Abstract
Background
Depression and stress promote obesity. This study addressed the impact of daily stressors and a history of major depressive disorder (MDD) on obesity-related metabolic responses to high-fat meals.
Methods
This double-blind, randomized crossover study included serial assessments of resting energy expenditure (REE), fat and carbohydrate oxidation, triglycerides, cortisol, insulin and glucose before and after two high-fat meals. During two separate 9.5 hour admissions, 58 healthy women (38 breast cancer survivors and 20 demographically-similar controls), mean age 53.1 years, received either a high saturated fat meal or a high oleic sunflower oil meal. Prior day stressors were assessed by the Daily Inventory of Stressful Events and MDD history by the Structured Clinical Interview for DSM-IV.
Results
Greater numbers of stressors were associated with lower post-meal REE (P=.008), lower fat oxidation (P=.04), and higher insulin (P=.01), with nonsignificant effects for cortisol (P=.25) and glucose (P=.33). Women with prior MDD had higher cortisol (P=.008), and higher fat oxidation (P=.004), without significant effects for REE (P=.26), insulin (P=.25), and glucose (P=.38). Women with a depression history who also had more prior day stressors had a higher peak triglyceride response than other participants (P=.01). The only difference between meals was higher postprandial glucose following sunflower oil compared to saturated fat (P=.03).
Conclusions
The cumulative 6-hour difference between one prior day stressor and no stressors translates into 104 kcal, a difference that could add almost 11 pounds/year. These findings illustrate how stress and depression alter metabolic responses to high-fat meals in ways that promote obesity.
Keywords: daily stressors, depression, resting energy expenditure, triglycerides, cortisol, insulin
INTRODUCTION
Depression and stress promote obesity (1–3). Depressed people have a 58% increased risk of becoming obese (1). In addition, a large prospective study showed that older depressed adults gained visceral fat over five years, while non–depressed adults lost visceral fat (4). Stressful events have also been associated with weight gain and adiposity (5, 6); longitudinal studies suggest that chronic stress and stressful life events enhance the development of the metabolic syndrome, which has central obesity as its cornerstone (2, 7–9).
Depression and stressful events can alter neurochemistry, neurobiology, and behavior, providing multiple pathways for metabolic alterations. For example, both depression and stress elevate cortisol production; higher cortisol fosters increased intake of calorie-dense “comfort” foods, and insulin secretion rises as cortisol increases (10). Persistent hypercortisolemia and higher insulin enhance visceral fat accumulation (4, 10).
Long-term weight maintenance or energy balance requires that caloric intake equals calories burned. Resting energy expenditure plays a key role in energy balance and weight control, accounting for 65% to 75% of the total daily energy expenditure (11). Lower daily energy expenditure increases risk for weight gain and obesity. In addition, metabolism of macronutrients, primarily fats and carbohydrates, also influences weight regulation (12), and lower fat oxidation rate clearly facilitates weight gain over time (13). Data from rodents have shown that psychological stressors provoke multiple metabolic changes including alterations in energy expenditure as well as fat, carbohydrate, and protein metabolism (10, 14, 15), but parallel human studies are scarce.
Stressors and depression have important biological consequences, both separately and together. Depression confers vulnerability to stressors. People with a history of depression experience more major and minor stressors than those without a similar history, and past depression can also boost emotional reactivity to daily stressors (16–18).
We assessed the impact of daily stressors and past depression on metabolic responses to two different high-fat meals. Based on the research that has linked stressors and depression to visceral fat and obesity, we hypothesized that both stressors on the prior day and a history of depression would be associated with lower post-meal energy expenditure and fat oxidation, as well as heightened postprandial triglycerides, cortisol, and insulin.
METHODS AND MATERIALS
Design and Overview
This double-blind, randomized crossover study assessed metabolic responses following high-fat meals. Women received one high saturated fat meal and one high oleic sunflower oil meal during two separate full-day visits to the Clinical Research Center (CRC), a hospital research unit, with the meal order randomized. Visits were spaced 1–4 weeks apart. The institutional review board approved this study, and each participant provided informed consent.
After fasting for 12 hours, a catheter was inserted in the arm on admission. Women had 20 minutes to eat the meal. Metabolic data and blood samples were obtained before and at intervals for 6–7 hours after the meal.
Glucose and insulin were sampled before the meal, and post-meal at 45 minutes, 1.5 hours, 2 hours, 2.5 hours, and then hourly (19). Triglycerides and salivary cortisol were assessed before the meal and then hourly afterwards (19).
Participants
The parent study was designed to assess whether a high-fat diet fuels fatigue in cancer survivors, and thus the 58 healthy women included 38 breast cancer survivors and 20 benign controls (women who had an initial abnormal mammogram). Demographic data did not differ between survivors and controls (Table 1). Survivors averaged 27.03 months (SD=17.45) since diagnosis and 19.87 months (SD=16.47) since treatment completion. Exclusions included a history of any other prior cancer, chronic obstructive pulmonary disease, symptomatic ischemic heart disease, alcohol/drug abuse, and immune-related conditions such as diabetes, autoimmune disease, and major inflammatory diseases (e.g., rheumatoid arthritis and ulcerative colitis). Medication exclusions included blood lipid medications (fibrates, statins, Xenical, and niacin), angiotensin type I receptor blockers, and regular use of medications with major immunological or endocrinological consequences, e.g., steroids.
Table 1.
Characteristics of Breast Cancer Survivors and Control Subjects
| Control subjects (N= 20) |
Breast Cancer Survivors (n=38) |
P-value | |
|---|---|---|---|
| Age, years | 54.9 (10.2) | 52.1 (7.3) | 0.22 |
| BMI, kg/m2 | 26.7 (4.1) | 28.9 (5.3) | 0.12 |
| Waist, cm | 91.2 (10.3) | 96.6 (12.8) | 0.11 |
| Trunk fat, g (DXA) | 13994.1 (5218.7) | 16983.8 (5758.6) | 0.06 |
| Lean body mass, g (DXA) | 40559.1 (3839.9) | 42541.1 (5239.7) | 0.14 |
| Activity, hours per week | 12.5 (6.9) | 11.1 (5.6) | 0.41 |
| Systolic blood pressure, mmHg | 127.3 (20.0) | 126.6 (21.5) | 0.90 |
| Diastolic blood pressure, mmHg | 73.5 (8.0) | 76.2 (9.0) | 0.27 |
| Total Cholesterol, mg/dL | 177.6 (40.3) | 181.4 (24.9) | 0.66 |
| HDL, mg/dL | 53.3 (17.6) | 52.0 (16.1) | 0.77 |
| LDL, mg/dL | 101.8 (37.6) | 102.6 (23.3) | 0.91 |
| Fasting triglyceride, mg/dL | 112.6 (78.3) | 133.1 (84.7) | 0.37 |
| Fasting glucose, mg/dL | 95.1 (8.3) | 96.8 (9.3) | 0.48 |
| Menopausal status | 0.09 | ||
| Pre-menopausal | 7 (35%) | 6 (16%) | |
| Post-menopausal | 13 (65%) | 32 (84%) | |
| Number of prior day stressors | 1.2 (1.1) | 1.1 (1.1) | 0.48 |
| History of major depression | 3 (15%) | 14 (37%) | 0.08 |
Data shown are mean (SD) or N (%).
Standardized Pre-Study Meals
Participants were instructed to avoid alcohol use the day prior to study, and any strenuous physical activity two days previously (19). Participants were also asked not to take aspirin, vitamins, antioxidants, or other dietary supplements for the 7 days prior to each admission.
On the day prior to each of the two study visits participants received three standardized meals from the CRC’s metabolic kitchen to reduce the variability associated with recent intake. Equations from the Dietary Reference Intakes were used to determine total kcal requirements for each participant based on age, height, weight, and physical activity (20). Macronutrient targets (as percent of total energy) for these research meals were 54.9 ± 2.68% carbohydrate, 27.6 ± 2.13% fat, and 17.6 ± 0.95% protein. The fat content was 9.10 ± 1.20 % saturated fats, 9.43 ± 1.55% monounsaturated fats, and 7.26 ± 1.25% polyunsaturated fats. Participants ate their last meal no later than 7:30 pm the night before admission; the dinner was light and low in fat (19). Compliance was good: women consumed 91.83 ± 8.41% of these meals.
Research Meals
Both research meals included eggs, turkey sausage, biscuits, and gravy for a total of 930 kcals, with 60 grams fat, 59 grams carbohydrate, and 36 grams protein (percent of total kcals = 60, 25, 15, respectively). However, following Poppitt et al. (21), the saturated:unsaturated fatty acid ratio varied between the meals; the high saturated fat meal contained 16.84 g palmitic and 13.5 g oleic (ratio=1.93), compared to 8.64 g palmitic and 31.21 g oleic for the high oleic sunflower oil meal (ratio=0.67). For the parent study we wanted to contrast two different kinds of high-fat meals because some human studies have suggested that high saturated fat meals may fuel fatigue-inducing inflammatory responses, although others have not found these effects (21–23).
Metabolic Data
Metabolic data were obtained using indirect calorimeters (Ultima CPX and CCM Express, MedGraphics, St. Paul, MN). Inspired and expired airflow of oxygen and carbon dioxide were measured after the participant had rested for 30 minutes in a thermoneutral room in a bed. During the 30 minute resting metabolic rate (RMR) measurement process, women reclined at a 30-degree angle and remained still but awake. RMR was determined during the steady state, defined during the measurement as the consecutive 5 minute interval in which energy expenditure was the lowest while oxygen consumption (VO2) and carbon dioxide production (VCO2) changed by <10% (24, 25). During the remainder of the admission metabolic data were obtained 20 minutes out of every hour.
Macronutrient Oxidation Rates
Fat and carbohydrate oxidation (g/min) were calculated from VO2 and VCO2 using the Weir formulas (26). Adjustments for the protein respiratory quotient were based on estimations of urinary urea nitrogen using the protein consumption from the standardized meals consumed the day prior to study (27).
Body Composition
Body composition was assessed by dual x-ray absorptiometry (DXA) (28). DXA data provided a way to assess both lean body mass (which explains 70–80% of the variance in resting metabolic rate (29) and trunk fat, which contributes to adverse metabolic meal-related responses and obesity-related disease risk (28).
Interview Data
The mood disorder modules of the Structured Clinical Interview for DSM-IV, nonpatient version (SCID), provided data on lifetime prevalence of major depressive disorder (MDD) (30). Interviews were administered by well-trained clinical psychology graduate students or staff, and regular consensus meetings with recorded interviews were used to obtain diagnoses and maintain consistent, reliable, and valid interview data. SCID data showed that 29% (n=17) had met MDD criteria. The average time since diagnosis was 8.32 years (SD=10.94).
The Daily Inventory of Stressful Events (DISE) provided data on the number of daily stressors within the last 24 hours (31). The DISE interview utilizes investigator-rated measures of stressor severity, which provides a way to reduce the bias inherent in self-ratings of stressor severity and appraisal (31); an extensive electronic “dictionary” specifies codable events. In our sample 31 women reported at least one recent stressor at one visit, 21 at both visits, and 6 women reported no stressors.
Questionnaires
Following CRC admission participants completed several questionnaires. The Center for Epidemiological Studies Depression Scale (CES-D) assessed depressive symptomatology in the last week (32). The Community Healthy Activities Model Program for Seniors questionnaire (CHAMPS) assessed the weekly frequency and duration of various physical activities (33, 34). These measures have well-established reliability and validity (32–34).
Dietary Assessment
Three 24-hour dietary recalls, administered via telephone interviews, used the validated USDA Multiple Pass Approach method (35, 36). Data were averaged across the three interviews (including one weekend day).
Statistical Methods
The primary analyses used linear mixed models, which allowed explicit modeling of the within-subject correlations both across visits and within a visit. Demographics and other pre-meal subject characteristics were compared across groups (cancer, control) using two-sample t-tests and chi-square tests for data collected at only one time point (e.g., trunk fat, menopausal status) and linear mixed models with a random subject effect for data collected before each meal (e.g., fasting triglycerides). Triglyceride, insulin, and cortisol data were right-skewed, thus all analyses for these outcomes used natural-log transformed values to better approximate normality of residuals. Initial exploratory data analysis revealed that the post-meal trajectories of post-meal energy expenditure, carbohydrate and fat oxidation, insulin, glucose, and cortisol were approximately linear, thus models for these outcomes included a linear fixed effect of time (hours since the meal). The exception was the post-meal triglyceride data, which showed an initial rise for the first 3–4 hours post-meal followed by a decline until the end of the measurement period. Thus in the triglyceride model time was modeled as a quadratic effect.
Of primary interest for all models were the effects of number of prior day stressors and history of depression on the post-meal responses, and thus we included the interaction of each of these effects with time along with the respective main effects. Interactions of prior day stressors and depression were also investigated. Non-significant interactions (p>0.05) were removed from the models. The number of prior day stressors was used as a continuous predictor in all models. All models controlled for the pre-meal outcome measurement by including it as a fixed effect. Additionally, to properly account for the design of the parent study all models included the three-way interaction of group (cancer vs. control) by meal type (high saturated fat vs. high oleic sunflower oil) by time, plus all lower-order terms. To assess whether the effect of stressors might differ between groups (cancer vs. control), we added the interaction of stressors by group to each model; it was not significant for any outcome (p>0.3 for all tests) and thus was not included in any final models. Additional covariates were controlled for in each model to guard against confounding; specific controlling variables for each model were selected a priori based on their relationships with target variables and are noted in the results section. Random effects included subject-specific meal effects that were allowed to be correlated. This accounted for the different magnitude of within-subject correlations within the meal types and across the meal types. The Kenward-Roger adjustment to the degrees of freedom was used to control type I error (37), and no adjustments were made to p-values to account for multiple testing.
Bang blinding indices were calculated using participant and experimenter guesses about the meal type, separately for the high oleic sunflower oil meal and the high saturated fat meal (38). All analyses were conducted in SAS version 9.3 (Cary, NC).
RESULTS
Primary Analyses
A larger number of stressors was associated with a steeper postprandial decline in postmeal energy expenditure (Figure 1), controlling for pre-meal resting energy expenditure (REE), age, lean body mass, trunk fat, physical activity, and past depression, as evidenced by a significant stressors by time interaction (p=0.008). For subjects with no prior day stressors, the estimated post-meal energy expenditure slope was −12.9 kcal per hour, and with each additional stressor the slope decreased by an estimated 5.0 kcal. There was no effect of depression on post-meal energy expenditure (p=0.25). Age (p=0.95), lean body mass (p=0.67), trunk fat (p=0.60) and physical activity (p=0.18) were not significant predictors of post-meal REE.
Figure 1.
Estimated postprandial decline in energy expenditure as a function of number of daily stressors on the previous day. Results are from a linear mixed model controlling for pre-meal RMR, age, lean body mass, trunk fat, physical activity, history of depression, cancer/control status, and meal type.
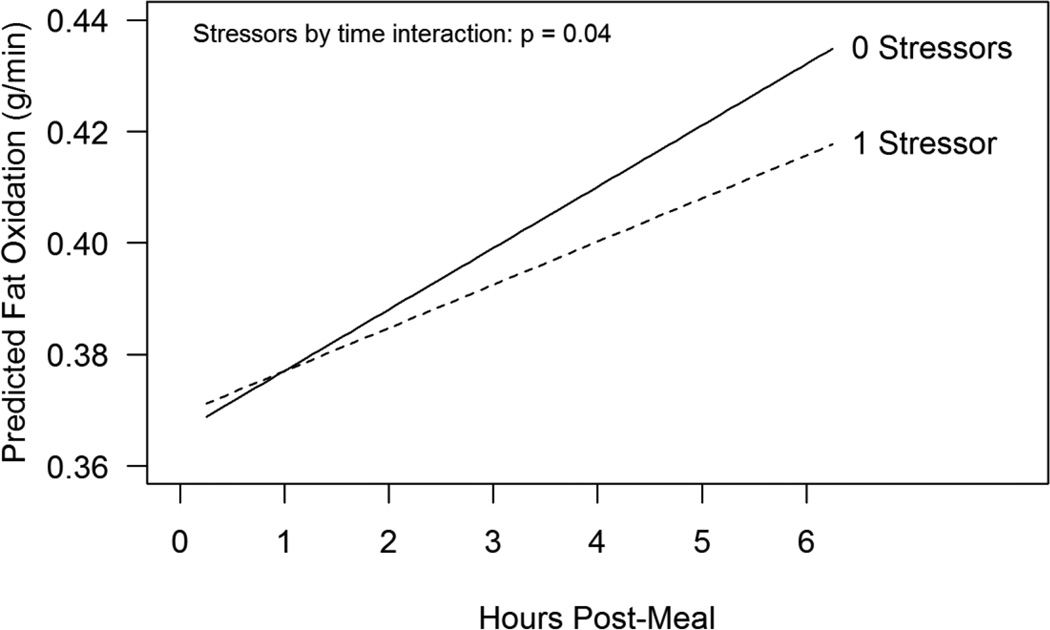
A larger number of stressors was also associated with decreased fat oxidation in the post-meal period, controlling for pre-meal fat oxidation, age, trunk fat, HOMA insulin resistance (13), physical activity, and depression (stressors by time interaction p=0.04). Fat oxidation increased at an estimated rate of 0.011 g/min per hour for subjects with no prior day stressors (Figure 2), and with each additional stressor this slope decreased an estimated 0.0033 g/min. The main effect of past depression was also significant in this model, after controlling for the effect of prior day stressors, with subjects who had a history of depression having higher fat oxidation than subjects without a history of depression (estimated mean difference = 0.069 g/min, p=0.004). Age (p=0.15), trunk fat (p=0.29), HOMA insulin resistance (p=0.18), and physical activity (p=0.67) were not significant predictors of post-meal fat oxidation. There was not a significant effect of either prior day stressors or history of depression on change in carbohydrate oxidation post-meal (p=0.94, p=0.27, respectively), controlling for pre-meal carbohydrate oxidation, age, trunk fat, HOMA insulin resistance, and physical activity. However, there was a marginal effect of depression on the average level of carbohydrate oxidation post-meal, with subjects who had a history of depression having lower carbohydrate oxidation (estimated difference = −0.041 g/min, p=0.07). The effect of HOMA insulin resistance was significant, with higher HOMA insulin resistance associated with lower post-meal carbohydrate oxidation (p=0.05). Age (p=0.31), trunk fat (p=0.30), and physical activity (p=0.46) were not significant in the model.
Figure 2.
Estimated postprandial increase in fat oxidation as a function of number of daily stressors on the previous day. Results are from a linear mixed model controlling for pre-meal fat oxidation, age, trunk fat, HOMA insulin resistance, physical activity, history of depression, cancer/control status, and meal type.
Prior day stressors were also a significant predictor of postprandial insulin, controlling for pre-meal insulin, age, trunk fat, physical activity, and depression, as evidenced by a significant stressors by time interaction (p=0.01). However, the pattern was different than for postprandial energy expenditure and fat oxidation, where the effect of stressors increased over time. For insulin, the effect of stressors was strongest at the first post-meal measurement, where each additional stressor was associated with an estimated 0.061 ln-uIU/ml increase in ln-insulin (p=0.04). By the next measurement (1.5 hours post-meal) there was not a significant effect of stressors on insulin levels. The effect of depression on insulin was not significant (p=0.25). Trunk fat was significantly related to postprandial insulin with larger trunk fat associated with higher insulin levels (p=0.05), while age (p=0.34) and physical activity (p=0.68) were not significant predictors. Neither stressors nor depression were significantly associated with postprandial glucose (p=0.33, p=0.38, respectively).
The effect of stressors and depression history on post-prandial triglycerides was complex, as there were significant three-way interactions of stressors by depression by time (p=0.01) and stressors by depression by time squared (p=0.02), controlling for pre-meal triglycerides, age, trunk fat, physical activity, and menopausal status (39). As shown in Figure 3, subjects with both a larger number of stressors and a history of depression had a steeper immediate rise in triglycerides coupled with a subsequent steeper decline post-peak compared to other subjects. The effects of age (p=0.95), trunk fat (p=0.42), physical activity (p=0.61), and menopausal status (p=0.25) were not significant.
Figure 3.
Estimated postprandial changes in triglycerides as a function of number of daily stressors on the previous day and history of depression. Results are from a mixed model controlling for pre-meal triglycerides, age, trunk fat, physical activity, menopausal status, cancer/control status, and meal type.
Finally, there was a significant effect of history of depression on changes in cortisol post-meal (depression by time interaction p=0.008), controlling for pre-meal cortisol, age, trunk fat, physical activity, and number of stressors (Figure 4). For subjects with a history of depression, the estimated decrease in ln-cortisol levels post-meal was 0.047 ln-ug/dl per hour, compared to a decrease of 0.093 ln-ug/dl for subjects without a history of depression, a twofold difference in slopes. There was not a significant effect of stressors on post-meal cortisol levels (p=0.25). The effects of age (p=0.16), trunk fat (p=0.46), and physical activity (p=0.27) were not significant.
Figure 4.
Estimated postprandial changes in cortisol as a function of history of depression. Results are from a mixed model controlling for pre-meal cortisol, age, trunk fat, physical activity, cancer/control status, and meal type.
There were no differences between cancer and control subjects in fasting levels of any outcome measure. The only group difference in postprandial measures was a difference in cortisol’s rate of decline after the meal, with cancer survivors experiencing a steeper decline. For cancer survivors, the estimated decrease in ln-cortisol levels post-meal was 0.10 ln-ug/dl per hour, compared to a decrease of 0.042 ln-ug/dl per hour for control subjects (p=0.01).
The only significant difference between the high saturated fat meal and the high oleic sunflower oil meal was in postprandial glucose. Average glucose levels were higher immediately following the high oleic sunflower oil meal (p=0.03 at 45 minutes post-meal) but this effect diminished over time (meal by time interaction p=0.02) and by two hours post-meal there was not a significant difference.
Ancillary Analyses
The average hours of sleep the night before admission was 6.2 (SD=1.3). Hours of sleep were not associated with either the number of stressors the day before (p=0.61) or a history of depression (p=0.52).
The most common medications taken regularly by participants were antidepressants (N=16), aromatase inhibitors (N=14), and selective estrogen receptor modulators (N=12). Low dose antidepressants are commonly prescribed for breast cancer survivors as a treatment for menopausal symptoms. Inclusion of these medications in analyses did not alter the findings.
The CES-D was included in all models to assess the possibility that current mood was responsible for metabolic differences. Current depressive symptoms were not a significant predictor of any outcome except post-meal triglycerides, where more depressive symptoms were associated with a steeper rise in triglycerides immediately post-meal (p=0.05). For all models reported in the primary analyses, the magnitude and significance of the effects were unchanged when the CES-D was included, except for the model for carbohydrate oxidation post-meal, where including CES-D actually increased the estimated effect of depression by 20%, causing the marginal effect (p=0.06) to become significant (p=0.03).
We evaluated each woman’s typical diet and its relationship with pre- and post-meal REE as well as recent stressors and history of depression. There were associations between reports of usual daily dietary calories from carbohydrates and fat with pre-meal REE levels, with a one gram increase in fat intake associated with a 3.4 kcal decrease in pre-meal REE (p=0.04) and a one gram increase in carbohydrate intake associated with a 1.2 kcal increase in pre-meal REE (p=0.04). There was no association between protein intake and pre-meal REE (p=0.55), and there were no significant associations between dietary variables and post-meal REE levels or changes. Diet was also not associated with either prior day stressors or history of depression.
The Bang blinding index for the high oleic sunflower oil meal was 0.18 (95% CI: −0.02, 0.37; n=56) and for the high saturated fat meal it was −0.28 (95% CI: −0.48, −0.08; n=57). For the experimenter these indices were 0.07 (95% CI: −0.02, 0.16; n=57) and 0.05 (95% CI: −0.03, 0.14; n=58) for the high oleic sunflower oil and high saturated fat meals, respectively. A blinding index of zero indicates perfect blinding, i.e., random guessing (38).
DISCUSSION
This study provides novel evidence of metabolic pathways through which prior day stressors and past depression facilitate weight gain over time. Greater numbers of prior day stressors were associated with decreased post-meal energy expenditure. The cumulative difference between one recent stressor and no stressors over 6 hours translates into 104 kcal, averaged across meal type and group and all controlling variables. This difference would add up to almost 11 pounds across a year.
Greater numbers of prior day stressors were also associated with lower fat oxidation as well as higher insulin production. People with lower fat oxidation are more likely to gain weight by storing fat than those with higher fat oxidation, and thus their risk for obesity is increased (40). Higher levels of insulin are lipogenic, further enhancing fat storage (10).
Women with a history of depression who had also experienced more stressors the prior day had a higher peak triglyceride response compared to other participants. Larger postprandial triglyceride responses are reliably associated with enhanced cardiovascular risk, with greater risks for women than men (41–47). The magnitude and duration of the postprandial triglyceride response is linked with the progression of atherosclerosis (41, 48, 49). Depression has well-established effects on cardiovascular morbidity and mortality, and these meal-related changes highlight a previously unrecognized depression-sensitive mechanistic pathway (50, 51).
Women with a depression history also had higher post-meal cortisol values compared to women who did not have a similar history. Cortisol promotes triglyceride accumulations in visceral fat through two pathways (52). Cortisol binds to glucocorticoid receptors which have a high density in visceral fat; cortisol increases lipoprotein lipase activity, stimulating triglyceride accumulation in visceral fat. Cortisol also restrains lipid mobilization in the presence of insulin, thereby fueling triglyceride accumulation and retention in visceral adipose tissue (4). Fat oxidation was significantly higher among women with an MDD history than those without, a surprising finding that was at odds with both the cortisol and triglyceride data.
Our study has several strengths. We controlled both the composition and energy content of each woman's diet on the day prior to admission as well as during admission (53). Women were studied under sedentary conditions with uniform activity restrictions. We used the protein data from the prior day to calculate fat and carbohydrate oxidation; nitrogen excretion data (27) would have provided a more precise measure of substrate utilization, one limitation of our study.
The inclusion of breast cancer survivors, even though they were healthy, could have affected the results, another limitation. However, we found no reliable differences between cancer survivors and controls. Furthermore, other researchers have not observed metabolic differences between healthy cancer survivors and controls, in accord with our data (54–58).
Our sample’s average body mass index (BMI) was 28.1, in accord with the age-adjusted mean of 28.7 for US women (59). Similarly, in line with the 35.8% obesity prevalence among adult women (59), 36.2% of our women were obese (BMIs 30–38). Thus the women in our sample are comparable to their age-mates, a significant detail since obesity has multiple effects on metabolism (7).
Importantly, the metabolic differences associated with recent stressors and past depression did not appear in fasting metabolic data, but were only observable in response to the high-fat meals. These data suggest that significant metabolic consequences of stress and depression may be missed in fasting assessments.
Both of our meals included 930 kcal and 60 g fat, values that are quite comparable to common fast food choices. For example, a Burger King Double Whopper with cheese has 990 kcal and 64 g fat, while a Big Mac cheeseburger and medium French fries contain 930 kcals and 58 g fat. Moreover, unlike our participants who were only given one meal, most people eat every 4–5 hours, in addition to having snacks that contain fat, and thus many of the adverse metabolic alterations including triglyceride elevations could persist throughout the day (19, 41).
Our two study meals were both high in fat, so we do not know if recent stressors and past depression might have different metabolic consequences following low-fat meals. However, during stressful times many people turn to calorie-dense high-fat “comfort” food (6, 60). While the influence of stress and depression on food choice is well-established, our novel data suggest that stressors and depression also affect metabolic responses to these meals.
The only meal-related difference was the transiently higher postprandial glucose observed following the high oleic sunflower oil meal compared to the saturated fat meal. Some randomized controlled feeding trials have suggested that diets rich in monounsaturated fatty acids have a more favorable impact on lipid profiles than high saturated fat diets, consistent with the broader health benefits attributed to Mediterranean-style diets (61, 62). The variability in customary background diets may make it difficult to demonstrate metabolic differences following a single high saturated fat meal compared to a high oleic sunflower oil meal, but this remains an important area for further studies.
Obesity increases the risk for many diseases including coronary artery disease, stroke, type 2 diabetes, metabolic syndrome, and cancer. Our findings illustrate novel pathways through which stress and depression contribute to obesity and obesity-related diseases.
Acknowledgments
The study was supported in part by NIH grants CA131029, CA154054, UL1TRR025755, and CA016058. The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 2.Raikkonen K, Matthews KA, Kuller LH. Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women - a comparison of World Health Organization, Adult Treatment Panel III. International Diabetes Foundation definitions. Diabetes Care. 2007;30:872–877. doi: 10.2337/dc06-1857. [DOI] [PubMed] [Google Scholar]
- 3.Williams LJ, Pasco JA, Henry MJ, Jacka FN, Dodd S, Nicholson GC, et al. Lifetime psychiatric disorders and body composition: A population-based study. J Affect Disord. 2009;118:173–179. doi: 10.1016/j.jad.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Vogelzangs N, Kritchevsky SB, Beekman ATF, Newman AB, Satterfield S, Simonsick EM, et al. Depressive symptoms and change in abdominal obesity in older persons. Arch Gen Psychiatry. 2008;65:1386–1393. doi: 10.1001/archpsyc.65.12.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block JP, He Y, Zaslavsky AM, Ding L, Ayanian JZ. Psychosocial stress and change in weight among us adults. Am J Epidemiol. 2009;170:181–192. doi: 10.1093/aje/kwp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinha R, Jastreboff AM. Stress as a common risk factor for obesity and addiction. Biol Psychiatry. 2013;73:827–835. doi: 10.1016/j.biopsych.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyrou I, Chrousos GP, Tsigos C. Stress, visceral obesity, and metabolic complications. Stress, obesity, and metabolic syndrome. 2006:77–110. doi: 10.1196/annals.1367.008. [DOI] [PubMed] [Google Scholar]
- 8.Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: Prospective study. Br Med J. 2006;332:521–524A. doi: 10.1136/bmj.38693.435301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Troxel WM, Matthews KA, Gallo LC, Kuller LH. Marital quality and occurrence of the metabolic syndrome in women. Arch Intern Med. 2005;165:1022–1027. doi: 10.1001/archinte.165.9.1022. [DOI] [PubMed] [Google Scholar]
- 10.Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21:159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lara J, Taylor MA, Macdonald IA. Clinical obesity in adults and children. Third ed. Wiley-Blackwell; 2010. Energy expenditure in humans: The influence of activity, diet and the sympathetic nervous system; pp. 151–163. [Google Scholar]
- 12.Flatt JP. Misconceptions in body weight regulation: Implications for the obesity pandemic. Crit Rev Clin Lab Sci. 2012;49:150–165. doi: 10.3109/10408363.2012.712904. [DOI] [PubMed] [Google Scholar]
- 13.Blaak EE, Hul G, Verdich C, Stich V, Martinez A, Petersen M, et al. Fat oxidation before and after a high fat load in the obese insulin-resistant state. J Clin Endocrinol Metab. 2006;91:1462–1469. doi: 10.1210/jc.2005-1598. [DOI] [PubMed] [Google Scholar]
- 14.Laugero KD. Filling in the gaps of chronic psychological stress disease models: What's metabolic profiling have to do with it? Endocrinology. 2008;149:2712–2713. doi: 10.1210/en.2008-0328. [DOI] [PubMed] [Google Scholar]
- 15.Moles A, Bartolomucci A, Garbugino L, Conti R, Caprioli A, Coccurello R, et al. Psychosocial stress affects energy balance in mice: Modulation by social status. Psychoneuroendocrinology. 2006;31:623–633. doi: 10.1016/j.psyneuen.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Hammen C. Generation of stress in the course of unipolar depression. J Abnorm Psychol. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- 17.Gunthert K, Cohen L, Butler A, Beck J. Depression and next-day spillover of negative mood and depressive cognitions following interpersonal stress. Cognit Ther Res. 2007;31:521–532. [Google Scholar]
- 18.Husky M, Mazure C, Maciejewski P, Swendsen J. Past depression and gender interact to influence emotional reactivity to daily life stress. Cognit Ther Res. 2009;33:264–271. [Google Scholar]
- 19.Lairon D, Lopez-Miranda J, Williams C. Methodology for studying postprandial lipid metabolism. Eur J Clin Nutr. 2007;61:1145–1161. doi: 10.1038/sj.ejcn.1602749. [DOI] [PubMed] [Google Scholar]
- 20.Dietary reference intakes for energy, carbohydrate, fiber fat fatty acids, cholesterol, protein and amino acids. Washington, DC: National Academy Press; 2002. [DOI] [PubMed] [Google Scholar]
- 21.Poppitt SD, Keogh GF, Lithander FE, Wang Y, Mulvey TB, Chan YK, et al. Postprandial response of adiponectin, interleukin-6, tumor necrosis factor-alpha, and C-reactive protein to a high-fat dietary load. Nutrition. 2008;24:322–329. doi: 10.1016/j.nut.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Manning PJ, Sutherland WHF, McGrath MA, de Jong SA, Walker RJ, Williams MJA. Postprandial cytokine concentrations and meal composition in obese and lean women. Obesity. 2008;16:2046–2052. doi: 10.1038/oby.2008.334. [DOI] [PubMed] [Google Scholar]
- 23.Herieka M, Erridge C. High-fat meal induced postprandial inflammation. Molecular Nutrition & Food Research. 2014;58:136–146. doi: 10.1002/mnfr.201300104. [DOI] [PubMed] [Google Scholar]
- 24.McClave S, Spain D, Skolnick J, Lowen C, Kleber M, Wickerham P, et al. Achievement of steady state optimizes results when performing indirect calorimetry. JPEN J Parenter Enteral Nutr. 2003;27:16–20. doi: 10.1177/014860710302700116. [DOI] [PubMed] [Google Scholar]
- 25.Reeves MM, Davies PSW, Bauer J, Battistutta D. Reducing the time period of steady state does not affect the accuracy of energy expenditure measurements by indirect calorimetry. J Appl Physiol. 2004;97:130–134. doi: 10.1152/japplphysiol.01212.2003. [DOI] [PubMed] [Google Scholar]
- 26.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonson DC, DeFronzo RA. Indirect calorimetry: Methodological and interpretative problems. Am J Physiol. 1990;258:E399–E412. doi: 10.1152/ajpendo.1990.258.3.E399. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy AP, Shea JL, Sun G. Comparison of the classification of obesity by BMI vs. dual-energy x-ray absorptiometry in the Newfoundland population. Obesity. 2009;17:2094–2099. doi: 10.1038/oby.2009.101. [DOI] [PubMed] [Google Scholar]
- 29.Nelson KM, Weinsier RL, Long CL, Schutz Y. Prediction of resting energy expenditure from fat-free mass and fat mass. Am J Clin Nutr. 1992;56:848–856. doi: 10.1093/ajcn/56.5.848. [DOI] [PubMed] [Google Scholar]
- 30.First M, Gibbon M, Spitzer R, Williams J. User’s guide for the Structured Clinical Interview for DSM-IV axis I disorders—research version. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 31.Almeida DM, Kessler RC. Everyday stressors and gender differences in daily distress. J Pers Soc Psychol. 1998;75:670–680. doi: 10.1037//0022-3514.75.3.670. [DOI] [PubMed] [Google Scholar]
- 32.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 33.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. Champs physical activity questionnaire for older adults: Outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Harada ND, Chiu V, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Med Sci Sports Exerc. 2001;33:962–970. doi: 10.1097/00005768-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Blanton CA, Moshfegh AJ, Baer DJ, Kretsch MJ. The USDA Automated Multiple-Pass Method accurately estimates group total energy and nutrient intake. J Nutr. 2006;136:2594–2599. doi: 10.1093/jn/136.10.2594. [DOI] [PubMed] [Google Scholar]
- 36.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88:324–332. doi: 10.1093/ajcn/88.2.324. [DOI] [PubMed] [Google Scholar]
- 37.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- 38.Bang H, Ni L, Davis CE. Assessment of blinding in clinical trials. Control Clin Trials. 2004;25:143–156. doi: 10.1016/j.cct.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 39.Kolovou GD, Bilianou HG. Influence of aging and menopause on lipids and lipoproteins in women. Angiology. 2008;59:54S–57S. doi: 10.1177/0003319708319645. [DOI] [PubMed] [Google Scholar]
- 40.Westerterp KR. Dietary fat oxidation as a function of body fat. Curr Opin Lipidol. 2009;20:45–49. doi: 10.1097/mol.0b013e3283186f6f. [DOI] [PubMed] [Google Scholar]
- 41.Teno S, Uto Y, Nagashima H, Endoh Y, Iwamoto Y, Omori Y, et al. Association of postprandial hypertriglyceridemia and carotid intima/media thickness in patients with type 2 diabetes. Diabetes Care. 2000;23:1401–1406. doi: 10.2337/diacare.23.9.1401. [DOI] [PubMed] [Google Scholar]
- 42.Pastromas S, Terzi AB, Tousoulis D, Koulouris S. Postprandial lipemia: An under-recognized atherogenic factor in patients with diabetes mellitus. Int J Cardiol. 2008;126:3–12. doi: 10.1016/j.ijcard.2007.04.172. [DOI] [PubMed] [Google Scholar]
- 43.Madec S, Corretti V, Santini E, Ferrannini E, Solini A. Effect of a fatty meal on inflammatory markers in healthy volunteers with a family history of type 2 diabetes. Br J Nutr. 2011;106:364–368. doi: 10.1017/S0007114511000286. [DOI] [PubMed] [Google Scholar]
- 44.Lindman AS, Veierod MB, Tverdal A, Pedersen JI, Selmer R. Nonfasting triglycerides and risk of cardiovascular death in men and women from the Norwegian counties study. Eur J Epidemiol. 2010;25:789–798. doi: 10.1007/s10654-010-9501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–219. [PubMed] [Google Scholar]
- 46.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 47.Stensvold I, Tverdal A, Urdal P, Graffiversen S. Nonfasting serum triglyceride concentration and mortality from coronary heart-disease and any cause in middle-aged norwegian women. Br Med J. 1993;307:1318–1322. doi: 10.1136/bmj.307.6915.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pollin TI, Damcott CM, Shen HQ, Ott SH, Shelton J, Horenstein RB, et al. A null mutation in human APOc3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boquist S, Ruotolo G, Tang R, Bjorkegren J, Bond MG, de Faire U, et al. Alimentary lipemia, postprandial triglyceride-rich lipoproteins, and common carotid intima-media thickness in healthy, middle-aged men. Circulation. 1999;100:723–728. doi: 10.1161/01.cir.100.7.723. [DOI] [PubMed] [Google Scholar]
- 50.Carney RM, Freedland KE. Depression in patients with coronary heart disease. Am J Med. 2008;121:S20–S27. doi: 10.1016/j.amjmed.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 51.Nemeroff CB, Goldschmidt-Clermont PJ. Heartache and heartbreak-the link between depression and cardiovascular disease. Nature Reviews Cardiology. 2012;9:526–539. doi: 10.1038/nrcardio.2012.91. [DOI] [PubMed] [Google Scholar]
- 52.Björntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obesity Reviews. 2001;2:73–86. doi: 10.1046/j.1467-789x.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 53.Ruddick-Collins LC, King NA, Byrne NM, Wood RE. Methodological considerations for meal-induced thermogenesis: Measurement duration and reproducibility. Br J Nutr. 2013;110:1978–1986. doi: 10.1017/S0007114513001451. [DOI] [PubMed] [Google Scholar]
- 54.Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: Prevalence, pattern and health consequences. Obesity Reviews. 2011;12:282–294. doi: 10.1111/j.1467-789X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 55.Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19:2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 56.Kutynec CL, McCargar L, Barr SI, Hislop TG. Energy balance in women with breast cancer during adjuvant treatment. J Am Diet Assoc. 1999;99:1222–1227. doi: 10.1016/s0002-8223(99)00301-6. [DOI] [PubMed] [Google Scholar]
- 57.Campbell KL, Lane K, Martin AD, Gelmon KA, McKenzie DC. Resting energy expenditure and body mass changes in women during adjuvant chemotherapy for breast cancer. Cancer Nurs. 2007;30:95–100. doi: 10.1097/01.NCC.0000265004.64440.5f. [DOI] [PubMed] [Google Scholar]
- 58.Foltz AT. Weight gain among stage II breast cancer patients: A study of five factors. Oncol Nurs Forum. 1985;12:21–26. [PubMed] [Google Scholar]
- 59.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among us adults, 1999–2010. JAMA-J Am Med Assoc. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 60.Tomiyama AJ, Dallman MF, Epel ES. Comfort food is comforting to those most stressed: Evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology. 2011;36:1513–1519. doi: 10.1016/j.psyneuen.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allman-Farinelli MA, Gomes K, Favaloro EJ, Petocz P. A diet rich in high-oleic-acid sunflower oil favorably alters low-density lipoprotein cholesterol, triglycerides, and factor vii coagulant activity. J Am Diet Assoc. 2005;105:1071–1079. doi: 10.1016/j.jada.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Bergouignan A, Momken I, Schoeller DA, Simon C, Blanc S. Metabolic fate of saturated and monounsaturated dietary fats: The mediterranean diet revisited from epidemiological evidence to cellular mechanisms. Prog Lipid Res. 2009;48:128–147. doi: 10.1016/j.plipres.2009.02.004. [DOI] [PubMed] [Google Scholar]