Summary
To defend against pulmonary infections, lung epithelial cells are equipped with complex innate immunity closely linked to inflammation. Dysregulated innate immunity / inflammation leads to self-perpetuating lung injury. The CpG motif in bacterial DNA is one of the factors involved in bacterial infection-associated inflammation. Bacterial DNA and synthetic CpG oligonucleotide (ODN) induced CCN1 secretion from lung epithelial cells, functioning as a potential “braking” signal to prevent uncontrolled inflammatory responses. CpG ODN-induced ER stress resulted in Src-Y527 phosphorylation (pY527) and Src/CCN1 vWF domain dissociation. Src-Y527 activated caveolin-1 (cav-1) phosphorylation at Y14 and then modulated CCN1 secretion via pCav-1 interaction with CCN1 IGFbp domain. Functionally, secreted CCN1 promoted anti-inflammatory cytokine IL-10 release from epithelial cells via integrin αVβ6 PKC, and this subsequently suppressed TNF-α, MIP-2 secretion and neutrophil infiltration in the lungs. Collectively, bacterial DNA/CpG ODN-stimulated CCN1 secretion via BiP/GRP78-Src(Y527)-JNK-Cav-1(Y14) pathway and CpG-induced CCN1 conferred anti-inflammatory roles. Our studies suggested a novel paradigm by which the lung epithelium maintains innate immune homeostasis after bacterial infection.
Keywords: CCN1, CpG motif, Bacterial DNA, inflammation
Introduction
Lung epithelial cells are essential component of the airway physiological barrier and the first-line innate immunity to microbial pathogens.1,2 One of the most important innate immune function of lung epithelial cells is to recruit immune cells to the lung micro-environment to maintain homeostasis.1,2 Lung epithelial cells directly recognize the pathogen-associated molecular patterns (PAMPs) via Toll like receptors (TLRs) to initiate and amplify the innate immune responses needed for pathogen clearance during infection.3,4 Unmethylated CpG motifs are mostly present in bacterial DNA but not in human genomic DNAs.5-8 CpG motifs in oligodeoxynucleotides (ODN) activate host innate and acquired immune responses via TLR9 receptor-mediated mitogen activated protein kinases (MAPKs) and NFκB pathways, not only in immune cells, but also in epithelial cells.5-7 The innate immunity is closely linked to inflammatory responses that include robust release of inflammatory cytokines/chemokines, which play a crucial role in bactericidal effects.9,10 However, dysregulated innate immunity and inflammatory responses can lead to damage of lung parenchyma, resulting in acute lung injury (ALI) and contributing to significant mortality and morbidity.11 Therefore, a self-limiting or “braking” system in host epithelium is needed to prevent this ‘runaway’ inflammation and maintain lung microenvironment homeostasis after microbial infections.
The IL-10 family cytokines derived from immune cells and lung epithelial cells are essential for maintaining the integrity and homeostasis of lung epithelium via limiting the lung injury caused by bacterial infection-associated inflammation.12,13 For example, the neutrophil counts in bronchoalveolar lavage fluid (BALF) and blood are much higher in IL-10 KO mice than wild-type mice. Furthermore, neutrophil infiltration and excessive inflammation are found in IL-10 KO mice, suggesting that the high mortality in IL-10 KO mice is associated with exaggerated inflammation.14 However, in a model of pre-existing sepsis followed by Pseudomonas aeruginosa pneumonia (“two-hit” model), inhibition of IL-10 improved survival and clearance of bacteria15, suggesting a central role of IL-10 in the fine balance of bactericidal /innate immunity and tissue-damaging inflammation.16 Therefore, it is important to understand the regulatory machinery on lung epithelial cell-mediated homeostasis and innate immunity in response to bacterial infection. In our current report, we investigated a novel paradigm on how lung epithelial cells self-limit the potential ‘runaway’ inflammation after bacterial infection via the regulation of pro-inflammatory and immunosuppressive cytokines.
CCN1 (Cyr61) is an immediate early response gene product ubiquitously expressed in lung cells, particularly in lung epithelial cells.17-20 CCN1 is secreted into the extracellular matrix (ECM) after stimulation by various stimuli and exhibits diverse cellular functions in a paracrine/autocrine manner.18 CCN1 plays diverse roles such as cell repair, tissue remodeling, cell migration, differentiation, proliferation, apoptosis and senescence.18 Studies in animal models have confirmed that disruption of CCN1 is embryonic lethal21 and deregulation of CCN1 is involved in various pathologies related to inflammation and tissue repair.18 However, despite ubiquitous expression in lung parenchyma, its secretion and function in lung diseases remain unexplored. Our current studies investigated the secretion and function of CCN1 in response to bacterial infection. We further delineated the pathways involved in CCN1 secretion and the underlying mechanisms by which CCN1 confers its cellular and protective functions in lung epithelial cells during infection.
Results
Bacterial DNA and its synthetic motif CpG ODN induced CCN1 secretion from lung epithelial cells
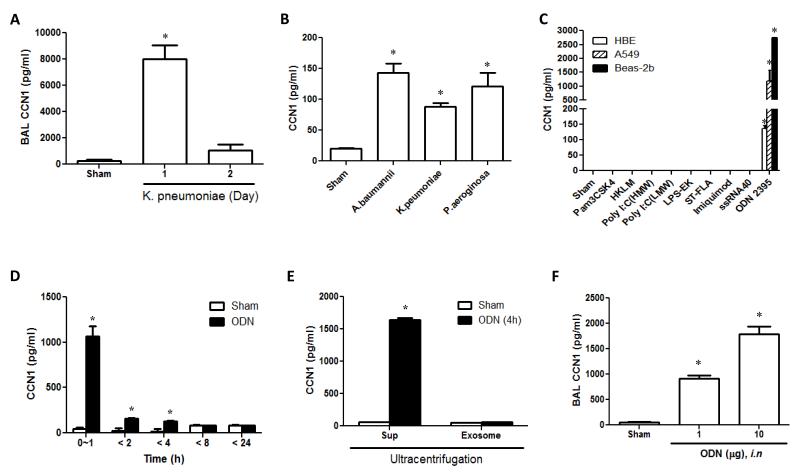
In vivo studies initially demonstrated that intranasal K. pneumoniae (103 CFU/mouse) infection rapidly increased CCN1 release in BALF and peaked around 24h after stimulation (Fig. 1A). We also stimulated the lung epithelial cells using bacterial genomic DNA obtained from K.pneumoniae, A.bauminnii and P.aerosinosa (Fig. 1B). Bacterial DNAs rapidly augmented the CCN1 secretion from cultured Beas2B epithelial cells 4h after stimulation (Fig. 1B). Given the critical roles of TLRs in mediating lung bacterial infection and inflammation, we next assessed the effects of TLR agonists on CCN1 secretion in epithelial cells. Class C TLR9 agonist, CpG ODN 2395, induced a robust CCN1 secretion in three different epithelial cells, in a dose- and time-dependent manner (Fig. 1C, S1A-B). Unmethylated CpG dinucleotides are more frequent in the genomes of bacteria than of humans.24 CpG ODNs are synthetic unmethylated bacterial DNA motif containing CpG dinucleotides which activate TLR9-mediated pathways.22,23 We found that all three major classes of stimulatory CpG ODNs (ODN2336, 2006 and 2395) increased the CCN1 secretion (Fig. S1C) and class C expressed the strongest effects on induction of CCN1 secretion (Fig. S1C). Interestingly, although CpG ODNs are well known TLR9 agonists, TLR9 silencing by siRNA and inhibition of NF-κB pathway by Bay11-7082 had no significant effects on CCN1 secretion (Fig. S1D-E), suggesting that the CpG ODN-induced CCN1 secretion was TLR9-independent. To determine the temporal dynamics of CCN1 secretion from lung epithelial cells after bacterial DNA/CpG ODNs, we evaluated the CCN1 protein level at various time points by ELISA. Majority of CCN1 were secreted within 1h after ODN stimulation (Fig. 1D). Furthermore, we collected the supernatant of Beas2B cells after stimulation with ODN. Exosome and soluble fraction were isolated using ultracentrifugation. We found that the majority of matrix CCN1 was secreted via soluble forms, rather than in exosome-encapsulated forms (Fig. 1E). To test CpG ODN-induced CCN1 secretion in vivo, we delivered ODN 2395 intranasally to mice. Consistently, ODN 2395 enhanced CCN1 level in mouse BAL fluid (BALF) in a dose-dependent manner (Fig. 1F). All the above observations supported that bacterial DNA/CpG ODN-stimulated CCN1 secretion from lung epithelial cells in soluble forms as an immediate early response to microbial infection/stimulation. These results prompted us to explore the functional roles of secreted CCN1 in lungs after bacterial infection.
Fig. 1. Bacterial DNA/CpG ODN induced CCN1 secretion in lung epithelial cells.
(A) Secreted CCN1 in BALF was measured using ELISA after administration of K.pneumoniae (103CFU/mouse) intra-nasally into C57/BL6 mice.
(B) Beas2B cells were treated with bacterial DNAs (1μg/ml). After 4h, CCN1 was measured using ELISA.
(C) HBE, A549 and Beas2B cells were treated with TLR agonists. After 24h, the secreted CCN1 was measured using ELISA. Pam3CSK4 (1 Pg/ml), HKLM (108 cells/ml), LPS-EK (1 μg/ml), ST-FLA (1 μg/ml), FSL-1 (1 μg/ml) and ODN2395 (1μM)
(D) Beas2B cells were treated with ODN (1 μM). After the designated time, the supernatant was collected and the CCN1 level was measured using ELISA.
(E) Beas2B cells were treated with ODN (1 μM). After 4h, the supernatant was collected and ultracentrifuged to separate the exosomes and soluble factors. CCN1 was determined in both portions.
(F) ODN was administered into C57/BL6 mice intra-nasally. BALF was collected after 24h. CCN1 was detected using ELISA.
In all Fig.s, *p < .05, compared to Sham; All Fig.s represented three independent experiments with identical results.
Bacterial DNA/CpG ODN–induced CCN1 secretion conferred anti-inflammatory effects both in vitro and in vivo
To explore the functions of CCN1 in bacterial lung infection-associated inflammation in vivo, we first used LPS-induced inflammation model in mice. We administrated intranasally CCN1 or ODN with and without LPS. The CCN1 or ODN was delivered at the same time along with LPS, and BAL fluid was collected after 24h. Differential cell counts including neutrophils and macrophages were conducted after Diff-Quick staining. We showed that co-treatment with either ODN or CCN1 with LPS attenuated the LPS-induced neutrophil infiltration, assessed by neutrophil count in BALF (Fig. 2A) and lung tissue (Fig. 2B). Macrophage inflammatory protein 2 (MIP-2), a chemokine which attracts neutrophils and normally has its secretion enhanced by bacterial infection-associated inflammation, was also enhanced by LPS. However, this effect was abolished after addition of ODN or CCN1 (Fig. 2C). TNF-α is a well-known inflammatory mediator of sepsis-induced lung injury.24,25 IL-10, on the contrary, represses pro inflammatory responses in lungs.26 Both ODN and CCN1 suppressed LPS-induced TNF-α, but up regulated the IL-10 level in BALF in both the absence or presence of LPS (Fig. 2D). Furthermore, co-treatment of bioactive CCN1 recombinant protein reduced the LPS-induced TNF-α and IL 6 secretion from alveolar macrophages in a dose-dependent manner (Fig. S2A).
Fig. 2. CpG ODN induced CCN1 limits neutrophil infiltration in vivo.
(A) CCN1 (5 μg), ODN (10 μg) and LPS (10 μg) was administered into C57/BL6 mice intra-nasally. After 24h, BALF was collected. Total cell counts and differentials were analyzed using Diff-Quick staining. *p < .05, compared to LPS
(B) Pre-treatment of ODN/CCN1 at the same dose as the above resulted in less neutrophil infiltration in mouse lung tissue after LPS inhalation. Markers used for neutrophils: Ly6G+F4/80-; for alveolar macrophages: Ly6G F4/80+ and for interstitial macrophages: Ly6G+F4/80+.
(C) C57/BL6 mice were exposed to sham, CCN1, ODN, LPS, LPS+CCN1 or LPS+ODN at the same dose as in (A). After 24h, MIP-2 level in BALF was measured using ELISA. *p < .05, compared to LPS treatment
(D) TNF-α and IL-10 level in BALF were measured using ELISA. *p < .05, compared to Sham, **p < .05 compared to LPS
(E) Anti-CCN1 (5 μg), ODN (10 μg) and LPS (10 μg) were administered into C57/BL6 mice intra nasally. After 24h, BALF was collected. Total cell counts and cell differentials were performed using Diff-Quick staining. *p < .05, compared to LPS
(F) C57/BL6 mice were exposed to sham, LPS, ODN+LPS or anti-CCN1+ODN+LPS at the same dose as in (E). After 24 h, neutrophil infiltration in mouse lung tissue was analyzed using flow cytometer. Markers used for neutrophils: Ly6G+F4/80−; For alveolar macrophages: Ly6G F4/80+; For interstitial macrophages: Ly6G+F4/80+.
(G) BAL MIP-2 level in same condition with (E). After 24h, MIP-2 level in BALF was measured using ELISA. *p < .05, compared to LPS+ODN
(H) TNF-α and IL-10 level in BALF were measured by ELISA. *p < .05, compared to LPS+ODN
(I) H & E stained lung tissue in the same condition with (E) (Left panel). Lung inflammation was scored in 10 different spots per slide and 5 slides per group (Right panel). *p < .05, compared to Sham, **p < .05, compared to LPS
All Fig.s represented three independent experiments with identical results.
To test whether the above effects of ODN were triggered by the induction of CCN1 secretion, we administered anti-CCN1 antibodies in ODN/LPS treated mice. ODN alone blocked the LPS induced neutrophil accumulation in BAL. Neutralization of CCN1 abolished the effects of ODN, resulted in significantly higher neutrophil counts in both BAL and lung tissues (Fig. 2E-F). Consistent with observations on neutrophil cell numbers and differentiations detected in BAL, neutralization of CCN1 notably reversed the inhibitory effects of ODN on LPS-induced TNF-α and MIP-2 level in BALF (Fig. 2G-H). We next confirmed this observation in vitro using alveolar macrophages. Neutralization of CCN1 using anti-CCN1 antibodies consistently reversed the effects of ODN on LPS activated alveolar macrophages, as determined by both IL-6 and TNF-α level (Fig. S2B). Furthermore, lung histological examination suggested that LPS-induced inflammation was reduced after ODN treatment. Anti-CCN1 antibodies reversed the anti-inflammatory effects of ODN (Fig. 2I).
CCN1 promoted IL-10 secretion via integrin αVβ6-mediated pathways
To determine which cells secreted IL-10 after CCN1 treatment, we isolated primary mouse alveolar macrophages and epithelial cells. CCN1 and ODN both induced IL-10 production, either alone or synergistically with LPS in primary type II lung epithelial cells, but not in alveolar macrophages (Fig. 3A). In human A549 lung epithelial cells, CCN1-induced IL-10 secretion in a dose-dependent manner (Fig. S3A). Previous reports have suggested that integrin αVβ6 affects IL-10 secretion in other disease models.27 Therefore, we assessed the expression of integrin αVβ6 in lung epithelial cells after CCN1 stimulation. We found that CCN1-induced the expression of both integrin αV and β6 (Fig. S3B). Blocking integrin αVβ6 using neutralizing antibodies prevented CCN1-induced IL-10 release from primary mouse lung type II epithelial cells (Fig. 3B). Furthermore, we confirmed that CCN1 induced IL-10 expression was down-regulated after integrin αVβ6 neutralization in lung tissue by immunofluorescence (Fig. 3C). To confirm the importance of integrin αVβ6 in mediating ODN/CCN1-induced IL-10 secretion, we administrated anti integrin αVβ6 antibodies intranasally with either ODN or CCN1 into mice. Neutralization of integrin αVβ6 reversed the inhibitory effects of both ODN and CCN1 on LPS-induced neutrophil infiltration and TNF-α level in BALF (Fig. 3D E). We next evaluated the down stream signaling pathways of integrin αVβ6 which potentially mediated the effects of CCN1 on IL-10 secretion. Inhibitors were used for various signaling pathways; Bay11-7082 for NK-κB, U0126 for MEK, JNK II inhibitor for JNK, Y27632 for p160 ROCK, watmannin for PI3K, SB 203580 for p38 MAPK and GO6850 for PKC. Cells were pre-treated with each inhibitor (10 μM) 1h prior to the treatment of CCN1 recombinant protein (1 μg/ml). We found that only GO6850 (PKC inhibitor) blocked CCN1-induced IL-10 secretion (Fig. 3F). Taken together, CpG ODN-induced CCN1 augmented IL-10 secretion via integrin αVβ6 – PKC pathway in lung epithelial cells
Fig. 3. CCN1 promoted IL-10 secretion via integrin αVβ6-PKC pathways in lung epithelial cells.
(A) Primary alveolar macrophage and type II lung epithelial cells were treated with LPS (100 ng/ml), CCN1 (1 μg/ml) and ODN (1 μM). IL-10 production after treatment was determined using ELISA *p < .05, compared to Sham, **p < .05, compared to LPS
(B) Inhibiting integrin αVβ6 using neutralizing antibodies (5 μg/ml) prevented IL-10 production in primary type II lung epithelial cells. *p < .05, compared to ODN and CCN1
(C) Neutralization of integrin αVβ6 reduced the CCN1 induced IL-10 expression in lung tissue. Recombinant CCN1 (5 μg) with or without anti-αVβ6 antibody (10 μg) was administered into intra nasally and samples were collected after 24h.
(D) Anti-αVβ6 (10 μg), ODN (10 μg), CCN1 (5 μg) and LPS (10 μg) was administered into C57/BL6 mice intra nasally. After 24h, BALF was collected. The cell counts and differentials were performed using Diff-Quick staining. *p < .05, compared to ODN and CCN1
(E) Levels of TNF-α and IL-10 in BALF were measured using ELISA. *p < .05, compared to ODN and CCN1
(F) Primary type II lung epithelial cells were pretreated with each integrin signaling inhibitor and recombinant CCN1 (1 Pg/ml) was added. After 4h, IL-10 level was measured using ELISA. *p < .05, compared to Sham
All Fig.s represented three independent experiments with identical results.
Cav-1 phosphorylation was crucial in regulating CCN-1 secretion
Our previous report suggests that Cav-1 is involved in the regulation of CCN1 secretion after oxidative stress.28 In this current study, we found that ODN promoted Cav-1 phosphorylation at Y14 in a time-dependent manner (Fig. 4A). Next, we isolated primary type II lung epithelial cells from WT and Cav-1−/− mice and stimulated these cells with ODN. CCN1 secretion and expression in Cav-1−/− cells were remarkably reduced compared to similarly treated WT cells (Fig. 4B). Pretreatment of daidzein, a Cav-1 inhibitor29, suppressed the ODN-stimulated CCN1 secretion in a dose-dependent manner as well (Fig. S4A). Consistently, over-expression of Cav-1 in Beas2B cells augmented CCN1 secretion (Fig. S4B). Using co-immunoprecipitation (Co-IP) assays, we demonstrated that CCN1 interacted with pCav-1 at a peak time between 15 min to 45 mins. This interaction dissociated after 60 min (Fig. 4C). The co-localization between CCN1 and pCav-1 was detected after ODN treatment (Fig. 3D). CCN1 protein consists of five specific domains (SP, IGFbp, vWF, TSP and CT) and each domain carries different protein binding motifs.18 To determine the specific binding domain for pCav-1/CCN1 interactions, we generated specific domain-depleted CCN1 constructs and transfected them into Beas2B cells. For each construct, one specific domain was deleted. The interactions between p-Cav-1 and CCN1 were diminished in the absence of domain 2 (IGFbp domain), suggesting that IGFbp is a crucial binding site of pCav-1 (Fig. 4E). To confirm the effects of Cav-1 on CCN1 secretion in vivo, we administrated LPS with/without ODN intranasally into WT and Cav-1−/− mice. Deletion of Cav-1 reversed the ODN-suppressed neutrophil infiltration (Fig. 4F) and reversed the effects of ODN of both TNF-α and IL-10 secretion (Fig. 4G). These results indicated that pCav-1 regulated CCN1 secretion and that pCav-1/IGFbp domain interaction was crucial in mediating CCN1 secretion.
Fig. 4. CCN1 secretion was regulated by Cav-1.
(A) Beas2B cells were treated with ODN (1 μM). After the designated time, CCN1 (approximately 42kDa), Cav-1, pCav-1 and β-actin was detected using Western Blot analysis.
(B) Primary type II lung epithelial cells were isolated from WT and Cav-1−/−mice and treated with ODN for 4h. CCN1 was detected using Western Blot analysis (upper panel) and ELISA (lower panel)
(C) Beas2B cells were stimulated with ODN (1 μM) in a time-dependent manner. Whole cell lysate was incubated with anti-CCN1, anti-Cav-1 and anti-pCav-1 antibodies, respectively, followed by incubating with IgG beads for 1h. Samples were then washed 3 times with RIPA buffer. Each precipitated protein was detected using Western Blot analysis.
(D) Co-localization CCN1 and pCav-1 after ODN (1 μM) stimulation. CCN1 (FITC) and pCav-1 (Cy3) were detected using confocal microscopy.
(E) Specific domain-depleted CCN1 constructs were transfected into Beas2B cells. After 2 days, cell lysate was subjected to Western Blot analysis using anti-V5, Cav-1 and pCav-1 antibodies.
(F) LPS±ODN was administered into the WT and Cav-1−/− mice intra-nasally. Differential cell count was performed using the Diff-Quick staining. *p < .05, compared to LPS
(G) TNF-α and IL-10 level in BALF were measured using ELISA. *p < .05, compared to LPS+ODN in WT
All Fig.s represented three independent experiments with identical results.
Cav-1 phosphorylation and CCN1 secretion were regulated by Src activation at Y527
We have shown that pCav-1 was crucial to CCN1 secretion. Previous report shows that the phosphorylation of Cav-1 is triggered by Src tyrosine kinase.30 Therefore, we assessed the expression of Src, Src-Y416 and Src-Y527 in Beas2B cells after ODN stimulation. Expression of Src-Y416 and Src-Y527 were increased within 5 min after ODN treatment (Fig. 5A). Deletion of Src reduced pCav-1 (Fig. 5B) and CCN1 secretion (Fig. 5C), suggesting that Src is important in the Cav-1-mediated CCN1 secretion. Next, to investigate whether the phosphorylation of Src is crucial in CCN1 secretion, we pre-treated Beas2B cells with Src phosphorylation inhibitor (PP2). PP2 suppressed Src phosphorylation at tyrosine 416 (Src-Y416) and 527 (Src-Y527), as well as Cav-1 phosphorylation (pCav-1) (Fig. 4D) and CCN1 secretion (Fig. 5E). To determine the relevance of Src-Y527 and Y416 with Cav-1 phosphorylation and CCN1 secretion, we evaluated the expression of Src-Y416 and Y527 after silencing both CCN1 and Cav-1 using siRNAs. Deletion of either CCN1 or Cav-1 diminished the expression of Src-Y527 but not Y416, suggesting that Y527 is important in the regulation of CCN1 and Cav-1 (Fig. 4F). The interactions between pCav-1 and Src increased in a time-dependent manner (Fig. 5G), while CCN1/Src interactions peaked at 15 min after ODN stimulation (Fig. 5G). Next, the domain-depleted CCN1 constructs were transfected into Beas2B cells. Deletion of domain 3 (vWF domain) resulted in significant less interaction between CCN1 and Src or Src-Y527 (Fig. 5H). To confirm our observations in vivo, we administered LPS with or without ODN to wild type mice intranasally in the presence or absence of intraperitoneal PP2 treatment. ODN inhibited LPS-induced neutrophil infiltration in BALF; however, PP2 treatment reversed the effects of ODN (Fig. 5I). PP2 also reversed the regulatory effects of ODN on TNF-α and IL-10, detected in BALF (Fig. 5J). These results further supported the fact that Src phosphorylation, particularly Src-pY527, plays a crucial role in Cav-1 phosphorylation and CCN1 secretion after ODN stimulation in lung epithelial cells.
Fig. 5. Cav-1 phosphorylation and CCN1 secretion were regulated by Src phosphorylation, particularly at Y527.
(A) Beas2B cells were treated with ODN (1 μM). Src, Src-Y416 and Src-Y527 were detected.
(B) Beas2B cells were transfected with Src siRNA. After 2 days, cells were treated with ODN (1 μM) for 45 and 60 min. β-actin, Cav-1, pCav-1 and Src were detected using Western Blot analysis.
(C) After Src silencing using siRNAs, Beas2B cells were treated with ODN (1 μM) for 4h. CCN1 was measured using ELISA.
(D) Beas2B cells were pre-treated with PP2, inhibitor of Src phosphorylation. After 45 or 60 min, β-actin, Cav-1, pCav-1, Src, Src-Y416 and Src-Y527 were detected.
(E) Beas2B cells were pre-treated with PP2, for 1h, and ODN for 4h. CCN1 was measured using ELISA
(F) Co-Immunoprecipitation (Co-IP) assays between Src or CCN1 and pCav-1 in Beas2B cells were performed after ODN stimulation in a time-dependent manner.
(G) Specific domain-depleted CCN1 constructs (with V5 tag) were transfected into Beas2B cells. After 2 days, V5 and Src were detected using Western Blot analysis.
(H) Beas2B cells were transfected with CCN1 or Cav-1 siRNAs. After 2 days, ODN (1 μM) was added. 45 and 60 min after adding ODN, β-actin, Cav-1, CCN1, Src, Src-Y416, Src-Y527 were detected.
(I) LPS with or without ODN (1 μM) were administered into C57/BL6 mice intra-nasally. PP2 (5 mg/kg) was administrated intra peritoneally. After 24h, BALF was collected. Cell counts were assessed. *p < .05, compared to LPS+ODN_Sham
(J) TNF-α and IL-10 level in BALF were measured using ELISA. *p < .05, compared to LPS+ODN_Sham
All Fig.s represented three independent experiments with identical results.
ER stress regulated CCN1 secretion induced by CpG ODN
To further delineate the mechanisms by which CpG ODN regulated CCN1 secretion, we first determined the localization of CpG ODN in major cell organelles. We found that CpG ODN-FITC co-localized with the ER associated protein BiP/GRP78 as observed using confocal microscopy (Fig. 6A). We next evaluated the effects of ODN on the expression of ER stress-associated proteins, including BiP/GRP78 and CCAAT-enhancer-binding protein homologous protein (CHOP). Expression of BiP/GRP78 or CHOP was increased at 1 min or 3 min, respectively, after ODN stimulation (Fig. 6B). To investigate the association of ER stress signaling with the CCN1 secretion, we treated the Beas2B cells with chemical inhibitors of various ER stress pathways. JNK (JNK inhibitor), MAPK (SB203580) and NF-κB (Bay11-7082) inhibitors were used for Ire-1-associated pathways; perk inhibitor was used for perk pathway and γ-secretase inhibitor for ATF6 cleavage pathway. Blocking JNK pathway significantly reduced the CCN1 secretion (Fig. 6C). Deletion of ER-stress protein BiP or JNK using siRNAs significantly suppressed the ODN-induced CCN1 secretion in Beas2B cells (Fig. 6D). Furthermore, deletion of ER stress associated protein BiP inhibited expression of pSrc-Y527, pSrc-Y416 (Fig. 6E), Cav-1 and pCav-1(Y14) (Fig. 6F). However, BiP expression was independent to Src and its phosphorylation (Fig. S5A-B). Inhibition of JNK diminished the level of pCav-1 (Fig. 6F, S5D). JNK activation (pJNK) was regulated by Src phosphorylation (Fig. S5E) but independent of Cav-1 (Fig. S5F). Dissociation of CCN1/Src was an important step for ODN-triggered CCN1 secretion, as presented in above data (Fig. 5). The dissociation of CCN1/Src interaction after ODN stimulation was suppressed by BiP depletion (Fig. 6G), suggesting the involvement of ER stress in this process. Taken together, ER stress is the initiator of ODN-induced CCN1 secretion, followed by Src-Y527 and Cav-1 phosphorylation (Fig. S6A, B), in lung epithelial cells.
Fig. 6. CpG ODN localized in ER and triggered ER stress.
(A) Beas2b cells were treated with CpG ODN-FITC (1 μM). The localization of CpG ODN was observed using confocal microscope. Markers for mitochondria, Bcl2; for endosome, EEA; for lysosome, LAMP-1; for Golgi, GM130; for ER, BiP/GRP78
(B) Beas2B cells were treated with ODN (1 μM). Within 5 min, calnexin, ERp94, ERp44, BiP, CHOP and β-actin was evaluated using Western Blot analysis
(C) Beas2B cells were exposed to each inhibitor. After 1h, cells were exposed to ODN (1 μM). After additional 4h, CCN1 was measured using ELISA. Inhibitors used for JNK, JNK inhibitor; for MAPK, SB 203580; for NF-κB, Bay 11-7082; for Perk, Perk inhibitor; for ATF6 activation, γ-secretase inhibitor
(D) Beas2B cells were transfected with BiP or JNK siRNAs. After 2days, cells were then treated with ODN (1 μM). After additional 4h, CCN1 was measured using ELISA
(E) Beas2B cells were transfected with BiP siRNA. After 2 days, BiP, Src, Src (Y416) and Src (Y527) were detected using Western Blot analysis
(F) Beas2B cells were transfected with BiP or JNK siRNAs, followed by treatment of ODN as the above. BiP, JNK, Cav-1, pCav-1 and β-actin were detected
(G) Beas2B cells were transfected with BiP siRNA. After 2 days, whole cell lysate was incubated with anti-CCN1 or anti-Src, respectively. Cells were then incubated with IgG beads for 1h and washed out 3 times with RIPA buffer. Each precipitated sample was detected using Western Blot analysis.
All Fig.s represented three independent experiments with identical results.
Discussion
Lung epithelial cells are constantly exposed to environmental toxicants and pathogens. Bacterial infections trigger the acute inflammatory responses which are bactericidal but potentially carry consequences of tissue damage leading to lung injury.3 To maintain the integrity of the lung epithelium and ensure effective physiological functions, lung epithelial cells are armed with ‘self-limiting’ systems to control ‘runaway’ inflammatory responses. Here, we identified a novel paradigm in which the homeostasis of innate immunity and inflammation are regulated by lung epithelial cells.
We showed that the CCN1 secretion from lung epithelial cells was robustly enhanced by CpG ODN/bacterial DNAs in a TLR9-independent manner. Currently, the potential receptors /pathways by which CpG ODN induces CCN1 secretion remain unclear and under further investigation. CCN1 gene expression and secretion are augmented by mechanical stretch, oxidative stress, growth factors and cytokines through diverse signaling pathways.18 To eradicate the bacteria, innate immune cells generate proteolytic enzymes, radical oxygen and granules, while epithelial cells secrete β-defensin.31-33 During this bactericidal process, we suspect that bacterial DNA was leaked to ECM from the damaged bacteria. At this point, the leaked bacterial DNAs in ECM were recognized by the surrounding lung epithelial cells and triggered a signaling cascade to secret CCN1. We will further investigate the cell type-specific bacterial clearance and bacterial DNA leakage in vitro and in vivo. This bacterial DNA-induced CCN1 exerted anti-inflammatory effects in a paracine/autocrine manner, including promoting IL-10 release, suppressing both MIP-2 and TNF-α level. Consequently, neutrophil infiltration, but not macrophage and dendritic cell infiltration, was inhibited in lung parenchyma (Not shown). Thus, CCN1 potentially functioned as a “braking” molecule to bacterial infection-associated lung inflammatory responses
ER stress often refers to both ER dysfunction and loss of its homeostasis in the presence of stressful stimuli, such as bacterial infection.34 The effects of pathogens on ER stress robustly affect the innate and adaptive immune responses. Previous studies have demonstrated that bacterial infection, particularly Gram-negative bacteria-derived LPS induces lung inflammation via triggering ER stress.35 Particularly, the CHOP-associated ER stress has been shown to participate in the pathogenesis of sepsis-associated inflammation, cell death and lung injury.35,36 However, how the host responds to pathogen-induced ER stress remains largely unclear. Interestingly, the ER stress-associated proteins (BiP, CHOP), besides involved in the inflammatory responses, also facilitated the secretion of CCN1 to matrix. In the presence of inflammatory inducers, such as bacterial DNAs and CpG ODN motifs, CCN1 exerts anti-inflammatory features, shown in our current studies. However, ER stress in lung epithelial cells can also be induced by a variety of non-infectious stimuli, such as cigarette smoke and hyperoxia.20,37 These environmental stimuli, such as cigarette smoke, are less likely to induce the similar inflammatory process after bacterial infection. In fact, we have shown that the cigarette smoke-induced CCN1 acts as a pro-inflammatory, rather than anti-inflammatory agent.20 In another example, CCN1 has been reported previously to promote inflammation and wound repair.38-40 In these studies, Bai et al demonstrated that a high dose of CCN1 (10 μg/ml) treatment leads to an up-regulation of pro-inflammatory cytokine/chemokine mRNAs in macrophages.38 CCN1 supports the adhesion of macrophages, activates NF-κB mediated transcription, and induces pro-inflammatory cytokines which are generated by classically activated M1 macrophages.38 It is not surprising to us that CCN1 plays a dual role in response to the inflammation triggered by different stimuli. Besides inflammation, numerous reports have also demonstrated that CCN1 carries dual roles in cell death. CCN1 has been shown to confer cytoprotection in many cell types including, but not limited to, lung epithelial cells, endothelial cells, intestinal epithelial cells and cardiomyocytes.18,19,41,42 On the other hand, CCN1 has also been reported to induce apoptosis in fibroblasts.43 One of the main explanations for the differential functions of CCN1 in cell death is associated with surface integrins that CCN1 binds to. For instance, CCN1 exerts its cytoprotective effects via integrin αVβ3, while causing cell death via integrin α6β1.41,43 Therefore, we hypothesized that CCN1 carries either pro-inflammatory or anti-inflammatory effects depending on the different cell surface integrins. In fact, in our current studies, CCN1 exerted its anti-inflammatory functions via integrin αVβ6, but not integrin αVβ2 which is known to mediate pro-inflammatory effects of CCN1.38 Taken together, to maintain the homeostasis of lung micro-environment, it is very possible that CCN1 functions as an anti-inflammatory factor in response to acute infection phase. The timing, route and amount of CCN1 secretion triggered by the “sterile” ER-stress can be significantly different compared to when it is infectious pathogen-triggered. The dual function of CCN1 may well be regulated according to multiple factors, including the stimuli, the cell type, the lung micro-environment and the amount of secretion. The detailed functions of CCN1, in response to each specific stimulus, require further exploration.
CCN1 promoted IL-10 release via integrin αVβ6–PKC pathway. In the promoter region of IL-10, there are multiple transcriptional factor binding sites which can be activated by CCN1. For instance, the NF-κB.44 CCN1 has been reported to exert its functions via both an immediate-early response resulting from direct activation of NF-κB and a delayed response resulting from CCN1-induced cytokines.38 Furthermore, the expression of integrin αVβ6 is rapidly increased in epithelial cells upon inflammation and involved in immune suppression.46 Importantly, previous studies demonstrate that the function of αVβ6 is through the activation of transforming growth factor β (TGF-β) by binding the latency associated peptide.47 TGF-β is known to drive the production of IL-10.48 Thrombospondin 1 (TSP 1) has been known as one of the major activators of TCGF-β.49 More interestingly, there is a TSP-1 like domain (domain 3, Fig. 4E) existing in the full-length CCN1.18 Therefore, it is possible that CCN1 also induces IL-10 via a latent / indirect pathway, via TSP-1 domain activated TGF-β. Furthermore, the production of IL-10 is regulated by PKCδ signaling after LPS stimulation in BMDM50. IL-10 is one of the major mediators involved in the host defense against the infection-induced inflammation.12,14 Consistently, our studies showed that CCN1-induced IL-10 contributed to preserve lung homeostasis by suppressing TNF-α, MIP-2 level and neutrophil counts in BALF (Fig. 2). However, lung epithelial cell probably is not the only cell type which secretes IL-10 after CpG ODN stimulation. Other immune cells (e.g., plasmacytoid dendritic cells) may also generate IL-10 in this circumstance. One of the limitations in this study is that we did not address IL-10 secretion from other cells.
Our studies focused on the lung epithelial cell-derived CCN1s induced by bacterial DNA or synthetic bacterial DNA motif in the form of CpG ODNs. One future direction will be to explore the direct interactions between CCN1 and bacteria, as well as the functions of CCN1 in bacterial adherence, invasion and proliferation. Moreover, we will investigate the virus triggered CCN1 secretion and its function. We observed influenza and RSV infection stimulated CCN1 secretion in in vivo infection model (not shown). Whether the virus-triggered CCN1 is pro- or anti-inflammatory remains unclear. The co-existence of both bacterial and viral infections is a common scenario seen in upper airway infections, pneumonia and pneumonitis. Exploration of the role of CCN1 in this complicated process potentially opens a novel area for lung infection research.
In summary, our studies demonstrated a novel paradigm by which hosts maintain the fine balance between the innate immunity / bactericidal effects and the ‘runaway’ inflammation which can cause lung injury. Bacterial DNA/CpG motif triggered CCN1 secretion via ER stess-Src-JNK-Cav-1 pathways. These lung epithelial cell-derived CCN1s exerted anti-inflammatory functions via promoting IL-10 and inhibiting multiple pro-inflammatory cytokines, as well as the neutrophil infiltrations in the lung.
Experimental procedures
Reagent
Human TLR agonist kit was purchased from Invivogen (San Diego, CA), Bay11-7082, daidzein, 2-bromopalmitate (2-BP) from Sigma (St. Louis, MO), LPS, SB203580, γ-secretase inhibitor and JNK inhibitor from Calbiochem (Darmstadt, Germany), PERK inhibitor from EMD Milloipre (Darmstadt, Germany), recombinant human CCN1 (with biological activities), anti-human and anti-mouse CCN1 from R&D systems, (Minneapolis, MN), anti-αVβ6 antibody from abcam (Cambridge, MA), A.bauminnii, K.pneumoniae and P.aerosinosa DNA from ATCC (Manassas, VA).
Lung inflammation mouse model
K.pneumoniae (103 cfu) was administered intra-tracheally. Mice were sacrificed at the designated time points. To investigate the functional role of ODN or CCN1 in vivo, ODN (10 μg) or CCN1 (5 μg) was simultaneously administered with LPS (10 μg) into intra-nasally in WT and Cav-1−/− mice. PP2 (5 mg/kg) was injected intra-peritoneally. After 24 h, BAL fluids were obtained. Total cell counts and differentials were analyzed as previously described (Moon HG et al., 2010). To detect neutrophil infiltration in lung tissue, lung tissues were prepared as previously described26. Neutrophil was stained with Ly6G+F4/80− (BD Bioscience, Franklin Lakes, New Jersey) and analyzed using Flow Cytometer.
ELISA
Human CCN1 (no cross-activity with CCN2), human IL-10, mouse TNF-α, mouse IL-10, mouse IL-6, mouse MIP-2 duoset ELISA were purchased from R&D systems (Minneapolis, MN) and mouse CCN1 ELISA (no cross-activity with CCN2) was purchased from MyBioSource (San Diego, CA). ELISA was performed per manufacturer’s instruction.
Western blot
Cells were harvested after cold PBS washing for twice. Cells were then re-suspended in RIPPA buffer with protease inhibitors (Roche, Indianapolis, IN). Total protein samples were fractioned using 4-12% NuPAGE gel (Invitrogen, Carlsbad, CA) and transferred to PVDF membranes (Bio-Rad, Hercules, CA). Membranes were blocked in 5% nonfat milk in PBST for 1 hour at room temperature and then incubated with primary antibodies at 4oC overnight. CCN1 and β-actin antibodies were purchased from Santa Cruz (Santa Cruz, CA) and TLR9 antibody from Sigma (St. Louis, MO), Cav-1 antibody from BD Bioscience (San Jose, CA), pCav-1 from Abcam (Cambridge, MA), Src, Src (Y416) and Src (527) from Cell Signaling Technology (Danvers, MA). Membranes were washed and incubated with appropriate secondary antibodies (Santa Cruz, CA). Detection was performed using the SuperSignal West Pico and Femto system (Pierce, IL) and exposed to Molecular Imager® chemi DocTM XRS+ (Bio-Rad, Hercules, CA). Normalization and relative quantification were performed using Image Lab software (Bio-Rad, Hercules, CA).
Immunofluoresence (IF) staining
Cells were grown on the cover slip and collected and fixed with 4% paraformaldehyde for 30 minutes and then permeabilized by 0.5% TritonX-100 for 1 hour at room temperature. Cells were blocked with 5% BSA in PBS for 2h at room temperature before incubating with primary antibodies at 4 °C overnight. Cells were then washed three times and secondary fluorescein and Cy3-conjugated antibodies (Santa. Cruz, CA) were applied at 1:200 dilution at room temperature for 1 hour. Images were captured using Olympus Fluoview BX 61 confocal microscope (Olympus, Center Valley, PA).
Small-interfere RNA transfection & CCN1 plasmid transfection
Human TLR9 siRNA purchased from Sigma (St. Louis, MO) and CCN1, Cav-1 siRNA from Santa Cruz Biotechnology (Santa Cruz, CA), Src siRNA from Origenes (Rockville, MD), JNK and BiP siRNA from Cell Signaling Technology (Danvers, MA) and INTERFERin® from Polyplus (Illkirch, FRANCE). Transfection procedure was performed per INTERFERin® manufacturer’s instruction.
Human CCN1 plasmid constructs were designed following pcDNA3.1/V5-His© TOPO® TA Expression Kit (Invitrogen, Grand Island, NY). CCN1 constructs were transfected using LipoD293 (SignaGen, Rockville, MD) and followed manufacturer’s instruction. Transfected CCN1 plasmids were detected using anti-V5 antibody (Invitrogen).
Statistics
The means of fold change in all figures were compared by using two-way analysis of variance to test the differences among independent samples. With p< 0.05 the difference was considered statistically significant. Error bars indicate standard deviation.
Supplementary Material
Supplemental figure legends
Supplement 1. CpG ODN-induced CCN1 in a time- and dose- dependent but TLR9-independent manner.
(A) Three different lung epithelial cells (Beas2B, A549 and HBE cells) were cultured and exposed to CpG ODN (1 μM). After the designated time, CCN1 secretion was determined using ELISA.
(B) Beas2B, A549 and HBE cells were cultured and exposed to CpG ODN at different doses. After 24h, CCN1 secretion was determined using ELISA.
(C) Beas2B cells were treated with CpG ODN (1 μM) for 4h. CCN1 was assessed using ELISA.
(D) Beas2B cells were transfected with TLR9 siRNA. After 2 days, siRNA efficiency was confirmed using Western Blot analysis.
(E) Beas2B cells were pre-treated with NF-κB signaling inhibitor, Bay 11-7082, for 1h, followed by ODN (1 μM) for 4h. CCN1 was measured using ELSIA
All Figures represented two independent experiments with identical results.
Supplement 2. CCN1 conferred anti-inflammatory effects in LPS stimulated alveolar macrophages.
(A) Alveolar macrophages (MH-s) were stimulated with LPS with and without CCN. Level of IL-6 and TNF-α were determined using ELISA. *p < .05, compared to LPS
(B) Neutralizing CCN1 with anti-CCN1 antibodies reversed the IL-6 and TNF-α secretion in LPS+CCN1 stimulated alveolar macrophages (MH-s). *p < .05, compared to LPS+CCN1
All Figures represented two independent experiments with identical results.
Supplement 3. CCN1 induced IL-10 secretion and integrin αVβ6 expression
(A) A549 cells were treated with CCN1(1 μg/ml). After 4h, IL-10 was measured using ELISA
(B) Beas2B cells were treated with CCN1 (1 μg/ml). After 6h, the mRNA expression of integrin αV and β6 was measured using real-time PCR and then normalized by HPRT
All Figures represented two independent experiments with identical results.
Supplement 4 Cav-1 and CCN1 secretion
(A) Beas2B cells were pre-treated with Cav-1 inhibitor, Daidzen, at the indicated doses. After 3h, cells were treated with ODN. CCN1 level was determined using ELISA after additional 4h.
(B) Beas2B cells were transfected with Cav-1 over-expression plasmid. After 2 days, CCN1 was measured using ELISA
All Figures represented two independent experiments with identical results.
Supplement 5. Expression of BiP and CHOP was independent to Src activation
(A) Beas2B cells were transfected with Src siRNA, followed by ODN (1 μM). BiP, CHOP, Src and β-actin were detected using Western Blot analysis
(B) Beas2B cells were pre-treated with PP2 for 1h, followed by ODN. BiP, CHOP, Src (Y527), Src and β-actin were detected
(C) Beas2B cells were transfected with BiP or JNK siRNAs and siRNA efficiency was confirmed using Western Blot analysis
(D) Beas2B cells were pre-treated with JNK inhibitor for 1h, JNK, pJNK, Cav-1, pCav-1 and β-actin were detected using Western Blot analysis.
(E) Beas2B cells were pre-treated with PP2 for 1h, followed by ODN treatment. Src (Y527), Src, JNK and pJNK were determined using Western Blot analysis.
(F) Beas2B cells were transfected with Cav-1 siRNA, followed by ODN treatment. JNK, pJNK, Cav-1and β-actin were detected using Western Blot analysis.
All Figures represented two independent experiments with identical results.
Supplement 6. Schemata
A. Schematic presentation of CCN1 secretion in response to CpG ODN stimulation: Bacterial DNA motif (CpG) -- ER stress (BiP) -- Src-pY527 -- dissociation of Src from CCN1 vWF domain -- pJNK -- Cav-1 pY14 -- Cav-1 interactions with CCN1 IGFbp domain -- CCN1 release from cell to ECM
B. Schematic presentation of the function of CCN1: CCN1 stimulated IL-10 secretion via Integrin αVβ6/PKC pathway, subsequently inhibited the TNF-α and MIP2 and suppressed neutrophil infiltration.
C. Summary: The bacterial DNA motif (CpG ODN) induced CCN1 secretion from lung epithelial cells, via the ER-stress-Src-JNK-Cav-1 mediated pathway. This bacterial DNA induced CCN1 exerted anti-inflammatory effects in a paracine/autocrine manner, including promoting IL-10 release, suppressing MIP-2 and TNF-α level. Consequently, neutrophil infiltration was inhibited in lung parenchyma. Through augmenting CCN1 secretion, the lung epithelium maintains its innate immunity homeostasis and limits the risks of overwhelming inflammatory responses triggered by bacteria. Thus, CCN1 potentially functioned as a “braking” molecule to bacterial infection-associated lung inflammatory responses.
Acknowledgments
This work is supported by National Institutes of Health R01 HL102076 (Y.J.). National Natural Science Foundation of China 81370103 (L.X.).
We thank Dr. Augustine MK Choi and Dr. Lester F Lau for their scientific discussions and support. We also thank Mr. Jincheng Yang for his help on editing the manuscript.
Footnotes
Conflict of Interest Statement: No Conflict
Disclosure
The authors declare no conflict of interests.
Reference
- 1.Eddens T, Kolls JK. Host defenses against bacterial lower respiratory tract infection. Curr Opin Immunol. 2012;24:424–430. doi: 10.1016/j.coi.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tam A, Wadsworth S, Dorscheid D, Man SF, Sin DD. The airway epithelium: more than just a structural barrier. Ther Adv Respir Dis. 2011;5:255–273. doi: 10.1177/1753465810396539. [DOI] [PubMed] [Google Scholar]
- 3.Grainge CL, Davies DE. Epithelial injury and repair in airways diseases. Chest. 2013;144:1906–1912. doi: 10.1378/chest.12-1944. [DOI] [PubMed] [Google Scholar]
- 4.Bauer S, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalpke A, Zimmermann S, Heeg K. CpG DNA in the prevention and treatment of infections. BioDrugs. 2002;16:419–431. doi: 10.2165/00063030-200216060-00003. [DOI] [PubMed] [Google Scholar]
- 6.Wooldridge JE, Ballas Z, Krieg AM, Weiner GJ. Immunostimulatory oligodeoxynucleotides containing CpG motifs enhance the efficacy of monoclonal antibody therapy of lymphoma. Blood. 1997;89:2994–2998. [PubMed] [Google Scholar]
- 7.Krug A, et al. Identification of CpG oligonucleotide sequences with high induction of IFNalpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Cardon LR, Burge C, Clayton DA, Karlin S. Pervasive CpG suppression in animal mitochondrial genomes. Proc Natl Acad Sci USA. 1994;91:3799–3803. doi: 10.1073/pnas.91.9.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore TA, Standiford TJ. Cytokine immunotherapy during bacterial pneumonia: from benchtop to bedside. Semin Respir Infect. 2001;16:27–37. doi: 10.1053/srin.2001.22726. [DOI] [PubMed] [Google Scholar]
- 10.Cazzola M, Matera MG, Pezzuto G. Inflammation--a new therapeutic target in pneumonia. Respiration. 2005;72:117–126. doi: 10.1159/000084039. [DOI] [PubMed] [Google Scholar]
- 11.Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annu Rev Physiol. 2014;76:467–492. doi: 10.1146/annurev-physiol-021113-170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinhauser ML, Hogaboam CM, Kunkel SL, Lukacs NW, Strieter RM, Standiford TJ. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol. 1999;162:392–399. [PubMed] [Google Scholar]
- 13.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi MH, Harmsen AG, Garvy BA. IL-10 modulates host responses and lung damage induced by Pneumocystis carinii infection. J Immunol. 2003;170:1002–1009. doi: 10.4049/jimmunol.170.2.1002. [DOI] [PubMed] [Google Scholar]
- 15.Muenzer JT, et al. Characterization and modulation of the immunosuppressive phase of sepsis. Infect Immun. 2010;78:1582–1592. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun L, et al. Dual role of interleukin-10 in the regulation of respiratory syncitial virus (RSV)-induced lung inflammation. Clin Exp Immunol. 2013;172:263–279. doi: 10.1111/cei.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schütze N, Rücker N, Müller J, Adamski J, Jakob F. 5′ flanking sequence of the human immediate early responsive gene ccn1 (cyr61) and mapping of polymorphic CA repeat sequence motifs in the human ccn1 (cyr61) locus. Mol Pathol. 2001;54:170–175. doi: 10.1136/mp.54.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin Y, Kim HP, Ifedigbo E, Lau LF, Choi AM. Cyr61 protects against hyperoxia induced cell death via Akt pathway in pulmonary epithelial cells. Am J Respir Cell Mol Biol. 2005;33:297–302. doi: 10.1165/rcmb.2005-0144OC. [DOI] [PubMed] [Google Scholar]
- 20.Moon HG, Zheng Y, An CH, Kim YK, Jin Y. CCN1 secretion induced by cigarette smoking extracts augments IL 8 release from bronchial epithelial cells. PLoS One. 2013;8:e68199. doi: 10.1371/journal.pone.0068199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol. 2002;22:8709–8720. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krieg A. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 23.Krieg A. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 24.Leeper-Woodford SK, et al. Tumor necrosis factor. Alpha and beta subtypes appear in circulation during onset of sepsis induced lung injury. Am Rev Respir Dis. 1991;145:1076–1082. doi: 10.1164/ajrccm/143.5_Pt_1.1076. [DOI] [PubMed] [Google Scholar]
- 25.Sheridan BC, McIntyre RC, Meldrum DR, Fullerton DA. Pentoxifylline treatment attenuates pulmonary vasomotor dysfunction in acute lung injury. J Surg Res. 1997;71:150–154. doi: 10.1006/jsre.1997.5144. [DOI] [PubMed] [Google Scholar]
- 26.Mosser DM, Zhang X. Interleukin 10: new perspectives on an old cytokine. Immunol Rev. 2008;226:208–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugimoto K, et al. The αvβ6 integrin modulates airway hyperresponsiveness in mice by regulating intraepithelial mast cells. J Clin Invest. 2012;122:748–758. doi: 10.1172/JCI58815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin Y, Kim HP, Cao J, Zhang M, Ifedigbo E, Choi AM. Caveolin-1 regulates the secretion and cytoprotection of Cyr61 in hyperoxic cell death. FASEB J. 2009;23:341–350. doi: 10.1096/fj.08-108423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodman OL, Missen MA, Boujaoude M. Daidzein and 17 beta estradiol enhance nitric oxide synthase activity associated with an increase in calmodulin and a decrease in caveolin 1. J Cadiovasc Pharmacol. 2004;44:155–163. doi: 10.1097/00005344-200408000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Li S, Seitz R, Lisanti MP. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem. 1996;274:3863–3868. [PubMed] [Google Scholar]
- 31.Segal AW. How Neutrophils Kill Microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baxt LA, Garza-Mayers AC, Goldberg MB. Bacterial subversion of host innate immune pathways. Science. 340:697–701. doi: 10.1126/science.1235771. [DOI] [PubMed] [Google Scholar]
- 33.Singh PK, Jia HP, Wiles K, Hesselberth J, Liu L, Conway BA, Greenberg EP, Valore EV, Welsh MJ, Ganz T, Tack BF, McCray PB., Jr. Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci USA. 95:14961–6. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinon F. The endoplasmic reticulum: a sensor of cellular stress that modulates immune responses. Microbes Infect. 2012;14:1293–1300. doi: 10.1016/j.micinf.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HJ, Jeong JS, Kim SR, Park SY, Chae HJ, Lee YC. Inhibition of endoplasmic reticulum stress alleviates lipopolysaccharide-induced lung inflammation through modulation of NF-κB/HIF-1α signaling pathway. Sci Rep. 2013;3:1142. doi: 10.1038/srep01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endo M, Oyadomari S, Suga M, Mori M, Gotoh T. The ER stress pathway involving CHOP is activated in the lungs of LPS-treated mice. J Biochem. 2005;138:501–507. doi: 10.1093/jb/mvi143. [DOI] [PubMed] [Google Scholar]
- 37.Gewandter JS, Staversky RJ, O’Reilly MA. Hyperoxia augments ER-stress-induced cell death independent of BiP loss. Free Radic Biol Med. 2009;47:1742–1752. doi: 10.1016/j.freeradbiomed.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bai T, Chen CC, Lau LF. Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages. J Immunol. 2010;184:3223–3232. doi: 10.4049/jimmunol.0902792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minhas U, Martin TA, Ruge F, Harding KG, Jiang WG. Pattern of expression of CCN family members Cyr61, CTGF and NOV in human acute and chronic wounds. Exp Ther Med. 2:641–5. doi: 10.3892/etm.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu PL, Su BC, Kuok QY, Mo FE. Extracellular matrix protein CCN1 regulates cardiomyocyte apoptosis in mice with stress-induced cardiac injury. Cardiovasc Res. 2013;98:64–72. doi: 10.1093/cvr/cvt001. [DOI] [PubMed] [Google Scholar]
- 42.Koon HW, Zhao D, Xu H, Bowe C, Moss A, Moyer MP, Pothoulakis C. Substance P-mediated expression of the pro-angiogenic factor CCN1 modulates the course of colitis. Am J Pathol. 2008;173:400–10. doi: 10.2353/ajpath.2008.080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Todorovic V, Chen CC, Hay N, Lau LF. The matrix protein CCN1 (CYR61) induces apoptosis in fibroblasts. J Cell Biol. 2005;171:559–568. doi: 10.1083/jcb.200504015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 45.Tabata T, et al. Induction of an epithelial integrin alphavbeta6 in human cytomegalovirus-infected endothelial cells leads to activation of transforming growth factor-beta1 and increased collagen production. Am J Pathol. 2008;172:1127–1140. doi: 10.2353/ajpath.2008.070448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu XM, Liao YW, Wang HY, Ji KQ, Li GF, Zang B. Integrin alphavbeta6 is involved in measles protein-induced airway immune suppression. Cytokine. 2012;59:59–64. doi: 10.1016/j.cyto.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Munger JS, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 48.McGeachy MJ, et al. TGF beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1309–1307. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 49.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokin Growth Factor Rev. 2000;11:59–69. doi: 10.1016/s1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 50.Noh KT, et al. Protein kinase C δ (PKCδ)-extracellular signal regulated kinase 1/2 (ERK1/2) signaling cascade regulates glycogen synthase kinase-3 (GSK-3) inhibition-mediated interleukin-10 (IL-10) expression in lipopolysaccharide (LPS)-induced endotoxemia. J Biol Chem. 2012;287:14226–14233. doi: 10.1074/jbc.M111.308841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.An CH, et al. TLR4 deficiency promotes autophagy during cigarette smoke-induced pulmonary emphysema. Am J Physiol Lung Cell Mol Physiol. 2012:303, L748–757. doi: 10.1152/ajplung.00102.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure legends
Supplement 1. CpG ODN-induced CCN1 in a time- and dose- dependent but TLR9-independent manner.
(A) Three different lung epithelial cells (Beas2B, A549 and HBE cells) were cultured and exposed to CpG ODN (1 μM). After the designated time, CCN1 secretion was determined using ELISA.
(B) Beas2B, A549 and HBE cells were cultured and exposed to CpG ODN at different doses. After 24h, CCN1 secretion was determined using ELISA.
(C) Beas2B cells were treated with CpG ODN (1 μM) for 4h. CCN1 was assessed using ELISA.
(D) Beas2B cells were transfected with TLR9 siRNA. After 2 days, siRNA efficiency was confirmed using Western Blot analysis.
(E) Beas2B cells were pre-treated with NF-κB signaling inhibitor, Bay 11-7082, for 1h, followed by ODN (1 μM) for 4h. CCN1 was measured using ELSIA
All Figures represented two independent experiments with identical results.
Supplement 2. CCN1 conferred anti-inflammatory effects in LPS stimulated alveolar macrophages.
(A) Alveolar macrophages (MH-s) were stimulated with LPS with and without CCN. Level of IL-6 and TNF-α were determined using ELISA. *p < .05, compared to LPS
(B) Neutralizing CCN1 with anti-CCN1 antibodies reversed the IL-6 and TNF-α secretion in LPS+CCN1 stimulated alveolar macrophages (MH-s). *p < .05, compared to LPS+CCN1
All Figures represented two independent experiments with identical results.
Supplement 3. CCN1 induced IL-10 secretion and integrin αVβ6 expression
(A) A549 cells were treated with CCN1(1 μg/ml). After 4h, IL-10 was measured using ELISA
(B) Beas2B cells were treated with CCN1 (1 μg/ml). After 6h, the mRNA expression of integrin αV and β6 was measured using real-time PCR and then normalized by HPRT
All Figures represented two independent experiments with identical results.
Supplement 4 Cav-1 and CCN1 secretion
(A) Beas2B cells were pre-treated with Cav-1 inhibitor, Daidzen, at the indicated doses. After 3h, cells were treated with ODN. CCN1 level was determined using ELISA after additional 4h.
(B) Beas2B cells were transfected with Cav-1 over-expression plasmid. After 2 days, CCN1 was measured using ELISA
All Figures represented two independent experiments with identical results.
Supplement 5. Expression of BiP and CHOP was independent to Src activation
(A) Beas2B cells were transfected with Src siRNA, followed by ODN (1 μM). BiP, CHOP, Src and β-actin were detected using Western Blot analysis
(B) Beas2B cells were pre-treated with PP2 for 1h, followed by ODN. BiP, CHOP, Src (Y527), Src and β-actin were detected
(C) Beas2B cells were transfected with BiP or JNK siRNAs and siRNA efficiency was confirmed using Western Blot analysis
(D) Beas2B cells were pre-treated with JNK inhibitor for 1h, JNK, pJNK, Cav-1, pCav-1 and β-actin were detected using Western Blot analysis.
(E) Beas2B cells were pre-treated with PP2 for 1h, followed by ODN treatment. Src (Y527), Src, JNK and pJNK were determined using Western Blot analysis.
(F) Beas2B cells were transfected with Cav-1 siRNA, followed by ODN treatment. JNK, pJNK, Cav-1and β-actin were detected using Western Blot analysis.
All Figures represented two independent experiments with identical results.
Supplement 6. Schemata
A. Schematic presentation of CCN1 secretion in response to CpG ODN stimulation: Bacterial DNA motif (CpG) -- ER stress (BiP) -- Src-pY527 -- dissociation of Src from CCN1 vWF domain -- pJNK -- Cav-1 pY14 -- Cav-1 interactions with CCN1 IGFbp domain -- CCN1 release from cell to ECM
B. Schematic presentation of the function of CCN1: CCN1 stimulated IL-10 secretion via Integrin αVβ6/PKC pathway, subsequently inhibited the TNF-α and MIP2 and suppressed neutrophil infiltration.
C. Summary: The bacterial DNA motif (CpG ODN) induced CCN1 secretion from lung epithelial cells, via the ER-stress-Src-JNK-Cav-1 mediated pathway. This bacterial DNA induced CCN1 exerted anti-inflammatory effects in a paracine/autocrine manner, including promoting IL-10 release, suppressing MIP-2 and TNF-α level. Consequently, neutrophil infiltration was inhibited in lung parenchyma. Through augmenting CCN1 secretion, the lung epithelium maintains its innate immunity homeostasis and limits the risks of overwhelming inflammatory responses triggered by bacteria. Thus, CCN1 potentially functioned as a “braking” molecule to bacterial infection-associated lung inflammatory responses.