Abstract
Coordinated migration of distinct classes of neurons to appropriate positions leads to the formation of functional neuronal circuitry in the cerebral cortex. Two major classes of cortical neurons, interneurons and projection neurons, utilize distinctly different modes (radial vs. tangential) and routes of migration to arrive at their final positions in the cerebral cortex. Here, we show that adenomatous polyposis coli (APC) modulates microtubule (MT) severing in interneurons to facilitate tangential mode of interneuron migration, but not the glial-guided, radial migration of projection neurons. APC regulates the stability and activity of the MT severing protein p60-katanin in interneurons to promote the rapid remodeling of neuronal processes necessary for interneuron migration. These findings reveal how severing and restructuring of MTs facilitate distinct modes of neuronal migration necessary for laminar organization of neurons in the developing cerebral cortex.
Keywords: Cerebral cortical development, APC, Katanin, microtubules, interneuron, projection neuron, neuronal migration
Introduction
Appropriate neuronal placement, the basis for the emergence of neuronal connectome or wiring, is achieved through a process of coordinated pattern of neuronal migration enabling distinct classes of neurons to navigate from their sites of birth to their final laminar and areal destinations in the cerebral cortex (Evsyukova et al., 2013; Kwan et al., 2012; Valiente and Marin, 2010; Rakic, 1972). The two major classes of cortical neurons, i.e., projection neurons (PN) and interneurons (IN), migrate in distinctly different ways (Evsyukova et al., 2013; Valiente and Marin, 2010). Projection neurons, generated in the dorsal germinal zones, migrate to the cortical plate, using somal translocation during initial stages of corticogenesis and radial glial-guided migration at later embryonic stages. Interneurons, generated in the ganglionic eminence of the ventral telencephalon, migrate tangentially into the developing cerebral wall, prior to radially oriented migration into the cortical plate. Coordination of these diverse patterns of neuronal migration leads to the placement of appropriate numbers of distinct classes of projection neurons and interneurons within specific areas or layers of the developing cerebral cortex. Disruptions in neuronal migration, resulting from genetic mutations or environmental insults, alter the positioning and thus the connectivity and function of cortical neurons (Kwan et al., 2012; Valiente and Marin, 2010; Metin et al., 2008). Area specific and neuronal type specific defects in neuronal migration and the resultant changes in neuronal connectivity are thought to contribute to a wide spectrum of neurological disorders, including autism, schizophrenia, epilepsy, mental retardation, and gross malformations such as lissencephaly, schizencephaly, microencephaly, and macro/microgyria (Batista-Brito and Fishell, 2009; Manzini and Walsh, 2011; Valiente and Marin, 2010; Wynshaw-Boris et al., 2010; Yizhar et al., 2011). In spite of its importance, how the two major classes of cortical neurons molecularly modulate their distinct modes of migration within the developing cerebral cortex remains unclear.
Adenomatous Polyposis Coli (APC) serves an essential function in the formation of cerebral cortex (Yokota et al., 2009; Ivaniutsin et al., 2012). APC is a multi-domain protein that is known to complex with and modulate the activities of microtubules (MTs), intermediate filaments, actin, β-catenin, Axin, and cytoskeletal regulators, EB1, mDia1, Asef1, and IQGAP1 (Aoki and Taketo, 2007; Sakamoto et al., 2013; Preitner et al., 2014). Mutational analysis of APC indicates that β-catenin and MTs are the two major targets of APC activity (Aoki and Taketo, 2007; McCartney and Nathke, 2008; Wen et al., 2004). During corticogenesis, APC is necessary for the formation of polarized radial progenitors (Yokota et al., 2009). Polarized radial progenitors from the dorsal and ventral proliferative zones give rise to projection neurons and interneurons, respectively. As they migrate, both populations elongate an actively probing leading process, trailed by pre-somal swellings into which nucleus and cell soma translocate while the trailing process retracts (Evsyukova et al., 2013; Metin et al., 2008; Polleux et al., 2002). However, interneuronal movement is highly dynamic compared to that of projection neurons. They rapidly extend, retract, and modify branches, change the orientation of the leading processes, and move in multiple different directions within the developing cerebral wall as they navigate towards their target layer (Anderson et al., 1997; Godin et al., 2012). In contrast, radially migrating projection neurons tend to have a single unbranched leading process and constantly modulate (maintain/break) adhesive contacts with radial glial guides as they move. APC is hypothesized to play a significant role in the polarization, migration, and axon growth of neurons in vitro (Barth et al., 2008; Chen et al., 2011), but the in vivo evidence for these functions or whether APC differentially regulates the migration and development of distinct classes of cortical neurons is unknown. Therefore, using interneuron or projection neuron-type specific inactivation of APC, we examined the roles of APC in the appropriate migration, placement, and differentiation of different classes of neurons in the cerebral cortex.
Here, we show surprisingly different roles for APC in interneuronal and projection neuron migration and identify a hitherto undefined mechanism underlying their characteristically different patterns of migration. APC modulates the stability of the MT severing enzyme p60-katanin differently in interneurons and projection neurons. APC-regulated MT severing, via p60-katanin, promotes branching intensive interneuron migration, whereas bipolar, glial-guided radial migration of projection neurons is not affected by APC. Dynamic regulation of MT severing may therefore promote distinct patterns of cortical neuronal migration within the developing cerebral cortex.
Results
Conditional Ablation of APC in the Developing Interneurons and Projection Neurons
To examine the function of APC in interneuron and projection neuron migration and differentiation, APC was inactivated in newborn cortical neurons using an APC floxed allele line known to yield APC loss of function after Cre-mediated recombination (Hasegawa et al., 2002; Sansom et al., 2004; Shibata et al., 1997). Dlx5/6-Cre-IRES-EGFP (Dlx5/6-Cre) line that drives Cre and EGFP expression in interneurons generated from the ganglionic eminence (GE) (Stenman et al., 2003) or Nex-Cre line that induces recombination in newborn projection neurons generated from the dorsal radial progenitors (Goebbels et al., 2006) were used for APC deletion. Both lines express Cre in respective neurons from around embryonic day 12 (Stenman et al., 2003; Goebbels et al., 2006; Higginbotham et al., 2012) and lead to neuron-type specific deletion of APC (Figure S1). In addition to Dlx5/6-Cre line, I12b-Cre (Potter et al., 2009) was also used to inactivate APC in developing interneurons.
Effect of APC Deletion in Developing Interneurons
The extent and pattern of migration of control and APC deficient interneurons were evaluated in embryonic day 14-P0 cerebral cortices. At E14.5, interneurons migrate in streams through the marginal zone (MZ), intermediate zone (IZ) and subventricular zone (SVZ). Significant reduction in the extent and patterns of interneuronal migration throughout the APC cKO cortex was evident at E14.5 (Figure. 1A–E). The extent of migration into the developing cerebral wall from the ganglionic eminence was reduced in APC cKO when compared with controls (Figure. 1C–E; compare neurons in areas indicated by asterisks in C and D). APC cKO neurons also display defective branching (Figure. 1F). The altered patterns of interneuronal migration in APC cKO persisted at E16.5, the height of interneuronal migration into the developing cerebral wall, and through P0 (Figure. 1G–L). Furthermore, similar changes in interneuronal migration in APC cKO were evident when migrating interneurons were labeled with multiple different interneuron-specific markers (GAD67, Dlx2, Lhx6, and Dlx5) (Figure. 1M–T). We next examined whether the reduction in cortical interneurons migrating in the cortex in APCLox/Lox;Dlx5/6-CIE mutants was due to changes in either interneuronal generation or survival. The number of proliferating progenitors (BrdU+ or PH3+) in the ventricular and subventricular zone of the ganglionic eminence at different embryonic stages was not altered in APC cKO (Figure. S2A–H). We found no differences in the number of cleaved caspase 3+ apoptotic cells in the GE of control and mutant mice at different embryonic stages (Figure. S3I–N). The radial progenitor organization in APC cKO is similar to that of control (Figure. S2U–V). These observations suggest that the loss of APC in APCLox/Lox;Dlx5/6-CIE mutants does not affect the initial generation or survival of post mitotic interneurons in GE. No differences in brain weight between control and mutants were detected at E18 (control, 8.6±0.66mg; APC cKO, 8.6±0.33mg). Average cortical thickness is also similar (control, 812.4±82.4μm; APC cKO, 829±76.4μm). Measurement of GFP+ interneuron density in the striatum at E18 indicates no significant changes (APCLox/+; Dlx5/6-CIE=116±2/10Kμm2; APCLox/Lox; Dlx5/6-CIE=111±8/10Kμm2), thus ruling out potential misrouting of interneurons into ventral telencephalon in APC cKO. However, we did notice an increase in apoptotic cells in the dorsal cortex (Figure. S2O–T), suggesting that some of the aberrantly migrating interneurons may undergo cell death in the cerebral wall. Collectively, these studies confirm that APC activity is essential for the directed migration of interneurons in the developing cerebral cortex.
Figure 1. Deletion of APC leads to interneuronal migration defects.
(A–L) Developmental analysis of E14.5-P0 APCLox/Lox; Dlx5/6-Cre mutants illustrate disrupted patterns of interneuron migration in APC mutants. (A–B) In E14.5 coronal sections of cerebral cortex, asterisk marks migratory streams in the IZ/SVZ and arrow marks the migration front. Comparison of indicated areas in control and mutants illustrate the migratory defects in mutants. (C–E) In higher magnification images of the cerebral wall (C–D), asterisks indicate streams of migrating interneurons. Significant changes in the migratory streams are evident in mutants (compare asterisks in C and D). (E) Quantification of interneuron distribution. E14.5 cerebral wall is divided into 10 equal bins and the number of GFP+ interneurons in each bin is measured. Compared to controls, APC mutants exhibit a significant decrease in the number of GFP+ interneurons migrating into the cerebral wall. Data shown are mean±SEM (n=7). (F) Camera lucida drawings of control and APC deficient interneurons illustrating branching defects in APC mutants. (G–L) interneuron defects in the cerebral wall persist at E16.5 (G, H), E18.5 (I, J), and P0 (K, L). (M–T) In situ hybridization labeling of control and APC cKO cortical (E14.5) sections with interneuronal markers showing similar migration defects. Interneurons are labeled with mRNA probes for GAD67 (M and N), Dlx2 (O and P), Lhx6 (Q and R), and Dlx5 (S and T). Arrows (M–T) mark the migration front. Compare green arrows (controls) to red arrows (mutants) to evaluate the deficit in the extent of migration. (U–W) Disrupted migration of APC deficient interneurons in vitro. (U–V) Dissociated, GFP+ interneurons from control (U) and APC cKO (V) were seeded on feeder layers of dorsal cortical cells and their movement was monitored. Time elapsed between observations are indicated in minutes and interneurons at each time point were pseudo colored in different colors. Green arrows in the merged image show the trajectory of different interneurons during observation. (W) Quantification of migration rate. *, p<0.05 (Student’s t test). Number of cells/group: Control (25), APC cKO (27). GE, ganglionic eminence; D.CX, dorsal cortex; CP, cortical plate; IZ, intermediate zone; VZ, ventricular zone. Scale bar= 430 μm (A, B), 130 μm (C, D), 115 μm (F), 100 μm (G–J), 120 μm (K, L), 357 μm (M–T), 30μm (V, W).
Altered Patterns and Dynamics of Migration of APC Deficient Interneurons
To further evaluate the role of APC in the dynamics of interneuronal movement into the developing cerebral wall, we performed real-time imaging of interneuron migration in control and mutant embryonic cortices (E14.5). Control interneurons normally stream through the MZ and the SVZ/IZ in the cerebral wall and the leading processes of migrating interneurons were primarily oriented dorsally, toward the net direction of their movement. Radially turning interneurons were also apparent in the control cerebral wall (Movie S1). In contrast, fewer APC deficient interneurons migrated through the MZ and the migratory streams in the SVZ/IZ were diffusely organized and scattered in APC cKO brains (Movie S2). The leading processes of APC cKO interneurons migrating through the SVZ/IZ were often oriented toward the ventricular zone. A 2.1±0.03 fold decrease in the number of interneurons migrating radially toward the MZ is evident in APC cKO cortex (Movie S2). Furthermore, compared to controls, the rate of migration of APC deficient interneurons is significantly reduced (control, 45.9±1.7 μm/h [n=127 cells]; APC deficient interneurons, 32.4±2.6 μm/h [n= 108 cells]; P<0.05, [Student’s t-test]). Further, APC null interneurons displayed longer leading processes (control, 50.97±1.9μm [n=30 cells]; APC deficient, 61.99±2.38μm [n=31 cells]; P<0.001 [Student’s t-test]), extended increased number of branches (control, 1.53±0.078 [n=29 cells]; APC deficient, 2.34±0.08 [n=30 cells]; P<0.001 [Student’s t-test]) at an accelerated rate (control, 1.57±0.1 branching events/100 min [n=30 cells]; APC deficient, 1.97±0.1 branching splits/100 min [n=30 cells]; P<0.05 [Student’s t-test]; Movies S1 and S2; Figure. 1F). Together, these real-time live imaging observations indicate that APC activity regulates the migration of interneurons in the developing cerebral cortex. Furthermore, the migration deficit was also evident in in vitro assays in which migration of isolated, individual post mitotic interneurons (control and APC deficient) was monitored (Figure. 1U–W). However, APC deficient interneurons are not impaired in their ability to respond to guidance cues (Figure S3).
Altered Placement of APC Deficient Interneurons
To evaluate how disrupted migration may have affected the final placement of interneurons essential for normal cortical connectivity, we analyzed postnatal APCLox/Lox; I12b-Cre; Ai9 mice (Madisen et al., 2010; Potter et al., 2009). APCLox/Lox; Dlx5/6-Cre mice have malformed lower jaw, preventing them from suckling and thus leading to early death soon after birth (On occasion, APCLox/Lox; Dlx5/6-Cre mice survive to P7 and show interneuron placement defects [Figure. 2A, B]). To circumvent this problem, we used APCLox/Lox; I12b-Cre; Ai9 mice to analyze the effects of APC deletion in late postnatal brains. I12b-Cre was previously used to selectively inactivate genes in embryonic interneurons (Potter et al., 2009) and Ai9 line enables the labeling of Cre recombined cells with tdTomato (Madisen et al., 2010). We analyzed the patterns of distribution of tdTomato+ interneurons in control and APC cKO somatosensory cortex (P30). tdTomato+ neurons in each of the cortical layers (I–VI) were quantified. APC deletion led to significantly fewer interneurons across cortical layers, consistent with the migratory defect (Figure. 2C–F). Together, these results indicate that APC deletion impairs the migration and the resultant final placement of interneurons in the cerebral cortex.
Figure 2. APC deletion disrupts interneuronal placement.
(A–B) Anti-GABA antibody labeling of APCLox/+; Dlx5/6-Cre (B) and APCLox/Lox; Dlx5/6-Cre (B) cortices indicates disrupted placement of INs in APCLox/Lox; Dlx5/6-Cre cortex. (C–D) tdTomato+ interneuron distribution in P30 APCLox/+; I12b-Cre; Ai9 (C) and APCLox/Lox; I12b-Cre; Ai9 (D) cortex. (E) Co-immunolabeling of tdTomato+ interneurons with pan- interneuron specific anti-GABA antibodies. Majority of tdTomato+ cells are GABA positive. (F) Quantification of changes in the distribution of tdTomato+ interneurons across the cortical layers. Sections are from somatosensory cortex and counterstained with DAPI (blue). Data shown are mean ± SEM; asterisk, significant when compared with controls at P<0.05 (Student’s t test). Scale bar= 157 μm (A–D), 20 μm (E).
The Effect of Conditional Ablation of APC in Developing Projection Neurons
To examine if APC function modulates projection neuron migration and connectivity, we generated APCLox/Lox; Nex-Cre; Tau-Lox-STOP-Lox-mGFP (Tau-mGFP) mice. Nex-Cre induces recombination in newborn projection neurons generated from the dorsal radial progenitors. Generation of mice with Nex-Cre specific inactivation of APC, which also contains the Tau-mGFP transgene, enabled us to inactivate APC in projection neurons and label these neurons’ processes with mGFP. We first analyzed changes in the patterns of radial neuronal migration of GFP+ APC-deficient neurons. Notably, at E14, 16, and P0, the extent of radial migration of projection neurons into the developing cortical plate was not affected by APC deletion (Figure 3). The morphology of the GFP+ projection neurons and their position within the dorsal cortex were similar in both controls and APC deficient mice (Figure. 3A–D). At P0, evaluation of the cortical laminar organization with antibodies to different layer specific markers, Cux-1 (Layers 2/3), Ctip-2 (layers 5/6) and Tbr-1 (layer5/6), indicates that neuronal positioning and layer formation was not affected in APC deficient brains (Figure. 3E–H). APC deficient deeper and superficial layer destined neurons, birthdated with EdU (E13.5) and BrdU (E16.5), respectively, arrived at appropriate layers at P7 (Figure. S4). We also examined APC function on deeper layer projection neuron migration and placement, using APCLox/Lox; Nex-Cre; golli-τ-GFP mice in which APC deleted pioneer cortical projection neurons express golli promoter driven GFP (Jacobs et al., 2007). Analysis of GFP+ neuronal positions in E16.5 cortex indicates that GFP+ projection neurons migrated normally in both control and APC cKO mice (Figure. S5A–D). Lastly, we also electroporated Cre expressing vectors into APCLox/Lox embryos and examined the pattern of radial migration of APC deficient neurons. Radial migration of Cre expressing APC deficient neurons was not affected (Figure S5G–K). Together, these observations suggest that APC activity is not necessary for the migration and positioning of cortical projection neurons.
Figure 3. Deletion of APC does not affect Projection neuron migration, placement, and growth.
(A–D) GFP-labeled E14.5 and E16.5 coronal sections from APCLox/+; Nex-Cre; Tau-mGFP (A and C) and APCLox/Lox; Nex-Cre; Tau-mGFP (B and D) mice illustrate similar patterns of migration of GFP+ projection neurons in control and mutant cortices. (E–H) P0 somatosensory cortex of control (E and G) or APC mutant (F and H) mice was immunolabeled with antibodies to Cux1 (layers II–IV), Ctip2 (layer V), and Tbr1 (layer VI). Layer formation and neuronal positioning are not altered following APC deletion in projection neurons. (I–J) Coronal sections from E16.5 control (I) or APC mutant (J) cortex were immunolabeled for GFP (green), L1 (red), and TAG1 (purple). No defects in the patterns of axon growth of APC-deficient projection neurons are evident. Sections in A–F and I–J are counterstained with DRAQ5 (nuclei). CC-corpus callosum, IC-internal capsule, FI-fimbria, AC-anterior commisure. Scale bar= 116 μm (A and B), 606 μm (C and D), 51 μm (E and F), 129 μm (G and H), 550 μm (I and J).
Once projection neurons reach their laminar destination, they establish functional connections by extending axons and dendrites. To examine if the mature patterns of connectivity of projection neurons emerge in the absence of APC, we examined major cortical projection neuron fiber tracts such as cortico-spinal, cortico-thalamic, and commissural projections (corpus callosum and anterior commisure) in the developing cerebral cortex (Figure. 3I–J). These fiber tracts in control and APC deficient brains were immunolabeled with anti-GFP and anti-cell adhesion molecule L1 or TAG1 antibodies. We found that the pattern of extension and the developmental progression of the major cortical fiber tracts (i.e., corticothalamic, cortico-spinal, and commissural projections) are not notably affected by deletion of APC in the APCLox/Lox; Nex-Cre; Tau-mGFP brains. In addition, we also observed no obvious difference in the developmental progression of GFP+ axonal extensions of deeper layer projection neurons in APCLox/Lox; Nex-Cre; golli-τ-GFP mice (Figure. S5E–F). Together, these observations suggest that APC activity alone is not sufficient for the post migratory growth or extension of cortical neuronal projections.
Mechanisms Underlying APC Effect in Interneurons: Altered Patterns of MT Severing in APC Deficient Interneurons
Our observation that the MT cytoskeletal regulator, APC, differentially modulates the migration of interneurons and projection neurons lead us to hypothesize that a selective influence of APC on interneuronal MT cytoskeleton remodeling may underlie its distinct role in interneuronal migration. In particular, MT severing facilitates rapid remodeling of MTs and cell morphology by seeding short MTs to newly forming cell processes (Baas et al., 2005; (McNally and Vale, 1993; Roll-Mecak and McNally, 2010; Sharp and Ross, 2012). Since interneurons undergo rapid and frequent changes in cell shape and process growth during migration as compared to projection neurons, we investigated whether MT severing is affected in APC deficient interneurons. The MT network in APC deficient interneurons or projection neurons from the E14.5 cortices were labeled with anti-acetylated tubulin antibodies and the number of MT breaks per unit length was measured (Sudo and Baas, 2010) as an index of MT severing (Figure. 4A–I). Serial optical scans obtained with super resolution microscopy were used to definitively identify MT breaks. Increased MT severing was noticed in APC deficient interneurons when compared to the controls (Control: 0.22±0.03 breaks/μm; APC cKO: 0.34±0.05 breaks/μm; P<0.05 [Student’s t-test]; Figure. 4A–F, I). In contrast, APC deficient projection neurons did not show any significant changes in MT severing when compared to the controls (Control: 0.10±0.04 breaks/μm; APC cKO: 0.12±0.03 breaks/μm; p=0.68 [Student’s t-test]; Figure. 4G–I). To ascertain the effect of APC deletion on MT severing in interneurons further, we assayed severing of MTs in vitro. Rhodamine-labeled tubulin was polymerized into MTs and extracts from control and APC deficient GE interneurons were tested for their effects on MT severing. Compared to control, APC deficient interneuron extracts enhanced MT severing, leading to an increase in short MT filaments (≤2 μm) in vitro (Figure S6A–F). Extracts from APC deficient projection neurons did not alter MT severing (Figure S6G–K). Together, these results suggest that depletion of APC promotes MT severing in interneurons, but not in projection neurons.
Figure 4. Deletion of APC disrupts MT severing in developing interneurons.
(A–H) MTs in E14.5 control and APC mutant interneurons (INs) and projection neurons (PNs) are immunolabeled with anti-acetylated tubulin antibodies and imaged using a super-resolution microscope. (A–F) APC deficient INs have more breakpoints (arrow heads) than controls, indicating increased MT severing. (G–H) Such differences in MT severing is not evident between control (G) and APC deficient projection neurons (H). (I) Quantification of MT severing in control and APC mutant INs and PNs. Data shown are mean ± SEM; n=40 cells from 4 brains per group; *, significant when compared with controls at p<0.05 (Student’s t test). (J) Comparisons of MT severing protein levels in INs and PNs isolated from the GE and D. Cx of respective control, APCLox/Lox; Dlx5/6-Cre, or APCLox/Lox; Nex-Cre mice. Immunoblot analysis indicates increased p60-katanin in APC deficient INs (arrowhead), but not in PNs. No changes in other MT severing protein levels are evident. (K) Densitometric quantification of p60-katanin levels in control and APC mutants. Data shown are mean ± SEM; n=4 brains per group; *, p<0.05 (Student’s t test). (L–M) Immunolabeling of E14.5 interneurons from APCLox/+; Dlx5/6-Cre (L) and APCLox/Lox; Dlx5/6-Cre (M) GE shows increased expression of p60-katanin in APC deficient interneurons. (N) Serine phosphorylated and ubiquitylated-p60-katanin (arrowhead) are reduced in APC deficient INs. Immunoprecipitated p60-katanin are immunoblotted with anti-phosphoserine and anti-ubiquitin antibodies, respectively. (O) Densitometric quantification of phosphoserine-p60-katanin levels in control and APC mutants. Data shown are mean ± SEM; n=3 brains per group; *, p<0.05 (Student’s t test). (P) APC affects the stability of p60Katanin. Control and APC mutant interneurons were treated with 5μg/ml protein synthesis inhibitor cyclohexamide (CHX) for 3 and 6 hours. APC deletion led to increased stability of p60Katanin. C, control; M, mutant [APC deficient]; IN, interneuron-specific; PN, projection neuron-specific; GE, ganglionic eminence; D.CX, dorsal cortex. Scale bar= 3.1 μm (A–D, G–H), 2.8 μm (E and F), 15 μm (L and M).
APC Regulates MT Severing Protein Activity in Interneurons
To identify the molecular basis of APC-regulated MT severing in interneurons as compared to projection neurons, we tested if the altered MT severing in APC deficient interneurons is due to changes in the levels of MT severing proteins, such as katanin, spastin, and fidgetin (McNally and Vale, 1993; Roll-Mecak and McNally, 2010; Sharp and Ross, 2012) known to be expressed in the developing cortex. Immunoblotting analysis was performed on extracts from GE and DC of APCLox/Lox; Dlx5/6-Cre and APCLox/Lox; Nex-Cre brains, respectively, and the relevant control brains. We found that APC deletion in interneurons significantly increased the levels of p60-katanin catalytic subunit by 23.3±1.4% in APC cKO (Figure. 4J–K). No significant changes were detected in other MT severing molecules, spastin, fidgetin, and p80-katanin regulatory subunit (Figure. 4J). Immunolabeling of isolated interneurons with anti-p60-katanin antibodies also confirms the increase in p60-katanin levels in APC deficient interneurons (Figure. 4L–M). In contrast, APC deletion in projection neurons did not affect the levels of any of the severing proteins (Figure. 4J–K).
p60-katanin activity and degradation is regulated by phosphorylation and subsequent ubiquitination (Maddika and Chen, 2009). Phosphorylation of p60-katanin leads to ubiquitination and degradation. Therefore we investigated whether the increased level of p60-katanin in APC deficient interneurons is due to changes in p60-katanin phosphorylation-mediated ubiquitination. We found that the levels of phosphorylated p60-katanin were significantly reduced (−30.9±4.4%; P<0.05 [Student’s t-test]) in APC deficient interneurons when compared to the controls (Figure. 4N–O). Consistent with changes in the levels of phosphorylated p60-katanin, the ubiquitin conjugation in p60-katanin was also significantly reduced in APC deficient interneurons (Figure. 4N–O). Additionally, cyclohexamide treatment of control and APC cKO interneurons show that APC deletion lead to more stable p60-katanin (Figure. 4P). Together, these results strongly suggest that APC may regulate MT severing in migrating interneurons by modulating p60-katanin stability and thus the level of active p60-katanin.
We also found that stabilizing microtubules with taxol did not affect p60-katanin levels in APC cKO, suggesting MT stability-independent contribution of katanin in APC cKO (Figure. S7A–B). Further, dynamic MTs are known to retain tyrosine side chains. Tubulin detyrosination is less prevalent at leading edges of APC cKO interneurons (Figure. S7C–G), consistent with enhanced branching dynamism noticed in mutants.
Knockdown of p60-katanin Rescues the Migratory Defect in APC Deficient Interneurons
To address whether the increased level of p60-katanin in APC deficient interneurons is responsible for the migration defect, we first tested whether reducing p60-katanin activity can rescue the migration defect seen in APC deficient interneurons. Validated p60-katanin specific shRNA plasmids were electroporated into the MGE of APCLox/+; Dlx5/6-Cre or APCLox/Lox; Dlx5/6-Cre E14.5 cortex and the pattern of migration of electroporated interneurons into the cortex was examined (Figure. 5A–H). Defects in both the extent and rate of migration in APC deficient interneurons were rescued by p60-katanin knockdown (Figure. 5G–H). p60-katanin knockdown also retarded the migration of control interneurons (Figure. 5A–B, I, J). We then analyzed the effect of p60-katanin over expression in wild-type interneurons. We focally electroporated control tdTomato or two p60-katanin constructs, wild-type and triple phospho-mutant (AAA mutant) of katanin (Maddika and Chen, 2009) tagged with tdTomato, into the MGE of wild-type E14.5 cortex and examined the extent of migration of electroporated interneurons into the cortex after 48 hours (Figure. 5K–Q). AAA mutant of p60-katanin contains point mutations at amino acid (aa) 42 (S–>A), aa109 (S–>A), and aa133 (T–>A), thus preventing the phosphorylation-mediated ubiquitination and degradation. Compared to control interneurons, significantly fewer p60-katanin over expressing interneurons entered the dorsal cortex (Figure. 5L, N, P). Further, p60-katanin over expressing interneurons exhibited increased number of branching (Figure. 5R–S), similar to APC deficient interneurons. Together, these results suggest that increased levels of p60-katanin resulting from APC deletion in interneurons disrupt interneuron migration in the cerebral cortex. Appropriate balance of microtubule severing activity regulated by APC is necessary for interneuron migration. In contrast, katanin knockdown or over expression did not affect radial migration of projection neurons (Figure. 5T–W).
Figure 5. p60-katanin mediates APC’s effects on interneuron migration.
(A–D) Migration defects seen in APC deficient interneurons are rescued by knockdown of p60-katanin. E14.5 Control or APCLox/Lox; Dlx5/6-Cre MGE are focally electroporated with control or p60-katanin shRNAs. Extent of electroporated tdTomato+ interneuron migration into dorsal cortex is quantified after 48 hr. Arrowheads mark the migration front. (E–H) Higher magnification images of dorsal cortex (D. Cx) from A, B, C, and D respectively. Katanin knockdown retarded control interneuron entry into D. Cx. Fewer interneurons entered the mutant D. Cx (compare E and G) and this deficit is rescued by p60-Katanin knockdown (compare G and H). (I) Quantification of the distribution of electroporated interneurons indicates diminished migration of interneurons into dorsal cortex in APC mutants. p60-katanin shRNA rescues this defect. p60-katanin shRNA expression in control cortices also retarded IN migration. Migration index are calculated as % of interneurons migrating in three equal sectors (1–3; A) along the dorso-ventral extent of the cerebral wall. Data shown are mean ± SEM; *, significant when compared with respective controls at p<0.05, (Student’s t test). Number of slices and brains: control shRNA (16, 14), p60-katanin shRNA+ control (12, 8), control shRNA+ mutant (8, 6), p60-katanin shRNA+ mutant (21, 10). (J) p60-katanin shRNA rescues the defect in the rate of migration in APC deficient interneurons. Data shown are mean ± SEM; *, significant when compared with respective controls at p<0.05, (Student’s t test). Number of interneurons and brains: control shRNA (54, 4), p60-katanin shRNA+ control (63, 7), control shRNA+ mutant (23, 3), p60-katanin shRNA+ mutant (24, 3). (K–P) The effect of p60-katanin over expression on interneuron migration. pCAG-IRES-tdTomato, p60-katanin wild-type (p60KatWT)-tdTomato, or p60-katanin AAA (p60KatAAA)-tdTomato were focally electroporated into the MGE of E14.5 coronal slices. Electroporated control interneurons leave the MGE and migrate into the dorsal cortex (K). In contrast, interneurons expressing p60KatWT or p60KatAAA display diminished migration into the dorsal cortex (M, O). Arrowheads (K–O) mark the migration front. (L, N, P) Higher magnification images of dorsal cortex (D. Cx) from K, M, and N respectively. Fewer interneurons expressing p60KatWT or p60KatAAA entered the mutant D. Cx (compare L to N and P). (Q) Quantification of the effect of p60-katanin over expression on IN migration. Data shown are mean ± SEM; *, significant when compared with controls at p<0.05 (Student’s t test). Number of slices and brains: Control (6, 5), p60KatWT (8, 7), p60KatAAA (12, 9). (R) Higher magnification images of control and p60-katanin over expressing interneurons. p60-katanin over expressing interneurons exhibit increased branching. (S) Quantification of the branching numbers in p60-katanin over expressing interneurons as compared to controls. Data shown are mean ± SEM; *, significant when compared with controls at p<0.05 (Student’s t test). Number of cells and brains: Control (116, 10) and p60Kat (105, 15). (T–W) The effect of p60-katanin knockdown or over expression on radial migration. (T–V) Control shRNA (T), p60-katanin shRNA (U), or p60-katanin wild-type (V) DNA were electroporated into E14.5 embryos. The maximum extent of radial migration of electroporated neurons into the developing cerebral wall (i.e., migration index) was measured at E17.5. Changes in katanin did not affect radial migration (W). Data shown are mean ± SEM (n=4 brains per group). CP, cortical plate; IZ, intermediate zone; VZ/SVZ, ventricular/subventricular zone. Scale bar= 400 μm (A–D), 275 μm (E–H), 300μm (K, M, O), 200μm (L, N, P), 100μm (R), 120 μm (T–V).
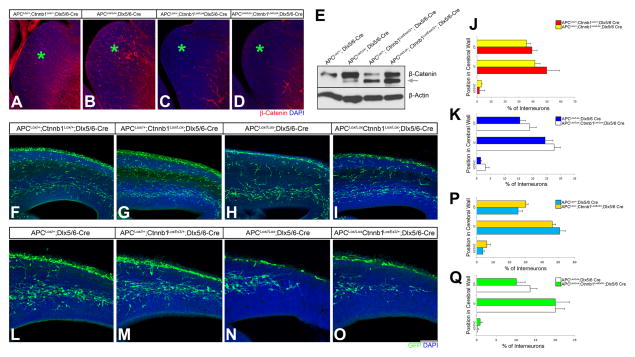
Deletion or Activation of β-catenin Does Not Affect APC Mediated Interneuron Migration
Aside from MTs, APC’s other major cellular target is β-catenin. To delineate whether APC modulated β-catenin signaling mediates any aspect of interneuron migration, we first tested the effect of loss or gain-of-function of β-catenin in interneuron migration. Towards this goal, we conditionally deleted β-catenin in interneurons (Ctnnb1Lox/Lox; Dlx5/6-Cre; Figure. 6A–D) or constitutively activated β-catenin in interneurons (Ctnnb1LoxEx3/+; Dlx5/6-Cre; Figure. 6E). Ctnnb1LoxEx3/+; Dlx5/6-Cre mice die soon after birth because of malformed lower jaw. Notably, neither the deletion (Figure. 6F–G, J) nor induction (Figure. 6L–M, P) of β-catenin affected interneuron migration. We then deleted β-catenin in APC deficient interneurons (APC Lox/Lox; Ctnnb1Lox/Lox; Dlx5/6-Cre) to examine if β-catenin contributed to aberrant patterns of interneuron migration in APC mutants. β-catenin deletion did not rescue or increase the severity of the migration defect seen in APC deficient interneurons (Figure. 6H–I, K). Activating β-catenin expression in APC deficient interneurons (APC Lox/Lox; Ctnnb1LoxEx3/+; Dlx5/6-Cre) also did not rescue APC deficient phenotype (Figure. 6N–O, Q). Together, these results suggest that APC modulated β-catenin signaling does not play a role in interneuron migration.
Figure 6. Effect of β-catenin Signaling on Interneuron Migration.
(A–D) Changes in β-catenin expression in GE after deletion of APC and/or β-catenin. Immunolabeling of E15.5 APCLox/+, Ctnnb1Lox/+, Dlx5/6-Cre (A); APCLox/Lox, Dlx5/6-Cre (B); Ctnnb1Lox/Lox, Dlx5/6-Cre (C); APCLox/Lox, Ctnnb1Lox/Lox, Dlx5/6-Cre (D) cortices with active β-catenin antibody illustrates that the levels of unphosphorylated β-catenin (red) expression in GE (asterisk). Compared to control (A), APC deletion alone leads to increased expression of β-catenin as expected (asterisk, B), whereas β-catenin deletion abolishes its expression (asterisk, C–D). (E) Induction of active β-catenin in GE. Immunoblot analysis indicates corresponding changes of β-catenin expression in E14.5 APCLox/+, Dlx5/6-Cre; APCLox/Lox; Dlx5/6-Cre; APCLox/+, Ctnnb1LoxExon3/+, Dlx5/6-Cre; and APCLox/Lox, Ctnnb1LoxExon3/+,Dlx5/6-Cre cortices. Lower molecular weight band (arrow) is unphosphorylated, active β-catenin, which is induced in Ctnnb1LoxExon3/+; Cre brains. Also note APC deletion upregulates β-catenin levels. (F–M) Deletion of β-catenin on its own or together with APC does not affect interneuron migration. Compared to controls (F), patterns of IN migration were not perturbed after deletion of β-catenin alone (G, J). Similarly, deletion of β-catenin in APC null interneurons (H–I, K) also did not affect the migration of APC deficient INs. (L–Q) Constitutive activation of β-catenin signaling in interneurons (Ctnnb1LoxEx3/+; Dlx5/6-Cre) on its own or in APC deficient interneurons (Ctnnb1LoxEx3/+; APCLox/Lox; Dlx5/6-Cre) also has no effect on interneuron migration. Compared to controls (L), induction of active β-catenin did not affect IN migration (M, P). Further, migration defect in APC deficient INs (N) was not rescued by induction of active β-catenin in APC null INs (O, Q). Panels F–I and L–O are from E15.5 and E14.5 cerebral wall, respectively. GFP+ interneuron distribution in the ventricular/subventricular zone (VZ/SVZ), intermediate zone (IZ), and cortical plate (CP) of the embryonic cerebral wall was quantified (J, K, P, Q). Data shown are mean±SEM (one-way ANOVA, Tukey-Kramer post hoc test, not significant [p>0.05]). Number of brains: 4 (APCLox/+, Ctnnb1Lox/+, Dlx5/6-Cre), 3 (APCLox/+, Ctnnb1Lox/Lox, Dlx5/6-Cre), 3 (APCLox/Lox, Dlx5/6-Cre), 3 (APCLox/Lox, Ctnnb1Lox/Lox, Dlx5/6-Cre), 5 (APCLox/+, Dlx5/6-Cre), 4 (APCLox/+, Ctnnb1LoxExon3/+, Dlx5/6-Cre), 3 (APCLox/Lox, Dlx5/6-Cre), and 4 (APCLox/Lox, Ctnnb1LoxExon3/+, Dlx5/6-Cre). Scale bar= 263 μm (A–D), 875 μm (F–I), 100 μm (J–M and O–R).
APC2 and projection neuron migration
APC2, a second APC-like molecule in mammals is expressed in radially migrating neurons. To define the relative roles of APC and APC2 in projection neuron migration and connectivity, we generated compound APCLox/Lox; APC2−/−; Nex-Cre mutants (Figure 7). Using this mouse genetic model in which developing projection neurons are deficient in both APC and APC2, we first evaluated patterns of neuronal migration and the resultant laminar organization. Immunolabeling of E16.5 cerebral cortex with antibodies to layer specific markers, Cux1 (Layers 2/3), Ctip2 (layers 5/6), and Tbr1 (layer5/6), indicates that, as noticed earlier (Shintani et al., 2012), neuronal migration and laminar organization were severely impaired in APC2 null cortexes (Figure 7), but not in APC deficient cortex (Figure. 7A–B, E–F). APC2 deficiency led to inversion of cortical layers and new neurons do not migrate past previously generated neurons as in control cortices (Figure. 7C–D, G–H). Importantly, APC/APC2 double null mice indicate that APC and APC2 do not exert any synergistic effects on neuronal migration and APC2 is the critical APC isoform for radial migration and placement of projection neurons. To examine if APC2 affects MT severing activity, we examined MT severing enzyme levels in APC2 null and control brains. We found that APC2 deletion did not affect the expression of any of the MT severing enzymes (p-60 katanin, p80-katanin, spastin, and fidgetin [Figure. 7I]), suggesting that APC2 effect on radial migration does not involve regulation of MT severing activity.
Figure 7. APC2, but not APC1 is required for projection neuron migration and placement.
(A–H) P0 somatosensory cortex of control Nex-Cre, APCLox/+, APC2+/− (A, E); Nex-Cre, APCLox/Lox, APC2+/− (B, F); Nex-Cre, APCLox/+, APC2−/− (C, G); and Nex-Cre, APCLox/Lox, APC2−/− (D, I) were immunolabled with antibodies to Cux1 (layers II-IV), Ctip2 (layer V/VI), and Tbr1 (layer VI). Neuronal migration and neuronal positioning are not altered following APC deletion in newborn projection neurons (A–B, E–F), but are compromised following APC2 deletion (C–D, G–H). Neuronal migration and laminar organization depends primarily on APC2, since no synergistic effect was noticed in APC/APC2 double mutants (compare C and D, G and H). Sections (E–H) were nuclear counterstained with DAPI. (I) The effect of APC2 on MT severing proteins. Immunoblot analysis of MT severing protein levels in the GE and DC of wild type, heterozygous, and homozygous null APC2 mice. No changes in MT severing protein levels are evident in APC2 null brains. β-actin was used as a loading control. Scale bar= 150μm. GE, ganglionic eminence; DC, dorsal cortex.
Discussion
Neuron specific deletion of APC shows that APC inactivation specifically interrupts interneuron migration and their final placement, but not projection neuron migration, placement and connectivity. The effects of APC in interneurons depend on p60-katanin mediated MT severing, but not on the β-catenin pathway. These observations indicate that interneurons employ dynamic severing and restructuring of MTs as a mechanism to modulate MT stability and facilitate their distinct mode of migration in the developing cerebral cortex.
APC’s modulation of MT dynamics and β-catenin activity during interneuron migration
The two major downstream components of APC signaling are β-catenin pathway and MT cytoskeleton. APC is an integral component of the destruction complex that normally promotes β-catenin phosphorylation and proteosomal degradation. APC activity is also known to be critical for MT organization and stability. Our results demonstrate a hitherto uncharacterized contribution of APC to MT severing in cortical interneurons. Deletion of β-catenin activity in APC mutant interneurons (APC Lox/Lox; Ctnnb1 Lox/Lox; Dlx5/6-Cre) did not alter the migration defect seen in APC deficient interneurons. Deletion of β-catenin alone in interneurons (Ctnnb1Lox/Lox; Dlx5/6-Cre) did not affect their migration (Figure 6). Further, conditional activation of β-catenin signaling in interneurons (Ctnnb1LoxEx3/+; Dlx5/6-Cre) also did not affect interneuron migration (Figure 6). Together, these results suggest that APC’s effect on interneuron migration does not depend on APC regulated β-catenin signaling, but on MT dynamics.
APC mediated regulation of MT severing in interneurons
Notably, our results indicate that MT severing is compromised in APC deficient interneurons, thus affecting their morphology, migration, and final placement in the developing brain. Katanin, composed of a p60 subunit, an ATPase that disassembles and severs MTs to tubulin dimers, and a non-enzymatic p80 subunit that targets katanin to the centrosome (McNally and Vale, 1993), is essential for regulating MT length and reorientation (Roll-Mecak and McNally, 2010; Sharp and Ross, 2012; Lindeboom et al., 2013). APC modulates MT stability and severing by regulating the activity of p60-katanin in interneurons, and thus enabling interneuronal MT networks to reorganize in a dynamic manner during migration. APC-regulated MT severing may enable interneurons to rapidly initiate and modify branches during migration.
MT severing by katanin in neurons depends on the level, phosphorylation status, and subcellular distribution of katanin. Phosphorylation of p60-katanin inhibits its MT severing activity (Loughlin et al., 2011; Maddika and Chen, 2009; Roll-Mecak and McNally, 2010; Sharp and Ross, 2012). Modification of the MTs, such as acetylation and tau binding, also determines the regions on MT filaments targeted by Katanin (Sudo and Baas, 2010). In the absence of APC, p60-katanin levels increase as a result of decreased p60-katanin serine phosphorylation and degradation. APC deletion also decreases the binding affinity of tau to the MTs (data not shown), which in turn can promote the efficiency of katanin’s severing activity during MT reorganization in interneurons. Thus, enhanced MT severing activity in the absence of APC may have led to aberrant patterns of migration of interneurons. Collectively, our studies indicate that a balanced regulation of microtubule severing activity is necessary for appropriate interneuron migration. Deletion of APC disrupts this balance and thus migration.
Mechanisms underlying the differences in the regulation of MT severing in interneurons and projection neurons
Migrating interneurons exhibit a highly stereotyped movement with rapid morphological changes of their leading processes. In contrast, projection neurons move uni-directionally along radial glial guides while maintaining a major leading and trailing process. The different patterns of migration of interneurons and projection neurons suggest that interneurons may require distinct mechanisms for dynamic alterations in the MT scaffolding as compared to projection neurons, which rely more on cell-cell adhesion mechanisms to move along radial glial guides. Neuronal migration based on rapid branching and remodeling of processes may require a highly dynamic pool of MTs to facilitate this activity. MT severing in interneurons serves this goal. The nature of the pathway through which APC regulates MT severing enzyme activity and how the balance between APC’s direct regulation of MT stability and indirect regulation of severing is achieved are still open questions. APC does not bind katanin. APC deletion may decrease the binding affinity of MAPs such as tau to the MTs, which in turn can promote the efficiency of katanin’s MT severing activity. APC deletion may also alter the activity of kinases (e.g., DYRK2) and phosphatases (e.g., PP4c), which can then modulate the stability, phosphorylation, or distribution of p60-katanin (Roll-Mecak and McNally, 2010; Sharp and Ross, 2012). Further, activity of enzymes such as tubulin acetyl transferase (TAT) acetylates α-tubulin and stabilizes microtubules. Enhanced severing of MTs in APC mutants may have prevented the appropriate stabilization of MTs by TAT necessary for oriented migration (Szyk et al., 2014) and led to more unstable MTs. APC’s direct role as an RNA-binding protein in specifying β2b-tubulin subcellular distribution and MT organization may also indirectly affect MT severing (Preitner et al., 2014). APC did not affect other MT severing enzymes, spastin and fidgetin. Further identification of other atypical regulators of MT severing (e.g., kinases or phosphatases that regulate serine phosphorylation of p60-katanin or tau) in the developing cortical neurons will help define the specific signaling pathways that differentiate MT dynamics in the developing projection neurons and interneurons. It will help identify the contributions of the dynamic interplay between MT severing and stability in the migration of these two types of neurons.
APC’s role in projection neuron migration
Contrary to APC’s role in interneuron migration and placement, its relative contribution to radial neuronal migration of projection neurons is not significant. In contrast to earlier in vitro studies with undefined cortical neurons (Chen et al., 2011), our data show that the migration and laminar positioning of cortical projection neurons in vivo are not influenced by APC activity (Fig. 3). However, APC2, a second APC-like molecule in mammals, is highly expressed in cortical neurons throughout the brain (van Es et al., 1999; Shintani et al., 2012). Migration and laminar defects in APC2-deficient brains (Figure 7; Shintani et al., 2012) and the lack of synergistic effects between APC and APC 2 (Figure 7) suggest that APC2, not APC, is essential in the radially migrating projection neurons. APC2, however, lacks the C-terminal MT binding domain present in APC and its influence on MT cytoskeleton, including tubulin localization and expression (Preitner et al., 2014), remains to be defined. Further, phosphorylation of cofilin, an actin-depolymerizing protein, and the resultant stabilization of the actin filaments is required for proper radial migration of projection neurons (Bellenchi et al. 2007; Chai X et al. 2009). It is conceivable that glial-guided radial migration of cortical neurons may rely on cytoskeletal mechanisms involving stabilization of the actin cytoskeleton, whereas tangential migration of INs may require rapid regulation of MT dynamics. In both cases, cytoskeletal regulators with severing activity on distinct cytoskeletal components are required.
Conclusions
Both interneurons and projection neurons migrate next to each other in the developing cerebral wall in distinctly different ways. How interneurons selectively convert extracellular signals in the cerebral wall to modulate MT severing via APC remains an open question. Importantly, what molecular triggers tweak the common cytoskeletal machinery in interneurons and projection neurons to achieve their distinctly different migratory behavior? Our studies demonstrate how interneurons employ MT severing as a means to achieve this goal. Recent studies indicate that MT severing enzyme family members KATNAL1 and 2 (KATANIN p60 subunit A-like 1 and 2) are candidate genes for human 13q12.3 microdeletion syndrome characterized by intellectual disability and autism spectrum disorders, respectively (Neale et al., 2012; Sanders et al., 2012; Bartholdi et al., 2014). Further understanding of the integration of dynamic intracellular mechanisms governing different types of neuronal migration will help decipher how the process of migration leads to the emergence of functional neuronal circuitry in the cerebral cortex. In addition, characterization of tubulin diversity and the dynamic interplay between MT stability and severing (Tischfield et al., 2011; Chen et al., 2013; Godin et al., 2012; Preitner et al., 2014) in interneurons and projection neurons will also help define how these two different types of cortical neurons build MT structures that subserve diverse patterns of cell migration.
Materials and Methods
Mice
Mice were cared for according to animal protocols approved by the University of North Carolina. APC was conditionally inactivated in interneurons or projection neurons by mating mice carrying an APC allele flanked by loxP site (Shibata et al., 1997) with either Dlx5/6-Cre (Stenman et al., 2003) or Nex-Cre; Tau-Lox-STOP-Lox-mGFP (Goebbels et al., 2006; Kramer et al., 2006) mice. Littermate APCLox/+; Dlx5/6-Cre or APCLox/+; Nex-Cre; Tau-Lox-STOP-Lox-mGFP mice served as controls. I12b-Cre; Ai9 (Potter et al., 2009; Madisen et al., 2010) was also used to inactivate APC in the GE. Ctnnb1Lox/Lox (Brault et al., 2001) and Ctnnb1LoxEx3/LoxEx3 (Zechner et al., 2003) mice were crossed with Dlx5/6-Cre to inactivate or activate β-catenin signaling in interneurons, respectively. Golli-τ-GFP mice (Jacobs et al., 2007) were gift from Dr. A. Campagnoni (UCLA).
Immunohistochemistry, Immunoprecipitation and In Situ Hybridization
Cerebral cortical sections and cortical cells were immunolabeled as previously described (Witte et al., 2008; Yokota et al., 2009). The following primary antibodies were used: calretinin, Ctip2, GFP, mRFP (Abcam); BrdU (BD Biosciences); EdU (Invitrogen); cleaved caspase 3 (Cell Signaling); GABA, (Sigma-Aldrich); TAG1 (Iowa Hybridoma Bank); acetylated tubulin, active-β-catenin, L1, PH3, Tbr1 (Millipore); APC, Cux1, p60-katanin, p80-katanin (Santa Cruz). Appropriate Cy2, Cy3, or Alexa dye conjugated secondary antibodies (Jackson ImmunoResearch, Molecular Probes) were used to detect primary antibody binding. DRAQ5 (Alexis) or DAPI (Invitrogen) was used as nuclear counterstain. In situ hybridization labeling of embryonic control and APC cKO cerebral cortical sections was performed as described in McKinsey et al., 2013. The following cDNA probes were used: Dlx2, Dlx5, Lhx6, and Gad67 (McKinsey et al., 2013). Immunoprecipitation and immunoblot analyses of control and APC cKO cortex were performed as described in Yokota et al., 2009 and Higginbotham et al., 2012. See supplemental data for details.
Electroporation of Medial Ganglionic Eminence
MGE of E14. 5 embryos were electroporated with control shRNA, p60-katanin shRNA, wild-type p60-katanin, or triple phospho-mutant p60-katanin cDNA as outlined in Higginbotham et al., 2012 and Yokota et al., 2007. Wild-type and triple phospho-mutant p60-katanin cDNAs were generously gifted by Dr. Maddika Subba Reddy (CDFD, India). See supplemental data for details.
Live Imaging of Neuronal Migration in Cortical Slices
E14.5 cortices were removed from the embryos, embedded in 3 % low-melting-point agarose in complete Hank’s Balanced Salt Solution and coronally sectioned (250 μm) on a vibratome (Leica VT 1000S). Sections were then mounted on Millicell-CM membrane filters (Millipore), placed in glass-bottom FluoroDish chambers (World Precision Instruments, Inc.) and maintained in MEM/10 % fetal bovine serum (FBS) at 37 °C and 5 % CO2. GFP-expressing interneurons were repeatedly imaged using a Zeiss LSM780 inverted confocal microscope attached to a live cell incubation chamber. Zeiss LSM Image Browser or ImageJ Software was used for quantification of migration patterns (Yokota et al., 2009, Stanco et al., 2009, and Higginbotham et al., 2012). Also see supplemental methods.
In Vitro Microtubule Severing Assay
Rhodamine-labeled MTs were generated as described in the manufacturer’s instructions(Cytoskeleton. Inc.) and used to test MT severing activity in control and APC deficient extracts. See supplemental data for details.
Supplementary Material
Supplemental Figure 1 (related to Figures 1 and 3). Cre-mediated ablation of APC in the developing cortical neurons. (A–B) GFP expression in E14.5 GE of APCLox/+; Dlx5/6-Cre (A) and APCLox/Lox; Dlx5/6-Cre (B) indicates Cre expression in newborn interneurons. Color separated images are shown underneath panels A and B. (C–D) APC immunolabeling (red) indicates the loss of APC in newborn, GFP+ interneurons in APCLox/Lox; Dlx5/6-Cre GE. High magnification images of newborn interneurons from GE (asterisk, A–B) of APCLox/+; Dlx5/6-Cre (C) and APCLox/Lox; Dlx5/6-Cre (D) illustrating the lack of APC expression in APC mutant interneurons (arrowhead, D). Arrow (C) indicates APC expressing control interneurons (red-APC, green-GFP, yellow-merge). (E, F) Migrating control interneurons (arrow, E) in the cerebral wall express APC, but not the mutant interneurons (arrowhead, F). (G–H) Interneurons from E14.5 GE of APCLox/+; Dlx5/6-Cre (H) and APCLox/Lox; Dlx5/6-Cre (H) illustrating the lack of APC expression in APC deficient interneurons (arrow, H). Arrowhead (H) indicates expression of APC in non-GFP positive control cells. (I) Immunoblot analysis of control and APCLox/Lox; Dlx5/6-Cre GE extracts confirms the loss of APC in APC mutant brains. (J–K) Comparison of APC expression in APCLox/+; Nex-Cre (J) and APCLox/Lox; Nex-Cre (K) cortical slices (E16.5) show reduced APC levels in mutant dorsal cortex (compare areas indicated by asterisk). Color separated images are shown underneath panels J and K. (C–D) High magnification images of migrating projection neurons from areas indicated by asterisks (L, M) in APCLox/+; Nex-Cre (L) and APCLox/Lox; Nex-Cre (M) cortex illustrating the lack of APC expression in APC deficient projection neurons (arrowhead, M). Arrow (L) indicates expression of APC in GFP+ control neurons. (N–O) Projection neurons isolated from E14.5 dorsal cortex of APCLox/+; Nex-Cre (N) and APCLox/Lox; Nex-Cre (O) mice illustrating the lack of APC expression in APC deficient projection neurons (arrow, O). Arrowhead (O) indicates expression of APC in GFP negative control neurons. (P) Immunoblot analysis of dorsal cortical extracts from control and APCLox/Lox; Nex-Cre mice illustrates the loss of APC in mutant brains. GFP and β-actin were used as loading controls. CP, cortical plate; IZ, intermediate zone; VZ, ventricular zone; GE, ganglionic eminence. GFP and β-actin were used as loading controls. Scale bar= 250 μm (A–B, J–K), 60 μm (C–D, L, M), 70 μm (E–H, N–O).
Supplemental Figure 2 (related to Figure 2). Analysis of cell proliferation and cell death in the APCLox/Lox; Dlx5/6-Cre brains. (A–H) Cell proliferation in the APCLox/Lox; Dlx5/6-Cre mice. Anti-BrdU antibody labelling of BrdU-pulsed E14.5 (A–C) and E16.5 (E–G) GE did not reveal significant differences in mitotically active, BrdU+ progenitors between APCLox/+; Dlx5/6-Cre control (A, C, E, G) and APCLox/Lox; Dlx5/6-Cre mutant (B, C, F, G). (D, H) Quantification of mitotically active progenitors with M-phase marker PH3 also did not reveal changes in APCLox/Lox; Dlx5/6-Cre GE. Data shown are mean ± SEM; n=3 brains per group. (I–T) Cell death in the APCLox/Lox; Dlx5/6-Cre mice. Cleaved caspase 3 expression in E14.5 (I–J) and E16.5 (K–L) GE from APCLox/+; Dlx5/6-Cre (I, K) and APCLox/Lox; Dlx5/6-Cre (J, L) brains. No significant changes in apoptotic cells were noticed in the VZ/SVZ of GE in the mutants (M, N). (O–T) Cleaved caspase 3 expression in E16.5 (O–P) and P0 (Q–R) dorsal cortex from APCLox/+; Dlx5/6-Cre (O, Q) and APCLox/Lox; Dlx5/6-Cre (P, R) brains. An increase in apoptotic cells (arrowheads) was noticed in the mutants (P, R, S, T). Data shown are mean ± SEM; n=3 brains per group. *, p<0.05 (Student’s t test). (U–V). Labeling with RC2 antibodies indicates normal radial progenitor organization in APCLox/Lox; Dlx5/6-Cre GE. Scale bar= 200μm (A, B, E, F); 175μm (I–L, O–R), 110μm (U, V). SVZ, subventricular zone; VZ, ventricular zone.
Supplemental Figure 3 (related to Figure 1). APC is not required for interneuron guidance towards endogenous guidance cues from dorsal cortex. (A) Schematic of microfluidic chamber migration assay. Dissociated wild-type dorsal cortical cells (DCx, blue) were plated in one side of a chamber and dissociated interneurons (INs) from the GE of control (APCLox/+; Dlx5/6-CIE) or mutant (APCLox/Lox; Dlx5/6-CIE) cortex were plated in the other side. Interneurons were exposed to a microfluidic gradient of cues released by dorsal cortical neurons. Interneurons navigate through the microgradient of guidance cues that is established in the microchannels (vertical lanes) that separate the two chambers towards the dorsal cortical cells. (B–D) No significant differences were evident between control and APC deficient interneurons. (D) Quantification of the extent of migration. Number of cells per group= 700. Data shown are mean ± SEM. Scale bar= B–C, 50μm.
Supplemental Figure 4 (related to Figure 3). APC does not affect projection neuron migration. (A and B) Cortical neurons born in E13.5 and E16.5 are birthdated with EdU and BrdU, respectively. The positions of birthdated neurons are analyzed in postnatal day 7 control (A) and APC deficient, APCLox/Lox; Nex-Cre, cortex (B). No significant defects in neuronal placement are evident in APC cKO. Scale bar=129 μm.
Supplemental Figure 5 (related to Figures 3 and 7). APC function in projection neuron migration and connectivity. (A–D) E16.5 APCLox/Lox; Nex-Cre; golli-τ-GFP and control mice are immunolabeled with anti-GFP, Tbr1 (layer5/6) antibodies and nuclear counterstained with DRAQ5. APC-deficient GFP+ deeper layer neurons migrated normally. (E and F) Placement and initial axon growth of projection neurons is not affected in APCLox/Lox; Nex-Cre; golli-τ-GFP mice. GFP-immunolabeling of sagittal sections from P0 control and APC mutant cortex indicates generally normal neuronal migration, placement, and developmental progression of axon growth in APC mutant (F) as compared to control (E). (G–K) APC deletion does not affect radial migration. E14 embryos (APCLox/+ [G, I] and APCLox/Lox [H, J]) were electroporated with Cre-EGFP and the radial migration of control and APC deficient neurons were analyzed at E16.5. Compared to control (I, arrow), cre expression in APC Lox/Lox cortex leads to loss of APC expression in radially migrating neurons (J, arrow; [arrowhead shows APC expression in Cre-GFP negative cell]). (K) Quantification of the distribution of electroporated cells in the cerebral wall indicates normal patterns of radial migration in APC Lox/Lox; Cre cortex. CP, cortical plate; SP, sub plate; IZ, intermediate zone; VZ, ventricular zone. Scale bar= 77 μm (A–D), 340 μm (E, F), 100μm (G, H), 60μm (I, J).
Supplemental Figure 6 (related to Figure 4). Increased MT severing activity in APC deficient interneurons. (A–D) Rhodamine labeled MT filaments (A, C) were treated with E14.5 GE extracts from APCLox/+; Dlx5/6-Cre (B) and APCLox/Lox; Dlx5/6-Cre (D) brains. Increased severing (arrowheads) was noticed in MTs treated with mutant extracts, but not with control extracts. (E) Percent changes in ≤2μm fragments of MTs resulting from MT severing was quantified and used as an index of severing activity. Data shown are mean ± SEM. n=11 (APCLox/+; Dlx5/6-Cre), 12 (APCLox/Lox; Dlx5/6-Cre). (F) Treatment of MT filaments with different concentrations of GE extracts. Increased severing activity in APCLox/Lox; Dlx5/6-Cre extracts led to shorter MT filaments (n=4). (G–L) Treatment of MT filaments (G, I) with E14.5 dorsal cortex extracts from APCLox/+; Nex-Cre (H) and APCLox/Lox; Nex-Cre (J) brains. No significant changes in severing (arrowheads) was noticed in MTs treated with APC deficient projection neuron extracts. (K) Quantification of severing activity of Nex-Cre extracts. (L) Effect of different concentrations of Nex-Cre extracts on MT filaments. Data shown are mean ± SEM. n=4. *, significant when compared with controls at p<0.05 (Student’s t test) *, significant when compared with controls at p<0.05 (Student’s t test). Scale bar= 2 μm.
Supplemental Figure 7 (related to Figure 5). Taxol stabilization of microtubules does not affect katanin levels in APC deficient interneurons and APC deletion increases tyrosinated tubulin at the tips of interneuronal processes. (A–B) Control [C] and APC mutant [M] interneurons were treated with Taxol (5nM) for 24 hours. Taxol stabilization did not affect Katanin levels in APC deficient interneurons. Levels of stable acetylated tubulin did increase as expected after Directions of the ganglionic eminence (GE) and dorsal cortex (D. CX) are indicated. P-pial surface, V-ventricular surface. Time length = 290 minutes.
Time lapse images of interneurons migrating toward the dorsal cortex (D.CX). Arrow heads (yellow) indicate streams of migrating interneurons in the MZ and IZ. A interneuron with asterisk (purple) shows its dynamic movements within the developing cerebral wall as interneurons navigate towards their target layer. Directions of the GE and dorsal cortex (D.CX) are indicated. P-pial surface, V-ventricular surface. Time length = 240 minutes.
APC deletion leads to disrupted, unorganized streams of tangentially migrating interneurons in the cerebral wall. Increased number and frequency of branching are noticed in APC deficient interneurons.
Acknowledgments
This research was supported by NIH grant MH060929 to EA. We thank Suriya Thompson for technical assistance and L. Pevny and S. A. Anderson (U Penn) for helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Aoki K, Taketo MM. Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci. 2007;120:3327–3335. doi: 10.1242/jcs.03485. [DOI] [PubMed] [Google Scholar]
- Baas PW, Karabay A, Qiang L. Microtubules cut and run. Trends Cell Biol. 2005;15:518–524. doi: 10.1016/j.tcb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Barth AI, Caro-Gonzalez HY, Nelson WJ. Role of adenomatous polyposis coli (APC) and microtubules in directional cell migration and neuronal polarization. Semin Cell Dev Biol. 2008;19:245–251. doi: 10.1016/j.semcdb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Fishell G. The developmental integration of cortical interneurons into a functional network. Curr Top Dev Biol. 2009;87:81–118. doi: 10.1016/S0070-2153(09)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholdi D, et al. A newly recognized 13q12.3 microdeletion syndrome characterized by intellectual disability, microcephaly, and eczema/atopic dermatitis encompassing the HMGB1 and KATNAL1 genes. Am J Med Genet A. 2014;164A:1277–1283. doi: 10.1002/ajmg.a.36439. [DOI] [PubMed] [Google Scholar]
- Bellenchi GC, Gurniak CB, Perlas E, Middei S, Ammassari-Teule M, Witke W. N-cofilin is associated with neuronal migration disorders and cell cycle control in the cerebral cortex. Genes Dev. 2007;21:2347–2357. doi: 10.1101/gad.434307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Chai X, Forster E, Zhao S, Bock HH, Frotscher M. Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine3. J Neurosci. 2009;29:288–299. doi: 10.1523/JNEUROSCI.2934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Tian X, Kim WY, Snider WD. Adenomatous polyposis coli regulates axon arborization and cytoskeleton organization via its N-terminus. PLoS One. 2011;6:e24335. doi: 10.1371/journal.pone.0024335. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Chen J, Shi H, Wei M, Castaneda-Castellanos DR, Bultje RS, Pei X, Kriegstein AR, Zhang M, Shi SH. Regulation of microtubule stability and organization by mammalian Par3 in specifying neuronal polarity. Dev Cell. 2013;24:26–40. doi: 10.1016/j.devcel.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evsuykova I, Plestant C, Anton ES. Integrative mechanisms of oriented neuronal migration in the developing brain. Annu Rev Cell Dev Biol. 2013;29:299–353. doi: 10.1146/annurev-cellbio-101512-122400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin JD, Thomas N, Laguesse S, Malinouskaya L, Close P, Malaise O, Purnelle A, Raineteau O, Campbell K, Fero M, Moonen G, Malgrange B, Chariot A, Metin C, Besson A, Nguyen L. p27(Kip1) is a microtubule-associated protein that promotes microtubule polymerization during neuron migration. Dev Cell. 2012;23:729–44. doi: 10.1016/j.devcel.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave KA. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis. 2006;44:611–621. doi: 10.1002/dvg.20256. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, et al. Apoptosis in neural crest cells by functional loss of APC tumor suppressor gene. Proc Natl Acad Sci U S A. 2002;99:297–302. doi: 10.1073/pnas.012264999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham H, Eom TY, Mariani LE, Bachleda A, Hirt J, Gukassyan V, Cusack CL, Lai C, Caspary T, Anton ES. Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev Cell. 2012;23:925–938. doi: 10.1016/j.devcel.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaniutsin U, Chen Y, Mason JO, Price DJ, Pratt T. Adenomatous polyposis coli is required for early events in the normal growth and differentiation of the developing cerebral cortex. Neural Dev. 2012;4 doi: 10.1186/1749-8104-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EC, et al. Visualization of corticofugal projections during early cortical development in a tau-GFP-transgenic mouse. Eur J Neurosci. 2007;25:17–30. doi: 10.1111/j.1460-9568.2006.05258.x. [DOI] [PubMed] [Google Scholar]
- Kramer I, Sigrist M, de Nooij JC, Taniuchi I, Jessell TM, Arber S. A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron. 2006;49:379–393. doi: 10.1016/j.neuron.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Sestan N, Anton ES. Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex. Development. 2012;139:1535–1546. doi: 10.1242/dev.069963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeboom JJ, Nakamura M, Hibbel A, Shundyak K, Gutierrez R, Ketelaar T, Emons AMC, Mulder BM, Kirik V, Ehrhardt DW. A mechanism for reorientation of cortical microtubule arrays driven by microtubule severing. Science. 2013;342:1245533. doi: 10.1126/science.1245533. [DOI] [PubMed] [Google Scholar]
- Loughlin R, Wilbur JD, McNally FJ, Nedelec FJ, Heald R. Katanin contributes to interspecies spindle length scaling in Xenopus. Cell. 2011;147:1397–1407. doi: 10.1016/j.cell.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddika S, Chen J. Protein kinase DYRK2 is a scaffold that facilitates assembly of an E3 ligase. Nat Cell Biol. 2009;11:409–419. doi: 10.1038/ncb1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzini MC, Walsh CA. What disorders of cortical development tell us about the cortex: one plus one does not always make two. Curr Opin Genet Dev. 2011;21:333–339. doi: 10.1016/j.gde.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney BM, Nathke IS. Cell regulation by the Apc protein Apc as master regulator of epithelia. Curr Opin Cell Biol. 2008;20:186–193. doi: 10.1016/j.ceb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- McKinsey GL, Lindtner S, Trzcinski B, Visel A, Pennacchio LA, Huylebroeck D, Higashi Y, Rubenstein JL. Dlx1&2-dependent expression of Zfhx1b (Sip1, Zeb2) regulates the fate switch between cortical and striatal interneurons. Neuron. 2013;77:83–98. doi: 10.1016/j.neuron.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally FJ, Vale RD. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell. 1993;75:419–429. doi: 10.1016/0092-8674(93)90377-3. [DOI] [PubMed] [Google Scholar]
- Metin C, Vallee RB, Rakic P, Bhide PG. Modes and mishaps of neuronal migration in the mammalian brain. J Neurosci. 2008;28:11746–11752. doi: 10.1523/JNEUROSCI.3860-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–245. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development. 2002;129:3147–3160. doi: 10.1242/dev.129.13.3147. [DOI] [PubMed] [Google Scholar]
- Potter GB, Petryniak MA, Shevchenko E, McKinsey GL, Ekker M, Rubenstein JL. Generation of Cre-transgenic mice using Dlx1/Dlx2 enhancers and their characterization in GABAergic interneurons. Mol Cell Neurosci. 2009;40:167–186. doi: 10.1016/j.mcn.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preitner N, Quan J, Nopwakowski DN, Hancock ML, Shi J, Tcherkezian J, Young-Pearse TL, Flanagan JG. APC is an RNA-binding protein, and its interactome provides a link to neural development and microtubule assembly. Cell. 2014;158:368–382. doi: 10.1016/j.cell.2014.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A, McNally FJ. Microtubule-severing enzymes. Curr Opin Cell Biol. 2010;22:96–103. doi: 10.1016/j.ceb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Boeda B, Etienne-Manneville S. APC binds intermediate filaments and is required for their reorganization during cell migration. J Cell Biol. 2013;200:249–258. doi: 10.1083/jcb.201206010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H, et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science. 1997;278:120–123. doi: 10.1126/science.278.5335.120. [DOI] [PubMed] [Google Scholar]
- Shintani T, Takeuchi Y, Fujikawa A, Noda M. Directional neuronal migration is impaired in mice lacking adenomatous polyposis coli 2. J Neurosci. 2012;32:6468–84. doi: 10.1523/JNEUROSCI.0590-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Ross JL. Microtubule-severing enzymes at the cutting edge. J Cell Sci. 2012;125:2561–9. doi: 10.1242/jcs.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003;23:167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanco A, Szekeres C, Patel N, Rao S, Campbell K, Kreidberg JA, Polleux F, Anton ES. Netrin-1-alpha3beta1 integrin interactions regulate the migration of interneurons through the cortical marginal zone. Proc Natl Acad Sci U S A. 2009;106:7595–600. doi: 10.1073/pnas.0811343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo H, Baas PW. Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. J Neurosci. 2010;30:7215–7226. doi: 10.1523/JNEUROSCI.0048-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyk A, Deaconescu AM, Spector J, Goodman B, Valenstein ML, Ziolkowska NE, Kormendi V, Grigorieff N, Roll-Mecak A. Molecular basis for age- dependent microtubule acetylation by tubulin acetyltransferase. Cell. 2014;157:1405–1415. doi: 10.1016/j.cell.2014.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischfield MA, Cederquist GY, Gupta ML, Jr, Engle EC. Phenotypic spectrum of the tubulin-related disorders and functional implications of disease-causing mutations. Curr Opin Genet Dev. 2011;21:286–294. doi: 10.1016/j.gde.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente M, Marin O. Neuronal migration mechanisms in development and disease. Curr Opin Neurobiol. 2010;20:68–78. doi: 10.1016/j.conb.2009.12.003. [DOI] [PubMed] [Google Scholar]
- van Es JH, Kirkpatrick C, van de Wetering M, Molenaar M, Miles A, Kuipers J, Destree O, Peifer M, Clevers H. Identification of APC2, a homologue of the adenomatous polyposis coli tumour suppressor. Curr Biol. 1999;9:105–108. doi: 10.1016/s0960-9822(99)80024-4. [DOI] [PubMed] [Google Scholar]
- Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Wallar BJ, Alberts AS, Gundersen GG. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol. 2004;6:820–830. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- Witte H, Neukirchen D, Bradke F. Microtubule stabilization specifies initial neuronal polarization. J Cell Biol. 2008;180:619–632. doi: 10.1083/jcb.200707042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynshaw-Boris A, Pramparo T, Youn YH, Hirotsune S. Lissencephaly: mechanistic insights from animal models and potential therapeutic strategies. Semin Cell Dev Biol. 2010;21:823–830. doi: 10.1016/j.semcdb.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Kim WY, Chen Y, Wang X, Stanco A, Komuro Y, Snider W, Anton ES. The adenomatous polyposis coli protein is an essential regulator of radial glial polarity and construction of the cerebral cortex. Neuron. 2009;61:42–56. doi: 10.1016/j.neuron.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, 3rd, Birchmeier W, Birchmeier C. β-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Zhang D, et al. Drosophila katanin is a microtubule depolymerase that regulates cortical-microtubule plus-end interactions and cell migration. Nat Cell Biol. 2011;13:361–370. doi: 10.1038/ncb2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 (related to Figures 1 and 3). Cre-mediated ablation of APC in the developing cortical neurons. (A–B) GFP expression in E14.5 GE of APCLox/+; Dlx5/6-Cre (A) and APCLox/Lox; Dlx5/6-Cre (B) indicates Cre expression in newborn interneurons. Color separated images are shown underneath panels A and B. (C–D) APC immunolabeling (red) indicates the loss of APC in newborn, GFP+ interneurons in APCLox/Lox; Dlx5/6-Cre GE. High magnification images of newborn interneurons from GE (asterisk, A–B) of APCLox/+; Dlx5/6-Cre (C) and APCLox/Lox; Dlx5/6-Cre (D) illustrating the lack of APC expression in APC mutant interneurons (arrowhead, D). Arrow (C) indicates APC expressing control interneurons (red-APC, green-GFP, yellow-merge). (E, F) Migrating control interneurons (arrow, E) in the cerebral wall express APC, but not the mutant interneurons (arrowhead, F). (G–H) Interneurons from E14.5 GE of APCLox/+; Dlx5/6-Cre (H) and APCLox/Lox; Dlx5/6-Cre (H) illustrating the lack of APC expression in APC deficient interneurons (arrow, H). Arrowhead (H) indicates expression of APC in non-GFP positive control cells. (I) Immunoblot analysis of control and APCLox/Lox; Dlx5/6-Cre GE extracts confirms the loss of APC in APC mutant brains. (J–K) Comparison of APC expression in APCLox/+; Nex-Cre (J) and APCLox/Lox; Nex-Cre (K) cortical slices (E16.5) show reduced APC levels in mutant dorsal cortex (compare areas indicated by asterisk). Color separated images are shown underneath panels J and K. (C–D) High magnification images of migrating projection neurons from areas indicated by asterisks (L, M) in APCLox/+; Nex-Cre (L) and APCLox/Lox; Nex-Cre (M) cortex illustrating the lack of APC expression in APC deficient projection neurons (arrowhead, M). Arrow (L) indicates expression of APC in GFP+ control neurons. (N–O) Projection neurons isolated from E14.5 dorsal cortex of APCLox/+; Nex-Cre (N) and APCLox/Lox; Nex-Cre (O) mice illustrating the lack of APC expression in APC deficient projection neurons (arrow, O). Arrowhead (O) indicates expression of APC in GFP negative control neurons. (P) Immunoblot analysis of dorsal cortical extracts from control and APCLox/Lox; Nex-Cre mice illustrates the loss of APC in mutant brains. GFP and β-actin were used as loading controls. CP, cortical plate; IZ, intermediate zone; VZ, ventricular zone; GE, ganglionic eminence. GFP and β-actin were used as loading controls. Scale bar= 250 μm (A–B, J–K), 60 μm (C–D, L, M), 70 μm (E–H, N–O).
Supplemental Figure 2 (related to Figure 2). Analysis of cell proliferation and cell death in the APCLox/Lox; Dlx5/6-Cre brains. (A–H) Cell proliferation in the APCLox/Lox; Dlx5/6-Cre mice. Anti-BrdU antibody labelling of BrdU-pulsed E14.5 (A–C) and E16.5 (E–G) GE did not reveal significant differences in mitotically active, BrdU+ progenitors between APCLox/+; Dlx5/6-Cre control (A, C, E, G) and APCLox/Lox; Dlx5/6-Cre mutant (B, C, F, G). (D, H) Quantification of mitotically active progenitors with M-phase marker PH3 also did not reveal changes in APCLox/Lox; Dlx5/6-Cre GE. Data shown are mean ± SEM; n=3 brains per group. (I–T) Cell death in the APCLox/Lox; Dlx5/6-Cre mice. Cleaved caspase 3 expression in E14.5 (I–J) and E16.5 (K–L) GE from APCLox/+; Dlx5/6-Cre (I, K) and APCLox/Lox; Dlx5/6-Cre (J, L) brains. No significant changes in apoptotic cells were noticed in the VZ/SVZ of GE in the mutants (M, N). (O–T) Cleaved caspase 3 expression in E16.5 (O–P) and P0 (Q–R) dorsal cortex from APCLox/+; Dlx5/6-Cre (O, Q) and APCLox/Lox; Dlx5/6-Cre (P, R) brains. An increase in apoptotic cells (arrowheads) was noticed in the mutants (P, R, S, T). Data shown are mean ± SEM; n=3 brains per group. *, p<0.05 (Student’s t test). (U–V). Labeling with RC2 antibodies indicates normal radial progenitor organization in APCLox/Lox; Dlx5/6-Cre GE. Scale bar= 200μm (A, B, E, F); 175μm (I–L, O–R), 110μm (U, V). SVZ, subventricular zone; VZ, ventricular zone.
Supplemental Figure 3 (related to Figure 1). APC is not required for interneuron guidance towards endogenous guidance cues from dorsal cortex. (A) Schematic of microfluidic chamber migration assay. Dissociated wild-type dorsal cortical cells (DCx, blue) were plated in one side of a chamber and dissociated interneurons (INs) from the GE of control (APCLox/+; Dlx5/6-CIE) or mutant (APCLox/Lox; Dlx5/6-CIE) cortex were plated in the other side. Interneurons were exposed to a microfluidic gradient of cues released by dorsal cortical neurons. Interneurons navigate through the microgradient of guidance cues that is established in the microchannels (vertical lanes) that separate the two chambers towards the dorsal cortical cells. (B–D) No significant differences were evident between control and APC deficient interneurons. (D) Quantification of the extent of migration. Number of cells per group= 700. Data shown are mean ± SEM. Scale bar= B–C, 50μm.
Supplemental Figure 4 (related to Figure 3). APC does not affect projection neuron migration. (A and B) Cortical neurons born in E13.5 and E16.5 are birthdated with EdU and BrdU, respectively. The positions of birthdated neurons are analyzed in postnatal day 7 control (A) and APC deficient, APCLox/Lox; Nex-Cre, cortex (B). No significant defects in neuronal placement are evident in APC cKO. Scale bar=129 μm.
Supplemental Figure 5 (related to Figures 3 and 7). APC function in projection neuron migration and connectivity. (A–D) E16.5 APCLox/Lox; Nex-Cre; golli-τ-GFP and control mice are immunolabeled with anti-GFP, Tbr1 (layer5/6) antibodies and nuclear counterstained with DRAQ5. APC-deficient GFP+ deeper layer neurons migrated normally. (E and F) Placement and initial axon growth of projection neurons is not affected in APCLox/Lox; Nex-Cre; golli-τ-GFP mice. GFP-immunolabeling of sagittal sections from P0 control and APC mutant cortex indicates generally normal neuronal migration, placement, and developmental progression of axon growth in APC mutant (F) as compared to control (E). (G–K) APC deletion does not affect radial migration. E14 embryos (APCLox/+ [G, I] and APCLox/Lox [H, J]) were electroporated with Cre-EGFP and the radial migration of control and APC deficient neurons were analyzed at E16.5. Compared to control (I, arrow), cre expression in APC Lox/Lox cortex leads to loss of APC expression in radially migrating neurons (J, arrow; [arrowhead shows APC expression in Cre-GFP negative cell]). (K) Quantification of the distribution of electroporated cells in the cerebral wall indicates normal patterns of radial migration in APC Lox/Lox; Cre cortex. CP, cortical plate; SP, sub plate; IZ, intermediate zone; VZ, ventricular zone. Scale bar= 77 μm (A–D), 340 μm (E, F), 100μm (G, H), 60μm (I, J).
Supplemental Figure 6 (related to Figure 4). Increased MT severing activity in APC deficient interneurons. (A–D) Rhodamine labeled MT filaments (A, C) were treated with E14.5 GE extracts from APCLox/+; Dlx5/6-Cre (B) and APCLox/Lox; Dlx5/6-Cre (D) brains. Increased severing (arrowheads) was noticed in MTs treated with mutant extracts, but not with control extracts. (E) Percent changes in ≤2μm fragments of MTs resulting from MT severing was quantified and used as an index of severing activity. Data shown are mean ± SEM. n=11 (APCLox/+; Dlx5/6-Cre), 12 (APCLox/Lox; Dlx5/6-Cre). (F) Treatment of MT filaments with different concentrations of GE extracts. Increased severing activity in APCLox/Lox; Dlx5/6-Cre extracts led to shorter MT filaments (n=4). (G–L) Treatment of MT filaments (G, I) with E14.5 dorsal cortex extracts from APCLox/+; Nex-Cre (H) and APCLox/Lox; Nex-Cre (J) brains. No significant changes in severing (arrowheads) was noticed in MTs treated with APC deficient projection neuron extracts. (K) Quantification of severing activity of Nex-Cre extracts. (L) Effect of different concentrations of Nex-Cre extracts on MT filaments. Data shown are mean ± SEM. n=4. *, significant when compared with controls at p<0.05 (Student’s t test) *, significant when compared with controls at p<0.05 (Student’s t test). Scale bar= 2 μm.
Supplemental Figure 7 (related to Figure 5). Taxol stabilization of microtubules does not affect katanin levels in APC deficient interneurons and APC deletion increases tyrosinated tubulin at the tips of interneuronal processes. (A–B) Control [C] and APC mutant [M] interneurons were treated with Taxol (5nM) for 24 hours. Taxol stabilization did not affect Katanin levels in APC deficient interneurons. Levels of stable acetylated tubulin did increase as expected after Directions of the ganglionic eminence (GE) and dorsal cortex (D. CX) are indicated. P-pial surface, V-ventricular surface. Time length = 290 minutes.
Time lapse images of interneurons migrating toward the dorsal cortex (D.CX). Arrow heads (yellow) indicate streams of migrating interneurons in the MZ and IZ. A interneuron with asterisk (purple) shows its dynamic movements within the developing cerebral wall as interneurons navigate towards their target layer. Directions of the GE and dorsal cortex (D.CX) are indicated. P-pial surface, V-ventricular surface. Time length = 240 minutes.
APC deletion leads to disrupted, unorganized streams of tangentially migrating interneurons in the cerebral wall. Increased number and frequency of branching are noticed in APC deficient interneurons.