Abstract
Interleukin-15 (IL-15) is a common γ-chain cytokine that has a significant role in the activation and proliferation of T and NK cells and holds great potential in fighting infection and cancer. We have previously shown that bioactive IL-15 in vivo comprises a complex of the IL-15 chain with the soluble or cell-associated IL-15 receptor alpha (IL-15Rα) chain, which together form the IL-15 heterodimer. We have generated DNA vectors expressing the heterodimeric IL-15 by optimizing mRNA expression and protein trafficking. Repeated administration of these DNA plasmids by intramuscular injection followed by in vivo electroporation in rhesus macaques resulted in sustained high levels of IL-15 in plasma, with no significant toxicity. Administration of DNAs expressing heterodimeric IL-15 also resulted in an increased frequency of NK and T cells undergoing proliferation in peripheral blood. Heterodimeric IL-15 led to preferential expansion of CD8+NK cells, all memory CD8+ T-cell subsets and effector memory CD4+ T cells. Expression of heterodimeric IL-15 by DNA delivery to the muscle is an efficient procedure to obtain high systemic levels of bioactive cytokine, without the toxicity linked to the high transient cytokine peak associated with protein injection.
Introduction
Cytokines of the γ-chain family have the potential to be used in different clinical settings, such as AIDS and cancer immunotherapy, due to their ability to regulate the homeostasis of the immune system and to boost host responses against pathogens and tumor antigens. Interleukin-15 (IL-15) supports the development, proliferation, survival and trafficking of several lymphocyte subsets, including NK, CD8+ and γδ T cells. It has also a non-redundant role in the establishment and maintenance of CD8+ T-cell memory.1, 2, 3, 4, 5 The efficient secretion of IL-15 requires co-expression of the IL-15-binding protein named IL-15 receptor alpha (IL-15Rα) in the same cell.6, 7, 8 Co-production of the two molecules leads to intracellular association of IL-15 and IL-15Rα in the endoplasmic reticulum, stabilization of both molecules and efficient transport to the cell surface.9, 10, 11 This complex anchored to the cell surface is trans-presented to cells expressing the β/γ subunits comprising the low-affinity IL-2 receptor and IL-15 receptor,12 or is secreted in a soluble form upon cleavage of the transmembrane domain of the IL-15Rα.13 Indeed, the circulating form of IL-15 in biological fluids is in complex with soluble IL-15Rα (sIL-15Rα) in both mice and humans.14 Several studies in mice showed that the soluble heterodimeric IL-15:sIL-15Rα (hetIL-15) has superior pharmacokinetics and a 10- to 100-fold increase in agonistic activity over single-chain IL-15 in vivo.10,13,15, 16, 17, 18
For therapeutic purposes, hetIL-15 can be provided in vivo as recombinant protein or by gene delivery. Systemic delivery of protein may cause significant toxicity, as it has also been reported for single-chain IL-15 (refs 19, 20) and other cytokines, such as IL-2 (refs. 21, 22) or IL-12.23 Toxicity may be reduced or eliminated through the delivery of cytokine genes, which are expressed for short periods of time. Among gene therapy approaches, the use of naked DNA is promising because of its simplicity, flexibility and possibility of repeated applications owing to the absence of immunity against the vector (for review see Ferraro et al.24). Successful gene therapy depends largely on the optimization of gene expression and on the method of delivery (for recent reviews see Kutzler and Weiner,25 Felber et al.26). We have previously described methods to develop efficient expression vectors for IL-15, combining mRNA optimization (RNA/codon optimization) of the IL-15-coding sequences and substitution of the signal peptide with other efficient secretory signals to optimize trafficking of the molecules.27 We also produced vectors expressing IL-15 in combination with either membrane-associated or soluble IL-15Rα, which resulted in stable, secreted heterodimeric IL-15:sIL-15Rα, with a final improvement of more than 1000-fold in the systemic level of bioactive IL-15 compared with vectors expressing the wild-type IL-15 cDNA.10 Substantial improvement in gene delivery using plasmid DNAs has been achieved by intramuscular injection followed by in vivo electroporation (EP).28,29,30 In vivo EP of naked DNAs results in increased DNA uptake and in enhanced gene expression by the cells at the injection site. Intramuscular injection followed by in vivo EP (IM/EP) has been widely used as delivery method to improve the expression and immunogenicity of human/simian immunodeficiency virus (HIV/SIV) DNA vaccines in macaques31, 32, 33, 34, 35 and humans.36, 37, 38 In addition, DNA EP was used to deliver cytokine genes, including IL-12 and IL-15, as vaccine adjuvants in preventive and therapeutic SIV DNA immunization in macaques.34,39, 40, 41, 42 Cytokine gene delivery by in vivo EP has also been successfully employed as cancer treatment in several preclinical and clinical studies. Intratumoral delivery of single-chain IL-15-expressing DNA by EP resulted in the complete regression of established B16 melanoma tumors in mice.43 In melanoma patients, the delivery of the IL-12-expressing DNA by EP has been shown to be safe, with lower toxicity in comparison to the systemic delivery of the recombinant protein.44
In the present work, optimized DNA vectors encoding human heterodimeric IL-15 were delivered in rhesus macaques by the IM/EP method. Elevated levels of IL-15 were detected in the plasma and were associated with an increased proliferation of NK and T cells, with no adverse effects. These results demonstrate that intramuscular administration of optimized IL-15 vectors in non-human primates results in systemic bioactive levels of heterodimeric IL-15, suggesting possible applications in vaccination regimens and immunotherapy protocols.
Results
Generation of optimized vectors expressing human IL-15 heterodimers
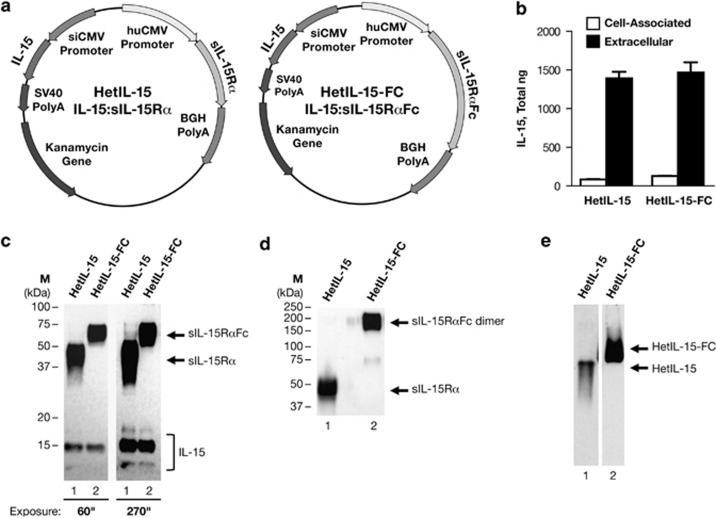
The optimization of DNA vectors expressing the heterodimeric IL-15 is essential for the efficient gene delivery of the cytokine in vivo. We have previously shown that combining two approaches, namely mRNA optimization (RNA/codon optimization) of the coding sequence and substitution of signal peptide with the granulocyte macrophage-colony stimulating factor secretory signal, results in synergistically improved expression and secretion of bioactive single-chain IL-15.27 As we have shown that the IL-15 circulating in the body is the heterodimeric form in association with the so-called IL-15Rα,14 we generated dual gene expression vectors producing the heterodimeric cytokine IL-15:IL-15Rα to assure expression of both chains upon in vivo DNA delivery. We produced and tested two plasmids encoding for heterodimeric IL-15:IL-15Rα cytokine, that is, IL-15:sIL-15Rα (hetIL-15, plasmid AG153) and IL-15:sIL-15RαFc (hetIL-15-FC, plasmid AG256). The hetIL-15 DNA produces the human soluble heterodimeric IL-15:sIL-15Rα comprising a truncated IL-15Rα that lacks the transmembrane and cytoplasmic regions (Figure 1a, left panel). The hetIL-15-Fc is a fusion protein comprising the human soluble heterodimeric IL-15, in which the sIL-15Rα chain is fused to the Fc region of human IgG1 (Figure 1a, right panel). The fusion to the Fc fragment results in a more stable form of IL-15 heterodimer15, 16, 17 and may also have the ability to act as a cell-associated cytokine in vivo, mimicking IL-15 trans-presentation. We studied the expression of these vectors after transient transfection in human HEK293 cells (Figure 1b). Co-expression of either sIL-15Rα or sIL-15RαFc with single-chain IL-15 resulted in similar levels of cytokine production measured by ELISA. More than 90% of total IL-15 was found in the cell supernatant, demonstrating efficient and comparable secretion of the cytokine upon its intracellular association with either sIL-15Rα or sIL-15RαFc. The secreted forms of IL-15 and sIL-15Rα were also visualized by western immunoblot (Figure 1c). sIL-15Rα encoded by hetIL-15 DNA migrated as a broad band of ~50 kDa (lane 1, exposure 60”), whereas sIL-15RαFc fusion protein encoded by hetIL-15-FC DNA migrated at ~75 kDa (lane 2, exposure 60”). In both cases, broad bands were indicative of extensive glycosylation, as previously reported.13 Human IL-15 is also glycosylated and the western immunoblot analysis revealed three forms of IL-15 upon transient transfection of hetIL-15 DNA (lane 1, exposure 270”) or hetIL-15-FC DNA (lane 2, exposure 270”), namely, unglycosylated ~13 kDa, partially glycosylated ~15 kDa and fully glycosylated ~17 kDa proteins. Western immunoblot analysis performed under non-reducing condition (SDS without β-mercaptoethanol) revealed the formation of disulfide bond between the Fc regions of two hetIL-15-FC molecules (Figure 1d, lane 2, band ~200 kDa), showing that hetIL-15Fc is secreted as dimers, as expected. The formation of IL-15:sIL-15Rα complexes was verified on native gels. No single-chain IL-15, sIL-15Rα or sIL-15RαFc was detected in the supernatants of HEK293 cells transfected with either DNA, suggesting secretion of the heterodimeric forms (Figure 1e).
Figure 1.
Generation of optimized vectors expressing human IL-15 heterodimers. (a) Maps of the dual promoter plasmids encoding two forms of the heterodimeric IL-15 cytokine, hetIL-15 (AG153; left panel) and hetIL-15-FC (AG256; right panel). The IL-15Rα and IL-15 genes are under the control of the human cytomegalovirus (hCMV) and the simian CMV (siCMV) promoter, respectively. The polyadenylation signals from bovine growth hormone (BGH) and the simian virus 40 (SV40) were used for the IL-15Rα and the IL-15 subunit, respectively. The plasmids contain the kanamycin resistance gene for selection in bacteria. The plasmid backbone is optimized for efficient growth in bacteria. (b) Human HEK293 cells were transiently transfected with hetIL-15 DNA-expressing IL-15:sIL-15Rα or hetIL-15-FC DNA-expressing IL-15:sIL-15RαFc. Cell-associated IL-15 and extracellular IL-15 were measured by ELISA 3 days after transfection. Bars indicate mean values of IL-15 production; standard deviation (s.d.) of three independent transfections is shown. The mean green fluorescent protein (GFP) values for the transfections in the figure were: 122 and 107 arbitrary GFP units. (c) Western immunoblot analyses of secreted IL-15Rα (sIL-15Rα, lane 1; sIL-15RαFc, lane 2) and IL-15 after transfections of HEK293 cells as described above, under denaturing and reducing conditions. One representative sample of triplicate plates is shown. The gel contained 1/200 of the culture supernatant loaded per lane. The panel on the right is a longer exposure of the left panel to visualize the three bands corresponding to non-glycosylated (13 kDa), partially glycosylated (15 kDa) and fully glycosylated (17 kDa) IL-15, indicated by bracket. Arrows indicate the position of sIL-15RαFc and sIL-15Rα. The position of molecular weight (MW) markers is shown on the left. (d) Western immunoblot analyses of secreted IL-15Rα (sIL-15Rα, lane 1; sIL-15RαFc, lane 2) after transfections of HEK293 cells as described above, under non-reducing conditions. Arrows indicate the position of sIL-15Rα and sIL-15RαFc dimer. The position of MW markers is shown on the left. (e) Western immunoblot analyses of secreted heterodimeric IL-15 under native conditions. Arrows indicate the position of hetIL-15 (lane 1) and hetIL-15-FC (lane 2).
Detection of IL-15 in the plasma of macaques upon intramuscular delivery followed by in vivo EP of DNAs expressing heterodimeric IL-15
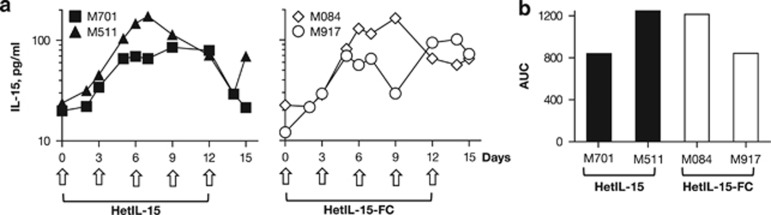
To test whether systemic levels of IL-15 could be achieved in vivo, the plasmids encoding the two heterodimeric forms of IL-15 were administered into rhesus macaques by IM/EP. We measured circulating human IL-15 in the plasma of two macaques (M701 and M511) injected with hetIL-15 DNA, and in two macaques (M084 and M917) injected with hetIL-15-FC DNA. Each macaque received a dose of 8 mg of DNA every 3 days. The plasma levels of the heterodimeric IL-15 were measured at the indicated time points using a commercially available human IL-15 ELISA. Endogenous levels of IL-15 before treatment ranged between 12 and 24 pg ml−1 in these macaques (Figure 2a). We have previously reported that a single administration of heterodimeric IL-15-expressing DNA via IM/EP results in IL-15 plasma levels that peak at day 3 or 4 after the injection, followed by a decline to baseline levels within 1 week.33 Plasma IL-15 levels increased approximately twofold over baseline 3 days after the first injection, and continued to increase upon subsequent injections. Peak levels of ~100 pg ml−1 were reached between day 7 and 12 (range=85–175 pg ml−1). In three of the four animals, no further increase in plasma IL-15 was observed after the fourth and fifth DNA injection. Plasma IL-15 levels were still higher than the baseline values at the time of the last measurement (day 15, 3 days after the last DNA injection), with the exception of animal M701 (Figure 2a). Interestingly, the plasmids encoding the two different heterodimeric forms of IL-15 produced similar IL-15 plasma levels overtime and similar area-under-the-curve values (Figure 2b).
Figure 2.
Plasma IL-15 levels after intramuscular delivery of IL-15 heterodimer DNAs followed by in vivo electroporation in rhesus macaques. (a) Two groups of macaques (N=2) were subjected to intramuscular injection followed by in vivo EP using hetIL-15 DNA or hetIL-15-FC DNA vectors at days 0, 3, 6, 9 and 12, as marked by arrows, using a dose of 8 mg DNA per injection. Plasma IL-15 levels were measured overtime before, during and after the IL-15 treatment by ELISA and reported for the animals receiving hetIL-15 DNA (left panel) or hetIL-15-FC DNA (right panel). (b) Area-under-the curve (AUC) of IL-15 plasma levels over the 15 days was determined for the individual animals.
Hematologic profile of rhesus macaques treated with DNA-encoded heterodimeric IL-15
The cytokine DNA injections were well tolerated with no adverse effects. The hematologic profile of the four animals was monitored at days 0, 2, 7 and 14 after the start of the treatment and no abnormalities were detected. Lymphocyte, monocyte, eosinophil, basophil and neutrophil counts were within the normal range reported for rhesus macaques throughout the study. The lymphocyte and neutrophil profiles are reported in Table 1. Similarly, no abnormalities were detected in the platelet count, hematocrit or hemoglobin content. Serum chemistry analysis was also performed at the same time points (Table 2). A transient increase in aspartate aminotransferase and creatinine phosphokinase was observed in all animals, whereas only one animal (M511) had a small increase in alanine aminotransferase. However, measurements in historical control macaques suggest that blood samples taken from sedated animals could have abnormally high creatinine phosphokinase values because of increased tonic muscular activity sometimes associated with the use of ketamine as anesthetic drug. Transient elevation of creatinine phosphokinase has been also previously noted following EP to the muscle.45 The majority of the values returned to normal range at day 14 of the study (Table 2).
Table 1. Absolute lymphocyte and neutrophil counts during IL-15 treatment.
| Macaque |
Cell count after initiation of IL-15 treatment (cells per μl blood) |
|||||
|---|---|---|---|---|---|---|
| Day 0 | Day 2 | Day 7 | Day 14 | |||
| Lymphocytes (cells per μl blood) | ||||||
| M701 | 3312 | 2360 | 1980 | 2856 | ||
| M511 | 4225 | 4161 | 2964 | 4628 | ||
| M084 | 2001 | 3116 | 2024 | 2552 | ||
| M917 | 3300 | 2592 | 1834 | 2146 | ||
| Neutrophils (cells per μl blood) | ||||||
| M701 | 3384 | 3127 | 2205 | 2240 | ||
| M511 | 2015 | 2701 | 4368 | 3827 | ||
| M084 | 4002 | 4182 | 6072 | 2204 | ||
| M917 | 3750 | 2322 | 3111 | 4958 | ||
Lymphocytes and neutrophil absolute counts were reported as cells per μl blood overtime, before and during the heterodimeric IL-15 treatment for the individual animals.
Abbreviation: IL, interleukin.
Table 2. Enzyme levels in blood during IL-15 treatment.
| Macaque | Enzyme |
Enzyme units |
|||
|---|---|---|---|---|---|
| Day 0 | Day 2 | Day 7 | Day 14 | ||
| M701 | AST (normal range 14–30 U l−1) | 28 | 51a | 72a | 81a |
| M511 | 24 | 221a | 76a | 33a | |
| M084 | 23 | 44a | 56a | 27 | |
| M917 | 32 | 44a | 68a | 30 | |
| M701 | ALT (normal range 0–82 U l−1) | 30 | 50 | 70 | 60 |
| M511 | 28 | 296a | 123a | 63 | |
| M084 | 18 | 39 | 39 | 23 | |
| M917 | 15 | 34 | 60 | 33 | |
| M701 | CPK (normal range 0–436 U l−1) | 317 | 581a | 1598a | 5428a |
| M511 | 324 | 1502a | 1538a | 244 | |
| M084 | 482a | 418 | 2728a | 288 | |
| M917 | 944a | 694a | 1964 | 351 | |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK, creatinine phosphokinase; IL, interleukin.
Values outside the normal range.
AST, ALT and CPK were reported as U l−1 in blood overtime, before and during the heterodimeric IL-15 treatment for the individual animals. Normal range for rhesus macaques are also provided.
We also examined the effect of the IL-15 treatment on the virological status of the animals. The macaques were infected with SIVsmE660 (M917, M701) or SHIV (M511, M084) and controlled viremia from 18 months to 7 years before enrollment in this study. The plasma viral loads were determined in all the animals before and after IL-15 treatment (Table 3). Macaques M917 and M511 had undetectable viral loads before treatment and no changes were observed throughout the study. Macaques M701 and M084 had low pre-treatment viremia (1.8 × 104 and 4 × 104, respectively), which could contribute to the increased baseline lymphocyte proliferation observed in peripheral blood (see below). No significant changes in viral load were observed during the study in these animals either (Table 3), suggesting that IL-15 treatment does not impact viremia significantly, which is in agreement with previous observations.42,46
Table 3. Viral load levels after initiation of IL-15 treatment.
| Macaque | Gender | Age at study onset (years) | Infected by | Time infected before study onset (years) |
Viral load (RNA copies per ml plasma) |
|
|---|---|---|---|---|---|---|
| Day 0 | Day 14 | |||||
| M701 | Male | 8 | SIVsmE660 | 1.5 | 18 809 | 40 810 |
| M511 | Male | 10 | SHIV163P3 | 5 | <50 | <50 |
| M084 | Male | 13 | SHIV 89.6P | 7 | 40 283 | 19 265 |
| M917 | Female | 9 | SIVsmE660 | 1.5 | <50 | <50 |
Viral load levels were reported as RNA copies per ml plasma overtime, before and during the heterodimeric IL-15 treatment for the individual animals.
Abbreviation: IL, interleukin.
The development of anti-IL-15 antibodies upon delivery of human IL-15 into rhesus macaques has been previously described.47 To determine whether administration of DNAs encoding human heterodimeric IL-15 induced an immune response against the human molecules, macaque plasma samples were screened for the presence of antibodies against human IL-15 or human IL-15Rα by western immunoblot analysis. The presence of antibodies was evaluated using plasma samples collected before the treatment, at day 14 after the initiation of the treatment cycle and at 7 months after termination of the study. All the samples scored negative (data not shown), suggesting that no immune responses against human IL-15 heterodimers were induced under this treatment protocol.
Collectively, these data demonstrate that it is feasible to obtain safe supraphysiological plasma levels of heterodimeric IL-15 in rhesus macaques upon IM/EP delivery of optimized-plasmid DNAs.
Elevated systemic levels of heterodimeric IL-15 result in proliferation of NK and T cells in peripheral blood in rhesus macaques
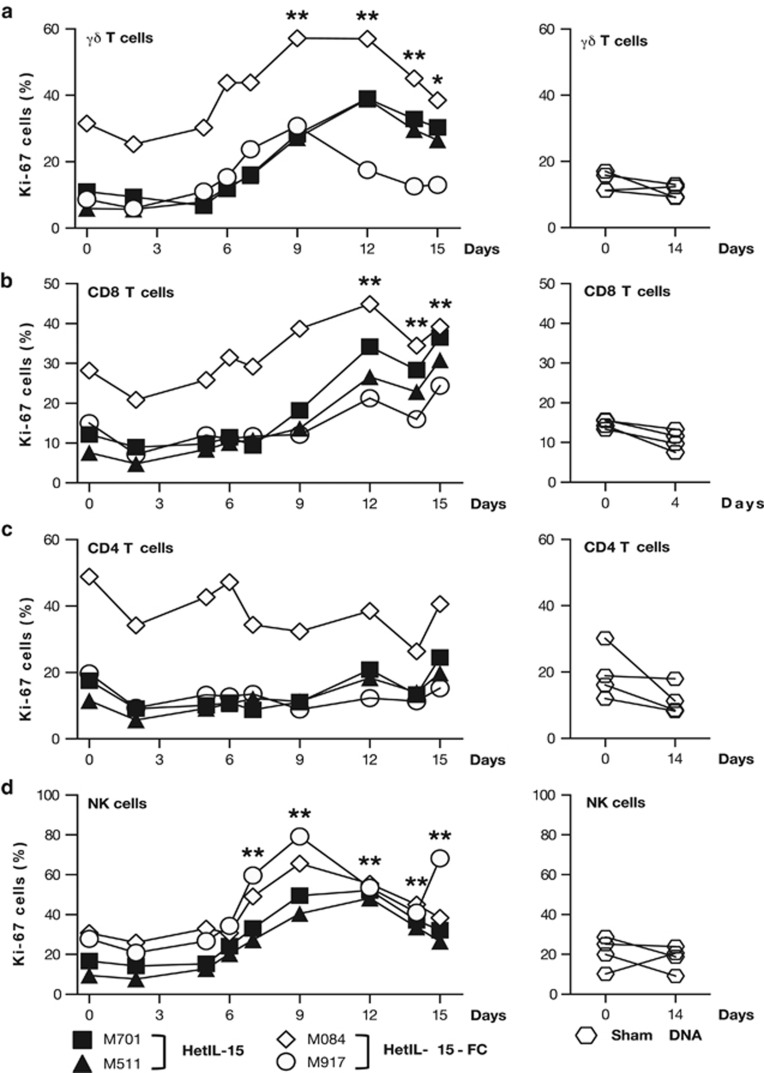
IL-15 promotes the survival and stimulates the proliferation of several leukocyte populations, including NK cells, canonical CD8+ and γδ T lymphocytes. Therefore, we evaluated the effect of heterodimeric IL-15 on the proliferation of lymphocytes in peripheral blood. Peripheral blood mononuclear cells isolated prior, during and after the IL-15 DNA administrations were stained for Ki-67, a nuclear marker expressed by proliferating cells. At baseline, ~5–10% of γδ T cells (Figure 3a), ~5–15% of CD8+ T cells (Figure 3b) and ~10–20% of CD4+ T cells (Figure 3c) were Ki-67+, with the exception of macaque M084. This animal had an abnormally elevated level of proliferating T cells (see below). Administration of DNAs expressing heterodimeric IL-15 stimulated the proliferation of γδ and CD8+ T cells in peripheral blood. The percentage of proliferating γδ T lymphocytes peaked between days 9 and 12, when 30–60% of the cells were Ki-67+. In the two animals that received hetIL-15 DNA, the proportion of proliferating γδ T cells was still elevated over the baseline after termination of the treatment (day 15; Figure 3a, left panel). Similarly, CD8+ T cells showed increased proliferation in response to IL-15 heterodimer starting at approximately day 9, and, by day 12, ~20–35% of CD8+ T cells were Ki-67+ (Figure 3b, left panel) and remained elevated up to day 15. No significant changes in the proportion of proliferating total CD4+ T cells were observed throughout the study in any of the IL-15-treated animals (Figure 3c, left panel). Macaque M084 showed significantly higher baseline levels of proliferating T cells (~30% of γδ T and CD8+ T cells, and ~50% of CD4+ T lymphocytes were Ki-67+; Figures 3a–c, diamonds), most likely as a consequence of the immune activation secondary to chronic viremia (Table 3). In this animal, IL-15 DNA administrations resulted in a modest peak increase (~2-fold) in the percentage of both γδ and CD8+ T proliferating cells, following kinetics similar to the other macaques. We also investigated the proliferation of NK cells, defined as CD3−CD16+, in response to heterodimeric IL-15. At baseline, the percentage of NK cells expressing Ki-67 was ~10–30% in the different animals. The proportion of proliferating NK cells peaked between days 9 and 12, when 50–80% of NK cells were Ki-67+ and remained elevated at day 15 (Figure 3d, left panel). In contrast, no significant changes in the percentage of proliferating lymphocytes were observed between day 0 and day 14 in the macaques treated with sham DNA by IM/EP (Figures 3a–d, right panels). These data show that the cell proliferation observed in the animals treated with heterodimeric IL-15 DNA was due to the specific effects of the IL-15 treatment.
Figure 3.
Effect of heterodimeric IL-15 on the proliferation of circulating NK and T cells. Peripheral blood mononuclear cells (PBMCs) were obtained from macaques M701 (square), M511 (triangle), M084 (diamond) and M917 (circle) prior and during the course of IL-15 DNA injections (left panels). PBMCs were also obtained from four control animals (hexagon) treated with sham DNA (right panels). PBMC samples were stained with monoclonal antobodies specific for CD3, CD4, CD8, γδ TCR and CD16 to distinguish the different lymphocyte populations. The percentage of dividing Ki-67+ cells within the CD3+ γδ TCR+ (a), CD3+CD8+ (b), CD3+CD4+ (c) and CD3−CD16+ (d) cell subsets is shown at the indicated time point during the study. *P<0.05; **P<0.01, in comparison to time point 0 (one-way analysis of variance test).
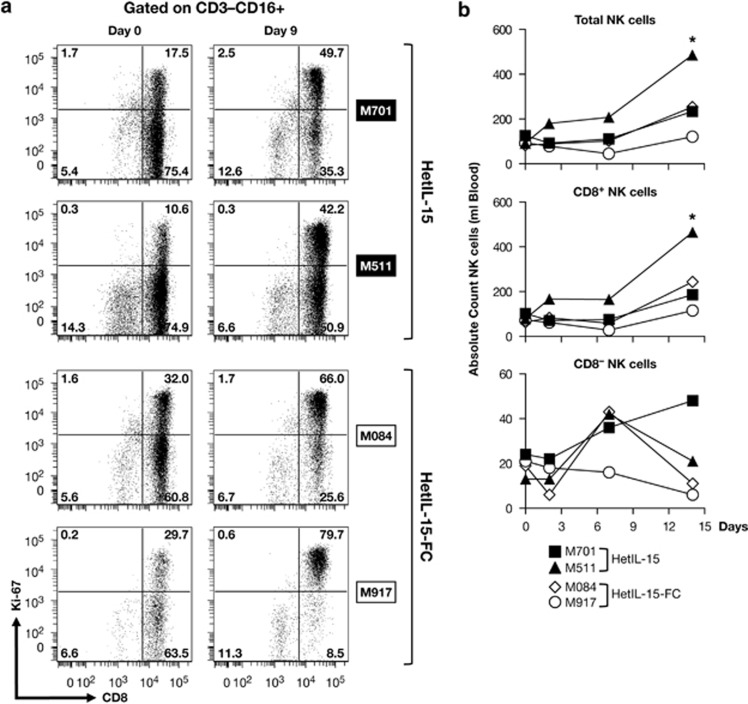
We further analyzed the phenotype of the proliferating NK cells to determine whether a specific subset preferentially responded to heterodimeric IL-15. In particular, we studied expression of CD8, as it has been reported that human NK cells expressing the CD8 antigen have more potent cytotoxic function compared with their CD8− counterpart.48 In all animals, the majority (>85%) of CD3−CD16+ NK cells expressed CD8 at baseline (Figure 4a). CD8+ NK cells preferentially expanded in response to IL-15 heterodimer, and by day 9, we found an approximately two- to fourfold increase in the proportion of proliferating CD8+ NK cells, whereas no significant increase in the frequency of Ki67+ cells was observed in the CD8− counterparts (Figure 4a). As reflection of the great proliferation of NK cells in response to systemic levels of heterodimeric IL-15 after repeated DNA injections (Figure 3d), the absolute NK cell count in blood increased by two- to sixfold (Figure 4b, top panel). Further analysis revealed that, indeed, the CD8+ NK cell subset accounted for this increase (Figure 4b, middle panel), whereas IL-15 had little effect on CD8− NK cells (bottom panel). Only a marginal increase in the absolute count of NK cells was observed in macaque M917, possibly as a result of a more rapid movement of cells to the tissues.
Figure 4.
Both forms of the heterodimeric IL-15 preferentially expand CD8+NK cells. (a) Expression of CD8 and Ki-67 within the CD3−CD16+ total NK cells subset is shown on day 0 and day 9 after the start of the treatment for the individual animals. (b) Blood samples were obtained at the indicated time points and stained with monoclonal antobodies binding to CD3, CD4, CD8 and CD16 and examined by flow cytometry. The data for CD3−CD16+ (Total NK cells, top panel), for CD3−CD16+CD8+ (CD8+NK cells, middle panel) and for CD3−CD16+CD8− (CD8−NK cells, bottom panel) are shown as absolute numbers per μl of blood on the indicated day relative to the absolute number per μl of blood of cells at the day of the start of the treatment. *P<0.05, in comparison to time point 0 (one-way analysis of variance test).
In conclusion, we noted a hierarchical proliferative response in peripheral blood of rhesus macaques treated with IL-15 DNA, with NK>γδ T>CD8+>CD4+ T cells, suggesting that the different lymphocytes have different threshold of response to systemic IL-15 levels. In agreement with the pharmacokinetics, no differences in biological effects were observed after delivery of either hetIL-15 or hetIL-15-FC DNAs.
Effects of IL-15 on different memory T-cell subsets
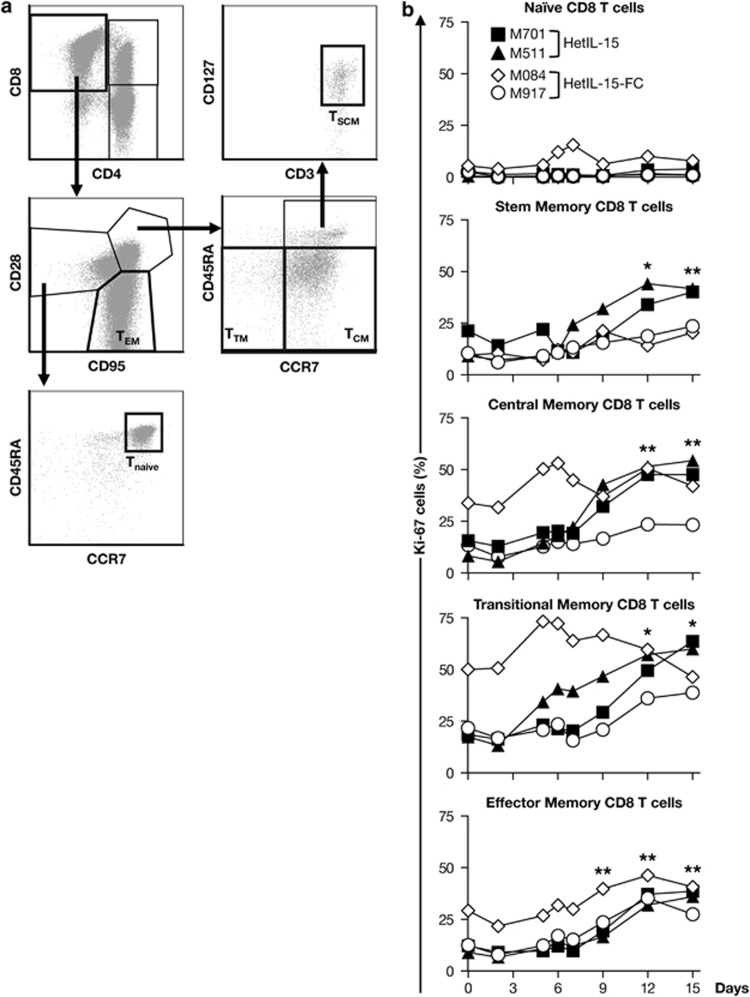
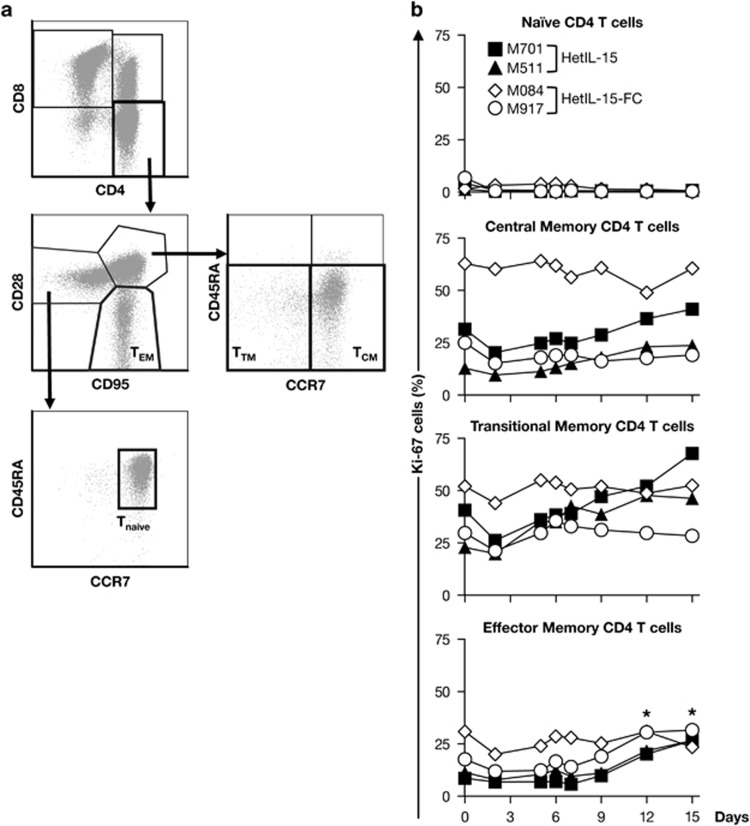
We also investigated the effects of both forms of the heterodimeric IL-15 on subsets of CD8+ and CD4+ T cells. Analysis of the surface markers CD45RA, CD28, CD95, CCR7 and CD127 allowed distinguishing among different memory T-cell subsets. For both CD8+ and CD4+ lymphocytes, naïve T cells (TN) were defined as CD95−CD28+CD45RA+CCR7+, central memory (TCM) as CD95+CD28+CD45RA−CCR7+, transitional memory (TTM) as CD95+CD28+CD45RA−CCR7− and effector memory (TEM) as CD95+CD28−, as previously reported and shown in representative flow plots (Figures 5a and 6a). In the case of CD8+ T cells, a population defined as T-stem memory cells (TSCM) has been described as CD95+CD28+CD45RA+CCR7+CD127+ (Figure 5a).49,50 This population comprising the least differentiated memory T cells is capable of self-renewal generating TCM and it is strictly dependent on IL-15.49,50 Before treatment, few CD8+ TN cells were Ki-67+ and their frequency did not change significantly as a consequence of the heterodimeric IL-15 treatment (Figure 5b). In contrast, the proportion of CD8+ TSCM, TCM, TTM and TEM fractions that expressed Ki-67 increased approximately two- to threefold during the course of the study, consistent with a proliferative response induced by the hetIL-15 treatment. The frequency of proliferating memory CD8+ T cells peaked between days 12 and 15, and all the memory compartments were affected similarly by the treatment (Figure 5b). Despite this elevated proliferation, no increase in the absolute count of CD8+ T cells in blood was observed in any of the animals (data not shown), suggesting either a rapid mobilization to the tissues or proliferation-linked apoptosis, as previously reported.19,51,52 The gating strategy used to identify the different CD4 T memory cells is depicted in Figure 6a. Heterodimeric IL-15 also induced proliferation of TEM CD4+ in all animals with the exception of M084, and of TTM CD4+ in two of the four animals, but to a lesser extent than CD8+ T cells (~1.5- to 2-fold; Figure 6b). A modest variation in the frequency of proliferating cells in the TCM CD4+ compartment was only observed in animal M511, whereas no changes were observed in the CD4+ TN subset in any of the animals (Figure 6b). Taken together, these data suggest that heterodimeric IL-15 induces the preferential proliferation and accumulation of lymphocytes characterized by the expression of CD8 antigen, either NK or T cells. In addition, heterodimeric IL-15 targets both CD8+ and CD4+ T cells, mainly those with a memory/effector phenotype.
Figure 5.
Heterodimeric IL-15 resulted in robust proliferation of memory CD8+ T cells subsets. (a) Gating strategy for the identification of naïve and memory CD8 T cells. Within the CD3+ T cells population, CD4+ and CD8+ cells were identified. The different subsets of CD8+ T cells were identified using a mixture of monoclonal antobodies that bind to CD95, CD28, CCR7, CD45RA and CD127. The T-cell subsets were defined as follows: Tnaïve, CD95−CD28+CCR7+CD45RA; TSCM, CD95+CD28+CCR7+CD45RA+CD127+; TCM, CD95+CD28+CCR7+CD45RA−; TTM, CD95+CD28+CCR7−CD45RA− and TEM, CD95+CD28−. (b) Expression of Ki-67 by the different CD8+ T cell subsets overtime for the individual animals is shown. *P<0.05; **P<0.01, in comparison to time point 0 (one-way analysis of variance test).
Figure 6.
Effects of heterodimeric IL-15 on the proliferation of memory CD4+ T-cell subsets. (a) Gating strategy for the identification of naïve and memory CD4 T cells. Within the CD3+ T-cell population, CD4+ and CD8+ cells were identified. The different subsets of CD4+ T cells were identified using a mix of monoclonal antobodies that bind to CD95, CD28, CCR7 and CD45RA. Tnaïve cells were identified as CD95-CD28+CCR7+CD45RA+, TCM as CD95+CD28+CCR7+CD45RA−, TTM as CD95+CD28+CCR7−CD45RA− and TEM as CD95+CD28−. (b) Expression of Ki-67 by the different CD4+ T-cell subsets overtime for the individual animals is shown. *P<0.05; **P<0.01, in comparison to time point 0 (one-way analysis of variance test).
Discussion
Several investigators have tested the effect of single-chain IL-15, provided as recombinant protein, in non-human primates.19,47,53,54 Among these studies, recombinant Escherichia coli-derived single-chain human IL-15 has been tested in preclinical studies in macaques upon intravenous injection,53 subcutaneous injection and continuous intravenous infusion.47 The subcutaneous and continuous intravenous protocols are currently being evaluated in phase I clinical studies in patients with refractory metastatic melanoma and metastatic renal cell carcinoma (NCT01572493 and NCT01727076). Administration of single-chain IL-15 resulted in an increase in the absolute number and proliferation of peripheral NK and memory CD8+ T cells. Increased proliferation and migration to peripheral tissues of effector memory CD4+ T cells was also reported,51 although no significant effects on CD4+Foxp3+ T-regulatory cells were observed.19,53,54 It has been shown that high-dose IL-15 is able to expand Tregs in vitro, although these cells were characterized by weak suppressive activity.55,56 Single-chain IL-15 delivery to macaques did not result in a preferential proliferation of Tregs as compared with the general CD4+ T-cell population.19 Although administration of the single-chain IL-15 was overall well tolerated, transient neutropenia and hypotension were observed,19,20 effects possibly related to the spike levels of IL-15 in serum.
Several reports suggested that gene therapy approaches could reduce or eliminate the toxicity associated with cytokine treatment. However, the major limitation of these preclinical and clinical studies was the undocumented efficiency of gene expression. This report explores the delivery, efficiency and safety of heterodimeric IL-15:sIL-15Rα gene therapy in rhesus macaques. Although single-chain IL-15 was shown to be bioactive in non-human primates,19,53 the use of heterodimeric IL-15 may provide additional advantages, as the heterodimeric IL-15:sIL-15Rα is a natural IL-15 form circulating in the blood,14 and is bioactive and more stable in vivo compared with monomeric IL-15.10,13 The physiological IL-15 plasma levels in rhesus macaques (~10–20 pg ml−1) were elevated 5- to 10-fold upon repeated delivery of DNA encoding heterodimeric IL-15:sIL-15Rα without any sign of toxicity. Elevated IL-15 levels were observed for more than 10 days, which suggests continuous production of IL-15 from the injected DNA vectors. Importantly, IL-15 delivered as DNA did not induce the high systemic cytokine spikes, likely responsible for the toxicity associated with that treatment, commonly observed after administration of recombinant proteins. Muscle is a physiologic location for IL-15 production57 and the IL-15 gene therapy did not cause any adverse effects in macaques. The achieved systemic levels of IL-15 were biologically relevant and had the expected effects on target populations. Heterodimeric IL-15 induced the proliferation of NK, γδ T cells, all memory CD8+ T cells, including stem cells memory, and effector memory CD4+ T cells in peripheral blood. Higher proliferation resulted in increased blood NK absolute cell counts. These results demonstrate that delivery of IL-15 as DNA or as recombinant protein induces similar effects in macaques. Interestingly, the systemic IL-15 peak levels achieved by DNA injections were similar to the ones obtained in macaques treated via the subcutaneous route with 1 μg kg−1 of purified human IL-15 heterodimer (unpublished data), but persisted for a longer period of time, indicative of continuous production of IL-15 from the injected DNA vectors in vivo. Therefore, heterodimeric IL-15 gene therapy using naked DNA delivered by IM/EP offers a versatile and effective way to obtain systemic-acting level of IL-15, without toxicity.
The use of SIV-infected macaques has also allowed us to evaluate the effects of IL-15 gene therapy on viral replication during chronic infection, in the absence of antiretroviral treatment. It has been reported that IL-15 administration during acute SIV infection resulted in increased viral set point and accelerated disease progression.58,59 However, other reports suggested that IL-15 treatment during chronic infection does not contribute to viral replication.42,46,51 In agreement with these findings, no differences were observed in the virological status of the animals before and after IL-15 DNA treatment, suggesting that IL-15 gene delivery is safe in the context of chronic HIV/SIV infection. In addition, heterodimeric IL-15 was able to promote the expansion of T cells, including effector memory CD4+ in infected animals.
In the present report, we have also compared the bioactivity of two different forms of heterodimeric IL-15. Heterodimeric IL-15:sIL-15RαFc has been shown to be superior to single-chain IL-15 in anti-cancer activity in several mouse studies.15,17,18,60,61 In macaques, both forms of the heterodimeric IL-15 expressed after DNA injection resulted in similar plasma levels and similar bioactivity. This is in contrast to the reported mouse data, where the IL-15:sIL-15RαFc form was characterized by increased bioactivity in comparison to the natural IL-15:sIL-15Rα as the result of its increased plasma half-life.15 The reason for this discrepancy could be either impaired entry into the blood stream of human IL-15:sIL-15RαFc from the site of production or, most likely, the high binding affinity of human IgG to the murine neonatal Fc receptor form.62,63 These results suggest important differences in the properties and biological activity of the two IL-15 heterodimers observed in mice and macaques.
In conclusion, improvements in vector design and delivery methods indicate that it is feasible to use IL-15 heterodimer-encoding DNA to produce systemic levels of bioactive cytokine, with minimal toxicity. This study provides a strong rationale for the evaluation of IL-15 heterodimer gene therapy for clinical applications, as an alternative to the recombinant protein administration.
Materials and methods
Generation of IL-15 heterodimer-encoding DNAs
Optimized dual promoter plasmids expressing the two chains of the human heterodimeric cytokine IL-15:IL-15Rα (hetIL-15) have been previously described.10 Briefly, the backbone vector used for the generation of the IL-15 heterodimer-expressing constructs, pDP, contains two expression cassettes, consisting of the human cytomegalovirus promoter linked to the bovine growth hormone polyadenylation signal; the simian cytomegalovirus promoter linked to the simian virus 40 (SV40) polyadenylation signal in a counter-clockwise orientation; and the kanamycin-resistance gene. The hetIL-15 DNA (AG153) used in this study contains RNA-optimized IL-15 under the control of simian cytomegalovirus promoter, and RNA-optimized sIL-15Rα without the transmembrane and cytoplasmic tail (amino acids 1–205) under the control of human cytomegalovirus promoter; the vector produces a soluble form of human heterodimeric IL-15. A similar dual promoter plasmid, in which the C-terminus of the sIL-15Rα region is genetically fused to the Fc region of human IgG1 immunoglobulin, was also generated (hetIL-15-FC, AG256).
In vitro transfection and IL-15 heterodimer measurement
Expression levels of human IL-15 heterodimer from hetIL-15 and hetIL-15-FC DNAs were tested by transient transfection of human HEK293 cells. Cells were plated in 60-mm tissue culture dishes at the density of 1 × 106 cells in complete Dulbecco's modified Eagle's medium with 10% fetal bovine serum and allowed to adhere overnight. The next day, the cells were transfected with 100 ng of IL-15 heterodimer-expressing DNAs, using the calcium phosphate co-precipitation technique. Cotransfection of 50 ng of the green fluorescent protein expression vector pFRED143 served as internal control. After 48 h, culture supernatant and cells, lysed in a 0.5% Triton buffer, were harvested. Human IL-15 levels were measured by colorimetric ELISA (Quantikine Human IL-15 immunoassay; R&D Systems, Minneapolis, MN, USA) or by western immunoblot (using the polyclonal goat anti-human IL-15 antibody AF315, R&D Systems). Human IL-15Rα expression was analyzed by western immunoblot using polyclonal goat anti-human IL-15Rα antibody AF247 (R&D Systems). For western immunoblot assays, aliquots (1/200) from the extracellular fractions were resolved on 12% TGX Bio-Rad gels (Bio-Rad, Hercules, CA, USA) under reducing conditions (treated with SDS and β-mercaptoethanol) or non-reducing condition (treated with SDS without β-mercaptoethanol). TGX Bio-Rad gels (4–15%) were used to analyze proteins under native conditions (no β-mercaptoethanol, no SDS). After transfer, the membranes were probed with a mixture of anti-IL-15 and anti-IL-15Rα antibodies (1:1000), followed by the anti-goat HRP secondary antibody (Calbiochem, Billerica, MA, USA). The protein bands were visualized on immunoblots by Enhanced Chemi-Luminescence (GE HealthCare, Pittsburgh, PA, USA) and imaged using autoradiography or the ChemiDoc XRS+ system (Bio-Rad). Green fluorescent protein levels in cell extracts were measured using a SpectraMax Gemini EM fluorimeter (Molecular Devices, Sunnyvale, CA, USA).
IL-15 DNA injection in rhesus macaques
Indian rhesus macaques (Macaca mulatta) were housed and handled in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care International, at the Advanced BioScience Laboratories, Inc. The animals received 8 mg of plasmid DNA expressing different forms of the heterodimeric IL-15 by IM/EP using the ELGEN electroporation device (Inovio Pharmaceuticals, Inc.). The plasmid DNA was delivered at four sites (left and right inner thighs and left and right upper arms), using 0.5 ml per site of an endotoxin-free plasmid DNA preparation (Qiagen, Vale, CA, USA) at the concentration of 4 mg ml−1. Macaques received IL-15-expressing plasmid DNA every 3 days for a total of five treatments. Animals M701 and M917 had been infected with SIV E660 for 18 months before initiation of this study. Animals M511 had been infected with SHIV 162P3 for 5 years. Animal M084 had been infected with SHIV 89.6P for 7 years. Viral loads were measured before and after the IL-15 treatment.
Plasma IL-15 measurements in rhesus macaques
Rhesus macaques were bled at different time points prior, during and after IL-15 heterodimer DNA administration (day 0, 2, 5, 6, 7, 9, 12, 14 and 15). IL-15 plasma levels were evaluated using a chemiluminescent immunoassay (Quantiglo, Q1500B; R&D Systems), according to manufacturer's instructions. This assay cross-reacts with rhesus macaque IL-15, allowing the determination of endogenous plasma IL-15 levels prior to treatment.
Analysis of lymphocyte subsets in peripheral blood mononuclear cell and BAL
Peripheral blood mononuclear cells were isolated by Ficoll density gradient centrifugation and cryopreserved in liquid nitrogen until analysis. Immunophenotypic analysis was performed using the following directly conjugated anti-human antibodies: APC-Cy7 CD3 (clone SP34-2), V500 CD4 (clone L200), PE-CD95 (clone DX2), PECy7-CD16 (clone 3G8) and PE-γδ TCR (clone B1) were obtained from BD Biosciences (San Diego, CA, USA); APC-CCR7 (R&D Systems; clone 150503); AF405-CD8 (Caltag, Buckingham, UK; clone 3B5), PerCpCy5.5-CD28 (Biolegend, San Diego, CA, USA; clone CD28.2), AF700-CD45RA (Abd Serotec, Raleigh, NC, USA; clone F8-11-13) and PECy7-CD127 (Biolegend; clone A019D5) were also used in these analysis. Cell proliferation was monitored by staining with AF700- or FITC-Ki-67 Ab in cells permeabilized with the Foxp3 Staining Buffer Set (eBioscience, San Diego, CA, USA). All the samples were acquired in a LSR II Flow Cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR, USA).
Determination of the immunogenicity of human IL-15 and IL-15Rα in macaques
Monkey plasma samples were analyzed for the development of antibodies against human IL-15 and human IL-15Rα using western Immunoblot. hetIL-15 protein (150 ng, dissolved in a buffer containing SDS and β-mercaptoethanol) was resolved on 12% TGX Bio-Rad polyacrylamide gels and transferred onto nitrocellulose membranes (Life Technologies, Grand Island, NY, USA), which were probed with plasma (1:1000 dilution) from DNA-treated animals followed by anti-monkey IgG-HRP labeled (1:10 000 dilution). The presence of antibodies reacting against human IL-15 or human IL-15Rα was evaluated using plasma samples collected before the treatment, on day 14 after the initiation of the treatment cycle and 7 months after termination of the study.
Statistical analysis
Statistical analysis was performed using Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA).
Acknowledgments
This work was funded in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health (NCI/NIH). We thank GR Pilkington for discussions and assistance and KA Goetz for technical assistance; D Weiss, J Treece, A Cristillo, M Ferrari and staff at Advanced Bioscience Laboratory for support; and T Jones for editorial assistance.
CB, AV, GNP and BKF are inventors on US Government-owned patents and patent applications related to DNA vaccines, IL-15 and gene expression optimization. NYS is a full-time employee of Inovio Pharmaceuticals and as such receives compensation in the form of salary and stock options. This does not alter our adherence to all the policies on sharing data and materials.
References
- Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci. 2012;33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka R, Burkett PR, Chien M, Chai S, Chan F, Lodolce JP, et al. Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R[alpha]-deficient mice. J Exp Med. 2003;197:977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandau MM, Schluns KS, Lefrancois L, Jameson SC. Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15R alpha by the same cells. J Immunol. 2004;173:6537–6541. doi: 10.4049/jimmunol.173.11.6537. [DOI] [PubMed] [Google Scholar]
- Bergamaschi C, Jalah R, Kulkarni V, Rosati M, Zhang GM, Alicea C, et al. Secretion and biological activity of short signal peptide IL-15 is chaperoned by IL-15 receptor alpha in vivo. J Immunol. 2009;183:3064–3072. doi: 10.4049/jimmunol.0900693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi C, Rosati M, Jalah R, Valentin A, Kulkarni V, Alicea C, et al. Intracellular interaction of interleukin-15 with its receptor alpha during production leads to mutual stabilization and increased bioactivity. J Biol Chem. 2008;283:4189–4199. doi: 10.1074/jbc.M705725200. [DOI] [PubMed] [Google Scholar]
- Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205:1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- Chertova E, Bergamaschi C, Chertov O, Sowder R, Bear J, Roser JD, et al. Characterization and favorable in vivo properties of heterodimeric soluble IL-15.IL-15Ralpha cytokine compared to IL-15 monomer. J Biol Chem. 2013;288:18093–18103. doi: 10.1074/jbc.M113.461756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi C, Bear J, Rosati M, Beach RK, Alicea C, Sowder R, et al. Circulating IL-15 exists as heterodimeric complex with soluble IL-15Ralpha in human and mouse serum. Blood. 2012;120:e1–e8. doi: 10.1182/blood-2011-10-384362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois S, Patel HJ, Zhang M, Waldmann TA, Muller JR. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol. 2008;180:2099–2106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, et al. Converting IL-15 to a superagonist by binding to soluble IL-15Ralpha. Proc Natl Acad Sci USA. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Ramlogan-Steel CA, Yu P, Black BA, Mannan P, Allison JP, et al. Vaccination with tumor cells expressing IL-15 and IL-15Ralpha inhibits murine breast and prostate cancer. Gene Therapy. 2014;21:393–401. doi: 10.1038/gt.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C, Berger M, Hackman RC, Gough M, Elliott C, Jensen MC, et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114:2417–2426. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann TA, Lugli E, Roederer M, Perera LP, Smedley JV, Macallister RP, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011;117:4787–4795. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474–484. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein M, Ettinghausen SE, Rosenberg SA. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986;137:1735–1742. [PubMed] [Google Scholar]
- Gollob JA, Mier JW, Veenstra K, McDermott DF, Clancy D, Clancy M, et al. Phase I trial of twice-weekly intravenous interleukin 12 in patients with metastatic renal cell cancer or malignant melanoma: ability to maintain IFN-gamma induction is associated with clinical response. Clin Cancer Res. 2000;6:1678–1692. [PubMed] [Google Scholar]
- Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. Clinical applications of DNA vaccines: current progress. Clin Infect Dis. 2011;53:296–302. doi: 10.1093/cid/cir334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzler MA, Weiner DB. DNA vaccines: ready for prime time. Nat Rev Genet. 2008;9:776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felber BK, Valentin A, Rosati M, Bergamaschi C, Pavlakis GN. HIV DNA Vaccine: Stepwise improvements make a difference. Vaccines. 2014;2:354–379. doi: 10.3390/vaccines2020354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalah R, Rosati M, Kulkarni V, Patel V, Bergamaschi C, Valentin A, et al. Efficient systemic expression of bioactive IL-15 in mice upon delivery of optimized DNA expression plasmids. DNA Cell Biol. 2007;26:827–840. doi: 10.1089/dna.2007.0645. [DOI] [PubMed] [Google Scholar]
- Hutnick NA, Myles DJ, Ferraro B, Lucke C, Lin F, Yan J, et al. Intradermal DNA vaccination enhanced by low-current electroporation improves antigen expression and induces robust cellular and humoral immune responses. Hum Gene Ther. 2012;23:943–950. doi: 10.1089/hum.2012.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol. 2011;23:421–429. doi: 10.1016/j.coi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flingai S, Czerwonko M, Goodman J, Kudchodkar SB, Muthumani K, Weiner DB. Synthetic DNA vaccines: improved vaccine potency by electroporation and co-delivered genetic adjuvants. Front Immunol. 2013;4:354. doi: 10.3389/fimmu.2013.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Jalah R, Kulkarni V, Valentin A, Rosati M, Alicea C, et al. DNA and virus particle vaccination protects against acquisition and confers control of viremia upon heterologous simian immunodeficiency virus challenge. Proc Natl Acad Sci USA. 2013;110:2975–2980. doi: 10.1073/pnas.1215393110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati M, Bergamaschi C, Valentin A, Kulkarni V, Jalah R, Alicea C, et al. DNA vaccination in rhesus macaques induces potent immune responses and decreases acute and chronic viremia after SIVmac251 challenge. Proc Natl Acad Sci USA. 2009;106:15831–15836. doi: 10.1073/pnas.0902628106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati M, Valentin A, Jalah R, Patel V, von Gegerfelt A, Bergamaschi C, et al. Increased immune responses in rhesus macaques by DNA vaccination combined with electroporation. Vaccine. 2008;26:5223–5229. doi: 10.1016/j.vaccine.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao LA, Wu L, Khan AS, Hokey DA, Yan J, Dai A, et al. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine. 2008;26:3112–3120. doi: 10.1016/j.vaccine.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laddy DJ, Yan J, Khan AS, Andersen H, Cohn A, Greenhouse J, et al. Electroporation of synthetic DNA antigens offers protection in nonhuman primates challenged with highly pathogenic avian influenza virus. J Virol. 2009;83:4624–4630. doi: 10.1128/JVI.02335-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalams SA, Parker S, Jin X, Elizaga M, Metch B, Wang M, et al. Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PLoS One. 2012;7:e29231. doi: 10.1371/journal.pone.0029231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalams SA, Parker SD, Elizaga M, Metch B, Edupuganti S, Hural J, et al. Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. J Infect Dis. 2013;208:818–829. doi: 10.1093/infdis/jit236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasan S, Hurley A, Schlesinger SJ, Hannaman D, Gardiner DF, Dugin DP, et al. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PLoS One. 2011;6:e19252. doi: 10.1371/journal.pone.0019252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MA, Chong SY, Megati S, Montefiori DC, Rose NF, Boyer JD, et al. Priming with plasmid DNAs expressing interleukin-12 and simian immunodeficiency virus gag enhances the immunogenicity and efficacy of an experimental AIDS vaccine based on recombinant vesicular stomatitis virus. AIDS Res Hum Retroviruses. 2005;21:629–643. doi: 10.1089/aid.2005.21.629. [DOI] [PubMed] [Google Scholar]
- Jalah R, Patel V, Kulkarni V, Rosati M, Alicea C, Ganneru B, et al. IL-12 DNA as molecular vaccine adjuvant increases the cytotoxic T cell responses and breadth of humoral immune responses in SIV DNA vaccinated macaques. Hum Vaccin Immunother. 2012;8:1620–1629. doi: 10.4161/hv.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Ayyavoo V, Bagarazzi ML, Chattergoon MA, Dang K, Wang B, et al. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J Immunol. 1997;158:816–826. [PubMed] [Google Scholar]
- Valentin A, von Gegerfelt A, Rosati M, Miteloudis G, Alicea C, Bergamaschi C, et al. Repeated DNA therapeutic vaccination of chronically SIV-infected macaques provides additional virological benefit. Vaccine. 2010;28:1962–1974. doi: 10.1016/j.vaccine.2009.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugen KE, Kutzler MA, Marrero B, Westover J, Coppola D, Weiner DB, et al. Regression of subcutaneous B16 melanoma tumors after intratumoral delivery of an IL-15-expressing plasmid followed by in vivo electroporation. Cancer Gene Ther. 2006;13:969–974. doi: 10.1038/sj.cgt.7700973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26:5896–5903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartikka J, Sukhu L, Buchner C, Hazard D, Bozoukova V, Margalith M, et al. Electroporation-facilitated delivery of plasmid DNA in skeletal muscle: plasmid dependence of muscle damage and effect of poloxamer 188. Mol Ther. 2001;4:407–415. doi: 10.1006/mthe.2001.0483. [DOI] [PubMed] [Google Scholar]
- Lugli E, Mueller YM, Lewis MG, Villinger F, Katsikis PD, Roederer M. IL-15 delays suppression and fails to promote immune reconstitution in virally suppressed chronically SIV-infected macaques. Blood. 2011;118:2520–2529. doi: 10.1182/blood-2011-05-351155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneller MC, Kopp WC, Engelke KJ, Yovandich JL, Creekmore SP, Waldmann TA, et al. IL-15 administered by continuous infusion to rhesus macaques induces massive expansion of CD8+ T effector memory population in peripheral blood. Blood. 2011;118:6845–6848. doi: 10.1182/blood-2011-09-377804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addison EG, North J, Bakhsh I, Marden C, Haq S, Al-Sarraj S, et al. Ligation of CD8alpha on human natural killer cells prevents activation-induced apoptosis and enhances cytolytic activity. Immunology. 2005;116:354–361. doi: 10.1111/j.1365-2567.2005.02235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugli E, Gattinoni L, Roberto A, Mavilio D, Price DA, Restifo NP, et al. Identification, isolation and in vitro expansion of human and nonhuman primate T stem cell memory cells. Nat Protoc. 2013;8:33–42. doi: 10.1038/nprot.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker LJ, Reed-Inderbitzin EF, Hagen SI, Edgar JB, Hansen SG, Legasse A, et al. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest. 2006;116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C, Berger M, Beard BC, Kiem HP, Gooley TA, Riddell SR. Proliferation-linked apoptosis of adoptively transferred T cells after IL-15 administration in macaques. PLoS One. 2013;8:e56268. doi: 10.1371/journal.pone.0056268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugli E, Goldman CK, Perera LP, Smedley J, Pung R, Yovandich JL, et al. Transient and persistent effects of IL-15 on lymphocyte homeostasis in nonhuman primates. Blood. 2010;116:3238–3248. doi: 10.1182/blood-2010-03-275438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller YM, Petrovas C, Bojczuk PM, Dimitriou ID, Beer B, Silvera P, et al. Interleukin-15 increases effector memory CD8+ t cells and NK Cells in simian immunodeficiency virus-infected macaques. J Virol. 2005;79:4877–4885. doi: 10.1128/JVI.79.8.4877-4885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamichi H, Sereti I, Lane HC. IL-15 acts as a potent inducer of CD4(+)CD25(hi) cells expressing FOXP3. Eur J Immunol. 2008;38:1621–1630. doi: 10.1002/eji.200737607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuest TY, Willette-Brown J, Durum SK, Hurwitz AA. The influence of IL-2 family cytokines on activation and function of naturally occurring regulatory T cells. J Leukoc Biol. 2008;84:973–980. doi: 10.1189/jlb.1107778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistilli EE, Quinn LS. From anabolic to oxidative: reconsidering the roles of IL-15 and IL-15Ralpha in skeletal muscle. Exerc Sport Sci Rev. 2013;41:100–106. doi: 10.1097/JES.0b013e318275d230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryniewicz A, Price DA, Moniuszko M, Boasso A, Edghill-Spano Y, West SM, et al. Interleukin-15 but not interleukin-7 abrogates vaccine-induced decrease in virus level in simian immunodeficiency virus mac251-infected macaques. J Immunol. 2007;178:3492–3504. doi: 10.4049/jimmunol.178.6.3492. [DOI] [PubMed] [Google Scholar]
- Mueller YM, Do DH, Altork SR, Artlett CM, Gracely EJ, Katsetos CD, et al. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. J Immunol. 2008;180:350–360. doi: 10.4049/jimmunol.180.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epardaud M, Elpek KG, Rubinstein MP, Yonekura AR, Bellemare-Pelletier A, Bronson R, et al. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008;68:2972–2983. doi: 10.1158/0008-5472.CAN-08-0045. [DOI] [PubMed] [Google Scholar]
- Xu W, Jones M, Liu B, Zhu X, Johnson CB, Edwards AC, et al. Efficacy and mechanism-of-action of a novel superagonist interleukin-15: interleukin-15 receptor alphaSu/Fc fusion complex in syngeneic murine models of multiple myeloma. Cancer Res. 2013;73:3075–3086. doi: 10.1158/0008-5472.CAN-12-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol. 2001;13:1551–1559. doi: 10.1093/intimm/13.12.1551. [DOI] [PubMed] [Google Scholar]
- Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]