Abstract
Background
Interindividual differences in estrogen metabolism may partially account for differences in risks of estrogen-responsive cancers. We conducted a proof-of-performance study to assess the reproducibility of a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for measurement of 15 serum estrogens and metabolites (all 15 termed EM) in total (conjugated+unconjugated) and unconjugated forms and describe interindividual variation.
Methods
Interindividual variation in serum EM profiles was evaluated for 20 premenopausal women, 15 postmenopausal women, and 10 men. Replicate aliquots from 10 premenopausal women, 5 postmenopausal women, and 5 men were assayed 8 times over 4 weeks. Components of variance were used to calculate coefficients of variation (CVs) and intraclass correlation coefficients (ICCs).
Results
In postmenopausal women and men, median EM concentrations were similar and substantially lower than in premenopausal women. Within each sex/menopausal group, the sum of all EM varied 5–7 fold across extreme deciles. Some EM had greater variation; total estrone varied ~12-fold in premenopausal and postmenopausal women. Unconjugated estradiol varied 17-fold in postmenopausal women but only 5-fold in premenopausal women and men. CVs reflecting variation across replicate measures for individuals were <5% for most EM, but higher in some individuals with a low EM concentration. Overall laboratory CVs for all but one EM were <2% and ICCs were >99% for all EM in each group.
Conclusions
The serum EM assay has excellent laboratory reproducibility. In premenopausal women, postmenopausal women, and men, interindividual variation in EM measures is substantially greater than laboratory variation.
Impact
The serum EM assay is suitable for epidemiologic application.
Keywords: Estradiol, Estrogens, Estrogen metabolism, Estrone, Hydroxylation, Methylation, Women, Men, Postmenopause, Premenopause, Liquid Chromatography-Mass Spectrometry
INTRODUCTION
Endogenous estrogens are known to play a causal role in breast and endometrial cancers and may also be important in male reproductive cancers, particularly male breast cancer, testicular cancer, and prostate cancer (1–3). Both physiologic and pathologic actions of estrogens are modulated by metabolic conversions which occur in the liver and kidney, and locally within target tissues. The parent estrogens, estrone and estradiol, can each be irreversibly hydroxylated at the C-2, C-4, or C-16 positions of the steroid ring; catechol estrogen metabolites, characterized by adjacent hydroxyl groups, can be further modified by methylation of one of the hydroxyl groups (4). Estrogens and their metabolites (jointly referred to as EM) can be further modified through addition of sulfate, glucuronide or glutathionate conjugates (5).
Because EM vary with respect to bioavailability (6), affinities for the estrogen receptors (7) and mutagenic potential (8, 9), many investigators have hypothesized that differences in estrogen metabolism profiles may partially explain interindividual differences in cancer risks. Substantial interindividual variability has been observed in absolute concentrations of creatinine-adjusted urinary EM (10), but interindividual variability has not been similarly investigated for serum EM. Similarly, reproducibility has been formally tested for the urine EM assay (10), but not a serum EM assay.
Circulating EM can be conjugated or unconjugated; in urine, practically all EM are conjugated. Therefore, serum measures of EM in both conjugated and unconjugated forms can provide information about their bioavailability; this information cannot be obtained from the urinary EM profile. In addition, circulating EM represent the net effects of estrogen production, metabolism, and excretion, and are a major determinant of endogenous estrogen exposure at the tissue level.
Hypotheses about the roles of estrogen metabolism profiles and particular estrogen metabolites in cancer etiology can now be tested in epidemiologic settings due to the development of an accurate, sensitive, and reasonably high through-put liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay that concurrently measures 15 serum EM (11). Data on the reproducibility and interindividual variability of these serum EM measures may be useful for investigators considering employing this assay. Whereas urine has been used to study metabolism in clinical studies, most epidemiologic cohorts have stored blood samples only. Three prospective studies using the serum EM assay to test hypotheses about the role of estrogen metabolism in postmenopausal breast cancer have been published (12–14), and future work will address other hormone-sensitive cancers in women, such as endometrial and ovarian cancer, and men.
Here, we present results from a reproducibility study of the LC-MS/MS method for measurement of serum concentrations of 15 EM. For each EM, the total concentration, which includes sulfated, glucuronidated, and unconjugated forms, and the unconjugated concentration were measured. We undertook this study to assess the reproducibility of the serum EM assay and to provide descriptive data on interindividual variability in EM profiles in serum samples from premenopausal women, postmenopausal women, and men.
MATERIALS AND METHODS
Subject and study design
Volunteers were recruited from the Research Donor Program (RDP) administered by the NCI-Frederick Occupational Health Services in Frederick, Maryland under an approved protocol. Donors were identified only by code and the specimens were further de-identified using laboratory accession numbers. A total of 45 participants, including 20 premenopausal women, 15 postmenopausal women, and 10 men, were recruited for the present study in 2009–2010. All participants, including women and men, were at least 18 years of age and denied current or recent (within the last 12 months) use of exogenous hormones. Premenopausal participants met the following additional criteria: they were ≤40 years of age, reported no current pregnancy or lactation; and reported a menstrual period within the previous 2 months. Postmenopausal participants met the following additional criteria: they were ≥50 years, reported natural rather than surgical menopause, and experienced their last menstrual period more than 12 months prior to blood collection.
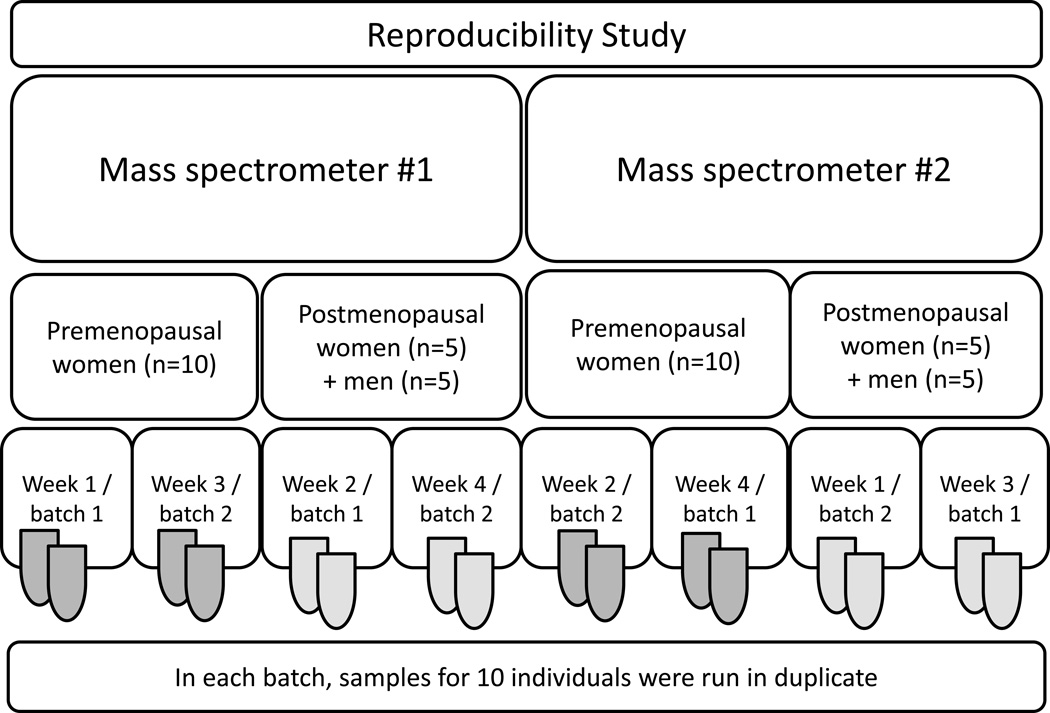
At the time of blood collection, premenopausal donors were given postcards to report the date of the following menstrual period. Menstrual cycle phase was determined by counting 28 days backwards from the first day of the menstrual period following sample collection; samples collected on days 1–12 of the menstrual cycle were considered to be in follicular phase; days 13–15 were considered periovulatory; and days 16–28 were considered to be luteal. Of 20 premenopausal women in the study, 7 were in follicular phase, 9 were in luteal phase, and 4 were periovulatory at the time of blood donation. For the laboratory reproducibility study, serum samples from 10 premenopausal women, 5 postmenopausal women, and 5 men were aliquoted, and eight aliquots from the single blood draw assayed. Two 1 mL aliquots were run for each participant in each of two batches on each of two mass spectrometers (see Figure 1).
Figure 1.
Design for the laboratory reproducibility study. In each batch, samples for 10 individuals were run in duplicate. Both total and unconjugated estrogens and estrogen metabolites were assayed for each sample.
The descriptive analyses included 20 premenopausal women, 15 postmenopausal women, and 10 men. For the 20 participants with repeated EM measures from the laboratory reproducibility study, a single measure was selected at random for inclusion. For the remaining 10 premenopausal women, 10 postmenopausal women, and 5 men a single serum aliquot was assayed for use in descriptive analyses.
Blood collection and sample processing
Whole blood from each donor was collected into nine 10 ml BD Vacutainer® non-additive, silicone coated, red-top blood collection tubes (BD Diagnostics, Franklin Lakes, NJ). The tubes were inverted 8 times at the point of collection before transfer to the processing laboratory and allowed to clot for 60 minutes at room temperature prior to centrifugation. The total time of processing was less than 2 hours. The samples were then centrifuged at 1,200 g for 15 minutes at 10 °C. Serum was immediately removed from the pellet using a serological pipet. All the serum from same donor was pooled into a 50 ml conical tube and transferred on wet ice to the aliquotting laboratory.
Pooled serum samples were subsequently vortexed, briefly centrifuged at 3,000 g and 10 °C, and immediately placed on a Tecan Freedom EVO® liquid handler (Tecan Group Ltd., Switzerland) to create 1.0 ml aliquots. Aliquots were frozen and stored at −80°C.
Estrogen/estrogen metabolite (EM) assay
Serum samples were analyzed at the Laboratory of Proteomics and Analytical Technologies (LPAT), Cancer Research Technology Program, Leidos Biomedical Research, Inc., Frederick National Laboratory for Cancer Research, Frederick, MD. Details of the analytic method for measuring serum EM have been published previously (11). Stable isotope dilution-high performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) was used to measure serum concentrations of 15 EM. Measured analytes include the parent estrogens (estrone and estradiol) and 13 estrogen metabolites, including 2-hydroxyestrone, 2-hydroxyestradiol, 2-methoxyestrone, 2-methoxyestradiol, 2-hydroxyestrone-3-methyl ether, 4-hydroxyestrone, 4-methoxyestrone, 4-methoxyestradiol, 16α-hydroxyestrone, estriol, 16-ketoestradiol, 16-epiestriol, and 17-epiestriol. All are found in serum in conjugated forms, attached to sulfate or glucuronide moieties; five (i.e., estrone, estradiol, 2-methoxyestrone, 2-methoxyestradiol, and estriol) also exist in unconjugated forms. The assay requires 0.5 mL serum for measurement of total EM (unconjugated EM + conjugated EM), and 0.5 mL serum for measurement of unconjugated EM.
Steps for measurement of serum EM included addition of stable isotopically-labeled standards, extraction with dichloromethane, derivatization with dansyl chloride, and LC-MS/MS. First, six stable isotopically labeled internal standards were added to each 1 mL aliquot of serum, including: estrone-13,14,15,16,17,18-13C6 and estradiol-13,14,15,16,17,18-13C6 (Cambridge Isotope Laboratories, Andover, MA); 2-hydroxyestradiol-1,4,16,16,17-d5, 2-methoxyestradiol-1,4,16,16,17-d5 and estriol-2,4,17-d3 (C/D/N Isotopes Inc, Pointe-Claire, QC, Canada); and 16-epiestriol-2,4,16-d3 (Medical Isotopes Inc, Pelham, NH). Then each sample was split and one aliquot underwent enzymatic hydrolysis using a preparation from Helix pomatia with β-glucuronidase and sulfatase activity (Sigma Chemical Co, St Louis, MO) — this additional step enables measurement of each EM in total (unconjugated + conjugated forms). Split samples were run adjacent to one another. Capillary LC- electrospray ionization (ESI)-MS/MS analysis was performed using an Agilent 1200 series nanoflow LC system (Agilent Technologies, Palo Alto, CA) coupled to a TSQ™ Quantum Ultra triple quadrupole mass spectrometer (Thermo Electron, San Jose, CA).
Statistical analysis
Serum concentrations of EM were expressed as picomoles per liter and log-transformed. We also assessed each EM concentration as a proportion of all EM combined (EM%). Concentrations of conjugated EM were calculated for each individual as the difference between the total EM concentration in an aliquot that underwent hydrolysis, and the unconjugated EM concentration measured in the paired aliquot that did not undergo hydrolysis.
Medians and ranges were calculated for each sex/menstrual status/menopausal group under study. Distributions of replicate measures for an individual and measures within and between populations were inspected using histograms and descriptive statistics. Analysis of variance (ANOVA) was used to compare distributions of EM% across sex and menopausal groups.
To characterize reproducibility of EM measures, PROC varcomp (Method = TYPE1, SAS Institute, Cary, NC) was used to estimate components of variance in log-transformed serum EM concentrations attributable to differences between individuals and across replicate measures for individuals in each population under study (premenopausal women, postmenopausal women, and men). These estimates were used to compute laboratory coefficients of variation (CVs) and intraclass correlation coefficients (ICCs) representing, respectively, the reproducibility of, and variation in, non-log transformed serum EM concentrations (15). CVs for each individual were calculated as the standard deviation / mean for the set of 8 replicate measures of non-log transformed serum EM concentration. Finally, we plotted, for each EM, the coefficient of variation across the 8 replicate measures for each individual participant by its mean value. Measures from a single aliquot from a male participant were excluded from these analyses as the aliquot had been mislabeled.
Analyses which attempted to simultaneously parse variance in EM measures to within batch variation, across batch variation, and across instrument variation, resulted in very small or negative estimates of components of variance for many EM, probably due to the very small variation observed overall across all of the replicate measures. Therefore we present overall laboratory CVs rather than measures attributing variation to any particular component of the measurement process.
RESULTS
EM profiles and interindividual variability
In Table 1 we present the median and interdecile range for the serum concentration of each EM, in picomoles per liter, for all participants in the descriptive study by sex / menopausal group. Median serum concentrations of each EM were similar in postmenopausal women and men and substantially higher in premenopausal women. Across their interdecile ranges and within each sex/menopausal group, the sum of all serum EM varied 5–7 fold, but some EM varied over a much wider range. For example total estrone varied ~12-fold across the interdecile range in premenopausal and postmenopausal women. Similarly, 17-epiestriol varied 54- and 42-fold, and 16-epiestriol varied 18- and 21-fold, in postmenopausal women and men, respectively. Serum concentrations of unconjugated estradiol varied 17-fold across the interdecile range in postmenopausal women, but only 5-fold in premenopausal women and men.
Table 1.
Serum concentrations* of 15 estrogens and estrogen metabolites (EM), in total (conjugated + unconjugated) and unconjugated forms (pmol/L), in 20 premenopausal women, 15 postmenopausal women, and 10 men.
| Premenopausal women | Postmenopausal women | Men | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Interdecile range | Interdecile range | Interdecile range | |||||||
| median | 10th | 90th | median | 10th | 90th | median | 10th | 90th | |
| All EM | 5406.4 | 2215.4 | 12967.4 | 1463.9 | 589.6 | 4384.6 | 1644.0 | 1044.9 | 4715.9 |
| Conjugated EM | 3143.4 | 1007.9 | 9083.1 | 828.8 | 403.9 | 2517.4 | 939.0 | 718.4 | 3039.9 |
| Unconjugated EM | 609.8 | 296.2 | 1277.9 | 170.8 | 82.0 | 404.0 | 199.6 | 122.0 | 334.0 |
| Parent estrogens | 2517.1 | 785.6 | 7422.0 | 533.2 | 169.4 | 2055.3 | 723.4 | 413.8 | 2147.9 |
| Estrone | 1986.2 | 538.2 | 6712.3 | 480.3 | 136.0 | 1611.5 | 614.5 | 379.3 | 1962.6 |
| Unconjugated Estrone | 186.7 | 84.6 | 362.9 | 76.9 | 35.6 | 170.3 | 77.6 | 47.9 | 154.6 |
| Estradiol | 432.2 | 199.6 | 1094.4 | 64.8 | 20.7 | 230.5 | 133.1 | 33.4 | 211.6 |
| Unconjugated estradiol | 267.4 | 126.9 | 666.0 | 16.7 | 5.4 | 91.5 | 54.0 | 16.3 | 87.8 |
| 2-Pathway EM | 777.5 | 353.7 | 2028.0 | 266.9 | 84.9 | 733.1 | 280.3 | 122.8 | 704.8 |
| 2-Hydroxyestrone | 471.4 | 169.5 | 1247.2 | 127.6 | 46.2 | 351.1 | 134.2 | 66.3 | 522.7 |
| 2-Hydroxyestradiol | 79.5 | 24.9 | 235.5 | 35.7 | 12.8 | 110.9 | 34.1 | 15.1 | 83.5 |
| 2-Methoxyestrone | 164.8 | 32.5 | 293.8 | 45.7 | 13.4 | 86.2 | 50.9 | 11.7 | 124.9 |
| Unconjugated 2-methoxyestrone | 55.6 | 11.3 | 127.6 | 16.9 | 7.6 | 26.1 | 12.2 | 5.2 | 21.6 |
| 2-Methoxyestradiol | 33.8 | 7.5 | 105.9 | 16.2 | 7.1 | 69.7 | 14.7 | 7.8 | 50.8 |
| Unconjugated 2-methoxyestradiol | 8.8 | 2.5 | 20.3 | 4.1 | 1.9 | 12.4 | 4.3 | 1.2 | 5.2 |
| 2-Hydroxyestrone-3-methyl ether | 25.8 | 10.9 | 62.2 | 12.3 | 2.6 | 24.0 | 11.4 | 3.2 | 24.7 |
| 4-Pathway EM | 91.6 | 36.6 | 208.8 | 30.5 | 12.0 | 75.9 | 32.8 | 23.5 | 93.0 |
| 4-Hydroxyestrone | 63.9 | 25.7 | 151.5 | 22.5 | 6.9 | 61.7 | 21.7 | 16.2 | 85.0 |
| 4-Methoxyestrone | 10.1 | 2.3 | 33.7 | 4.5 | 3.4 | 10.8 | 5.3 | 2.4 | 11.4 |
| 4-Methoxyestradiol | 7.2 | 3.1 | 16.0 | 3.1 | 1.6 | 5.7 | 2.9 | 1.4 | 6.1 |
| 16-Pathway EM | 1704.6 | 750.0 | 4599.2 | 548.2 | 314.1 | 1249.4 | 555.9 | 484.0 | 1770.2 |
| 16α-Hydroxyestrone | 251.7 | 98.7 | 642.9 | 74.0 | 37.6 | 227.8 | 72.4 | 36.0 | 310.7 |
| Estriol | 1089.2 | 405.2 | 2894.2 | 427.7 | 247.8 | 846.8 | 441.5 | 292.7 | 1091.3 |
| Unconjugated estriol | 90.8 | 30.1 | 133.6 | 52.5 | 18.9 | 75.0 | 46.8 | 25.8 | 74.2 |
| 16-Ketoestradiol | 291.1 | 105.4 | 718.3 | 83.6 | 24.0 | 179.8 | 77.2 | 29.5 | 255.8 |
| 16-Epiestriol | 73.0 | 32.1 | 145.6 | 24.8 | 3.5 | 63.2 | 32.4 | 3.3 | 69.3 |
| 17-Epiestriol | 45.9 | 10.5 | 131.1 | 21.7 | 1.4 | 75.6 | 16.3 | 1.3 | 54.5 |
Medians and ranges were calculated using a single, arbitrarily selected aliquot for each of the 10 premenopausal women, 5 postmenopausal women, and 5 men whose samples were assayed in replicate in the laboratory reproducibility study, and the single aliquot assayed for the additional 10 premenopausal women, 10 postmenopausal women, and 5 men.
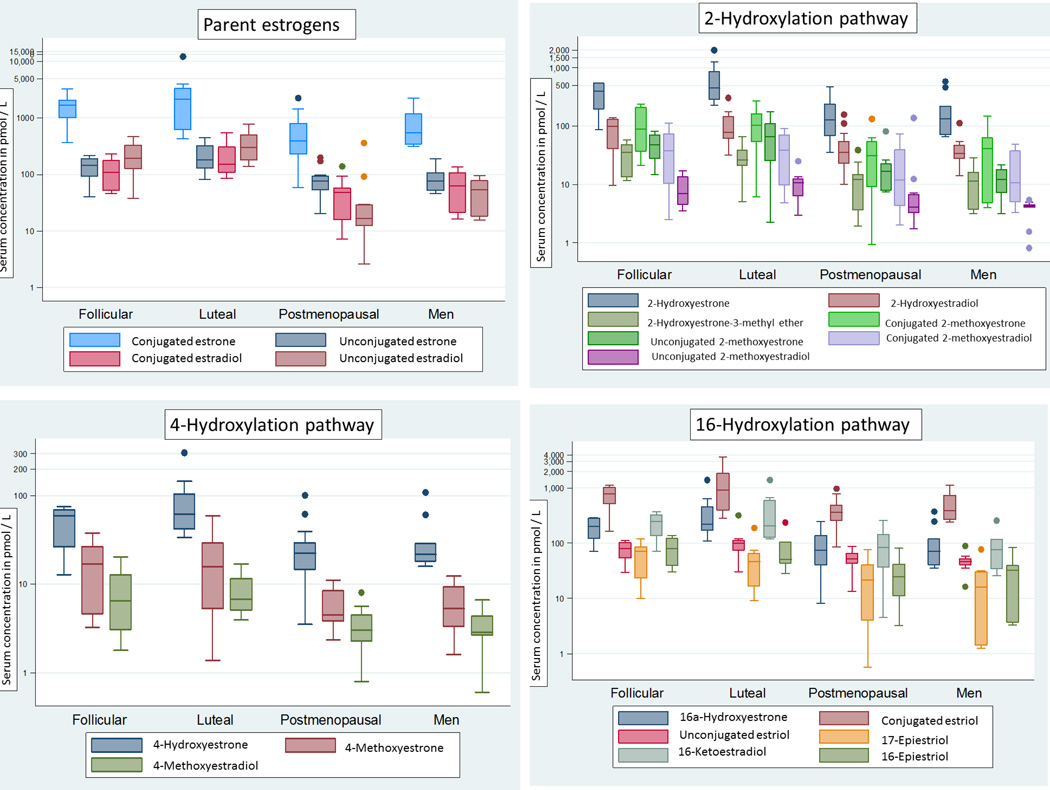
In Figure 2 distributions of serum concentrations are presented on a log-scale for parent estrogens and their metabolites and are grouped by pathway for premenopausal women determined to be in follicular and luteal phases of the menstrual cycle at the time of sample collection (n=7 and 9, respectively), postmenopausal women (n=15), and men (n=10). Premenopausal women found to be periovulatory (n=4) are not included in this figure. Relative EM concentrations were broadly similar across the groups defined by sex, menopausal status, and menstrual phase. In each of the four groups, estrone represented the largest fraction of the all EM combined, while estriol and 2-hydroxyestrone ranked second and third. For premenopausal women, postmenopausal women, and men, parent estrogens comprised, on average, 38–50% of all EM and 2-hydroxylation pathway metabolites represented 15–17%; 4-hydroxylation pathway metabolites accounted for 1.8–2.1% in all three groups and 16-hydroxylation pathway metabolites represented 33–43% (data not shown). While most estrone was found in conjugated rather than unconjugated forms (>80% of total estrone in all sex/menopausal groups), approximately half of total estradiol and total estriol were present in the unconjugated form. In premenopausal women, compared with postmenopausal women and men, the parent estrogens represented a significantly greater proportion of all EM combined (50%, 38%, and 44% respectively, P<0. 001) while 16-pathway metabolites accounted for a significantly smaller proportion (33%, 43%, and 39% respectively, P<0.001). There were no statistically significant differences in the proportion of 2-pathway metabolites or 4-pathway metabolites.
Figure 2.
Box and whisker plots of the distributions of estrogens and estrogen metabolites (EM) in serum samples of premenopausal women collected during the follicular (n=7) and luteal (n=9) phases of the menstrual cycle, postmenopausal women (n=15), and men (n=10). Periovulatory samples are not included due to small sample size (n=4) and the high variability in serum estrogen levels during this period of the menstrual cycle. The y-axis shows serum concentrations in pmol/L on a logarithmic scale. The horizontal line within the box is the median value of the distribution. The top and bottom of the box are the 75th and 25th percentiles of the distribution, respectively. The top and bottom vertical whiskers outside the box represent the data within 1.5 times the interquartile range of the top and bottom quartiles, respectively. Outliers, defined as >1.5 the interquartile range, are represented by dots.
Laboratory and population variability
In Table 2 we present data on the reproducibility of the laboratory assay and its ability to distinguish among individual EM measures for premenopausal women, postmenopausal women and men. Total laboratory CVs, including within- and between-batch variation, were calculated across individuals in each sex/menopausal group and for individual participants. Overall laboratory CVs for each EM were <2% in each sex/menopausal group with a single exception; among men, the overall CV for 4-hydroxyestrone was 5.6%. In each sex/menopausal group, ICCs were uniformly high, ≥99.5% for all EM (data not shown), demonstrating that interindividual variation in serum EM concentrations is much greater than laboratory variability.
Table 2.
Reproducibility of serum estrogens and estrogen metabolites, measured in total (conjugated + unconjugated) and unconjugated forms, in 10 premenopausal women, 5 postmenopausal women, and 5 men.
| Premenopausal women | Postmenopausal women | Men | ||||
|---|---|---|---|---|---|---|
| Overall CVs | Range of individual CVs |
Overall CVs | Range of individual CVs |
Overall CVs | Range of individual CVs | |
| Estrone | 0.30% | 0.06%–0.54% | 0.04% | 0.01%–0.07% | 0.03% | 0.02%–0.04% |
| Unconjugated estrone | 0.22% | 0.07%–0.49% | 0.09% | 0.02%–0.15% | 0.10% | 0.07%–0.15% |
| Estradiol | 0.88% | 0.08%–2.21% | 0.12% | 0.04%–0.22% | 0.12% | 0.04%–0.24% |
| Unconjugated estradiol | 0.53% | 0.03%–1.18% | 0.27% | 0.01%–0.50% | 0.12% | 0.06%–0.19% |
| 2-Hydroxyestrone | 0.24% | 0.08%–0.30% | 0.05% | 0.02%–0.07% | 0.05% | 0.01%–0.07% |
| 2-Hydroxyestradiol | 0.43% | 0.09%–1.03% | 0.14% | 0.01%–0.22% | 0.16% | 0.03%–0.27% |
| 2-Methoxyestrone | 0.17% | 0.04%–0.43% | 0.17% | 0.03%–0.23% | 0.14% | 0.06%–0.19% |
| Unconjugated 2-methoxyestrone | 0.40% | 0.09%–0.93% | 0.26% | 0.03%–0.40% | 0.52% | 0.23%–0.97% |
| 2-Methoxyestradiol | 0.32% | 0.08%–0.56% | 0.28% | 0.02%–0.52% | 0.35% | 0.19%–0.54% |
| Unconjugated 2-methoxyestradiol | 0.85% | 0.15%–2.17% | 0.35% | 0.03%–0.44% | 1.38% | 0.31%–2.11% |
| 2-Hydroxyestrone-3-methyl ether | 0.50% | 0.09%–1.01% | 0.54% | 0.14%–0.75% | 0.49% | 0.19%–0.77% |
| 4-Hydroxyestrone | 0.25% | 0.04%–0.62% | 0.45% | 0.11%–0.93% | 5.64% | 0.04%–11.59% |
| 4-Methoxyestrone | 1.15% | 0.15%–2.57% | 0.57% | 0.33%–0.77% | 0.80% | 0.46%–1.46% |
| 4-Methoxyestradiol | 0.84% | 0.24%–1.72% | 1.34% | 0.33%–2.57% | 1.66% | 0.74%–3.06% |
| 16α-Hydroxyestrone | 0.28% | 0.06%–0.54% | 0.10% | 0.01%–0.16% | 0.08% | 0.04%–0.11% |
| Estriol | 0.26% | 0.02%–0.59% | 0.02% | 0.01%–0.02% | 0.02% | 0.01%–0.03% |
| Unconjugated estriol | 0.26% | 0.09%–0.42% | 0.12% | 0.06%–0.18% | 0.28% | 0.04%–0.60% |
| 16-Ketoestradiol | 0.31% | 0.02%–0.78% | 0.40% | 0.04%–0.87% | 0.10% | 0.05%–0.14% |
| 16-Epiestriol | 0.31% | 0.06%–0.63% | 0.47% | 0.23%–0.72% | 0.55% | 0.15%–0.75% |
| 17-Epiestriol | 0.41% | 0.11%–0.78% | 1.73% | 0.19%–3.35% | 1.35% | 0.31%–1.92% |
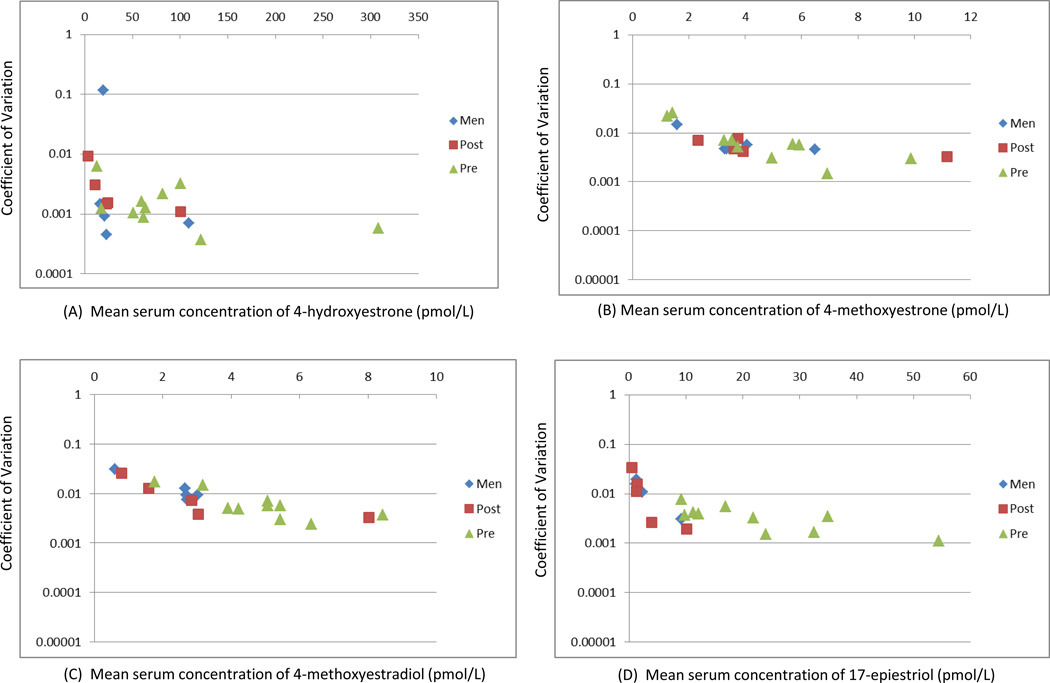
Individual CVs were <5% for most EM and most people. However, the individual CV for 4-hydroxyestrone was observed to exceed 10% in one man (11.6%). In Figure 3 we present plots of laboratory variation in replicate measures of selected EM for each individual by the mean concentration. The figure includes the four EM with the highest observed individual CV’s: 4-hydroxyestrone, 4-methoxyestrone, 4-methoxyestradiol, and 17-epiestriol. These data suggest that with the assay methodology used in this study, measures of serum EM show adequate reproducibility even in samples with the lowest concentrations.
Figure 3.
The coefficient of variation across eight replicate measurements of serum concentration of 4-hydroxyestrone (A), 4-methoxyestrone (B), 4-methoxyestradiol (C), and 17-epiestriol (D) are plotted for each individual participant on the y-axis. The mean serum concentration, in pmol/L, is plotted on the x-axis. Included are 10 premenopausal women, 5 postmenopausal women, and 5 men.
DISCUSSION
In this study we observed substantial interindividual variability in circulating levels of 15 EM in premenopausal women, postmenopausal women, and men. Further, the LC-MS/MS assay showed excellent reproducibility, with overall CV’s for all but one EM of <2%, when used to measure serum concentrations of EM in each of the three populations sampled. Within sex/menopausal groups, interindividual variability was much greater than laboratory variation, as demonstrated by ICCs >99% for all 15 EM, which suggests excellent discrimination across individuals in each population. While we observed a modestly elevated laboratory CV for 4-hydroxyestrone (6%), most of this variability could be attributed to one man's serum, which had for this EM, an individual CV of 12%, while individual CVs for other men and women in this study did not exceed 1%.
Results of a previous study of assay reproducibility and interindividual variation in urinary EM had broadly similar findings (10). CVs for urinary concentrations of the same 15 EM, measured using a similar LC-MS/MS technique but an older mass spectrometer model, were somewhat larger (most below 5%) than those observed here for serum EM (most below 2%). However, similar to the current study, interindividual variation in creatinine-adjusted urinary EM concentrations was observed to be substantially larger than laboratory variation, resulting in ICCs ≥95% in each sex/menstrual group for all but one EM (10). It is reassuring that CVs and ICCs for measuring the 15 EM in serum were at least as good as these measures for urinary EM since serum is a more challenging matrix due to substantially higher concentrations of protein and lipid.
Based on the descriptive information from this study and the previous urinary study (10), the mean concentration of all EM combined, in both serum and urine, was approximately four times higher in premenopausal women than postmenopausal women and similar in men compared to postmenopausal women. In serum, the most abundant metabolic group was the parent estrogens, while the next most abundant, the 16-hydroxylation pathway is substantially lower. In contrast, in urine, the 16-hydroxylation pathway was the most abundant metabolic group in all sex/menopausal populations (10). A better understanding of the relationships between serum and urinary EM measures will be obtained when EM profiles can be compared in serum and urine collected from the same individuals at close to the same time.
Previously, we reported that only five of the 15 EM---estrone, estradiol, 2-methoxyestrone, 2-methoxyestradiol, and estriol---were present in circulation in both conjugated and unconjugated forms among postmenopausal women (12). The other 10 EM were detectable in circulation only in conjugated form. In the current study, we find the same is true for premenopausal men and women. Of note, the serum concentration of unconjugated estradiol, the bioactive form of estrogen, is less than that of conjugated estradiol in postmenopausal women and men. Nearly all clinical and laboratory assays for estradiol measure only the unconjugated form; the importance of conjugated estradiol is rarely investigated.
Since both urinary and serum EM assays can perform well in premenopausal and postmenopausal women and in men, the choice of which assay to use may be based upon other considerations, which may include the logistics of sample collection or sample availability, the relevance of the measures to the target tissue or physiologic processes of interest, or perhaps the intraindividual reliability of these measures over time (16). While urinary EM are mostly found in conjugated form, serum EM can be found in both conjugated and unconjugated forms. Therefore the serum assay has been designed to measure each of these forms and allows, importantly, the measurement of unconjugated estradiol which is thought to be the most bioactive of the EM.
To date we have applied this assay in three prospective studies of serum EM profiles and postmenopausal breast cancer risk (12–14). Differences were noted across these studies in the degree of interindividual variability observed. While most EM varied by only 20% across their interdecile ranges in non-cases from the PLCO cohort (12), the same serum measures in non-cases in the B~FIT cohort varied 3 to 8-fold across their interdecile ranges (14), and 2 to 11-fold in controls drawn from the Columbia Missouri cohort (13). Interindividual variability in EM measures in the present study, while based on small numbers of participants, is most similar to the latter two studies. The reasons for variation in EM ranges across population-based studies are unclear, but may result from differences in the populations studied, or perhaps from differences in sample collection or handling protocols or assay procedures. Ongoing efforts to standardize estrogen measures across laboratories will make it easier to pool data, compare study findings, and translate research results into clinical guidance (17, 18).
Given the single blood draw for study participants, we were unable to address intraindividual variation in EM profiles, which is also an important consideration relevant to the validity of a measure at a single point in time as a marker of usual or recent levels of endogenous hormones. Sample sizes in this study were limited but this is the first presentation of serum EM profiles in premenopausal women, in both luteal and follicular phase, and in men. We had limited information about donor characteristics, including sex, menopausal status, and menstrual cycle phase, and were thus unable to evaluate other potential correlates of EM profiles.
Our findings, specifically excellent laboratory reproducibility and substantial interindividual variation in EM concentrations in premenopausal women, postmenopausal women and men, suggest that this serum assay is suitable for use in epidemiologic research. Investigators should be able to use this EM assay to investigate the importance of estrogen metabolism in epidemiologic studies of cancer, heart disease, bone health, and other conditions in both women and men.
Acknowledgements
This study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and National Cancer Institute federal funds awarded under Contract HHSN261200800001E to SAIC-Frederick, Inc. The authors thank Norma A. Diaz-Mayoral, Munira Gunja, Deesha Patel, and Noel Keith for research assistance. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services; nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Disclosure of Potential Conflicts of Interest:
X. Xu, T. Veenstra, L. Keefer, R. Ziegler; coinventors on relevant government patent.
Other authors report no potential conflicts of interest.
References
- 1.Eliassen AH, Hankinson SE. Endogenous hormone levels and risk of breast, endometrial and ovarian cancers: prospective studies. Advances in experimental medicine and biology. 2008;630:148–165. [PubMed] [Google Scholar]
- 2.Risbridger GP, Davis ID, Birrell SN, Tilley WD. Breast and prostate cancer: more similar than different. Nature reviews Cancer. 2010;10:205–212. doi: 10.1038/nrc2795. [DOI] [PubMed] [Google Scholar]
- 3.Depue RH, Pike MC, Henderson BE. Estrogen exposure during gestation and risk of testicular cancer. Journal of the National Cancer Institute. 1983;71:1151–1155. [PubMed] [Google Scholar]
- 4.Jefcoate CR, Liehr JG, Santen RJ, Sutter TR, Yager JD, Yue W, et al. Tissue-specific synthesis and oxidative metabolism of estrogens. Journal of the National Cancer Institute Monographs. 2000:95–112. doi: 10.1093/oxfordjournals.jncimonographs.a024248. [DOI] [PubMed] [Google Scholar]
- 5.Raftogianis R, Creveling C, Weinshilboum R, Weisz J. Estrogen metabolism by conjugation. Journal of the National Cancer Institute Monographs. 2000:113–124. doi: 10.1093/oxfordjournals.jncimonographs.a024234. [DOI] [PubMed] [Google Scholar]
- 6.Sasano H, Suzuki T, Miki Y, Moriya T. Intracrinology of estrogens and androgens in breast carcinoma. J Steroid Biochem Mol Biol. 2008;108:181–185. doi: 10.1016/j.jsbmb.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Zhu B, Han G, Shim J, Wen Y, Jiang X. Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology. 2006;147:4132–4150. doi: 10.1210/en.2006-0113. [DOI] [PubMed] [Google Scholar]
- 8.Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents--DNA adducts and mutations. Journal of the National Cancer Institute Monographs. 2000:75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- 9.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. The New England journal of medicine. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 10.Falk RT, Xu X, Keefer L, Veenstra TD, Ziegler RG. A liquid chromatography-mass spectrometry method for the simultaneous measurement of 15 urinary estrogens and estrogen metabolites: assay reproducibility and interindividual variability. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:3411–3418. doi: 10.1158/1055-9965.EPI-08-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Analytical chemistry. 2007;79:7813–7821. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 12.Fuhrman BJ, Schairer C, Gail MH, Boyd-Morin J, Xu X, Sue LY, et al. Estrogen metabolism and risk of breast cancer in postmenopausal women. Journal of the National Cancer Institute. 2012;104:326–339. doi: 10.1093/jnci/djr531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falk RT, Brinton LA, Dorgan JF, Fuhrman BJ, Veenstra TD, Xu X, et al. Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast cancer research : BCR. 2013;15:R34. doi: 10.1186/bcr3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dallal CM, Tice JA, Buist DS, Bauer DC, Lacey JV, Jr, Cauley JA, et al. Estrogen metabolism and breast cancer risk among postmenopausal women: a case-cohort study within B~FIT. Carcinogenesis. 2014 doi: 10.1093/carcin/bgt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gail MH, Fears TR, Hoover RN, Chandler DW, Donaldson JL, Hyer MB, et al. Reproducibility studies and interlaboratory concordance for assays of serum hormone levels: estrone, estradiol, estrone sulfate, and progesterone. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1996;5:835–844. [PubMed] [Google Scholar]
- 16.Eliassen AH, Ziegler RG, Rosner B, Veenstra TD, Roman JM, Xu X, et al. Reproducibility of fifteen urinary estrogens and estrogen metabolites over a 2- to 3-year period in premenopausal women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:2860–2868. doi: 10.1158/1055-9965.EPI-09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how, and when? Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:1713–1719. doi: 10.1158/1055-9965.EPI-06-0765. [DOI] [PubMed] [Google Scholar]
- 18.Rosner W, Hankinson SE, Sluss PM, Vesper HW, Wierman ME. Challenges to the measurement of estradiol: an endocrine society position statement. The Journal of clinical endocrinology and metabolism. 2013;98:1376–1387. doi: 10.1210/jc.2012-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]