Abstract
Objectives/Background
Studies from several laboratories have implicated intracellular Ca2+ dynamics in the modulation of electrical activity. We have reported that abnormal Ca2+ wave activity is the underlying cause of afterdepolarization-induced electrical activity in subendocardial Purkinje cells that survive in the 48-hour infarcted canine heart. These cells form the focus of arrhythmias at this time postcoronary artery occlusion.
Methods
We studied the effects of agonists and antagonists on the abnormal Ca2+ release activity of Purkinje cell aggregates dispersed from the subendocardium 48 hours postcoronary artery occlusion (IZPCs). Studies were completed using epifluorescent microscopy of Fluo-3 loaded Purkinje cells.
Results
Similar to our previous report, highly frequent traveling micro Ca2+ transients(μCaiTs) and cell-wide Ca2+ waves were seen in IZPCs in the absence of any drug. Isoproterenol (ISO) increased μCaiTs and cell-wide Ca2+ waves in Purkinje cells dispersed from the normal heart (NZPCs). In IZPCs, ISO increased cell-wide wave frequency but had no effect on the already highly frequent micro Ca2+ wave transient activity, suggesting that ISO lowers the threshold of cell-wide generators responding to micro Ca2+ transients. Drugs that block inward sodium or calcium currents (verapamil, tetrodotoxin) had no effect on Ca2+ activity in Purkinje cells. Antagonists of intracellular Ca2+ release channels [ryanodine, JTV519(K201)] greatly suppressed spontaneous Ca2+ release events in IZPCs. 2APB, an agent that blocks IP3 receptors, greatly reduced the frequency of Ca2+events in IZPCs.
Conclusions
In arrhythmogenic Purkinje cells that survive in the infarcted heart, agents that block or inhibit intracellular Ca2+ release channel activity reduced Ca2+ waves and could be antiarrhythmic.
Keywords: Purkinje, Action potentials, Ca2+ waves, Cai transients, Automaticity, Myocardial infarction
Introduction
A portion of rapid ventricular arrhythmias occurring after coronary artery occlusion in the canine arise from ectopic foci (triggered or abnormally automatic) within the subendocardial Purkinje fiber network located in the subendocardium of the infarct zone in the left ventricle.1,2 Spontaneously occurring arrhythmias predictably occur between 24 to 48 hours after occlusion of the coronary artery. We previously have shown that the density/function of several sarcolemmal ion channels are significantly altered at this time in Purkinje cells dispersed from the subendocardium of the infarct zone (IZPCs).3 We recently showed that abnormal Ca2+ wave activity was the underlying cause of afterdepolarization-induced activity in IZPCs.4 This Ca2+ wave activity originates in a region immediately adjacent to the sarcolemma of the Purkinje cell.5 Thus, the occurrence of nondriven electrical activity in the Purkinje cells from the subendocardial infarct border zone is secondary to both remodeling of ion channels and changes in intracellular Ca2+ transport. The goal of this study was to determine the pharmacologic sensitivity of arrhythmogenic Ca2+ waves that occur in subendocardial Purkinje cells from the infarcted canine heart.
Methods
This investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Publication No. 85-23, 1996).
Healthy male mongrel dogs (12–15 kg, age 1–2 years) were used in these studies. Under isoflurane anesthesia (30 mg/kg), myocardial infarction was produced by a two-step total occlusion of the left coronary artery using the Harris procedure.6 Forty-eight hours after occlusion, infarcted hearts were used for the myocyte study.
Aggregates of 2 to 6 Purkinje cells were enzymatically dispersed from the subendocardial Purkinje network of the normal (NZPCs) or of the infarcted canine left ventricle (IZPCs) using previously described techniques.7 Briefly, small strips (4 × 2 × 2 mm) of left ventricular endocardium containing longitudinally oriented Purkinje fiber bundles were carefully dissected from larger preparations removed from specific regions in the control and infarcted hearts. Strips prepared from infarcted hearts were taken from subendocardium directly overlying the infarcted portion of the left ventricle.7 Cell aggregates were studied in a chamber on the stage of an epifluorescence microscope and superfused with normal Tyrode's solution containing the following (in mM): NaCl 137, NaHCO3 24, NaH2PO4 1.8, MgCl2 0.5, CaCl2 2.0, KCl 4.0, and dextrose 5.5 (pH 7.4; 24°–25°C). We previously established that this model is suitable for fluorescence studies of intracellular [Ca2+].4,8
Aggregate/cell selection for fluorescence measurements
Cell aggregates were selected for study based on previously determined criteria,4,7,8 that is, only Purkinje cell aggregates that were rod shaped with typical junctional ends (fingerlike projections),7 clear striations, and surface membrane free of blebs were used. For some experiments, we selected aggregates of Fluo-3/AM loaded Purkinje cells that exhibited nondriven rhythmic activity. Aggregates of cells of inhomogeneous thickness or with branches out of the field of view were not imaged.
Fluorescence measurements: data acquisition
The single-wavelength Ca2+-sensitive dye Fluo-3 was used for these experiments. Fluo-3 is excited at longer wavelengths; thus, the light results in minimal ultraviolet damage to the cells during prolonged experiments. Although use of a single-wavelength dye could permit motion artifacts, it provides the temporal resolution essential for this study. For imaging experiments, cells were allowed to settle onto the glass bottom of the superfusion chamber and then were loaded with 4 μM Fluo-3AM for 20 minutes without superfusion. Aggregates then were superfused for at least 15 minutes before measurements were made.
For imaging, the excitation light from a xenon lamp (485 nm) was passed into the fluorescent port of an inverted Nikon microscope (20×). The fluorescent light (at 525 nm) was projected through a 520-nm barrier filter onto the surface of an image intensifier plate coupled to a CCD camera (Higain Videoscope Camera, Technology International, Washington, DC). For maximal temporal resolution without loss of spatial quality associated with video recordings, images were recorded directly using a Panasonic optical disk recorder at video frame rate (30 frames/s) and analyzed offline (Photon Technology International, Princeton, New Jersey).
Data analysis
For some analyses, a portion of consecutive images recorded during control or in the presence of drug was selected for a movie (300–400 frames). Then the intensity of fluorescence (F) in regions of interest (ROIs) of cells was determined (PTI software) from these movies of selected frames. F was divided by baseline fluorescence (Fo) for that ROI. Parameters of movement of Ca2+ waves were determined from intensity profiles of the fluorescence (F/Fo) images. Cell-wide wave velocity (Vprop) was calculated from the position of the steepest rise of the Ca2+ transient over time. Peak F/Fo and t1/2max of cell-wide Ca2+ waves were determined in various ROIs for aggregates in each group as described previously.4 RMSF ( over time was taken as the variance of the fluctuations of the average F/Fo in each ROI over a subset of the movie frames (300–450 frames) and was used to quantify traveling micro Ca2+ transient (μCaiT) activity. Number of frames with events/total frames and the actual number of events/total frames were determined by observing all frames and calculating the number of frames with μCaiTs, cell-wide waves and/or cell-wide waves that led to spontaneous action potentials (characterized by a synchronized Ca2+ release4) or by determining the number of observable μCaiTs in all frames (approximately 2,000 frames/cell/drug free or drug conditions). At our frame rate (30 frames/s), this latter value gave a frequency of μCaiTs (number/s).
Drugs
All drugs were added to Tyrode's solution and then used to superfuse the aggregate. Isoproterenol (Sigma, St. Louis, MO) was made into a stock solution (0.2 mg/mL), and aliquots were used on the day of the experiment. Verapamil (Sigma) was made into a stock solution (1 mg/mL), and aliquots were used on the day of the experiment to achieve the needed concentration. Tetrodotoxin (TTX) was made fresh each day as stock solution. 2-Aminoethoxy-diphenyl borate (2APB; Calbiochem, San Diego, CA) was made into stock of 100 mM (DMSO) and frozen in 100-μL aliquots. On the day of the experiment, fresh solution was made from stock by adding to external recording solution. JTV519(K-201) was a kind gift from Aetas Pharm Co. (Tokyo, Japan; Dr. Matsumoto). A stock solution was made (5 mM, DMSO) and an aliquot used for each experiment. Ryanodine (Sigma) and thapsigargin (Sigma) were each made as stock and an aliquot added to fresh solution daily.
Data are expressed as mean ± SEM. Comparisons between groups were made using a unpaired or paired Student's t-test. P <0.05 was considered statistically significant.
Results
Effects of isoproterenol on NZPCs and IZPCs
Cell-wide Ca2+ waves lead to nondriven electrical activity in NZPCs and aggregates from the 48-hour infarcted heart (IZPCs).4 Subcellular micro (<8 μm) Ca2+ traveling transients (μCaiTs) occur with increased frequency in IZPCs and cause cell-wide waves (Fig. 1), membrane depolarizations, and nondriven electrical activity in the form of action potentials.4 Therefore, we studied the effects of 100 nM isoproterenol (ISO) on spontaneous Ca2+ release events in 7 NZPCs and 11 IZPCs in the absence (CON) and then in the presence of isoproterenol. We calculated the RMSF of F/Fo for several ROIs in CON and isoproterenol images of aggregates from both groups to estimate the micro Ca2+ wave (μCaiT) frequency. In the absence of drug, RMSF was greater in IZPCs (0.168 ± 0.01, n = 116) versus NZPCs (0.099 ± 0.014, n = 78) consistent with an increased frequency of imaged μCaiTs in IZPCs.4 Isoproterenol increased RMSF in NZPCs by 44% (to 0.143 ± 0.014; P < 0.05) but not to the level of IZPCs in the presence of isoproterenol (by 15%, to 0.193 ± 0.013). Most ROIs of NZPCs responded with an increase in RMSF (Fig. 2), whereas in IZPCs, isoproterenol caused, on average, no increase in RMSF. Isoproterenol increased both the frequency of cell-wide waves (by 73 and 47% in 5,981 NZPCs and 5,575 IZPCs frames, respectively) and those leading to action potentials in IZPCs (by 13%). Cell-wide waves + isoproterenol traveled more slowly in NZPCs (CON 127 ± 6.4, n = 54; ISO 113 ± 5 μm/s, n = 53) and faster in IZPCs (89 ± 6.1, n = 67, to 111 ± 6.4 μm/s, n = 83, P < 0.05). Isoproterenol had no significant effect on peak F/Fo and relaxation rate of cell-wide waves in NZPCs, but cell-wide waves in isoproterenol in IZPCs relaxed more rapidly (Table 1).
Figure 1.
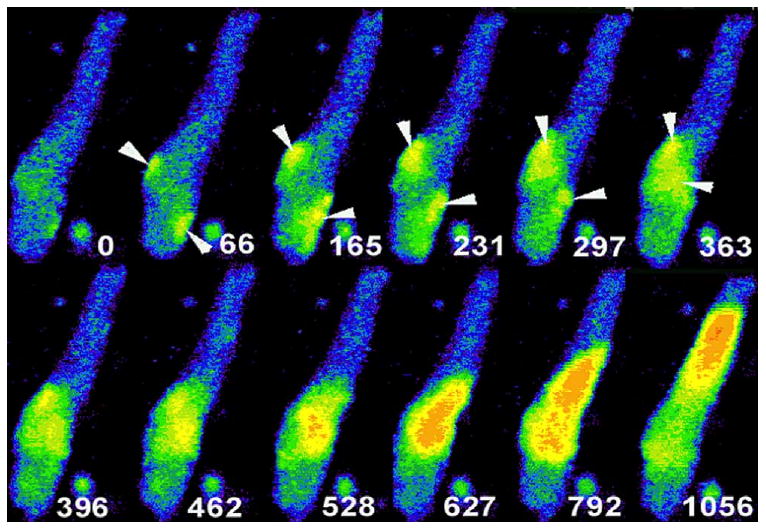
Selected image frames from an IZPC showing spontaneous micro Ca2+ transients and cell-wide Ca2+ waves. White number in each frame is time relative to t = 0 of the first frame of this sequence. Note the occurrence of small micro μCaiTs (arrowheads) at t = 66 to 363 ms. μCaiTs propagate but not throughout the entire aggregate. In t = 396 to 528 ms, μCaiTs coalesce to form a larger Ca2+ wave, a cell-wide Ca2+ wave (t = 627ms). The cell-wide Ca2+ wave propagates through the remainder of the aggregate and, in some cases, gives rise to nondriven electrical activity.4
Figure 2.
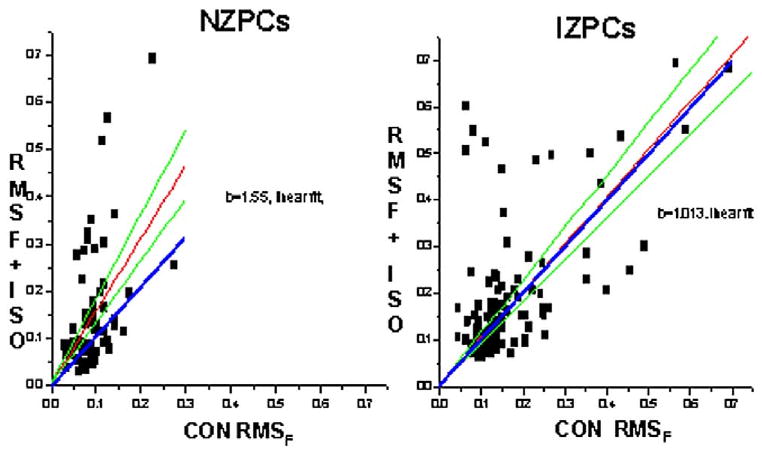
Effects of isoproterenol (100 nM) on RMSF in NZPCs (left) and IZPCs (right). For each region-of-interest data point, the RMSF value in the absence (CON RMSF) is plotted against that in the presence of isoproterenol (RMSF + ISO). Blue lines indicate line of unity; red and green lines represent best linear fit (red) and upper/lower confidence intervals. Note that for NZPCs, most data lie above the unity line.
Table 1.
Effects of isoproterenol on NZPCs and IZPCs
| Cell-wide Ca2+ waves | Peak F/Fo | t1/2 (ms) | Vprop (μm/s) |
|---|---|---|---|
| NZPCs | |||
| Con (54) | 6.94 ± 0.33 | 209 ± 4.6 | 127 ± 6.4 |
| ISO (53) | 6.51 ± 0.35 | 217 ± 6.4 | 113 ± 4.7* |
| IZPCs | |||
| Con (67) | 5.36 ± 0.23 | 235 ± 5.9 | 89 ± 6.1 |
| ISO (83) | 4.90 ± 0.19 | 205 ± 5.3* | 111 ± 6.4* |
| mean ± SEM |
Number in parentheses is number of regions of interest.
Values are given as mean ± SEM.
Abbreviations as defined in the text.
P < .05.
Thus, isoproterenol increased μCaiTs and cell-wide Ca2+ waves, consistent with increased automaticity in NZPCs. In IZPCs, isoproterenol increased cell-wide wave frequency but had no effect on the already highly frequent micro Ca2+ wave activity, suggesting that isoproterenol may lower the threshold of cell-wide generators responding to μCaiTs. Alternatively, in IZPCs, isoproterenol may increase μCaiTs in regions of the cell with initial low RMSF values such that the threshold for cell-wide waves is exceeded (see Discussion).
Effects of verapamil on IZPCs
To determine the role of Ca2+ flux via the L-type Ca2+ channel activity in initiating or perpetuating μCaiTs waves in IZPCs, we tested the effects of verapamil (VER; 10 μM) on μCaiTs in four IZPCs. There is no effect after 10 minutes of verapamil on RMSF of ROIs in IZPCs (CON RMSF: 0.098 ± 0.004, n = 41; VER; 0.100 ± 0.005, n = 41; P > 0.05; Fig. 3). This is consistent with work showing that mibefradil, a blocker of both T- and L-type Ca2+ currents in Purkinje cells, has no effect on the rate of spontaneous electrical activity of Purkinje cells.9
Figure 3.
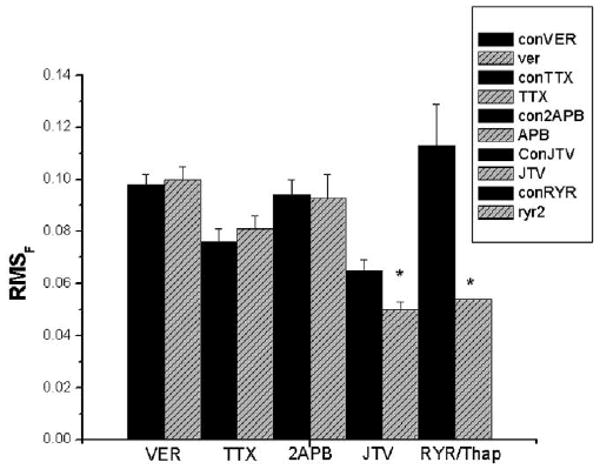
Summary bar graph showing the effects of various agents on the spontaneous Ca2+ events in several groups of IZPCs. The height of each bar indicates average RMSF value (+SEM) of regions of interest (ROI) obtained from imaged aggregates in the absence (CON, black bars) and presence of drug (gray bars) noted. Number of ROIs on average ranges from 25 to 46 (see text). VER = verapamil 10 μM, 10 minutes; TTX = tetrodotoxin 30 μM, 15 minutes; 2APB = 2-aminoethoxy-diphenyl borate 3 μM, 10 minutes; JTV = JTV519 1 μM, 15 minutes; RYR = ryanodine 2 μM and thapsigargin 5 μM, 6–10 minutes. *P < 0.05 vs appropriate CON.
Effects of TTX on IZPCs
To determine the role of Na+ flux via the Na+ channel in initiating or perpetuating μCaiTs waves in IZPCs, we tested the effects of the Na+ channel blocker TTX (30 μM) in three IZPCs. We found that there was no significant effect of TTX (+15 minutes) (CON RMSF: 0.076 ± 0.005, n = 25; TTX: 0.081 ± 0.005, n = 26; P > 0.05; Fig. 3). Thus, TTX-sensitive Na+ channel influx does not contribute to spontaneously occurring Ca2+ wave transients.
Effects of 2APB on IZPCs
Next we sought to determine the effects of 2APB, a membrane-permeant IP3R antagonist,10 on μCaiTs in five IZPCs. Figure 4 shows the effects of the drug. In the absence of drug (CON), a region of this IZPC aggregate (as shown in panel C (ROI1 and ROI2) initiated cell-wide Ca2+ waves. This region also was rich in μCaiTs, similar to our previous report.4 In this IZPC, the incidence of μCaiTs was 0.018% (total no. events/2,000 frames), and a few gave rise to cell-wide waves (0.0025%). With 2APB, this aggregate had a reduced incidence of spontaneous Ca2+ events (0.0085%), which were reduced in total amplitude (Fig. 4B). However, overall, the average amplitude of all spontaneous Ca2+ events in IZPCs in the presence of 2APB (2.29 ± 0.093 F/Fo units, n = 90) was not significantly different from that in the absence of drug (2.37 ± 0.139 F/Fo units, n = 94). Superfusion of drug (3 μM) produced a reduction in the incidence of spontaneous μCaiTs events in IZPCs (total frames with events/total frames, where total frames = 7,200 frames in CON and 7,200 frames in 2APB) (CON frequency 0.0166 ± 0.002; 2APB 0.01 ± 0.002; P < 0.05) and a decrease in the frequency of μCaiTs (1.82 ± 0.5 to 1.4 ± 0.6; P < 0.05) and cell-wide waves (0.093 ± 0.02 to 0.078 ± 0.01). However, there was no significant effect of 2APB on RMSF (CON 0.094 ± 0.006, n = 46, +2APB 0.093 ± 0.005, n = 45). Thus, the effects of 2APB, a blocker of IP3-mediated Ca2+ release, on spontaneously occurring micro Ca2+ wave activity suggest to us that IP3 activation in diseased Purkinje cells may modulate spontaneous Ca2+ release to initiate larger spontaneous Ca2+ release events that propagate.
Figure 4.
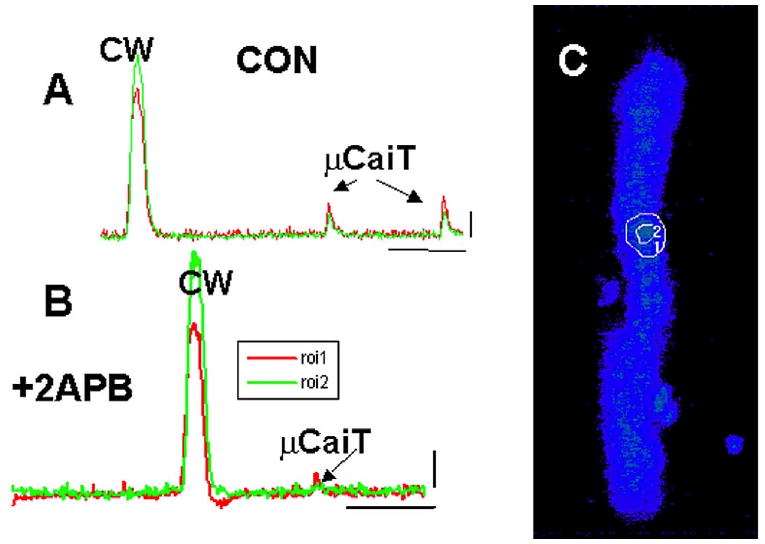
Effects of 2APB on spontaneous Ca2+ events in one IZPC. A: In this IZPC, one area of aggregate (C) generated micro Ca2+ transients (μCaiTs) that led to cell-wide Ca2+ waves (CW). B: In the presence of 2APB, μCaiTs were diminished in frequency and amplitude, particularly at regions depicted in C. Calibration bars in panels A and B: 1 F/Fo, 3.3 s.
Effects of JTV519(K201) on IZPCs
JTV519(K201) 1 μM has been shown to reduce Ca2+ leak from canine sarcoplasmic reticulum (SR) vesicles of ventricular cells from both normal and failing hearts.11 Thus, we determined the effects of JTV519(K201) superfusion on μCaiTs in five IZPCs. Figure 5 shows the effects of the drug. Superfusion of the drug significantly reduced the incidence of μCaiTs events in IZPCs (total events/total frames for 8,600 frames in CON and for 7,800 frames in JTV519; CON 0.012 ± 0.003; JTV519 0.008 ± 0.002), RMSF (CON 0.065 ± 0.004, n = 41, JTV519 0.05 ± 0.004, n = 4; P < 0.05, Fig. 3), and frequency of μCaiTs (1.04 ± 0.14 to 0.56 ± 0.16, P < 0.05) and cell-wide waves (0.124 ± 0.04 to 0.12 ± 0.05). The average amplitude (F/Fo) of all spontaneous Ca2+ events in IZPCs in the presence of JTV519 was reduced by 13.5% (Con 2.44 ± 0.084, n = 269, and 2.11 ± 0.083, n = 165, P < 0.05).
Figure 5.
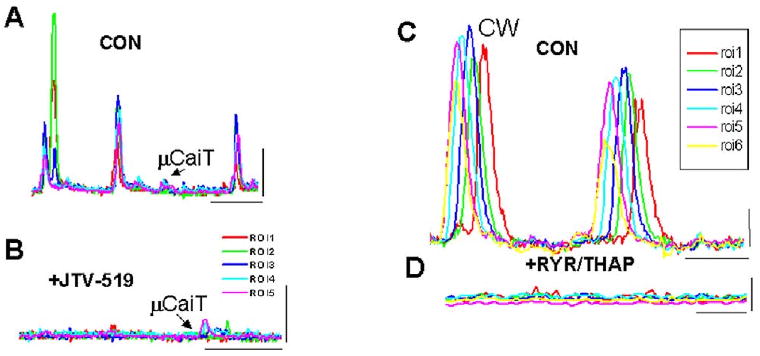
A, B: Effects of JTV519 on spontaneous Ca2+ events in one IZPC. A: Spontaneous Ca2+ events (both CW and μCaiTs) were observed in several regions of interest (ROIs) in this aggregate during CON. B: Their frequency and amplitude decreased in the presence of JTV519. Calibration bars: 2 F/Fo, 3.3 s. C, D: Effects of ryanodine (RYR) and thapsigargin (THAP) on spontaneous Ca2+ events in another IZPC. C: Spontaneous Ca2+ events (both CW and μCaiTs) were observed in several ROIs in this aggregate during CON. D: Their frequency and amplitude decreased in the presence of RYR/THAP. Calibration bars: 0.5 F/Fo, 1.67 s (C), 0.67 s (D).
Effects of ryanodine/thapsigargin on IZPCs
Similar to previous data,8 ryanodine (RYR; 2 μM) together with thapsigargin (Thap; 5 μM) inhibited cell-wide waves and μCaiTs in the five IZPCS tested. This was accompanied by a significant reduction in RMSF (CON 0.113 ± 0.016, n = 43, +RYR/Thap 0.054 ± 0.007, n = 40; Fig. 3).
Discussion
We have used Fluo-3 epifluorescence from aggregates of undialyzed normal and diseased Purkinje cells to address the effects of one agonist and several antagonists on spontaneous Ca2+ release events. In our previous reports, we defined these Ca2+ release events as underlying membrane depolarization in Purkinje cell aggregates.4,8 The mechanism of abnormal Ca2+ release is not known at this time, but these results shed new light on which sarcolemmal and/or intracellular ion channels do or do not contribute to these abnormal spontaneous Ca2+ release events.
Recently, several studies have suggested that beta-adrenergic stimulation synchronizes Ca2+ release events12 leading to an increase in pacemaker activity in sinoatrial nodal cells.13 It also can restore coordination of Ca2+ sparks in myocytes from an infarcted heart.14 Others have suggested that isoproterenol has no effect on spark frequency or distribution, that it can or cannot increase spark amplitude but can accelerate its decay and increase the maximal rate of release15,16 (but see references 17–19). To date, there have been no reports directly showing the effects of isoproterenol on Purkinje cell Ca2+ release events. However, most data presented in a recent rabbit Purkinje study were obtained in the presence of high [Ca2+]o and isoproterenol.20 It is widely accepted that isoproterenol increases the rate of firing of canine Purkinje fibers. In this study, we report that isoproterenol increased both μCaiT and cell-wide Ca2+ waves in normal Purkinje cell aggregates, consistent with observations that isoproterenol enhances membrane depolarization and the amplitude of delayed afterdepolarizations in normal canine Purkinje fibers.21 However, isoproterenol produced these effects in NZPCs without a significant effect on peak F/Fo or relaxation of the spontaneous cell-wide wave. Such an effect may have been expected because isoproterenol can produce this effect in evoked transients. The rate of uptake of Ca2+ by the SR is increased by isoproterenol because of phospholamban (PLB) phosphorylation. A PLB effect may explain accelerated relaxation of the Ca2+ transients, and this increased relaxation rate will blunt the Ca transients even in the presence of increased release flux. If, in this scenario, protein kinase A–induced phosphorylation of the RYR reduces the probability of opening (W.R.S. Chen personal communication), propagation velocity of Ca2+ waves would decrease and Ca2+ loss through the RYR would be reduced. However, filling of the SR may or may not19 change, and thus such would modify the isoproterenol-induced effect on probability of RYR opening and retain both spontaneous opening rate and propagation velocity of a wave.
If, on the other hand, RYR phosphorylation increases channel opening probability,22 isoproterenol would be expected to increase both propagation velocity of Ca2+ waves and the rate of opening events. Eventually, the SR content may or may not decrease. The combination of events could modify the amplitude, frequency, and propagation velocity of the events. Our data do not allow precise discrimination between these possibilities at this time.
In IZPCs, we found that beta-adrenergic stimulation increased cell-wide activity without a significant effect on μCaiT activity. Thus, it appears that with beta-adrenergic stimulation of IZPCs, there maybe “enhanced coupling” between μCaiT events to a cell-wide wave. Furthermore, we conclude that the generators for both μCaiT and cell-wide Ca2+ waves are different and that the threshold of cell-wide generators is reduced with isoproterenol superfusion. In IZPCs + isoproterenol, spontaneous cell-wide waves were not larger than control. However, there was an increase in the frequency of cell-wide waves leading to synchronized action potential-driven Ca2+ release. This latter effect may be due to an adrenergic effect on membrane excitability. In sum, beta-adrenergic stimulation affects Ca2+ release dynamics in Purkinje cell aggregates and can account for nondriven activity.
Our findings with verapamil, a blocker of the L-type Ca2+ channel, and with TTX, a cardiac sodium channel blocker, suggest that neither of these sarcolemmal ion channels contributes significantly to μCaiTs events in Purkinje cells.
Our studies with modulators of intracellular Ca2+ release channels (2APB, JTV519(K201) and RYR/Thap) reveal both expected and unexpected findings. First, quite expectedly, the combination of ryanodine and thapsigargin reduced RMSF. Thus, both μCaiT and cell-wide Ca2+ waves need functioning SR units. Ryanodine type 2 receptors have been reported to be in the core of rabbit and canine Purkinje cells20,23; thus, cell-wide Ca2+ waves in Purkinje cells depend highly on the propagation of Ca2+ between units of ryanodine-mediated release. Sensitivity of μCaiTs and cell-wide waves to ryanodine/thapsigargin in IZPCs is consistent with the effects of ryanodine alone or caffeine in reducing the firing frequency of subendocardial Purkinje fibers from the infarcted heart.24
JTV519(K-201) is a 1,4-benzothiazepine derivative25 that has been shown to protect against Ca2+ overload.26 Two recent reports have suggested that a major effect of this annexin blocker is to improve defective ryanodine receptor gating by enhancing the coupling between FKBP and RYR2 proteins to stabilize the release channel.11,27 Other reports have shown that it can affect several sarcolemmal ion channels, e.g., L-type Ca2+ channel.25,28,29 In these studies, we found that JTV519(K201) had a potent effect on abnormal Ca2+ release events in Purkinje cells. The drug reduced RMSF by 20% (Fig. 3). We think it unlikely that this effect is due to its ion channel blocking effects (e.g., L-type block), because the Ca2+ channel blocker verapamil had no significant effect. Thus, like ryanodine, we expect that JTV519(K201), by reducing spontaneous Ca2+ release events such as μCaiTs, could be antiarrhythmic in this setting postmyocardial infarction.
Finally, we unexpectedly found that 2APB had an effect on initial spontaneous Ca2+ release events that occur just previous to cell-wide waves (Fig. 4). Similar to normal rabbit Purkinje cells with isoproterenol,20 we found that in IZPCs (in the absence of isoproterenol), a cell-wide wave is preceded by highly frequent μCaiTs (Fig. 1).4 These micro Ca2+ events often occur at the Purkinje cell's periphery. In fact, recent confocal studies have shown that Ca2+ sparks/events occur principally in this region, and μCaiTs propagate from this subsarcolemmal (SubSL) region to the cell's core.5 Thus, 2APB reduces the generation of μCaiTs and in so doing cell-wide waves. Interestingly, 2APB in these Purkinje cells did not abolish all micro Ca2+ events (it had no significant effect on RMSF), suggesting that its target receptor (i.e., the IP3 receptor10) is not found in all regions of the canine Purkinje cell. Although IP3 receptors have been defined in Purkinje cells,30,31 we propose that they have a specific subcellular location, perhaps in a defined subsarcolemmal region as observed by confocal microscopy5 (Fig. 6).
Figure 6.
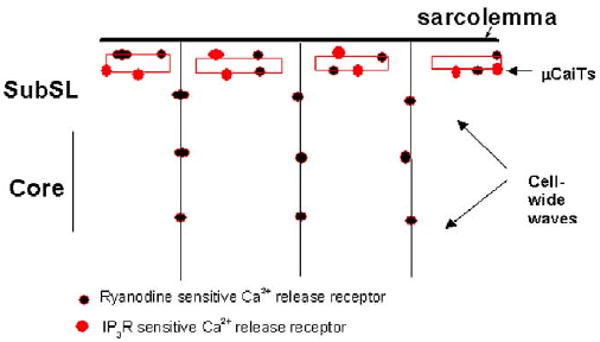
Schematic diagram illustrating a proposed view of the relationship between the location of the two types of Ca2+ release receptors (IP3 and RYR) and the μCaiTs and cell-wide Ca wave in a Purkinje cell aggregate.
Implications of findings
The idea that there is spontaneous Ca2+ release came from the observations of Fabiato and Fabiato32 in mechanically skinned fibers. Subsequently, spontaneous contractions, spontaneous oscillations in both current and potential, were described in both multicellular and myocyte preparations.33–35 The amplitude of spontaneous Ca2+ oscillations depends on the level of intracellular Ca2+ much like the triggered oscillations in Cai that have been shown to be concomitant with delayed afterdepolarizations.36–38 Thus, spontaneous Ca2+ oscillations are not due to transmembrane potential changes but, given the correct initiating conditions, cause traveling Ca2+ waves, depolarizations, and nondriven action potentials.4,8,37,39 Although several investigators have identified the nature of the sarcolemmal currents that may underlie these depolarizations,40 to date no specific antiarrhythmic agents directed toward these underlying currents (e.g., Iti) have been developed. Thus, we propose that, at least for Purkinje cells from infarcted heart, agents directed toward eliminating aberrant spontaneous Ca2+ release, which leads to micro Ca2+ transients, cell-wide waves, and action potentials, may be effective antiarrhythmic agents.
Interestingly, an agent known to block IP3 receptors, 2APB, also was effective in reducing the number of frames showing spontaneous micro Ca2+ activity, as well as the number of μCaiTs. Although IP3 receptors have been identified in normal Purkinje cells,23,30,31 little is known about their functional role, particularly in Purkinje cells that have survived in infarcted heart. Indirect evidence suggests that IP3 generation in cells can be facilitated by a rise in Ca2+ that accompanies ischemia and reperfusion. In fact, there is a relationship between an increase in IP3 in ventricular cells and the incidence of arrhythmias41 and IP3 inhibition and antiarrhythmic effect in reperfused myocardium.42 The role of Purkinje cells in initiating these IP3-dependent arrhythmias was not stated. In this study, by isolating and studying spontaneous Ca2+ release events in Purkinje cells that are the substrate for the arrhythmias in this canine model, we have shown that an agent effective in blocking IP3 receptors also is effective in reducing spontaneous Ca2+ release events.
Acknowledgments
Supported by Grants HL-58860 from the National Heart, Lung, and Blood Institute, Bethesda, Maryland; CIHR; and Alberta Heritage Foundation for Medical Research.
References
- 1.Wit AL, Janse MJ. The ventricular arrhythmias of ischemia and infarction: electrophysiological mechanisms. Mount Kisco, NY: Futura Publishing Co; 1993. [Google Scholar]
- 2.Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989;69:1049–1169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- 3.Pinto JMB, Boyden PA. Electrophysiologic remodeling in ischemia and infarction. Cardiovasc Res. 1999;42:284–297. doi: 10.1016/s0008-6363(99)00013-9. [DOI] [PubMed] [Google Scholar]
- 4.Boyden PA, Barbhaiya C, Lee T, Ter Keurs HEDJ. Nonuniform Ca2+ transients in arrhythmogenic Purkinje cells that survive in the infarcted canine heart. Cardiovasc Res. 2003;57:681–693. doi: 10.1016/s0008-6363(02)00725-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuyvers BD, Boyden PA, Ter Keurs HEDJ. A subsarcolemmal Ca-compartment triggers Ca-events in the core of canine Purkinje cells. Biophys J. 2003;84:199a. [Google Scholar]
- 6.Harris AS. Delayed development of ventricular ectopic rhythms following experimental coronary occlusion. Circulation. 1950;1:1318–1328. doi: 10.1161/01.cir.1.6.1318. [DOI] [PubMed] [Google Scholar]
- 7.Boyden PA, Albala A, Dresdner K. Electrophysiology and ultrastructure of canine subendocardial Purkinje cells isolated from control and 24 hour infarcted hearts. Circ Res. 1989;65:955–970. doi: 10.1161/01.res.65.4.955. [DOI] [PubMed] [Google Scholar]
- 8.Boyden PA, Pu J, Pinto JMB, Ter Keurs HEDJ. Ca2+ transients and Ca2+ waves in Purkinje cells: role in action potential initiation. Circ Res. 2000;86:448–455. doi: 10.1161/01.res.86.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinto JMB, Sosunov EA, Gainullin RZ, Rosen MR, Boyden PA. The effects of mibefradil, a T type calcium channel current antagonist, on the electrophysiology of Purkinje fibers that have survived in the infarcted heart. J Cardiovasc Electrophysiol. 1999;10:1224–1235. doi: 10.1111/j.1540-8167.1999.tb00300.x. [DOI] [PubMed] [Google Scholar]
- 10.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 11.Yano M, Kobayashi S, Doi M, Tokuhisa T, Okuda S, Suetsugu M, Hisaoka T, Obayashi M, Ohkusa T, Kohno M, Matsuzaki M. FKBP12.6 mediated stabilization of calcium release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circ. 2003;107:477–484. doi: 10.1161/01.cir.0000044917.74408.be. [DOI] [PubMed] [Google Scholar]
- 12.Song LS, Wang SQ, Xiao RP, Spurgeon H, Lakatta EG, Cheng H. Beta-adrenergic stimulation synchronizes Intracellular Ca2+ release during excitation-contraction coupling in cardiac myocytes. Circ Res. 2001;88:794–801. doi: 10.1161/hh0801.090461. [DOI] [PubMed] [Google Scholar]
- 13.Vinogradova TM, Bogdanov KY, Lakatta EG. Beta-adrenergic stimulation modulates ryanodine receptor Ca2+ release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res. 2002;90:73–79. doi: 10.1161/hh0102.102271. [DOI] [PubMed] [Google Scholar]
- 14.Litwin SE, Zhang D, Bridge JHB. Dyssynchronous Ca2+ sparks in myocytes from infarcted hearts. Circ Res. 2000;87:1040–1047. doi: 10.1161/01.res.87.11.1040. [DOI] [PubMed] [Google Scholar]
- 15.Gomez AM, Cheng H, Lederer WJ, Bers DM. Ca2+ diffusion and sarcoplasmic reticulum transport both contribute to [Ca2+]i decline during Ca2+ sparks in rat ventricular myocytes. J Physiol. 1996;496:575–581. doi: 10.1113/jphysiol.1996.sp021708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka H, Nishimuru K, Sekine T, Kawanishi T, Nakamura R, Yamagaki K, Shigenobu K. Two dimensional millisecond analysis of intracellular Ca2+ sparks in cardiac myocytes by rapid scanning confocal microscopy increase in amplitude by isoproterenol. Biochem Biophys Res Commun. 1997;233:413–418. doi: 10.1006/bbrc.1997.6470. [DOI] [PubMed] [Google Scholar]
- 17.Reiken S, Gaburjakova M, Guatimosim S, Gomez AM, D'Armiento J, Burkhoff D, Wang J, Vassort G, Lederer WJ, Marks AR. PKA phosphorylation of the cardiac calcium release channel (ryanodine receptor) in normal and failing hearts: role of phosphatases and response to isoproterenol. J Biol Chem. 2003;278:444–453. doi: 10.1074/jbc.M207028200. [DOI] [PubMed] [Google Scholar]
- 18.Ziolo MT, Katoh H, Bers DM. Positive and negative effects of nitric oxide on Ca(2+) sparks: influence of beta-adrenergic stimulation. Am J Physiol Heart Circ Physiol. 2001;281:H2295–H2303. doi: 10.1152/ajpheart.2001.281.6.H2295. [DOI] [PubMed] [Google Scholar]
- 19.Ginsburg KS, Bers DM. Modulation of excitation-coupling by isoproterenol in cardiomyocytes with controlled SR load and ICa trigger. J Physiol. 2004 doi: 10.1113/jphysiol.2003.055384. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordeiro JM, Spitzer KW, Giles W, Ershler PR, Cannell MB, Bridge JHB. Location of the initiation of calcium transients and sparks in rabbit Purkinje cells. J Physiol. 2001;531:301–314. doi: 10.1111/j.1469-7793.2001.0301i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cranefield PF, Aronson RS. Cardiac arrhythmias: the role of triggered activity. Mount Kisco, NY: Futura Publishing Co.; 1988. [Google Scholar]
- 22.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 23.Boyden PA, Dun W, Stuyvers B, Matkovich SJ, Ter Keurs HEDJ. 2APB sensitive micro Ca2+ transients in arrhythmogenic Purkinje cells that have survived in the infarcted heart: a role for IP3 Ca2+ release in Ca2+ wave initiation. Biophys J. 2004;86:223a. [Google Scholar]
- 24.Boutjdir M, El-Sherif N, Gough WB. Effects of caffeine and ryanodine on delayed afterdepolarizations and sustained rhythmic activity in the 1-day old myocardial infarction in the dog. Circulation. 1990;81:1393–1400. doi: 10.1161/01.cir.81.4.1393. [DOI] [PubMed] [Google Scholar]
- 25.Kimura J, Kawahara M, Sakai E, Yatabe J, Nakanishi H. Effects of a novel cardioprotective drug, JTV-519 on membrane currents of guinea pig ventricular myocytes. Jpn J Pharmacol. 1999;79:275–281. doi: 10.1254/jjp.79.275. [DOI] [PubMed] [Google Scholar]
- 26.Kaneko N. New 1,4-benzothiazepine derivative, K201, demonstrates cardioprotective effects against sudden cardiac death and intracellular calcium blocking action. Drug Dev Res. 1994;33:429–438. [Google Scholar]
- 27.Kohno M, Yano M, Kobayashi S, Doi M, Oda T, Tokuhisa T, Okuda S, Ohkusa T, Kohno M, Matsuzaki M. A new cardioprotective agent, JTV519, improves defective channel gating of ryanodine receptor in heart failure. Am J Physiol. 2003;284:H1035–H1042. doi: 10.1152/ajpheart.00722.2002. [DOI] [PubMed] [Google Scholar]
- 28.Nakaya H, Furusawa Y, Ogura T, Tamagawa M, Uemara H. Inhibitory effects of JTV-519, a novel cardioprotective drug, on potassium currents and experimental atrial fibrillation in guinea pig hearts. Br J Pharmacol. 2000;131:1363–1372. doi: 10.1038/sj.bjp.0703713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiriyama K, Kiyosue T, Wang JC, Dohl K, Arita M. Effects of JTV-519 a novel anti-ischemic drug on the delayed rectifier K current in guinea pig ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:646–653. doi: 10.1007/s002100000230. [DOI] [PubMed] [Google Scholar]
- 30.Gorza L, Schiaffino S, Volpe P. Inositol 1,4,5-triphosphate receptor in heart: evidence for its concentration in Purkinje myocytes of the conduction system. J Cell Biol. 1993;121:345–352. doi: 10.1083/jcb.121.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipp P, Laine M, Tovey SC, Burrell KM, Berridge MJ, Li W, Bootman MD. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Curr Biol. 2000;10:939–942. doi: 10.1016/s0960-9822(00)00624-2. [DOI] [PubMed] [Google Scholar]
- 32.Fabiato A, Fabiato F. Calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cells from adult human, dog, cat, rabbit, rat, and frog hearts and from fetal and new-born rat ventricles. Ann N Y Acad Sci. 1978;307:491–522. doi: 10.1111/j.1749-6632.1978.tb41979.x. [DOI] [PubMed] [Google Scholar]
- 33.Kass RS, Tsien RW, Weingart R. Ionic basis of transient inward current induced by strophanthidin in cardiac Purkinje fibers. J Physiol. 1978;281:209–226. doi: 10.1113/jphysiol.1978.sp012417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cannell MB, Lederer WJ. The arrhythmogenic current ITI in the absence of electrogenic sodium-calcium exchange in sheep cardiac Purkinje fibres. J Physiol. 1986;374:201–219. doi: 10.1113/jphysiol.1986.sp016075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kort AA, Lakatta EG. Calcium-dependent mechanical oscillations occur spontaneously in unstimulated mammalian cardiac tissues. Circ Res. 1984;54:396–404. doi: 10.1161/01.res.54.4.396. [DOI] [PubMed] [Google Scholar]
- 36.Kass RS, Tsien RW. Fluctuations in membrane current driven by intracellular calcium in cardiac Purkinje fibers. Biophys J. 1982;38:259–269. doi: 10.1016/S0006-3495(82)84557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuda H, Noma A, Kurachi Y, Irisawa H. Transient depolarization and spontaneous voltage fluctuations in isolated single cells from guinea-pig ventricles. Circ Res. 1982;51:142–151. doi: 10.1161/01.res.51.2.142. [DOI] [PubMed] [Google Scholar]
- 38.Berlin JR, Cannell MB, Lederer WJ. Cellular origins of the transient inward current in cardiac myocytes: role of fluctuations and waves of elevated intracellular calcium. Circ Res. 1989;65:115–126. doi: 10.1161/01.res.65.1.115. [DOI] [PubMed] [Google Scholar]
- 39.Capogrossi MC, Houser SR, Bahinski A, Lakatta EG. Synchronous occurrence of spontaneous localized calcium release from the sarcoplasmic reticulum generates action potentials in rat cardiac ventricular myocytes at normal resting membrane potential. Circ Res. 1987;61:489–503. doi: 10.1161/01.res.61.4.498. [DOI] [PubMed] [Google Scholar]
- 40.Volders PG, Vos MA, Szabo B, Sipido KR, Marieke de Groot SH, Gorgels APM, Wellens HJJ, Lazzara R. Progress in the understanding of cardiac early afterdepolarizations and torsades de pointes: time to revise current concepts. Cardiol Res. 2000;46:376–392. doi: 10.1016/s0008-6363(00)00022-5. [DOI] [PubMed] [Google Scholar]
- 41.Woodcock EA, Matkovich SJ, Binah O. Ins(1,4,5,)P3 and cardiac dysfunction. Cardiovasc Res. 1998;40:251–256. doi: 10.1016/s0008-6363(98)00187-4. [DOI] [PubMed] [Google Scholar]
- 42.Woodcock EA, Reyes N, Jacobsen AN, Du XJ. Inhibition of inositol(1,4,5) triphosphate generation by endothelin-1 during postischemic reperfusion: a novel antiarrhythmic mechanism. Circulation. 1999;99:823–828. doi: 10.1161/01.cir.99.6.823. [DOI] [PubMed] [Google Scholar]


