Abstract
Cefoxitin is a new, cephalosporin-like antibiotic which is highly resistant to hydrolysis by β-lactamase. Ninety-one cultures were selected either for their general resistance to cephalosporin antibiotics or for their ability to produce β-lactamase. Some of these cultures were resistant to cefoxitin. The capacity of each of the 91 strains to hydrolyze cefoxitin with β-lactamase was determined. Only seven of the cultures degraded the antibiotic as determined by a general assay for β-lactamase. Several others were able to hydrolyze cefoxitin after enzyme was induced by low concentrations of the antibiotic. The role of the constitutive and inducible enzyme in bacterial resistance to the antibiotic was investigated. Enzymatic destruction of cefoxitin was found to be an important factor contributing to bacterial resistance. However, the complete and rapid degradation of cefoxitin is not essential to resistance since one strain, Enterobacter cloacae 1316, hydrolyzed the antibiotic very slowly but was able to grow unaffected in the presence of cefoxitin. The presence of the enzyme is not necessarily sufficient to confer resistance since another culture, Klebsiella D535, readily hydrolyzed the antibiotic but was susceptible to it.
Full text
PDF
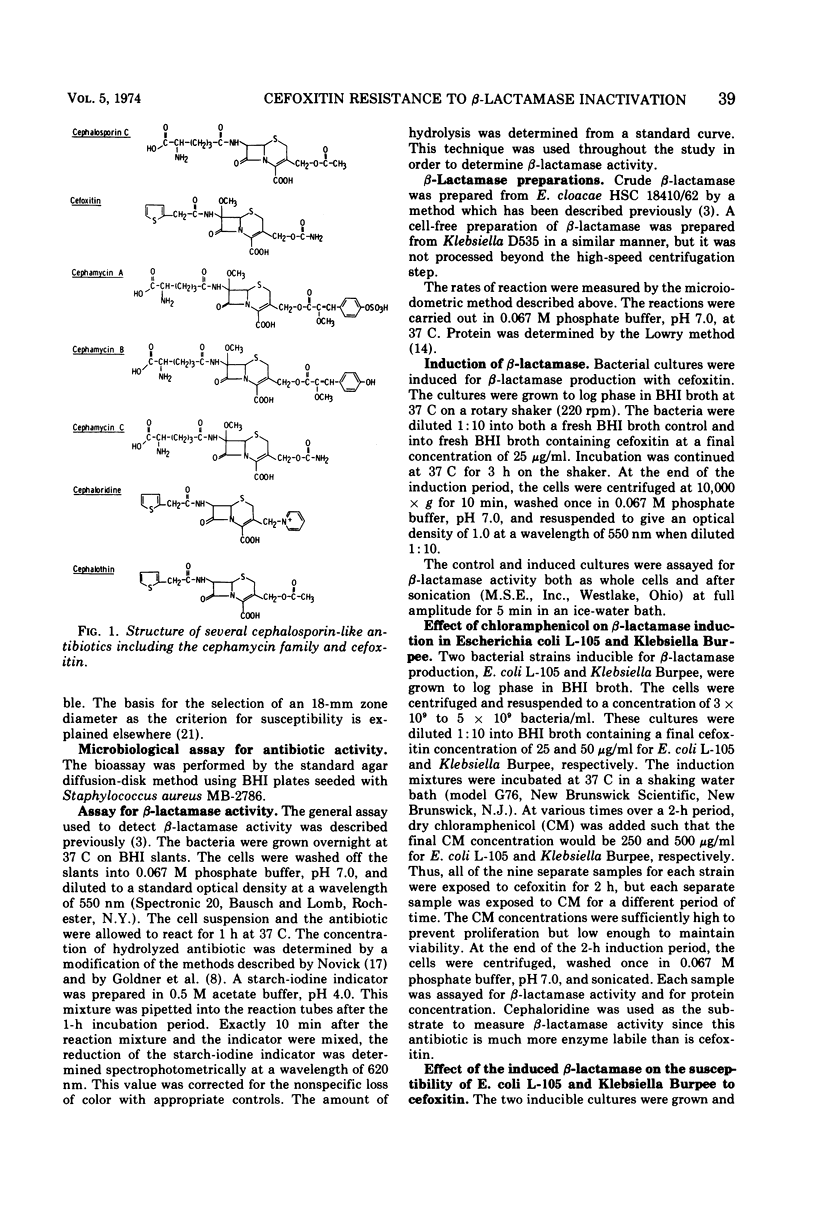

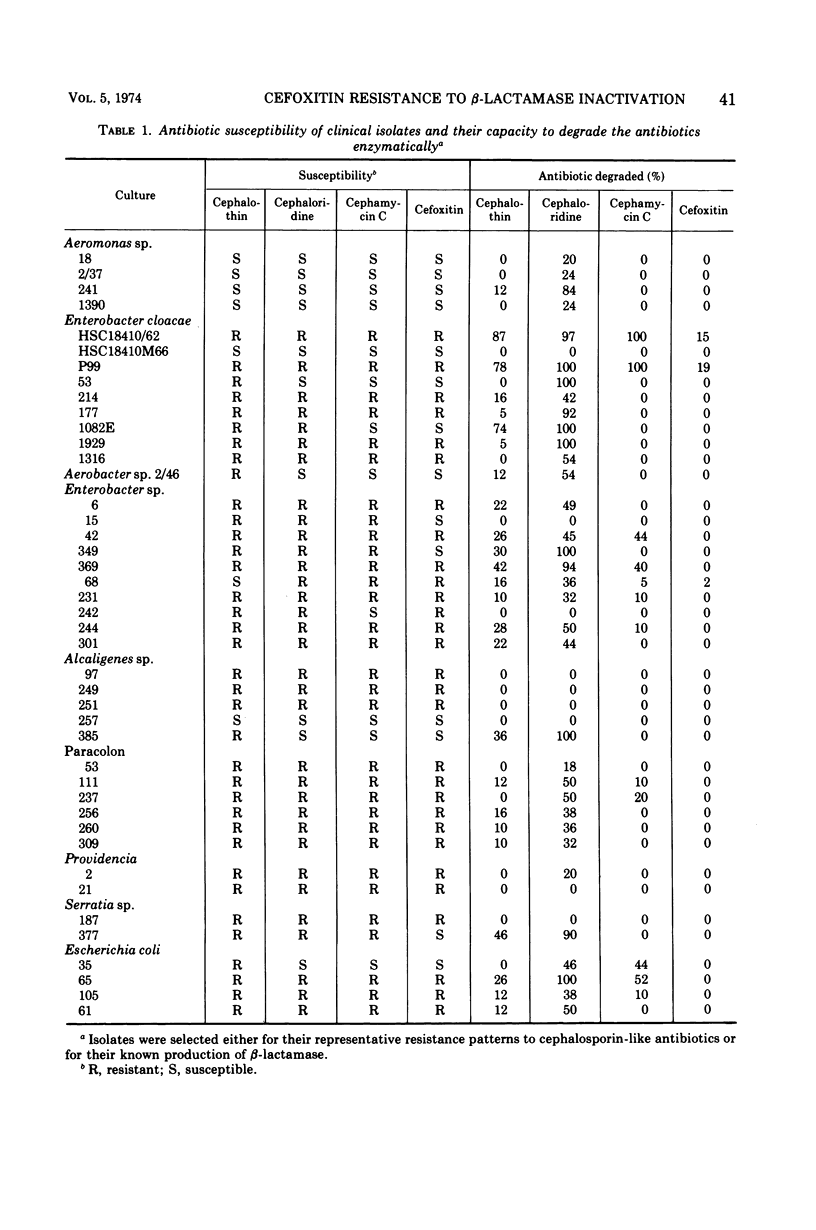
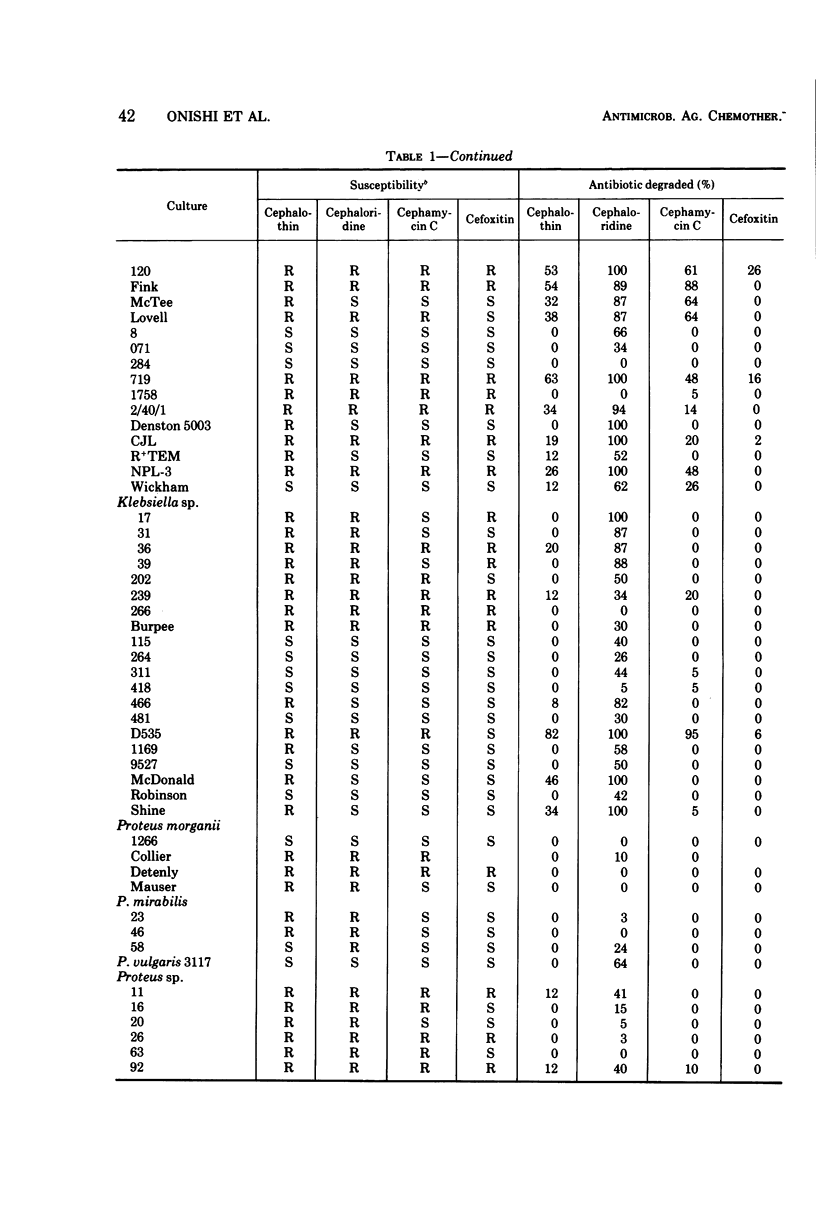
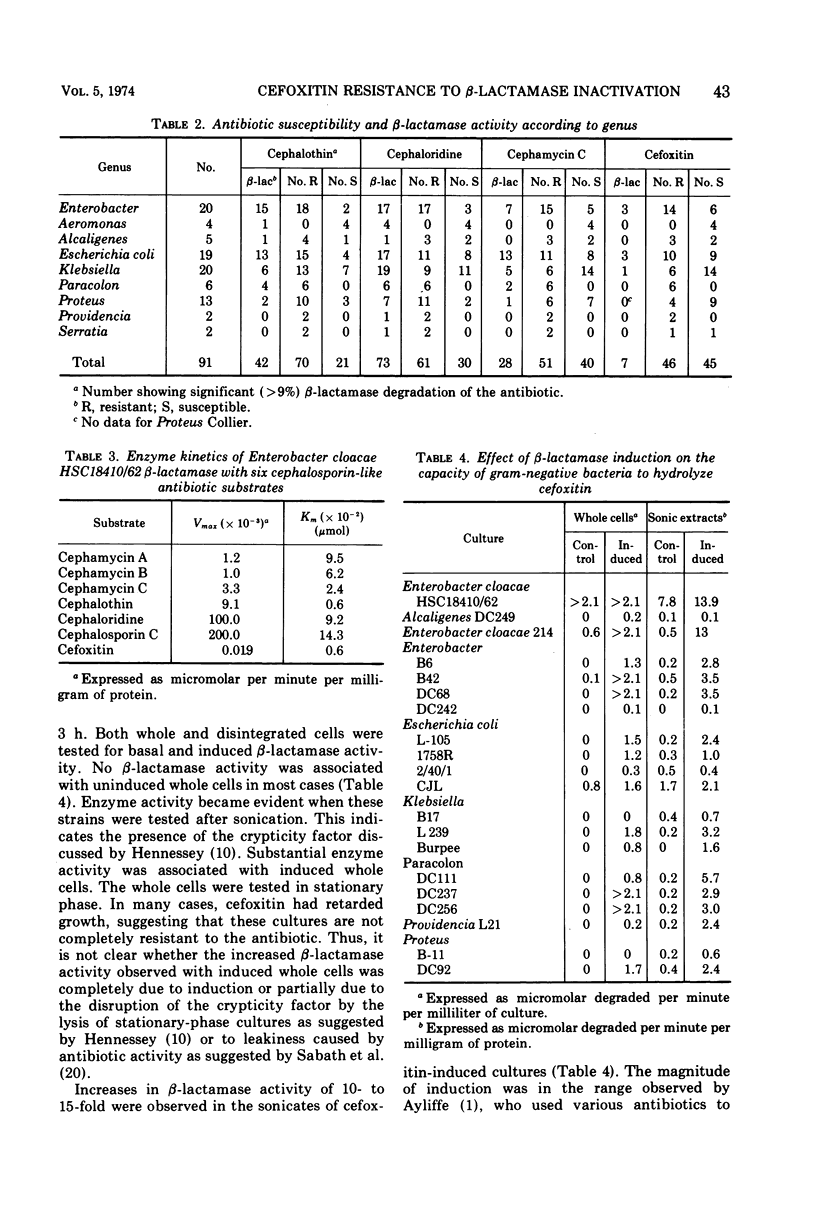



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayliffe G. A. Cephalosporinase and penicillinase activity of Gram-negative bacteria. J Gen Microbiol. 1965 Jul;40(1):119–126. doi: 10.1099/00221287-40-1-119. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Daoust D. R., Onishi H. R., Wallick H., Hendlin D., Stapley E. O. Cephamycins, a new family of beta-lactam antibiotics: antibacterial activity and resistance to beta-lactamase degradation. Antimicrob Agents Chemother. 1973 Feb;3(2):254–261. doi: 10.1128/aac.3.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEMING P. C., GOLDNER M., GLASS D. G. Observations on the nature, distribution, and significance of cephalosporinase. Lancet. 1963 Jun 29;1(7296):1399–1401. doi: 10.1016/s0140-6736(63)92051-8. [DOI] [PubMed] [Google Scholar]
- Farrar W. E., Krause J. M. Relationship Between beta-Lactamase Activity and Resistance of Enterobacter to Cephalothin. Infect Immun. 1970 Nov;2(5):610–616. doi: 10.1128/iai.2.5.610-616.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber N., Friedman J. Beta-lactamase and the resistance of Pseudomonas aeruginosa to various penicillins and cephalosporins. J Gen Microbiol. 1970 Dec;64(3):343–352. doi: 10.1099/00221287-64-3-343. [DOI] [PubMed] [Google Scholar]
- Goldner M., Glass D. G., Fleming P. C. Spontaneous mutant with loss of beta-lactamase in Aerobacter cloacae. J Bacteriol. 1969 Feb;97(2):961–961. doi: 10.1128/jb.97.2.961-.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessey T. D. Inducible beta-lactamase in Enterobacter. J Gen Microbiol. 1967 Nov;49(2):277–285. doi: 10.1099/00221287-49-2-277. [DOI] [PubMed] [Google Scholar]
- Jack G. W., Richmond M. H. A comparative study of eight distinct beta-lactamases synthesized by gram-negative bacteria. J Gen Microbiol. 1970 Apr;61(1):43–61. doi: 10.1099/00221287-61-1-43. [DOI] [PubMed] [Google Scholar]
- Karady S., Pines S. H., Weinstock L. M., Roberts F. E., Brenner G. S., Hoinowski A. M., Cheng T. Y., Sletzinger M. Semisynthetic cephalosporins via a novel acyl exchange reaction. J Am Chem Soc. 1972 Feb 23;94(4):1410–1411. doi: 10.1021/ja00759a090. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller A. K., Celozzi E., Kong Y., Pelak B. A., Hendlin D., Stapley E. O. Cefoxitin, a semisynthetic cephamycin antibiotic: in vivo evaluation. Antimicrob Agents Chemother. 1974 Jan;5(1):33–37. doi: 10.1128/aac.5.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. Effect of beta-lactamase location in Escherichia coli on penicillin synergy. Appl Microbiol. 1969 Jun;17(6):783–786. doi: 10.1128/am.17.6.783-786.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Jack G. W., Sykes R. B. Mechanisms of drug resistance. The beta-lactamases of gram-negative bacteria including pseudomonads. Ann N Y Acad Sci. 1971 Jun 11;182:243–257. doi: 10.1111/j.1749-6632.1971.tb30661.x. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Finland M. Resistance of penicillins and cephalosporins to beta-lactamases from Gram-negative bacilli: some correlations with antibacterial activity. Ann N Y Acad Sci. 1967 Sep 27;145(2):237–247. doi: 10.1111/j.1749-6632.1967.tb50222.x. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Jago M., Abraham E. P. Cephalosporinase and penicillinase activities of a beta-lactamase from Pseudomonas pyocyanea. Biochem J. 1965 Sep;96(3):739–752. doi: 10.1042/bj0960739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallick H., Hendlin D. Cefoxitin, a semisynthetic cephamycin antibiotic: susceptibility studies. Antimicrob Agents Chemother. 1974 Jan;5(1):25–32. doi: 10.1128/aac.5.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]



