Abstract
PURPOSE
A magnetic resonance imaging-ultrasonography (MRI-US) fusion-guided prostate biopsy increases detection rates compared to an extended sextant biopsy. The imaging characteristics and pathology outcomes of subsequent biopsies in patients with initially negative MRI-US fusion biopsies are described in this study.
MATERIALS AND METHODS
We reviewed 855 biopsy sessions of 751 patients (June 2007 to March 2013). The fusion biopsy consisted of two cores per lesion identified on multiparametric MRI (mpMRI) and a 12-core extended sextant transrectal US (TRUS) biopsy. Inclusion criteria were at least two fusion biopsy sessions, with a negative first biopsy and mpMRI before each.
RESULTS
The detection rate on the initial fusion biopsy was 55.3%; 336 patients had negative findings. Forty-one patients had follow-up fusion biopsies, but only 34 of these were preceded by a repeat mpMRI. The median interval between biopsies was 15 months. Fourteen patients (41%) were positive for cancer on the repeat MRI-US fusion biopsy. Age, prostate-specific antigen (PSA), prostate volume, PSA density, digital rectal exam findings, lesion diameter, and changes on imaging were comparable between patients with negative and positive rebiopsies. Of the patients with positive rebiopsies, 79% had a positive TRUS biopsy before referral (P = 0.004). Ten patients had Gleason 3+3 disease, three had 3+4 disease, and one had 4+4 disease.
CONCLUSION
In patients with a negative MRI-US fusion prostate biopsy and indications for repeat biopsy, the detection rate of the follow-up sessions was lower than the initial detection rate. Of the prostate cancers subsequently found, 93% were low grade (≤3+4). In this low risk group of patients, increasing the follow-up time interval should be considered in the appropriate clinical setting.
Prostate cancer is the most common cancer in males, with an estimated 238 590 new diagnoses annually in the USA, and it is the second leading cause of cancer-related mortality in males (1). One in six males will develop prostate cancer in his lifetime (1). The current standard of care for diagnosing and grading prostate cancer is a 12-core extended sextant biopsy obtained with transrectal ultrasonography (TRUS) guidance (2, 3). As magnetic resonance imaging (MRI) has superior contrast resolution than ultrasonography (US), it is possible for multiparametric MRI (mpMRI) to detect prostate cancer with high reliability (4). Since clinically insignificant cancer is often invisible to magnetic resonance (MR), prostate MRI preferentially detects more aggressive cancers (5–9). MRI can be used to guide the prostate biopsy, either using a direct “in-gantry” approach or by using MRI-US fusion, which was developed as an office-based alternative (10). MRI-US targeted biopsies have about twice the per-core detection rate of sextant biopsies (11), and have been shown to be particularly useful for prostates measuring greater than 40 mL, which typically have lower rates of cancer detection than smaller prostate glands (12).
Since TRUS-guided biopsies have a relatively low sensitivity, many patients with a rising prostate-specific antigen (PSA), but an initial negative biopsy, undergo additional biopsies with progressively lower yields. In a study of sequential systematic biopsies in 1051 males, the detection rate of successive biopsies was 22%, 10%, 5%, and 4%, respectively (13). The third and fourth TRUS-guided biopsy sessions detected lower grade cancers and were found to have higher morbidity than the first two biopsies. Recently, MRI-US fusion biopsy has been reported to increase cancer detection rates in the setting of a prior negative TRUS biopsy (14, 15).
While MRI-US fusion biopsy is promising in the setting of previous negative random sampling, the response to a negative MRI-US fusion biopsy is less clear. Since a MRI-US fusion biopsy increases prostate cancer detection, this population should have a lower disease burden than patients with an initial negative TRUS-guided biopsy alone. Now that MRI-US fusion biopsies have been available for several years, such data are beginning to accumulate. Here, we investigate the detection rates of subsequent biopsies in patients with an initial negative MRI-US fusion prostate biopsy.
Materials and methods
Study design
This was a retrospective review of patients who underwent MRI-US fusion prostate biopsy at the National Cancer Institute from June 2007 to March 2013. All patients provided written informed consent as part of the Institutional Review Board-approved clinical protocol. Patients were referred for clinical suspicion of prostate cancer due to an elevated PSA or abnormal digital rectal exam, or with a prior prostate cancer diagnosis with a Gleason grade or volume not deemed consistent with their absolute serum PSA level or PSA kinetics. Patients underwent a diagnostic prostate mpMRI, and patients with no lesions seen on MRI were not eligible to participate in the study protocol and returned to their referring urologist for standard-of-care clinical management.
The fusion platforms used for the MRI-US fusion were research versions of a commercial platform (UroNav system, Invivo, Philips Healthcare, Cleveland, Ohio, USA). Standard-of-care 12-core TRUS-guided extended sextant biopsies and MRI-US fusion targeted biopsies were performed in all eligible patients. Two cores (lateral and medial) were obtained bilaterally from the base, middle, and apex of the prostate for the 12-core extended sextant, and two cores in the axial and sagittal planes on TRUS imaging were obtained from each MRI-identified lesion. All biopsy specimens were reviewed by a single genitourinary pathologist (M.J.M.) with over 25 years of experience.
MRI acquisition and interpretation
Images were acquired using a 3.0 Tesla MRI scanner (Achieva, Philips Healthcare), with a 6- or 16-channel body coil (SENSE, Philips Healthcare) and endorectal coil (BPX-30, Medrad, Pittsburgh, Pennsylvania, USA). The mpMRI consisted of triplanar T2-weighted, diffusion-weighted imaging (from which apparent diffusion coefficient maps were generated) (16), fast field echo dynamic contrast enhanced MRI, and multi-voxel three-dimensional localized MR spectroscopy. The mpMRI was evaluated prospectively by two genitourinary radiologists (B.T. and P.L.C., with six and 13 years of experience, respectively). The detailed criteria for determining if each mpMRI sequence was positive has been described previously, along with the grading system assigning a level of suspicion based on the number of positive imaging sequences (17). This grading system has been validated and found to correlate with the D’Amico risk stratification and cancer detection rates (8, 18). Prostate volumes were computed planimetrically by manually contouring the prostate on each slice of the axial T2-weighted sequence.
Data analysis
The data analysis was performed using JMP 9.0 (SAS Institute, Cary, North Carolina, USA). As the continuous variables typically had right-skewed distributions, medians and interquartile ranges were computed. Counts and percentages were provided for categorical variables. Statistical testing was performed using the two-tailed Wilcoxon rank-sum test for continuous variables and the two-tailed Fisher’s exact test for categorical variables. A significance level of P < 0.05 was used.
Results
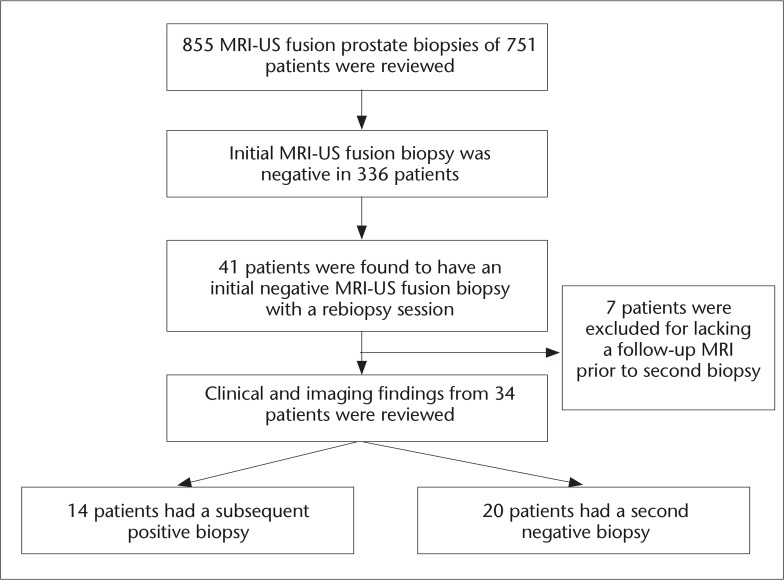
From June 2007 to March 2013, 855 MRI-US fusion prostate biopsies were performed in 751 patients (Fig. 1). On the initial fusion biopsy, 336 patients were negative, corresponding to a detection rate of 55.3% in our entire cohort. Forty-one of these patients had a subsequent follow-up fusion biopsy. Seven of these patients did not have a follow-up mpMRI before their second fusion biopsy and were excluded. The final group of 34 patients had a median age of 58 years and PSA of 6.07 ng/mL. In two patients, one additional biopsy core was directed at a hypoechoic lesion seen on US at the discretion of the interventional radiologist; these two cores were excluded from the analysis as they were not a standardized part of the biopsy protocol and would not have been performed otherwise. At the repeat fusion biopsy session, 14 patients (41%) had a biopsy-positive finding of prostate cancer: ten with Gleason 6 (3+3), three with Gleason 7 (3+4), and one with Gleason 8 (4+4) disease (Table 1).
Figure 1.
Patient flow-chart.
Table 1.
Characteristics of the patient population
| Characteristics | Descriptive statistics |
|---|---|
| Age (years) | 58 (54–64) |
| Initial PSA (ng/mL) | 6.07 (4.3–8.0) |
| Initial whole prostate volume (mL) | 54.5 (41–65.5) |
| Initial PSA density (ng/mL2) | 0.10 (0.072–0.145) |
| History of prostate biopsy outside our institution | |
| None | 7 (21%) |
| Prior negative | 11 (32%) |
| Prior positive | 16 (47%) |
| Interval between biopsies (months) | 15 (14–22.5) |
| Detection rate on second MRI-US fusion biopsy | 14/34 (41.2%) |
| Gleason scores | |
| 6 (3+3) | 10 (71%) |
| 7 (3+4) | 3 (21%) |
| 7 (4+3) | 0 (0%) |
| 8 (4+4) | 1 (7%) |
MRI-US, magnetic resonance imaging-ultrasonography; PSA, prostate-specific antigen.
Data are given as median (interquartile range) or n (%).
Comparing patients with positive and negative second fusion biopsies, there were no significant differences in age, PSA, prostate volume, time interval between biopsies, or digital rectal exam findings (Table 2). However, the history of prior TRUS biopsy differed substantially between the two groups: 79% of the patients with a positive repeat fusion biopsy had a prior diagnosis of prostate cancer predating the referral for a fusion biopsy, versus only 25% of the patients with a negative repeat fusion biopsy. A prior positive biopsy had an odds ratio of 11 in favor of having a positive cancer diagnosis on the subsequent fusion biopsy. A history of negative TRUS biopsies was associated with an odds ratio of 13 in favor of a negative repeat fusion biopsy compared to those without any prior biopsies or prior positive biopsy. In patients without a prior cancer diagnosis, the negative predictive value of an initial negative biopsy was 83% (15 of 18) when compared to the rebiopsy findings.
Table 2.
Clinical and imaging parameters in patients with a negative rebiopsy vs. patients with a positive rebiopsy
| Characteristics | Negative rebiopsy | Positive rebiopsy | P |
|---|---|---|---|
| Number of patients | 20 | 14 | - |
| Age (years) | 57 (54–63.5) | 61.5 (55.3–64.8) | 0.472 |
| Initial PSA (ng/mL) | 6.18 (4.85–8.08) | 5.09 (2.88–8.83) | 0.302 |
| Initial whole prostate volume (mL) | 57.5 (45–66.5) | 45.5 (33.5–61.8) | 0.132 |
| Initial PSA density (ng/mL2) | 0.116 (0.086–0.146) | 0.086 (0.063–0.214) | 0.270 |
| Follow-up PSA (ng/mL) | 7.04 (5.00–9.12) | 5.03 (2.85–9.55) | 0.241 |
| Follow-up whole prostate volume (mL) | 59.5 (50.8–84.0) | 48 (39.5–64.3) | 0.146 |
| Follow-up PSA density (ng/mL2) | 0.102 (0.079–0.155) | 0.106 (0.056–0.198) | 0.793 |
| Time interval between biopsies (months) | 14.5 (12.25–20.0) | 16.5 (15–25.3) | 0.105 |
| Initial DRE findings | |||
| Normal | 18 (90%) | 12 (86%) | 1.000 |
| Abnormal | 2 (10%) | 2 (14%) | |
| Follow-up DRE findings | |||
| Normal | 16 (80%) | 14 (100%) | 0.126 |
| Abnormal | 4 (20%) | 0 (0%) | |
| Number of lesions on initial MRI | |||
| 1 | 9 (45%) | 6 (43%) | 0.502 |
| 2 | 9 (45%) | 5 (36%) | |
| 3 | 1 (5%) | 3 (21%) | |
| 4 | 1 (5%) | 0 (0%) | |
| Index lesion diameter on initial MRI (mm) | 11 (8–14) | 1.1 (8–14) | 0.792 |
| Change in highest MRI suspicion level | |||
| Stable | 13 (65%) | 12 (86%) | 0.250 |
| Increase | 7 (35%) | 2 (14%) | |
| New lesions seen on follow-up MRI | |||
| No new lesion | 17 (85%) | 13 (93%) | 0.627 |
| New lesion | 3 (15%) | 1 (7%) | |
| History of prostate biopsy outside our institution | |||
| None | 5 (25%) | 2 (14%) | 0.004 |
| Prior negative | 10 (50%) | 1 (7%) | |
| Prior positive | 5 (25%) | 11 (79%) |
DRE, digital rectal examination; MRI, magnetic resonance imaging; PSA, prostate-specific antigen.
Data are given as median (interquartile range) or n (%).
One patient was previously treated with high-intensity focused US, and this patient underwent only a targeted biopsy, which was positive. Seven patients were positive on the 12-core TRUS biopsies only, three were positive on the targeted biopsies only, and four were positive on both. Of the 11 patients with cancer noted on a TRUS biopsy, seven had one positive core, three had two, and only one had three. Of the 16 positive sextant biopsy cores, only four corresponded to the anatomical regions where suspicious lesions were visualized on MRI.
Of the 11 patients who were positive on 12-core systematic biopsies, eight met Epstein’s criteria for very-low-risk prostate cancer (Gleason 6, no more than two positive cores, no core >50% involved) based on their sextant biopsy results.
Discussion
MRI-US fusion technology is emerging as a superior method of sampling the prostate because it allows image guidance for what was previously a blind, random procedure. In addition, the locations of prior biopsies can be recorded to allow repeat sampling during future biopsy sessions (19). When performed in conjunction with a systematic biopsy, a targeted fusion biopsy improves prostate cancer detection rates (11, 20), especially in patients with prior negative TRUS-guided biopsies (Fig. 2) (14, 15).
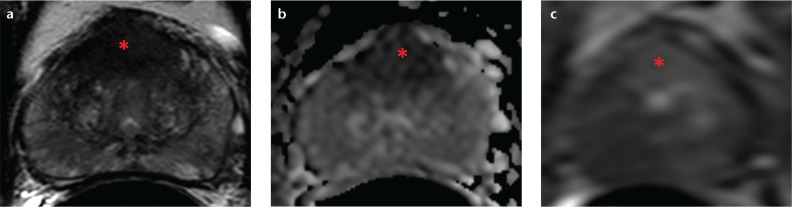
Figure 2. a–c.
A 53-year-old male with a serum PSA of 17 ng/mL and two prior negative biopsies. Axial T2-weighted MRI (a), the apparent diffusion coefficient map from diffusion-weighted MRI (b), and dynamic contrast-enhanced MRI (c) depicting a midline apical-mid anterior transitional zone lesion (asterisks). The MRI-US fusion-guided biopsy of the prostate revealed Gleason 4+4 tumor in the lesion (90% core involvement).
We found that the positive detection rate of repeat fusion biopsies was lower (41% vs. 55%) than for the initial fusion biopsy. Of the lesions detected in the repeat biopsy, most patients had low-grade Gleason 6 (3+3) disease or intermediate-grade Gleason 7 (3+4) disease. Only one of 14 patients with fusion-positive biopsies had a Gleason 8 tumor on repeat fusion biopsy. Interestingly, most of the low-grade cancers were detected on the 12-core TRUS biopsy and only three of the 14 patients had cancer detected exclusively by targeted biopsies. Therefore, a negative fusion biopsy followed by continued elevation of PSA tends to be negative on follow-up, and even when positive this is usually detected on the extended routine cores and not the targeted part of the biopsy. This is likely because the lesions are small and below the detection limit of MRI and are therefore detected only on systematic, unguided biopsies.
Gleason 8 disease was detected in one patient in an anterior central gland lesion on repeat biopsy. He was one of the first 10 patients subjected to the protocol, and in the initial biopsy session this location was considered to represent benign prostate hyperplasia on imaging and was not selected as a biopsy target. A retrospective comparison of the images showed an interval increase in the size of this lesion, with extracapsular extension. This highlights the learning curve of interpreting mpMRI findings in the anterior transitional zone.
A prior diagnosis of prostate cancer is much more common in patients who had a subsequent positive repeat fusion biopsy session after an initial negative MRI-US fusion biopsy. These patients were most commonly referred to our institution due to low-grade, low-volume cancer detected on a systematic biopsy by their referring urologists, who believed that the pathology was not concordant with the patient’s elevated PSA levels or PSA dynamics. In addition, some patients being managed with active surveillance were referred for further characterization and confirmation of minimal disease burden.
Although repeat fusion biopsy sessions had a relatively high detection rate of 41.2% in this patient series, note that 47% of the patients had a prior prostate cancer diagnosis, and their initial fusion biopsy was intended to better characterize the extent of their disease. This detection rate is still lower than our initial detection rate and that of a fusion-biopsy-naïve cohort (11, 15). The optimal biopsy strategy would maximize only the detection rate of clinically significant cancers. However, many cancers detected in this scenario are low-grade, low-volume disease that would unequivocally qualify for active surveillance under the Epstein criteria based on the 12-core biopsy (21). The discordance between the positive sextant locations and the imaging locations of lesions indicates that such foci are not visualized on MRI, but are detected only coincidentally on systematic biopsy. In patients with a prior diagnosis of cancer on surveillance, a repeat biopsy after a negative fusion biopsy might help to characterize the extent of the disease. However, further repeat biopsy sessions are of particularly low yield in patients with a history of prior negative TRUS biopsies.
A prostate biopsy is generally considered to be safe, but it is still invasive and involves significant morbidity, such as hematuria, pain, and infection (22). It is associated with short-term exacerbation of urinary symptoms and erectile dysfunction, as well as anxiety over the procedure (23). mpMRI is a relatively costly imaging modality compared to traditional US, and requires expertise to interpret. Cancer might be detected on a subsequent biopsy, but the risk of missing cancer must be weighed against possible burdens of over-diagnosis and overtreatment of clinically insignificant disease. A negative biopsy session integrating MRI-US fusion biopsies in conjunction with systematic 12-core extended sextant biopsy appears to select for a subpopulation that might benefit from more conservative management options.
The main limitation of this study was the small sample size. This is because patients are usually either treated (after a positive prior biopsy) or have not yet developed indications for a repeat biopsy. Therefore, only a minority return for repeat MRI and fusion biopsy. Other limitations include its retrospective design, potential selection bias amongst patients with multiple prior standard-of-care biopsies, and a patient population from a single tertiary referral center where patients enroll in specific research protocols.
Therefore, in patients with an initial negative fusion biopsy, a repeat fusion biopsy with an extended sextant biopsy is most likely to yield low-grade disease. Patients with a prior positive systematic biopsy (negative fusion biopsy) are most likely to have positive extended sextant biopsies and these are almost always low grade. A repeat fusion biopsy is most useful when there is a suspicious lesion on the second scan that was smaller or missed on the first. Such tumors can represent clinically significant disease that escaped initial detection.
In conclusion, cancer management remains a personalized decision between the patient and his urologist. The findings of this exploratory study should be validated in larger longitudinal studies, which will be feasible when MRI-US fusion biopsy becomes more commonplace in the clinical setting.
Footnotes
Conflict of interest disclosure
The study institution, National Institutes of Health, and Philips Healthcare have a cooperative research and development agreement and may have intellectual property in this field. Invivo Corp. is a subsidiary of Philips Healthcare.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Hodge KK, McNeal JE, Stamey TA. Ultrasound-guided transrectal core biopsies of the palpably abnormal prostate. J Urol. 1989;142:66–70. doi: 10.1016/s0022-5347(17)38663-9. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Türkbey B, Bernardo M, Merino MJ, Wood BJ, Pinto PA, Choyke PL. MRI of localized prostate cancer: coming of age in the PSA era. Diagn Interv Radiol. 2012;18:34–45. doi: 10.4261/1305-3825.DIR.4478-11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Mazaheri Y, Zhang J, Ishill NM, Kuroiwa K, Hricak H. Assessment of biologic aggressiveness of prostate cancer: correlation of mr signal intensity with gleason grade after radical prostatectomy. Radiology. 2008;246:168–176. doi: 10.1148/radiol.2461070057. [DOI] [PubMed] [Google Scholar]
- 6.Turkbey B, Shah VP, Pang Y, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011;258:488–495. doi: 10.1148/radiol.10100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurhanewicz J, Swanson MG, Nelson SJ, Vigneron DB. Combined magnetic resonance imaging and spectroscopic imaging approach to molecular imaging of prostate cancer. J Magn Reson Imaging. 2002;16:451–463. doi: 10.1002/jmri.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rastinehad AR, Baccala AA, Jr, Chung PH, et al. D’Amico risk stratification correlates with degree of suspicion of prostate cancer on multiparametric magnetic resonance imaging. J Urol. 2011;185:815–820. doi: 10.1016/j.juro.2010.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64:713–719. doi: 10.1016/j.eururo.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu S, Kruecker J, Turkbey B, et al. Real-time MRI-TRUS fusion for guidance of targeted prostate biopsies. Comput Aided Surg. 2008;13:255–264. doi: 10.1080/10929080802364645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281–1285. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walton-Diaz A, Hoang AN, Turkbey B, et al. Can MR-US fusion biopsy improve cancer detection in enlarged prostates? J Urol. 2013;190:2020–2025. doi: 10.1016/j.juro.2013.05.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Djavan B, Ravery V, Zlotta A, et al. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop? J Urol. 2001;166:1679–1683. [PubMed] [Google Scholar]
- 14.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65:809–815. doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vourganti S, Rastinehad A, Yerram NK, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol. 2012;188:2152–2157. doi: 10.1016/j.juro.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turkbey B, Aras O, Karabulut N, et al. Diffusion-weighted MRI for detecting and monitoring cancer: a review of current applications in body imaging. Diagn Interv Radiol. 2012;18:46–59. doi: 10.4261/1305-3825.DIR.4708-11.2. [DOI] [PubMed] [Google Scholar]
- 17.Stamatakis L, Siddiqui MM, Nix JW, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer. 2013;119:3359–3366. doi: 10.1002/cncr.28216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rais-Bahrami S, Siddiqui MM, Turkbey B, et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol. 2013;190:1721–1727. doi: 10.1016/j.juro.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turkbey B, Xu S, Kruecker J, et al. Documenting the location of prostate biopsies with image fusion. BJU Int. 2011;107:53–57. doi: 10.1111/j.1464-410X.2010.09483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonn GA, Natarajan S, Margolis DJA, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013;189:86–91. doi: 10.1016/j.juro.2012.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185–2190. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 22.Ilic D, Neuberger MM, Djulbegovic M, Dahm P. Screening for prostate cancer. Cochrane Database Syst Rev. 2013;1:CD004720. doi: 10.1002/14651858.CD004720.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glaser AP, Novakovic K, Helfand BT. The impact of prostate biopsy on urinary symptoms, erectile function, and anxiety. Curr Urol Rep. 2012;13:447–454. doi: 10.1007/s11934-012-0277-6. [DOI] [PubMed] [Google Scholar]