Summary
Both strands of human mitochondrial DNA (mtDNA) are transcribed in continuous, multi-genic units that are cleaved into the mature rRNAs, tRNAs, and mRNAs required for respiratory chain biogenesis. We sought to systematically identify nuclear-encoded proteins that contribute to processing of mitochondrial RNAs (mt-RNAs) within the organelle. First, we devised and validated a multiplex “MitoString” assay that quantitates 27 mature and precursor mtDNA transcripts. Second, we applied MitoString profiling to evaluate the impact of silencing each of 107 mitochondrial-localized, predicted RNA-binding proteins. With the resulting dataset, we rediscover the roles of recently identified RNA processing enzymes, detect unanticipated roles of known disease genes in RNA processing, and identify new regulatory factors. We demonstrate that one such factor, FASTKD4, modulates half-lives of a subset of mt-mRNAs and associates with mitochondrial RNAs in vivo. MitoString profiling may be useful in diagnosing and deciphering the pathogenesis of mtDNA disorders.
Introduction
Human mitochondrial DNA (mtDNA) encodes 13 protein subunits of the respiratory chain, as well as the 2 rRNAs and 22 tRNAs required for their translation. All protein factors required for mtDNA replication, transcription, and translation are nuclear-encoded and imported into the mitochondrion. Mutations in either nuclear- or mitochondrial-encoded genes cause a range of heritable disorders with overlapping phenotypes. Expression of nuclear and mtDNA-encoded oxidative phosphorylation (OXPHOS) complex proteins is coordinated by nuclear transcription factors and co-activators (Scarpulla et al., 2012). However, local control of mtDNA expression is also possible, as suggested by chemical perturbations capable of decoupling the expression of the two genomes (Wagner et al., 2008).
The 16.5 kB human mitochondrial genome is highly compact and transcribed by mitochondrial RNA polymerase as two continuous polycistrons, one for each strand. The “heavy strand” expresses 2 rRNAs, 14 tRNAs and 12 mRNAs, while the “light strand” expresses the ND6 mRNA and 8 tRNAs (Figure 1AB). These precursor RNAs primarily contain mt-mRNAs “punctuated” by tRNAs, whose structure is proposed to guide the cleavage responsible for liberating individual mt-mRNAs and tRNAs (Anderson et al., 1981; Ojala et al., 1981). Cleavage at the 5′ end of tRNAs is catalyzed by the RNase P complex, comprised of three recently identified proteins, MRPP1/2/3 (Holzmann et al., 2008). An alternative RNase P containing an imported catalytic RNA has also been described (Puranam and Attardi, 2001). Cleavage at the 3′ end of tRNAs is catalyzed by the nuclease ELAC2 (Brzezniak et al., 2011; Lopez Sanchez et al., 2011). After cleavage, the mitochondrial RNA poly-A polymerase (MTPAP) polyadenylates the mt-mRNAs (Nagao et al., 2008; Piechota et al., 2006). Mt-mRNA abundance is regulated by the SLIRP- LRPPRC complex, although the exact mechanism is debated. LRPPRC has been implicated in mt-mRNA transcription, polyadenylation, translation and degradation suppression (Baughman et al., 2009; Chujo et al., 2012; Gohil et al., 2010; Liu et al., 2011; Ruzzenente et al., 2012; Sasarman et al., 2010).
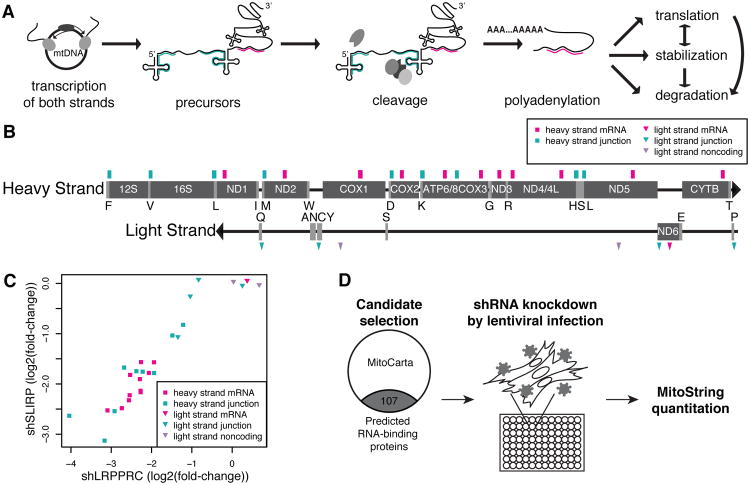
Figure 1. MitoString screen for regulators of mitochondrial RNA processing.
(A) Schematic depicting mtDNA transcription (in two continuous units, the heavy and light strands), followed by cleavage into individual mRNAs, tRNAs, and rRNAs. Pink and turquoise lines represent MitoString probes targeting mRNAs and junctions, respectively. (B) Location of MitoString probes on the mtDNA heavy and light strands. rRNAs and mRNAs encoded by the mtDNA are labeled in white text. tRNAs are demarcated by their one letter symbols. The location of mRNA probes are noted in pink, junction probes in turquoise, and noncoding probes in grey. Rectangles indicate probes targeting the heavy strand, while triangles indicate probes targeting the light strand. (C) Comparison of the effects of shLRPPRC and shSLIRP on MitoString probes, shown as log2(fold-change) with respect to shGFP. Probe colors and shapes are depicted as in (B). (D) Overview of MitoString screening approach. Candidates for lentiviral knockdown were selected by identifying MitoCarta proteins containing RNA-binding domains. Candidate genes were knocked down in WI-38 fibroblasts and the mitochondrial RNA levels were assessed six days later by the MitoString probes described in (B).
Despite the concurrent transcription of heavy strand genes, their cognate mt-mRNAs reach distinct steady-state levels. These 10 transcripts have distinct half-lives that fall into two categories: complex I transcripts ND1-3, and 5 and complex III transcript CYTB are short-lived (τ1/2= 68-94 min), whereas complex IV transcript COX1-3, complex V bicistronic transcript ATP6/8, and complex I bicistronic transcript ND4/4L are long-lived (τ1/2= 138-231 min) (Nagao et al., 2008). These differential mt-mRNA stabilities are consistent with early observations (Gelfand and Attardi, 1981) and recent RNA sequencing analysis (Mercer et al., 2011), but remain unexplained by transcript length, polyadenylation, known degradation pathways, or characterized stability factors. Although the purpose of these differential half-lives is unexplored, we note that concentrations of OXPHOS protein complexes (reviewed by Lenaz and Genova, 2010) are correlated to reported mt-mRNA half-lives (Nagao et al., 2008). Complex I is the least abundant complex and contains subunits encoded by the short-lived mt-mRNAs. Thus, differences in mt-mRNA abundance may help establish OXPHOS protein stoichiometry.
Because all mt-mRNA transcripts but one originate from a single heavy strand promoter, the observed steady-state levels are expected to be highly dependent on transcript degradation rates (Chujo et al., 2012). The helicase SUPV3L1, in complex with polynucleotide phosphorylase (PNPT1), has been implicated in the degradation of the light-strand transcripts, and a dominant negative form of either gene stabilizes light strand noncoding transcripts and some short-lived heavy strand mt-mRNAs (Borowski et al., 2013; Szczesny et al., 2010). However, both RNAi and dominant negative experiments targeting SUPV3L1 or PNPT1 actually decrease levels of the long-lived mt-mRNA COX1, indicating additional degradation or feedback mechanisms may exist.
Our goal was to systematically identify mitochondrial proteins that contribute to mitochondrial RNA processing. We begin with a scalable, accurate method to measure multiple mitochondrial RNAs throughout the processing stages. Past approaches to measuring mitochondrial RNA levels, such as Northern blots, quantitative PCR, and GE-HTS (Wagner et al., 2008), have been valuable, but limited by scalability, strand specificity, or dynamic range, respectively. Here, we report simultaneous, strand-specific measurement of multiple precursor and mature mtDNA-encoded RNAs following stable genetic silencing of nuclear factors predicted to play a role in mitochondrial RNA biology. We produce a focused compendium of mitochondrial RNA expression across a set of targeted genetic perturbations, which we mine to probe the identity and role of nuclear-encoded factors in mitochondrial RNA processing. In the process, we identify FASTKD4, a novel factor that regulates the stability of a subset of mt-mRNAs.
Results
MitoString: a multiplexed assay for precursor and mature mt-RNAs
We developed a “MitoString” assay for interrogating four types of transcripts using the nCounter Analysis System (Geiss et al., 2008), in which fluorescent RNA probes quantitate unamplified RNA within a crude cell lysate sample. First, we designed a probe targeting each of the highly abundant mt-mRNA transcripts (Figure 1B), achieving a coefficient of variation (CV) of 7-14% for all but one probe across 10 independent infections of the control hairpin shGFP (Figure S1AB). Second, we designed probes to two regions of the light strand precursor that are transcribed, but are not believed to encode a functional protein. Third, we designed probes overlapping the junctions of two adjacent genes. These junction probes only produce signal when bound to the unprocessed precursor transcript. Probes assessing the precursor strands have lower signal at baseline, and are thus noisier (Figure S1C), but their levels can be reproducibly induced in response to perturbation. In addition, we designed probes to detect nuclear-encoded genes important to mitochondrial function, as well as the nuclear-encoded candidate genes, with the vast majority having a CV of <20% (Figure S1D).
To verify that mtRNA perturbations are reliably measured, we profiled cells following transduction with PGC-1α, which induces mitochondrial biogenesis, and shLRPPRC, which depletes mtRNAs. PGC-1α overexpression increases mature and immature transcripts (Figure S2AB), as well as nuclear-encoded, mitochondrial-localized transcripts (Figure S2C), as expected. LRPPRC silencing depletes heavy strand mt-mRNAs as found previously (Figure 1C). We further note that knockdowns of the individual components of the LRPPRC-SLIRP complex have well-correlated effects on all transcripts measured by MitoString (Figure 1C), suggesting that hierarchical clustering of MitoString profiles may be valuable for predicting gene function.
Prioritizing candidate mt-RNA binding proteins for knockdown
With a facile assay for quantifying immature and mature mitochondrial transcripts in hand, we proceeded to select genes that might be involved in mitochondrial RNA processing for RNAi-based silencing. We focused on members of MitoCarta, a high-confidence collection of mitochondrial-localized proteins (Pagliarini et al., 2008), and prioritized proteins with known or predicted RNA-binding domains, based on Pfam, GO annotation, or manual curation (Figure 1D) (Finn et al., 2008). We excluded components of the mitochondrial ribosome and in total prioritized 107 candidates.
For each of these genes, we selected the three most effective lentiviral shRNAs available from the RNAi Consortium (Table S1) (Methods). We screened WI-38 fetal lung fibroblasts, which are untransformed and viable after six days of strong LRPPRC knockdown. Cells were infected with hairpins in duplicate, and lysed for MitoString analysis after six days of antibiotic selection. Using our nuclear NanoString probes, we were able to measure knockdown efficiency for most hairpins within the same assay (Table S2). On each 96-well screening plate, we included five hairpins targeting non-human sequences as RNAi controls, a hairpin targeting LRPPRC known to deplete mt-mRNAs (Figure 1C), and a PGC-1α overexpression construct to stimulate mitochondrial biogenesis (Figure S2A-C).
A compendium of perturbational profiles for mitochondrial RNA
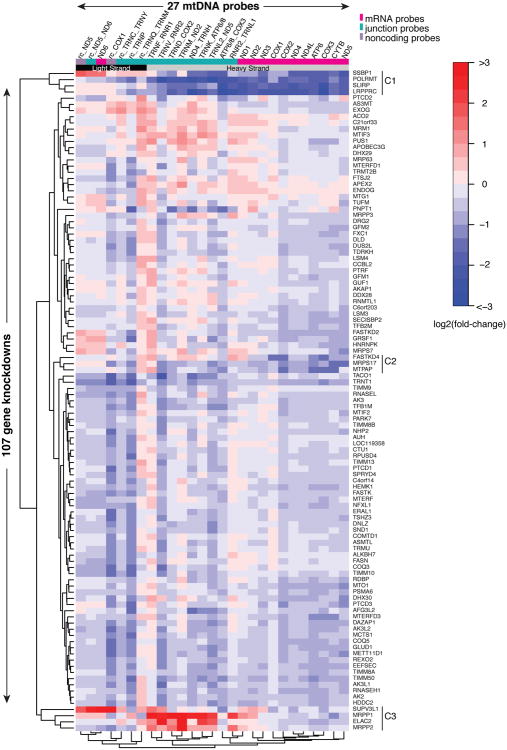
MitoString quantitation of knockdown perturbations provides a valuable compendium for gene function prediction and mtDNA transcript characterization. Each expression value is normalized to a set of endogenous controls and calculated as a fold-change compared to shGFP, one of our controls targeting non-human sequences. Since PGC-1α induction and LRPPRC knockdown produce the expected mt-mRNA perturbations (Figure 1C, S2A-C), we have confidence that our dataset can identify novel regulators. By hierarchically clustering the average perturbational profile across hairpins for each of 107 gene knockdowns along each dimension, we can identify genes and mitochondrial transcripts that may have similar functions (Figure 2, Table S3,S4). Unsupervised hierarchical clustering automatically groups LRPPRC and SLIRP together with POLRMT, which encodes the RNA polymerase responsible for mtDNA transcription, and SSBP1, which encodes a protein required for mtDNA replication (Figure 2, cluster C1). Separately, genes with roles in mtRNA precursor cleavage, including ELAC2 and MRPP1, also form a strong group based on the signature of junction probe enrichment (Figure 2, cluster C3). Unsupervised hierarchical clustering of the MitoString probes results in automated segregation of light strand probes, heavy strand mRNAs, and heavy strand junctions (Figure 2). Principal component analysis further demonstrates the power of our dataset to differentiate these distinct transcript types (Figure S1F). In particular, the heavy strand junction and mRNA probes are separated by Principal Component 2, which explains 19% of the variance. The lone mRNA-mRNA junction, ATP6_COX3, is the sole outlier junction, as it behaves like its mature products, ATP6 and COX3. These unsupervised analyses indicate that many genes targeted for RNAi are grouped by function and that mtDNA transcripts respond to perturbations in modules.
Figure 2. MitoString profiles for 107 gene knockdowns.
Heatmap depicts the log2(fold-change) expression level of each of 27 mtDNA probes across 107 gene knockdowns. mtDNA probes are noted along the top, and ordered by hierarchical clustering (Euclidean distance). Each gene was targeted by three distinct lentiviral hairpins and measured in duplicate. The mean of all hairpins for a given gene is displayed and the genes are hierarchically clustered (Euclidean distance). Red represents increased and blue represents decreased expression with respect to shGFP. Identifiers starting with ‘TRN’ denote tRNAs. Probe colors are as in Figure 1B.
Knockdown of LRPPRC or SLIRP depletes all heavy strand mt-RNAs
By mining the compendium, we gain insight into the action of LRPPRC and SLIRP, which are the strongest regulators tested. Knockdown of LRPPRC or SLIRP results in depletion of the heavy strand precursor and all its resulting mt-mRNAs (Figure 2, cluster C1). In addition to LRPPRC and SLIRP, POLRMT and SSBP1 also universally deplete heavy strand mt-RNAs when knocked down (Figure 2, C1), consistent with our expectation that depletion of both precursor and mature transcripts should reflect reduced transcription or mtDNA content. SLIRP and LRPPRC form a complex known to bind mitochondrial RNA and affect mt-mRNA levels (Mili and Piñol-Roma, 2003; Sasarman et al., 2010), but reports both supporting and contesting a role for LRPPRC in transcription exist (Harmel et al., 2013; Sondheimer et al., 2010). Although all heavy strand probes are impacted by shLRPPRC and shSLIRP, some junction probes are less strongly affected than the mRNA probes, suggesting a dual role for these regulatory proteins in post-transcriptional stabilization and transcription (Figure 1C). One theory consistent with our results is that the complex stabilizes the nascent transcript, as suggested elsewhere (Harmel et al., 2013). Because we could quantitate junction probes strand-specifically, we were able to observe that ND6 and most light strand transcripts are unaffected by LRPPRC or SLIRP knockdown.
MitoString highlights distinct regulatory mechanisms for coding and noncoding antisense transcripts
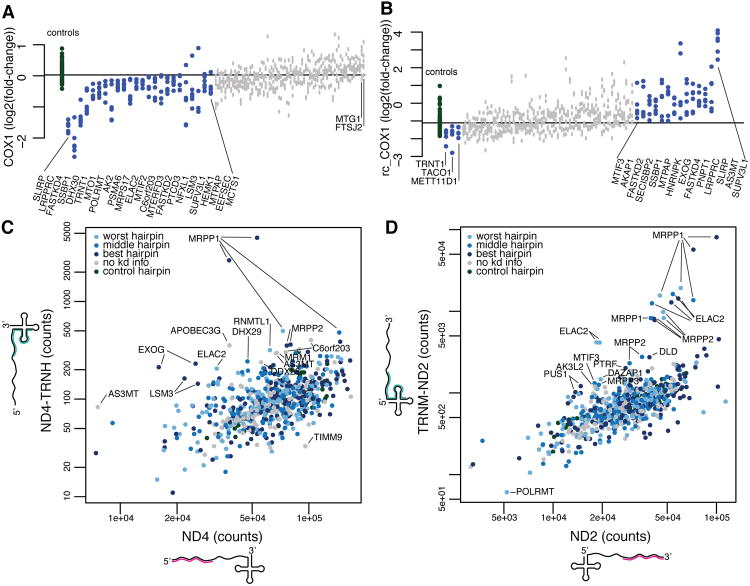
Querying the MitoString compendium for specific transcripts, such as the heavy strand mRNA COX1, uncovers distinct regulation for heavy strand coding and light strand noncoding RNAs. We identify nuclear genes whose knockdown alters COX1 expression relative to the control hairpins, using a rank-sum statistic at a nominal p-value <0.01 (Figure 3A). On the left tail of the distribution, we recover the aforementioned LRPPRC and SLIRP. COX1 is also depleted by knockdown of FASTKD4 (also known as TBRG4), an uncharacterized protein containing a RAP domain with predicted RNA-binding abilities (Lee and Hong, 2004). Hairpins targeting the uncharacterized nucleoid component DHX30 also deplete COX1. Interestingly, depletion of SUPV3L1, which is implicated in mtRNA degradation, also decreases COX1 levels, possibly due to decreased mtDNA copy number, as described previously (Khidr et al., 2008). Silencing of MTPAP (mitochondrial poly-A polymerase) also diminishes COX1 levels markedly, with the exception of one hairpin (Figure 3A). No knockdowns significantly increased COX1 expression, perhaps due to its extremely high baseline abundance.
Figure 3. Discordant expression of junction and noncoding probes highlight genes required for processing and degradation.
(A) Expression of the COX1 probe as a log2(expression/shGFP expression) value is plotted for each control (green) and knockdown hairpin (blue denotes nominal p-value <0.01 by Mann-Whitney rank-sum test when comparing six gene-targeted hairpins to the control hairpins shown, grey p-value >0.01). The knockdown of LRPPRC, SLIRP and FASTKD4 caused the strongest depletion of COX1. (B) Expression of the rc_COX1 probe, which targets a noncoding region on the light strand is plotted as in (A). (C) The probe count for each knockdown hairpin was plotted for the ND4 mRNA and ND4_TRNH junction probes. Genes that disproportionally affect one probe are found offset from the diagonal and labeled (Supplemental experimental procedures). Hairpins are colored based on knockdown strength (light blue for worst hairpin, blue for middle hairpin, dark blue for best hairpin, grey for no knockdown information, and green for control). (D) The probe count for each knockdown shRNA hairpin plotted for the ND2 mRNA and TRNM_ND2 junction probe as in (C)(see also Figure S3).
A similar analysis, focusing on the light strand probe rc_COX1, reveals that the majority of significant knockdowns increase rc_COX1 expression (Figure 3B). Since the noncoding portion of the light strand exists transiently, measuring these regions can identify degradation machinery. For the rc_COX1 probe, knockdown of SUPV3L1 induces a strong increase in transcript levels, confirming the role of SUPV3L1 in this process (Figure 3B). However, SUPV3L1 silencing actually decreases the level of some mt-mRNA transcripts, most notably COX1, COX3, and ND4 (Figure 2, cluster C3), suggesting that its role in degradation may be selective. The response of COX1 and rc_COX1 transcripts to systematic RNAi perturbation highlights the distinct regulation of heavy strand and light strand transcripts shown in Figure 2.
Identifying negative regulators of mt-mRNA abundance
We next sought to identify factors that function to repress the abundance of mt-mRNAs at steady state, in contrast to LRPPRC and SLIRP, which stabilize transcripts, by further mining our compendium. Loss of MTG1 (Mitochondrial GTPase 1) expression results in an increase of all mt-mRNAs except ND5 (Figure S2D), and it is the second strongest scoring gene on the right tail of the COX1 distribution (Figure 3A). Although it is not significant when summarized by three hairpins for COX1 individually (Figure 3A), measuring multiple genes results in an intriguing phenotype (Figure S2D). MTG1 is linked to respiration and translation in human cell lines, but the effect of MTG1 knockdown on mt-mRNA levels had not been measured (Barrientos et al., 2003; Kotani et al., 2013). Knockdown of the uncharacterized gene C21orf33 increases the mt-mRNAs ND1-3, with excellent knockdown-phenotype correlation, but has minimal effect on the other transcripts (Figure S2E). In a complementary analysis using the GNF Mouse GeneAtlas (Lattin et al., 2008), mRNA expression of the C21orf33 mouse homolog is well-correlated with nuclear-encoded OXPHOS gene expression (Figure S2F). Our data offers tantalizing clues, but more research is required to elucidate the exact roles of these genes.
Identifying factors that cleave precursor transcripts
A key advantage of the MitoString approach is that it simultaneously monitors both precursor and mature transcripts across a battery of perturbations, allowing identification of specific cleavage and processing factors. Within our dataset, known cleavage factors form a distinct cluster marked by increased junction expression concomitant with stable mt-mRNA expression (Figure 2, cluster C3). These recently discovered factors include mitochondrial RNase P proteins 1 and 2 (MRPP1 and MRPP2), which encode subunits of the tRNA 5′ end cleavage machinery, and ELAC2, which cleaves the tRNA 3′ end (Brzezniak et al., 2011; Holzmann et al., 2008; Lopez Sanchez et al., 2011).
By comparing the abundance of specific mt-mRNAs to their unprocessed precursor, over all perturbations, we observe a background distribution from which outliers represent candidate cleavage factors (Figure 3CD). The activity of mitochondrial RNase P is estimated by comparing the ND4 transcript with its neighboring ND4_TRNH transcript (Figure 3C). If the junction is less efficiently cleaved, due to gene silencing, more unprocessed RNA will remain (Figure 3C). Hairpins targeting MRPP1 and MRPP2 have this effect and are consistently distinct from the background distribution in this case and in others tested (Figure 3C, S3A-C). We investigate the 3′ cleavage site similarly, by comparing ND2 counts to TRNM_ND2 counts (Figure 3D). In this case, we identify ELAC2, MRPP1, and MRPP2, suggesting that knockdown of RNase P components can disrupt 3′ cleavage, as found previously for some junctions (Brzezniak et al., 2011; Lopez Sanchez et al., 2011).
We also interrogate junctions cleaved by non-canonical mechanisms. We find that silencing MRPP1-3 or ELAC2 does not influence the ATP6/8_COX3 junction cleavage (Figure 2, cluster C3 and S3D-F). Further, outlier hairpins for this distribution are driven by mt-mRNA depletion, not ATP6/8_COX3 transcript accumulation, leaving the cleavage factor for this junction unidentified (Figure S3D-F). The 5′ junction of TRNQ on the light strand abuts noncoding DNA and is unexpectedly more strongly affected by knockdown of ELAC2 than knockdown of MRPP1/2 (Figure 2, cluster C3). Conversely, TRNF_RNR1 is more strongly regulated by silencing of MRPP1/2 and SUPV3L1 than silencing of ELAC2, although one ELAC2 hairpin still has a strong effect (Figure 2, C3). Both cases suggest noncanonical processing.
Impact of known disease genes on mt-mRNA levels
Our knockdown inventory includes twelve genes implicated in Mendelian mitochondrial disease (Table S5). Disease genes MTO1, PUS1, and TRMU encode enzymes with well-established roles in tRNA modification and efficient translation, but to our knowledge, their role in regulating human mt-mRNA abundance has never been tested (Patton et al., 2005; Wang et al., 2010b; Yan and Guan, 2004; Zeharia et al., 2009). For the strongest MTO1 knockdown hairpin (13% remaining), our compendium shows depletion of multiple mt-mRNAs (Figure 2), confirming similar findings in yeast (Wang et al., 2010b). Similarly, strong PUS1 knockdown decreased ATP6, COX3, and CYTB mtmRNA abundance (Figure 2). On the other hand, silencing of TRMU has a negligible effect on mt-mRNAs (Figure 2). In combination, these data suggest that some tRNA modifications may be necessary for proper mt-mRNA stability. Disease mechanisms may include both improper tRNA modification and mt-mRNA depletion.
Our compendium also expands our understanding of a number of other disease genes (Table S5). A mutation in FASTKD2 has been implicated in cytochrome c oxidase (COX) deficiency (Ghezzi et al., 2008), and we now find a 2-fold decrease of COX2-3,CYTB, ATP6/8, and ND3-4/4L-5 mt-mRNA expression under FASTKD2 silencing by at least one hairpin (Figure 2). PNPT1, which is implicated in both RNA degradation (Borowski et al., 2013) and 5S rRNA, RNase P RNA and MRP RNA import (Wang et al., 2010a), has also been recently associated with hearing loss and respiratory chain deficiency (Vedrenne et al., 2012; von Ameln et al., 2012). Our data reveals moderate increases in short-lived transcripts CYTB and ND2 as well as moderate decreases in long-lived transcripts COX1-3 (Figure 2) in PNPT1 silenced cells, consistent with previous reports and highlighting the complexity of this protein's role in the cell (Borowski et al., 2013). Knockdown of TIMM8A, a known inner-mitochondrial membrane translocase component targeted in the current study because it contains a zinc finger-like motif, is correlated with moderately decreased mt-mRNA levels for almost all transcripts (Figure 2), and has been previously implicated in deafness-dystonia (Aguirre et al., 2006; Swerdlow et al., 2004).
Identifying genes that influence mature mt-mRNA abundance
To identify genes that specifically stabilize mature heavy strand mt-mRNAs, we developed an ordered list that incorporates all of our heavy strand probe data. We examined the ratio of the average heavy strand mature mt-mRNA probes to the average heavy strand precursor probes for each knockdown gene (Figure 4A). Using this measurement, we ordered each gene based on the rank-sum of all hairpins targeting that gene (Figure 4A). This procedure is complementary to Figure 3CD, which focuses on two individual junctions. Proteins identified through this method might play a role in post-transcriptional processing, stability, and degradation.
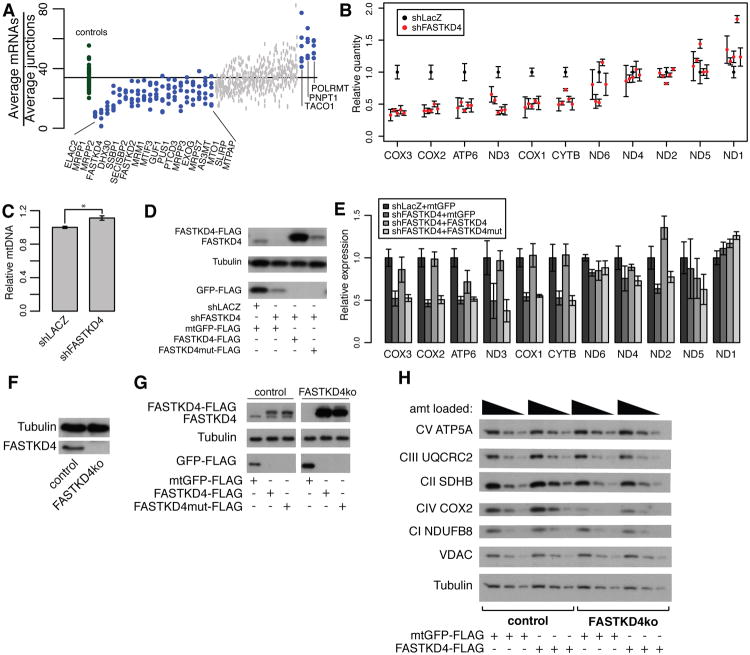
Figure 4. Loss of FASTKD4 leads to loss of a subset of steady-state mtDNA gene products.
(A) The geometric mean of heavy strand mRNA probe counts divided by the geometric mean of heavy strand junction probe counts is plotted for each hairpin. Control hairpins are in green and knockdown hairpins are in blue or grey (blue represents nominal p-value <0.01 by Mann-Whitney rank-sum test). (B) MitoString results normalized to shLacZ for five distinct shFASTKD4 hairpins in HEK-293T cells. Control hairpin shown in black, FASTKD4 hairpins shown in red. (C) Relative mtDNA content in shLACZ knockdown cells compared to shFASTKD4 knockdown cells as measured by qPCR, * indicates p <.05 (two-tailed unpaired t-test). (D) Western blot showing expression of FASTKD4-FLAG and FASTKD4 (detected by FASTKD4 antibody, top), tubulin (loading control, middle), and GFP-FLAG (overexpression control, bottom) in stable HEK-293T cell lines. Cells are overexpressing RNAi resistant FASTKD4-FLAG and FASTKD4mut-FLAG (with RAP domain point mutations), or mitoGFP-FLAG in the presence of shLACZ (control) or shFASTKD4 knockdown, as indicated. (E) Relative expression of each mt-mRNA probe quantified by MitoString, shown for the four cell lines depicted in (D). (F) Western blot showing expression of FASTKD4 and tubulin in the FASTKD4ko CRISPR/Cas9 cell line. (G) Western blot showing expression of FASTKD4-FLAG and FASTKD4 (detected by FASTKD4 antibody, top), tubulin (loading control, middle), and GFP-FLAG (overexpression control, bottom) in control and FASTKD4ko cell lines with overexpression of FASTKD4-FLAG, FASTKD4mut-FLAG, and mtGFP-FLAG as indicated (samples run on same gel with irrelevant lane removed). (H) Western blot showing respiratory complex protein expression in control and FASTKD4ko cell lines with mtGFP-FLAG or FASTKD4-FLAG overexpression as indicated. Three concentrations of each cell lysate was loaded as indicated. In all panels, error bars represent s.e.m., n=3.
POLRMT, which encodes the mitochondrial mtRNA polymerase, is amongst the highest scoring hits, causing the ratio of mature mt-mRNA to precursor RNA to increase when silenced (Figure 4A). POLRMT is well-studied in vitro, but to our knowledge, our study is the first to perturb it in vivo. This result suggests that when mtDNA transcription is reduced, the cell's response is to stabilize mature mt-mRNAs, via an uncharacterized regulatory mechanism.
A number of genes, when silenced, decrease the ratio of mt-mRNAs to precursor transcripts, suggesting that either the nascent strand is stabilized and/or the mt-mRNAs are destabilized. As expected, RNase P- and RNase Z-encoding genes (MRPP1, MRPP2, ELAC2) are represented here because they stabilize nascent transcripts. An uncharacterized gene, FASTKD4, has a similar effect size as these known regulators, since its silencing destabilizes mature transcripts (Figure 4A). FASTKD4 clusters in our study with MTPAP and the ribosomal protein-encoding MRPS17 (Figure 2, cluster C2). In general, silencing of genes in this cluster affects the mt-mRNA probes more strongly than the junction probes and does not affect all mt-mRNAs uniformly. As FASTKD4 has not been previously linked to mt-mRNA expression, we pursued its function in more depth.
Silencing of FASTKD4 depletes some, but not all, mt-mRNAs
FASTKD4 belongs to a metazoan-specific cohort of proteins, defined by the presence of FAST (Fas-activated serine/threonine kinase-like) and RAP (RNA-binding domain abundant in Apicomplexans) domains. The human genome encodes six of these proteins (FASTK, FASTKD1-5), all of which localize to the mitochondrion (Simarro et al., 2010), although not all were identified in the initial MitoCarta compendium (Pagliarini et al., 2008). Mutations in FASTKD2 are associated with cytochrome c oxidase deficiency (Ghezzi et al., 2008) and FASTKD3 knockdown inhibits respiration in cultured cells (Simarro et al., 2010). FASTK encodes a kinase, whose mitochondrial localization is disrupted during UV- or anti-Fas antibody-induced apoptosis (Li et al., 2004). FASTK also plays a role in alternative splicing in the nucleus, which requires the RAP domain (Izquierdo and Valcárcel, 2007; Simarro et al., 2007). Unlike FASTK, FASTKD4 does not have an identifiable kinase active site.
To confirm our screening result that shFASTKD4 depletes a subset of mt-mRNAs, we began by validating the FASTKD4 knockdown phenotype in an independent cell line (HEK-293T). We used five distinct hairpins against FASTKD4 and used MitoString to measure expression of the mitochondrial transcripts after eight days of stable knockdown (Figure 4B). In agreement with our initial screen, only a subset of the mt-mRNA transcripts were affected (COX1-3, ATP6/8, CYTB, ND3), and we show that mtDNA is actually increased, ruling out mtDNA depletion as a mechanism (Figure 4C). ND1 was also consistently upregulated in both cases, while ND2, ND4/4L and ND5 remained unchanged. Thus, the impact of FASTKD4 silencing on mt-mRNA abundance is robust across five distinct hairpin sequences and two cell lines. To confirm that this is an on-target effect of FASTKD4 knockdown, we overexpressed a FLAG-tagged RNAi-resistant FASTKD4 (FASTKD4-FLAG), as well as FASTKD4 with four characteristic RAP domain residues mutated to alanines (FASTKD4mut-FLAG) (Figure 4DE). FASTKD4-FLAG expression, but not FASTKD4mut-FLAG expression, is accompanied by recovery in RNA levels for affected transcripts (Figure 4E), proving that the FASTKD4 knockdown is responsible for the observed phenotype.
We next used a complementary gene knockout strategy to perturb FASTKD4 and interrogate its impact on OXPHOS protein production. Although the knockdown produced a reliable RNA phenotype, residual FASTKD4 RNA is translated. We generated a FASTKD4 knockout (FASTKD4ko) in HEK-293T cells using the CRISPR/Cas9 system (Hsu et al., 2013), which enabled assays in the complete absence of FASTKD4. In our FASTKD4ko cell line, we identified three indels at the FASTKD4 locus that generate protein-terminating frame shifts, resulting in the absence of full-length protein (Figure 4F). In FASTKD4ko, we observe reduced abundance of complex IV subunit COX2 protein that is rescued by FASTKD4-FLAG overexpression (Figure 4GH). Complex I, II, III, and V nuclear subunits were unaffected, consistent with our screening results and the dependence of these complexes on mtDNA-encoded subunits (Figure 4H). Thus, two independent genetic strategies indicate that FASTKD4 is required for the proper expression of a specific subset of mitochondrial RNAs, and in the case of COX2, the protein product.
Stability of a subset of mt-RNA transcripts requires FASTKD4
Assuming all heavy strand transcripts are transcribed concurrently, we hypothesize that the differential abundance of mt-mRNAs following silencing of FASTKD4 is due to differential degradation rates. We can measure RNA stability within the mitochondrion by blocking POLRMT-mediated transcription with media containing a high concentration of ethidium bromide (ETBR) (Nagao et al., 2008).
We quantitated the mt-mRNAs using qPCR following six hours of ETBR treatment, during which time period mitochondrial transcription is suspended. The fraction of RNA remaining can be determined by comparing the ETBR-treated RNA to the untreated RNA. Of the six transcripts downregulated at steady state in FASTKD4 knockdown cells (Figure 4B), we find that COX2, ND3, and COX1 also have a strong decrease in the fraction of RNA remaining under ETBR treatment (Figure 5A), suggesting an increased degradation rate. CYTB also trends downward, but not significantly. The only transcripts that are depleted (Figure 4B) but not destabilized in our assay are COX3 and ATP6/8 (Figure 5A): regulation of these two transcripts, which are part of the stable ATP6/8_COX3 precursor, appears to be more complicated. By performing the same assay in our FASTKD4ko, we observed destabilized COX1, and unchanged ND1, consistent with shFASTKD4 (Figure 5BC). Further, to rule out off-target RNAi effects, we rescued the COX1 destabilization with FASTKD4-FLAG overexpression, but not with overexpression of FASTKD4mut-FLAG (Figure 5B).
Figure 5. FASTKD4 is required for stability of a subset of mt-mRNAs.
(A) The fraction of RNA remaining after ethidium-bromide (ETBR) transcription inhibition for 6-hours is plotted for each mt-mRNA as measured by qPCR in shLACZ and shFASTKD4 cell lines. This fraction is also plotted specifically for (B) COX1 and (C) ND1 in control or knockout cell lines expressing FLAG-tagged mtGFP, FASTKD4, and FASTKD4mut. Error bars represent s.e.m., n=3. * represents p<=0.05 (two-tailed unpaired t-test).
FASTKD4 associates with mitochondrial RNAs in the matrix
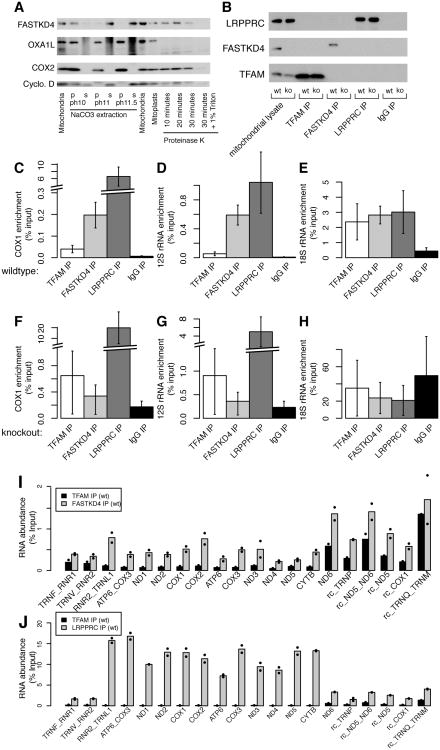
We sought to define the sub-organellar localization of FASTKD4 relative to the mt-mRNAs. FASTKD4, alongside soluble protein Cyclophilin D, was extractable with alkaline carbonate treatment of isolated mitochondria (Figure 6A). Additionally, FASTKD4 in mitoplasts was resistant to proteinase K treatment, alongside matrix protein Cyclophilin D (Figure 6A). All measured proteins are substrates for Proteinase K. Collectively, these studies indicate that FASTKD4 is a soluble protein residing within the mitochondrial matrix.
Figure 6. FASTKD4 is localized to the matrix and physically associates with mitochondrial RNAs.
(A) Western blot showing the presence of FASTKD4, OXA1L, COX2, and Cyclophilin D in isolated mitochondria, with carbonate extraction, and with proteinase K digestion of mitoplasts. (B) Western blot showing presence of LRPPRC, FASTKD4, and TFAM after immunoprecipitation of each protein or IgG control in control (wt) and FASTKD4ko (ko) cells. (C-E) qPCR was used to measure enrichment of (C) COX1 mRNA and (D) 12S rRNA, and (E) 18S rRNA with immunoprecipitation of TFAM, FASTKD4, LRPPRC, and IgG from wild-type cells. (F-H) qPCR-measured enrichment of transcripts measured in C-E with identical immunoprecipitation conditions, but in FASTKD4ko cells. Error bars represent s.e.m., n=3. (I) RNA abundance (% input) after FASTKD4 immunoprecipitation or TFAM immunoprecipitation in wild type cells measured by MitoString. (J) RNA abundance (as % input) after LRPPRC immunoprecipitation or TFAM immunoprecipitation in wild type cells. Points represent each duplicate.
Because FASTKD4 appears to stabilize mt-mRNAs, we investigated its binding to mtRNA by immunoprecipitating endogenous FASTKD4 from mitochondrial lysates and testing for mitochondrial RNA enrichment using qPCR. As controls, we immunoprecipitated TFAM, a known DNA-binding protein which is not expected to bind RNA, and LRPPRC, a known RNA-binding protein, alongside FASTKD4 in both wildtype and FASTKD4ko HEK-293T cells (Figure 6B). In agreement with published results (Chujo et al., 2012; Sasarman et al., 2010), LRPPRC is associated with COX1 mRNA (Figure 6C). In addition, LRPPRC associates with 12S rRNA but not nuclear 18S rRNA (Figure 6DE). In comparison, TFAM is not appreciably enriched for either RNA (Figure 6C). Similar to LRPPRC, FASTKD4 immunoprecipitation enriches both COX1 mRNA and 12S rRNA, as compared to TFAM immunoprecipitation, in wild type cells, but does not enrich nuclear 18S rRNA (Figure 6CDE). Intriguingly, FASTKD4 seems to bind 12S almost as strongly as LRPPRC, while it is a weaker binder of COX1. In FASTKD4ko cells, RNA enriched from FASTKD4 immunoprecipitation is indistinguishable from that enriched for TFAM and IgG immunoprecipitation, proving that the RNA enrichment of COX1 and 12S rRNA is specifically due to FASTKD4 immunoprecipitation and is not an antibody artifact (Figure 6FGH).
In addition to qPCR, we assessed our TFAM, FASTKD4, and LRPPRC immunoprecipitations with MitoString (Figure 6IJ). MitoString is valuable in this context as it allows strand-specific multiplexed measurement with minimal sample. We found that the majority of heavy strand abundant transcripts were enriched in both LRPPRC and FASTKD4 immunoprecipitations in comparison to TFAM immunoprecipitation (Figure 6IJ). In contrast, light strand probes targeting ND6 and light strand intergenic space (rc_ND5, rc_ND5_ND6, and rc_TRNQ_TRNM) had less specific enrichment (Figure 6I). The heavy strand junction probes spanning the RNR2_TRNL1 junction and the ATP6_COX3 junction are enriched in both FASTKD4 and LRPPRC immunoprecipitations. These results for LRPPRC are well correlated with previous findings using qPCR (Chujo et al., 2012). In addition, our approach interrogates new junction transcripts, especially on the light strand, which do not appear enriched relative to TFAM immunoprecipitation. RNR2_TRNL1, which we measure for the first time, is enriched in both LRPPRC and FASTKD4 immunoprecipitations at the same level as many of the mRNA genes. Our distinct approach and the inclusion of TFAM as a negative control demonstrate that FASTKD4 associates with mitochondrial RNAs and further validates LRPPRC as a mitochondrial RNA-binding protein.
Discussion
The expression of mtDNA requires nuclear-encoded proteins, yet at present, we lack a full molecular description of this system. Previous approaches to mitochondrial RNA measurement, including northern blots, qPCR, and next-generation sequencing, have been valuable, but are limited by lack of strand-specificity or sample throughput. In the current study, we establish a facile assay, MitoString, to systematically monitor precursor and mature mitochondrial RNA species following silencing of over 100 candidate nuclear-encoded factors. MitoString enables us to identify and classify mitochondrial RNA regulators based on their roles in transcription, cleavage, and stability. Through this compendium, we have expanded our understanding of previously characterized mitochondrial RNA processing proteins and implicated new players in this pathway, including the novel regulator FASTKD4.
Our approach, which measures both mature and precursor transcripts (Figure 1), implicates the LRPPRC-SLIRP complex in production or stability of the heavy strand precursor. Previous reports have implicated LRPPRC in promoting transcription (Liu et al., 2011; Sondheimer et al., 2010) or the stability of individual mt-mRNAs (Chujo et al., 2012; Ruzzenente et al., 2012). We find that silencing of SLIRP or LRPPRC primarily affects abundance of the heavy strand precursor, indicating a role for the complex in either transcription or stabilization of the precursor (Figure 2). This decrease is accompanied by a more pronounced downregulation of mature heavy strand mt-mRNAs, which may suggest a secondary role in mature transcript stability. In contrast, a previously reported precursor measurement in LRPPRC-silenced cells found no change in precursor abundance using qPCR, which is not strand-specific (Gohil et al., 2010). Our new method assesses just the heavy strand and finds a 4-fold decrease in the same TRNM_ND2 junction upon LRPPRC silencing (Figure 2). LRPPRC or SLIRP silencing has no effect on the light strand mRNA ND6 by our assay (Figure 2), consistent with strand-specific Northern blot measurements (Ruzzenente et al., 2012) and in contrast with strand-insensitive qPCR results (Gohil et al., 2010). Recently, GRSF1 has been implicated as a binder and regulator of ND6 and its precursor strand, suggesting a distinct mechanism operates on the light strand (Antonicka et al., 2013; Jourdain et al., 2013). Additionally, we and others find that LRPPRC binds heavy strand transcripts at a higher abundance and specificity than light strand transcripts (Chujo et al., 2012), which is consistent with our steady-state mtRNA findings.
We confirm the role of proteinaceous RNase P in the cleavage of tRNA 5′ junctions and identify noncanonical cleavage sites. The MitoString profiles of RNase P subunits MRPP1 and MRPP2 knockdowns are well correlated, while the effect of MRPP3 is weaker (Figure 2, 3CD). Our comprehensive approach builds on earlier findings focused on MRPP1 and a subset of junctions, (Holzmann et al., 2008) as well as work focused on MRPP1 and MRPP3 (Lopez Sanchez et al., 2011). We also identified a few exceptions to this canonical cleavage process. On the light strand, ELAC2 plays a role in the 5′ cleavage of TRNQ, which excises it from surrounding noncoding DNA (Figure 2, cluster C3). Also, TRNF_RNR1, which we expect to be regulated by ELAC2, is more strongly regulated by silencing of MRPP1/2 and SUPV3L1 (Figure 2, cluster C3). Consistent with this, this same TRNF_RNR1 junction was previously reported to be unaffected by ELAC2 siRNA knockdown (Brzezniak et al., 2011). Sequencing results had suggested that knockdown of ELAC2 and RNase P components did not affect all tRNA junctions (Lopez Sanchez et al., 2011), but for all of our tested junctions on the heavy strand (excepting ATP6/8_COX3), either one or both were involved (Figure 2). The discrepancy may stem from the prior study's exclusion of large precursor fragments due to size selection for tRNA-sized transcripts (Lopez Sanchez et al., 2011). Our assay found no obvious candidate for processing of the ATP6/8_COX3 junction, as all outlier hairpins are driven by a decrease in mt-mRNA levels, not an increase in junction content (Figure S3D-F).
Although SUPV3L1 clearly plays a role in the degradation of light strand transcripts, we find it has contrasting effects on different classes of mt-mRNAs (Figure 2). The short-lived ND1 and ND2 transcripts are increased, whereas the long-lived COX1-3 transcripts are actually decreased, suggesting that SUPV3L1 may not be responsible for all degradation within the mitochondria. These contrasting effects have been previously demonstrated (Szczesny et al., 2010), but only for a subset of mt-mRNAs, and we present here data for all transcripts simultaneously. For the TRNF_RNR1 and TRNV_RNR2 junctions, which we expect to be a readout for transcriptional initiation, hairpins targeting SUPV3L1 are the second strongest inducers of expression (Figure 2,C3). This increased transcription may pertain only to early termination transcripts, which end after the second rRNA, since the RNR2_TRNL1 junction and other heavy strand junctions are not increased (Figure 2, cluster C3). Therefore, SUPV3L1 depletion may increase ribosome production.
We find that FASTKD4 regulates a specific subset of mt-mRNAs. FASTKD4 silencing in general depletes those transcripts that are long-lived while sparing the precursor strand (Figure 4B). The sole exceptions are ND3, a short-lived depleted transcript, and ND4/4L, a long-lived unaffected transcript. Most of the affected mt-mRNAs also have an increased degradation rate in both FASTKD4 knockdown or knockout cells (Figure 5AB). Thus, we hypothesize that FASTKD4 may be serving to shield a specific set of mt-mRNAs from degradation by SUPV3L1 or an as-yet unidentified degradation machinery. ATP6 and COX3 unexpectedly do not have an increased degradation rate by our assay (Figure 5A), however the ATP6/8_COX3 precursor is the sole mtDNA gene-gene junction and is relatively stable compared to other precursors, as estimated by probe counts. The factor responsible for ATP6/8_COX3 cleavage remains undetermined and we expect a full understanding of this mechanism will resolve the discrepancy we observe.
We cement a role for FASTKD4 in mitochondrial RNA metabolism based upon the above findings, past studies and our immunoprecipitation of FASTKD4-associated mt-RNA. FASTKD4 contains a RAP domain in common with experimentally-validated RNA-binding proteins (Lee and Hong, 2004) and is found in a genome-wide mRNA-binding resource (Castello et al., 2012). Here, we show that FASTKD4 is localized to the mitochondrial matrix (Figure 6A) and associates with mitochondrial RNAs in a specific way (Figure 6C-I). Our immunoprecipitation assay does not distinguish among the heavy strand transcripts in terms of strength of association with FASTKD4, as it associates with all heavy strand transcripts. However, mt-RNA susceptibility to degradation may be set in part by the activity of another unidentified protein that requires FASTKD4 binding.
Why the mitochondrion maintains mt-mRNAs at such disparate levels is unknown, but we propose that it may provide a mechanism for ensuring proper OXPHOS complex stoichiometry. Across tissues, the molar ratios of different complexes is robust, but the mechanism that generates this stability is unknown (Benard et al., 2006). Complex I is consistently the least abundant among the OXPHOS complexes (Lenaz and Genova, 2010), and with the exception of the bicistronic ND4/4L transcript, mtDNA-encoded Complex I transcripts have half-lives of less than 90 minutes (Nagao et al., 2008). In contrast, Complex IV and V are present at 3-12 times the concentration of Complex I, a stoichiometry that we suggest could be metered in part by higher levels of Complex IV and V component transcripts (COX1-3, ATP6/8), whose half-lives range 138-231 minutes. Thus, the precise regulation of mt-mRNA transcript levels by factors like FASTKD4 and SUPV3L1 may be important for ensuring proper OXPHOS complex stoichiometry and function.
In the future we anticipate that MitoString may prove useful for interrogating the role of mt-mRNA processing directly in tissues from patients with mitochondrial disease. Our current study includes the analysis of 12 previously established disease genes (Table S5). Of these genes, three had previously been linked to aberrant expression of mt-mRNAs in humans. Our current compendium demonstrates that MTO1, PUS1, and FASTKD2 loss of function may also cause aberrant expression of mitochondrial RNAs. Our results point to additional modes of pathogenesis that may be at play in these disorders. As new genetic variants in mitochondrial RNA processing factors are identified in patients, a key challenge will lie in proving their pathogenicity. MitoString is a highly quantitative and systematic measure of mitochondrial gene expression that in principle can be easily applied to patient biopsy specimens. MitoString may therefore be a useful companion diagnostic for detecting aberrant expression of the mtDNA genome in human diseases.
Experimental Procedures
Cell culture
WI-38 fibroblasts and HEK-293T cells were cultured in high glucose DMEM (Invitrogen Cat. No. 11995) supplemented with 10% fetal bovine serum (Sigma Cat. No. F6178) and 1X penicillin, streptomycin, and glutamine (Invitrogen Cat. No. 10378-016) at 37° C with 5% CO2.
Immunoblotting
Protein was electrophoresed on a 12% Novex Tris-Glycine polyacrylamide gel at constant voltage (125V). The gel was transferred to a polyvinylidene difluoride membrane (Trans-Blot Turbo Transfer System). Membranes were blocked for one hour at room temperature in tris-buffered saline with 0.1% Tween-20 and 5% BSA (TBST-BSA). Membranes were incubated with primary antibody in TBSTBSA overnight at 4°C (Table S6). Secondary antibody was used at (1:5,000) for one hour at room temperature. Membranes were developed using WesternLightning Plus-ECL.
Screen
The three best hairpins per gene were selected, and produced and arrayed by the RNAi consortium (TRC) as previously described (Moffat et al., 2006).
WI-38 cells were seeded, infected after 24-hours, and selected 24-hours later. 6 days post-infection the cells were lysed with RLT buffer (Invitrogen) and β-Mercaptoethanol (1:100) and frozen. RLT lysates were processed with a custom MitoString codeset from NanoString technologies per manufacturer's instructions (Geiss et al., 2008). Screen details and data processing were as described in supplemental experimental procedures.
Protein expression
plexFLAG was generated from plex-TRC-983 (TRC) by molecular cloning. pDONR223-FASTKD4 from a previously described cDNA library (Pagliarini et al., 2008) was mutagenized via QuikChange mutagenesis (Agilent) using the primers described (Table S7). mtGFP was a gift from Y. Sancak. All cDNAs were cloned into plexFLAG via the Gateway system (Invitrogen).
Lentiviral production and infection
For follow-up experiments, shRNA lentiviral vectors (TRC) and FLAG-tagged expression constructs were used to produce virus and infect HEK-293T cells as previously described (Baughman et al., 2009). 4 ug/ml puromycin or 5 ug/ml blasticidin was used for selection.
mtDNA quantitation
mtDNA quantification was as previously described (Baughman et al., 2009)
Mitochondrial Isolation / Na2CO3 extraction / Proteinase K protection
HEK-293T cells were suspended in isolation buffer (220 mM mannitol, 70 mM sucrose, 5mM HEPES, 1 mM EGTA, pH 7.4 + complete, EDTA-free Protease Inhibitor Cocktail (Roche)) and lysed using a Cell Disruption Vessel (Parr Instrument Company) pressurized to 800 psi with Nitrogen followed by homogenization with five strokes of a Teflon Potter Elvehjem homogenizer. Crude mitochondria were isolated by differential centrifugation at 600g and 8000g. Mitoplasts were created by swelling with H2O on ice and were stabilized in 137 mM KCl, 2.5mM MgCl2, 10 mM HEPES pH 7.2, pelleted at 8000g and resuspended in isolation buffer. Na2CO3 extraction was performed on crude mitochondria and proteinase K protection was performed on mitoplasts as previously described (Ryan et al., 2001).
Measurement of RNA half-lives
HEK-293T cell lines were seeded the day before exposure to media with and without 1 ug/ml ethidium bromide. After six hours, the cells were lysed with RLT (Qiagen) for RNA extraction.
RNA isolation and qPCR
RNA was isolated using the RNeasy protocol with DNase I treatment (Qiagen). cDNA was transcribed using Superscript III First-Strand Synthesis Supermix (Invitrogen). qPCR was performed with a 7500-FAST ABI instrument and 2× Taqman FAST Advanced master-mix (Applied Biosystems) with mt-mRNA probes as described previously (Gohil et al., 2010).
RNA Immunoprecipitation
Isolated crude mitochondria were lysed in lysis buffer (50 mM HEPES (pH 7.4), 150 mM NaCl, 2 mM EDTA, 1% Triton, RNase Inhibitor). Lysates were treated with Turbo DNase for 1 hour at 37C. After spinning at 16000g 10 min at 4C, the supernatant was precleared with Protein A agarose beads for 40 min at 4C with rotation. The beads were spun out and the supernatant incubated with antibody overnight at 4C with rotation. Fresh Protein A agarose beads were blocked overnight with 1% BSA, 100 ug/ml Heparin, 100 ug/ml yeast tRNA in Lysis buffer. The next day, the beads were washed 3× with lysis buffer, and added to the lysate-antibody mix, and incubated with rotation for 2 hours at 4C. The bead complex was washed 3× with lysis buffer, and 3× with 500 mM NaCl lysis buffer. RNA samples were eluted with 300 ul 1M NaHCO3, 1% SDS at room temperature for 15 minutes. Beads were spun down and the supernatant was added to 12 ul 5M NaCl. 700 ul of RLT buffer was added for RNA isolation. Protein samples were boiled in sample buffer for Immunoblotting.
CRISPR knockout cell line generation
sgRNA and U6 primers (Table S7) were used to amplify the pX064 plasmid (gift from F. Zhang), as previously described (Hsu et al., 2013). This PCR product was cotransfected with the Cas9 plasmid (gift from F. Zhang) into HEK-293T cells with XTreme Gene transfection reagent per manufacturer's instructions. Cells were single-cell cloned and screened by Western blot for FASTKD4 protein. The identified knockout was verified by PCR followed by subcloning and Sanger sequencing.
Supplementary Material
Highlights.
MitoString directly measures mtDNA-encoded precursor and mature RNA species
We report MitoString profiles after silencing 107 mitochondrial RNA-binding factors
MitoString provides insights into the roles of MRPP1-3, ELAC2, LRPPRC, and SLIRP
FASTKD4 stabilizes a subset of mt-mRNAs and associates with mt-mRNAs
Acknowledgments
We thank J. Baughman, X.R. Bao, S. Calvo, Y. Sancak, L. Strittmatter, I. Jain, N. Delaney, E. Kovacs-Bogdan, A. Li, S. Vafai, M. Staller and N.M. Cabili for comments and helpful discussions. We thank the Broad Institute RNAi Platform for shRNA reagents and S. Silver and S. Gopal for advice; R. Boykin and G.
Geiss of Nanostring Technologies for technical assistance; the Regev and Shamji groups for access to the nCounter Analysis system. We thank M. Guttman, D. Shechner, J. Rinn, D. Scott, and F. Zhang for guidance on experimental protocols. This work was supported by an NSF graduate research fellowship to A.R.W. and an NIH grant (GM077465) to V.K.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre LA, del Castillo I, Macaya A, Medá C, Villamar M, Moreno-Pelayo MA, Moreno F. A novel mutation in the gene encoding TIMM8a, a component of the mitochondrial protein translocase complexes, in a Spanish familial case of deafness-dystonia (Mohr-Tranebjaerg) syndrome. Am J Med Genet A. 2006;140:392–397. doi: 10.1002/ajmg.a.31079. [DOI] [PubMed] [Google Scholar]
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Antonicka H, Sasarman F, Nishimura T, Paupe V, Shoubridge EA. The mitochondrial RNA-binding protein GRSF1 localizes to RNA granules and is required for posttranscriptional mitochondrial gene expression. Cell Metab. 2013;17:386–398. doi: 10.1016/j.cmet.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Barrientos A, Korr D, Barwell KJ, Sjulsen C, Gajewski CD, Manfredi G, Ackerman S, Tzagoloff A. MTG1 codes for a conserved protein required for mitochondrial translation. Mol Biol Cell. 2003;14:2292–2302. doi: 10.1091/mbc.E02-10-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman JM, Nilsson R, Gohil V, Arlow DH, Gauhar Z, Mootha V. A computational screen for regulators of oxidative phosphorylation implicates SLIRP in mitochondrial RNA homeostasis. PLoS Genetics. 2009;5:e1000590. doi: 10.1371/journal.pgen.1000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard G, Faustin B, Passerieux E, Galinier A, Rocher C, Bellance N, Delage JP, Casteilla L, Letellier T, Rossignol R. Physiological diversity of mitochondrial oxidative phosphorylation. Am J Physiol Cell Physiol. 2006;291:C1172–1182. doi: 10.1152/ajpcell.00195.2006. [DOI] [PubMed] [Google Scholar]
- Borowski LS, Dziembowski A, Hejnowicz MS, Stepien PP, Szczesny RJ. Human mitochondrial RNA decay mediated by PNPase-hSuv3 complex takes place in distinct foci. Nucleic Acids Res. 2013;41:1223–1240. doi: 10.1093/nar/gks1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezniak LK, Bijata M, Szczesny RJ, Stepien PP. Involvement of human ELAC2 gene product in 3′ end processing of mitochondrial tRNAs. RNA biology. 2011;8:616–626. doi: 10.4161/rna.8.4.15393. [DOI] [PubMed] [Google Scholar]
- Castello A, Fischer B, Eichelbaum K, Horos R, Beckmann BM, Strein C, Davey NE, Humphreys DT, Preiss T, Steinmetz LM, et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149:1393–1406. doi: 10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- Chujo T, Ohira T, Sakaguchi Y, Goshima N, Nomura N, Nagao A, Suzuki T. LRPPRC/SLIRP suppresses PNPase-mediated mRNA decay and promotes polyadenylation in human mitochondria. Nucleic Acids Res. 2012;40:8033–8047. doi: 10.1093/nar/gks506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Tate J, Mistry J, Coggill PC, Sammut SJ, Hotz HR, Ceric G, Forslund K, Eddy SR, Sonnhammer EL, et al. The Pfam protein families database. Nucleic Acids Res. 2008;36:D281–288. doi: 10.1093/nar/gkm960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- Gelfand R, Attardi G. Synthesis and turnover of mitochondrial ribonucleic acid in HeLa cells: the mature ribosomal and messenger ribonucleic acid species are metabolically unstable. Mol Cell Biol. 1981;1:497–511. doi: 10.1128/mcb.1.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi D, Saada A, D'Adamo P, Fernandez-Vizarra E, Gasparini P, Tiranti V, Elpeleg O, Zeviani M. FASTKD2 nonsense mutation in an infantile mitochondrial encephalomyopathy associated with cytochrome c oxidase deficiency. Am J Hum Genet. 2008;83:415–423. doi: 10.1016/j.ajhg.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohil VM, Nilsson R, Belcher-Timme CA, Luo B, Root DE, Mootha VK. Mitochondrial and nuclear genomic responses to loss of LRPPRC expression. J Biol Chem. 2010;285:13742–13747. doi: 10.1074/jbc.M109.098400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmel J, Ruzzenente B, Terzioglu M, Spahr H, Falkenberg M, Larsson NG. The leucine-rich pentatricopeptide repeat-containing protein (LRPPRC) does not activate transcription in mammalian mitochondria. J Biol Chem. 2013;288:15510–15519. doi: 10.1074/jbc.M113.471649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann J, Frank P, Löffler E, Bennett KL, Gerner C, Rossmanith W. RNase P without RNA: identification and functional reconstitution of the human mitochondrial tRNA processing enzyme. Cell. 2008;135:462–474. doi: 10.1016/j.cell.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo JM, Valcárcel J. Fas-activated serine/threonine kinase (FAST K) synergizes with TIA-1/TIAR proteins to regulate Fas alternative splicing. J Biol Chem. 2007;282:1539–1543. doi: 10.1074/jbc.C600198200. [DOI] [PubMed] [Google Scholar]
- Jourdain AA, Koppen M, Wydro M, Rodley CD, Lightowlers RN, Chrzanowska-Lightowlers ZM, Martinou JC. GRSF1 regulates RNA processing in mitochondrial RNA granules. Cell Metab. 2013;17:399–410. doi: 10.1016/j.cmet.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khidr L, Wu G, Davila A, Procaccio V, Wallace D, Lee WH. Role of SUV3 helicase in maintaining mitochondrial homeostasis in human cells. J Biol Chem. 2008;283:27064–27073. doi: 10.1074/jbc.M802991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani T, Akabane S, Takeyasu K, Ueda T, Takeuchi N. Human G-proteins, ObgH1 and Mtg1, associate with the large mitochondrial ribosome subunit and are involved in translation and assembly of respiratory complexes. Nucleic Acids Res. 2013;41:3713–3722. doi: 10.1093/nar/gkt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattin JE, Schroder K, Su AI, Walker JR, Zhang J, Wiltshire T, Saijo K, Glass CK, Hume DA, Kellie S, et al. Expression analysis of G Protein-Coupled Receptors in mouse macrophages. Immunome Res. 2008;4:5. doi: 10.1186/1745-7580-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Hong W. RAP--a putative RNA-binding domain. Trends Biochem Sci. 2004;29:567–570. doi: 10.1016/j.tibs.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Lenaz G, Genova ML. Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. Antioxid Redox Signal. 2010;12:961–1008. doi: 10.1089/ars.2009.2704. [DOI] [PubMed] [Google Scholar]
- Li W, Simarro M, Kedersha N, Anderson P. FAST is a survival protein that senses mitochondrial stress and modulates TIA-1-regulated changes in protein expression. Mol Cell Biol. 2004;24:10718–10732. doi: 10.1128/MCB.24.24.10718-10732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Sanosaka M, Lei S, Bestwick ML, Frey JH, Surovtseva YV, Shadel GS, Cooper MP. LRP130 protein remodels mitochondria and stimulates fatty acid oxidation. J Biol Chem. 2011;286:41253–41264. doi: 10.1074/jbc.M111.276121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Sanchez MIG, Mercer TR, Davies SMK, Shearwood AJ, Nygård KKA, Richman TR, Mattick JS, Rackham O, Filipovska A. RNA processing in human mitochondria. Cell Cycle. 2011;10:2904–2916. doi: 10.4161/cc.10.17.17060. [DOI] [PubMed] [Google Scholar]
- Mercer TR, Neph S, Dinger ME, Crawford J, Smith MA, Shearwood AJ, Haugen E, Bracken CP, Rackham O, Stamatoyannopoulos JA, et al. The human mitochondrial transcriptome. Cell. 2011;146:645–658. doi: 10.1016/j.cell.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mili S, Piñol-Roma S. LRP130, a pentatricopeptide motif protein with a noncanonical RNA-binding domain, is bound in vivo to mitochondrial and nuclear RNAs. Mol Cell Biol. 2003;23:4972–4982. doi: 10.1128/MCB.23.14.4972-4982.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G, Piqani B, Eisenhaure TM, Luo B, Grenier JK, et al. A Lentiviral RNAi Library for Human and Mouse Genes Applied to an Arrayed Viral High-Content Screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Nagao A, Hino-Shigi N, Suzuki T. Measuring mRNA decay in human mitochondria. Methods Enzymol. 2008;447:489–499. doi: 10.1016/S0076-6879(08)02223-4. [DOI] [PubMed] [Google Scholar]
- Ojala D, Montoya J, Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981;290:470–474. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- Pagliarini DJ, Calvo SE, Chang B, Sheth S, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JR, Bykhovskaya Y, Mengesha E, Bertolotto C, FischelGhodsian N. Mitochondrial myopathy and sideroblastic anemia (MLASA): missense mutation in the pseudouridine synthase 1 (PUS1) gene is associated with the loss of tRNA pseudouridylation. J Biol Chem. 2005;280:19823–19828. doi: 10.1074/jbc.M500216200. [DOI] [PubMed] [Google Scholar]
- Piechota J, Tomecki R, Gewartowski K, Szczesny R, Dmochowska A, Kudła M, Dybczyńska L, Stepien PP, Bartnik E. Differential stability of mitochondrial mRNA in HeLa cells. Acta Biochim Pol. 2006;53:157–168. [PubMed] [Google Scholar]
- Puranam RS, Attardi G. The RNase P associated with HeLa cell mitochondria contains an essential RNA component identical in sequence to that of the nuclear RNase P. Mol Cell Biol. 2001;21:548–561. doi: 10.1128/MCB.21.2.548-561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzzenente B, Metodiev MD, Wredenberg A, Bratic A, Park CB, Camara Y, Milenkovic D, Zickermann V, Wibom R, Hultenby K, et al. LRPPRC is necessary for polyadenylation and coordination of translation of mitochondrial mRNAs. EMBO J. 2012;31:443–456. doi: 10.1038/emboj.2011.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MT, Voos W, Pfanner N. Assaying protein import into mitochondria. Methods Cell Biol. 2001;65:189–215. doi: 10.1016/s0091-679x(01)65012-x. [DOI] [PubMed] [Google Scholar]
- Sasarman F, Brunel-Guitton C, Antonicka H, Wai T, Shoubridge EA, Consortium L. LRPPRC and SLIRP interact in a ribonucleoprotein complex that regulates posttranscriptional gene expression in mitochondria. Mol Biol Cell. 2010;21:1315–1323. doi: 10.1091/mbc.E10-01-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23:459–466. doi: 10.1016/j.tem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simarro M, Gimenez-Cassina A, Kedersha N, Lazaro JB, Adelmant GO, Marto JA, Rhee K, Tisdale S, Danial N, Benarafa C, et al. Fast kinase domain-containing protein 3 is a mitochondrial protein essential for cellular respiration. Biochem Biophys Res Commun. 2010;401:440–446. doi: 10.1016/j.bbrc.2010.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simarro M, Mauger D, Rhee K, Pujana MA, Kedersha NL, Yamasaki S, Cusick ME, Vidal M, Garcia-Blanco MA, Anderson P. Fas-activated serine/threonine phosphoprotein (FAST) is a regulator of alternative splicing. Proc Natl Acad Sci U S A. 2007;104:11370–11375. doi: 10.1073/pnas.0704964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondheimer N, Fang JK, Polyak E, Falk MJ, Avadhani NG. Leucine-Rich Pentatricopeptide-Repeat Containing Protein Regulates Mitochondrial Transcription. Biochemistry. 2010;49:7467–7473. doi: 10.1021/bi1008479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Juel VC, Wooten GF. Dystonia with and without deafness is caused by TIMM8A mutation. Adv Neurol. 2004;94:147–154. [PubMed] [Google Scholar]
- Szczesny RJ, Borowski LS, Brzezniak LK, Dmochowska A, Gewartowski K, Bartnik E, Stepien PP. Human mitochondrial RNA turnover caught in flagranti: involvement of hSuv3p helicase in RNA surveillance. Nucleic Acids Res. 2010;38:279–298. doi: 10.1093/nar/gkp903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedrenne V, Gowher A, De Lonlay P, Nitschke P, Serre V, Boddaert N, Altuzarra C, Mager-Heckel AM, Chretien F, Entelis N, et al. Mutation in PNPT1, which encodes a polyribonucleotide nucleotidyltransferase, impairs RNA import into mitochondria and causes respiratory-chain deficiency. Am J Hum Genet. 2012;91:912–918. doi: 10.1016/j.ajhg.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ameln S, Wang G, Boulouiz R, Rutherford MA, Smith GM, Li Y, Pogoda HM, Nürnberg G, Stiller B, Volk AE, et al. A mutation in PNPT1, encoding mitochondrial-RNA-import protein PNPase, causes hereditary hearing loss. Am J Hum Genet. 2012;91:919–927. doi: 10.1016/j.ajhg.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner BK, Kitami T, Gilbert TJ, Peck D, Ramanathan A, Schreiber SL, Golub TR, Mootha VK. Large-scale chemical dissection of mitochondrial function. Nat Biotechnol. 2008;26:343–351. doi: 10.1038/nbt1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Chen HW, Oktay Y, Zhang J, Allen EL, Smith GM, Fan KC, Hong JS, French SW, McCaffery JM, et al. PNPASE regulates RNA import into mitochondria. Cell. 2010a;142:456–467. doi: 10.1016/j.cell.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yan Q, Guan MX. Combination of the loss of cmnm5U34 with the lack of s2U34 modifications of tRNALys, tRNAGlu, and tRNAGln altered mitochondrial biogenesis and respiration. J Mol Biol. 2010b;395:1038–1048. doi: 10.1016/j.jmb.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Guan MX. Identification and characterization of mouse TRMU gene encoding the mitochondrial 5-methylaminomethyl-2-thiouridylatemethyltransferase. Biochim Biophys Acta. 2004;1676:119–126. doi: 10.1016/j.bbaexp.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Zeharia A, Shaag A, Pappo O, Mager-Heckel AM, Saada A, Beinat M, Karicheva O, Mandel H, Ofek N, Segel R, et al. Acute infantile liver failure due to mutations in the TRMU gene. Am J Hum Genet. 2009;85:401–407. doi: 10.1016/j.ajhg.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.