text, the 16-week group is referred to as “young” and the 64-week group referred to as “old”. All procedures were approved of by the Institutional Animal Care and Use Committees of Rutgers University and the University of Missouri.
Measurements of Aortic Pressure and Vascular Stiffness
Aortic (thoracic) pressure was directly measured in anesthetized (35 mg/kg ketamine and 5 mg/kg xylazine) rats using a Millar catheter (SPR-320; Millar Instruments Inc., Houston, TX), advanced from the left carotid artery. A total of 8 rats per strain and age group were studied (total = 32). Upon sacrificing the rats by pentobarbital overdose, the thoracic aorta was harvested and placed into ice-cold PBS. Segments of the descending thoracic aorta were cut into rings and mounted onto a system of wire hooks connected to an isometric force transducer (model 52-9545, Harvard Apparatus, South Natick, MA), and subjected to uniaxial tensile testing, as described previously20. The force response from stepwise stress-relaxation tests was used to determine the circumferential stress developed in the ring 1 minute after the stretch step over a range of stretched lengths. These data were processed using a custom script developed in MATLAB (version 7.10.0).A stress-strain plot was generated from these experiments, and used to compute the tangential elastic stiffness from the slope of the curve.
Stiffness and Adhesion Properties of Isolated Vascular Smooth Muscle Cells
VSMCs were obtained by enzymatic digestion of thoracic aortic tissue, as described previously21 and were plated onto glass-bottomed cell culture dishes. Primary (non-passaged) VSMCs were studied after less than 1 week in culture, and stiffness and adhesion properties of individual VSMCs were determined by atomic force microscopy (AFM) nanoindentation. A microcantilever tip was mounted to an AFM (Bruker Bio Scope Catalyst) that was coupled to a confocal microscope (Olympus IX81). In experiments to study adhesion properties, the microcantilever tip was functionalized by coating the tip with fibronectin (0.5 mg/ml) to promote the formation of VSMC adhesion attachments upon contact with the cell surface. Individual VSMCs were probed at 0.5 Hz approach-retraction cycle resulting in a cell indentation depth of 100-300 nm. For each cell, force curves were continuously collected for four minutes. The force signals were analyzed using proprietary software, N Force R (copyright 2006, Registration Number TXu1-328-659), and a custom MATLAB script. The approach curve was used to compute the stiffness of VSMCs using a modified Hertz model, where the indentation force was computed from Hooke's law, as described previously21. The presence of VSMC adhesions to the fibronectin functionalized cantilever tip were computationally identified from the retraction curves. For each retraction curve, the number of adhesions and the force required to rupture all adhesions were assessed. For each group of rats, measurements of stiffness, adhesion events, and adhesion force were obtained from averages of 60 cells from 3 animals per group.
Histological Analysis of the Thoracic Aorta
Freshly dissected segments of the thoracic aorta were fixed in neutral buffered 10% formalin solution, and subsequently embedded into paraffin blocks. Aortic cross-sections slices were cut and mounted onto glass slides. Masson's Trichrome staining was used to visualize the overall aortic morphology and architecture. Tissue dimensions were determined in Image J using digital calipers. Picrosirus red staining under circularly polarized light was used to visualize collagen, and Van Gieson's staining was used to visualize elastin within the aortic wall. Collagen and elastin contents were determined within each sampled field (15 fields per ring)by direct pixel quantification using custom MATLAB image analysis scripts. These contents were computed for the entire tissue ring by proportioning the sampled field area to the total tissue area. Collagen and elastin densities were computed by normalizing the content within a field to the underlying area of tissue within that field.
Statistical Analysis
The results are presented as mean ± SEM. Comparisons among the four groups were determined by 2 × 2 multi factorial ANOVA, with factors defined as hypertension (SHR and WKY) and age (16 weeks and 64 weeks), and the interaction term determined the additive effect of these two factors. Selected post-hoc comparisons were done by independent sample t-tests using a Bonferroni correction, and are displayed in graphical figures. Computations were performed using SPSS 20.0 software. A value of p < 0.05 was considered significant.
Results
Aortic Pressure Increases in SHR
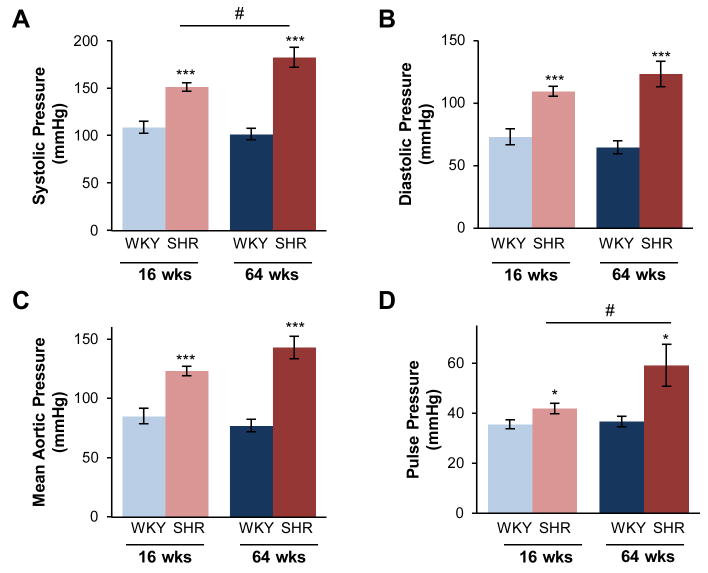
Measurement of thoracic aortic pressures in both young and old animals, revealed significantly elevated pressures within SHR (Fig. 1). ANOVA results were significant for the SHR strain, as evaluated for systolic, diastolic, mean aortic, and pulse pressures (p < 0.001 for all) compared to age-matched WKY. Aortic pressure was unchanged by age in WKY, as verified by post-hoc comparison, while it continued to increase from young to old in SHR. Importantly, a significant interaction between hypertension and age was found for systolic (p < 0.05) and pulse pressure (p < 0.001), suggesting a more than additive, or synergistic, effect of hypertension and age in SHR.
Figure 1.
Hypertension advances from young to old SHR. Thoracic aortic pressure was measured in the descending thoracic aorta in young (16 weeks old) and old (64 weeks old) WKY and SHR. In both age groups, (A) systolic pressure, (B) diastolic pressure, (C) mean aortic pressure, and (D) pulse pressure is increased in the SHR. Systolic and pulse pressures were significantly increased with age in the SHR, but not WKY. Data are shown as mean ± SEM, with n = 8 animals per group. Post-hoc comparisons, * p < 0.05, *** p < 0.001, compared to WKY; # p < 0.05, compared to young.
Vascular and VSMC Stiffness Increases with Hypertension and Aging
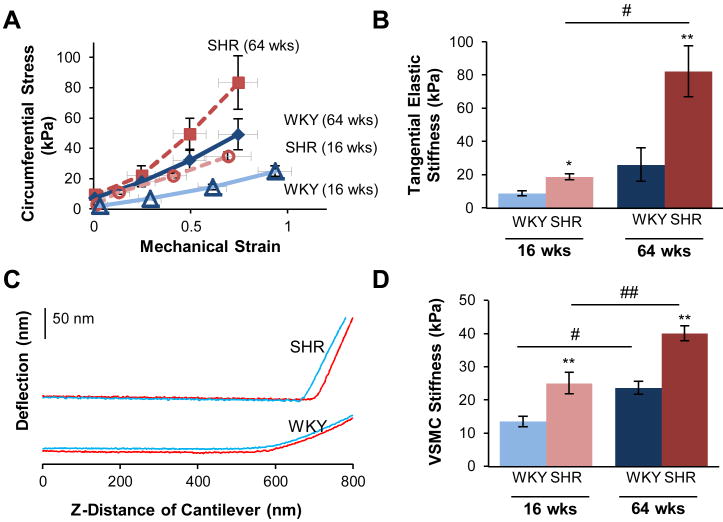
Vascular stiffness was determined by tensile testing of excised aortic rings. The circumferential stress was determined across a range of stretched lengths, and the mechanical strain across this range was used to generate stress-strain curves, as shown for representative rings for each strain group (Fig. 2A). The tangential elastic stiffness was evaluated from the stress-strain relationship, within a physiological range of vessel diameter (2.5-3.5 mm). In young animals, tangential elastic stiffness was increased by 2-fold in the young SHR and also the aging WKY, but rose by 4-fold in old SHR animals compared to young WKY (Fig. 2B). Significant differences were found for comparisons by ANOVA between SHR and WKY (p < 0.001) and between the age groups (p < 0.001). The comparisons again showed a significant interaction between hypertension and age (p < 0.01).
Figure 2.
Stiffness of the thoracic aorta and individual VSMCs increase with hypertension and aging. Excised aortic rings from young (16 weeks old) and old (64 weeks old) WKY and SHR were mechanically stretched, and stiffness was determined from the stress-strain relationship, as shown for representative rings (A) (SHR = dashed; WKY = solid; Old = filled symbols: Young = open symbols). Stiffness was increased in the SHR at the young age, and further augmented with age (B). The stiffness of VSMCs derived from the thoracic aorta was determined by AFM nanoindentation. Each cell was individually probed with a microcantilever, and the force response was determined from the laser deflection of the microcantilever as it touched the cell surface, as shown for representative approach (blue) and retraction (red) curves (C). The stiffness was increased in SHR at both young and old ages (D). Data are shown as mean ± SEM. Post-hoc comparisons, parisons among the four groups were determined * p < 0.05, ** p < 0.01, compared to WKY; # p < 0.05, ## p < 0.01, compared to young.
The mechanical properties of individual aortic VSMCs were evaluated by AFM nanoindentation for young and old SHR and WKY. The deflection signal from the AFM cantilever was recorded as it approached the surface of the cell, made contact and indented the cell as shown in representative force curves (Fig. 2C).The deflection signal from the cantilever can be seen to increase more steeply in the SHR compared to the WKY (i.e., reflecting increased stiffness and more rapid cantilever bending). This deflection signal was calibrated against sensitivity and used to determine the stiffness (elastic modulus) of the VSMCs. Stiffness of SHR VSMCs roughly doubled with simple aging and increased by more than 3-fold from young WKY baseline in hypertensive aging. Both factors of SHR strain (p < 0.0001) and age (p < 0.0001) were significant, with no significant interaction indicated.
Adhesion Properties of Individual VSMCs Increases with Hypertension and Age
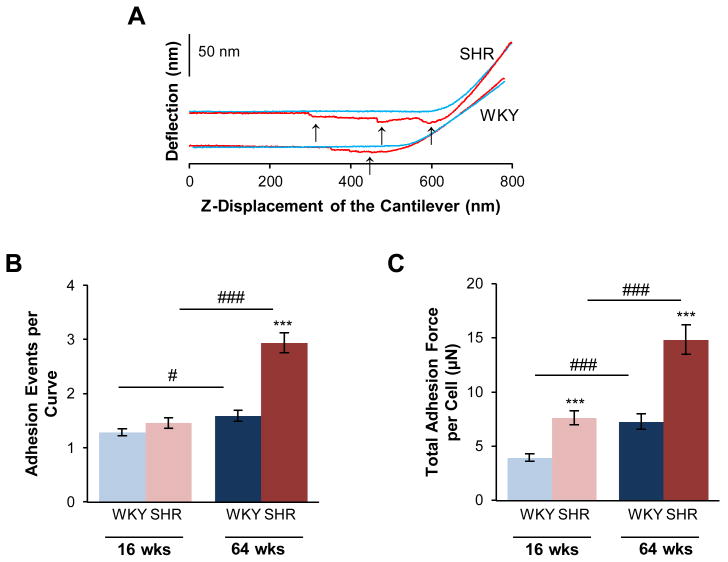
The adhesive properties of the VSMCs were determined from adhesions that occurred between the cell and the fibronectin functionalized AFM probe tip. As the probe was retracted from the cell surface, a cell-probe adhesive interaction was detected as downward deflection of the AFM cantilever followed by abrupt upward steps in deflection signal, which represented rupture of an adhesion. This is shown in representative force curves (Fig. 3A). The numbers of adhesion events were determined from each retraction curve for every approach-retraction cycle that was obtained during a 4-minute indentation experiment. Aging in WKY and SHR significantly increased the number of adhesions with the AFM probe. However, the increase in adhesion was much more pronounced in old SHR compared to either young SHR or to WKY (Fig. 3B).Both factors of hypertension (p < 0.001) and aging (p < 0.001), and their interaction (p < 0.001) were significant. The summed force required to rupture all adhesions in a given retraction curve was increased by hypertension and aging (Fig. 3C). In young animals, this sum increased by 93% in the SHR, compared to age-matched WKY; in old animals, it was increased by 104% in the SHR, compared to age-matched WKY. Both factors hypertension (p < 0.001) and aging (p < 0.001), and their interaction (p < 0.05) were significant.
Figure 3.
Adhesion properties of VSMCs are increased in SHR. In separate atomic force microscopy experiments, the microcantilever probe was coated with fibronectin, to promote the formation of VSMC surface attachments to the probe. This resulted in several stepwise adhesion breaks (arrows), as the cantilever was pulled away from the cell surface, as shown for representative approach (blue) and retraction (red) curves (A). SHR cells formed a greater number of adhesion attachments per approach-retraction cycle (B). The total force generated from these adhesion attachments was also greater in the SHR (C). Data are shown as mean ± SEM, with n = 60 cells per group. Post-hoc comparisons, * p < 0.05, ** p < 0.01, **** p < 0.0001 compared to WKY; # p < 0.05, ## p < 0.01, ### p < 0.001 compared to young.
Aortic ECM Remodeling in the Presence of Aging and Hypertension
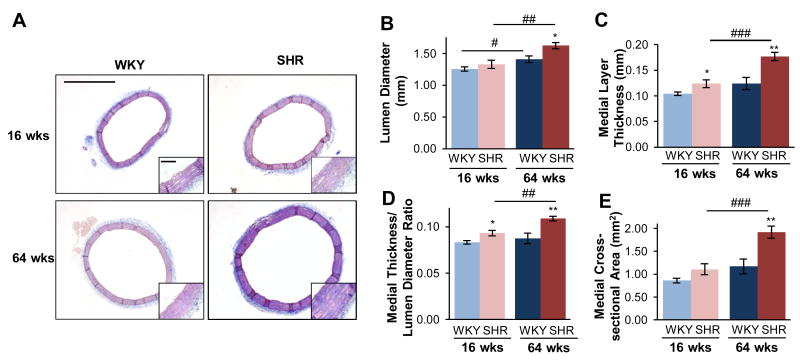
ECM composition and remodeling of the aortic wall from these groups was determined from Mason's Trichrome, Picrosirus Red, and Van Gieson's staining of the aortic tissue rings. Morphometric comparisons between the four groups were assessed by Masson's Trichrome staining (Fig. 4A). The aorta from the SHR had 5% greater lumen diameter (Fig. 4B) and 19% increase in medial layer thickness (Fig. 4C) in young animals compared to WKY. In old animals, the SHR had a 15% greater lumen diameter (Fig. 4B) and a 42% increase in medial thickness (Fig. 4C) compared to WKY. The ratio of the medial layer thickness to lumen diameter (Fig. 4D), a measure of mechanical load, experienced at the region of VSMCs, was also increased by 12% in young SHR, and by 25% in old SHR, compared to age-matched WKY. Both factors, hypertension and aging, were significant (p < 0.001 and p < 0.01), with no significant interaction. Additionally, the medial cross-sectional area was significantly increased in old SHR by 64%, compared to age-matched WKY, and by 74% compared to young SHR. Both factors, hypertension and aging, were significant (p < 0.001 and p < 0.001), with no significant interaction.
Figure 4.
Aortic structural remodeling in hypertension differs from that of age. Overall morphology was compared from Mason's Trichrome staining of the thoracic aorta (scale bar = 1.0 mm), shown with high-magnification insert (scale bar = 0.1 mm) (A). At both ages, the SHR had increased lumen diameter (B), medial layer thickness (C), and medial thickness-diameter ratio (D), and medial cross-sectional area (E), and these changes further increased with age. Data are shown as mean ± SEM, with n = 8 animals per group. Post-hoc comparisons, * p < 0.05, ** p < 0.01, compared to WKY; # p < 0.05, ## p < 0.01, ### p < 0.001, compared to young.
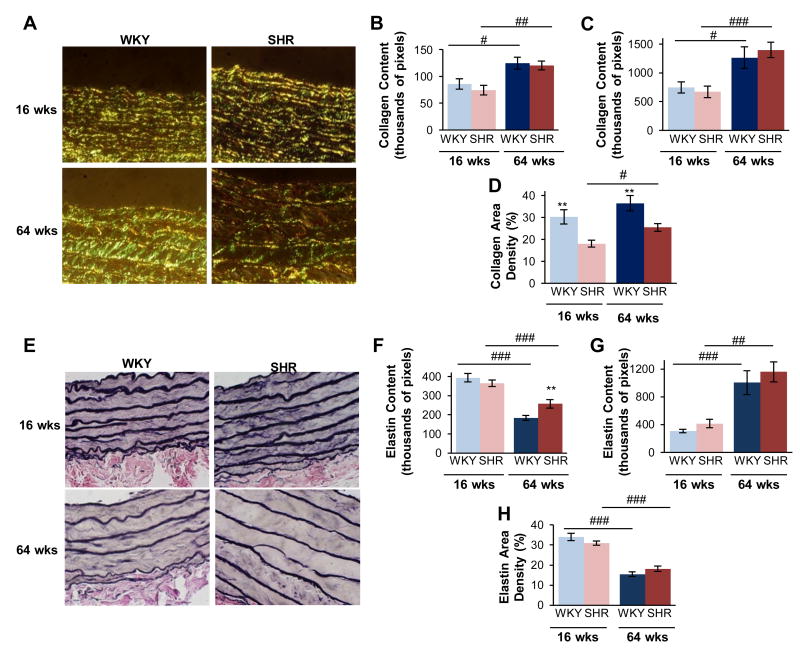
Collagen fibers were visualized under circularly polarized light (Fig. 5A), and the content of collagen was determined within sampled image fields (Fig. 5B) and computed for the whole tissue cross-section (Fig. 5C). Collagen content significantly increased with age in both WKY and SHR (Figs. 5B and C). However, there were no differences in total collagen content between WKY and SHR for the young or old groups (Figs. 5B and C). It was noted that the density of collagen (i.e. normalizing collagen content to area) was decreased in young SHR by 40%, compared to young WKY, and by 30% in old SHR (Fig. 5D), compared to old WKY. For both measures of collagen content and density, only the factor of aging was significant (p < 0.001). Within individual image fields, we observe visibly thinner elastin fibers in old animals(Fig. 5E) and deceased content(Fig. 5F), though for the overall ring cross-sections the content was significantly increased with age (Fig. G) in both SHR and WKY. Elastin density decreased with age (Fig. 5H) in both SHR and WKY (38% and 54%, respectively). However, comparison of elastin content and density did not reveal any significant differences between the SHR and WKY. Only the factor of aging was significantly (p < 0.001) affected elastin content and density.
Figure 5.
Collagen and elastin composition in hypertension differs from that of age. Collagen fibers were visualized by under circularly-polarized light (A), and neither the content within individual image fields (B) nor that computed for the total tissue (C) was not significantly different in hypertension. The collagen content was normalized to the tissue area within that field to determine a tissue density, which was decreased in the SHR (D). Elastin was quantified from Van Gieson's staining (E). Older age decreased elastin content within individual fields (F), but overall this was increased for the total tissue ring(G). Elastin density was decreased by age, and not hypertension (H). Data are shown as mean ± SEM, with n = 8 animals per group. Post-hoc comparisons, * p < 0.05, ** p < 0.01, compared to WKY; # p < 0.05, ## p < 0.01, ### p < 0.001, compared to young.
Discussion
The major finding in this investigation is that the observed amplification of aortic pressure and vascular stiffness when hypertension is superimposed on aging parallels and correlates with the increase in VSMC stiffness and adhesion properties, and that this increase in vascular stiffness is not mediated by the content or density of collagen and elastin. This finding contrasts with other studies of aging hypertension that have ascribed accelerated vascular mechanical changes to the ECM7,9.
We found that vascular collagen is rarefied (especially between lamina) in the SHR and this is likely due, in part, to hypertrophy of the VSMCs, particularly in the old animals. As shown in Figure 5, we found that the amount of collagen in the thoracic aorta of SHR is unchanged compared to WKY, at either young or old ages. Some previous studies of the SHR have also similarly concluded that hypertension does not increase the absolute collagen content6,15,16 or collagen concentration6,10,22 in the thoracic aorta. Similar observations and findings have been made in the abdominal aorta12,23, mesenteric16,24 and cerebral24 arteries. However, it is noted that in other studies of the SHR, contradictory results have been reported for the thoracic aorta13,14,25 and mesenteric artery26. In fact, of the numerous studies surveyed on the effect of hypertension, per se, on vascular collagen, inconsistency seems to be the common feature of the collective studies. In further support of the controversy, conflicting trends in vascular collagen content have also been noted within other hypertension models, such as renal clip models27-30, and DOCA-salt models31,32.
Numerous reasons may exist for discrepancies among these studies. For example, there are differences in quantification methodologies (histological vs. biochemical), as well as the variable ways that samples are reported and normalized (e.g., weight vs. length of the tissue, protein content vs. density, medial vs. total wall region, quantification from image field vs. total ring). Another reason may be innate differences in the hypertension model, stage of hypertension development, the vessel studied and the age at the time of sampling. A time-course study of an aortic coarctation model of hypertension by Hu et al. reported that the concentration of collagen in the aorta was only increased at 2- and 4- weeks post-surgery, and that by week 6 the concentration had returned to the levels of the sham control33, suggesting that remodeling of ECM may be quite dynamic. Thus, it is possible acute induction of hypertension, either surgically or by short-term infusions of vasopressor agents, only temporarily alters the levels of certain ECM proteins as an acute adaptive response that is followed by de-adaptation and continued changes in content. It is worth noting that such animal models are often sacrificed for tissue measurements within a few weeks of the induction of the hypertension, and therefore, may only record these early dynamic changes in the remodeling of the vessels. By contrast, the SHR is a model that develops and adapts to hypertension gradually, which may affect the temporal nature of its remodeling process. In our experiments we have chosen to sample SHR at a young age (after hypertension was fully established), and also at an older age to assess longer-term vascular remodeling events. Of relevance to the observations reported in our present investigation, studies of human essential hypertension that assessed aortic collagen post-mortem found that the collagen content was unchanged34,35 with hypertension or to have a similar concentration36 compared to age-matched normotensive patients.
Our findings do not support changes in content of collagen and elastin as an explanation for increased stiffness in hypertension. However, other types of ECM remodeling such as changes in ECM protein type, cross-linking, glycation, and structural or architectural rearrangement may contribute to altering the mechanical properties of the of the vascular wall. Although the directionality of the changes in vascular collagen associated with hypertension is controversial, it is widely accepted that vascular collagen does increase with aging. It may be that vascular collagen plays a larger role in increasing vascular stiffness as part of the aging process than it does in hypertension. To ultimately define these relationships, we suggest that it is important to distinguish between the overlapping and unique cellular mechanisms underlying vascular stiffness in hypertension and aging.
We postulate that VSMCs play a much larger role in hypertensive vascular stiffness than previously considered. In the SHR, we observe increased medial thickness hypertrophy of VSMCs (Fig. 4), which is particularly striking within the old SHR animals, and may be attributable to VSMC hypertrophy. Hypertrophy of VSMCs is commonly observed in hypertension37, and may also contribute to increases in VSMC stiffness. Furthermore, in the SHR we observe increases in the underlying increased mechanical load in the medial layer (Fig. 4D). VSMCs undergo major physiological adaptations to hypertension and thus we propose they may represent a larger contribution to the mechanical characteristics of the aortic wall than has been recognized. Already, we know that VSMCs readily adjust behaviors such as, proliferation, differentiation, and migration, in response to mechanical stimuli38. Furthermore, the inherent phenotypic plasticity of VSMCs is a well-recognized major contributor to the progression of several vascular diseases, including atherosclerosis and aging-related pathology39.
Our unique finding that the stiffness of individual VSMCs are augmented by hypertension (Fig. 2D) and that cellular stiffness continues to rise in older hypertensive animals suggests that increased VSMC stiffness is an important part of the disease process and plays a critical role in the stiffening process taking place in the vascular wall. Of course, it possible that inherent genetic differences between SHR and WKY may introduce variables in addition to hypertension, and that the blood pressure dependencies of stiffness and adhesion have not been completely defined by our studies. However, we note that the aging WKY, which do not undergo any change in blood pressure, do show an increase in VSMC stiffness and adhesion that correlate with overall vascular stiffening. This supports the conclusion that intrinsic changes in VSMC mechanical and adhesive properties are associated with vascular stiffening. Our studies of young hypertensive rats20 and aging monkeys18,19 also support this concept.
Furthermore, it was observed that the adhesive properties of VSMCs are also increased by hypertension(Figs. 3B-D). A stiffer cell combined with a larger number of cell adhesions to the ECM would allow the cellular cytoskeletal elements to play a greater role in influencing overall mechanical characteristics of the vascular wall. Importantly, the observed increase in attachment force of the SHR VSMC cytoskeletal connections could contribute to exert greater forces on ECM proteins. It is reasonable to envision that the larger pulling forces from these adhesive connections may by themselves contribute to stiffness of the vessel. Indeed the augmentation of adhesion force in the old SHR VSMCs correlates with the accelerated vascular stiffness. This finding of increased adhesion force to fibronectin is also significant in light of several reports of increased presence of fibronectin and alpha 5 integrin in the vessels of several hypertension models: SHR12,26,40, deoxycorticosterone/salt40, angiotensin II-infusion40, as well as recently described non-hypertensive vascular stiffness models41. Furthermore, treatment with anti hypertensive drugs has been reported to decrease vascular fibronectin levels13,26,42. While many of these studies have supported the notion that cell focal adhesions to fibronectin may be correlated with vascular stiffness, our measurements are the first to confirm using biophysical assessment tools that stiffness and increased attachment to fibronectin occur and are modified in the aging hypertensive model. This strongly supports the hypothesis that that cell stiffness and adhesion should be considered as alternative therapeutic targets for anti-hypertensive therapy.
Additionally, it is important to note is that the adhesive properties of VSMCs are related to VSMC stiffness. It was observed that similar values of VSMC stiffness are obtained in AFM experiments with and without adhesion (fibronectin-coated vs. uncoated cantilever probes). Therefore the presence of fibronectin on the probe was not influencing the stiffness of the VSMC. Conversely, the VSMC stiffness level may be influencing the adhesion properties, as recently published work supports a mechanistic link between, stiffness and adhesion. Hong et al.43,44 demonstrated that treatment of VSMCs with the vasoactive agents angiotensin II or adenosine, respectively increase and decrease VSMC stiffness and adhesion in a coordinated manor. It may be that the interplay of these two mechanisms, VSMC stiffness and VSMC adhesion, contributes to the interaction of hypertension and aging in accelerating vascular stiffness.
In summary, the current investigation is the first to identify that the stiffness and adhesion properties of VSMCs strongly correlate with increased vascular stiffness within an aging hypertension model. Future studies are needed to better understand the underlying mechanisms that increase VSMC stiffness in hypertension, and also how VSMC stiffness enhances VSMC adhesion to ECM proteins. These mechanisms, VSMC stiffness and VSMC adhesion, may elucidate potential therapeutic targets that directly control vascular stiffness in hypertension.
Perspectives
Whereas much of the prior work in this field has focused on changes in the ECM (collagen deposition and cross linking, elastin breakdown) as well as endothelial damage (impaired nitric oxide release), this study demonstrates that the intrinsic mechanical properties and adhesive behavior of VSMCs are associated with vascular stiffness in hypertension and aging. Furthermore, the interplay between VSMC stiffness and VSMC adhesion may be structurally responsible to the acceleration of vascular stiffness in hypertensive aging. VSMC stiffness and VSMC adhesion are emerging and novel concepts that provide new possibilities for elucidating mechanisms involved in hypertension, and these may guide us towards direct therapeutic targets for vascular stiffness.
Novelty and Significance.
-
What Is New?
The intrinsic stiffness and adhesion properties of VSMCs are increased in hypertension, and these may contribute to accelerated aortic stiffness observed with age in this disease.
VSMC stiffness and adhesion are emerging and novel concepts in the understanding of aortic stiffness in cardiovascular disease.
These concepts contrast to our previous understanding of aortic stiffness, which has largely focused on extracellular matrix and endothelial cell changes.
-
What Is Relevant?
Hypertension is a major health problem in the United States.
The novel mechanisms of VSMC stiffness and VSMC adhesion suggest the potential for new pharmacological targets to control hypertension and aortic stiffness, directed at VSMCs themselves.
Summary.
Our findings in the SHR have revealed that aortic VSMC stiffness and VSMC adhesion are increased in hypertension and aging, and that superimposing these two conditions leads to significant interaction. This supports the novel hypothesis that the mechanical properties of VSMCs contribute to aortic stiffness in hypertension.
Conversely, data in this study demonstrate that collagen and elastin content do not correlate with the increase in vascular stiffness.
The mechanisms underlying VSMC stiffness and VSMC adhesion may provide new therapeutic targets and strategies for selectively targeting aortic stiffness in hypertension.
Acknowledgments
None.
Sources of Funding: This study was supported in part by AHA 13PRE16980042 to N.L. Sehgel; NIH R01HL092241 to M.A. Hill, NIH P01HL069020, NIH R01HL093415, NIH R01HL095888 to D.E. Vatner; NIH 5R01HL102472, NIH P01AG027211, NIH P01HL069020, NIH T32HL069752, NIH R01HL093481, NIH R01HL106511 to S.F. Vatner, and NIH P01HL095486 to G.A. Meininger.
Footnotes
Conflicts of Interest/Disclosure Statements: None.
References
- 1.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D'Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham heart study. JAMA : The Journal of the American Medical Association. 2002;287:1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 2.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: A longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 3.Franklin SS, Gustin Wt, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham heart study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Cross-sectional correlates of increased aortic stiffness in the community: The framingham heart study. Circulation. 2007;115:2628–2636. doi: 10.1161/CIRCULATIONAHA.106.667733. [DOI] [PubMed] [Google Scholar]
- 5.Verwoert GC, Franco OH, Hoeks AP, Reneman RS, Hofman A, CM VD, Sijbrands EJ, Witteman JC, Mattace-Raso FU. Arterial stiffness and hypertension in a large population of untreated individuals: The Rotterdam Study. Journal of Hypertension. 2014;32:1606–1612. doi: 10.1097/HJH.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 6.van Gorp AW, Schenau DS, Hoeks AP, Boudier HA, de Mey JG, Reneman RS. In spontaneously hypertensive rats alterations in aortic wall properties precede development of hypertension. American Journal of Physiology Heart and Circulatory Physiology. 2000;278:H1241–1247. doi: 10.1152/ajpheart.2000.278.4.H1241. [DOI] [PubMed] [Google Scholar]
- 7.Chamiot-Clerc P, Renaud JF, Safar ME. Pulse pressure, aortic reactivity, and endothelium dysfunction in old hypertensive rats. Hypertension. 2001;37:313–321. doi: 10.1161/01.hyp.37.2.313. [DOI] [PubMed] [Google Scholar]
- 8.Isabelle M, Simonet S, Ragonnet C, Sansilvestri-Morel P, Clavreul N, Vayssettes-Courchay C, Verbeuren TJ. Chronic reduction of nitric oxide level in adult spontaneously hypertensive rats induces aortic stiffness similar to old spontaneously hypertensive rats. Journal of Vascular Research. 2012;49:309–318. doi: 10.1159/000337470. [DOI] [PubMed] [Google Scholar]
- 9.Labat C, Cunha RS, Challande P, Safar ME, Lacolley P. Respective contribution of age, mean arterial pressure, and body weight on central arterial distensibility in shr. American Journal of Physiology Heart and Circulatory Physiology. 2006;290:H1534–1539. doi: 10.1152/ajpheart.00742.2005. [DOI] [PubMed] [Google Scholar]
- 10.Marque V, Kieffer P, Atkinson J, Lartaud-Idjouadiene I. Elastic properties and composition of the aortic wall in old spontaneously hypertensive rats. Hypertension. 1999;34:415–422. doi: 10.1161/01.hyp.34.3.415. [DOI] [PubMed] [Google Scholar]
- 11.Tsamis A, Krawiec JT, Vorp DA. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: A review. Journal of the Royal Society Interface. 2013;10:20121004. doi: 10.1098/rsif.2012.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bezie Y, Lamaziere JM, Laurent S, Challande P, Cunha RS, Bonnet J, Lacolley P. Fibronectin expression and aortic wall elastic modulus in spontaneously hypertensive rats. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18:1027–1034. doi: 10.1161/01.atv.18.7.1027. [DOI] [PubMed] [Google Scholar]
- 13.Koffi I, Lacolley P, Kirchengaast M, Pomies JP, Laurent S, Benetos A. Prevention of arterial structural alterations with verapamil and trandolapril and consequences for mechanical properties in spontaneously hypertensive rats. European Journal of Pharmacology. 1998;361:51–60. doi: 10.1016/s0014-2999(98)00691-8. [DOI] [PubMed] [Google Scholar]
- 14.Koffi I, Safar ME, Labat C, Lacolley P, Benetos A, Mourad JJ. Arterial structural changes with verapamil in spontaneously hypertensive rats. American Journal of Hypertension. 1999;12:732–738. doi: 10.1016/s0895-7061(99)00050-3. [DOI] [PubMed] [Google Scholar]
- 15.Cox RH. Basis for the altered arterial wall mechanics in the spontaneously hypertensive rat. Hypertension. 1981;3:485–495. doi: 10.1161/01.hyp.3.4.485. [DOI] [PubMed] [Google Scholar]
- 16.Mizutani K, Ikeda K, Kawai Y, Yamori Y. Biomechanical properties and chemical composition of the aorta in genetic hypertensive rats. Journal of Hypertension. 1999;17:481–487. doi: 10.1097/00004872-199917040-00005. [DOI] [PubMed] [Google Scholar]
- 17.Bezie Y, Lacolley P, Laurent S, Gabella G. Connection of smooth muscle cells to elastic lamellae in aorta of spontaneously hypertensive rats. Hypertension. 1998;32:166–169. doi: 10.1161/01.hyp.32.1.166. [DOI] [PubMed] [Google Scholar]
- 18.Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RR, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA, Vatner SF. Short communication: Vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circulation Research. 2010;107:615–619. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y, Qiu H, Trzeciakowski JP, Sun Z, Li Z, Hong Z, Hill MA, Hunter WC, Vatner DE, Vatner SF, Meininger GA. Temporal analysis of vascular smooth muscle cell elasticity and adhesion reveals oscillation waveforms that differ with aging. Aging Cell. 2012;11:741–750. doi: 10.1111/j.1474-9726.2012.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sehgel NL, Zhu Y, Sun Z, Trzeciakowski JP, Hong Z, Hunter WC, Vatner DE, Meininger GA, Vatner SF. Increased vascular smooth muscle cell stiffness: A novel mechanism for aortic stiffness in hypertension. American Journal of Physiology Heart and Circulatory Physiology. 2013;305:H1281–1287. doi: 10.1152/ajpheart.00232.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Z, Martinez-Lemus LA, Hill MA, Meininger GA. Extracellular matrix-specific focal adhesions in vascular smooth muscle produce mechanically active adhesion sites. American Journal of Physiology Cell Physiology. 2008;295:C268–278. doi: 10.1152/ajpcell.00516.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrhart LA, Ferrario CM. Collagen metabolism and reversal of aortic medial hypertrophy in spontaneously hypertensive rats treated with methyldopa. Hypertension. 1981;3:479–484. doi: 10.1161/01.hyp.3.4.479. [DOI] [PubMed] [Google Scholar]
- 23.Ricci MA, Slaiby JM, Hendley ED, Stirewalt W, Cloutier L, Nichols P, Evans JN. Hemodynamic and biochemical characteristics of the aorta in the wky, shr, wkht, and wkha rat strains. Annals of the New York Academy of Sciences. 1996;800:121–130. doi: 10.1111/j.1749-6632.1996.tb33303.x. [DOI] [PubMed] [Google Scholar]
- 24.Brayden JE, Halpern W, Brann LR. Biochemical and mechanical properties of resistance arteries from normotensive and hypertensive rats. Hypertension. 1983;5:17–25. doi: 10.1161/01.hyp.5.1.17. [DOI] [PubMed] [Google Scholar]
- 25.Mourlon-Le Grand MC, Poitevin P, Benessiano J, Duriez M, Michel JB, Levy BI. Effect of a nonhypotensive long-term infusion of anp on the mechanical and structural properties of the arterial wall in wistar-kyoto and spontaneously hypertensive rats. Arteriosclerosis and Thrombosis : AJournal of Vascular Biology / American Heart Association. 1993;13:640–650. doi: 10.1161/01.atv.13.5.640. [DOI] [PubMed] [Google Scholar]
- 26.Intengan HD, Thibault G, Li JS, Schiffrin EL. Resistance artery mechanics, structure, and extracellular components in spontaneously hypertensive rats : Effects of angiotensin receptor antagonism and converting enzyme inhibition. Circulation. 1999;100:2267–2275. doi: 10.1161/01.cir.100.22.2267. [DOI] [PubMed] [Google Scholar]
- 27.Wolinsky H. Response of the rat aortic media to hypertension. Morphological and chemical studies. Circulation Research. 1970;26:507–522. doi: 10.1161/01.res.26.4.507. [DOI] [PubMed] [Google Scholar]
- 28.Wolinsky H. Long-term effects of hypertension on the rat aortic wall and their relation to concurrent aging changes. Morphological and chemical studies. Circulation Research. 1972;30:301–309. doi: 10.1161/01.res.30.3.301. [DOI] [PubMed] [Google Scholar]
- 29.Ceron CS, Rizzi E, Guimaraes DA, Martins-Oliveira A, Cau SB, Ramos J, Gerlach RF, Tanus-Santos JE. Time course involvement of matrix metalloproteinases in the vascular alterations of renovascular hypertension. Matrix Biology : Journal of the International Society for Matrix Biology. 2012;31:261–270. doi: 10.1016/j.matbio.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Cox RH, Bagshaw RJ. Effects of hypertension and its reversal on canine arterial wall properties. Hypertension. 1988;12:301–309. doi: 10.1161/01.hyp.12.3.301. [DOI] [PubMed] [Google Scholar]
- 31.Spector S, Ooshima A, Iwatsuki K, Fuller G, Cardinale G, Udenfriend S. Increased vascular collagen biosynthesis by hypertension and reversal by antihypertensive drugs. Blood Vessels. 1978;15:176–182. doi: 10.1159/000158163. [DOI] [PubMed] [Google Scholar]
- 32.Cox RH. Contribution of salt to arterial wall changes in doca hypertension in the rat. Journal of Hypertension. 1987;5:611–619. doi: 10.1097/00004872-198710000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Hu JJ, Ambrus A, Fossum TW, Miller MW, Humphrey JD, Wilson E. Time courses of growth and remodeling of porcine aortic media during hypertension: A quantitative immunohistochemical examination. The Journal of Histochemistry and Cytochemistry : Official Journal of the Histochemistry Society. 2008;56:359–370. doi: 10.1369/jhc.7A7324.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoshino H, Takahashi M, Kushida K, Ohishi T, Kawana K, Inoue T. Quantitation of the crosslinks, pyridinoline, deoxypyridinoline and pentosidine, in human aorta with dystrophic calcification. Atherosclerosis. 1995;112:39–46. doi: 10.1016/0021-9150(94)05395-y. [DOI] [PubMed] [Google Scholar]
- 35.Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: Implications for dissecting aortic aneurysm. The American Journal of Cardiology. 1977;39:13–20. doi: 10.1016/s0002-9149(77)80004-0. [DOI] [PubMed] [Google Scholar]
- 36.Faber M, Oller-Hou G. The human aorta. V. Collagen and elastin in the normal and hypertensive aorta. Acta Pathologica et Microbiologica Scandinavica. 1952;31:377–382. [PubMed] [Google Scholar]
- 37.Imanishi M, Tomita S, Ishizawa K, Kihira Y, Ueno M, Izawa-Ishizawa Y, Ikeda Y, Yamano N, Tsuchiya K, Tamaki T. Smooth muscle cell-specific hif-1alpha deficiency suppresses angiotensin ii-induced vascular remodelling in mice. Cardiovascular Research. 2014;102:460–468. doi: 10.1093/cvr/cvu061. [DOI] [PubMed] [Google Scholar]
- 38.Liu WF. Mechanical regulation of cellular phenotype: Implications for vascular tissue regeneration. Cardiovascular Research. 2012;95:215–222. doi: 10.1093/cvr/cvs168. [DOI] [PubMed] [Google Scholar]
- 39.Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB. The vascular smooth muscle cell in arterial pathology: A cell that can take on multiple roles. Cardiovascular Research. 2012;95:194–204. doi: 10.1093/cvr/cvs135. [DOI] [PubMed] [Google Scholar]
- 40.Takasaki I, Chobanian AV, Sarzani R, Brecher P. Effect of hypertension on fibronectin expression in the rat aorta. The Journal of Biological Chemistry. 1990;265:21935–21939. [PubMed] [Google Scholar]
- 41.Bouissou C, Lacolley P, Dabire H, Safar ME, Gabella G, Duchatelle V, Challande P, Bezie Y. Increased stiffness and cell-matrix interactions of abdominal aorta in two experimental nonhypertensive models: Long-term chemically sympathectomized and sinoaortic denervated rats. Journal of Hypertension. 2014;32:652–658. doi: 10.1097/HJH.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kakou A, Bezie Y, Mercier N, Louis H, Labat C, Challande P, Lacolley P, Safar ME. Selective reduction of central pulse pressure under angiotensin blockage in shr: Role of the fibronectin-alpha5beta1 integrin complex. AmericanJournal of Hypertension. 2009;22:711–717. doi: 10.1038/ajh.2009.87. [DOI] [PubMed] [Google Scholar]
- 43.Hong Z, Sun Z, Li Z, Mesquitta WT, Trzeciakowski JP, Meininger GA. Coordination of fibronectin adhesion with contraction and relaxation in microvascular smooth muscle. Cardiovascular Research. 2012;96:73–80. doi: 10.1093/cvr/cvs239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong Z, Sun Z, Li M, Li Z, Bunyak F, Ersoy I, Trzeciakowski JP, Staiculescu MC, Jin M, Martinez-Lemus L, Hill MA, Palaniappan K, Meininger GA. Vasoactive agonists exert dynamic and coordinated effects on vascular smooth muscle cell elasticity, cytoskeletal remodelling and adhesion. The Journal of Physiology. 2014;592:1249–1266. doi: 10.1113/jphysiol.2013.264929. [DOI] [PMC free article] [PubMed] [Google Scholar]