Abstract
This study aims to assess in residual periodontal pockets the clinical, microbiological, and local biological effects of antimicrobial photodynamic therapy (PDT), delivered after ultrasonic instrumentation either once or twice in a 1-week interval. A single center, three-arm randomized longitudinal study was carried out for 6 months. Twenty-eight systemically healthy patients on periodontal maintenance with residual pockets (pocket depth (PD) ≥5 mm, clinical attachment loss ≥2 mm, and bleeding upon probing (BOP+)) were included. Residual pockets on three teeth, separated from each other by at least two other teeth, served as study sites. After ultrasonic debridement, they were randomly assigned to either PDT delivered twice within 1 week (group A), PDT delivered only once (group B), or sham treatment without activating the laser (group C). Methylene blue was applied with a blunt irrigator tip into the pockets. Sites were irradiated with laser light at a wavelength of 670 nm using a light-diffusing tip introduced into the pocket. Initial PD was 5.9 ± 0.9, 6.3 ± 1.3, and 6.3 ± 1.5 mm in groups A, B, and C, respectively, differences being nonsignificant. PD was significantly reduced in all groups. At month 3, PD was significantly lower in groups A (2.9 ± 1.1 mm; p = 0.04) and B (2.8 ± 1.1 mm; p = 0.03) compared to group C (3.5 ± 1.2 mm). At month 6, none of the sites in group A had persisting pockets PD >4 mm and BOP+, whereas two sites in group B and four sites in group C stayed in this category. Detection frequencies of the studied microorganisms at >1,000 and >100.000 cells/ml did not change significantly from baseline to months 3 or 6 in any group. A significant overall decrease was observed from baseline to month 6 for C-reactive protein, serum amyloid A, fibrinogen, procalcitonin, and α-2 macroglobulin. When looking at the groups separately, C-reactive protein was significantly lower only if the laser had been activated twice (p < 0.05). Other differences between groups were not significant. A single or double episodes of PDT had some additional benefit over ultrasonic instrumentation alone.
Keywords: Photodynamic, Laser, Scaling/root planing, Nonsurgical/mechanical, Therapy/treatment, Periodontitis
Introduction
Mechanical removal of bacterial biofilm and calculus from root surfaces is a well-established procedure in the treatment of periodontal disease. However, mechanical protocols alone are unable to eliminate all incriminated bacteria completely, and the persistence of a limited number of residual pockets with bleeding on probing (BOP+) after therapy is therefore a reality. Repeated instrumentation in residual pockets with metal instruments removes a substantial amount of tooth substance over time [1–4], may cause gingival recession [5, 6], and may induce hypersensitivity of teeth to thermal and physical stimuli [7]. Various local and systemic antimicrobial regimens have been proposed for better suppression of periodontopathic microorganisms. The repeated use of antibiotics in residual pockets during maintenance care is, however, not advisable.
Photodynamic therapy (PDT), because of its bactericidal and detoxification effect, may be a beneficial adjunct to mechanical periodontal therapy [8]. PDT is based on the principle that a photoactive substance, the photosensitizer, binds to the target cell and can be activated by a light of suitable wavelength [9]. During the process, free oxygen radicals are produced which react with the bacteria and their products and are toxic for them. Gram-positive bacteria are the most susceptible to PDT, but, by selecting the appropriate photosensitizer and wavelength, Gram-negative bacteria can be annihilated as well [10].
Efforts were made in three systematic reviews to evaluate the benefit of PDT for the treatment of periodontitis, either as a primary mode of treatment or as an adjunct to mechanical debridement [11–13]. Results were nondefinitive and in part contradictory with regards to the clinical and microbiological effects. Clearly, more primary research is necessary to assess the value of PDT and to determine the factors influencing the outcomes. While in most studies carried out so far the procedure was applied just once, in some, PDT was provided multiple times, e.g., five times in a 2-week period [14] or three times in a 1-week period [15]. It is unclear if repeated PDT yields better results than a single application.
One approach for evaluating these effects is by monitoring the biomarkers in the gingival crevicular fluid (GCF) [16, 17]. Among many inflammatory and immune mediators identified in the GCF, cytokines have attracted particular attention and are suspected to be involved in both inflammation-related alteration and repair of the periodontal tissues. Several acute-phase reactants may be affected by successful periodontal therapy as well, even though they are produced in tissues remote from the infection.
In the current study, we tested the benefit of PDT delivered as adjunct to ultrasonic debridement once or twice in a 1-week interval in patients previously treated for periodontitis with residual periodontal pockets.
Materials and methods
This was a single center, examiner-masked, randomized clinical trial of 6 months in duration with a three-arm, within-subject parallel design. The Ethical Committee of the University Hospitals of Geneva approved the protocol. Research was conducted according to the principles outlined in the Declaration of Helsinki on human medical experimentation. All participants were informed about the procedures and signed a consent form in advance of their inclusion in the study.
Patients
Between September 2009 and June 2011, 28 patients were recruited in a private dental practice in Geneva. They had previously been treated for active periodontal disease in the same practice. Eligible participants were all adults aged 18 or over, in maintenance after completion of comprehensive periodontal therapy since 3 to 12 months, and with at least one site in each of three dentition quadrants with a probing pocket depth (PD) >4 mm, clinical attachment loss (CAL) >1 mm, and BOP+. The root surfaces at these sites had to be smooth and free of detectable calculus, and the teeth had to be without retention factors for plaque (overhanging margins of restorations or ill-fitting crowns). Appropriate sites had to be located in the region between the incisors and the mesial aspect of the first molar and separated from each other by at least two teeth.
The exclusion criteria were systemic illnesses (i.e., diabetes mellitus, cancer, HIV, bone metabolic diseases or disorders that compromise wound healing), radiation or immunosuppressive therapy, pregnancy or lactating, systemic antibiotics taken within the previous 2 months, the use of nonsteroid anti-inflammatory drugs, and confirmed or suspected allergy/hypersensitivity to methylene blue. Subjects with physical limitations or restrictions that might preclude normal oral hygiene procedures were excluded as well.
The sample size was chosen taking into consideration reported mean differences in PD in the order of 0.5 to 1 mm between nonsurgical periodontal therapies with or without adjunctive antibiotics [18, 19]. Assuming that the common standard deviation is 1 mm, a sample of 30 per group would provide 80 % power to detect a true difference of 0.75 mm between groups.
Test products and randomization
PDT was carried out in residual pockets using the Periowave™ System (Ondine Biomedical Inc., Vancouver, Canada). The photosensitizing agent was methylene blue. Approximately 0.2 ml of the solution was applied over 60 s to each pocket with a blunt-ended side-port irrigator. The site was illuminated for 60 s to activate the agent using a disposable, light-diffusing tip that was introduced into the pocket and was connected via a fiber optic cable to a diode laser (λ 670 nm, 280 mW of output power, stable to within ±0.2 dB over 60 s, and within ±0.3 dB over successive cycles). The control treatment consisted of the same procedure, except that the light-diffusing tip was kept in the pocket for 60 s without activating the laser. All sites received two rounds of treatment in an interval of 1 week. The following protocols were randomly assigned using a computer-generated table: A, the laser was activated during both treatments; B, the laser was activated during the first treatment only; and C, the laser was never activated.
Two clinicians performed the procedures involving a contact with the patients. The examiner (GC) enrolled the participants, recorded all data, and provided preliminary treatments; the operator (VMC) delivered the test treatments. The treatment assignments were concealed to the patient and the examiner. The operator was unaware of previously recorded data except the pocket depth measurements and was not involved in the posttreatment evaluations.
Clinical protocol
In the first visit (pre-baseline), the examiner recorded the medical history, removed supragingival deposits, and, if necessary, gave oral hygiene instructions. Three quadrants were selected as study quadrants. In each study quadrant, the deepest pocket in the area between the first incisor and the mesial aspect of the first molar with PD >4 mm, clinical attachment loss (CAL) >1 mm, and BOP+ was designated as the study site. Study sites in contralateral quadrants had to be separated by at least two teeth.
The second visit (baseline) was scheduled within the next 7 to 10 days. The examiner first recorded the Plaque Index (PlI) [20]. Then, he carefully removed supragingival plaque, isolated the study sites from saliva with cotton rolls, and collected a 20-s sample of GCF from each study site with a strip of Durapore membrane (Millipore, Bedford, MA, USA) as previously described [16]. Next, the Gingival Index (GI) [21] was assessed. A subgingival plaque sample was obtained by inserting one paper point (Dentsply-Maillefer ISO 035) into each study site. Finally, he recorded PD, BOP, and recession (REC; positive if gingival margin located apical, negative if located coronal to the cemento–enamel junction). Once completed, the operator took over. She opened an envelope with the subject’s number to reveal the instructions for activating the laser in the three study quadrants and for the sequence of treatment. The residual pockets were debrided with an ultrasonic scaler (EMS, Nyon, Switzerland), and PDT was provided according to the instructions. Treatments were performed under local anesthesia to the discretion of the patient. After each quadrant, the operator noted the time and asked the patient to rate pain/discomfort on a visual analog scale (VAS) by placing a mark on a horizontal line, 100 mm long, labeled with “no pain” at one end and with “worst pain” at the other. The third session was scheduled after 1 week. The operator applied the photosensitizer once again and activated the laser according to protocols A–C.
The examiner reassessed the participants 3 and 6 months after the treatment. Medical history, any concomitant medication, and all adverse events were recorded. Clinical parameters were measured, and subgingival plaque and GCF samples were obtained the same way as at baseline. The samples were stored at −20 °C until processing.
Microbiological procedures
Genomic DNA was extracted using the GenElute Bacterial Genomic DNA Kit (Sigma-Aldrich Co., St. Louis MO, USA) in accordance with the manufacturer’s instructions. Quantitative real-time PCR was performed to detect and quantify six specific bacteria (Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Tannerella forsythia, Treponema denticola, Prevotella intermedia, Parvimonas micra) using species-specific primers [22, 23]. SYBR Green (Life Technologies, Carlsbad CA, USA) was used as nucleic acid stain. Real-time PCR was carried out using an ABI Prism® 7900HT Sequence Detection System (Applied Biosystems, Foster City CA, USA). Bacterial counts were calculated by comparison with homologous reference. The detection limit was 1,000 cells/ml.
GCF analysis
GCF levels of 20 different biomarkers were determined using a multiplex fluorescent bead-based immunoassay and the Bio-Plex 200 suspension array system (BioRad Laboratories, Hercules, CA, USA) as previously described [16]. Cytokines and acute-phase proteins were measured using the human cytokine 11-plex (Kit M5000HIVRK, BioRad Laboratories, Hercules, CA, USA), the acute-phase protein 5-plex (Kit 171A4009M, BioRad Laboratories, Hercules, CA, USA), and the acute-phase protein 4-plex (Kit 171A4007M, BioRad Laboratories, Hercules, CA, USA). The first panel included the following 11 cytokines: Interleukin (IL)-1β, IL-1 receptor antagonist (IL-1ra), IL-8, IL-17, basic fibroblast growth factor (b-FGF), granulocyte colony stimulating factor (G-CSF), granulocyte macrophage colony stimulating factor (GM-CSF), interferon γ (IFN-γ), macrophage inflammatory protein 1β (MIP-1β), tumor necrosis factor α (TNF-α), and vascular endothelial growth factor (VEGF). The second panel included nine human acute-phase biomarkers in two assays: α 2 macroglobulin (α2M), C-reactive protein (CRP), haptoglobin, serum amyloid P, ferritin, fibrinogen, procalcitonin, serum amyloid A, and tissue plasminogen activator. For the 11-plex panel, the lowest limit of detection of the assay varied between 1 and 2.24 pg/ml, except IL-1ra and TNF-α, where the limits were 5.63 and 6.63 pg/ml, respectively. For the acute-phase protein panel, the limit of detection was below 1.1 ng/ml, except for ferritin (3.0 pg/ml), procalcitonin (0.9 pg/ml), and tissue plasminogen activator (0.16 pg/ml). Readings below these limits were recorded as zero.
Statistical analysis
The primary endpoint was the presence or absence of PD >4 mm with BOP+ after 6 months (persisting pockets >4 mm of bleeding upon probing are commonly perceived as needing further treatment in clinical practice). Secondary outcomes included changes in PD, BOP+, CAL (=PD + REC), the presence or absence of target microorganisms above >1,000 (detection threshold), and >100.000 cells/ml after 3 and 6 months. The Kruskal–Wallis one-way analysis of variance, the Mann–Whitney U test, or Fisher’s exact test was used to determine differences between sites treated with different procedures. The longitudinal changes were analyzed using the Wilcoxon matched-pairs signed-ranks test. One statistical program package (IBM SPSS Statistics 19, IBM Corporation, Somers, NY, USA) was used for all statistical analyses. P values <0.05 were accepted for statistical significance.
Results
Twenty-eight persons gave informed consent, were enrolled in the study, and received treatment as allocated. One participant was excluded shortly after therapy due to moving abroad. All other 27 subjects completed the study. The 3-month data could not be recorded for one participant due to temporary unavailability.
The mean age of the 27 participants was 62.8 years (range 37 to 77). There were 14 males and 13 females. Fourteen subjects were nonsmokers, and one was a former smoker who quit more than 10 years ago. Four subjects smoked less than ten cigarettes per day, while eight participants smoked 20 or more cigarettes daily.
Table 1 shows the baseline characteristics of the 27 participants by treatment protocol. All 81 sites included in the study had a PD >4 mm; 51 (63 %) of them were bleeding upon probing in the visit the baseline data were recorded. Ten out of the 81 sites (12 %) showed a PlI of 2 and none a value of 3. Twelve sites scored GI = 2 (15 %) and none higher. The clinical status of the three groups was homogeneous and revealed no statistically significant differences. Similarly, the six studied microorganisms showed no significant difference with regards to the frequency of detection at >1,000 or >100.000 cells/ml between the three groups.
Table 1.
Clinical and microbiological baseline characteristics of the study sites per treatment protocol. A, two irradiations; B, one irradiation; C, no irradiation
| A | B | C | p valuea | |
|---|---|---|---|---|
| Total n b | 27 | 27 | 27 | – |
| PD (mm)c | 5.9 (0.9) | 6.3 (1.3) | 6.3 (1.5) | n.s. |
| CAL (mm)c | 7 (1.6) | 7.9 (2.2) | 7.6 (2) | n.s. |
| PD >4 and BOP+b | 16 | 20 | 15 | n.s. |
| BOP+b | 16 | 20 | 15 | n.s. |
| PlI >0b | 17 | 19 | 19 | n.s. |
| GI >0b | 22 | 22 | 20 | n.s. |
| AA >1,000; >100.000b | 2; 1 | 2; 1 | 2; 2 | n.s.; n.s. |
| TF >1,000; >100.000b | 26; 13 | 24; 15 | 26; 16 | n.s.; n.s. |
| PG >1,000; >100.000b | 27; 15 | 25; 17 | 27; 14 | n.s.; n.s. |
| TD >1,000; >100.000b | 24; 14 | 24; 14 | 26; 17 | n.s.; n.s. |
| PI >1,000; >100.000b | 22; 7 | 19; 12 | 19; 11 | n.s.; n.s. |
| PM >1,000; >100.000b | 27; 18 | 26; 15 | 27; 18 | n.s.; n.s. |
PD probing pocket depth; CAL clinical attachment level; BOP+ bleeding on probing; PlI Plaque Index; GI Gingival Index; AA A. actinomycetemcomitans; TF T. forsythia; PG P. gingivalis; TD T. denticola; PI P. intermedia; PM P. micra
aDifference between groups
bNumber of sites
cMean (standard deviation)
From baseline to month 3, therapy resulted in significant and clinically relevant changes of PD (p < 0.001), CAL (p < 0.001), and BOP+ (p < 0.001). Table 2 shows the clinical and microbiologic status in the study sites after 3 months by treatment protocol. The mean PD amounted to 2.9, 2.8, and 3.5 mm in the three groups, respectively, the difference being statistically significant between A and C (p = 0.04) and between B and C (p = 0.03). No site in group A was deeper than 4 mm and bled upon probing, whereas three sites in group B, and three sites in group C, were still deeper than 4 mm and bled upon probing. However, these differences did not reach a level of statistical significance. No further significant clinical or microbiological differences between protocols were observed.
Table 2.
Clinical and microbiological findings 3 months after treatment. A, two irradiations; B, one irradiation; C, no irradiation
| A | B | C | p valuea | |
|---|---|---|---|---|
| Total n b | 26 | 26 | 26 | – |
| PD (mm)c | 2.9 (1.1) | 2.8 (1.1) | 3.5 (1.2) | A–C, 0.04; B–C, 0.03 |
| CAL (mm)c | 3.7 (1.8) | 4 (1.9) | 4.7 (2) | n.s. |
| PD >4 and BOP+b | 0 | 3 | 3 | n.s. |
| BOP+b | 7 | 9 | 8 | n.s. |
| PlI >0b | 8 | 11 | 12 | n.s. |
| GI >0b | 11 | 11 | 14 | n.s. |
| AA >1,000; >100.000b | 3; 1 | 4; 4 | 2; 2 | n.s.; n.s. |
| TF >1,000; >100.000b | 26; 13 | 24; 10 | 24; 15 | n.s.; n.s. |
| PG >1,000; >100.000b | 26; 10 | 25; 8 | 25; 11 | n.s.; n.s. |
| TD >1,000; >100.000b | 24; 13 | 24; 10 | 24; 12 | n.s.; n.s. |
| PI >1,000; >100.000b | 23; 8 | 19; 10 | 20; 8 | n.s.; n.s. |
| PM >1,000; >100.000b | 26; 17 | 25; 12 | 25; 17 | n.s.; n.s. |
PD probing pocket depth; CAL clinical attachment level; BOP+ bleeding on probing; PlI Plaque Index; GI Gingival Index; AA A. actinomycetemcomitans; TF T. forsythia; PG P. gingivalis; TD T. denticola; PI P. intermedia; PM P. micra
aDifference between groups
bNumber of sites
cMean (standard deviation)
Table 3 shows the clinical and microbiologic status after 6 months. The changes in PD, CAL, and BOP+ obtained after 3 months were maintained; the differences of PD between groups were, however, no more significant. No site of group A was deeper than 4 mm and bled upon probing. Two sites belonging to group B and four sites belonging to group C were deeper than 4 mm and bled upon probing. Each of these sites was found in a different person (five individuals were male and one was female; four were smokers, two were nonsmokers). Detection frequencies and frequencies of sites with counts >100.000 cells/ml of the studied microorganisms did not change between baseline and 3 or 6 months in any of the three treatment groups.
Table 3.
Clinical and microbiological findings 6 months after treatment. A, two irradiations; B, one irradiation; C, no irradiation
| A | B | C | p valuea | |
|---|---|---|---|---|
| Total n b | 27 | 27 | 27 | – |
| PD (mm)c | 3.1 (1.0) | 2.9 (1.8) | 3.4 (1.5) | n.s. |
| CAL (mm)c | 4.1 (1.6) | 4.2 (2.8) | 4.6 (2.2) | n.s. |
| PD >4 and BOP+b | 0 | 2 | 4 | n.s. |
| BOP+b | 10 | 7 | 10 | n.s. |
| PlI >0b | 11 | 12 | 12 | n.s. |
| GI >0b | 14 | 10 | 13 | n.s. |
| AA >1,000; >100.000b | 4; 2 | 4; 1 | 5; 3 | n.s.; n.s. |
| TF >1,000; >100.000b | 24; 12 | 22; 15 | 25; 14 | n.s.; n.s. |
| PG >1,000; >100.000b | 24; 11 | 25; 10 | 25; 15 | n.s.; n.s. |
| TD >1,000; >100.000b | 23; 13 | 23; 10 | 24; 13 | n.s.; n.s. |
| PI >1,000; >100.000b | 23; 10 | 22; 3 | 18; 11 | n.s.; n.s. |
| PM >1,000; >100.000b | 25; 14 | 25; 20 | 25; 18 | n.s.; n.s. |
PD probing pocket depth; CAL clinical attachment level; BOP+ bleeding on probing; PlI Plaque Index; GI Gingival Index; AA A. actinomycetemcomitans; TF T. forsythia; PG P. gingivalis; TD T. denticola; PI P. intermedia; PM P. micra
aDifference between groups
bNumber of sites
cMean (standard deviation)
The average total treatment time necessary per tooth at visit 2 was 9 min, with a range between 6 and 15 min depending on the accessibility of the tooth and the morphology of the pocket. This included local anesthesia, when requested, ultrasonic debridement and PDT. At visit 3, when only PDT was applied, the average treatment time was 7 min, with a range between 3 and 12 min.
The perception of pain/discomfort, assessed on a VAS from 1 to 100 mm, was 12 mm on average at the second visit. Only two out of 27 patients rated pain/discomfort >40 mm. One incidence was attributed to discomfort associated with mechanical debridement and one to a feeling of illumination in the eye, when a pocket mesial of a first maxillary molar was irradiated. At visit 3, when patients had only the PDT, the mean VAS score was 6 mm, and only one subject requested anesthesia.
Table 4 shows the overall mean GCF levels of the 11 biomarkers assessed using the human cytokine 11-plex. No significant changes were observed between baseline and 3 or 6 months after treatment, and the differences between groups were not significant. Table 5 shows the overall mean GCF levels of the nine biomarkers assessed using the acute-phase protein panels. A significant decrease was observed between baseline and 6 months after treatment for CRP, serum amyloid A, fibrinogen, procalcitonin, and α2M. When looking at the groups separately, CRP was significantly lower at month 6 only after treatment according to protocol A, the laser being activated twice (p < 0.05), the other differences between groups being nonsignificant.
Table 4.
GCF levels of 11 biomarkers assessed using the human cytokine 11-plex, expressed as mean (standard deviation) per 20-s sample
| Baseline | Month 3 | Month 6 | p valuea | |
|---|---|---|---|---|
| IL-1β (pg) | 71 (109) | 70 (103) | 75 (101) | n.s. |
| IL-1ra (pg) | 6,795 (7214) | 7,528 (6817) | 7,859 (7423) | n.s. |
| IL-8 (pg) | 134 (179) | 135 (166) | 164 (204) | n.s. |
| IL-17 (pg) | 1.9 (3.1) | 2.0 (3.2) | 2.1 (3.0) | n.s. |
| b-FGF (pg) | 3.0 (4.3) | 3.2 (3.9) | 3 (3.5) | n.s. |
| G-CSF (pg) | 16.4 (43.5) | 13.1 (28.5) | 7.18 (15.8) | n.s. |
| GM-CSF (pg) | 10.5 (14.7) | 11.6 (15.1) | 11.6 (14.9) | n.s. |
| IFN-γ (pg) | 18.8 (14.7) | 20.5 (20.4) | 19.9 (13.9) | n.s. |
| MIP-1β (pg) | 7.0 (11.3) | 6.3 (8.5) | 6.6 (9.2) | n.s. |
| TNF-α (pg) | 2.9 (3.3) | 2.7 (2.7) | 3.2 (3.5) | n.s. |
| VEGF (pg) | 155 (170) | 177 (245) | 155 (133) | n.s. |
IL-1β interleukin 1β; IL-1ra interleukin 1 receptor antagonist; IL-8 interleukin 8; IL-17 interleukin 17; b-FGF basic fibroblast growth factor; G-CSF granulocyte colony stimulating factor; GM-CSF granulocyte macrophage colony stimulating factor; IFN-γ interferon γ; MIP-1β macrophage inflammatory protein-1β; TNF-α tumor necrosis factor α; VEGF vascular endothelial growth factor
aDifference between baseline and month 3 or 6
Table 5.
GCF levels of nine acute-phase proteins, expressed as mean (standard deviation) per 20-s sample
| Baseline | Month 3 | Month 6 | p valuea | |
|---|---|---|---|---|
| α-2 Macroglobulin (ng) | 255 (516) | 157 (341) | 104 (177) | <0.05 |
| C-reactive protein (ng) | 0.3 (0.5) | 0.2 (0.4) | 0.1 (0.2) | <0.05 |
| Haptoglobin (ng) | 141 (324) | 183 (372) | 140 (309) | n.s. |
| Serum amyloid P (ng) | 28 (51) | 22 (45) | 14 (36) | n.s. |
| Ferritin (pg) | 4,643 (6,513) | 3,646 (5187) | 3,829 (4,802) | n.s. |
| Fibrinogen (ng) | 86 (48) | 90 (76) | 73 (46) | <0.05 |
| Procalcitonin (pg) | 19 (15) | 18 (17) | 14 (12) | <0.05 |
| Serum amyloid A (ng) | 3.6 (1.8) | 3.5 (1.5) | 3.0 (1.2) | <0.05 |
| TP activator (pg) | 160 (150) | 158 (168) | 175 (214) | n.s. |
TP tissue plasminogen
aDifference between baseline and month 6
Discussion
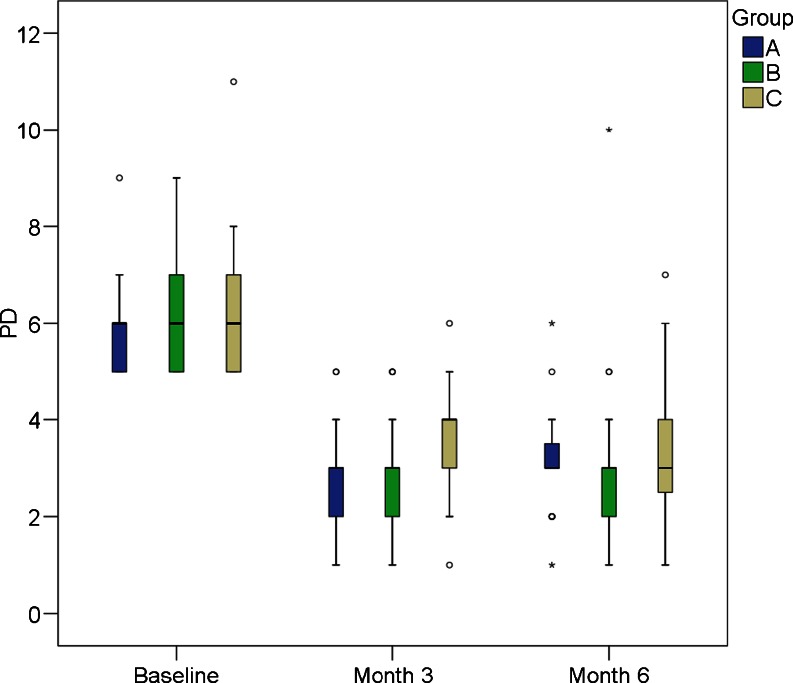
The aim of this study was to evaluate the benefit of PDT delivered as adjunct to ultrasonic debridement once or twice in a 1-week interval. The trial was carried out in patients with residual pockets, defined as sites with PD >4 mm and BOP+ after prior periodontal treatment. Because the root surfaces at these sites had been subjected to repeated thorough scaling and root planing previously, and were evaluated to be smooth and free of detectable calculus, scaling and root planing was not performed as part of the protocol of this study. The primary endpoint, i.e., the elimination of sites with PD >4 mm and BOP+, was achieved to 100 % by treatment according to protocol A: no such site was still present after two irradiations with the laser after 3 and 6 months. Small numbers of sites with PD >4 mm and BOP+, however, persisted in the other two groups. Significant reductions in PD (Fig. 1) and BOP+ could be shown after all the treatment procedures. Comparing the three modalities, the only significant difference related to PD after 3 months, with better results observed in sites that received active laser irradiation, either once (B) or twice (A), as compared to no irradiation (C). However, at month 6, these differences were no more significant. These results are in accordance with one systematic review [12] revealing statistically significant PD reductions and clinical attachment gain for adjunctive PDT after 3 months but not after 6 months.
Fig. 1.
Longitudinal development of PD (probing pocket depth in millimeter). n = 26 participants. A Two irradiations. B One irradiation. C No irradiation
Most of the previous trials included patients with untreated chronic [24–28] or aggressive [17, 29] periodontitis. The participants of our trial, however, presented residual pockets despite previous therapy. Conceptually, it is more challenging to obtain a further clinical improvement in such sites than in previously untreated pockets. In an earlier study, our group compared the effects of PDT in residual pockets to thorough scaling and root planing or diode soft laser therapy [30]. While all three treatments resulted in a significant clinical improvement, PDT and scaling and root planing resulted in fewer persisting pockets after 6 months than diode soft laser application.
The concept of repeated application and activation of the photosensitizer has been addressed in only few studies. In one trial [15], three rounds of PDT within 1 week had no significant effect on clinical and microbiological parameters. In another trial [14], however, five rounds of adjunctive PDT yielded better clinical outcomes than mechanical debridement only in residual pockets of maintenance patients; reductions of PD and BOP+, and gains in clinical attachment level were greater in the test as compared to the control group.
In the present study, the detection frequencies of the studied microorganisms, and frequencies of elevated counts, were not different before and after treatment, or between treatment groups. Microbiological changes have been analyzed in other studies as well and were found to be heterogeneous. Two studies [24, 31] showed no group differences, while two others [32, 33] detected significant differences for the investigated microorganisms. In our previous study [30], PDT and scaling and root planing suppressed P. gingivalis, T. forsythia, and T. denticola stronger than diode soft laser therapy, but the results for PDT and scaling and root planing did not differ significantly. In the present and previous trials of our group, antibiotic treatment was not allowed during 2 months prior to baseline. To what extent a longer period of confirmed nonexposure to antibiotics would have changed the outcome is unknown.
We found a significant decrease between baseline and month 6 for CRP, serum amyloid A, fibrinogen, procalcitonin, and α2M. Significant reductions in GCF levels of selected cytokines after nonsurgical periodontal treatment with PDT have been reported previously [16, 17, 34], corresponding to the clinical improvement seen in the periodontal status. However, similar levels were reported when treatments with or without PDT were compared. In the present study, when looking at the groups separately, CRP was significantly lower only at 6 months after treatment in sites treated according to protocol A, the other differences between groups being nonsignificant. CRP is not produced locally in the periodontal tissues; thus, its presence in GCF and/or periodontal tissues is indicative of systemic inflammation [35]. During periodontitis, bacteria and their products enter the systemic circulation, eliciting the production of a series of inflammatory biomarkers, such as CRP, which eventually arrives at the site of inflammation. Activating the laser twice may have further reduced the effect of periodontal inflammation on systemic CRP.
The evaluation of pain/discomfort yielded an average score of 12 at visit 2. One subject complained specifically about feeling the irradiation in the eye, when the mesial site of a first maxillary molar was treated with the laser. At visit 3, when patients had only the PDT, mean VAS scores were very low, and only one subject requested local anesthesia. However, repeated mechanical instrumentation may cause significant hard tissue damage cumulatively, whereas PDT only destroys the biofilm, thus potentially inducing less dentine hypersensitivity. In one study, it was even observed that laser irradiation might reduce dentine hypersensitivity [36].
The average treatment time per tooth of 7 to 9 min resulted in a cumulative session length that appears to be compatible with a common recall session for periodontal maintenance care, where typically one has to deal with isolated residual pockets only. Following the Periowave™ operating procedure as advised, a 45–60-min session allows treating five to seven teeth.
Within the limits of our study, we conclude that all three treatments resulted in significant clinical improvement when used in patients on periodontal maintenance. More studies with sufficient statistical power should be conducted in order to assess the possibilities of using PDT in various forms of periodontal disease.
Acknowledgments
The authors gratefully acknowledge the support of Ondine Biomedical Inc., Vancouver, Canada, which provided the material for the photodynamic therapy free of charge.
Conflict of interest
The authors declare that there are no conflicts of interest in this study.
References
- 1.Kocher T, Fanghanel J, Sawaf H, Litz R. Substance loss caused by scaling with different sonic scaler inserts—an in vitro study. J Clin Periodontol. 2001;28:9–15. doi: 10.1034/j.1600-051x.2001.280102.x. [DOI] [PubMed] [Google Scholar]
- 2.Flemmig TF, Petersilka GJ, Mehl A, Hickel R, Klaiber B. The effect of working parameters on root substance removal using a piezoelectric ultrasonic scaler in vitro. J Clin Periodontol. 1998;25:158–163. doi: 10.1111/j.1600-051X.1998.tb02422.x. [DOI] [PubMed] [Google Scholar]
- 3.Ritz L, Hefti AF, Rateitschak KH. An in vitro investigation on the loss of root substance in scaling with various instruments. J Clin Periodontol. 1991;18:643–647. doi: 10.1111/j.1600-051X.1991.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 4.Zappa U, Smith B, Simona C, Graf H, Case D, Kim W. Root substance removal by scaling and root planing. J Periodontol. 1991;62:750–754. doi: 10.1902/jop.1991.62.12.750. [DOI] [PubMed] [Google Scholar]
- 5.Badersten A, Nilvéus R, Egelberg J. Effect of nonsurgical periodontal therapy. I. Moderately advanced periodontitis. J Clin Periodontol. 1981;8:57–72. doi: 10.1111/j.1600-051X.1981.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 6.Badersten A, Nilvéus R, Egelberg J. Effect of nonsurgical periodontal therapy. II. Severely advanced periodontitis. J Clin Periodontol. 1984;11:63–76. doi: 10.1111/j.1600-051X.1984.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 7.von Troil B, Needleman I, Sanz M. A systematic review of the prevalence of root sensitivity following periodontal therapy. J Clin Periodontol. 2002;29(Suppl 3):173–177. doi: 10.1034/j.1600-051X.29.s3.10.x. [DOI] [PubMed] [Google Scholar]
- 8.Wilson M, Dobson J, Sarkar S. Sensitization of periodontopathogenic bacteria to killing by light from a low-power laser. Oral Microbiol Immunol. 1993;8:182–187. doi: 10.1111/j.1399-302X.1993.tb00663.x. [DOI] [PubMed] [Google Scholar]
- 9.Wilson M. Bactericidal effect of laser light and its potential use in the treatment of plaque-related diseases. Int Dent J. 1994;44:181–189. [PubMed] [Google Scholar]
- 10.Kömerik N, Nakanishi H, MacRobert AJ, Henderson B, Speight P, Wilson M. In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model. Antimicrob Agents Chemother. 2003;47:932–940. doi: 10.1128/AAC.47.3.932-940.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azarpazhooh A, Shah PS, Tenenbaum HC, Goldberg MB. The effect of photodynamic therapy for periodontitis: a systematic review and meta-analysis. J Periodontol. 2010;81:4–14. doi: 10.1902/jop.2009.090285. [DOI] [PubMed] [Google Scholar]
- 12.Sgolastra F, Petrucci A, Gatto R, Marzo G, Monaco A (2013) Photodynamic therapy in the treatment of chronic periodontitis: a systematic review and meta-analysis. Lasers Med Sci 28:669–682 [DOI] [PubMed]
- 13.Atieh MA. Photodynamic therapy as an adjunctive treatment for chronic periodontitis: a meta-analysis. Lasers Med Sci. 2010;25:605–613. doi: 10.1007/s10103-009-0744-6. [DOI] [PubMed] [Google Scholar]
- 14.Lulic M, Leiggener Gorog I, Salvi GE, Ramseier CA, Mattheos N, Lang NP. One-year outcomes of repeated adjunctive photodynamic therapy during periodontal maintenance: a proof-of-principle randomized-controlled clinical trial. J Clin Periodontol. 2009;36:661–666. doi: 10.1111/j.1600-051X.2009.01432.x. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz S, Kuru B, Kuru L, Noyan U, Argun D, Kadir T. Effect of gallium arsenide diode laser on human periodontal disease: a microbiological and clinical study. Lasers Surg Med. 2002;30:60–66. doi: 10.1002/lsm.10010. [DOI] [PubMed] [Google Scholar]
- 16.Giannopoulou C, Cappuyns I, Cancela J, Cionca N, Mombelli A. Effect of photodynamic therapy, diode laser and deep scaling on cytokine and acute-phase protein levels in gingival crevicular fluid of residual periodontal pockets. J Periodontol. 2012;83:1018–1027. doi: 10.1902/jop.2011.110281. [DOI] [PubMed] [Google Scholar]
- 17.de Oliveira RR, Schwartz-Filho HO, Novaes AB, Garlet GP, de Souza RF, Taba M, Scombatti de Souza SL, Ribeiro FJ. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: cytokine profile in gingival crevicular fluid, preliminary results. J Periodontol. 2009;80:98–105. doi: 10.1902/jop.2009.070465. [DOI] [PubMed] [Google Scholar]
- 18.Haffajee AD, Socransky SS, Gunsolley JC. Systemic anti-infective periodontal therapy: a systematic review. Ann Periodontol. 2003;8:115–181. doi: 10.1902/annals.2003.8.1.115. [DOI] [PubMed] [Google Scholar]
- 19.Herrera D, Sanz M, Jepsen S, Needleman I, Roldán S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J Clin Periodontol. 2002;29:136–159. doi: 10.1034/j.1600-051X.29.s3.8.x. [DOI] [PubMed] [Google Scholar]
- 20.Silness J, Löe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 21.Löe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 22.Kozarov E, Sweier D, Shelburne C, Progulske-Fox A, Lopatin D. Detection of bacterial DNA in atheromatous plaques by quantitative PCR. Microbes Infect. 2006;8:687–693. doi: 10.1016/j.micinf.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Shelburne CE, Prabhu A, Gleason RM, Mullally BH, Coulter WA. Quantitation of Bacteroides forsythus in subgingival plaque comparison of immunoassay and quantitative polymerase chain reaction. J Microbiol Methods. 2000;39:97–107. doi: 10.1016/S0167-7012(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 24.Christodoulides N, Nikolidakis D, Chondros P, Becker J, Schwarz F, Rossler R, Sculean A. Photodynamic therapy as an adjunct to non-surgical periodontal treatment: a randomized, controlled clinical trial. J Periodontol. 2008;79:1638–1644. doi: 10.1902/jop.2008.070652. [DOI] [PubMed] [Google Scholar]
- 25.Andersen R, Loebel N, Hammond D, Wilson M. Treatment of periodontal disease by photodisinfection compared to scaling and root planing. J Clin Dent. 2007;18:34–38. [PubMed] [Google Scholar]
- 26.Braun A, Dehn C, Krause F, Jepsen S. Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: a randomized clinical trial. J Clin Periodontol. 2008;35:877–884. doi: 10.1111/j.1600-051X.2008.01303.x. [DOI] [PubMed] [Google Scholar]
- 27.Ge L, Shu R, Li Y, Li C, Luo L, Song Z, Xie Y, Liu D. Adjunctive effect of photodynamic therapy to scaling and root planing in the treatment of chronic periodontitis. Photomed Laser Surg. 2011;29:33–37. doi: 10.1089/pho.2009.2727. [DOI] [PubMed] [Google Scholar]
- 28.Meisel P, Kocher T. Photodynamic therapy for periodontal diseases: state of the art. J Photochem Photobiol B. 2005;79:159–170. doi: 10.1016/j.jphotobiol.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 29.de Oliveira RR, Schwartz-Filho HO, Novaes AB, Jr, Taba M., Jr Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: a preliminary randomized controlled clinical study. J Periodontol. 2007;78:965–973. doi: 10.1902/jop.2007.060494. [DOI] [PubMed] [Google Scholar]
- 30.Cappuyns I, Cionca N, Wick P, Giannopoulou C, Mombelli A. Treatment of residual pockets with photodynamic therapy, diode laser, or deep scaling: a randomized, split-mouth controlled clinical trial. Lasers Med Sci. 2012;27:979–986. doi: 10.1007/s10103-011-1027-6. [DOI] [PubMed] [Google Scholar]
- 31.Rühling A, Fanghänel J, Houshmand M, Kuhr A, Meisel P, Schwahn C, Kocher T. Photodynamic therapy of persistent pockets in maintenance patients—a clinical study. Clin Oral Investig. 2010;14:637–644. doi: 10.1007/s00784-009-0347-4. [DOI] [PubMed] [Google Scholar]
- 32.Polansky R, Haas M, Heschl A, Wimmer G. Clinical effectiveness of photodynamic therapy in the treatment of periodontitis. J Clin Periodontol. 2009;36:575–580. doi: 10.1111/j.1600-051X.2009.01412.x. [DOI] [PubMed] [Google Scholar]
- 33.Chondros P, Nikolidakis D, Christodoulides N, Rossler R, Gutknecht N, Sculean A. Photodynamic therapy as adjunct to non-surgical periodontal treatment in patients on periodontal maintenance: a randomized controlled clinical trial. Lasers Med Sci. 2009;24:681–688. doi: 10.1007/s10103-008-0565-z. [DOI] [PubMed] [Google Scholar]
- 34.Lui J, Corbet EF, Jin L. Combined photodynamic and low-level laser therapies as an adjunct to nonsurgical treatment of chronic periodontitis. J Periodontal Res. 2011;46:89–96. doi: 10.1111/j.1600-0765.2010.01316.x. [DOI] [PubMed] [Google Scholar]
- 35.Megson E, Fitzsimmons T, Dharmapatni K, Bartold PM. C-reactive protein in gingival crevicular fluid may be indicative of systemic inflammation. J Clin Periodontol. 2010;37:797–804. doi: 10.1111/j.1600-051X.2010.01603.x. [DOI] [PubMed] [Google Scholar]
- 36.Yilmaz HG, Cengiz E, Kurtulmus-Yilmaz S, Leblebicioglu B. Effectiveness of Er, Cr:YSGG laser on dentine hypersensitivity: a controlled clinical trial. J Clin Periodontol. 2011;38:341–346. doi: 10.1111/j.1600-051X.2010.01694.x. [DOI] [PubMed] [Google Scholar]