Abstract
Anemia is now recognized as a risk factor for a number of adverse outcomes in the elderly, including hospitalization, morbidity, and mortality. What constitutes appropriate evaluation and management for an elderly patient with anemia, and when to initiate a referral to a hematologist, are significant issues. Attempts to identify suggested hemoglobin levels for blood transfusion therapy have been confounded for elderly patients with their co-morbidities. Since no specific recommended hemoglobin threshold has stood the test of time, prudent transfusion practices to maintain hemoglobin thresholds of 9–10 g/dl in the elderly are indicated, unless or until evidence emerges to indicate otherwise.
Keywords: Anemia, Elderly, Blood Transfusion
INTRODUCTION
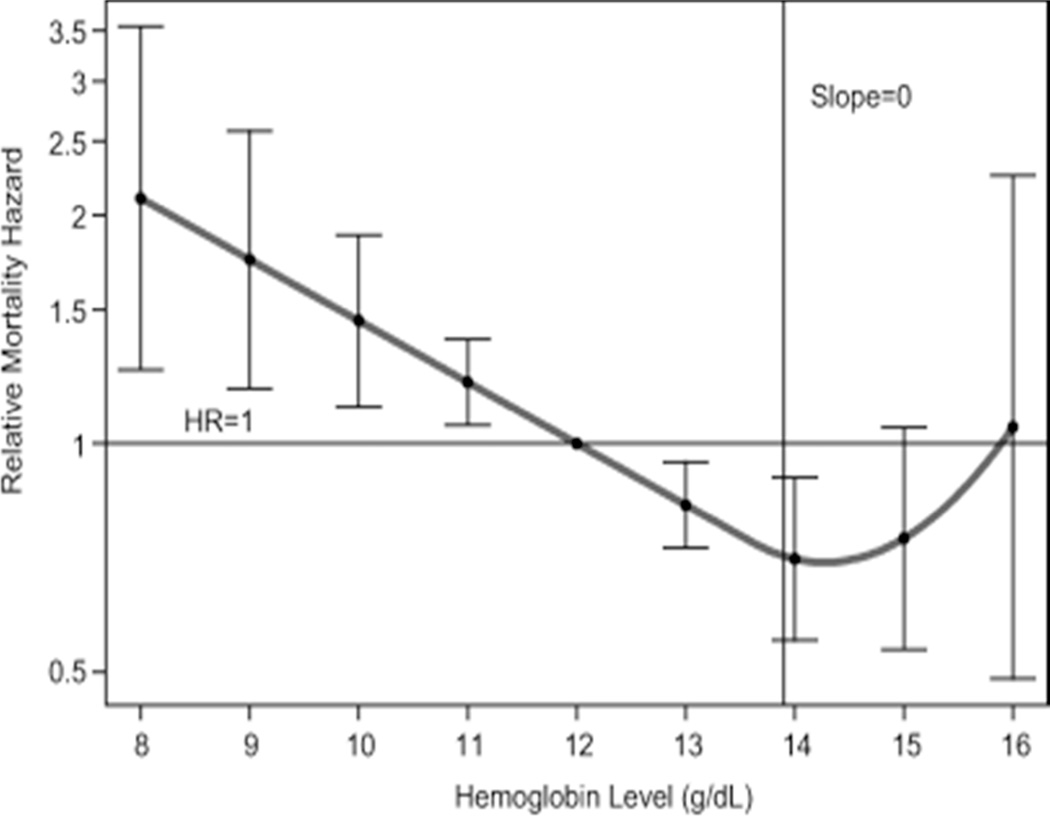
Anemia is now recognized as a risk factor for a number of adverse outcomes in older adults, including hospitalization, morbidity, and mortality.1–7 The elderly is an important demographic population that is growing rapidlyi in the context of increasing prevalence of anemia with age.8 An analysis of two databases (NHANES-III, Third U.S. National Health and Nutrition Examination Survey; and SCRIPPS-Kaiser Data, 1998–2002) found that normal ranges for hemoglobin values are unchanged for aging populations, with the exceptions of minor adjustments for males (Table 1).9 More than 10% of community-dwelling adult ≥ 65 years of age have a World Health Organization (WHO) – defined anemia (hemoglobin < 12 g/dL in women and < 13 g/dL in men). After 50 years of age, prevalence of anemia increases with advancing age and exceeds 20% in those ≥ 85 years of age.10 As illustrated in Figure 1,11 there is a Jshaped correlation of anemia with mortality in older men and women. A recent study has analyzed the impact of declines (rather than an absolute level) in hemoglobins in 3,759 nonanemic elderly participants from the Cardiovascular Health Study,12 a prospective randomized cohort of community-dwelling elderly patients ≥ 65 years of age followed for up to 16 years. The authors found that hemoglobin decreases identified a large group of elderly individuals at risk for subsequent adverse outcomes (worse cognitive function) who would not be identified using the World Health Organization (WHO) anemia criteria.
Table 1.
Lower limits of normal for hemoglobin concentration for white and black adults
| Group | Hemoglobin, g/dL |
|---|---|
| White men, y | |
| 20–59 | 13.7 |
| 60+ | 13.2 |
| White women, y | |
| 20–49 | 12.2 |
| 50+ | 12.2 |
| Black men, y | |
| 20–59 | 12.9 |
| 60+ | 12.7 |
| Black women, y | |
| 20–49 | 11.5 |
| 50+ | 11.5 |
From Beutler E, et al.9
Figure 1.
Relationship between hemoglobin (Hb) concentration and 5-year all-cause mortality in community-dwelling, disabled older women. Graphical display of relative risk estimates for the mortality linked to specific Hb concentrations compared with the risk linked to Hb of 12 g/dL. The curve represents smoothed relative mortality hazards across Hb concentrations, and the bars indicate their 95% confidence intervals. Indicated also is the 13.9 g/dL Hb threshold at which the slope of mortality risk decline was no longer statistically significant (i.e., the 95% confidence interval for the slope of the tangent included 0). The y-axis was transformed so that, for example, the graphical display of an increase in risk of the magnitude of two (hazard ratio (HR)52) would be equivalent to that of a decrease in risk of the same magnitude (HR50.5) in terms of scale size.
Reproduced, From Chaves PH, et al.11
With increasing recognition of the importance of anemia in the general population, guidelines have been published for the detection, evaluation, and management of anemia in medical13 and surgical14 patients. However for elderly patients, attempts to identify suggested hemoglobin levels for management of anemia, including blood transfusion therapy, have been confounded by increased risks from anemia, along with additional co-morbidities. What constitutes an appropriate work-up for an elderly patient with anemia; and when to refer the patient to a hematologist, given the potentially large number of subjects involved, are significant costs-benefit issues.15 In this review, we summarize our approach for management of anemia in the elderly, with a focus on transfusion therapy.
CHARACTERIZATION OF ANEMIA IN THE ELDERLY
An important contribution was made by the NHANES III investigators who did a laboratory evaluation of over 5000 community dwelling elderly subjects, 10% of whom had anemia according to the WHO criteria. For the most part the anemia is mild, with hemoglobin levels infrequently less than 10 g/dL.8 Nevertheless, this mild anemia has been associated with significant negative outcomes, including decreased physical performance,16 increased number of falls,17 increased frailty,18 decreased cognition,18 increased dementia,19 increased hospitalization,1 and increased mortality.7 The NHANES III investigators used fixed laboratory measures to determine that about one third of these anemic patients have evidence of a nutritional deficiency, primarily that of iron; one third have chronic inflammation or chronic kidney disease (CKD); and one third have unexplained anemia.8
Unexplained anemia of the elderly (UAE) is a real entity characterized by a hypoproliferative normocytic anemia that is not due to nutritional deficiency, CKD or inflammatory disease; and in which the erythropoietin response to anemia appears to be blunted. In a study of 124 anemic elderly (≥65 years) persons, 42 (37%) had UAE.20 These patients had significantly lower C-reactive protein (CRP) levels than non-anemic controls. Additionally, hepcidin levels do not seem to increase with age in the general population. Hepcidin levels in the anemia of aging change with comorbid conditions (low in iron-deficiency anemia, higher in inflammatory conditions); however in patients with UAE, who have no identifiable comorbidities, hepcidin levels remain in the normal range.20–22 These observations could be because UAE is heterogeneous, in which diverse underlying causes such as impaired erythropoietin response to anemia and or an underlying stem cell disorder, may confound an effect from hepcidin.21 The role of testosterone deficiency in males is currently being studied by an NIA (National Institute of Aging) funded consortium.
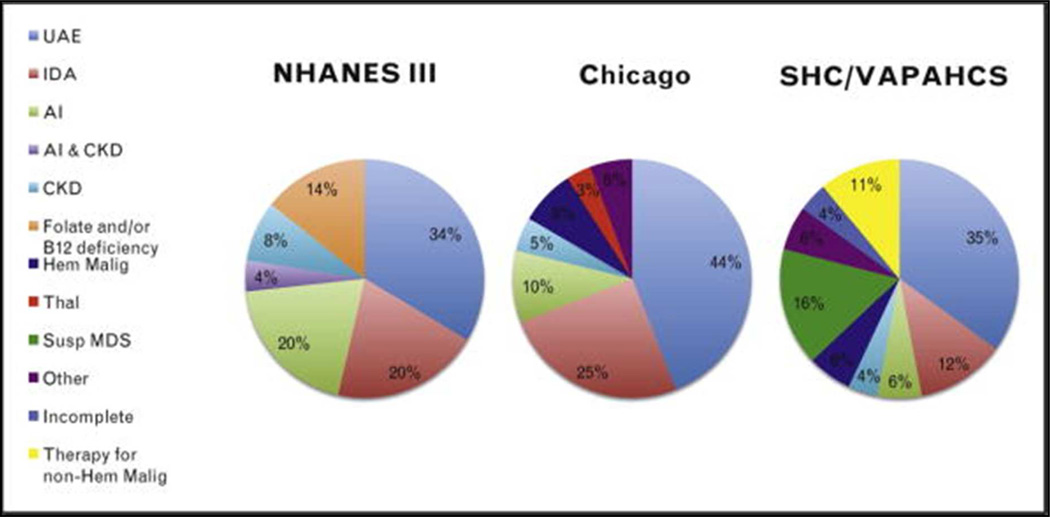
Typically, in persons 65 years of age or older there is an underlying etiology for the anemia such as chronic disease, iron deficiency, or myelodysplastic syndromes that can be identified by further investigation.23 In a study of 232 patients aged 65 to 98 (median 81) years of age, 24% were found to anemic.24 Of these, after a comprehensive workup 17% did not have an identifiable underlying cause. The major causes of anemia and their prevalence in the elderly are illustrated in Figure 2:25 prevalence ranges from three studies8,26,27 are for UAE (34–44%); iron deficiency (12–25%); CKD (4–8%); MDS (9–16%); malignant hematologic (e.g., chronic lymphocytic leukemia) disorder (2%); or inflammation (6–20%). Interestingly, folic acid deficiency has disappeared in the U.S. population, probably as a result of fortification of flour.26,27 While 10–20% of elderly patients have been described as Vitamin B12 deficient (defined by reduced serum levels of Vitamin B12),28,29 clinically significant Vitamin B12 deficiency is uncommon, diagnosed in only 1/19026 and 1/174 subjects27 in two studies, respectively. For emphasis that means that as a cause of macrocytosis, Vitamin B12 deficiency is much less common than MDS or ethanol abuse.
Figure 2. Prevalence of Anemia in the Elderly.
Prevalence of anemia in the elderly by cause identified in three studies. Studies shown are National Health and Nutrition Survey (NHANES) III,8 Chicago,27 and Stanford Hospital & Clinics, VA Palo Alto Health Care System (SHC/VAPAHCS).26 AI, anemia of inflammation; CKD, anemia secondary to renal disease; Hem Malig, hematologic malignancy; IDA, iron-deficiency anemia; Susp MDS, suspicious for myelodysplastic syndrome; Thal, thalassemia; UAE, unexplained anemia of the elderly.
Reproduced, From Pang, Schrier.25
Studies at two academic institutions using comprehensive clinical and hematologic analyses have refined the prevalence and causes of anemia in the elderly.26,27 Iron deficiency anemia represented the most common cause of anemia in the elderly, at 25.3%.27 Iron deficiency may be due to nutritional deficiency or blood loss; in the relatively affluent Western world, blood loss is the major issue. The cause of blood loss must be identified since in this patient group, since iron deficiency could be a sign of a serious disorder such as colon cancer.30–33 Furthermore, in this patient group one cannot use simple laboratory tests based on transferrin saturation and ferritin levels to readily differentiate between iron deficiency and inflammation as a cause of anemia.
A number of studies have demonstrated that IL-6 levels increase with aging, and are correlated with the development of anemia in the elderly,23 including healthy women,34 the Framingham Heart Study,35 and the EPESE (Established Populations for Epidemiologic Studies of the Elderly.36 More recently, studies of IL-6 and hepcidin levels have not been found these to be elevated in elderly patients who do not have comorbid diseases.20–22 There may be several reasons for these discrepant results. First, if aging is associated with only small increases in IL-6, one would a need large sample size for detection. Additionally, currently these assays measure the monomeric form of IL-6, which also exists as a multimer complicating the analysis.
EVALUATION OF ANEMIA
Commonly identified when the elderly are scheduled for elective surgical procedures. Anemia is a common condition in surgical patients and is independently associated with increased perioperative mortality.37 When pre-admission testing prior to elective surgery reveals anemia, it should therefore be viewed as a significant and treatable medical condition, rather than as simply an abnormal laboratory value.38 The diagnosis of an unexpected anemia in patients scheduled for elective surgery in which significant blood loss is anticipated, should be considered an indication for rescheduling elective surgery until evaluation and management of anemia is accomplished.
In traditionally taught approaches to evaluating a patient with anemia, the mean corpuscular volume (MCV) typically has been used as a starting indice,39 followed by biochemical analysis. The MCV has been shown to add value to the RDW (red cell distribution width) for evaluation of macrocytosis.40 But for microcytic anemias, MCV is of less value, particularly for patients with iron deficiency who have comorbidities. Twenty-two percent of elderly patients can be identified as having iron deficiency anemia by their response to a course of ORAL iron therapy, despite not having the typical laboratory findings of transferrin saturation less than 16% and ferritin less than 30 ng/mL.25 When transferrin saturation is low (<16%) and the ferritin level is high (>200ng/ml), the diagnosis of anemia of inflammation is generally considered.41 However, the MCV is normal in 70% of patients with anemia of inflammation,42 despite limited iron delivery to red cell precursors as indicated by the low transferrin saturation. This overlap of these two common causalities of anemia (iron deficiency and inflammation) has made the use of the traditional markers: MCV, transferrin saturation, and ferritin, difficult to interpret in routine practice.43
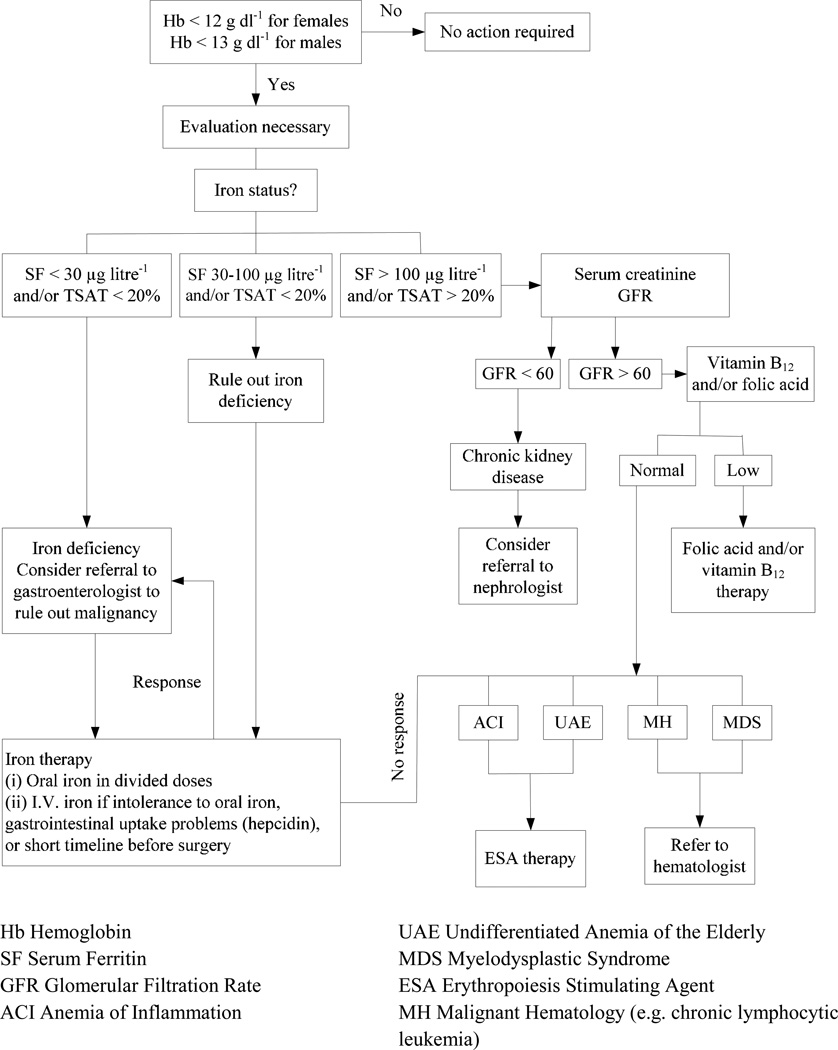
An algorithm for the evaluation and management of anemia in the elderly is presented in Figure 3. Iron-restricted erythropoiesis can cause anemia due to an absolute iron deficiency; iron sequestration which is mediated by hepcidin;44 and/or a functional iron deficiency due to erythropoietin-stimulated erythropoiesis.45 The evaluation of anemia must also consider unexpected diagnoses including chronic kidney disease (CKD)46 or occult malignancy.30,31 If absolute iron deficiency is diagnosed, in the elderly post-menopausal population it is mandatory to rule out gastrointestinal pathology, including malignancy as a source of chronic blood loss. Referral to a gastroenterologist may be the most effective way to proceed. However, in one-third to two-thirds of such patients, work-up of the gastrointestinal tract is negative.30–33,47 Serum creatinine and GFR must be determined in order to evaluate for CKD, in which case referral to a nephrologist may be appropriate. The suggested cut-off of glomerular filtration rate (GFR) < 60 mL/min for consideration that the anemia is secondary to end stage renal disease (ESRD), follows CMS guidelines on reimbursement for erythropoietic stimulating agent (ESA) therapy in patients with ESRD; but between 30–60 mL/min GFR, other causes for anemia may be possible.
Figure 3. Evaluation and Management of Anemia in the Elderly.
Once the screening blood count demonstrates anemia, an evaluation is necessary and begins with an assessment of iron status. When ferritin and/or iron saturation levels indicate absolute iron deficiency, referral to a gastroenterologist to rule out a gastrointestinal malignancy as a source of chronic blood loss may be indicated.
When ferritin and/or iron saturation values rule out absolute iron deficiency, serum creatinine and glomerular filtration rate (GFR) determination may indicate chronic kidney disease (CKD) and the need for referral to a nephrologist.
When ferritin and/or iron saturation values are in determinant, further evaluation to rule out absolute iron deficiency versus inflammation/chronic disease is necessary. A therapeutic trial of iron would confirm absolute iron deficiency. No response to iron therapy would indicate the anemia of chronic disease, suggesting that ESA therapy be initiated.
When serum ferritin and transferrin saturation values are inconclusive, further evaluation is necessary to rule out absolute iron deficiency or inflammation/chronic disease. As noted above, a response to a therapeutic trial of oral iron confirms absolute iron deficiency. However, lack of response to oral iron therapy may require a trial of IV iron, since in the presence of hepcidin, absorption of iron from the intestinal tract is impaired.13 In a study of patients with a clinical diagnosis of iron deficiency anemia based on ferritin and transferrin saturation assays, only 21% responded to 4 weeks of oral iron therapy compared to 65% of patients given IV iron therapy.48
A lack of response to iron therapy suggests a diagnosis of the anemia of inflammation or UAE. At this point, clinical evaluation for inflammatory conditions as well as measurement of C-reactive protein, fibrinogen, erythrocyte sedimentation rate (ESR), IL6, and hepcidin levels (if clinically available) may be useful.44 If abnormal, management of the underlying condition supplemented with an erythropoiesis stimulating agent (ESA) may be appropriate for further management.49 A careful history for alcohol use/abuse particularly in patients with an MCV>100 may be in order contributing either to poor marrow reserve or folate deficiency in the elderly. An early study by Cash and Sears of 90 patients (mean age 50.9 ± 16.5, not confined to elderly individuals) with anemia of chronic disease observed that there was a broader spectrum of associated diseases with ACD than had previously been recognized.50 A more recent study by Waalen et al.21 compared a large cohort of UAE cases in the elderly with a matched, non-anemic control group and found that IL-6 and hepcidin levels did not differ significantly; whereas testosterone levels were lower in men and erythropoietin levels were inappropriately low for the degree of anemia.
The diagnosis of UAE is usually considered when other causes of anemia in the elderly have been eliminated. The diagnosis of UAE is based on the findings of a hypoproliferative anemia: a low reticulocyte index and an inadequate erythropoietin level for the degree of anemia. The management of these patients is a serious and recurrent issue, since in the absence of an etiology there is no proven efficacious intervention. When such patients are symptomatic, when they find themselves in clinical situations involving blood loss, or when surgical intervention is required, consideration of transfusion therapy is necessary, as described below.
MANAGEMENT OF ANEMIA
As detailed above, management of anemia is determined by results of the evaluation. While folate deficiency is increasingly being made a non-entity with folate supplementation in flour, particular attention needs to be paid in certain settings: e.g. poor diet combined with alcoholism, or compliance failure with folate supplementation in a dialysis patient. Similarly, Vitamin B12 deficiency may be infrequently diagnosed, but a diagnostic trial of Vitamin B12 therapy may be necessary if the constellation of symptoms and signs are consistent with Vitamin B12 deficiency Further assays for, methylmalonic acid and homocysteine may be helpful but can also be inconclusive.51
As indicated above, a diagnostic or therapeutic trial including IV iron therapy may be necessary to rule out absolute iron deficiency.49,52,53 Even the “gold standard” diagnostic bone marrow aspirate indicating the presence of some stainable iron may obscure a deficiency of storage iron, since in some of these cases the patients’ anemia has been shown to be responsive to iron therapy.49,54
Thirdly, therapy with an erythropoiesis stimulating agent (ESA) may be indicated for treatment of one of several causes of anemia in the elderly. ESA’s can be useful in treating the anemia in patients with end stage kidney disease;55 anemia in patients with inflammation/chronic disease who are scheduled for elective surgery;49 and in patients with MDS56 who have moderate to severe anemia, in which the only other alternative may be chronic blood transfusions.
ESAs were first demonstrated and approved for use to increase the hemoglobin levels in patients with end-stage chronic kidney disease (CKD) undergoing dialysis57 and subsequently in such subjects who did not require dialysis.58 Based on prospective randomized trials that demonstrated reduced allogeneic blood transfusion,59 ESAs have subsequently been approved in patients undergoing elective surgery60 and in oncology patients with chemotherapy-induced anemia.
Subsequent to FDA approval, clinical trials were undertaken in an attempt to demonstrate long term improved patient outcomes with ESA therapy.61–67 Table 2 lists five such clinical trials in patients undergoing elective spine surgery;61 patients with chronic kidney disease (predialysis);62–66 and in patients with stage II-IV (New York Heart Association) congestive heart failure.67 Each of these studies were designed to evaluate aggressive anemia management (to a target Hgb within normal range) vs. conservative Hgb correction. In patients scheduled for elective spine surgery, outcomes were compared for ESA and placebo-treated cohorts who did not receive anticoagulation prophylaxis. In this study a higher incidence of deep vein thrombosis was found in patients receiving epoetin alfa compared to placebo. According to the subsequently revised prescribing information for ESA therapy, antithrombotic prophylaxis should be considered when ESA’s are used in elective surgical patients who do not receive perioperative anticoagulation. In anemic patients with congestive heart failure (CHF) a large, randomized trial67 of patients whose median age was 72.0 (range 63–78), long-term ESA therapy was successful in correcting anemia and reducing blood transfusions, but did not improve long-term clinical outcomes (composite outcome of death or any cause of hospitalization from worsening CHF). The American Society of Hematology/American Society of Clinical Oncology Clinical Practice guideline update68 has recommended use of ESAs in patients with low risk MDS, in order to avoid blood transfusions. In clinical practice, the potential for increased risks of death and thromboembolic events should be balanced against the benefits of treatment with ESAs, including avoidance of allogeneic blood transfusions.69
TABLE 2.
SELECTED POST-APPROVAL CLINICAL TRIALS OF ESA
| Trial | Age (Years) | Target vs. Control (Hgb) |
Reference (Year) |
Clinical Outcomes |
|---|---|---|---|---|
| I. Surgery | ||||
| SPINE | 60 ± 14 | <13 | Stowell, 200961 | Inc Thromb |
| II. CKD* | ||||
| CHOIR | 66 ± 14 | 13.5 vs 11.3 | Singh 200662 | Dec OS |
| CREATE | 59 ± 14 | 13–15 vs. 10.5–11.5 | Drueke 200663 | Dec OS |
| TREAT | 68 (60–75)** | 13 vs. 9 | Pfeffer 200664–66 | Dec OS |
| III. CHF | 71.5 (63–78)** | ≥ 13 | Swedberg 201367 | Dec OS |
ESA: Erythropoiesis Stimulating Agents. CHF: Congestive Heart Failure. Inc: Increase. Dec: decreased. OS: Overall Survival. CHOIR: Correction of Hemoglobin and Outcomes in Renal. CREATE = Cardiovascular Risk Reduction by Early Treatment with Epoetin beta. TREAT = Trial to Reduce Cardiovascular Events with Aranesp Therapy.
CKD: Chronic Kidney Disease pre-dialysis
Median (interquartile range)
BLOOD TRANSFUSION THERAPY
Patients with moderate anemia have few associated symptoms, due to significant compensatory mechanisms that preserve oxygen transport in the setting of a reduced hemoglobin. These compensatory mechanisms include increased blood flow due to decreased blood viscosity, increased oxygen unloading to tissues due to increased red cell bisphosphoglycerate, (2,3 BPG) increased plasma volume, and redistribution of blood flow.70 Generally, symptomatic manifestations occur only when the hemoglobin is below two-thirds of normal (i.e., less than 9 to 10 g/dL) as basal cardiac output increases in the anemic patient and is manifested clinically by symptoms of fatigue, dyspnea and tachycardia.71 However, the normal compensatory mechanisms of tachycardia and increased stroke volume may be impaired in the elderly, particularly in those with cardiovascular disease.
The recognition that transmission of non A, non B hepatitis was one of the complications for patients undergoing cardiac bypass surgery, along with the advent of HIV transmission by blood transfusion, led to the realization that rather than reacting to the cardiovascular compensatory response to anemia with blood transfusion therapy,72,73 a more appropriate response for stable patients would be to allow the normal compensatory cardiovascular response to anemia to preserve oxygen transport in patients who were otherwise stable and did not have risk factors.74 Based on Level 1 evidence it is now generally agreed that a more restrictive transfusion practice is safe, with equivalent patient outcomes compared to transfusion practice to maintain hemoglobin levels above 10g/dL.
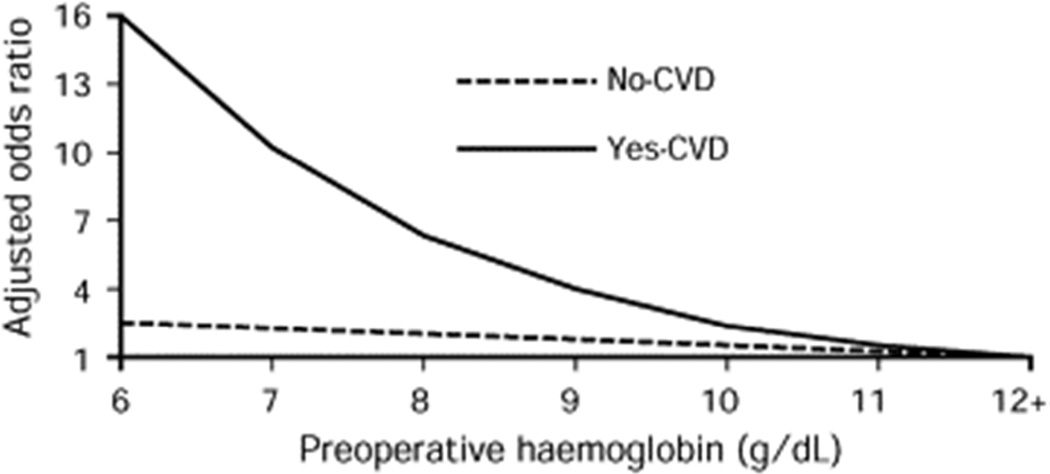
Blood transfusion strategies to treat anemia in the elderly and patients with cardiovascular disease, are poorly understood.75 For patients known to have risk factors such as age or cardiovascular disease (CVD), the odds adjusted ratio of postoperative mortality has been observed to increase significantly when compared with patients not known to have CVD (Figure 4),76 illustrating that the management of anemia, including blood transfusion therapy, should be different for patients known to have CVD. In this study of 1,958 Jehovah’s Witness patients undergoing surgery without blood transfusions, overall mortality was 3.2%. Five hundred forty seven (28%) of patients had Hgb <12 g/dl preoperatively. Ten deaths (16.1%) occurred within one day of surgery and 25 (40.3%) occurred within one week. Patients with cardiovascular disease had a 10.0% death rate, which was 4.3 times (95% CI, 2.6–7.1) higher than in patients without cardiovascular disease. This assessment echoes a clinical practice guideline that concluded that ‘the presence of coronary artery disease likely constitutes an important factor in determining a patient’s tolerance to low hemoglobin.77
Figure 4. Adjusted Odds Ratio for Mortality in Patients Undergoing Surgery.
Overall 30 day mortality for 1,958 Jehovah’s Witness patients undergoing surgery without blood transfusions was 3.2% (n=62;95% CI 2.4 – 4.0) Patients with cardiovascular disease had a 10.0% death rate, which was 4.3 times (95% CI, 2.6 – 7.1) higher than in patients without cardiovascular disease, according to preoperative hemoglobin.
Reproduced with permission from Carson JL, et al.76
Acute Coronary Syndromes (ACS)
Perioperative myocardial ischemic episodes are associated with hematocrit levels less than 28% in patients undergoing radical prostatectomy.78 A retrospective, analysis of CMS (Center for Medicare Services) data on 79,000 elderly patients (> 65 years of age) hospitalized with acute myocardial infarction in the United States., found that blood transfusion in patients whose admission hematocrit values were less than 33%, were associated with significantly lower mortality rates.79 Anemia (defined as a hematocrit of less than 39 percent) was present on admission to the hospital in nearly half the patients (43.4 percent) and was clinically significant (i.e., 33 percent or lower) in 10.4 percent of the patients. It was concluded that a more proactive management of anemia in elderly patients including blood transfusion therapy, was warranted.80
There is substantial variation in the use of blood transfusions in patients with ACS. An analysis of 24,000 patients in 27 countries found an association between non-ST segment ACS and a lower likelihood of blood transfusion.81 Rates of 30 day and 60 day clinical outcomes did not correspond to rates of transfusion, suggesting that the geographic variability in transfusion practices represented either underuse or the overuse of transfusions. A study of 44,000 patients with ACS in 400 U.S. hospitals found that 22.2% of patients were anemic and 10.4% received transfusions;82 of those with a nadir hematocrit ≤ 24%, transfusions had a beneficial impact on mortality; and for patients with hematocrit levels between 24–27%, transfusions had a neutral impact on mortality. However, more recently a systemic review83 of 10 published studies of blood transfusion strategies in patients with anemia associated with myocardial infarction, found that patients receiving blood transfusions had a higher all-cause mortality rate associated with blood transfusion vs no blood transfusion (18.2% vs 10.2%, risk ratio 2.9, p< 0.001). The difficulty in interpreting these retrospective, observational studies is that any conclusion that blood transfusions are causative, is confounded by both the underlying anemia and the underlying cause of the anemia as driving causes of the poor outcomes.84
A small pilot trial randomized 45 patients with acute myocardial infarction (MI) and hematocrit level ≤ 30% to a liberal cohort receiving transfusions to maintain hematocrit ≥30% or a conservative cohort (transfusions to maintain 24% to 27%).85 The primary clinical safety measurement of in-hospital death, recurrent MI or worsening congestive heart failure occurred in 8 (38%) of the liberal cohort vs 3 (13%) in the conservative cohort (p=0.046). These results suggested that more liberal transfusions practices in patients with AMI was associated with worse clinical outcomes. An addendum to the British Committee for Standards in Haematology (BCSH) guidelines86 dealt with measures to avoid over-transfusion, particularly transfusion-associated circulatory overload (TACO) in vulnerable patients such as the elderly. It was noted that there were complex issues including lack of attention to fluid balance and prescription of diuretics.
CLINICAL TRIALS
There is limited but emerging Level I evidence to guide blood transfusion practices. A Cochrane systematic review of prospective randomized trials up to 201187 compared “high” versus “low” hemoglobin thresholds of 19 trials involving a total of 6264 patients. The authors found that 1) “low” hemoglobin thresholds were well tolerated; 2) blood transfusions were reduced by 34% (CI 24 to 45%) in the “low” hemoglobin cohorts; and 3) blood transfusions were reduced by 1.2 units (CI 0.5 to 1.8 units) in the “low” hemoglobin cohorts. However, the authors have subsequently concluded that there are insufficient data available regarding transfusion thresholds for high risk patients, such as patient with acute coronary syndrome or brain injury.88
There are currently five Level I evidence clinical trials of liberal versus restrictive transfusion strategies (Table 3). These prospective, randomized trials include patients in the intensive care setting;89 patients undergoing cardiothoracic surgery;90 elderly patients (mean >80 years of age) undergoing repair of hip fracture;91 medical patients with gastrointestinal bleeds;92 and patients with symptomatic cardiovascular disease undergoing cardiac catheterization.93 Each trial investigated whether patients could tolerate a restrictive transfusion strategy with hemoglobin thresholds for blood transfusion between 7 and 8 g/dL, compared to a liberal strategy to maintain hemoglobin levels above 9 to 10 g/dL. As noted in Table 3, except for the FOCUS trial,91 eligibility for trial participation was not centered on the elderly; nevertheless, the mean ages approximate the lower end of the range for the elderly, particularly in the trial for patients with symptomatic coronary artery disease.93
Table 3.
Five Key Clinical Trials of Blood Transfusion in Adults
| Clinical Setting (Ref) |
Hemoglobin Threshold (g/dL) |
Age (Years) | Patients Transfused |
Deviation from Transfusion Protocol |
Mean Hemoglobin at Transfusion (g/dL) |
Participation of Eligible Patients |
|---|---|---|---|---|---|---|
| Intensive Care89 | 7 vs 10 | 57.1 ± 18.1 58.1 ± 18.3 |
67% 99% |
1.4% 4.3% |
8.5 ± 0.7* 10.7 ± 0.7* |
41% |
| CT Surgery90 | 8 vs 10 | 58.6 ± 12.5 vs 60.7 ± 12.5 |
47% 78% |
1.6% 0.0% |
9.1 (9.0–9.2) 10.5 (10.4–10.6) |
75% |
| Hip Fracture Repair91 | 8 vs 10 | 81.5 ± 9.0 vs 81.8 ± 8.8 |
41% 97% |
9.0% 5.6% |
7.9 ± 0.6 9.2 ± 0.5 |
56% |
| Acute Upper GI Bleeding92 | 7 vs 9 | NA NA |
49% 86% |
9.0% 3.0% |
7.3 ± 1.4 8.0 ± 1.5 |
93% |
| Symptomatic Coronary Artery Disease93 | 8 vs 10 | 74.3 ± 11.1 vs 67.3 ± 13.6 |
28.3% NA** |
1.8% 9.1% |
7.9 ± 0.8 9.3 ± 7.9 |
12.2% |
Average daily Hemoglobin.
NA: Not Available
The Transfusion Requirements in Critical Care (TRICC) trial89 found that intensive care patients could tolerate a restrictive transfusion strategy (hemoglobin range 7 to 9 g/dL, 8.2 g/dL on average) as well as patients transfused more liberally (10 to 12 g/dL, 10.5 g/dL on average), with no differences in 30 day mortality rates. In contrast, a retrospective study of 2393 patients consecutively admitted to the ICU has found that an admission hematocrit <25%, in the absence of transfusion, was associated with long-term mortality; suggesting that there may be hematocrit levels below which, the risk to benefit imbalance reverses.94
The TRACS (Transfusion Requirements after Cardiac Surgery) trial90 was a large, single center study of patients randomized to receive either restrictive (hematocrit > 24%) or liberal (hematocrit > 30%) red blood cell transfusions postoperatively. Thirty day all-cause mortality was not different (10% vs 11%, respectively) between the two cohorts. The FOCUS trial found that elderly, high risk (co-factors for cardiovascular disease) patients who underwent repair of hip fracture surgery, tolerated a hemoglobin trigger without red blood cell transfusions postoperatively to as low as 8 g/dL (or higher with transfusions, if symptomatic).91 Of the four large trials, only the FOCUS trial targeted a clinical setting that specifically evaluated elderly patients (mean age approximately 81 years of age). The age of the patients in the clinical trial of patients with acute gastrointestinal bleeds92 was not specified.
The MINT trial93 was a pilot, feasibility study of liberal (hemoglobin ≥ 10 g/dL) versus restrictive (hemoglobin < 8 g/dL) transfusion thresholds was initiated for a planned enrollment of 200 patients with symptomatic coronary artery disease (acute coronary syndrome or stable angina undergoing cardiac catheterization), but was terminated at the end of 18 months after enrollment of only 110 patients; of eligible screened patients, only 12% were enrolled (Table 3). The primary, composite outcome (death, myocardial infarction or revascularization) occurred in 10.9% of the liberal transfusion cohort, compared to 25.9% of the restrictive cohort (p=0.054); and mortality occurred in 1.8% and 13.0%, respectively (p=0.032). The MINT trial93 provides evidence that a more liberal transfusion practice to maintain hemoglobin thresholds above 10 g/dL, represents prudent management of high risk patients with symptomatic coronary artery disease.
It is noteworthy that one of the limitations of prospectively, randomized clinical trials is that patients who are eligible and who agree to participate in the study may not be particularly reflective of all patients in these clinical settings. Only 30% of the TRACS trial and 41% of the TRICC trial patients89 who were eligible, actually enrolled in the study. This leads to a concern over a selection bias; did the treating physicians accurately predict prior to enrollment, which patients would survive the study, thereby insuring that survival outcomes would be equivalent between treatment groups? It is reassuring that the patients who did participate were treated according to the clinical trial design in more than 90% of cases (defined according to authors’ published criteria), as indicated in Table 3.
Another limitation is the interpretation of the ‘transfusion trigger’ in these studies. While the target hemoglobin level in the restrictive group in the TRACS trial was 8.0 g/dL, the mean pretransfusion hemoglobin for these patients was 9.1 g/dL (Table 3). Yet a clinical practice guideline95 has interpreted this study to conclude that a hemoglobin level of 8 g/dL is appropriate for use as the ‘transfusion trigger’ in coronary care patients. Similarly, while the target hemoglobin level in the restrictive group for the TRICC trial was 7.0 g/dL, the mean pretransfusion hemoglobin for these patients was 8.5 g/dL; yet the authors interpreted this study to recommend a hemoglobin of 7 g/dL as appropriate for use as the transfusion trigger in critical care patients.96 The authors of the FOCUS trials concluded that “it is reasonable to withhold transfusion in patients who have undergone surgery in the absence of symptoms of anemia, or a decline in the hemoglobin level below 8 g/dL, even in elderly patients with underlying cardiovascular disease or other risk factors”.91 However, during the FOCUS study, mean hemoglobin values were 9.0 to 9.5 g/dL for the restrictive cohort, about 1.5 g/dL higher than the suggested thresholds during the study. An accompanying editorial97 to the FOCUS trial concluded that no specific hemoglobin level could be recommended for blood transfusion; rather, the constellation of patient symptoms, signs, clinical variables, and co-morbidities should be considered. This kind of scrutiny needs to be taken into account in interpreting results of clinical trials, and in management of the acute postsurgical care of elderly patients who have increased cardiovascular risks.
Clinical Practice Guidelines
The number of clinical practice guidelines for blood transfusions55,77,86,95,98–111 by professional societies (listed in Table 4) attests to the increasing interest in appropriate blood utilization. The published guidelines acknowledge the consideration of patient co-variables (including age) or other patient-specific criteria for making transfusion decisions. Among these guidelines, it is generally agreed that transfusion is not of benefit when the hemoglobin is greater than 10 g/dL, but may be beneficial when the hemoglobin is less than 6 to 7 g/dL. However, the selection of a discrete hemoglobin as a ‘trigger’ for transfusion has been controversial. For example, the initial guidelines by The American Society of Anesthesiology (ASA) in 1996109 identified hemoglobin < 6 g/dL as a transfusion trigger for acute blood loss, whereas the updated ASA guidelines in 2006102 noted that “although multiple trials have evaluated transfusion thresholds on patient outcome, the literature is insufficient to define a transfusion trigger in surgical patients with substantial blood loss.” Five guidelines98,101,107,109,112 have specified a hemoglobin threshold for patients only in those with acute bleeding. The attempt to define an empiric hemoglobin concentration as a transfusion trigger for patients who are stable clinically, has been contested by seven published clinical practice guidelines55,77,86,99,102,110,111 that do not recommend a specific hemoglobin threshold for transfusion. A recent study of elderly population with hip fracture found that while blood transfusion was not associated with changes in mortality, blood transfusion was associated with an increased rate of postoperative infection.113 The authors concluded that these data add to the wider literature about adverse clinical outcomes in patient receiving blood transfusions, and emphasizes the need for prospective trials to evaluate the role of transfusion in the elderly.
Table 4.
Published Clinical Practice Guidelines for Blood Transfusion
| Red Blood Cell Transfusion | |||
|---|---|---|---|
| Year | Society | Recommendations | Reference |
| 1988 | NIH Consensus Conference | < 7 g/dl (acute)* | JAMA 1988;260:270098 |
| 1992 | Am Coll Physicians (ACP) | No number | Ann Int Med 1992;116:393–40299 |
| 1996/2006 | AmerSocAnesth (ASA) | <6 g/dl (acute)* No number |
Anesth 1996;84:732–747109 Anesth 2006;105:198–208102 |
| 1997/1998 | Can Med Assoc (CMA) | No Number | Can Med Assoc J 1997;156: S1-2477 J Emerg Med 1998;16:129-31100 |
| 1998 | Coll Amer Path (CAP) | 6 g/dl (acute)* | Arch Path Lab Med 1998;122:130-8107 |
| 2001/2012 | Br Com Stand Haematol | No number 7–8 g/dl* |
http://www.bcshguidelines.com/documents/BCSH_Blood_Admin_-_addendum_August_2012.pdf Br J Haematol 2001;113:24–31101 |
| 2001 | Australasian Soc Blood Trans | 7 g/dl | http://www.nhmrc.health.gov.au |
| 2007/2011 | Soc Thor Surg (STS) SocCardvascAnesth (CVA) |
7 g/dl or 8 g/dl* |
Ann ThoracSurg 2007;83:S27-86103 Ann ThoracSurg 2011;91:944-82112 |
| 2009 | ACCM SCCM |
7 g/dl 7 g/dl |
Crit Care Med 2009;37:3124-57104 J Trauma 2009;67:1439-42105 |
| 2011 | SABM | 8 g/dl | Trans Med Rev 2011;232–246108 |
| 2012 | National Blood Authority, Australia | No number | http://www.nba.gov.au/guidelines/review.html |
| 2012 | AABB | 7–8 g/dl or 8 g/dl** | Ann Int Med 2012;157:49–5895 |
| 2012 | KDIGO*** | No number | Kid Int 2012;2:311–31655 |
| 2012 | National Cancer Center Network (NCCN) | 7 g/dl | JNCCN 2012;10:628-53106 |
For patients with acute blood loss
For patients with symptoms of end-organ ischemia.
KDIGO: Kidney Disease: Improving Global Outcomes
From Goodnough LT, et al.75
CONCLUSION
Arbitrary laboratory values are inadequate to define when red blood cell transfusions are appropriate. Each patient must be evaluated individually, and patient-specific anaemia management strategies be employed.75 This dilemma is illustrated by serial recommendations for a variety of integers for hemoglobin levels recommended as transfusion triggers, based on the Level 1 evidence-based literature over time: no number;55,77,86,99,102,110,111 7–8 g/dL;95 and 6–8 g/dL.87,88 This conflict between guideline-based medicine and a more personalized approach to elderly patients, predominantly occurs when considering withholding or providing a therapy that is not addressed by the guidelines, but may be beneficial for an individual patient group.114 For elderly patients, prudent transfusion practices to maintain hemoglobin thresholds of 9–10 g/dL are indicated, unless or until evidence emerges to indicate otherwise.
Acknowledgments
Disclosures
LTG: consultant for Amgen, Venofer, Luitpold, CSL Behring
SLS: is a recipient of NIH funding: RO1 AG029124; UO1 AG034661
Footnotes
References
- 1.Culleton BF, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Hemmelgarn BR. Impact of anemia on hospitalization and mortality in older adults. Blood. 2006;107:3841–3846. doi: 10.1182/blood-2005-10-4308. [DOI] [PubMed] [Google Scholar]
- 2.Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med. 2006;119:327–334. doi: 10.1016/j.amjmed.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Price EA, Schrier SL. Anemia in the elderly: introduction. Semin Hematol. 2008;45:207–209. doi: 10.1053/j.seminhematol.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Izaks GJ, Westendorp RG, Knook DL. The definition of anemia in older persons. JAMA. 1999;281:1714–1717. doi: 10.1001/jama.281.18.1714. [DOI] [PubMed] [Google Scholar]
- 5.Patel KV, Harris TB, Faulhaber M, et al. Racial variation in the relationship of anemia with mortality and mobility disability among older adults. Blood. 2007;109:4663–4670. doi: 10.1182/blood-2006-10-055384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penninx BW, Pahor M, Woodman RC, Guralnik JM. Anemia in old age is associated with increased mortality and hospitalization. J Gerontol A Biol Sci Med Sci. 2006;61:474–479. doi: 10.1093/gerona/61.5.474. [DOI] [PubMed] [Google Scholar]
- 7.Zakai NA, Katz R, Hirsch C, et al. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: the Cardiovascular Health Study. Arch Intern Med. 2005;165:2214–2220. doi: 10.1001/archinte.165.19.2214. [DOI] [PubMed] [Google Scholar]
- 8.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 9.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747–1750. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel KV. Epidemiology of anemia in older adults. Semin Hematol. 2008;45:210–217. doi: 10.1053/j.seminhematol.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaves PH, Xue QL, Guralnik JM, Ferrucci L, Volpato S, Fried LP. What constitutes normal hemoglobin concentration in community-dwelling disabled older women? J Am Geriatr Soc. 2004;52:1811–1816. doi: 10.1111/j.1532-5415.2004.52502.x. [DOI] [PubMed] [Google Scholar]
- 12.Zakai NA, French B, Arnold AM, et al. Hemoglobin decline, function, and mortality in the elderly: the Cardiovascular Health Study. Am J Hematol. 2013;88:5–9. doi: 10.1002/ajh.23336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood. 2010;116:4754–4761. doi: 10.1182/blood-2010-05-286260. [DOI] [PubMed] [Google Scholar]
- 14.Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106:13–22. doi: 10.1093/bja/aeq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrier SL, Hematology ASH. the anemia of the aged. Blood. 2005;106:3341–3342. doi: 10.1182/blood-2005-07-2816. [DOI] [PubMed] [Google Scholar]
- 16.Penninx BW, Guralnik JM, Onder G, Ferrucci L, Wallace RB, Pahor M. Anemia and decline in physical performance among older persons. Am J Med. 2003;115:104–110. doi: 10.1016/s0002-9343(03)00263-8. [DOI] [PubMed] [Google Scholar]
- 17.Penninx BW, Pluijm SM, Lips P, et al. Late-life anemia is associated with increased risk of recurrent falls. J Am Geriatr Soc. 2005;53:2106–2111. doi: 10.1111/j.1532-5415.2005.00491.x. [DOI] [PubMed] [Google Scholar]
- 18.Chaves PH, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women's Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60:729–735. doi: 10.1093/gerona/60.6.729. [DOI] [PubMed] [Google Scholar]
- 19.Hong CH, Falvey C, Harris TB, et al. Anemia and risk of dementia in older adults: Findings from the Health ABC study. Neurology. 2013;81:528–533. doi: 10.1212/WNL.0b013e31829e701d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrucci L, Guralnik JM, Bandinelli S, et al. Unexplained anaemia in older persons is characterised by low erythropoietin and low levels of pro-inflammatory markers. Br J Haematol. 2007;136:849–855. doi: 10.1111/j.1365-2141.2007.06502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waalen J, von Lohneysen K, Lee P, Xu X, Friedman JS. Erythropoietin, GDF15, IL6, hepcidin and testosterone levels in a large cohort of elderly individuals with anaemia of known and unknown cause. Eur J Haematol. 2011;87:107–116. doi: 10.1111/j.1600-0609.2011.01631.x. [DOI] [PubMed] [Google Scholar]
- 22.Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, et al. Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood. 2011;117:e218–e225. doi: 10.1182/blood-2011-02-337907. [DOI] [PubMed] [Google Scholar]
- 23.Joosten E, Pelemans W, Hiele M, Noyen J, Verhaeghe R, Boogaerts MA. Prevalence and causes of anaemia in a geriatric hospitalized population. Gerontology. 1992;38:111–117. doi: 10.1159/000213315. [DOI] [PubMed] [Google Scholar]
- 24.Ershler WB. Biological interactions of aging and anemia: a focus on cytokines. J Am Geriatr Soc. 2003;51:S18–S21. doi: 10.1046/j.1532-5415.51.3s.2.x. [DOI] [PubMed] [Google Scholar]
- 25.Pang WW, Schrier SL. Anemia in the elderly. Curr Opin Hematol. 2012;19:133–140. doi: 10.1097/MOH.0b013e3283522471. [DOI] [PubMed] [Google Scholar]
- 26.Price EA, Mehra R, Holmes TH, Schrier SL. Anemia in older persons: etiology and evaluation. Blood Cells Mol Dis. 2011;46:159–165. doi: 10.1016/j.bcmd.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Artz AS, Thirman MJ. Unexplained anemia predominates despite an intensive evaluation in a racially diverse cohort of older adults from a referral anemia clinic. J Gerontol A Biol Sci Med Sci. 2011;66:925–932. doi: 10.1093/gerona/glr090. [DOI] [PubMed] [Google Scholar]
- 28.Pennypacker LC, Allen RH, Kelly JP, et al. High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc. 1992;40:1197–1204. [PubMed] [Google Scholar]
- 29.Carmel R, Green R, Rosenblatt DS, Watkins D. Update on cobalamin, folate, and homocysteine. Hematology Am Soc Hematol Educ Program. 2003:62–81. doi: 10.1182/asheducation-2003.1.62. [DOI] [PubMed] [Google Scholar]
- 30.Rockey DC, Cello JP. Evaluation of the gastrointestinal tract in patients with iron-deficiency anemia. N Engl J Med. 1993;329:1691–1695. doi: 10.1056/NEJM199312023292303. [DOI] [PubMed] [Google Scholar]
- 31.Raje D, Mukhtar H, Oshowo A, Ingham Clark C. What proportion of patients referred to secondary care with iron deficiency anemia have colon cancer? Dis Colon Rectum. 2007;50:1211–1214. doi: 10.1007/s10350-007-0249-y. [DOI] [PubMed] [Google Scholar]
- 32.Joosten E, Ghesquiere B, Linthoudt H, et al. Upper and lower gastrointestinal evaluation of elderly inpatients who are iron deficient. Am J Med. 1999;107:24–29. doi: 10.1016/s0002-9343(99)00162-x. [DOI] [PubMed] [Google Scholar]
- 33.Gordon SR, Smith RE, Power GC. The role of endoscopy in the evaluation of iron deficiency anemia in patients over the age of 50. Am J Gastroenterol. 1994;89:1963–1967. [PubMed] [Google Scholar]
- 34.McKane WR, Khosla S, Peterson JM, Egan K, Riggs BL. Circulating levels of cytokines that modulate bone resorption: effects of age and menopause in women. J Bone Miner Res. 1994;9:1313–1318. doi: 10.1002/jbmr.5650090821. [DOI] [PubMed] [Google Scholar]
- 35.Roubenoff R, Harris TB, Abad LW, Wilson PW, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci. 1998;53:M20–M26. doi: 10.1093/gerona/53a.1.m20. [DOI] [PubMed] [Google Scholar]
- 36.Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 37.Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet. 2011;378:1396–1407. doi: 10.1016/S0140-6736(11)61381-0. [DOI] [PubMed] [Google Scholar]
- 38.Nissenson AR, Goodnough LT, Dubois RW. Anemia: not just an innocent bystander? Arch Intern Med. 2003;163:1400–1404. doi: 10.1001/archinte.163.12.1400. [DOI] [PubMed] [Google Scholar]
- 39.Goodnough LT, Shander A. In reply. Anesthesiology. 2013;118:223–224. doi: 10.1097/ALN.0b013e318276c2c8. [DOI] [PubMed] [Google Scholar]
- 40.Lam AP, Gundabolu K, Sridharan AA, et al. Multiplicative interaction between mean corpuscular volume and red cell distribution width in predicting mortality of elderly patients with and without anemia. Am J Hematol. doi: 10.1002/ajh.23529. Epub 7/6/2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keel SB, Abkowitz JL. The microcytic red cell and the anemia of inflammation. N Engl J Med. 2009;361:1904–1906. doi: 10.1056/NEJMcibr0906391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Means R. Amnemia of chronic disease. In: Young NS, Gerson SL, High KA, editors. Clinical Hematology. Philadelphia: Mosby Elesevier; 2006. [Google Scholar]
- 43.Skikne BS, Punnonen K, Caldron PH, et al. Improved differential diagnosis of anemia of chronic disease and iron deficiency anemia: a prospective multicenter evaluation of soluble transferrin receptor and the sTfR/log ferritin index. Am J Hematol. 2011;86:923–927. doi: 10.1002/ajh.22108. [DOI] [PubMed] [Google Scholar]
- 44.Ferrucci L, Semba RD, Guralnik JM, et al. Proinflammatory state, hepcidin, and anemia in older persons. Blood. 2010;115:3810–3816. doi: 10.1182/blood-2009-02-201087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodnough LT. Iron deficiency syndromes and iron-restricted erythropoiesis. Transfusion. 2012;52:1584–1592. doi: 10.1111/j.1537-2995.2011.03495.x. [DOI] [PubMed] [Google Scholar]
- 46.Artz AS, Fergusson D, Drinka PJ, et al. Mechanisms of unexplained anemia in the nursing home. J Am Geriatr Soc. 2004;52:423–427. doi: 10.1111/j.1532-5415.2004.52116.x. [DOI] [PubMed] [Google Scholar]
- 47.Niv E, Elis A, Zissin R, Naftali T, Novis B, Lishner M. Iron deficiency anemia in patients without gastrointestinal symptoms--a prospective study. Fam Pract. 2005;22:58–61. doi: 10.1093/fampra/cmh705. [DOI] [PubMed] [Google Scholar]
- 48.Bregman DB, Morris D, Koch TA, He A, Goodnough LT. Hepcidin levels predict nonresponsiveness to oral iron therapy in patients with iron deficiency anemia. Am J Hematol. 2013;88(2):97–101. doi: 10.1002/ajh.23354. [DOI] [PubMed] [Google Scholar]
- 49.Goodnough LT, Shander A. Current status of pharmacologic therapies in patient blood management. Anesth Analg. 2013;116:15–34. doi: 10.1213/ANE.0b013e318273f4ae. [DOI] [PubMed] [Google Scholar]
- 50.Cash JM, Sears DA. The anemia of chronic disease: spectrum of associated diseases in a series of unselected hospitalized patients. Am J Med. 1989;87:638–644. doi: 10.1016/s0002-9343(89)80396-1. [DOI] [PubMed] [Google Scholar]
- 51.Carmel R. How I treat cobalamin (vitamin B12) deficiency. Blood. 2008;112:2214–2221. doi: 10.1182/blood-2008-03-040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bregman DB, Morris D, Koch TA, He A, Goodnough LT. Hepcidin levels predict nonresponsiveness to oral iron therapy in patients with iron deficiency anemia. Am J Hematol. 2013;88:97–101. doi: 10.1002/ajh.23354. [DOI] [PubMed] [Google Scholar]
- 53.Liu K, Kaffes AJ. Iron deficiency anaemia: a review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol. 2012;24:109–116. doi: 10.1097/MEG.0b013e32834f3140. [DOI] [PubMed] [Google Scholar]
- 54.Mast AE, Blinder MA, Gronowski AM, Chumley C, Scott MG. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem. 1998;44:45–51. [PubMed] [Google Scholar]
- 55.KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney International Supplements. 2012;2:311–316. [Google Scholar]
- 56.Jansen AJ, Essink-Bot ML, Beckers EA, Hop WC, Schipperus MR, Van Rhenen DJ. Quality of life measurement in patients with transfusion-dependent myelodysplastic syndromes. Br J Haematol. 2003;121:270–274. doi: 10.1046/j.1365-2141.2003.04272.x. [DOI] [PubMed] [Google Scholar]
- 57.Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987;316:73–78. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- 58.Eschbach JW, Abdulhadi MH, Browne JK, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med. 1989;111:992–1000. doi: 10.7326/0003-4819-111-12-992. [DOI] [PubMed] [Google Scholar]
- 59.Seidenfeld J, Piper M, Flamm C, et al. Epoetin treatment of anemia associated with cancer therapy: a systematic review and meta-analysis of controlled clinical trials. J Natl Cancer Inst. 2001;93:1204–1214. doi: 10.1093/jnci/93.16.1204. [DOI] [PubMed] [Google Scholar]
- 60.Goodnough LT, Monk TG, Andriole GL. Erythropoietin therapy. N Engl J Med. 1997;336:933–938. doi: 10.1056/NEJM199703273361307. [DOI] [PubMed] [Google Scholar]
- 61.Stowell CP, Jones SC, Enny C, Langholff W, Leitz G. An open-label, randomized, parallel-group study of perioperative epoetin alfa versus standard of care for blood conservation in major elective spinal surgery: safety analysis. Spine. 2009;34:2479–2485. doi: 10.1097/BRS.0b013e3181bd163f. [DOI] [PubMed] [Google Scholar]
- 62.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 63.Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 64.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 65.Solomon SD, Uno H, Lewis EF, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med. 2010;363:1146–1155. doi: 10.1056/NEJMoa1005109. [DOI] [PubMed] [Google Scholar]
- 66.Lewis EF, Pfeffer MA, Feng A, et al. Darbepoetin alfa impact on health status in diabetes patients with kidney disease: a randomized trial. Clin J Am Soc Nephrol. 2011;6:845–855. doi: 10.2215/CJN.06450710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013;368:1210–1219. doi: 10.1056/NEJMoa1214865. [DOI] [PubMed] [Google Scholar]
- 68.Rizzo JD, Brouwers M, Hurley P, et al. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood. 2010;116:4045–4059. doi: 10.1182/blood-2010-08-300541. [DOI] [PubMed] [Google Scholar]
- 69.Goodnough LT, Shander AS. Erythropoiesis stimulating agents, blood transfusion, and the practice of medicine. Am J Hematol. 2010;85:835–837. doi: 10.1002/ajh.21870. [DOI] [PubMed] [Google Scholar]
- 70.Goodnough LT, Despotis GJ, Hogue CW, Jr, Ferguson TB., Jr On the need for improved transfusion indicators in cardiac surgery. Ann Thorac Surg. 1995;60:473–480. doi: 10.1016/0003-4975(95)98960-3. [DOI] [PubMed] [Google Scholar]
- 71.Finch CA, Lenfant C. Oxygen transport in man. N Engl J Med. 1972;286:407–415. doi: 10.1056/NEJM197202242860806. [DOI] [PubMed] [Google Scholar]
- 72.Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–1616. doi: 10.1097/01.CCM.0000217920.48559.D8. [DOI] [PubMed] [Google Scholar]
- 73.Adams RC, Lundy JS. Anesthesia in cases of poor surgical risk: some suggestions for decreasing the risk. Surg Gynecol Obstet. 1941;71:1011–1014. [Google Scholar]
- 74.Madjdpour C, Spahn DR, Weiskopf RB. Anemia and perioperative red blood cell transfusion: a matter of tolerance. Crit Care Med. 2006;34:S102–S108. doi: 10.1097/01.CCM.0000214317.26717.73. [DOI] [PubMed] [Google Scholar]
- 75.Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet. 2013;381:1845–1854. doi: 10.1016/S0140-6736(13)60650-9. [DOI] [PubMed] [Google Scholar]
- 76.Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060. doi: 10.1016/S0140-6736(96)04330-9. [DOI] [PubMed] [Google Scholar]
- 77.Expert Working Group. Guidelines for red blood cell and plasma transfusion for adults and children. Can Med Assoc J. 1997;156(Suppl 11):S1–S24. [Google Scholar]
- 78.Hogue CW, Jr, Goodnough LT, Monk TG. Perioperative myocardial ischemic episodes are related to hematocrit level in patients undergoing radical prostatectomy. Transfusion. 1998;38:924–931. doi: 10.1046/j.1537-2995.1998.381098440856.x. [DOI] [PubMed] [Google Scholar]
- 79.Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230–1236. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- 80.Goodnough LT, Bach RG. Anemia, transfusion, and mortality. N Engl J Med. 2001;345:1272–1274. doi: 10.1056/NEJM200110253451711. [DOI] [PubMed] [Google Scholar]
- 81.Rao SV, Chiswell K, Sun JL, et al. International variation in the use of blood transfusion in patients with non-ST-segment elevation acute coronary syndromes. Am J Cardiol. 2008;101:25–29. doi: 10.1016/j.amjcard.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 82.Alexander KP, Chen AY, Wang TY, et al. Transfusion practice and outcomes in non-ST-segment elevation acute coronary syndromes. Am Heart J. 2008;155:1047–1053. doi: 10.1016/j.ahj.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 83.Chatterjee S, Wetterslev J, Sharma A, Lichstein E, Mukherjee D. Association of blood transfusion with increased mortality in myocardial infarction: a meta-analysis and diversity-adjusted study sequential analysis. JAMA Intern Med. 2013;173:132–139. doi: 10.1001/2013.jamainternmed.1001. [DOI] [PubMed] [Google Scholar]
- 84.Wallis JP. Disentangling anemia and transfusion. Transfusion. 2011;51:8–10. doi: 10.1111/j.1537-2995.2010.02976.x. [DOI] [PubMed] [Google Scholar]
- 85.Cooper HA, Rao SV, Greenberg MD, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study) Am J Cardiol. 2011;108:1108–1111. doi: 10.1016/j.amjcard.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 86.British Committee for Standards in Haematology (BCSH) Guideline on the Administration of Blood Components. [Accessed: 7/1/2013]; http://www.bcshguidelines.com/documents/BCSH_Blood_Admin_-_addendum_August_2012.pdf. [Google Scholar]
- 87.Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;4:CD002042. doi: 10.1002/14651858.CD002042.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carson JL, Carless PA, Hebert PC. Outcomes using lower vs higher hemoglobin thresholds for red blood cell transfusion. JAMA. 2013;309:83–84. doi: 10.1001/jama.2012.50429. [DOI] [PubMed] [Google Scholar]
- 89.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 90.Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559–1567. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 91.Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–2462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 93.Carson JL, Hebert PC. Here we go again--blood transfusion kills patients?: comment on "Association of blood transfusion with increased mortality in myocardial infarction: a meta-analysis and diversity-adjusted study sequential analysis". JAMA Intern Med. 2013;173:139–141. doi: 10.1001/jamainternmed.2013.2855. [DOI] [PubMed] [Google Scholar]
- 94.Mudumbai SC, Cronkite R, Hu KU, et al. Association of admission hematocrit with 6-month and 1-year mortality in intensive care unit patients. Transfusion. 2011;51:2148–2159. doi: 10.1111/j.1537-2995.2011.03134.x. [DOI] [PubMed] [Google Scholar]
- 95.Carson JL, Grossman BJ, Kleinman S, et al. Red Blood Cell Transfusion: A Clinical Practice Guideline From the AABB. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 96.Hebert PC, Yetisir E, Martin C, et al. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit Care Med. 2001;29:227–234. doi: 10.1097/00003246-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 97.Barr PJ, Bailie KE. Transfusion thresholds in FOCUS. N Engl J Med. 2011;365:2532–2533. doi: 10.1056/NEJMe1110087. [DOI] [PubMed] [Google Scholar]
- 98.Consensus conference. Perioperative red blood cell transfusion. JAMA. 1988;260:2700–2703. [PubMed] [Google Scholar]
- 99.Welch HG, Meehan KR, Goodnough LT. Prudent strategies for elective red blood cell transfusion. Ann Intern Med. 1992;116:393–402. doi: 10.7326/0003-4819-116-5-393. [DOI] [PubMed] [Google Scholar]
- 100.Innes G. Guidelines for red blood cells and plasma transfusion for adults and children: an emergency physician's overview of the 1997 Canadian blood transfusion guidelines. Part 1: red blood cell transfusion. Canadian Medical Association Expert Working Group. J Emerg Med. 1998;16:129–131. doi: 10.1016/s0736-4679(97)00253-9. [DOI] [PubMed] [Google Scholar]
- 101.Murphy MF, Wallington TB, Kelsey P, et al. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24–31. doi: 10.1046/j.1365-2141.2001.02701.x. [DOI] [PubMed] [Google Scholar]
- 102.Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 103.Ferraris VA, Ferraris SP, Saha SP, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83:S27–S86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 104.Napolitano LM, Kurek S, Luchette FA, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37:3124–3157. doi: 10.1097/CCM.0b013e3181b39f1b. [DOI] [PubMed] [Google Scholar]
- 105.Napolitano LM, Kurek S, Luchette FA, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. J Trauma. 2009;67:1439–1442. doi: 10.1097/TA.0b013e3181ba7074. [DOI] [PubMed] [Google Scholar]
- 106.Rodgers GM, 3rd, Becker PS, Blinder M, et al. Cancer- and chemotherapy-induced anemia. J Natl Compr Canc Netw. 2012;10:628–653. doi: 10.6004/jnccn.2012.0064. [DOI] [PubMed] [Google Scholar]
- 107.Simon TL, Alverson DC, AuBuchon J, et al. Practice parameter for the use of red blood cell transfusions: developed by the Red Blood Cell Administration Practice Guideline Development Task Force of the College of American Pathologists. Arch Pathol Lab Med. 1998;122:130–138. [PubMed] [Google Scholar]
- 108.Shander A, Fink A, Javidroozi M, et al. Appropriateness of allogeneic red blood cell transfusion: The International Consensus Conference on Transfusion Outcomes. Transfus Med Rev. 2011;25:232–246. doi: 10.1016/j.tmrv.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 109.Practice Guidelines for blood component therapy: A report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology. 1996;84:732–747. [PubMed] [Google Scholar]
- 110.Australasian Society of Blood Transfusion. Clinical Practice Guidelines: Appropriate Use of Red Blood Cells. [Accessed: 7/1/2013]; http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cp78.pdf.
- 111.National Blood Authority, Australia. [Accessed 7/1/2013];Patient Blood Management Guidelines. http://www.nba.gov.au/guidelines/review.html.
- 112.Ferraris VA, Brown JR, Despotis GJ, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–982. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 113.Shokoohi A, Stanworth S, Mistry D, Lamb S, Staves J, Murphy MF. The risks of red cell transfusion for hip fracture surgery in the elderly. Vox Sang. 2012;103:223–230. doi: 10.1111/j.1423-0410.2012.01606.x. [DOI] [PubMed] [Google Scholar]
- 114.Goldberger JJ, Buxton AE. Personalized medicine vs guideline-based medicine. JAMA. 2013;309:2559–2560. doi: 10.1001/jama.2013.6629. [DOI] [PubMed] [Google Scholar]