Abstract
Background
Functional dyspepsia (FD) is a common problem affecting up to 10–25% of individuals. FD accounts for significant health care costs and affects quality of life but has no definitive treatment.
Objectives
The Functional Dyspepsia Treatment Trial (FDTT) aims to test whether treatment with an antidepressant (amitriptyline or escitalopram) leads to improvement of symptoms in patients with moderate to severe FD.
Design
The FDTT is an international multicenter, parallel group, randomized, double-blind, placebo-controlled trial to evaluate whether 12 weeks of treatment with escitalopram or amitriptyline improves FD symptoms compared to treatment with placebo. Secondly, it is hypothesized that acceleration of solid gastric emptying, reduction of postprandial satiation, and enhanced gastric volume change with a meal will be significant positive predictors of short- and long-term outcomes for those on antidepressants vs. placebo. The third aim is to examine whether polymorphisms of GNβ3 and serotonin reuptake transporter influence treatment outcomes in FD patients receiving a tricyclic antidepressant, selective serotonin reuptake inhibitor therapy, or placebo.
Methods
The FDTT enrollment began in 2006 and is scheduled to randomize 400 patients by the end of 2012 to receive an antidepressant or placebo for 12 weeks, with a 6-month post-treatment follow-up. The study incorporates multiple validated questionnaires, physiological testing, and specific genetic evaluations. The protocol was approved by participating centers' Institutional Review Boards and an independent Data Safety Monitoring Board was established for monitoring to ensure patient safety and a single interim review of the data in December 2010 (ClinicalTrials.gov number NCT00248651).
Keywords: Amitriptyline, Antidepressive agents, Citalopram, Dyspepsia, Clinical trial, National Institute of Diabetes and Digestive and Kidney Diseases
1. Introduction
In the U.S., 10 to 25% of people have symptoms suggestive of functional dyspepsia (FD) [1–4]. About a quarter of these people seek medical assistance [2,5]; FD and irritable bowel syndrome (IBS) account for over half of all GI consultations in the U.S. and remain the most frequent GI problems in primary care [2,5]. In addition to substantially impairing quality of life [2,5], health care costs for FD have been calculated to be enormous, exceeding several billion dollars annually in the U.S. despite there being no approved treatments [2,6,7].
FD is currently considered to be a bio-psychosocial disorder with disturbances of motor function, heightened visceral sensitivity, and possibly a central nervous system disturbance [8,9]. Psychosocial factors can alter motility and/or enhance sensation and influence the timing of patients’ presentation to physicians [8,9]. The current study addresses the efficacy of drugs that act at the level of the peripheral gut and central nervous system, and the impact that physiological and psychological disturbance, and genetic variation may play.
Current clinical treatment of FD is considered unsatisfactory [8,9]. In systematic reviews, it has been concluded that the only drugs established to be better than placebo in FD are possibly antisecretory and prokinetic agents [10–12]. However, a Cochrane meta-analysis suggested that the positive cisapride data might simply reflect publication bias, based on a funnel plot [10]. Of the prokinetics, only metoclopramide is available in the U.S. since the withdrawal of cisapride and side effects limit its use.
Psychosocial factors are potentially key modulators of experience and behavior. Patients with FD have been reported to have significantly higher levels of psychiatric illness than healthy controls [13–15] and patients with organic GI diseases [16]. Others have confirmed higher levels of psychological distress in those with FD presenting in primary care [17] and in the general population [18], compared with healthy controls.
Antidepressants are used in the treatment of FD and IBS based on three propositions. First, antidepressants could reduce the severity of psychological symptoms, particularly anxiety and depression, which are thought to exacerbate the symptoms of FD although this is controversial [9,16–21]. Second, antidepressants have central analgesic actions [22], and there is increasing evidence of central nervous system dysfunction in IBS [23]. Antidepressants reduce affective arousal and have sleep restorative actions [24,25]. Thirdly, these drugs have been shown to have local pharmacological actions on the upper gut, specifically altering transit and gastric accommodation [24–29].
The purpose of this study is to investigate whether antidepressant medications are efficacious in FD. Although widely used in FD, the use of antidepressants is not evidence-based. There have been no adequate randomized controlled trials with tricyclics or selective serotonin reuptake inhibitors (SSRIs) in FD. This paper illustrates a clinically relevant, investigator-initiated multi-site trial that proposes to answer this significant clinical question.
2. Methods
2.1. Design overview
The FDTT is an international, multi-center, parallel group, double blind, randomized, placebo-controlled, three-arm trial, comparing escitalopram, amitriptyline, and placebo in people with FD. The screening period for evaluating eligibility and collection baseline data is approximately 2 weeks before randomization. Baseline data collection includes health history, physical examination, physiological testing, and completion of validated surveys. Baseline data include screening measures used for randomization. A standardized and validated symptom diary is completed during this baseline period [30,31]. Patients are required to have at least 4 days of moderate epigastric pain or discomfort on the validated Gastrointestinal Symptoms Ratings Scale (GSRS; a score of >3 on a 7 graded Likert scale), in order to ensure that change over time will be detectable (avoid a floor effect) [32,33]. No placebo run-in is included as this strategy may increase the inclusion of atypical, resistant patients and is not recommended by methodological experts in the field [12,34].
Eligible patients are randomized to escitalopram (10 mg), amitriptyline (50 mg), or placebo for 12 weeks. The escitalopram dose was chosen based on standard antidepressant dosing in clinical practice. The amitriptyline dose was chosen based on the concept that low-dose tricyclic therapy may be efficacious in IBS [35] and preliminary data that this dose improves symptoms after a nutrient drink test [36].
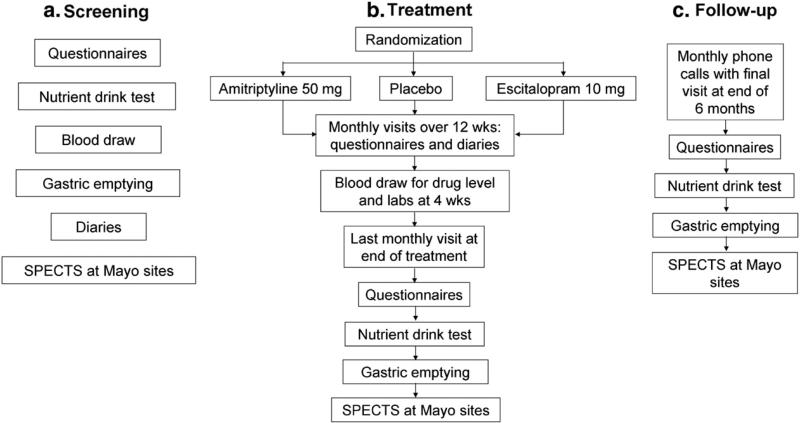
Patients are followed an additional 6 months after the end of the treatment phase to assess potential long-term treatment effects. The primary comparisons will be made using an intent-to-treat analysis (all patients randomized) of the global symptom score collected weekly while on medication, at the end of treatment, and monthly after treatment. A schematic of the trial design is presented in Fig. 1.
Fig. 1.
Study design schematic and study measures.
2.2. Treatment groups
Patients who have signed informed consent and who meet eligibility criteria at the completion of the 2-week baseline period are randomized using a dynamic allocation randomization method. Treatment assignments are made to ensure (marginal) balance on a set of relevant stratification factors (including e.g. gender, subtype of dyspepsia [ulcer-like vs. dysmotility-like], gastric emptying status [delayed vs. non-delayed], satiety status [normal vs. abnormal]). Each assignment is thus based on the distribution of assignments across the stratification factors among the patients randomized up to that point (see Randomization section below).
Amitriptyline hydrochloride is a tricyclic antidepressant; it has a half-life of 9–25 h. It is administered as a single caplet beginning at 25 mg for 2 weeks, then increasing to a single 50 mg caplet for the remaining 10 weeks. Escitalopram (S-citalopram), S-enantiomer of citalopram, binds with high affinity to the human serotonin transporter [37]. It has a half-life of 27–32 h, and is therefore generally administered once daily. Forest Pharmaceuticals has provided escitalopram and identical placebo without charge for this trial. This medication is also taken orally once per day.
Mayo Clinic Research Pharmacy packages the medications and each participant is required to take 1 caplet and 1 capsule daily to assure that the participant and study personnel are completely blinded to the medication. Patients are randomized to either amitriptyline, escitalopram, or placebo. To assure blinding since a capsule could be opened and the patient unblinded, each patient received a capsule and a caplet to take. Either the capsule or caplet or both are placebo for any given patient. Written and verbal instructions are provided to improve proper dosing and increase compliance. Doses are to be taken at night (before bedtime), although patients experiencing potential side effects of the medications may choose to take them in the morning if approved by the study team.
All subjects in the study are provided with the same baseline level of psychological support encompassing reassurance and education. They are provided education related to serotonin syndrome including medications and foods to avoid while in the trial. They are instructed to call the study coordinator if there are any changes in their medications (prescription, over the counter, or herbal medicines) although they are asked not to make changes. Participants are also provided a list of possible side effects and basic health information for dealing with them if mild in nature. All side effects are reported to the study coordinator during either weekly phone calls or additional calls if side effects are bothersome or severe. The trial is being conducted according to the Rome guidelines for clinical trials for the functional GI disorders published in 1999 [34], and adheres to the updated CONSORT reporting guidelines [38].
2.3. Outcome measures
A self-report global symptom score, the primary outcome, is widely accepted and has been tested for responsiveness in FD [39,40]. Patients are asked to answer the following yes/no question once a week: “In the past 7 days, have you had adequate relief of your stomach symptoms?” It is evaluated weekly as part of the phone call over the 12 weeks on therapy, and monthly over the 6 months of follow-up after therapy. A responder is defined as a study patient who answers “yes” for at least 50% or greater of weeks 3–12 of treatment (10 weeks). The first two weeks of treatment are not included to allow for steady state to be established [39,41]. The adequate relief measure is considered a clinically relevant and robust endpoint [40,42,43], and this type of measure is widely accepted by consensus panels in IBS trials as the primary outcome [34].
The second aim is to examine whether gastric emptying and the nutrient drink test are altered by antidepressant therapy. A sub-study will also examine whether gastric accommodation and symptom response, e.g. pain, nausea and bloating, are altered by antidepressants. The outcomes for these secondary aims are solid gastric emptying, postprandial satiation, and gastric volume changes.
Gastric emptying is a validated, standard test [44] using a standardized meal. Assessment of gastric emptying of solids is performed at baseline (all recruited) and at 12 weeks on therapy after an overnight fast, applying a standard meal and acquisition protocol at 0, 0.5, 1, 2, and 4 h. The meal consists of 2 large eggs, wheat bread and 8 oz of white skim milk accounting for 350 kcal (29.8% carbohydrate, 32.4% fat, and 37.8% protein). The 99mTc sulfur colloid is used. Although of uncertain relevance, a baseline gastric emptying study is performed at the beginning of the menstrual cycle in menstruating women to standardize the timing of transit and other physiological measurements [45].
Assessment of early postprandial sensations is conducted using a standardized liquid nutrient meal test. All subjects complete this test at baseline and on therapy at 12 weeks [46].
The third physiologic measure is a measurement of gastric volume and accommodation response by a non-invasive imaging method using 99mTc-single photon emission computed tomography (SPECT). The SPECT is undertaken only at the Mayo Clinic sites in a sub-study (n=20 in each arm of the trial); it is performed at baseline and at week 12 on therapy [47,48]. The randomization will be stratified across the sites, to balance treatment assignments at each site.
The third aim is to assess whether polymorphisms of GNβ3 and the serotonin reuptake transporter are associated with differential treatment outcome. The objective of the third aim is to test whether the genotype of specific polymorphisms alter treatment and potentially identify which patients would have the best response to receiving antidepressants. This aim uses the global symptom relief as the primary outcome.
2.4. Sample size justification and data analysis
The planned sample size for the FDTT is 400 patients with equal allocation to each of the three treatment groups (133 per group). Sample size estimates were based on the assumption that treatment effects would be homogeneous across study centers. An overall pooled comparison of each active treatment vs. placebo would require the number of subjects per treatment group listed in Table 1 to achieve 80% power at a two-sided alpha level of 0.025 (i.e., adjusting for two pair-wise tests, each active drug against placebo). A therapeutic gain of 20% or greater over placebo is considered clinically significant. The logistic regression model analysis incorporating potentially important covariates (gender, psychiatric status, dyspepsia symptom subtype, gastric emptying, satiety, and race) should provide similar or better power to detect comparable treatment group effects assuming no substantial interactions with covariates or differential site effects. A 25% dropout rate in each arm was anticipated, although to date there has only been a total dropout rate of 17%. Therefore, the planned recruitment of 133–134 patients per arm (total 400 patients) should provide sufficient power in the ITT analyses (Table 1). Assigning the ‘dropouts’ as treatment failures could attenuate the treatment differences, but an ITT analysis must include all subjects randomized, and it seems the dropouts would more likely be treatment failures anyway in this setting. Since the dropouts would be unlikely to report symptom relief, little misclassification bias should result.
Table 1.
Sample size calculations for intent to treat analysis.
| Placebo response rate | Active treatment response rate | N per group required | Placebo response rate | Active treatment response rate | N per group required |
|---|---|---|---|---|---|
| 20% | 40% | 98 | 35% | 55% | 116 |
| 45% | 65 | 60% | 75 | ||
| 50% | 46 | 65% | 52 | ||
| 25% | 45% | 107 | 40% | 60% | 118 |
| 50% | 70 | 65% | 75 | ||
| 55% | 49 | 70% | 51 | ||
| 30% | 50% | 113 | 45% | 65% | 116 |
| 55% | 73 | 70% | 73 | ||
| 60% | 51 | 75% | 49 |
Adequate relief will be compared overall among the treatment groups incorporating the sites as strata using the Cochran–Mantel–Haensel (CMH) test statistic for general association (on 2 degrees of freedom). Individual site assessments of the direction of treatment effects will be informally examined for homogeneity by computing site-specific odds ratios (ORs) and 95% confidence intervals (CI) for symptom relief in each active treatment relative to the placebo group. In addition, a logistic regression model with global symptom relief (based on the last 10 weeks of treatment as described above) as the binary dependent variable will be used to test for an increased odds for relief in the active treatment groups (each relative to the placebo reference group) adjusting for the covariates used in the dynamic allocation randomization. Specific two factor interactions of each active treatment group (dummy variable coding) with each of the primary (binary coded) covariates will also be examined in a further model.
An exploratory analysis will assess symptom subgroups and treatment outcome. The proposed analyses for potential interactions among subgroups and treatment assignment are exploratory analyses aimed at identifying potentially more responsive subsets of patients. It is conceivable that the differences in response rates (placebo vs. active treatment) may be much larger in some subgroups (e.g. 30% vs. 60% in ulcer-like dyspepsia) in contrast to other subgroups (e.g. 20% vs. 30% in dysmotility-like dyspepsia). Assuming an equal number of both types of patients in each treatment arm would then reflect an overall difference in response rates of 20% (e.g. 25% vs. 45%) for placebo vs. active drug. It is only these rather large “differential” response rates (interactions) that would be clinically relevant to detect. Similar analyses incorporating specific genotypes (separately) will also be examined to assess the pharmacogenetic aim.
To examine whether the clinical benefit persists longer following an antidepressant than placebo over 6 months of follow-up, the date of ‘symptom relapse’ (see definition above of adequate relief) will be ascertained from the monthly interviews. The date of last follow-up at 6 months, if no relapse occurs, will be considered a censoring date (or last contact date if the subject is lost to follow-up before 6 months). A proportional hazards regression analysis (for interval censored data) in only those subjects with global symptom relief at the end of the 12-week active treatment phase will be included in this analysis. The primary focus of this analysis is to identify potential covariates associated with time to relapse and estimate the ‘hazard ratios’ (with 95% CI) for each active treatment (relative to placebo) used in the 12-week treatment phase.
Assuming roughly 40% of the 400 total subjects over all three treatment groups achieve global symptom relief at the completion of the 12-week active treatment phase, there should be approximately 160 subjects to assess the association of specific covariates with ‘survival free of relapse’ (roughly 133 who were on antidepressant therapy and 27 on placebo). If the event rate (relapse) in subjects on active treatment was 20% and was 80% for subjects on placebo, there would be roughly 48 events available to assess the association of potential covariates with relapse-free survival. Assuming an exponential survival free of relapse, there is approximately 90% power (e.g., using the log rank test at α=0.05) to detect a difference in the proportions without relapse by 6 months (combined active treatment groups vs. placebo) of 0.8 vs. 0.35, or 0.7 vs. 0.25.
The second aim is to assess whether either the gastric emptying test (motor dysfunction) or the nutrient drink test (a test of gastric hypersensitivity and/or gastric accommodation) is altered by antidepressant therapy with a tricyclic or SSRI, and whether variation in physiologic responses is associated with treatment outcome. The effect of treatment on gastric emptying will be assessed using an analysis of covariance incorporating gender, site, and the baseline gastric emptying summary as covariates. The anticipated endpoint to be used will be the proportion (prop2 h) of meal emptied from the stomach at 2 h (first transforming to scale). A similar analysis of the (log) max-tolerated volume and the aggregate symptom score in each subject will be examined to assess treatment effects on satiety as measured using the nutrient drink test. The association of post-treatment gastric emptying with global symptom relief will be based on a multiple linear regression model with the gastric emptying summary as the dependent variable and symptom relief as the primary predictor variable, with other covariates included as potential confounders (e.g., gender, site, and dyspepsia subgroup). Similarly, the association of (log) max-tolerated volumes and aggregate symptom scores with global symptom relief will be assessed using multiple linear regression.
Table 2 summarizes data for the primary response measures and assumes a relative coefficient of variation (CV%) slightly greater than in previously observed data to accommodate site-to-site variation. CV% values are based on data using the same methods. The estimated effect size detectable with 80% power using a two sample z-test (i.e., assuming the variation values are known) at a two-sided alpha level of 0.05 is listed for a number of potential group sizes. The effect size is the difference in group means as a percentage of the overall mean for each response and assumes 80–100 subjects per group for the gastric emptying and nutrient drink test outcome measures. The analysis of covariance (using body mass index as a covariate) should provide 80% power to detect similar differences across all the groups.
Table 2.
Sample size calculations for aim 2.
| Response | Mean (SD)† | CV§ (%) | N per group‡ | Effect size# (%) | Corresponding alternative means |
|---|---|---|---|---|---|
| Solid gastric emptying (% emptied by 2 h) | 0.58% (0.16) | 29% | 80 | 14 | ≤50%, ≥66% |
| 100 | 13 | ≤ 51%, ≥ 65% | |||
| 120 | 12 | ≤ 51%, ≥ 65% | |||
| Solid gastric emptying (% emptied by 4 h) | 0.96% (0.08) | 8% | 80 | 3.9 | ≤92.3%,≥99.7% |
| 100 | 3.5 | ≤92.6%,≥99.4% | |||
| 120 | 3.2 | ≤92.9%,≥99.1% | |||
| Maximum tolerated vol. (nutrient drink test) | 1306 (373) | 35% | 80 | 17 | ≤ 1084, ≥ 1528 |
| 100 | 15 | ≤ 1110, ≥ 1502 | |||
| 120 | 14 | ≤ 1123, ≥ 1489 | |||
| Aggregate symptom score (nutrient drink test) | 166 (72) | 45% | 80 | 22 | ≤ 129, ≥ 203 |
| 100 | 20 | ≤ 133, ≥ 199 | |||
| 120 | 18 | ≤ 136, ≥ 193 | |||
| Fasting gastric volume (SPECT) | 236 (69) | 30% | 15 | 34 | ≤ 156, ≥ 316 |
| 20 | 29 | ≤ 168, ≥ 304 |
Based on previous data.
Based on a two sample t-test using a 2-sided alpha level of 0.025.
Inflated for potential site to site variation.
Effect size is the difference between (2) groups as a percentage of an overall (both groups) mean.
Aim 3 is to examine whether polymorphisms of the heterotrimeric G protein and serotonin reuptake transporter predict outcome in FD patients receiving antidepressant therapy. Allele frequencies (%) for the L and S allele (for 5-HTTLPR) and C and T allele (for C825T) will be generated. Genotype frequencies (%) for LL, LS, and SS (for 5-HTTLPR) and CC, CT, and TT (for C825T) will also be generated. The association between specific polymorphisms of the G protein and serotonin reuptake transporter vs. global symptom relief in response to treatment will be assessed based on a logistic regression model. The primary focus of this aim is to estimate the ORs (and 95% CIs) for global symptom relief in subjects with a specific polymorphism relative to those without the polymorphism and to assess potential differential treatment effects associated with specific polymorphisms. The ORs (and 95% CIs) for global symptom relief in those with the specific polymorphism (relative to those without) will be estimated (as well as interaction terms with treatment) using the coefficients from the logistic model to predict symptom relief. These will be graphically displayed; a similar graphical assessment for other subgroups will also be examined.
In addition, the association between specific physiologic responses (gastric emptying, gastric accommodation, and maximum tolerated volume in nutrient drink test) vs. the particular polymorphisms will be explored using multiple linear regression analyses. The physiologic responses will be considered as the dependent variables in these models and tests of the partial R2-values for the polymorphisms adjusting for gender, BMI and race will be examined.
It is anticipated that approximately 55% of Caucasian patients with FD will be CC (based on our pilot data) [49,50], and 35% will be LL (based on studies in IBS) [51]. Table 3 lists the power for a two-sample comparison of the proportions of patients with global symptom relief (those with a specific polymorphism vs. those without). Within each treatment group (N=130), it is assumed that 55% will be CC and 35% will be LL providing sample sizes of N=70 vs. 60 and 45 vs. 85, respectively. The power listed in Table 3 to detect several possible rates of global symptom relief is based on a two-sample test for proportions using a two-sided alpha level of 0.017 (i.e., adjusted for separate tests within each of the 3 treatment groups). Hence, the study will have good power for the anticipated difference of 25–30% (e.g. 15% vs. 45%). Moreover, a logistic regression model with global symptom relief as the binary dependent variable and treatment group, gender, race, BMI and polymorphism status as predictor variables should provide somewhat better power to detect corresponding associations between genotype and symptom relief by including all subjects in the same analysis assuming homogeneous associations across treatment groups.
Table 3.
Power to detect several possible rates of global symptom relief.
| Relief rate in Group 1* | Relief rate in Group 2* | Power (%)† |
||
|---|---|---|---|---|
| OR | N = 70 vs. N = 60 | N = 45 vs. N = 85 | ||
| 15% | 35% | 3.0 | 61 | 57 |
| 15% | 40% | 3.8 | 81 | 77 |
| 15% | 45% | 4.6 | 93 | 90 |
| 20% | 40% | 2.7 | 55 | 50 |
| 20% | 45% | 3.3 | 76 | 71 |
| 20% | 50% | 4.0 | 90 | 87 |
| 25% | 45% | 2.5 | 51 | 46 |
| 25% | 50% | 3.0 | 72 | 67 |
| 25% | 55% | 3.7 | 88 | 84 |
e.g. CC (Group 1) vs. TT/TC (Group 2), or separately LL (Group 1) vs. SS/SL (Group 2); OR=odds ratio.
Based on a two-sample test for proportions (after arcsin square root transformation) using a two-sided alpha level of 0.017 (i.e., adjusted for separate tests within each of the 3 treatment groups).
2.5. Conduct of the trial
2.5.1. Patient selection
Recruitment of eligible adults (≥18 and ≤75 years of age) began at participating clinical centers between October 2005 and 2006. Originally, six sites participated in the study. An additional two sites have subsequently been added to aid recruitment. Of the eight centers currently recruiting, seven are in the US and one is in Canada. To date, 189 patients have been enrolled. Determination of eligibility is based on standard of care tests and procedures, some of which are done clinically or completed during screening. Each patient signed the consent prior to or at the screening visit to obtain the tests and procedures needed to finalize eligibility. Screening includes a history and physical examination to identify any contraindications for participation.
2.5.2. Inclusion criteria
Patients suffering from FD, as defined by the “gold standard” Rome III criteria [52], are being recruited. Patients can have ulcer-like or dysmotility-like dyspepsia. A minimum 2-week washout of other medications for dyspepsia is required before entry (and a minimum of 4 weeks for any tricyclic or SSRI). Patients have to have had a normal esophagogastroduodenoscopy (EGD) (no esophagitis of any grade, Barrett's esophagus, cancer, erosions, or ulcer disease) within the last five years and to have been diagnosed with FD after specialist consultation. Patients who had previously failed to respond adequately to antisecretory therapy for FD are suitable. However, a previous good response to an antisecretory agent, which remains first line therapy, suggests underlying GERD [52]; such patients are excluded.
Symptom status is comprehensively assessed at baseline through questionnaires in order to characterize patients thoroughly. (See Table 4 for timing of questionnaires.) In addition, patients are subcategorized into symptom subgroups as recommended by the Rome II criteria based on a semi-structured interview [52]. They are subcategorized as having ulcer-like dyspepsia (predominant epigastric pain) or dysmotility-like dyspepsia (predominant non-painful symptom including early satiety, epigastric fullness, epigastric bloating, or nausea). Symptom subgroup is used for randomization stratification and will be considered in the analyses.
Table 4.
Study schedule.
| Evaluation and visit schedule | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Evaluation | Visit | 1 | 2 | 3 | 4 | 5 | 6 | 7-11 | 12 |
| Day | –14 | – 7 | 1 | 28 | 56 | 84 | 112 | 252 | |
| Month | 0 | 0 | 0 | 1 | 2 | 3 | 4 | 9 | |
| Informed consent | X | ||||||||
| Inclusion/exclusion criteria | X | ||||||||
| Demography/background information | X | ||||||||
| Past/current medical/surgical conditions | X | ||||||||
| Functional dyspepsia history | X | ||||||||
| Physical examination | X | X | |||||||
| Vital signs | X | X | X | X | X | X | X | ||
| Electrocardiogram (≥50 y.o.) | X | ||||||||
| Blood count and serum chemistry | X | X | X | ||||||
| Blood for polymorphisms | X | ||||||||
| H. pylori blood test | X | ||||||||
| Urine pregnancy test | Xa | Xa | |||||||
| Blood levels for study drugs | X | ||||||||
| Dispense study medication | X | X | X | ||||||
| Study medication/compliance | X | X | X | ||||||
| Symptom diaryb | X | X | X | X | X | X | |||
| FBD Severity Index (FBDSI) | X | X | X | ||||||
| Talley Bowel Disease Questionnaire© | X | X | X | ||||||
| Semi-structured interview | X | X | X | ||||||
| Gastrointestinal Symptoms Ratings Scale (GSRS) | X | X | X | X | X | X | X | ||
| Health status (physician visits, other medications) | X | X | X | X | X | X | |||
| Adverse events | X | X | X | X | X | ||||
| Global Evaluation–Adequate relief (weekly) | X | X | X | X | X | X | |||
| Clinical Global Impression (CGI) | X | X | X | X | X | X | |||
| Composite Diagnostic Interview | X | ||||||||
| Eysenck Personality Questionnaire | X | X | X | ||||||
| Profile of Moods State (POMS) | X | X | X | ||||||
| State Trait Anxiety Inventory (STAI) | X | X | X | ||||||
| Pittsburgh Sleep Quality Index (PSQI) | X | X | X | ||||||
| Eating Disorder Examination Questionnaire (EDE-Q) | X | ||||||||
| The Early Trauma Inventory Self Report–Short Form (ETISR-SF) | X | ||||||||
| Adult Trauma questions from BDQ 4 | X | ||||||||
| Hospital Anxiety Depression Scale (HADS) | X | X | X | ||||||
| Symptom Check List 90 (SCL-90) | X | X | X | ||||||
| SF-36 | X | X | X | X | X | X | |||
| Nepean Dyspepsia Index | X | X | X | X | X | X | |||
| Gastric emptying | X | X | |||||||
| Liquid nutrient test | X | X | |||||||
| Gastric accommodation sub study (SPECT) | X | X | |||||||
Women of childbearing potential.
To maximize diary compliance, study coordinators will call patients weekly with a reminder to update the daily diary.
IBS is not an exclusion criterion but all patients are required to have predominant dyspepsia (as identified on the semi-structured interview); IBS overlaps with FD in at least 30% of cases [8,52]. IBS status (by Rome III criteria) will be considered in the analyses.
2.5.3. Exclusion criteria
Reasons for exclusion of patients are significantly documented esophageal or gastric disease (e.g., esophagitis, predominant heartburn or acid regurgitation, GERD, peptic ulcer), use of non-steroidal anti-inflammatory drugs (with the exception of low dose aspirin), alcohol or drug abuse within the last year, major abdominal surgery, underlying major physical illness such as diabetes, history of clinical depression or use of antidepressants, and insufficient literacy skills in English. Patients who do not have frequent or severe symptoms as recorded on a two-week symptom diary are also excluded. Criteria are assessed through patient history, medical record review, and physical examination.
All eligible patients over age 50 have an electrocardiogram (ECG) before randomization. Those with significant arrhythmias, conduction defects or a previous myocardial infarction are excluded, as are those with a family history of long QT interval.
Helicobacter pylori is tested using a H. pylori antibody, IgG, serum test. As H. pylori is highly unlikely to contribute to symptoms in FD [53–55], this is not an exclusion, but infection status will be considered in the analyses as a possible confounder. The results will be disclosed at the end of the study to patients and the study personnel. Participants who are H. pylori-positive are treated for the infection after study participation.
2.5.4. Screening period
Patients are not allowed to take selected medications while in the trial. These include systemically acting cholinergics and anticholinergics (e.g., atropine, clidinium bromide, propantheline), prokinetics (e.g., metoclopramide), macro-lide antibiotics (e.g., erythromycin, azithromycin), aspirin (>325 mg per day), spasmolytics (e.g., dicyclomine), and antidepressants. Patients must have at least a 30-day wash out period. Patients must also be on stable doses of medication such as thyroid medication for at least 3 months prior to randomization.
Patients complete a two-week (14 day) diary and must have moderate to severe symptoms for at least four days during the two-week screening period to qualify. Since symptoms may be cyclical, patients may complete the diaries for an additional two weeks if necessary.
2.5.5. Study visit overview
The patient-related activities of the FDTT are divided into four phases: screening, randomization, treatment and follow-up. The screening phase includes two to three visits. The first visit includes the physical and surveys but may be combined with the nutrient drink test if the patient is fasting. A second visit is required for the gastric emptying with a second day needed for the gastric accommodation test at select sites. Randomization consists of one visit at which time patients receive medication and instructions. The treatment phase consists of three monthly visits over 12 weeks or 84 days. The follow-up phase consists of six visits (some of which can be done by phone) over six months. The visit and data collection schedule is summarized in Table 4 Study schedule.
2.5.6. Randomization
The FDTT uses a web-based randomization system. In order to ensure balance on a number of important covariates (e.g., gender, dyspepsia subtype, and psychiatric status), a dynamic allocation randomization method is used. Treatment assignment is determined directly as a result of the distribution of assignments given the prior patients. The treatment assignation for any patient is to the treatment group with the smallest number of patients having that unique combination of stratification factors. This method guarantees that the total number on each treatment is almost always balanced when the number of patients on study is a multiple of the number of treatments involved. This approach was described by Therneau [56], based on work of Pocock and Simon [57]. The general rule is that the number of categories of stratification factor combinations cannot exceed one-half of the treatment group sample size (i.e., n/2). The dynamic allocation procedure works by ensuring that, as accrual proceeds, no imbalance occurs along the marginal distributions of the stratification factors across treatment arms. Allocation indications are given by the relative frequency in each treatment by category combination for a given stratification factor. The first patient, and any situation where a tied situation exists, results in simple random allocation. Qualified subjects who meet entry criteria at the completion of the two-week baseline period will be assigned to one of the three treatments using the dynamic allocation randomization described above. This procedure will aim to provide treatment groups balanced on several important covariates: gender, anxiety (normal vs. present as identified by a score of 11 or higher on the Hospital Anxiety Depression Scale), dyspepsia subtype (ulcer-like vs. dysmotility-like), gastric emptying (delayed vs. non-delayed), satiety on the nutrient drink test (early vs. normal), BMI (non-obese vs. obese), and race (Caucasian vs. non-Caucasian), stratified by center (8 sites). Concealed allocation is assured by the use of a central web-based system developed at Mayo Clinic.
2.5.7. Follow-up visits
Participants return monthly while on drug to complete surveys, return diaries, and get the next month's supply of drug/placebo. One month after starting drug, blood for a drug level is taken. Results are reviewed by a qualified person in the laboratory and are reported back as normal or not. If drug levels are abnormal, the safety officer (physician) for the study is notified and follows up with the study team as he/she deems necessary. At the end of the 12 weeks of drug therapy, participants return to complete physiological testing, surveys, and a physical exam. After drug treatment, participants are followed through monthly telephone calls and complete surveys approximately six months after treatment has ended.
2.5.8. Standardized questionnaires
In addition to the primary outcome measure, subjects complete a set of measures to assess global improvement, symptom severity, quality of life, anxiety, depression (Hospital Anxiety Depression Screen score of ≥11is used to screen for depression), sleep, physiological measures and genotyping, as summarized in Table 5. Experience with all of these assessments by the team has shown that respondent burden has not been an issue in this trial.
Table 5.
Study questionnaires.
| Global evaluation | |
| Global Evaluation–Adequate relief (weekly) | Single question of adequate symptom relief [39,40] |
| Clinical Global Impression (CGI) | Global measure of treatment improvement or deterioration in antidepressant trials [58] |
| Symptoms and diagnostic evaluation | |
| Talley Bowel Disease Questionnaire© | Based on Rome II criteria [59] |
| Semi-structured interview | Determines symptom subgroup according to Rome II criteria [9,30] |
| Symptom diary | Record of symptoms [30,60] |
| Gastrointestinal Symptoms Rating Scale (GSRS) | Uses 7 graded Likert scales to assess dyspepsia and IBS symptoms [32,33] |
| Side effect (adverse events) | Side effects and adverse events |
| Health status (physician visits, other medications) | Number of physician visits and new medications |
| FBD Severity Index (FBDSI) | Quantifies disorder severity over the last 6 months [61] |
| Quality of life | |
| Nepean Dyspepsia Index | Measures impact of functional dyspepsia on 5 dimensions of health [32,62,63] |
| Medical Outcomes Study Short Form 36 (SF-36) | 36 item questionnaire with 8 subscales based on multidimensional model of health [64,65] |
| Composite Diagnostic Interview | Medical interview by physician |
| Psychological measures and psychiatric diagnoses | |
| Symptom Check List 90 (SCL-90) | Questions use a 5-point Likert scale. There are 9 scales; somatization, obsessive-compulsive, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychoticism [66] |
| Somatic Symptom Checklist (SSC) | Measures frequency and bothersomeness of 12 non-gastrointestinal symptoms [67] |
| Hospital Anxiety Depression Scale (HADS) | 14 item scale recommended for detecting mild mood disorders [68,69] |
| Profile of Moods State (POMS) | 65 item survey with 6 subscales [70] |
| State Trait Anxiety Inventory (STAI) | A self-report inventory of baseline and situational anxiety measuring both positive and negative emotional states [71] |
| Pittsburgh Sleep Quality Index (PSQI) | 19 self-rated questions with 7 component scores [72] |
| Eating Disorder Examination Questionnaire (EDE-Q) | Self-administered to assess for binge eating [73] |
| The Early Trauma Inventory Self Report–Short Form (ETISR-SF) | A 29 item scale that has been reported to be a valid and reliable measure of childhood trauma [74] |
| Adult Trauma questions from BDQ4 | Abuse questionnaire [75,76] |
2.5.9. Physiologic and genetic measurements
Genetic analysis for detection of polymorphisms will use a functional candidate gene approach. We considered two gene disorders to be potentially important in treatment response in FD as they may affect gut function and are linked to psychiatric co-morbidity: 5HTTLPR and GNβ3.
2.5.9.1. Genetic analysis
Approximately 15 ml of blood is drawn by a trained phlebotomist and collected into a standard tube with ethylenediaminestetraacetic acid (EDTA) at baseline. Fresh whole blood will be extracted using Pure-gene™ Reagents from the Gentra Corporation using either manual methodology or the Gentra Autopure™ automated DNA extractor. When possible, we will also prepare a blood spot card from leftover whole blood. After extraction, all DNA samples will be tested by spectrophotometry using the SPECTRAmax PLUS 384 spectrophotometer from Molecular Devices. All samples will be diluted to a standard concentration of 0.25 μg/μl. Molecular analysis will be performed using previously described primers specific for 5-HTTLPR (5HTT) and C825T (GNβ3) polymorphisms. The 5-HTTLPR polymorphism will be typed by polymerase chain reaction (PCR) using flanking primers (forward 5′-GGCGTTGCCGC TCTGAATGC-3′ and reverse 5′-GAGGGACTGAGCTGGACAAC CAC-3′) (78). The C825T polymorphism will be typed using the forward primer, 5′TGACCCACTTGCCACCCGTGC-3′ and 5′-GCAGCAGCCAGGGCTGGC-3′ (143). PCR will be performed by the Mayo Clinic Cancer Center Microarray and Molecular Epidemiology Shared Resources core facility using Applied Biosystems (TaqMan®) technologies. All patients recruited at baseline will have DNA extracted if they agree in order to assess the generalizability of the trial participants, but patients will not be excluded if they do not wish to undertake this part. (Refusal rates have been very low based on our current experience.)
2.5.9.2. Safety data.
A complete blood count and chemistry panel is collected at baseline, 4 and 12 weeks for safety monitoring, and processed centrally at Mayo Medical laboratories. Pulse and blood pressure are measured at each monthly visit. Data on adverse events and serious adverse events are collected, as is standard.
2.5.10. Specimen banking
Blood specimens are collected and stored in a central repository for use as approved by the Steering Committee of the FDTT. Blood is collected at screening, randomization, and completion of drug.
3. Results
Enrollment in the FDTT began in early 2006 and will continue through 2012. To date (1/1/2011), 3770 patients have been contacted, 211 patients have been screened and of those, 175 have been randomized. The most frequent reasons for ineligibility were a lack of adequate symptom severity while approximately 10–12% have been excluded because of pre-existing antidepressant use.
3.1. Summary
The FDTT is an international multicenter, randomized, placebo-controlled therapeutic study of two selected antidepressants in FD in adults.
4. Writing committee
Members consist of Nicholas J. Talley, G. Richard Locke, III, Alan R. Zinsmeister, Linda M. Herrick, Vickie M. Silvernail, Charlene Prather, Brian E. Lacy, John K. DiBaise, Ernest P. Bouras, Hashem El-Serag, Bincy Abraham, and Paul Moyeddi. These authors take full responsibility for the contents of this manuscript.
Acknowledgments
The FDTT is supported by the National Institute for Diabetes and Digestive and Kidney Disease (DK065713). Additionally, Forrest Pharmaceuticals provided escitalopram and placebo for the study.
Footnotes
The following members have been instrumental in the design and conduct of the FDTT:
Baylor College of Medicine, Houston, TX: Bincy Abraham, M.D., M.S., Hashem El-Serag, M.D., M.P.H., Stephanie Fitzgerald
Dartmouth-Hitchcock Medical Center, Lebanon, NH: Brian E. Lacy, M.D., Ph.D., Rebecca Husband
Northwestern University, Chicago, IL: Michael P. Jones, M.D.; Colin W. Howden, M.D.; Darren Brenner, M.D.; Laura Yun, M.D.; Jason Bratten.
Saint Louis University, Saint Louis, MO: Charlene Prather, M.D. M.P.H.; Debra L. King, R.N.
Mayo Clinic, Rochester, MN: Nicholas J. Talley, M.D., Ph.D.; G. Richard Locke III, M.D.; Alan Zinsmeister, Ph.D., Linda M. Herrick, Ph.D., R.N., Vickie M. Silvernail, L.P.N., CCRP; Lawrence A. Szarka, M.D., Annie Almazar
Mayo Clinic Florida, Jacksonville, FL: Ernest P. Bouras, M.D., Verna Skinner
Mayo Clinic Arizona, Scottsdale AZ: John K. DiBaise, M.D., M. Machiko (Tina) Anderson, RN
McMaster University Medical Centre, Hamilton, Ontario, Canada, Paul Moayyedi, M.D.; Melanie Wolfe
National Institutes of Diabetes, Digestive and Kidney Diseases, Bethesda, MD: Patricia R. Robuck, Ph.D., M.P.H., Rebekah Van Raaphorst, Rebecca Torrance.
References
- 1.Talley NJ, Zinsmeister AR, Schleck CD, Melton LJ., III Dyspepsia and dyspepsia subgroups: a population-based study. Gastroenterology. 1992;102:1259–68. [PubMed] [Google Scholar]
- 2.Locke GR. The epidemiology of functional gastrointestinal disorders in North America. Gastroenterol Clin North Am. 1996;25:1–19. doi: 10.1016/s0889-8553(05)70362-9. [DOI] [PubMed] [Google Scholar]
- 3.Koloski NA, Talley NJ, Boyce PM. Epidemiology and health care seeking in the functional GI disorders: a population-based study. Am J Gastroenterol. 2002;97:2290–9. doi: 10.1111/j.1572-0241.2002.05783.x. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Talley NJ. Systematic review: the prevalence and clinical course of functional dyspepsia. Aliment Pharmacol Ther. 2004;19:643–54. doi: 10.1111/j.1365-2036.2004.01897.x. [DOI] [PubMed] [Google Scholar]
- 5.Koloski N, Talley N, Boyce P. Predictors of health care seeking for irritable bowel syndrome and nonulcer dyspepsia: a critical review of the literature on symptom and psychosocial factors. Am J Gastroenterol. 2001;96:1340–9. doi: 10.1111/j.1572-0241.2001.03789.x. [DOI] [PubMed] [Google Scholar]
- 6.Sandler R, Everhart J, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–11. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 7.Agreus L, Borgquist L. The cost of gastro-oesophageal reflux disease, dyspepsia and peptic ulcer disease in Sweden. Pharmacoeconomics. 2002;20:347–55. doi: 10.2165/00019053-200220050-00006. [DOI] [PubMed] [Google Scholar]
- 8.Talley NJ, Silverstein MD, Agréus L, et al. AGA technical review: evaluation of dyspepsia. Gastroenterology. 1998;114:582–95. doi: 10.1016/s0016-5085(98)70542-6. [DOI] [PubMed] [Google Scholar]
- 9.Talley NJ, Stanghellini V, Heading R. Functional gastroduodenal disorders: a working team report for the Rome II consensus on functional gastrointestinal disorders. Gut. 1999;45(Suppl 2):II37–42. doi: 10.1136/gut.45.2008.ii37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delaney B, Moayyedi P, Forman D. Initial management strategies for dyspepsia. Cochrane Database Syst Rev. 2003:2. doi: 10.1002/14651858.CD001961. [DOI] [PubMed] [Google Scholar]
- 11.Veldhuyzen van Zanten SJ, Jones MJ, Verlinden M, Talley NJ. Efficacy of cisapride and domperidone in functional (nonulcer) dyspepsia: a meta-analysis. Am J Gastroenterol. 2001;96:689–96. doi: 10.1111/j.1572-0241.2001.03521.x. [DOI] [PubMed] [Google Scholar]
- 12.Veldhuyzen van Zanten SJ, Cleary C, Talley NJ. Drug treatment of functional dyspepsia: a systematic analysis of trial methodology with recommendations for design of future trials. Am J Gastroenterol. 1996;91:660–73. [PubMed] [Google Scholar]
- 13.Mishra DN, Shukla GD, Agarwal AK, Saxena HN, Jain PK. Non-organic dyspepsia: a controlled clinico-psychiatric study. J Assoc Physicians India. 1984;32:399–402. [PubMed] [Google Scholar]
- 14.Magni G, di Mario F, Bernasconi G, Mastropaolo G. DSM-III diagnoses associated with dyspepsia of unknown cause. Am J Psychiatry. 1987;144:1222–3. doi: 10.1176/ajp.144.9.1222. [DOI] [PubMed] [Google Scholar]
- 15.Haug TT, Wilhelmsen I, Ursin H, Berstad A. What are the real problems for patients with functional dyspepsia? Scand J Gastroenterol. 1995;30:97–100. doi: 10.3109/00365529509093244. [DOI] [PubMed] [Google Scholar]
- 16.Haug TT, Svebak S, Wilhelmsen I, Berstad A, Ursin H. Psychological factors and somatic symptoms in functional dyspepsia. A comparison with duodenal ulcer and healthy controls. J Psychosom Res. 1994;38:281–91. doi: 10.1016/0022-3999(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 17.Quartero AO, Post MW, Numans ME, de Melker RA, de Wit NJ. What makes the dyspeptic patient feel ill? A cross sectional survey of functional health status, Helicobacter pylori infection, and psychological distress in dyspeptic patients in general practice. Gut. 1999;45:15–9. doi: 10.1136/gut.45.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talley NJ, Boyce P, Jones M. Dyspepsia and health care seeking in a community: how important are psychological factors? Dig Dis Sci. 1998;43:1016–22. doi: 10.1023/a:1018878717715. [DOI] [PubMed] [Google Scholar]
- 19.Colgan S, Creed F, Klass H. Symptom complaints, psychiatric disorder and abnormal illness behaviour in patients with upper abdominal pain. Psychol Med. 1988;18:887–92. doi: 10.1017/s003329170000982x. [DOI] [PubMed] [Google Scholar]
- 20.Porcelli P, Taylor GJ, Bagby RM, De Carne M. Alexithymia and functional gastrointestinal disorders. A comparison with inflammatory bowel disease. Psychother Psychosom. 1999;68:263–9. doi: 10.1159/000012342. [DOI] [PubMed] [Google Scholar]
- 21.Talley NJ, Phillips SF, Bruce B, et al. Relation among personality and symptoms in non-ulcer dyspepsia and the irritable bowel syndrome. Gastroenterology. 1990;99:327–33. doi: 10.1016/0016-5085(90)91012-u. [DOI] [PubMed] [Google Scholar]
- 22.Lynch ME. Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci. 2001;26:30–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Mertz H, Morgan V, Tanner G, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118:842–8. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- 24.Clouse RE. Antidepressants for functional gastrointestinal syndromes. Dig Dis Sci. 1994;39:2352–63. doi: 10.1007/BF02087651. [DOI] [PubMed] [Google Scholar]
- 25.Clouse RE, Lustman PJ, Geisman RA, Alpers DH. Antidepressant therapy in 138 patients with irritable bowel syndrome: a five-year clinical experience. Aliment Pharmacol Ther. 1994;8:409–16. doi: 10.1111/j.1365-2036.1994.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 26.Prakash C, Lustman PJ, Freedland KE, Clouse RE. Tricyclic antidepressants for functional nausea and vomiting: clinical outcome in 37 patients. Dig Dis Sci. 1998;43:1951–6. doi: 10.1023/a:1018878324327. [DOI] [PubMed] [Google Scholar]
- 27.Jackson JL, O'Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108:65–72. doi: 10.1016/s0002-9343(99)00299-5. [DOI] [PubMed] [Google Scholar]
- 28.Gorelick AB, Koshy SS, Hooper FG. Differential effects of amitriptyline on perception of somatic and visceral stimulation in healthy humans. Am J Physiol. 1998:G460–6. doi: 10.1152/ajpgi.1998.275.3.G460. [DOI] [PubMed] [Google Scholar]
- 29.Drossman D, Toner BB, Whitehead WE, et al. Cognitive–behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 30.Talley NJ, Meineche-Schmidt V, Pare P, et al. Efficacy of omeprazole in functional dyspepsia: double-blind, randomized, placebo-controlled trials (the Bond and Opera studies). Aliment Pharmacol Ther. 1998;12:1055–65. doi: 10.1046/j.1365-2036.1998.00410.x. [DOI] [PubMed] [Google Scholar]
- 31.Junghard O, Lauritsen K, Talley NJ, Wiklund IK. Validation of seven graded diary cards for severity of dyspeptic symptoms in patients with non ulcer dyspepsia. Eur J Surg Suppl. 1998;(583):106–11. doi: 10.1080/11024159850191355. [DOI] [PubMed] [Google Scholar]
- 32.Talley NJ, Verlinden M, Jones M. Validity of a new quality of life scale for functional dyspepsia: a United States multicenter trial of the Nepean Dyspepsia Index. Am J Gastroenterol. 1999;94:2390–7. doi: 10.1111/j.1572-0241.1999.01363.x. [DOI] [PubMed] [Google Scholar]
- 33.Wiklund IK, Junghard O, Grace E. Quality of life in reflux and dyspepsia patients. Psychometric documentation of a new disease-specific questionnaire (QOLRAD). Eur J Surg Suppl. 1998;(583):41–9. [PubMed] [Google Scholar]
- 34.Veldhuyzen van Zanten SJ, Talley NJ, Bytzer P. Design of treatment trials for functional gastrointestinal disorders. Gut. 1999;45(Suppl 2):II69–77. doi: 10.1136/gut.45.2008.ii69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ford AC, Talley NJ, Schoenfeld PS, Quigley EMM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58:367–78. doi: 10.1136/gut.2008.163162. [DOI] [PubMed] [Google Scholar]
- 36.Choung RS, Cremonini F, Thapa P, Zinsmeister AR, Talley NJ. The effect of short-term, low-dose tricyclic and tetracyclic antidepressant treatment on satiation, postnutrient load gastrointestinal symptoms and gastric emptying: a double-blind, randomized, placebo-controlled trial. Neurogastroenterol Motil. 2008;20:220–7. doi: 10.1111/j.1365-2982.2007.01029.x. [DOI] [PubMed] [Google Scholar]
- 37.Holtmann G, Gschossmann J, Mayr P, Talley NJ. A randomised placebo-controlled trial of simethicone and cisapride for the treatment of patients with functional dyspepsia. Aliment Pharmacol Ther. 2002;16:1641–8. doi: 10.1046/j.1365-2036.2002.01322.x. [DOI] [PubMed] [Google Scholar]
- 38.Moher D, Schulz KF, Altman DG, CONSORT GROUP The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134:657–62. doi: 10.7326/0003-4819-134-8-200104170-00011. [DOI] [PubMed] [Google Scholar]
- 39.Tack J, Delia T, Ligizio G. A phase II placebo controlled randomized trial with tegaserod in functional dyspepsia patients with normal gastric emptying. Gastroenterology. 2002;122(Suppl 1):A20. [Google Scholar]
- 40.Talley NJ, Van Zanten SV, Saez LR, et al. A dose-ranging, placebo-controlled, randomized trial of alosetron in patients with functional dyspepsia. Aliment Pharmacol Ther. 2001;15:525–37. doi: 10.1046/j.1365-2036.2001.00941.x. [DOI] [PubMed] [Google Scholar]
- 41.Fawcett J, Epstein P, Fiester SJ, Elkin I, Autry JH. Clinical management—imipramine/placebo administration manual. NIMH Treatment of Depression Collaborative Research Program. Psychopharmacol Bull. 1987;23:309–24. [PubMed] [Google Scholar]
- 42.Camilleri M, Chey WY, Mayer EA, et al. A randomized controlled clinical trial of the serotonin type 3 receptor antagonist alosetron in women with diarrhea-predominant irritable bowel syndrome. Arch Intern Med. 2001;161:1733–40. doi: 10.1001/archinte.161.14.1733. [DOI] [PubMed] [Google Scholar]
- 43.Lembo A, Weber HC, Farraye FA. Alosetron in irritable bowel syndrome: strategies for its use in a common gastrointestinal disorder. Drugs. 2003;63:1895–905. doi: 10.2165/00003495-200363180-00002. [DOI] [PubMed] [Google Scholar]
- 44.Tougas G, Eaker EY, Abell T, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456–62. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 45.Degen LP, Phillips SF. Variability of gastrointestinal transit in healthy women and men. Gut. 1996;39:299–305. doi: 10.1136/gut.39.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chial HJ, Camilleri C, Delgado-Aros S, et al. A nutrient drink test to assess maximum tolerated volume and postprandial symptoms: effects of gender, body mass index and age in health. Neurogastroenterol Motil. 2002;14:249–53. doi: 10.1046/j.1365-2982.2002.00326.x. [DOI] [PubMed] [Google Scholar]
- 47.Liau SS, Camilleri M, Kim DY, et al. Pharmacological modulation of human gastric volumes demonstrated noninvasively using SPECT imaging. Neurogastroenterol Motil. 2001;13:533–42. doi: 10.1046/j.1365-2982.2001.00287.x. [DOI] [PubMed] [Google Scholar]
- 48.Kim DY, Delgado-Aros S, Camilleri M, et al. Noninvasive measurement of gastric accommodation in patients with idiopathic nonulcer dyspepsia. Am J Gastroenterol. 2001;96:3099–105. doi: 10.1111/j.1572-0241.2001.05264.x. [DOI] [PubMed] [Google Scholar]
- 49.Holtmann G, Grote E, Braun-Lang U. G-protein β 3 subunit (GNβ3) 825 CC genotype and the manifestation of functional gastrointestinal disorders. Gastroenterology. 2004;126(Suppl 2):A–162. [Google Scholar]
- 50.Holtmann G, Siffert W, Haag S, et al. G-protein beta 3 subunit 825 CC genotype is associated with unexplained (functional) dyspepsia. Gastroenterology. 2004;126:971–9. doi: 10.1053/j.gastro.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Camilleri M, Atanasova E, Carlson PJ, et al. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425–32. doi: 10.1053/gast.2002.34780. [DOI] [PubMed] [Google Scholar]
- 52.Talley NJ, Stanghellini V, Heading RC, et al. Functional gastroduodenal disorders. In: Drossman DA, editor. Rome II: the functional gastrointestinal disorders. Degnon; McLean, VA: 2000. pp. 299–350. [Google Scholar]
- 53.Talley NJ, Vakil N, Ballard ED, Fennerty MB. Absence of benefit of eradicating Helicobacter pylori in patients with nonulcer dyspepsia. N Engl J Med. 1999;341:1106–11. doi: 10.1056/NEJM199910073411502. [DOI] [PubMed] [Google Scholar]
- 54.Blum AL, Talley NJ, O'Morain C, et al. Lack of effect of treating Helicobacter pylori infection in patients with nonulcer dyspepsia. N Engl J Med. 1998;339:1875–81. doi: 10.1056/NEJM199812243392602. [DOI] [PubMed] [Google Scholar]
- 55.Talley NJ, Janssens J, Lauritsen K, Racz I, Bolling-Sternevald E. Eradication of Helicobacter pylori in functional dyspepsia: randomised double blind placebo controlled trial with 12 months’ follow up. The Optimal Regimen Cures Helicobacter Induced Dyspepsia (ORCHID) Study Group. BMJ. 1999;318:833–7. doi: 10.1136/bmj.318.7187.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Therneau T. How many stratification factors are “too many” to use in a randomization plan? Control Clin Trials. 1993;14:98–108. doi: 10.1016/0197-2456(93)90013-4. [DOI] [PubMed] [Google Scholar]
- 57.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–15. [PubMed] [Google Scholar]
- 58.Guy W. ECDEU assessment manual for psychopharmacology publication ADM 76–338. U. S. Department of Health Education and Welfare; Washington, DC: 1976. pp. 217–22. [Google Scholar]
- 59.Talley NJ, Phillips SF, Melton J, III, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–4. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 60.Talley NJ, Verlinden M, Snape WJ, et al. Failure of a motilin receptor agonist (ABT-229) to relieve the symptoms of functional dyspepsia in patients with and without delayed gastric emptying: a randomized double-blind placebo-controlled trial. Aliment Pharmacol Ther. 2000;14:1653–61. doi: 10.1046/j.1365-2036.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- 61.Drossman DA, Li Z, Toner BB, et al. Functional bowel disorders. A multicenter comparison of health status and development of illness severity index. Dig Dis Sci. 1995;40:986–95. doi: 10.1007/BF02064187. [DOI] [PubMed] [Google Scholar]
- 62.Talley NJ, Haque M, Wyeth JW, et al. Development of a new dyspepsia impact scale: the Nepean Dyspepsia Index. Aliment Pharmacol Ther. 1999;13:225–35. doi: 10.1046/j.1365-2036.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- 63.Talley NJ, Verlinden M, Jones M. Quality of life in functional dyspepsia: responsiveness of the Nepean Dyspepsia Index and development of a new 10-item short form. Aliment Pharmacol Ther. 2001;15:207–16. doi: 10.1046/j.1365-2036.2001.00900.x. [DOI] [PubMed] [Google Scholar]
- 64.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 65.Bensoussan A, Chang SW, Menzies RG, Talley NJ. Application of the general health status questionnaire SF36 to patients with gastrointestinal dysfunction: initial validation and validation as a measure of change. Aust N Z J Public Health. 2001;25:71–7. doi: 10.1111/j.1467-842x.2001.tb00554.x. [DOI] [PubMed] [Google Scholar]
- 66.Locke GR, III, Weaver AL, Melton LJ, III, Talley NJ. Psychosocial factors are linked to functional gastrointestinal disorders: a population based nested case–control study. Am J Gastroenterol. 2004;99:350–7. doi: 10.1111/j.1572-0241.2004.04043.x. [DOI] [PubMed] [Google Scholar]
- 67.Kalantar JS, Locke GR, III, Zinsmeister AR, Beighley CM, Talley NJ. Familial aggregation of irritable bowel syndrome: a prospective study. Gut. 2003;52:1703–7. doi: 10.1136/gut.52.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 69.Andrews H, Barczak P, Allan RN. Psychiatric illness in patients with inflammatory bowel disease. Gut. 1987;28:1600–4. doi: 10.1136/gut.28.12.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McNair DM, Loor M, Droppleman LF. Profile of Mood States Manual. Multi Health Systems; New York: 2003. [Google Scholar]
- 71.Speilberger CD, Gorusch RI, Luschene RE. STAI Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- 72.Welstein L, Dement DC, Redington D, Guillemnault C. Sleep/wake disorders: natural history, epidemiology, and long-term evolution. Raven Press; New York: 1983. Insomnia in the San Francisco Bay area: a telephone survey. pp. 73–85. [Google Scholar]
- 73.Mond JM, Hay PJ, Rodgers B, Owen C, Beumont PJV. Temporal stability of the Eating Disorder Examination Questionnaire. Int J Eat Disord. 2004;36:195–203. doi: 10.1002/eat.20017. [DOI] [PubMed] [Google Scholar]
- 74.Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. J Nerv Ment Dis. 2007;195:211–8. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drossman DA, Leserman J, Nachman G, et al. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med. 1990;113:828–33. doi: 10.7326/0003-4819-113-11-828. [DOI] [PubMed] [Google Scholar]
- 76.Drossman DA, Talley NJ, Leserman J, Olden KW, Barreiro MA. Sexual and physical abuse and gastrointestinal illness. Review and recommendations. Ann Intern Med. 1995;123:782–94. doi: 10.7326/0003-4819-123-10-199511150-00007. [DOI] [PubMed] [Google Scholar]