Abstract
Background
BRCA1 promoter methylation has been detected in DNA from peripheral blood cells of both breast cancer patients and cancer-free females. However, the pathological significance of this epigenetic change in white blood cells (WBC) remains an open question. In this study, we hypothesized that if constitutional BRCA1 methylation reflects an elevated risk for developing breast cancer (BC), WBC that harbor methylated BRCA1 in both cancer-free females and BC patients should exhibit similar molecular changes.
Methods
BRCA1 promoter methylation was examined by methylation-specific PCR in WBC from 155 breast cancer patients and 143 cancer-free females. The Human Breast Cancer EpiTect Methyl II Signature PCR Array and The Human Breast Cancer RT2 Profiler™ PCR Array were used to study the methylation status and the expression profile of several breast cancer-related genes, respectively. In addition, we used label-free MS-based technique to study protein expression in plasma.
Results
We have shown that 14.2% of BC patients and 9.1% of cancer-free females (carriers) harbored methylated BRCA1 promoter in their WBC. Interestingly, 66.7% of patients harbored methylated BRCA1 promoter in both WBC and tumors. Importantly, we have shown the presence of epigenetic changes in 9 other BC-related genes in WBC of both patients and carriers. Additionally, BRCA1 and 15 other important cancer –related genes were found to be differentially expressed in WBC from patients and carriers as compared to controls. Furthermore, we have shown that the carriers exhibited a unique plasma protein pattern different from those of BC patients and controls, with 10 proteins similarly differentially expressed in patients and carriers as compared to controls.
Conclusions
The present results suggest the presence of a strong link between aberrant methylation of the BRCA1 promoter in WBC and breast cancer –related molecular changes, which indicate the potential predisposition of the carriers for developing breast cancer. This informs the potential use of the aberrant methylation of BRCA1 promoter in WBC as a powerful non-invasive molecular marker for detecting predisposed individuals at a very early age.
Keywords: Breast cancer, BRCA1, Methylation, White blood cells, Gene expression
Background
Epigenetic is the inheritance of information on the basis of gene expression rather than direct changes to sequence composition [1]. Errors in epigenetic regulation, which result in aberrant transcriptional silencing of a normally active gene or reactivation of a normally silent gene, are termed epimutations[2]. In human cancers, this heritable yet non-genetic modification is a powerful mechanism responsible for the inhibition of different types of genes, including tumor suppressor genes [3]. Epimutation that is found in all tissues of the body could be either germline, with evidence of inheritance, or constitutional, no evidence of inheritance. While it is still controversial whether germline epimutations occur in humans [4–6], constitutional epimutation is increasingly being considered as a mechanism for cancer predisposition.
Breast Cancer Associated gene1, BRCA1, was identified in 1994 as the first gene associated with familial breast cancer predisposition [7]. Since then, germline mutations of BRCA1 have been found to be responsible for the hereditary type of breast cancer, which accounts for about 5-10% of all breast cancers. Individuals carrying germline BRCA1 mutations are more likely to develop aggressive breast tumors at an early age (<50) [8]. These tumors are characterized by aneuploidy, high grade, poor histologic differentiation, and the majorities are of the triple-negative subtype, which is negative for estrogen receptor (ER), progesterone receptor (PR) and HER2 expression [8]. Gene silencing by epigenetic mechanisms is an alternative mechanism for BRCA1 inactivation during sporadic carcinogenesis [9]. Results from various studies revealed that 9-44% of sporadic breast cancer samples harbor hypermethylated BRCA1 promoter [9, 10]. The pathological features of these tumors are similar to those with inherited mutated BRCA1. Indeed, both occur at an early age and present poor histological differentiation, aneuploidy, ER and PR negativity, as well as similarities in their global gene expression profiles [11, 12].
Lately, BRCA1 promoter methylation has been detected in DNA from both white blood cells and tumor tissues in 3 out of 7 breast cancer patients from breast-ovarian cancer families [13]. This suggested that BRCA1 promoter methylation occurring in normal tissue of the body is associated with the development of BRCA1-like breast cancer [13]. Similarly, we have recently reported BRCA1 promoter hypermethylation in WBC genomic DNA of 2 out of 7 (28.5%) breast cancer patients, whose tumors showed BRCA1-like characteristics [14]. Furthermore, we have shown BRCA1 methylation in WBC of 8 out of 73 (10.9%) cancer-free women. The presence of methylated BRCA1 promoter in healthy females may reveal predisposition of these individuals to develop breast cancer. Indeed, the functional equivalence between the effect and significance of the epigenetic silencing of BRCA1 and the inheritance of BRCA1 mutations [15–17], has supported the notion that BRCA1 promoter methylation may serve as a first hit, much like an inherited germline mutation.
Although several studies have indicated the association between the presence of methylated BRCA1 promoter in WBC and the risk of developing breast cancer [18–20], the pathological significance of methylated BRCA1 promoter in WBC of cancer-free women remains still unclear. In the present study, we provide clear evidences that females with methylated BRCA1 in their WBC have, epigenetic changes, modulated gene expression profile and changes in protein expression in plasma similar to that seen in BRCA1-methylated breast cancer patients, advocating the possible involvement of BRCA1 constitutional epimutation as an alternative breast cancer predisposition mechanism.
Methods
Study samples
Breast cancer: 10 ml fresh blood samples were collected from 155 breast cancer female patients coming to the oncology department in King Faisal Specialist Hospital and Research Centre in Riyadh, Saudi Arabia. Paraffin embedded breast cancer tissues were obtained from the Department of Pathology. The age of the patients at diagnosis ranged from 23 to 73 years. Clinicopathological data (age, histological grade and ER and PR status) were provided by the Department of Pathology. Control samples: 10 ml fresh blood samples were collected from 143 healthy cancer-free female volunteers with ages ranged from 15 to 47 years. All patients and controls gave written informed consent to participate in the study. The study was approved by the Human Research Ethics Committee of King Faisal Specialist Hospital and Research Centre.
Isolation of DNA and RNA from WBC
Fresh blood was collected in 2 EDTA blood collection tubes. The tubes were centrifuged immediately at 3000 rpm for 10 min at 4°C. The WBC layers were carefully collected and transferred into two 2 ml Eppendorf tubes, one containing 900 mls RBC Lysis solution for subsequent DNA extraction using the Gentra Puregene Blood Kit, and the other tube contained 1.2 ml RNALater solution for subsequent RNA extraction using RiboPure Blood Kit (Ambion).
Isolation of DNA from paraffin embedded tissues
Genomic DNA was isolated from two to three 10 μM thick paraffin sections using Puregene kit (Gentra).
Methylation specific PCR
DNA methylation was assessed by methylation-specific PCR of sodium bisulfate treated DNA. 1 μg of genomic DNA was treated with sodium bisulfite and purified using EpiTect Bisulfite Kit (Qiagen) following the manufacturer’s recommendations. Modified DNA was amplified with published PCR primers for BRCA1 and MGMT[9, 21] that distinguish methylated and unmethylated DNA. PCR products were electrophoresed on 2% agarose gels and stained with Ethidium bromide. SssI methylase treated and untreated bisulfite modified DNA was used as positive and negative controls, respectively. All PCR reactions were done in replicates.
Methylation PCR array
The Human Breast Cancer EpiTect Methyl II Signature PCR Array (Qiagen) was used to study the methylation status of 24 different breast cancer-related genes. 1 μg of genomic DNA isolated from WBC was used in the array following the manufacturer protocol. Data analysis was done using integrated Excel-based templates provided by the manufacturer, which provide gene methylation status as percentage unmethylated (UM) and percentage methylated (M) fraction of input DNA. M" represents the fraction of input genomic DNA containing two or more methylated CpG sites in the targeted region of a gene. 2.5-fold or greater change relative to controls was determined to be the threshold cut-off point for what is considered a change in gene methylation.
RT2 Profiler™ PCR array
The Human Breast Cancer RT2 Profiler™ PCR Array was used to profile the expression of 84 different breast cancer-related genes. 1 μg of total RNA isolated from WBC was revers-transcribed into cDNA using the RT2 first strand kit (SABiosciences) following the manufacturer’s instructions, which was then used in the Array. The Array was set following the manufacturer’s instructions and was performed according to the manufacturer’s protocol. Data analysis was done using online software PCR Array Data Analysis Web Portal provided by the manufacturer. Gene expression levels were normalized against five housekeeping genes included in the Array. Fold changes in gene expression were calculated using the 2-∆∆Ct method by the software. 1.5-fold or greater change was determined to be the threshold cut-off point for what is considered a change in gene expression.
In solution-digestion and protein identification by mass spectrometry: LC-MSE analysis
Plasma samples from 4 breast cancer patients, 4 carriers and 4 controls were handled and prepared similarly. Equal amount of proteins was taken from each sample to generate a pool of patients as one group, a pool of carriers, and a third pool of controls. For each analysis sample group, 200 μg complex protein mixtures was subjected to in-solution digestion for mass spectrometry analysis as previously described [22, 23]. We have used the 1-Dimensional Nano Acquity liquid chromatography coupled with tandem mass spectrometry on Synapt G2 (Waters, Manchester, UK) to generate expression proteomics data based on quantitative protein changes between the three sample groups. The ESI- MS analysis and instrument settings were optimized on the tune page as previously described [22, 23]. All samples were analyzed in triplicate runs and data were acquired using the Mass Lynx programs (version. 4.1, SCN833, Waters, Manchester, UK) operated in resolution and positive polarity modes. Protein Lynx Global Server (PLGS) 2.5 (Waters, Manchester, UK) was used for all automated data processing and database searching. The generated peptide masses were searched against Uniprot Human protein sequence database using the PLGS 2.5 for protein identification (Waters, UK). TransOmics Informatics (Waters Corporation, UK) was used to process and search the data. The principle of the search algorithm is described [24]. The following criteria were used for the search 1 missed cleavage, Max protein mass 1000 kDa, Trypsin, Carbamidomethyl C fixed and Oxidation M variable modifications. Normalized label-free quantification was achieved using Progenesis QI software, (Nonlinear Dynamics (Newcastle, UK). The data was filtered to show only statistically significantly regulated proteins (ANOVA), (p ≤0.001) and a fold change >1.5. The data set was subjected to unsupervised PCA analysis.
Statistical analysis
The Chi-square and Fisher’s exact tests were performed to determine the statistical significance for the correlation between BRCA1 promoter methylation and age, and BRCA1 promoter methylation and cancer family history. T-test was performed to determine the statistical significance between the different groups for methylation (Carriers vs controls, Patients vs controls and Carriers vs patients). ANOVA test with multiple comparisons using SAS version 9.3(SAS Institute, Cary, NC, USA) was performed for confirmation. T-test was performed to determine the statistical significance between the carriers and the patients groups for gene methylation and expression levels. All observed differences were considered to be significant when associated with a P value <0.05.
Results
Identification of breast cancer patients and cancer-free females harboring methylated BRCA1promoter in their WBC
In order to identify breast cancer patients and cancer-free females harboring methylated BRCA1 promoter in their WBC, we screened 155 patients and 143 cancer-free females using the methylation-specific PCR (MSP) assay. We identified 22 (14.2%) breast cancer patients harboring methylated BRCA1 promoter (Figure 1A). Interestingly, 20 out of 22 were <50 years (90.9%), and only 2 were >50 years (9.1%) (Table 1). This indicates a strong association between the presence of methylated BRCA1 promoter in WBC and early onset of breast cancer (p = 0.032). In addition, we identified 13 cancer-free healthy females (9.1%) harboring methylated BRCA1 promoter in their WBC (Figure 1A). Importantly, 11 out the 13 were <40 years (84.6%), and only 2 were >40 years (15.4%) (Table 2). Additionally, we have found a significant association between the incidence of cancer in the family of those subjects and the presence of BRCA1 promoter methylation in their WBC (10/13) 77% (p = 0.036). 7 of those families 7/10 (70%) have breast/ and or ovarian cancer history. For simplification, we termed cancer-free females harboring methylated BRCA1 as “Carriers”.
Figure 1.
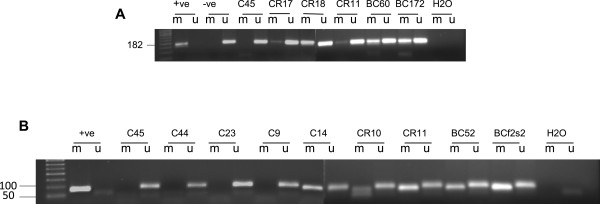
MSP analysis assay, (A) MSP analysis of the BRCA1 promoter region, (B) MSP analysis of the MGMT promoter region in WBC DNA. SssI methylase-treated and -untreated bisulfite-modified DNA was used as positive (+ve) and negative (-ve) controls, respectively. BC; breast cancer, CR; carriers and C; controls; M, methylated product; U; unmethylated product.
Table 1.
Clinical characterizations of BRCA1 methylated breast cancer positive cases
| Breast Cancer samples (BC) | Age | BRCA 1 status in tumor tissue | ER | PR | HER-2 | Breast cancer tumor type | Histological grade |
|---|---|---|---|---|---|---|---|
| 13 | 67 | + | +ve | +ve | -ve | ILC | G1 |
| 138 | 40 | + | +ve | +ve | -ve | IDC | G2 |
| 172 | 41 | + | +ve | -ve | -ve | IDC | G3 |
| 154 | 46 | + | +ve | +ve | -ve | IDC | G2 |
| 176 | 49 | + | -ve | -ve | -ve | IDC | G3 |
| 116 | 41 | + | -ve | -ve | -ve | metaplastic | |
| 181 | 42 | - | +ve | +ve | -ve | IDC | G2 |
| 171 | 37 | ND | -ve | -ve | -ve | IDC | G2 |
| 52 | 69 | - | +ve | +ve | -ve | ILC | G1 |
| 81 | 27 | - | +ve | -ve | +ve | IDC | G2 |
| 130 | 39 | + | -ve | -ve | -ve | IDC | G3 |
| 60 | 40 | + | -ve | -ve | -ve | IDC | G2 |
| 183 | 36 | + | -ve | -ve | -ve | IDC | G2 |
| 111 | 44 | ND | +ve | +ve | -ve | IDC | G2 |
| 161 | 48 | ND | +ve | +ve | -ve | IDC | G2 |
| 187 | 33 | ND | -ve | -ve | -ve | IDC | G3 |
| 54 | 40 | - | +ve | +ve | -ve | ILC | |
| 58 | 39 | + | +ve | +ve | -ve | IDC | G2 |
| 86 | 23 | Fibro adenoma benign | |||||
| S3 | 40 | - | +ve | +ve | -ve | ILC | |
| S2 | 43 | ND | ND | ND | ND | ||
| F2S2 | 39 | ND | ND | ND | ND |
IDC; Invasive ductal carcinoma.
ILC; Invasive lobular carcinoma.
ND; No data.
Table 2.
Cancer family history of the carriers
| Carriers Samples (CR) | Age | Affected family members | Type of cancer in affected relative | BRCA1 methylation in affected family member |
|---|---|---|---|---|
| CR7 | 25 | Cousin | Breast cancer | ND |
| CR9 | 48 | Grandmother, two aunts and cousin | Breast cancer | |
| CR18 | 41 | Aunt (mother side) | Ovarian cancer | ND |
| CR21 | 17 | Mother | Breast cancer | Yes |
| CR25 | 27 | Aunt | Breast cancer | ND |
| CRF2D3 | 15 (Twin) | Mother and two aunts (mother side) | Breast cancer | One aunt |
| CR39 | 22 | Great grandmother (mother side) | Breast cancer | ND |
| Mother | Ovarian cancer | ND | ||
| CR5 | 28 | ND | Cancer (other than breast and ovarian) | ND |
| CR10 | 27 | No cancer in the family | ||
| CR11 | 26 | ND | Cancer (other than breast and ovarian) | ND |
| CR17 | 26 | No cancer in the family | ||
| CR35 | 22 | ND | Cancer (other than breast and ovarian) | ND |
| CR59 | 27 | NO cancer in the family |
ND; No data.
Strong correlation between BRCA1methylation in WBC and matched breast tumors
Next, we sought to study the correlation between BRCA1 promoter methylation in WBC and their paired tumors. To this end, the methylation status of the BRCA1 promoter was studied, by the MSP assay, in genomic DNA isolated from matched tumor samples obtained from the above BRCA1 methylated positive breast cancer cases. Out of the 22 BRCA1 methylated positive cases, only 19 archived tumor tissues were available for BRCA1 methylation analysis. The BRCA1 promoter was hypermethylated in 10 samples, unmethylated in 5 samples and no results could be determined for 4 samples (Table 1). This indicates that 66.7% (10/15) of patients harbored methylated BRCA1 promoter in both WBC and tumors, which reveals strong correlation between the presences of methylated BRCA1 promoter in WBC and matched tumor tissues. Out of these 10 cases 5 had tumors of the triple negative type (50%).
WBC with methylated BRCA1show similar epigenetic changes in both breast cancer cases and carriers
Since BRCA1 has been found to be methylated in WBC genomic DNA, we hypothesized that other breast cancer related genes may also be epigenetically affected. Hence, we made use of the Human Breast Cancer EpiTect Methyl II Signature PCR Array, which profiles the promoter methylation status of 24 genes, whose hypermethylation is known to play a role in breast carcinogenesis. WBC from 17 cases, 13 carriers and 10 controls were used in these experiments. On the basis of a cut-off value of +2.5 fold relative to controls, 9 different breast cancer-related genes, other than BRCA1, were highly methylated in the breast cancer cases compared to controls (Figure 2), HIC1 (p = 0.093), CDH13 (p = 0.014), CDH1 (p = 0.011), CDKN2A (p = 0.167), MGMT (p = 0.067), SLIT2 (p = 0.013), CCNA1 (p = 0.075), TNFRSF10C (p = 0.029) and PYCARD (p = 0.269). Interestingly, the same genes, except SLIT2, were also highly methylated in the carriers compared to controls (Figure 2), HIC1 (p = 0.02), CDH13 (p = 0.01), CDH1 (p = 0.025), CDKNA2 (p = 0.002), MGMT (p = 0.002), CCNA1 (p = 0.037), TNFRSF10C (p = 0.005) and PYCARD (p = 0.02). This indicates that WBC harboring BRCA1 promoter methylation exhibit similar epigenetic changes in both breast cancer patients and cancer-free females. On the other hand, WBC from 5 breast cancer cases and 4 carriers did not show any changes in methylation levels for the 9 genes as compared to controls (Figure 2A).
Figure 2.
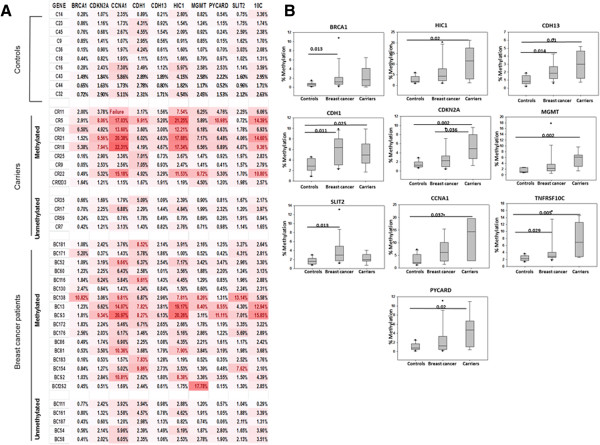
WBC with methylated BRCA1 show similar epigenetic changes in both breast cancer cases and carriers (A) a heat map comparing the methylation status of a panel of 10 cancer-related genes in WBC from controls C, breast cancer BC, and carriers CR as determined using Human Breast Cancer EpiTect Methyl II Signature PCR Array. The red color represents values of +2.5 fold relative to controls (B) Comparison between quantitative analysis of methylation for the candidate genes in the studied cohort; Controls (n = 10), Breast cancer (n = 17) and Carriers (n = 9). Significant correlation; Chi square. Numbers represent p values. Error bars represent the mean ± SD.
In order to validate the signature PCR Array data, the methylation status of MGMT was analyzed by MSP in five control samples, two breast cancer cases, and two carriers. The MGMT methylated band was detected in WBC of all breast cancer cases and carrier samples. However, only one control sample showed the presence of a methylated MGMT band (Figure 1B).
Similar gene expression pattern in WBC with methylated BRCA1from breast cancer cases and carriers
Next, we sought to assess the expression of cancer-related genes, including BRCA1, at the mRNA level, in the WBC that harbor methylated BRCA1 promoter. To this end, we made use of the Human Breast Cancer RT2 Profiler™ PCR Array that profiles the expression of 84 key genes, commonly involved in breast carcinogenesis, using RNA isolated from WBC of 8 breast cancer cases, 9 carriers and 5 controls. Out of 84 genes present in the array, 17 were not detected in the WBC. Importantly, we have found a strong correlation between the expression level of BRCA1 in breast cancer cases and its methylation levels detected by the Methyl II Signature PCR Array (Figure 3A). However, only 4 samples from the carriers group showed such correlation, CR10, CR11, CR18 and CR25 (Figure 3B). On the basis of the cut-off value ±1.5 fold relative to controls, 16 genes, including BRCA1, were differentially expressed in breast cancer cases and carriers as compared to controls (Figure 4A). Interestingly, no significant differences were detected between the breast cancer cases and the carriers for the expression of 11 different genes:, ADAM23 (p = 0.343), BCL2 (0.213), EGF (p = 0.90), CDKN1A (p = 0.567), CTSD (0.397), GSTP1 (0.186), MAPK3 (p = 0.058), MGMT (p = 0.165), MMP9 (p = 0.463), TGFB1 (p = 0.084) and TP53 (p = 0.334) (Figure 4B). This indicates that WBC from cancer-free females with methylated BRCA1 has abnormal gene expression profile similar to that seen in WBC from breast cancer cases. On the other hand, 5 genes were differentially expressed with significant difference between the breast cancer patients and carriers, BRCA1 (p = 0.03), BIRC5 (p = 0.035), CCND2 (p0.034), ATM (p = 0.012) and IGF1R (p = 0.025) (Figure 4B).
Figure 3.
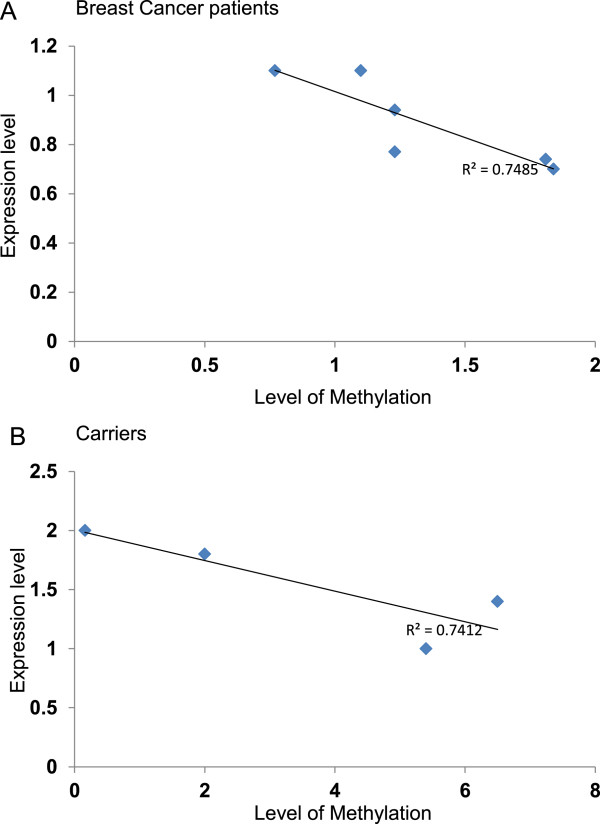
Correlations of the mRNA and methylation levels of BRCA1 . Correlation between the methylation levels of BRCA1 in WBC determined by Human Breast Cancer EpiTect Methyl II Signature PCR Array and the mRNA levels measured by Human Breast Cancer RT2 Profiler™ PCR Array (A) breast cancer group (B) carriers group, R2; correlation coefficient.
Figure 4.
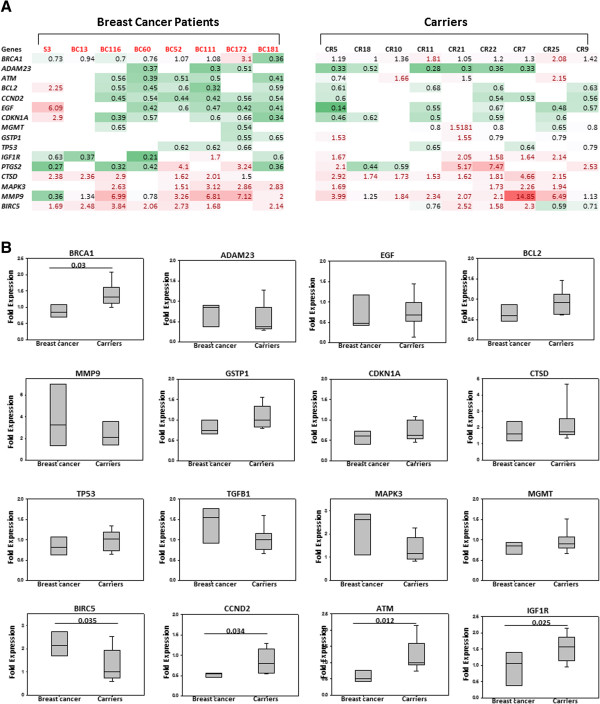
Similar gene expression pattern in WBC with methylated BRCA1 from breast cancer cases and carriers (A) a heat map comparing the differential expression of16 cancer-related genes in WBC RNA from breast cancer patients and carriers as compared to controls determined by using Human Breast Cancer RT 2 Profiler™ PCR Array. (B) Comparison between quantitative analysis of expression level for the candidate genes in the studied cohort; Breast cancer (n = 8), Carriers (n = 9) against controls (n = 5). Significant correlation; Chi square. Numbers represent p values. Error bars represent the mean ± SD.
Similar changes in protein expression pattern in plasma from patients and carriers with WBC harboring methylated BRCA1
It is anticipated that combination of different analyses platforms would give better and more reliable information to answer complex biological questions. Hence, we sought to investigate the protein plasma signature in the carriers group and compare it with those from breast cancer cases and controls. To this end, we have used the label-free MS-based technique as a tool for quantitative and comparative expression analysis. Approximately 400–450 proteins were identified during each run across all sample groups. This resulted in 91 unique protein species from all three sample groups. Only 35 of these proteins were found to be differentially expressed with significant expression changes of at least 1.5 fold and with a probability of ≥0.95-1, between patient, carrier and control sample groups. This dataset discriminated the samples into three distinct groups by unsupervised principal component analysis (PCA) and Hierarchical Cluster Analysis (Figure 5A, B). This indicates that the carriers group exhibits a unique plasma protein pattern different from those of breast cancer patients and controls. Importantly, 10 out of the 35 differentially expressed proteins showed similar expression patterns in patients and carriers as compared to controls (Figure 5C). This indicates that cancer-free females with WBC methylated BRCA1 have abnormal plasma protein expression profile with great similarities with that seen in plasma from the breast cancer cases.
Figure 5.

Similar changes in protein expression pattern in plasma from patients and carriers (A) Principal Component Analysis using the 35 identified proteins with significant difference in expression between plasma samples from breast cancer patients, carrier and controls. The accession numbers of the identified proteins are indicated in the grey color, while Blue, cancer, Purple, carrier, and Orange, control. (B) Hierarchical Cluster Analysis using the expression profiles of the 35 identified proteins with significant difference between plasma samples from breast cancer patients, carrier and controls. (Blue, cancer, Purple, carrier, and Orange, control). (C) Histograms showing relative expression (quantitation), based on intensities, of10 out of the 35 differentially expressed proteins (The expression changes/ abundance ratio were calculated based on the averaged intensities of all identified peptides corresponding to an identification of a particular protein). (The proteins were identified by LC/MS/MS/Synapt G2 and the expression data was generated using Progenesis LC-MS/QI by Nonlinear Dynamics)”. The numbers on the x axis represent Normalized Averaged Intensities.
Discussion
Up to date, the molecular and the pathological significance of the presence of methylated BRCA1 promoter in WBC has not been investigated. The main aim of the present study was to investigate, at the molecular level, whether women with methylated BRCA1 promoter in their WBC are indeed at an elevated risk of developing breast cancer. We have shown that the BRCA1 promoter is methylated in WBC of 14.2% of breast cancer patients and that this methylation is significantly associated with the early onset of the disease. These results are in agreement with previous studies, which showed that BRCA1 epimutations were significantly enriched in woman with early-onset breast cancer [20].
While there is significant correlation between BRCA1 mutation and development of breast cancer, it is still controversial whether or not there is a correlation between the presence of methylated BRCA1 promoter in WBC and paired tumor DNA from breast cancer patients [25]. In our study, we have shown that 10 out of 15 (66.7%) breast cancer patients, with methylated BRCA1 in their WBC, displayed BRCA1 methylation in paired tumor DNA. This suggests that methylated BRCA1 in WBC may also trigger the formation of breast cancer. Indeed, it has been hypothesized that constitutional BRCA1 methylation may constitute the “first-hit” predisposing and initiating tumorigenesis with morphologic features similar to those related to BRCA1 germline mutations [19]. However, in patients with no methylated BRCA1 in paired tumour DNA, BRCA1 promoter methylation is only specific to WBC and does not directly contribute to breast cancer of those patients [25]. Nonetheless, the fact that we have observed similar molecular changes, in the WBC from the two groups of patients, suggests a strong link between aberrant methylation of BRCA1 promoter in WBC and breast carcinogenesis, regardless of its presence or absence in breast tissue.
To further appreciate the link between BRCA1 promoter methylation in WBC and breast carcinogenesis we searched for BRCA1 methylation in WBC from cancer-free women. Since, in our previous study, we have found a strong association between BRCA1 promoter methylation and young age (≤40 years) at diagnosis [14], it was crucial to screen for methylated BRCA1 promoter in cancer-free control cohort of young age. Interestingly, we have shown that BRCA1promoter is methylated in WBC of 9.1% cancer-free females. Importantly, the majority of those carriers (84%) are <40 years, which is to the contrary of the breast cancer cases where the majority of the patients are >40 (91%). This might be an indication for the potential predisposition of those individuals for developing breast cancer. Interestingly, we have found that 10/13 (77%) (p = 0.036) of the carriers have cancer family history. Importantly, 7 of those families 7/10 (70%) have breast and/ or ovarian cancer history.
In this study, we hypothesized that if BRCA1 methylation in WBC reflects an elevated risk for developing breast cancer, WBC from the carriers should exhibit molecular changes similar, to some extent, to those described in BRCA1-methylted WBC from breast cancer patients. Indeed, we have shown significant epigenetic changes in 9 different breast cancer-related genes, other than BRCA1, in WBC from both the carriers and breast cancer patients. All of these genes are known to be involved in different aspects of breast carcinogenesis, which includes tumor suppression, HIC1[26], CDH1[27], CDH13[28], CDKN2[29], DNA repair, MGMT[30], apoptosis, PYCARD[31], TNFRSF10C[32], and cell cycle regulation, CCNA1[33]. In addition, we report the differential expression of another fifteen cancer-related genes, other than BRCA1, in the WBC from the carriers and breast cancer patients. This is very interesting as it has always been known that many of the “cancer-related” genes are tissue-specific and seem unlikely to pop out of WBC analyses. Nevertheless, our results demonstrate the potential use of the WBC as an important source for profiling cancer-related gene expression.
In order to validate our results, we first assessed the methylation status and the expression level of BRCA1 in WBC from the two studied groups. As expected, the level of BRCA1 methylation was significantly higher in WBC from breast cancer group than from the carriers. This was further confirmed by showing that the BRCA1 mRNA was significantly lower in the breast cancer group than in the carriers. This is in concordance with the fact that constitutional methylation is mono allelic [25], hence only one allele of the BRCA1 gene is methylated in the carriers, however, in the breast cancer patients, the two alleles are affected (according to Knudsen’s hypothesis of tumor suppressor deactivation) [34].
One of the important genes that we have shown to be differentially expressed in the WBC from the carriers and breast cancer patients is ATM, which is a risk factor for breast cancer [35]. This gene is down- regulated in breast tumours confirming its potential role in the development of such tumours [35, 36]. Intriguingly, we have shown a significant increase in the mRNA level of ATM in the WBC from the carries as compared to that in the breast cancer cases. In fact, ATM is also up regulated in cases of sclerosing adenosis (SA) [37], a benign proliferative disease of the breast, which is an independent risk factor for subsequent invasive ductal carcinomas [38]. This suggests that the increase in the mRNA level of ATM in the WBC of the carriers could be an indication for the potential predisposition of those individuals for developing breast cancer.
The insulin-like growth factor receptor (IGF1R) is another important gene that we have shown to be differentially expressed in WBC from the carriers and breast cancer patients. An elevation in the expression of IGF1R in normal breast tissue is known to be associated with an increase in the risk of subsequent breast cancer [39]. However, this gene is down-regulated in advanced human breast cancer [40]. Similarly, the expression of IGF1R mRNA was found to be down-regulated in peripheral blood cells and stimulated monocytes from patients with advanced stages of colorectal carcinoma (CRC) [41]. Conversely, IGF1R mRNA was found to be up-regulated in tumour tissue and stimulated CRC monocytes from early stages revealing a role of IGF1R in tumour initiation [41]. Importantly, we have shown that the expression of IGF1R mRNA is up-regulated in WBC from the carriers and down-regulated in WBC from the breast cancer patients (p = 0.025). This gives further evidence for the potential predisposition of the carriers for developing breast cancer.
To shed more light on this possible predisposition to breast cancer, we investigated the protein plasma signature in the carriers group and compare it to that in the breast cancer cases and controls. We have identified 35 differentially expressed proteins in plasma from the carriers, breast cancer patients and controls. 10 out of those proteins showed similar expression pattern between the carriers and the patients. The expression of one of these proteins, Apolipoprotein CIII, was found to be reduced in the plasma from pancreatic cancer patients as compared to controls making it a potential marker for the early detection of this disease [42, 43]. Intriguingly, we have shown here that the expression of Apolipoprotein CIII is down regulated more than 3 fold in plasma from breast cancer patients and about 1.5 fold in plasma from the carriers as compared to its level in the controls plasma. This provides another evidence for the potential predisposition of the carriers for developing breast cancer.
Conclusions
We have shown her that healthy women with BRCA1 methylation in their WBC have, besides BRCA1 methylation, modulation in the methylation and expression of other breast cancer-related genes, in addition to the secretion of important cancer- related proteins. We propose the presence of a strong correlation between aberrant methylation of BRCA1 promoter in WBC, regardless of its presence or absence in breast tissue, and breast cancer-related molecular changes, which may advocates the risk for developing breast cancer. Our findings, together with previous study [19], strongly suggest the potential use of BRCA1 methylation as a powerful biomarker for detecting predisposed women at a far early age.
Acknowledgements
We are grateful to the patients and controls who participated in this study. The help of Zahra breast cancer association in sample collection is greatly appreciated. This work was supported by The National Comprehensive Plan for Science and Technology. Project number 10-BIO1353-20.
Footnotes
Competing interests
The authors declare they have no competing interests.
Authors’ contributions
NM designed and performed the research, analyzed data and wrote the manuscript. AN and SM and SA helped the collection of blood and isolation of white blood cells. NY helped the RNA extraction from blood. TT and AT contributed clinical specimen. BK contributed the data analysis, AYA and ZS helped the proteomic analysis. AA contributed the data analysis and manuscript revision. All authors read and approved the final manuscript.
Contributor Information
Nisreen Al-Moghrabi, Email: nisreen@kfshrc.edu.sa.
Asmaa Nofel, Email: asmaanofel@kfshrc.edu.sa.
Nujoud Al-Yousef, Email: nyousef@kfshrc.edu.sa.
Safia Madkhali, Email: msafia@kfshrc.edu.sa.
Suad M Bin Amer, Email: suad@kfshrc.edu.sa.
Ayodele Alaiya, Email: AAlaiya@kfshrc.edu.sa.
Zakia Shinwari, Email: SZakia@kfshrc.edu.sa.
Taher Al-Tweigeri, Email: ttweigeri@kfshrc.edu.sa.
Bedri Karakas, Email: bkarakas@kfshrc.edu.sa.
Asma Tulbah, Email: tulbah@kfshrc.edu.sa.
Abdelilah Aboussekhra, Email: aboussekhra@kfshrc.edu.sa.
References
- 1.Wolffe AP, Matzke MA. Epigenetics: regulation through repression. Science. 1999;286:481–486. doi: 10.1126/science.286.5439.481. [DOI] [PubMed] [Google Scholar]
- 2.Cropley JE, Martin DI, Suter CM. Germline epimutation in humans. Pharmacogenomics. 2008;9(12):1861–1868. doi: 10.2217/14622416.9.12.1861. [DOI] [PubMed] [Google Scholar]
- 3.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev. 2002;3(6):415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 4.Chong S, Youngson NA, Whitelaw E. Heritable germline epimutation is not the same as transgenerational epigenetic inheritance. Nat Genet. 2007;39(5):574–575. doi: 10.1038/ng0507-574. [DOI] [PubMed] [Google Scholar]
- 5.Horsthemke B. Heritable germline epimutations in humans. Nat Genet. 2007;39(5):573–574. doi: 10.1038/ng0507-573b. [DOI] [PubMed] [Google Scholar]
- 6.Suter CM, Martin DI. Inherited epimutation or a haplotypic basis for the propensity to silence? Nat Genet. 2007;39(5):573. doi: 10.1038/ng0507-573a. [DOI] [PubMed] [Google Scholar]
- 7.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, Bell R, Rosenthal J, Hussey C, Tran T, McClure M, Frye C, Hattier T, Phelps R, Haugen-Strano A, Katcher H, Yakumo K, Gholami Z, Shaffer D, Stone S, Bayer S, Wray C, Bogden R, Dayananth P, Ward J, Tonin P, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science (New York, NY) 1994;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 8.Arver B, Du Q, Chen J, Luo L, Lindblom A. Hereditary breast cancer: a review. Semin Cancer Biol. 2000;10(4):271–288. doi: 10.1006/scbi.2000.0325. [DOI] [PubMed] [Google Scholar]
- 9.Birgisdottir V, Stefansson OA, Bodvarsdottir SK, Hilmarsdottir H, Jonasson JG, Eyfjord JE. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res. 2006;8(4):R38. doi: 10.1186/bcr1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butcher DT, Rodenhiser DI. Epigenetic inactivation of BRCA1 is associated with aberrant expression of CTCF and DNA methyltransferase (DNMT3B) in some sporadic breast tumours. Eur J Cancer. 2007;43(1):210–219. doi: 10.1016/j.ejca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Hedenfalk I, Duggan D, Chen Y, Radmacher M, Bittner M, Simon R, Meltzer P, Gusterson B, Esteller M, Kallioniemi OP, Wilfond B, Borg A, Trent J, Raffeld M, Yakhini Z, Ben-Dor A, Dougherty E, Kononen J, Bubendorf L, Fehrle W, Pittaluga S, Gruvberger S, Loman N, Johannsson O, Olsson H, Sauter G. Gene-expression profiles in hereditary breast cancer. N Engl J Med. 2001;344(8):539–548. doi: 10.1056/NEJM200102223440801. [DOI] [PubMed] [Google Scholar]
- 12.van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AAM, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 13.Snell C, Krypuy M, Wong EM, Con Fabi K, Loughrey MB, Dobrovic A. BRCA1 promoter methylation in peripheral blood DNA of mutation negative familial breast cancer patients with a BRCA1 tumour phenotype. Breast Cancer Res. 2008;10(1):R12. doi: 10.1186/bcr1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Moghrabi N, Al-Qasem AJ, Aboussekhra A. Methylation-related mutations in the BRCA1 promoter in peripheral blood cells from cancer-free women. Int J Oncol. 2011;39(1):129–135. doi: 10.3892/ijo.2011.1021. [DOI] [PubMed] [Google Scholar]
- 15.Esteller M, Fraga MF, Guo M, Garcia-Foncillas J, Hedenfalk I, Godwin AK, Trojan J, Vaurs-Barrière C, Bignon YJ, Ramus S, Benitez J, Caldes T, Akiyama Y, Yuasa Y, Launonen V, Canal MJ, Rodriguez R, Capella G, Peinado MA, Borg A, Aaltonen LA, Ponder BA, Baylin SB, Herman JG. DNA methylation patterns in hereditary human cancers mimic sporadic tumorigenesis. Hum Mol Genet. 2001;10(26):3001–3007. doi: 10.1093/hmg/10.26.3001. [DOI] [PubMed] [Google Scholar]
- 16.Esteller M. Epigenetic lesions causing genetic lesions in human cancer: promoter hypermethylation of DNA repair genes. Eur J Cancer. 2000;36(18):2294–2300. doi: 10.1016/S0959-8049(00)00303-8. [DOI] [PubMed] [Google Scholar]
- 17.Esteller M, Silva JM, Dominguez G, Bonilla F, Matias-Guiu X, Lerma E, Bussaglia E, Prat J, Harkes IC, Repasky EA, Gabrielson E, Schutte M, Baylin SB, Herman JG. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst. 2000;92(7):564–569. doi: 10.1093/jnci/92.7.564. [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto T, Yamamoto N, Taguchi T, Tamaki Y, Noguchi S. BRCA1 promoter methylation in peripheral blood cells is associated with increased risk of breast cancer with BRCA1 promoter methylation. Breast Cancer Res Treat. 2011;129(1):69–77. doi: 10.1007/s10549-010-1188-1. [DOI] [PubMed] [Google Scholar]
- 19.Wong EM, Southey MC, Fox SB, Brown MA, Dowty JG, Jenkins MA, Giles GG, Hopper JL, Dobrovic A. Constitutional methylation of the BRCA1 promoter is specifically associated with BRCA1 mutation-associated pathology in early-onset breast cancer. Cancer Prev Res (Phila) 2011;4(1):23–33. doi: 10.1158/1940-6207.CAPR-10-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansmann T, Pliushch G, Leubner M, Kroll P, Endt D, Gehrig A, Preisler-Adams S, Wieacker P, Haaf T. Constitutive promoter methylation of BRCA1 and RAD51C in patients with familial ovarian cancer and early-onset sporadic breast cancer. Hum Mol Genet. 2012;21(21):4669–4679. doi: 10.1093/hmg/dds308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pejovic T, Yates JE, Liu HY, Hays LE, Akkari Y, Torimaru Y, Keeble W, Rathbun RK, Rodgers WH, Bale AE, Ameziane N, Zwaan CM, Errami A, Thuillier P, Cappuccini F, Olson SB, Cain JM, Bagby GC., Jr Cytogenetic instability in ovarian epithelial cells from women at risk of ovarian cancer. Cancer Res. 2006;66(18):9017–9025. doi: 10.1158/0008-5472.CAN-06-0222. [DOI] [PubMed] [Google Scholar]
- 22.Wetzig A, Alaiya A, Al-Alwan M, Pradez CB, Pulicat MS, Al-Mazrou A, Shinwari Z, Sleiman GM, Ghebeh H, Al-Humaidan H, Gaafar A, Kanaan I, Adra C. Differential marker expression by cultures rich in mesenchymal stem cells. BMC Cell Biol. 2013;14:54. doi: 10.1186/1471-2121-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alaiya A, Fox J, Bobis S, Matic G, Shinwari Z, Barhoush E, Marquez M, Nilsson S, Holmberg AAR. Proteomic analysis of soft tissue tumor implants treated with a novel polybisphosphonate. Cancer Genomics Proteomics. 2014;11:39–50. [PubMed] [Google Scholar]
- 24.Li GZ, Vissers JP, Silva JC, Golick D, Gorenstein MV, Geromanos SJ. Database searching and accounting of multiplexed precursor and product ion spectra from the data independent analysis of simple and complex peptide mixtures. Proteomics. 2009;9(6):1696–1719. doi: 10.1002/pmic.200800564. [DOI] [PubMed] [Google Scholar]
- 25.Wojdacz TK, Thestrup BB, Overgaard J, Hansen LL. Methylation of cancer related genes in tumor and peripheral blood DNA from the same breast cancer patient as two independent events. Diagn Pathol. 2011;6:116. doi: 10.1186/1746-1596-6-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wales MM, Biel MA, el Deiry W, Nelkin BD, Issa JP, Cavenee WK, Kuerbitz SJ, Baylin SB. p53 activates expression of HIC-1, a new candidate tumour suppressor gene on 17p13.3. Nat Med. 1995;1(6):570–577. doi: 10.1038/nm0695-570. [DOI] [PubMed] [Google Scholar]
- 27.Guilford PJ, Hopkins JB, Grady WM, Markowitz SD, Willis J, Lynch H, Rajput A, Wiesner GL, Lindor NM, Burgart LJ, Toro TT, Lee D, Limacher JM, Shaw DW, Findlay MP, Reeve AE. E-cadherin germline mutations define an inherited cancer syndrome dominated by diffuse gastric cancer. Hum Mutat. 1999;14(3):249–255. doi: 10.1002/(SICI)1098-1004(1999)14:3<249::AID-HUMU8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Lee SW. H-cadherin, a novel cadherin with growth inhibitory functions and diminished expression in human breast cancer. Nat Med. 1996;2(7):776–782. doi: 10.1038/nm0796-776. [DOI] [PubMed] [Google Scholar]
- 29.Lukas J, Parry D, Aagaard L, Mann DJ, Bartkova J, Strauss M, Peters G, Bartek J. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995;375(6531):503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- 30.Pegg AE. Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res. 1990;50(19):6119–6129. [PubMed] [Google Scholar]
- 31.Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z, Alnemri ES. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem. 2002;277(24):21119–21122. doi: 10.1074/jbc.C200179200. [DOI] [PubMed] [Google Scholar]
- 32.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2(6):420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 33.Yang R, Muller C, Huynh V, Fung YK, Yee AS, Koeffler HP. Functions of cyclin A1 in the cell cycle and its interactions with transcription factor E2F-1 and the Rb family of proteins. Mol Cell Biol. 1999;19(3):2400–2407. doi: 10.1128/mcb.19.3.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knudson AG. Hereditary cancer: two hits revisited. J Cancer Res Clin Oncol. 1996;122(3):135–140. doi: 10.1007/BF01366952. [DOI] [PubMed] [Google Scholar]
- 35.Angele S, Hall J. The ATM gene and breast cancer: is it really a risk factor? Mutat Res. 2000;462(2–3):167–178. doi: 10.1016/S1383-5742(00)00034-X. [DOI] [PubMed] [Google Scholar]
- 36.Waha A, Sturne C, Kessler A, Koch A, Kreyer E, Fimmers R, Wiestler OD, von Deimling A, Krebs D, Schmutzler RK. Expression of the ATM gene is significantly reduced in sporadic breast carcinomas. Int J Cancer. 1998;78(3):306–309. doi: 10.1002/(SICI)1097-0215(19981029)78:3<306::AID-IJC8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 37.Clarke RA, Kairouz R, Watters D, Lavin MF, Kearsley JH, Lee CS. Upregulation of ATM in sclerosing adenosis of the breast. Mol Pathol. 1998;51(4):224–226. doi: 10.1136/mp.51.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen RA, Page DL, Dupont WD, Rogers LW. Invasive breast cancer risk in women with sclerosing adenosis. Cancer. 1989;64(10):1977–1983. doi: 10.1002/1097-0142(19891115)64:10<1977::AID-CNCR2820641002>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 39.Tamimi RM, Colditz GA, Wang Y, Collins LC, Hu R, Rosner B, Irie HY, Connolly JL, Schnitt SJ. Expression of IGF1R in normal breast tissue and subsequent risk of breast cancer. Breast Cancer Res Treat. 2011;128(1):243–250. doi: 10.1007/s10549-010-1313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schnarr B, Strunz K, Ohsam J, Benner A, Wacker J, Mayer D. Down-regulation of insulin-like growth factor-I receptor and insulin receptor substrate-1 expression in advanced human breast cancer. Int J Cancer. 2000;89(6):506–513. doi: 10.1002/1097-0215(20001120)89:6<506::AID-IJC7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 41.Karakolev Expression of insuline-like growth factor-1 receptor mrna in colorectal carcinoma patients. Biotechnol Biotec Eq SPECIAL EDITION/ON-LINE. 2011;26(Number 1):89–95. [Google Scholar]
- 42.Chen J, Anderson M, Misek DE, Simeone DM, Lubman DM. Characterization of apolipoprotein and apolipoprotein precursors in pancreatic cancer serum samples via two-dimensional liquid chromatography and mass spectrometry. J Chromatogr A. 2007;1162(2):117–125. doi: 10.1016/j.chroma.2007.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Honda K, Okusaka T, Felix K, Nakamori S, Sata N, Nagai H, Ioka T, Tsuchida A, Shimahara T, Shimahara M, Yasunami Y, Kuwabara H, Sakuma T, Otsuka Y, Ota N, Shitashige M, Kosuge T, Büchler MW, Yamada T. Altered plasma apolipoprotein modifications in patients with pancreatic cancer: protein characterization and multi-institutional validation. PLoS One. 2012;7(10):e46908. doi: 10.1371/journal.pone.0046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pre-publication history
- The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2407/14/830/prepub


