Abstract
Background
Interest in improving care for the growing number of individuals with chronic conditions is rising. However, access to care is limited by distance, disability, and distribution of doctors. Small-scale studies in Parkinson disease, a prototypical chronic condition, have suggested that delivering care using video house calls is feasible, offers similar clinical outcomes to in-person care, and reduces travel burden.
Methods/Design
We are conducting a randomized comparative effectiveness study (Connect.Parkinson) comparing usual care in the community to usual care augmented by virtual house calls with a Parkinson disease specialist. Recruitment is completed centrally using online advertisements and emails and by contacting physicians, support groups, and allied health professionals. Efforts target areas with a high proportion of individuals not receiving care from neurologists. Approximately 200 individuals with Parkinson disease and their care partners will be enrolled at 20 centers throughout the United States and followed for one year. Participants receive educational materials, then are randomized in a 1:1 ratio to continue their usual care (control arm) or usual care and specialty care delivered virtually (intervention arm). Care partners are surveyed about their time and travel burden and their perceived caregiver burden. Participants are evaluated via electronic survey forms and videoconferencing with a blinded independent rater at baseline and at 12 months. All study activities are completed remotely.
The primary outcomes are: (1) feasibility, as measured by the proportion of visits completed, and (2) quality of life, as measured by the 39-item Parkinson’s Disease Questionnaire. Secondary outcomes include measures of clinical benefit, quality of care, time and travel burden, and caregiver burden.
Discussion
Connect.Parkinson will evaluate the feasibility and effectiveness of using technology to deliver care into the homes of individuals with Parkinson disease. The trial may serve as a model for increasing access and delivering patient-centered care at home for individuals with chronic conditions.
Trial registration
This trial was registered on clinicaltrials.gov on January 8, 2014 [NCT02038959].
Electronic supplementary material
The online version of this article (doi:10.1186/1745-6215-15-465) contains supplementary material, which is available to authorized users.
Keywords: Health care delivery, Health care disparities, House call, Parkinson disease, Telemedicine, Videoconferencing
Background
Chronic conditions affect more than 147 million Americans and account for 85% of U.S. health care expenditures [1]. By 2030, chronic conditions will affect 171 million Americans, or more than half of the U.S. population [1]. Current care for chronic conditions in the United States is costly, ineffectual, often leads to poor outcomes [1–7], and increases burden on caregivers [1, 8, 9]. Many studies have shown that coordinated, multidisciplinary specialty care delivered more frequently can reduce the incidence of acute complications of chronic illness and improve patient and caregiver quality of life [2, 4, 10–14]. Home visits - once a standard mode of care delivery [15] - and care delivered into the home have shown particular promise, especially in caring for older people [16–19]. However, access to such care is frequently limited by distance, disability, and the distribution of specialists (Figure 1), and varies with race and gender [1, 20–22]. Simple, inexpensive videoconferencing technology can alleviate these barriers and provide care to these individuals in their homes.
Figure 1.
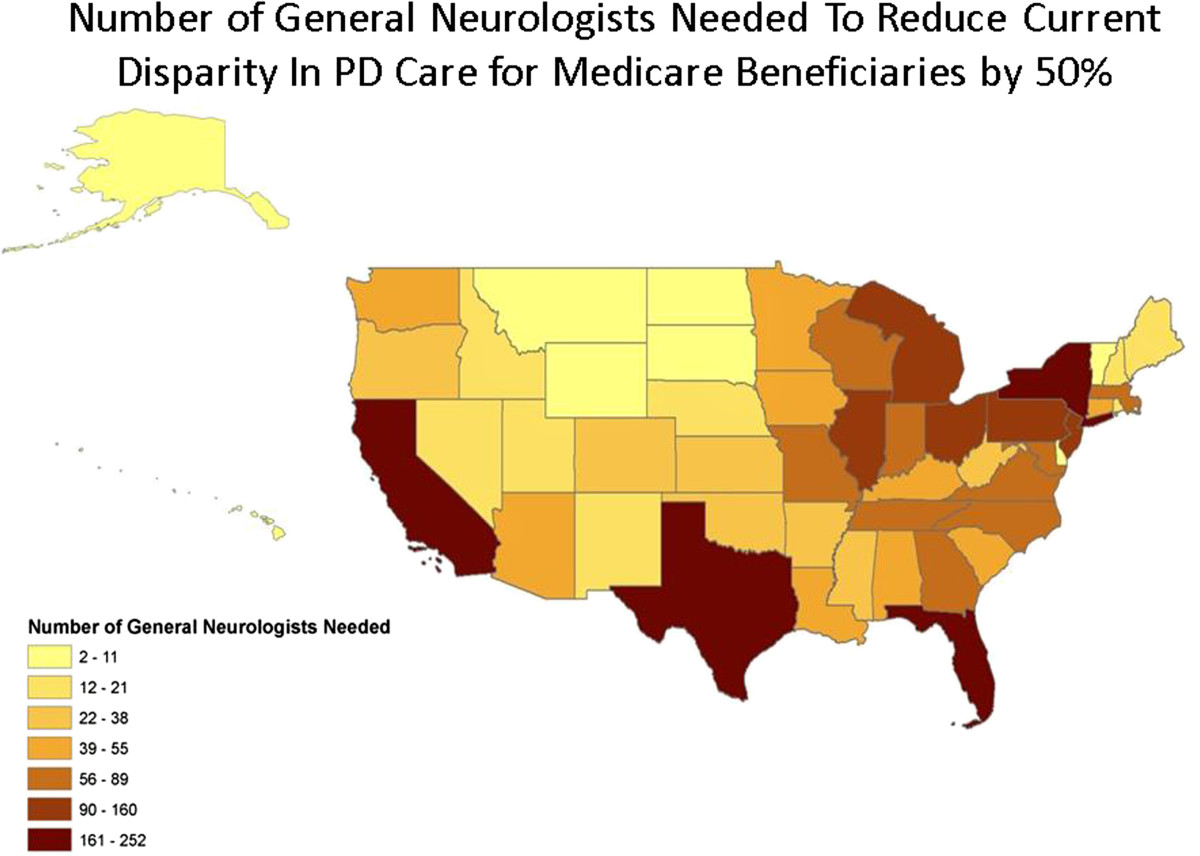
Number of typical full-time neurologist practices that would need to open to reduce the current disparity in Parkinson disease care by 50%. Estimates assume that a typical neurologist has 10% of office visits for Parkinson disease patients; that in one year, that neurologist sees patients every six months; and that each neurologist works full-time, five days per week, minus federal holidays and standard vacation.
As videoconferencing technology has become more available, reliable, and less expensive [23–26], interest in using telemedicine to deliver chronic illness care has been increasing [23, 27]. A 2000 Cochrane review of studies comparing telemedicine to face-to-face care concluded, among other things, that ‘Studies of effectiveness, efficiency and appropriateness of telematics applications to health care urgently need to be performed, but technology may permit provision of care which is presently not possible by conventional means’ [27]. Telemedicine interventions including videoconferencing and telemonitoring for veterans with chronic conditions [28], individuals with severe asthma [29], diabetes [30–32], and heart failure [14, 33–43] have all shown promise. In a recent analysis of telemedicine’s applications in chronic disease management, Dr. Richard Wootton reviewed studies of all forms of telemedicine intervention, including remote monitoring and telephone calls, and identified significant problems with the published literature [44]. Studies exploring the use of telemedicine to enable physicians to make virtual house calls have been conducted in a variety of conditions, but have not yet been conducted in Parkinson disease at this scale. We conducted a PubMed search using ‘telemedicine AND home AND randomized’, (336 total results), ‘randomized AND video AND home’ (241 total results), ‘virtual AND visits AND home’ (29 results), and ‘videoconferencing’ AND ‘randomized’ (168 total results), to identify randomized controlled trials reporting on uses of home-based videoconferencing and reviewed the references of Dr. Wootton’s review [44]. Of the 774 search results and 141 studies identified by Dr. Wootton, a total of 16 randomized controlled trials involving remote delivery of care from a physician directly to a patient in the home were identified (Table 1). The present study will be the longest randomized, controlled trial of telemedicine for Parkinson disease of which the authors are aware.
Table 1.
Randomized controlled trials involving video-based virtual house calls from physicians
| Study | Year | Sample size | Study population | Intervention(s) | Duration | Primary outcomes | Results |
|---|---|---|---|---|---|---|---|
| Dorsey ER et al. [45] | 2013 | 20 | Individuals with Parkinson disease | Randomized to (1) in-person care or (2) care via telemedicine | 7 months | • Feasibility | • Virtual house calls were feasible |
| • Quality of life | • As effective as in-person care | ||||||
| Fortney JC et al. [46] | 2013 | 364 | Individuals with depression | Randomized to practice-based or telemedicine-base collaborative care | 18 months | • Clinical | • Telemedicine-based collaborative care yielded better outcomes for depressed patients |
| McCrossan B et al. [47] | 2012 | 83 | Infants with congenital heart defects | Participants randomized to (1) videoconferencing support, (2) telephone support, or (3) control | 10 weeks | • Acceptability | • Clinicians were more confident in treating patients in video visits vs. telephone |
| • Health care resource utilization | |||||||
| • Parents were satisfied with video visits | |||||||
| • Health care resource utilization was lower in videoconferencing group | |||||||
| Moreno FA et al. [48] | 2012 | 167 | Hispanic adults with depression | Randomized to telemedicine care from a psychiatrist or usual care from a primary care physician | 6 months | • Clinical | • All participants improved on clinical measures |
| • Quality of life | |||||||
| • Time to improvement was shorter in telemedicine group | |||||||
| Leon A et al. [49] * | 2011 | 83 | Individuals with HIV | Randomized to (1) usual care of (2) Virtual Hospital care for one year, then crossed over after one year | 2 years | • Clinical | • Satisfaction with Virtual Hospital was high |
| • Health care resource utilization | |||||||
| • Quality of life | |||||||
| • Satisfaction | |||||||
| • Clinical outcomes were similar for both groups | |||||||
| Ferrer-Roca O et al. [50] | 2010 | 800 | Primary care patients referred for specialized care | Randomized to face-to-face hospital referral or telemedicine from specialist | 6 months | • Quality of life | • Telemedicine care was comparable to face-to-face care |
| • Diagnosis and examination to start treatment were faster in the telemedicine group | |||||||
| Stahl JE, Dixon RF [51] | 2010 | 175 | Patients in a general primary care practice | Interviewed face to face and via videoconferencing, order randomized | 2 visits | • Satisfaction | • Patients and providers highly satisfied with videoconferencing but preferred face to face |
| • Willingness to pay | |||||||
| • Technical quality of video calls had significant impact on satisfaction | |||||||
| Dorsey ER et al. [52] | 2010 | 14 | Individuals with Parkinson disease | Randomized to (1) usual care or (2) care via telemedicine | 6 months | • Feasibility | • Virtual house calls were feasible |
| • Virtual house calls improved disease-specific measures significantly compared to usual care. | |||||||
| Dixon RF, Stahl JE [53] | 2009 | 175 | Patients in a general primary care practice | Randomized to one virtual visit and one face to face visit, or two face to face visits. | 2 visits | • Diagnostic agreement | • Physicians and patients highly satisfied with virtual visits |
| • Diagnostic agreement between virtual and in-person evaluation was similar to comparison of two in-person evaluations | |||||||
| • Satisfaction | |||||||
| Ahmed SN et al. [54] | 2008 | 41 | Epilepsy patients | Randomized to telemedicine follow-up or conventional | 1 visit | • Cost-effectiveness | • 90% of patients in both groups satisfied with quality of services |
| • Cost to patients and caregivers | |||||||
| • Cost of telemedicine production was similar to patient savings | |||||||
| • Satisfaction | |||||||
| Morgan GJ et al. [55] | 2008 | 30 | Parents of children with severe congenital heart disease | Randomized to telephone or videoconferencing follow-up | 6 weeks | • Anxiety | • Videoconferencing decreased anxiety levels compared to telephone and allowed better clinical information |
| • Clinical | |||||||
| O’Reilly R et al. [56] | 2007 | 495 | Patients referred for psychiatric consult | Randomized to face to face or telepsychiatry | 4 months | • Clinical | • Similar outcomes were seen in both arms |
| • Cost-effectiveness | |||||||
| • Telepsychiatry was at least 10% less expensive than in-person care | |||||||
| • Satisfaction | |||||||
| • Both groups expressed similar satisfaction | |||||||
| De Las Cuevas C et al. [57] | 2006 | 140 | Psychiatric outpatients | Randomized to face to face or telepsychiatry | 24 weeks | • Clinical | • Telepsychiatry had equivalent efficacy to face-to-face care |
| Ruskin PE et al. [58] | 2004 | 119 | Veterans with depression | Randomized to telepsychiatry or in-person psychiatrist visits | 6 months | • Clinical | • Both groups were equivalent in clinical outcomes, cost, patient adherence, and patient satisfaction. |
| • Cost-effectiveness | |||||||
| • Health care resource utilization | |||||||
| • Satisfaction | |||||||
| Bishop JE et al. [59] | 2002 | 19 | Psychiatric patients | Randomized to videoconference or face to face | 4 months | • Satisfaction | • Similar satisfaction observed in both groups |
*Study evaluates an intervention that includes virtual house calls, but also includes other telemonitoring or electronic communication methodologies.
Parkinson disease is a prototypical chronic condition in which to test this health care delivery method. Like many chronic conditions, Parkinson disease has an incidence that increases with age [60], a long duration (average survival of approximately 14 years after diagnosis) that results in progressive disability [61], impairs driving ability [62, 63], burdens caregivers [64], often requires institutional care [61, 65–68], generates high health care costs to private and public payers [69], and, importantly, benefits from specialized care [12, 21, 22, 70]. However, over 40% of Medicare beneficiaries with Parkinson disease do not receive neurologic care within four years of diagnosis, and those who have not are at a yearly 14% increased risk of hip fracture, 21% increased risk of placement in skilled nursing facilities within the first year, and a 22% increased risk of death within six years compared to those who see a neurologist [21, 22]. Because many of its symptoms can be readily assessed visually, interest in using telemedicine to facilitate care began over 20 years ago [71] and has increased to the present [72]. Pilot studies using web-based videoconferencing have previously shown the efficacy, value, and acceptability of virtual house calls from specialists to people with Parkinson disease [45, 52, 73]. Virtual house calls can also incorporate multidisciplinary care and education from a team of health care providers, which has been shown to be highly effective for Parkinson disease [10, 11, 74–79]. The present study will add to understanding of the promise and limitations of virtual house calls for the treatment of Parkinson disease.
The trial was registered on clinicaltrials.gov in January 2014 (NCT02038959).
Methods
Trial design
We are conducting a randomized comparative effectiveness study (Connect.Parkinson) comparing usual care in the community to usual care augmented by video house calls with a Parkinson disease specialist [80]. Approximately 200 individuals with Parkinson disease and their care partners will be enrolled at 20 centers throughout the United States and followed for one year. Participants receive educational materials, then are randomized in a 1:1 ratio to continue their usual care (control arm) or usual care and specialty care delivered virtually (intervention arm). Care partners are surveyed about their time and travel burden and their perceived caregiver burden. A blinded independent rater and a study coordinator conduct baseline and end-of-study assessments. All study activities are completed remotely. The specific aims of the study are: (1) to demonstrate the feasibility of using virtual house calls to deliver specialty care into the homes of individuals with Parkinson disease who have limited access to care; (2) to show that such an approach can improve quality of life; (3) to establish that virtual house calls can enhance the quality of care; and (4) to demonstrate that this remote approach to care saves time, reduces travel, and decreases care partner burden.
To conduct the study, we have partnered with the largest Parkinson disease patient organization in the country, the National Parkinson Foundation, and formed a Patient Advisory Board with patients and patient advocates who have contributed to the design of the trial and continue to be involved with the project. Finally, we have assembled a Dissemination and Implementation Advisory Board to assist in disseminating the results of the research and drive broader adoption.
This study was approved by the Research Subjects Review Board of the University of Rochester as a coordinating center (January 2014) and an enrolling site (March 2014). As of October 26, 2014, the study has been approved at 16 sites and is under review at four additional sites (Additional file 1).
Participants
Eligibility criteria were designed to permit broad participation in the study. Individuals with clinically diagnosed idiopathic Parkinson disease, who have access to a non-public, internet-enabled device with the capacity for videoconferencing, who are physically located in a state where a participating site investigator is licensed to practice medicine when visits are conducted, and are willing and able to provide informed consent, may enroll. Participants must also have a local health care provider (for example primary care physician, nurse practitioner) who the study team can contact to provide recommendations from the site investigators, and must live at home, in a senior housing complex or assisted living facility. Individuals who are currently hospitalized, enrolled in another telemedicine study, or who have a condition (for example, prominent psychosis) that precludes study participation will be excluded from study participation.
Care partners must be adults who are able and willing to provide informed consent to be enrolled. Their participation is optional.
Procedures
Individuals with Parkinson disease will be recruited and enrolled remotely and sent educational materials about Parkinson disease created by the National Parkinson Foundation. They will also be asked to identify their regular care partners (friends or family members who provide regular assistance with daily activities and are not paid caregivers), who will be invited to enroll. Enrollment is completed in two parts; first, a central study coordinator reviews an interest survey and contacts interested individuals to verify their eligibility and complete a screening form, then enrolling site staff contacts the potential participant to obtain consent. Consent is obtained with a written signature on a printed consent form. All study activities are completed remotely, using email, phone, fax, mail, and videoconferencing modalities to enable individuals to participate from home. Study data are collected and managed using Research Electronic Data Capture (REDCap) tools hosted at the University of Rochester [81]. REDCap is a secure, web-based application designed to support data capture for research studies, providing an interface for validated data entry; audit trails for tracking data manipulation and export procedures, automated export procedures for seamless data downloads to common statistical packages; and procedures for importing data from external sources if needed. REDCap supports the use of electronic patient surveys and automated email invitations, which are used in this study to allow participant-completed assessments to be done securely from home, with the aid of a family member if needed. A complete schedule of activities is included in Additional file 2.
Participants who enroll will be emailed a link to download secure Health Insurance Portability and Accountability Act-compliant virtual visit software from SBR Health (Cambridge, MA, USA). The software embeds videoconferencing software from Vidyo (Hackensack, NJ, USA) that is hosted by ID Solutions (Indianapolis, IN, USA), which uses two-way encrypted video transmission to ensure privacy. SBR Health also creates a virtual waiting room that allows patients to ‘check in’ for appointments. If participants do not have access to a webcam, a Creative Labs Live! Cam Chat HD camera is mailed to them prior to their baseline assessment virtual visit. A study coordinator at the University of Rochester performs a test connection with the participants, providing technical support by phone if needed. No in-person technical support is sent to the participant’s home.
Participants will be evaluated via videoconferencing and via electronically administered surveys at baseline and at 12 months. Blinded independent raters complete remote baseline and end-of-study (12-month) assessments of Parkinson disease using the Movement Disorder Society Unified Parkinson Disease Rating Scale (MDS-UPDRS) [82] modified (excludes assessment of tone and balance) for remote assessment [83]. Individuals who the independent rater believes not to have Parkinson disease are withdrawn at the baseline visit prior to randomization. A study team member also completes a remote Montreal Cognitive Assessment (MoCA) [84, 85] at this visit. Additional baseline assessments are completed by the participant/care partner and study staff as detailed in Additional file 2. Care partners are surveyed at baseline and at the end of the study about the time and travel required to help the participant with their Parkinson disease appointments, and the perceived burden of caring for the individual with Parkinson disease. All participant-completed study assessments are completed via secure survey links sent to their email addresses using REDCap, and study teams enter data from each visit directly into the study database.
Randomization
After the initial evaluation, participants are randomized to either continue with their usual care throughout the year or to continue their usual care and receive virtual visits from a Parkinson disease specialist licensed to practice in the state in which they reside. The randomization allocation sequence was generated by C.A.B. using R version 3.0.2. Randomization is conducted in the study’s REDCap database after the baseline assessments have been completed and Parkinson disease diagnosis confirmed. The randomization plan is stratified by enrolling site and contains blocking to ensure approximately even distribution of control/treatment arm participants.
Interventions
Participants with Parkinson disease are randomly assigned to either continue with their usual care or continue with usual care supplemented by virtual house calls. The care received by the usual care (control) group will be variable but will be a reflection of the status quo for Parkinson disease care in the United States. This group is free to seek out specialty care over the course of the study, and we anticipate that some may do so. Those assigned to usual care are given the opportunity to have a one-time virtual visit with a Parkinson disease specialist after their final study assessment. For the telemedicine (intervention) group, the visit schedule is set by the investigator in consultation with the patient and will include at least four virtual visits over one year. Visits are similar to regular in-person clinical visits for Parkinson disease, and investigators provide a clinical note summarizing the visit and any recommendations for treatment to the patients and their local health care providers at the conclusion of each visit.
Outcome measures
Primary outcomes include (1) feasibility, defined as the percentage of telemedicine participants who complete at least one telemedicine visit, and the overall percentage of completed telemedicine visits, and (2) quality of life, measured by the change in the 39-item Parkinson’s Disease Questionnaire (PDQ-39) [86] from baseline to 12 months.
Secondary outcomes include quality of care, as measured by the change from baseline in the Patient Assessment of Chronic Illness Care (PACIC) [87], and time and travel savings from remote appointments, and change in caregiver burden as measured by the Multidimensional Caregiver Strain Index (MCSI) [88]. Additional secondary outcomes have been selected to determine the impact of telemedicine specialist care on Parkinson disease-specific outcomes and global quality of life. The change in Parkinson disease symptoms and signs will be assessed by the change in the MDS-UPDRS from baseline to 12 months. In addition, changes in depression and cognition, common comorbidities with Parkinson disease [89–92], will be identified as the change in the 15-item Geriatric Depression Scale (GDS-15) [93] and the MoCA. Additional quality-of-life metrics are the Patient Global Impression of Change [94] and the European Quality of Life Five Dimension Five Level Scale (EuroQoL-5D-5 L) [95]. Patient-reported utilization of health care services such as hospitalizations, emergency room visits, and visits to primary care doctors [96] will also be compared between the control and intervention arms.
Planned statistical analyses
The aims of the study are to evaluate the feasibility, quality of life, clinical benefit, quality of care, and value of using telemedicine to deliver specialty care to patients in their home. Primary measures of feasibility will be summarized using descriptive statistics. We will consider telemedicine to be feasible if 80% of participants in the telemedicine arm complete at least one telemedicine visit, and at least 80% of all telemedicine visits are completed as scheduled. Generalized linear mixed models will be used to determine what factors affect the probability of completing telemedicine visits as scheduled.
The primary efficacy outcome measure of this study is the PDQ-39. For this outcome, we will fit an analysis of covariance model with the change in PDQ-39 from baseline to one year as the response, treatment group as the factor of interest, participating physician as a stratification factor, and baseline PDQ-39 as a covariate. A t test will be performed to compare the adjusted treatment group means. Secondary measures of quality of life, clinical benefit, quality of care, and value to patients and care partners will be analyzed similarly. Additional analyses will examine the relationships among outcome variables. All statistical tests will be performed at the two-sided significance level of 5%, and no corrections will be made for multiple testing.
Sample size
The sample size of 200 Parkinson disease patients was selected to ensure adequate power (80 to 90%) to detect a moderate effect size on the PDQ-39 (Cohen’s d of 0.5) using a two-sided t test at a significance level of 5% allowing for an anticipated dropout rate of up to 20%.
Recruitment
Recruitment for the study began in February 2014. Recruitment methods were designed to reach the large number of patients with Parkinson disease who do not currently see a neurologist. To address disparities in access to care, we have identified and continue to target underserved areas nationally, defining counties as ‘underserved’ as those in which a majority of Medicare beneficiaries diagnosed with Parkinson disease have not seen a neurologist [20]. We have created targeted Google AdWords to display for searches related to Parkinson disease in these defined geographies. We have also identified primary care providers who may see a large proportion of Parkinson disease patients and will send study materials to these practices in eligible states to recruit patients from these areas. We have built a website (Connect.Parkinson.org) based on the study flier and created an informational video featuring a member of our Patient Advisory Board. Interested individuals contact the National Parkinson Foundation PD Helpline (800.4PD.INFO) for information about the study and can submit their contact information to the coordinating center through the Helpline or directly through a survey form on the website. Additional methods of recruitment include outreach to support groups and trained allied health professionals (for example, physical therapists) in underserved areas. We supplement these efforts by announcing the study through communications to the National Parkinson Foundation’s distribution list, a Clinical Trial Announcement through the patient social networking site PatientsLikeMe, and by posting the study in online patient communities such as the Michael J. Fox Foundation’s Fox Trial Finder. Based on our objective to reach those with limited access to care, we will prioritize enrollment of individuals who are not seeing a neurologist or come from an underserved region.
Discussion
Telemedicine holds tremendous promise for increasing access and quality of care and decreasing cost for chronic conditions. Video visits into the home represent a new generation of house calls, poised to bring about the return of this personalized, convenient, and accessible care model [18]. The Connect.Parkinson study aims to demonstrate the feasibility and efficacy of using home telemedicine for individuals with Parkinson disease. This effort is one of the largest and longest randomized controlled trials assessing this care delivery model for a chronic condition and will involve providers and patients with little previous experience of telemedicine. Large-scale implementation of this method of care will depend in part on physician and patient adoption of this care model [97, 98]. Even though the means of communication used in this study are common in everyday life (for example, grandparents use videoconferencing to connect to their grandchildren), the use of this technology to deliver care may still appear foreign to many.
Interest in the study has been robust. In the first month (February 2014 to March 2014) in response to limited outreach, over 1,400 individuals visited the Connect.Parkinson website from all over the United States and the world (Figure 2) and over 300 completed an online survey expressing interest in participating in the study. Efforts to reach those with limited access to care have been more challenging. Most interested individuals in the first four months of recruiting efforts came from individuals in underserved areas (Figure 3); however, most of the respondents are seeing a neurologist regularly, suggesting that time and travel burden may be driving interest. Considering the known differences between the demographics of clinical trial participants and those of the general population and Medicare beneficiaries in particular, these data are not surprising [99, 100]. We will continue efforts to facilitate inclusion of individuals who may be having difficulty accessing neurologists.
Figure 2.
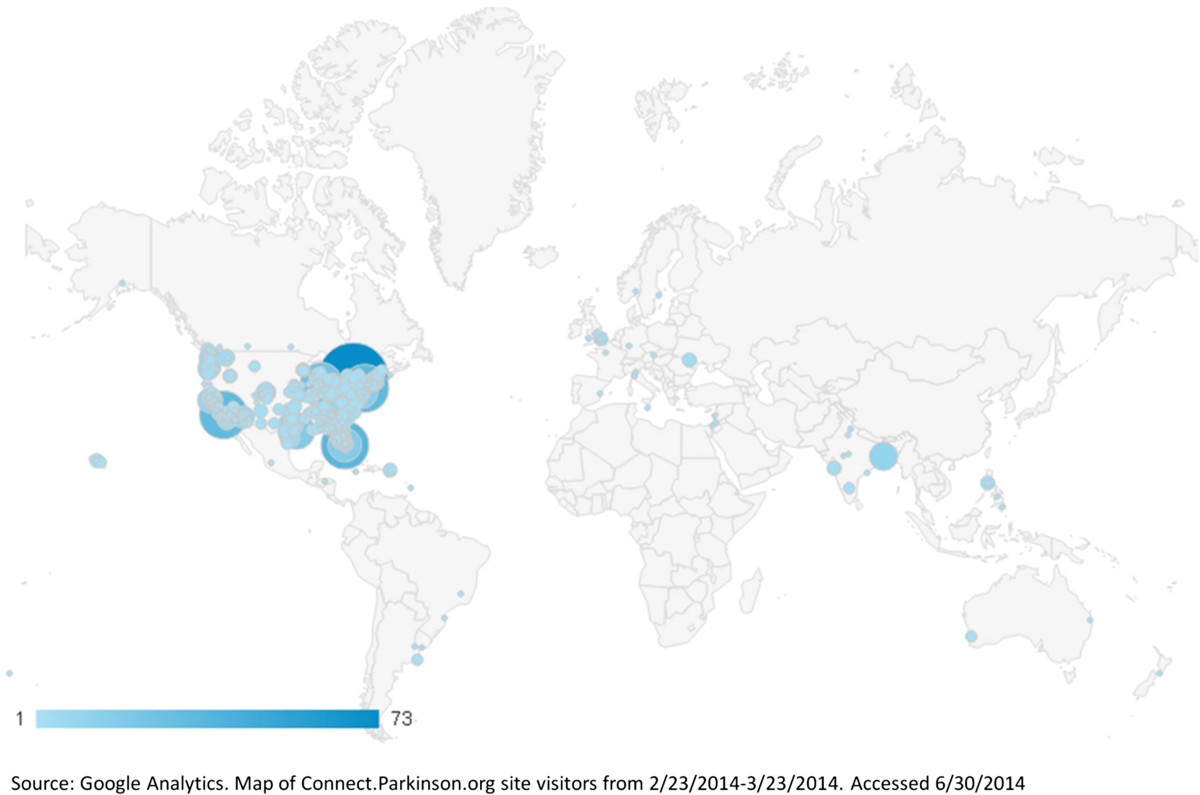
Individuals from all over the world have accessed the Connect.Parkinson study website at . http://connect.parkinson.org
Figure 3.
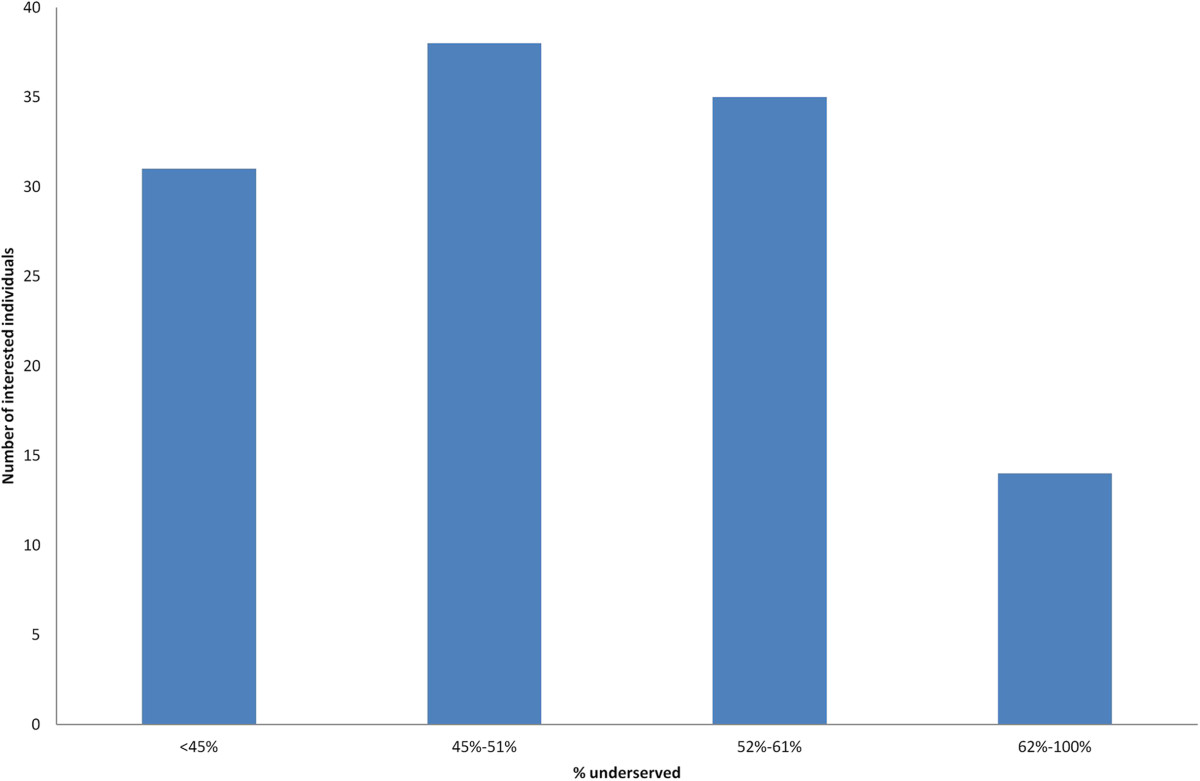
Potential Connect.Parkinson participants in underserved zip codes. Distribution of interested individuals by the proportion of underserved patients with Parkinson disease in their zip code. Data current as of May 20, 2014.
The study has additional limitations related to the availability of the technology and the nature of the visits. While broadband access is increasingly common [101, 102], a digital divide still exists [25]. In particular, individuals with chronic medical conditions report significantly less internet access than those without [103]. This divide may indeed limit our ability to connect to many of the patients in underserved areas who we are trying to reach. Even for those who are able to connect, the quality of connection is often highly variable and dependent on the speed of the patient’s internet connection. This issue may be more prominent in older people. While 86% of American adults use the internet, only 59% of adults over 65 do so, and only 47% have a broadband internet connection at home [104]. Only 15% of adults over 65 reported using videoconferencing in 2010 [105], and the technology is still foreign to many, especially when applied to health care. Even those who do have internet connectivity may be using older hardware and operating systems that do not readily support the newer videoconferencing software, or may have limited familiarity with installing software, both of which can cause delays in setting up and conducting visits and interfere with the quality of the assessments. These delays and the participants’ baseline familiarity with the internet and related technologies are being measured as part of this study. The necessity of obtaining written signatures for consent forms (in lieu of electronic signatures) also introduces a delay in the enrollment process. Signed consent forms must be sent to sites via mail or email, creating unnecessary delays. This has been the case with other primarily internet-based clinical trials [106]. With the continuing integration of internet-based communications into all aspects of medicine and research, methods of obtaining electronic signatures securely should increasingly become part of standard research practice, as they have been implemented successfully in other primarily internet-based clinical trials [107–109].
In addition to the study’s technological limitations, the nature of the remote visits is limited. While several studies have demonstrated that the standard Parkinson disease rating scale can be administered remotely and that remote assessments closely correlate with in-person assessments [83, 110–112], the quality of the examination is not as good as in person. As such, assessments of tone (for example, for cogwheel rigidity) and balance (for example, a ‘pull’ test in which patients are pulled backward by an examiner) are not feasible. Similarly, assessments of more subtle signs, such as eye movements, can be more difficult remotely. Notwithstanding these limitations, it should be noted that the seminal description of the disease nearly two centuries ago by Dr. James Parkinson in 1817 including the cardinal features of rest tremor, bradykinesia (slowness in movement), and gait imbalance was based almost exclusively on his visual observations of individuals walking in a London park [113]. Beyond the technical assessment, the personal connection between a patient and physician is limited by the absence of physical touch. However, studies of telemedicine have largely found the quality of the interpersonal connection between patients and physicians to be high [52, 74, 114] and patients with Parkinson disease experiencing virtual visits for the first time have highlighted care (including access to specialists), convenience (absence of travel), and comfort (including privacy) as benefits of telemedicine [73], suggesting that remote visits are qualitatively different and not necessarily inferior to in-person visits.
Broader adoption of telemedicine is also limited by regulatory and reimbursement barriers. Currently, most state licensing boards require that physicians be licensed in the state where the patient is physically located when services are provided [115]. Consequently, many patients often cannot access care from specialists simply because of where they live. The state of Delaware, for example, has no Parkinson disease specialists, leaving patients who desire such care having to drive hours to major urban centers (for example, Baltimore or Philadelphia). In addition, payers have been slow to reimburse for telemedicine. While an increasing number of states mandate that private insurers cover telemedicine to the extent they cover in-person care [116], many of these mandates do not extend to care in the home. In addition, Medicare does not cover care provided virtually in the home. In fact, Medicare pays more for care provided in high-cost, often patient-unfriendly, institutions (for example, hospitals) than it does for care in the community [117]. Where such licensing and reimbursement barriers do not exist (for example, Canada [118, 119], prisons [120–125], and the Department of Veterans Affairs [28, 126]) telemedicine in its various forms, including care into the home, has flourished.
The Connect.Parkinson study aims to contribute valuable information about the feasibility, effectiveness, and value of using technology to deliver care to patients with Parkinson disease directly in their home. The dissemination of the results, aided by the Dissemination and Implementation Advisory Board, will help break down many of the barriers to adoption of this care model and bring us closer to enabling anyone anywhere to receive care.
Trial status
Currently recruiting participants.
Electronic supplementary material
Additional file 1: Connect.Parkinson participating sites.(DOC 44 KB)
Additional file 2: Schedule of activities.(DOC 46 KB)
Below are the links to the authors’ original submitted files for images.
Acknowledgements
We would like to acknowledge the contributions of our Patient Advisory Board members, including SSR and RBS, and Dennis Leebel, Steven DeMello, and Dr. Paul Wicks for their work on the design of the study and contributions to recruitment planning and study start-up.
Funding
This research is supported by an award from the Patient-Centered Outcomes Research Institute (AD-12-11-4701), and the National Parkinson Foundation is collaborating in the conduct of the study. Software used in the study is provided and supported by SBR Health (Cambridge, MA, USA), Vidyo (Hackensack, NJ, USA), and ID Solutions (Indianapolis, IN, USA).
The project described in this publication was supported by the University of Rochester CTSA award number UL1 TR000042 from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authors’ sources of funding
Meredith A. Achey is an employee of the University of Rochester and receives a salary.
Christopher A. Beck has received research support from Amarin Corporation, Guidant Corporation, Boston Scientific, Lundbeck, Auspex Pharmaceuticals, Cure Huntington’s Disease Initiative Foundation, the National Parkinson Foundation, Patient-Centered Outcomes Research Institute, the Food and Drug Administration, and the National Institutes of Health.
Denise B. Beran is the Manager of Professional Programs for the National Parkinson Foundation and receives a salary.
Cynthia M. Boyd is an author for a chapter of UptoDate on multimorbidity for which she receives a royalty. She has received grant funding from Patient-Centered Outcomes Research Institute, National Institutes of Health, Agency for Healthcare Research and Quality, the John A. Hartford Foundation, the Center for Health Care Strategies, the Robert Wood Johnson Foundation, the Starr Foundation, the Commonwealth Foundation and the Langeloth Foundation.
Peter N. Schmidt is the Chief Information Officer and Vice President of Research Programs for the National Parkinson Foundation and receives a salary.
Allison W. Willis receives grant support from the National Institutes of Health (National Institute of Neurological Disorders and Stroke) to study the impact of comorbidities on specialty care utilization in Parkinson disease, and has lectured for the National Parkinson Foundation patient advocate training program (honorarium was declined). She has also received grant support from the St. Louis Chapter of the American Parkinson Disease Foundation, non-restricted funding from Teva Pharmaceuticals to study prescription utilization in Medicare beneficiaries with Parkinson disease.
Sara S. Riggare has received grant funding from the Swedish Governmental Agency for Innovation Systems, VINNOVA.
Richard B. Simone is a member of the Patient Advisory Board of the Connect.Parkinson study.
Kevin M. Biglan is a consultant to Lundbeck, UCB, and KJT consulting, has received grant support from the Michael J. Fox Foundation, National Institutes of Health, National Parkinson Foundation, Huntington’s Disease Society of America, Google, and Excellus BlueCross BlueShield, and has telemedicine contracts with the Presbyterian Home for Central New York and the Susquehanna Nursing Home and Rehabilitation Center.
E. Ray Dorsey has stock options in Grand Rounds, has filed a patent application related to telemedicine and neurology, is a consultant to Amgen, Avid Radiopharmaceuticals, Clintrex, Lundbeck, Medtronic, and Transparency Life Sciences, has provided medical malpractice services, is an unpaid advisor to SBR Health and Vidyo, has received honoraria for continuing medical education lectures, and has received grant support from the Agency for Healthcare Research and Quality, National Institutes of Health, Davis Phinney Foundation, Macklin Foundation, Michael J. Fox Foundation, National Parkinson Foundation, Verizon Foundation, Patient-Centered Outcomes Research Institute, Avid Radiopharmaceuticals, Biogen, Great Lakes NeuroTechnologies, Lundbeck, Medtronic, and Prana Biotechnology.
Abbreviations
- EQ-5D-5 L/EuroQoL-5D-5 L
European Quality of Life Five Dimension Five Level Scale
- GDS-15
15-item Geriatric Depression Scale
- MCSI
Multidimensional Caregiver Strain Index
- MDS-UPDRS
Movement Disorders Society Unified Parkinson Disease Rating Scale
- MoCA
Montreal Cognitive Assessment
- PACIC
Patient Assessment of Chronic Illness Care
- PDQ-39
39-item Parkinson’s Disease Questionnaire
- REDCap
Research Electronic Data Capture.
Footnotes
Competing interests
Meredith A. Achey is an employee of the University of Rochester and the study coordinator for the study. Christopher A. Beck has received research support from Amarin Corporation, Guidant Corporation, Boston Scientific, Lundbeck, Auspex Pharmaceuticals, Cure Huntington’s Disease Initiative Foundation, the National Parkinson Foundation, Patient-Centered Outcomes Research Institute, the Food and Drug Administration, and the National Institutes of Health. Denise B. Beran is the Manager of Professional Programs for the National Parkinson Foundation. Cynthia M. Boyd is an author for a chapter of UptoDate on multimorbidity for which she receives a royalty. She has received grant funding from Patient-Centered Outcomes Research Institute, National Institutes of Health, Agency for Healthcare Research and Quality, the John A. Hartford Foundation, the Center for Health Care Strategies, the Robert Wood Johnson Foundation, the Starr Foundation, the Commonwealth Foundation and the Langeloth Foundation. Peter N. Schmidt is the Chief Information Officer and Vice President of Research Programs for the National Parkinson Foundation. Allison W. Willis receives grant support from the National Institutes of Health (National Institute of Neurological Disorders and Stroke) to study the impact of comorbidities on specialty care utilization in Parkinson disease, and has lectured for the National Parkinson Foundation patient advocate training program (honorarium was declined). She has also received grant support from the St. Louis Chapter of the American Parkinson Disease Foundation, non-restricted funding from Teva Pharmaceuticals to study prescription utilization in Medicare beneficiaries with Parkinson disease. Sara S. Riggare is a member of the Patient Advisory Board of the Connect.Parkinson study. Richard B. Simone is a member of the Patient Advisory Board of the Connect.Parkinson study. Kevin Biglan has filed a patent application related to telemedicine and neurology, is a consultant to Lundbeck, UCB, and KJT consulting, has received grant support from the Michael J. Fox Foundation, National Institutes of Health, National Parkinson Foundation, Huntington’s Disease Society of America, Google, and Excellus BlueCross BlueShield, and has telemedicine contracts with the Presbyterian Home for Central New York and the Susquehanna Nursing Home and Rehabilitation Center. E. Ray Dorsey has stock options in Grand Rounds, has filed a patent application related to telemedicine and neurology, is a consultant to Amgen, Avid Radiopharmaceuticals, Clintrex, Lundbeck, Medtronic, and Transparency Life Sciences, has provided medical malpractice services, is an unpaid advisor to SBR Health and Vidyo, has received honoraria for continuing medical education lectures, and has received grant support from the Agency for Health Care Research and Quality, National Institutes of Health, Davis Phinney Foundation, Macklin Foundation, Michael J. Fox Foundation, National Parkinson Foundation, Verizon Foundation, Patient-Centered Outcomes Research Institute, Avid Radiopharmaceuticals, Biogen, Great Lakes NeuroTechnologies, Lundbeck, Medtronic, and Prana Biotechnology.
Authors’ contributions
MAA contributed to the development of the study protocol, developed the study databases, serves as the primary study coordinator and project manager, and drafted, edited, and critiqued the manuscript. ERD, KMB, PNS, CMB, CAB, and AWW serve on the study’s steering committee, conceived of and designed the study, contributed to the development of the protocol, provide continuing oversight for the study, and drafted, edited, and critiqued the manuscript. DBB works with the National Parkinson Foundation and has contributed to the design and conduct of the study and study recruitment, and drafted, edited, and critiqued the manuscript. CAB serves as the study’s biostatistician and developed the statistical analysis plan. SSR and RBS serve on the patient advisory board of the study, contributed to study design and planning, and drafted, edited, and critiqued the manuscript. All authors read and approved the final manuscript.
Contributor Information
Meredith A Achey, Email: meredith.achey@chet.rochester.edu.
Christopher A Beck, Email: beck@bst.rochester.edu.
Denise B Beran, Email: dberan@parkinson.org.
Cynthia M Boyd, Email: cyboyd@jhmi.edu.
Peter N Schmidt, Email: pschmidt@parkinson.org.
Allison W Willis, Email: Allison.Willis@uphs.upenn.edu.
Sara S Riggare, Email: sara@riggare.se.
Richard B Simone, Email: rbsimone@pacbell.net.
Kevin M Biglan, Email: kevin_biglan@urmc.rochester.edu.
E Ray Dorsey, Email: ray.dorsey@chet.rochester.edu.
References
- 1.Chronic care: making the case for ongoing care. [http://www.rwjf.org/en/research-publications/find-rwjf-research/2010/01/chronic-care.html]
- 2.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff. 2001;20:64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 3.Schaefer J, Davis C. Case management and the chronic care model: a multidisciplinary role. Lippincotts Case Manag. 2004;9:96–103. doi: 10.1097/00129234-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Boult C, Green AF, Boult LB, Pacala JT, Snyder C, Leff B. Successful models of comprehensive care for older adults with chronic conditions: evidence for the institute of medicine's “retooling for an aging America” report. J Am Geriatr Soc. 2009;57:2328–2337. doi: 10.1111/j.1532-5415.2009.02571.x. [DOI] [PubMed] [Google Scholar]
- 5.Dentzer S. Reform chronic illness care? Yes, we can. Health Aff. 2009;28:12–13. doi: 10.1377/hlthaff.28.1.12. [DOI] [PubMed] [Google Scholar]
- 6.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition—multimorbidity. JAMA. 2012;307:2493–2494. doi: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson W, Committee on Quality of Health Care in America . Crossing the quality chasm: a new health system for the 21st century. Washington, DC: The National Academies Press; 2001. [Google Scholar]
- 8.National Alliance for Caregiving in collaboration with AARP: Caregiving in the US 2009. [http://www.caregiving.org/data/Caregiving_in_the_US_2009_full_report.pdf]
- 9.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 10.Van der Marck MA, Bloem BR, Borm GF, Overeem S, Munneke M, Guttman M. Effectiveness of multidisciplinary care for Parkinson's disease: a randomized, controlled trial. Mov Disord. 2013;28:605–611. doi: 10.1002/mds.25194. [DOI] [PubMed] [Google Scholar]
- 11.Wade D, Gage H, Owen C, Trend P, Grossmith C, Kaye J. Multidisciplinary rehabilitation for people with Parkinson’s disease: a randomised controlled study. J Neurol Neurosurg Psychiatry. 2003;74:158–162. doi: 10.1136/jnnp.74.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willis AW, Schootman M, Tran R, Kung N, Evanoff BA, Perlmutter JS, Racette BA. Neurologist-associated reduction in PD-related hospitalizations and health care expenditures. Neurology. 2012;79:1774–1780. doi: 10.1212/WNL.0b013e3182703f92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boult C, Leff B, Boyd CM, Wolff JL, Marsteller JA, Frick KD, Wegener S, Reider L, Frey K, Mroz TM, Karm L, Scharfstein DO. A matched-pair cluster-randomized trial of guided care for high-risk older patients. J Gen Intern Med. 2013;28:612–621. doi: 10.1007/s11606-012-2287-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 15.Leff B, Burton JR. The future history of home care and physician house calls in the United States. J Gerontol A Biol Sci Med Sci. 2001;56:M603–M608. doi: 10.1093/gerona/56.10.m603. [DOI] [PubMed] [Google Scholar]
- 16.Stuck AE, Egger M, Hammer A, Minder CE, Beck JC. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022–1028. doi: 10.1001/jama.287.8.1022. [DOI] [PubMed] [Google Scholar]
- 17.Leff B, Burton L, Mader SL, Naughton B, Burl J, Inouye SK, Greenough WB, III, Guido S, Langston C, Frick KD, Steinwachs D, Burton JR. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely Ill older patients. Ann Intern Med. 2005;143:798–808. doi: 10.7326/0003-4819-143-11-200512060-00008. [DOI] [PubMed] [Google Scholar]
- 18.Landers SH. Why health care is going home. N Engl J Med. 2010;363:1690–1691. doi: 10.1056/NEJMp1000401. [DOI] [PubMed] [Google Scholar]
- 19.Meyer GS, Gibbons RV. House calls to the elderly–a vanishing practice among physicians. N Engl J Med. 1997;337:1815–1820. doi: 10.1056/NEJM199712183372507. [DOI] [PubMed] [Google Scholar]
- 20.Wright Willis A, Evanoff BA, Lian M, Criswell SR, Racette BA. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology. 2010;34:143–151. doi: 10.1159/000275491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology. 2011;77:851–857. doi: 10.1212/WNL.0b013e31822c9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorsey ER, George BP, Leff B, Willis AW. The coming crisis: obtaining care for the growing burden of neurodegenerative conditions. Neurology. 2013;80:1989–1996. doi: 10.1212/WNL.0b013e318293e2ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Federal Communications Commission: National Broadband Plan: Connecting America. [http://transition.fcc.gov/national-broadband-plan/national-broadband-plan-action-agenda.pdf] Accessed November 13, 2014
- 24.Demographics of internet users. [http://www.pewinternet.org/Trend-Data-(Adults)/Whos-Online.aspx]
- 25.Norris P. Digital Divide: Civic Engagement, Information Poverty, and the Internet Worldwide. Cambridge; New York: Cambridge University Press; 2001. [Google Scholar]
- 26.Duggan M. Cell Phone Activities 2013. Washington, DC: Pew Research Center; 2013. [Google Scholar]
- 27.Currell RUC, Wainwright P, Lewis R. Telemedicine versus face to face patient care: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2000;2:CD002098. doi: 10.1002/14651858.CD002098. [DOI] [PubMed] [Google Scholar]
- 28.Darkins A, Ryan P, Kobb R, Foster L, Edmonson E, Wakefield B, Lancaster AE. Care Coordination/Home Telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14:1118–1126. doi: 10.1089/tmj.2008.0021. [DOI] [PubMed] [Google Scholar]
- 29.McLean S, Chandler D, Nurmatov U, Liu J, Pagliari C, Car J, Sheikh A. Telehealthcare for asthma. Cochrane Database Syst Rev. 2010;10:CD007717. doi: 10.1002/14651858.CD007717.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shea S, Weinstock RS, Teresi JA, Palmas W, Starren J, Cimino JJ, Lai AM, Field L, Morin PC, Goland R, Izquierdo RE, Ebner S, Silver S, Petkova E, Kong J, Eimicke JP, IDEATel Consortium A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus: 5 year results of the IDEATel study. J Am Med Inform Assoc. 2009;16:446–456. doi: 10.1197/jamia.M3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verhoeven F, van Gemert-Pijnen L, Dijkstra K, Nijland N, Seydel E, Steehouder M. The contribution of teleconsultation and videoconferencing to diabetes care: a systematic literature review. J Med Internet Res. 2007;9:e37. doi: 10.2196/jmir.9.5.e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shulman RM, O'Gorman CS, Palmert MR. Int J Pediatr Endocrinol. 2010. The impact of telemedicine interventions involving routine transmission of blood glucose data with clinician feedback on metabolic control in youth with type 1 diabetes: a systematic review and meta-analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyne JJ, Vrijhoef HJ, Crijns HJ, De Weerd G, Kragten J, Gorgels AP. Tailored telemonitoring in patients with heart failure: results of a multicentre randomized controlled trial. Eur J Heart Fail. 2012;14:791–801. doi: 10.1093/eurjhf/hfs058. [DOI] [PubMed] [Google Scholar]
- 34.Clark RA, Inglis SC, McAlister FA, Cleland JG, Stewart S. Telemonitoring or structured telephone support programmes for patients with chronic heart failure: systematic review and meta-analysis. BMJ. 2007;334:942. doi: 10.1136/bmj.39156.536968.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarke M, Shah A, Sharma U. Systematic review of studies on telemonitoring of patients with congestive heart failure: a meta-analysis. J Telemed Telecare. 2011;17:7–14. doi: 10.1258/jtt.2010.100113. [DOI] [PubMed] [Google Scholar]
- 36.Dansky KH, Vasey J, Bowles K. Use of telehealth by older adults to manage heart failure. Res Gerontol Nurs. 2008;1:25–32. doi: 10.3928/19404921-20080101-01. [DOI] [PubMed] [Google Scholar]
- 37.Dansky KH, Vasey J, Bowles K. Impact of telehealth on clinical outcomes in patients with heart failure. Clin Nurs Res. 2008;17:182–199. doi: 10.1177/1054773808320837. [DOI] [PubMed] [Google Scholar]
- 38.De Lusignan S, Wells S, Johnson P, Meredith K, Leatham E. Compliance and effectiveness of 1 year's home telemonitoring: the report of a pilot study of patients with chronic heart failure. Eur J Heart Fail. 2001;3:723–730. doi: 10.1016/s1388-9842(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 39.Hebert PL, Sisk JE, Wang JJ, Tuzzio L, Casabianca JM, Chassin MR, Horowitz C, McLaughlin MA. Cost-effectiveness of nurse-led disease management for heart failure in an ethnically diverse urban community. Ann Intern Med. 2008;149:540–548. doi: 10.7326/0003-4819-149-8-200810210-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jerant AF, Azari R, Martinez C, Nesbitt TS. A randomized trial of telenursing to reduce hospitalization for heart failure: patient-centered outcomes and nursing indicators. Home Health Care Serv Q. 2003;22:1–20. doi: 10.1300/J027v22n01_01. [DOI] [PubMed] [Google Scholar]
- 41.Jerant AF, Azari R, Nesbitt TS. Reducing the cost of frequent hospital admissions for congestive heart failure: a randomized trial of a home telecare intervention. Med Care. 2001;39:1234–1245. doi: 10.1097/00005650-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Pekmezaris R, Mitzner I, Pecinka KR, Nouryan CN, Lesser ML, Siegel M, Swiderski JW, Moise G, Younker R, Sr, Smolich K. The impact of remote patient monitoring (telehealth) upon Medicare beneficiaries with heart failure. Telemed J E Health. 2012;18:101–108. doi: 10.1089/tmj.2011.0095. [DOI] [PubMed] [Google Scholar]
- 43.Wakefield BJ, Ward MM, Holman JE, Ray A, Scherubel M, Burns TL, Kienzle MG, Rosenthal GE. Evaluation of home telehealth following hospitalization for heart failure: a randomized trial. Telemed J E Health. 2008;14:753–761. doi: 10.1089/tmj.2007.0131. [DOI] [PubMed] [Google Scholar]
- 44.Wootton R. Twenty years of telemedicine in chronic disease management–an evidence synthesis. J Telemed Telecare. 2012;18:211–220. doi: 10.1258/jtt.2012.120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dorsey ER, Venkataraman V, Grana MJ, Bull MT, George BP, Boyd CM, Beck CA, Rajan B, Seidmann A, Biglan KM. Randomized controlled clinical trial of "virtual house calls" for Parkinson disease. JAMA Neurol. 2013;70:565–570. doi: 10.1001/jamaneurol.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fortney JC, Pyne JM, Mouden SB, Mittal D, Hudson TJ, Schroeder GW, Williams DK, Bynum CA, Mattox R, Rost KM. Practice-based versus telemedicine-based collaborative care for depression in rural federally qualified health centers: a pragmatic randomized comparative effectiveness trial. Am J Psychiatry. 2013;170:414–425. doi: 10.1176/appi.ajp.2012.12050696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCrossan B, Morgan G, Grant B, Sands AJ, Craig BG, Doherty NN, Agus AM, Crealey GE, Casey FA. A randomised trial of a remote home support programme for infants with major congenital heart disease. Heart. 2012;98:1523–1528. doi: 10.1136/heartjnl-2012-302350. [DOI] [PubMed] [Google Scholar]
- 48.Moreno FA, Chong J, Dumbauld J, Humke M, Byreddy S. Use of standard Webcam and Internet equipment for telepsychiatry treatment of depression among underserved Hispanics. Psychiatr Serv. 2012;63:1213–1217. doi: 10.1176/appi.ps.201100274. [DOI] [PubMed] [Google Scholar]
- 49.Leon A, Caceres C, Fernandez E, Chausa P, Martin M, Codina C, Rousaud A, Blanch J, Mallolas J, Martinez E, Blanco JL, Laguno M, Larrousse M, Milinkovic A, Zamora L, Canal N, Miró JM, Gatell JM, Gómez EJ, García F. A new multidisciplinary home care telemedicine system to monitor stable chronic human immunodeficiency virus-infected patients: a randomized study. PLoS One. 2011;6:e14515. doi: 10.1371/journal.pone.0014515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrer-Roca O, Garcia-Nogales A, Pelaez C. The impact of telemedicine on quality of life in rural areas: the Extremadura model of specialized care delivery. Telemed J E Health. 2010;16:233–243. doi: 10.1089/tmj.2009.0107. [DOI] [PubMed] [Google Scholar]
- 51.Stahl JE, Dixon RF. Acceptability and willingness to pay for primary care videoconferencing: a randomized controlled trial. J Telemed Telecare. 2010;16:147–151. doi: 10.1258/jtt.2009.090502. [DOI] [PubMed] [Google Scholar]
- 52.Dorsey ER, Deuel LM, Voss TS, Finnigan K, George BP, Eason S, Miller D, Reminick JI, Appler A, Polanowicz J, Viti L, Smith S, Joseph A, Biglan KM. Increasing access to specialty care: a pilot, randomized controlled trial of telemedicine for Parkinson's disease. Mov Disord. 2010;25:1652–1659. doi: 10.1002/mds.23145. [DOI] [PubMed] [Google Scholar]
- 53.Dixon RF, Stahl JE. A randomized trial of virtual visits in a general medicine practice. J Telemed Telecare. 2009;15:115–117. doi: 10.1258/jtt.2009.003003. [DOI] [PubMed] [Google Scholar]
- 54.Ahmed SN, Mann C, Sinclair DB, Heino A, Iskiw B, Quigley D, Ohinmaa A. Feasibility of epilepsy follow-up care through telemedicine: a pilot study on the patient's perspective. Epilepsia. 2008;49:573–585. doi: 10.1111/j.1528-1167.2007.01464.x. [DOI] [PubMed] [Google Scholar]
- 55.Morgan GJ, Craig B, Grant B, Sands A, Doherty N, Casey F. Home videoconferencing for patients with severe congential heart disease following discharge. Congenit Heart Dis. 2008;3:317–324. doi: 10.1111/j.1747-0803.2008.00205.x. [DOI] [PubMed] [Google Scholar]
- 56.O'Reilly R, Bishop J, Maddox K, Hutchinson L, Fisman M, Takhar J. Is telepsychiatry equivalent to face-to-face psychiatry? Results from a randomized controlled equivalence trial. Psychiatr Serv. 2007;58:836–843. doi: 10.1176/ps.2007.58.6.836. [DOI] [PubMed] [Google Scholar]
- 57.De Las CC, Arredondo MT, Cabrera MF, Sulzenbacher H, Meise U. Randomized clinical trial of telepsychiatry through videoconference versus face-to-face conventional psychiatric treatment. Telemed J E Health. 2006;12:341–350. doi: 10.1089/tmj.2006.12.341. [DOI] [PubMed] [Google Scholar]
- 58.Ruskin PE, Silver-Aylaian M, Kling MA, Reed SA, Bradham DD, Hebel JR, Barrett D, Knowles F, III, Hauser P. Treatment outcomes in depression: comparison of remote treatment through telepsychiatry to in-person treatment. Am J Psychiatry. 2004;161:1471–1476. doi: 10.1176/appi.ajp.161.8.1471. [DOI] [PubMed] [Google Scholar]
- 59.Bishop JE, O'Reilly RL, Maddox K, Hutchinson LJ. Client satisfaction in a feasibility study comparing face-to-face interviews with telepsychiatry. J Telemed Telecare. 2002;8:217–221. doi: 10.1258/135763302320272185. [DOI] [PubMed] [Google Scholar]
- 60.Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 61.Mitchell SL, Kiely DK, Kiel DP, Lipsitz LA. The epidemiology, clinical characteristics, and natural history of older nursing home residents with a diagnosis of Parkinson's disease. J Am Geriatr Soc. 1996;44:394–399. doi: 10.1111/j.1532-5415.1996.tb06408.x. [DOI] [PubMed] [Google Scholar]
- 62.Dubinsky RM, Gray C, Husted D, Busenbark K, Vetere-Overfield B, Wiltfong D, Parrish D, Koller WC. Driving in Parkinson's disease. Neurology. 1991;41:517–520. doi: 10.1212/wnl.41.4.517. [DOI] [PubMed] [Google Scholar]
- 63.Heikkila VM, Turkka J, Korpelainen J, Kallanranta T, Summala H. Decreased driving ability in people with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1998;64:325–330. doi: 10.1136/jnnp.64.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schrag A, Hovris A, Morley D, Quinn N, Jahanshahi M. Caregiver-burden in Parkinson's disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism Relat Disord. 2006;12:35–41. doi: 10.1016/j.parkreldis.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 65.Larsen JP. Parkinson's disease as community health problem: study in Norwegian nursing homes: the Norwegian Study Group of Parkinson's disease in the elderly. BMJ. 1991;303:741–743. doi: 10.1136/bmj.303.6805.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goetz CG, Stebbins GT. Risk factors for nursing home placement in advanced Parkinson's disease. Neurology. 1993;43:2227–2229. doi: 10.1212/wnl.43.11.2227. [DOI] [PubMed] [Google Scholar]
- 67.Factor SA, Feustel PJ, Friedman JH, Comella CL, Goetz CG, Kurlan R, Parsa M, Pfeiffer R. Longitudinal outcome of Parkinson's disease patients with psychosis. Neurology. 2003;60:1756–1761. doi: 10.1212/01.wnl.0000068010.82167.cf. [DOI] [PubMed] [Google Scholar]
- 68.Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson's disease: a population-based, prospective study. J Am Geriatr Soc. 2000;48:938–942. doi: 10.1111/j.1532-5415.2000.tb06891.x. [DOI] [PubMed] [Google Scholar]
- 69.Noyes K, Liu H, Li Y, Holloway R, Dick AW. Economic burden associated with Parkinson's disease on elderly Medicare beneficiaries. Mov Disord. 2006;21:362–372. doi: 10.1002/mds.20727. [DOI] [PubMed] [Google Scholar]
- 70.Cheng EM, Swarztrauber K, Siderowf AD, Eisa MS, Lee M, Vassar S, Jacob E, Vickrey BG. Association of specialist involvement and quality of care for Parkinson's disease. Mov Disord. 2007;22:515–522. doi: 10.1002/mds.21311. [DOI] [PubMed] [Google Scholar]
- 71.Hubble JP, Pahwa R, Michalek DK, Thomas C, Koller WC. Interactive video conferencing: a means of providing interim care to Parkinson's disease patients. Mov Disord. 1993;8:380–382. doi: 10.1002/mds.870080326. [DOI] [PubMed] [Google Scholar]
- 72.Achey M, Aldred JL, Aljehani N, Bloem BR, Biglan KM, Chan P, Cubo E, Ray Dorsey E, Goetz CG, Guttman M, Hassan A, Khandhar SM, Mari Z, Spindler M, Tanner CM, Van den Haak P, Walker R, Wilkinson JR, on behalf of the International Parkinson and Movement Disorder Society Telemedicine Task Force The past, present, and future of telemedicine for Parkinson's disease. Mov Disord. 2014;29:871–883. doi: 10.1002/mds.25903. [DOI] [PubMed] [Google Scholar]
- 73.Venkataraman V, Donohue SJ, Biglan KM, Wicks P, Dorsey ER. Virtual visits for Parkinson disease: a case series. Neurol: Clin Pract. 2014;4:146–152. doi: 10.1212/01.CPJ.0000437937.63347.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Constantinescu G, Theodoros D, Russell T, Ward E, Wilson S, Wootton R. Treating disordered speech and voice in Parkinson's disease online: a randomized controlled non-inferiority trial. Int J Lang Commun Disord. 2011;46:1–16. doi: 10.3109/13682822.2010.484848. [DOI] [PubMed] [Google Scholar]
- 75.Constantinescu GA, Theodoros DG, Russell TG, Ward EC, Wilson SJ, Wootton R. Home-based speech treatment for Parkinson's disease delivered remotely: a case report. J Telemed Telecare. 2010;16:100–104. doi: 10.1258/jtt.2009.090306. [DOI] [PubMed] [Google Scholar]
- 76.Delprato U, Greenlaw R, Cristaldi M. PARKSERVICE: Home support and walking aid for people with Parkinson's disease. Stud Health Technol Inform. 2006;121:1–6. [PubMed] [Google Scholar]
- 77.Howell S, Tripoliti E, Pring T. Delivering the Lee Silverman Voice Treatment (LSVT) by web camera: a feasibility study. Int J Lang Commun Disord. 2009;44:287–300. doi: 10.1080/13682820802033968. [DOI] [PubMed] [Google Scholar]
- 78.Marzinzik F, Wahl M, Doletschek CM, Jugel C, Rewitzer C, Klostermann F. Evaluation of a telemedical care programme for patients with Parkinson's disease. J Telemed Telecare. 2012;18:322–327. doi: 10.1258/jtt.2012.120105. [DOI] [PubMed] [Google Scholar]
- 79.Tindall LR, Huebner RA, Stemple JC, Kleinert HL. Videophone-delivered voice therapy: a comparative analysis of outcomes to traditional delivery for adults with Parkinson's disease. Telemed J E Health. 2008;14:1070–1077. doi: 10.1089/tmj.2008.0040. [DOI] [PubMed] [Google Scholar]
- 80.Achey MA, Beck CA, Biglan KM, Boyd C, Dorsey ER, Schmidt P, Willis AW. Design of a randomized, controlled trial of virtual care visits for Parkinson's disease: the Connect.Parkinson's study. Mov Disord. 2014;Suppl 1:607. [Google Scholar]
- 81.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, Van Hilten JJ, LaPelle N, Movement Disorder Society UPDRS Revision Task Force Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 83.Abdolahi A, Scoglio N, Killoran A, Dorsey ER, Biglan KM. Potential reliability and validity of a modified version of the unified Parkinson's disease rating scale that could be administered remotely. Parkinsonism Relat Disord. 2013;19:218–221. doi: 10.1016/j.parkreldis.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 85.Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal Cognitive Assessment as a screening tool for cognitive impairment in Parkinson's disease. Mov Disord. 2008;23:1043–1046. doi: 10.1002/mds.22017. [DOI] [PubMed] [Google Scholar]
- 86.Peto V, Jenkinson C, Fitzpatrick R. PDQ-39: a review of the development, validation and application of a Parkinson's disease quality of life questionnaire and its associated measures. J Neurol. 1998;Suppl 1:S10–S14. doi: 10.1007/pl00007730. [DOI] [PubMed] [Google Scholar]
- 87.Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the patient assessment of chronic illness care (PACIC) Med Care. 2005;43:436–444. doi: 10.1097/01.mlr.0000160375.47920.8c. [DOI] [PubMed] [Google Scholar]
- 88.Stull D. The Multidimensional Caregiver Strain Index (MCSI): its measurement and structure. J Clin Geropsychol. 1996;2:175–196. [Google Scholar]
- 89.Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2010;289:18–22. doi: 10.1016/j.jns.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 90.Thompson AW, Liu H, Hays RD, Katon WJ, Rausch R, Diaz N, Jacob EL, Vassar SD, Vickrey BG. Diagnostic accuracy and agreement across three depression assessment measures for Parkinson's disease. Parkinsonism Relat Disord. 2011;17:40–45. doi: 10.1016/j.parkreldis.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Latoo J, Mistry M, Dunne FJ. Depression in Parkinson's disease: diagnosis and management. Br J Hosp Med (Lond) 2012;73:331–334. doi: 10.12968/hmed.2012.73.6.331. [DOI] [PubMed] [Google Scholar]
- 92.Weerkamp NJ, Tissingh G, Poels PJ, Zuidema SU, Munneke M, Koopmans RT, Bloem BR. Nonmotor symptoms in nursing home residents with Parkinson's disease: prevalence and effect on quality of life. J Am Geriatr Soc. 2013;61:1714–1721. doi: 10.1111/jgs.12458. [DOI] [PubMed] [Google Scholar]
- 93.Brink TLYJ, Lum O, Heersema P, Adey MB, Rose TL. Screening tests for geriatric depression. Clin Gerontol. 1982;1:37–44. [Google Scholar]
- 94.Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther. 2004;27:26–35. doi: 10.1016/j.jmpt.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 95.Herdman M, Gudex C, Lloyd A, Janssen MF, Kind P, Parkin D, Bonsel G, Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5 L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mauldin PD, Guimaraes P, Albin RL, Ray Dorsey E, Bainbridge JL, Siderowf A. Optimal frequency for measuring health care resource utilization in Parkinson's disease using participant recall: The FS-TOO resource utilization substudy. Clin Ther. 2008;30:1553–1557. doi: 10.1016/j.clinthera.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Topol E. The Creative Destruction of Medicine: How the Digital Revolution will Create Better Health Care. New York, NY: Basic Books; 2012. [Google Scholar]
- 98.Clayton M, Christensen JHG, Hwang J. The Innovator's Prescription: a Disruptive Solution for Health. 1. New York, NY: McGraw-Hill; 2008. [Google Scholar]
- 99.Dhruva SS, Redberg RF. Variations between clinical trial participants and medicare beneficiaries in evidence used for medicare national coverage decisions. Arch Intern Med. 2008;168:136–140. doi: 10.1001/archinternmed.2007.56. [DOI] [PubMed] [Google Scholar]
- 100.Bloge SC, Mills DL, Princeton NJ, Consumer Health Sciences . A comparison of clinical trial participants to the general patient population [poster] New York, NY: Kantar Health; 2006. [Google Scholar]
- 101.Pew Research Center . Internet use over time. 2014. [Google Scholar]
- 102.Broadband technology fact sheet. [http://www.pewinternet.org/fact-sheets/broadband-technology-fact-sheet/]
- 103.Fox S, Duggan M. The diagnosis difference. 2013. [Google Scholar]
- 104.Smith A. Older adults and technology use. 2014. [Google Scholar]
- 105.Rainie L, Zickuhr K. Video calling and video chat. 2010. [Google Scholar]
- 106.Eilenberg KL, Hoover AM, Rutherford ML, Melfi CA, Segal S. From informed consent through database lock: an interactive clinical trial conducted using the internet. Drug Inf J. 2004;38:239–251. [Google Scholar]
- 107.Jacobs BP, Bent S, Tice JA, Blackwell T, Cummings SR. An internet-based randomized, placebo-controlled trial of kava and valerian for anxiety and insomnia. Medicine. 2005;84:197–207. doi: 10.1097/01.md.0000172299.72364.95. [DOI] [PubMed] [Google Scholar]
- 108.Orri M, Lipset CH, Jacobs BP, Costello AJ, Cummings SR. Web-based trial to evaluate the efficacy and safety of tolterodine ER 4 mg in participants with overactive bladder: REMOTE trial. Contemp Clin Trials. 2014;38:190–197. doi: 10.1016/j.cct.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 109.Dobscha SK, Corson K, Solodky J, Gerrity MS. Use of videoconferencing for depression research: enrollment, retention, and patient satisfaction. Telemed J E Health. 2005;11:84–89. doi: 10.1089/tmj.2005.11.84. [DOI] [PubMed] [Google Scholar]
- 110.Goetz CG, Stebbins GT, Wolff D, DeLeeuw W, Bronte-Stewart H, Elble R, Hallett M, Nutt J, Ramig L, Sanger T, Wu AD, Kraus PH, Blasucci LM, Shamim EA, Sethi KD, Spielman J, Kubota K, Grove AS, Dishman E, Taylor CB. Testing objective measures of motor impairment in early Parkinson's disease: feasibility study of an at-home testing device. Mov Disord. 2009;24:551–556. doi: 10.1002/mds.22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cubo E, Trejo Gabriel-Galán JM, Seco Martínez J, Rioja Alcubilla C, Yang C, Fernández Arconada O, Mariscal Pérez N. Comparison of office-based versus home web-based clinical assessments for Parkinson's disease. Mov Disord. 2012;27:308–311. doi: 10.1002/mds.24028. [DOI] [PubMed] [Google Scholar]
- 112.Grana MJ, Bull MT, Venkataraman V, Dorsey ER, Biglan KM. Web-based clinical assessments for Parkinson's disease: reliable and feasible. Mov Disord. 2012;27:1466. doi: 10.1002/mds.25121. [DOI] [PubMed] [Google Scholar]
- 113.Parkinson J. An Essay on the Shaking Palsy. London: Sherwood, Neely, and Jones; 1817. [Google Scholar]
- 114.Samii A, Ryan-Dykes P, Tsukuda RA, Zink C, Franks R, Nichol WP. Telemedicine for delivery of health care in Parkinson's disease. J Telemed Telecare. 2006;12:16–18. doi: 10.1258/135763306775321371. [DOI] [PubMed] [Google Scholar]
- 115.American Telemedicine Association . Medical licensure and practice requirements. 2011. [Google Scholar]
- 116.State Coverage for Telehealth Services: National Conference of State Legislatures. [http://www.ncsl.org/research/health/state-coverage-for-telehealth-services.aspx]
- 117.Centers for Medicare and Medicaid Services . Medicare Benefit Policy Manual. 2013. Chapter 15 - covered medical and other health services. [Google Scholar]
- 118.Pearl R. Kaiser Permanente Northern California: current experiences with internet, mobile, and video technologies. Health Aff. 2014;33:251–257. doi: 10.1377/hlthaff.2013.1005. [DOI] [PubMed] [Google Scholar]
- 119.Brown EM. The Ontario telemedicine network: a case report. Telemed J E Health. 2013;19:373–376. doi: 10.1089/tmj.2012.0299. [DOI] [PubMed] [Google Scholar]
- 120.Antonacci DJ, Bloch RM, Saeed SA, Yildirim Y, Talley J. Empirical evidence on the use and effectiveness of telepsychiatry via videoconferencing: implications for forensic and correctional psychiatry. Behav Sci Law. 2008;26:253–269. doi: 10.1002/bsl.812. [DOI] [PubMed] [Google Scholar]
- 121.Lavrentyev V, Seay A, Rafiq A, Justis D, Merrell RC. A surgical telemedicine clinic in a correctional setting. Telemed J E Health. 2008;14:385–388. doi: 10.1089/tmj.2007.0061. [DOI] [PubMed] [Google Scholar]
- 122.Ellis DG, Mayrose J, Jehle DV, Moscati RM, Pierluisi GJ. A telemedicine model for emergency care in a short-term correctional facility. Telemed J E Health. 2001;7:87–92. doi: 10.1089/153056201750279584. [DOI] [PubMed] [Google Scholar]
- 123.Brecht RM, Gray CL, Peterson C, Youngblood B. The University of Texas Medical Branch-Texas Department of Criminal Justice Telemedicine Project: findings from the first year of operation. Telemed J. 1996;2:25–35. doi: 10.1089/tmj.1.1996.2.25. [DOI] [PubMed] [Google Scholar]
- 124.Zaylor C, Nelson EL, Cook DJ. Clinical outcomes in a prison telepsychiatry clinic. J Telemed Telecare. 2001;Suppl 1:47–49. doi: 10.1177/1357633X010070S119. [DOI] [PubMed] [Google Scholar]
- 125.Patel MC, Young JD. Delivering HIV subspecialty care in prisons utilizing telemedicine. Dis Mon. 2014;60:196–200. doi: 10.1016/j.disamonth.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 126.Darkins A. Changing the location of care: management of patients with chronic conditions in Veterans Health Administration using care coordination/home telehealth. J Rehabil Res Dev. 2006;43:vii–xii. doi: 10.1682/jrrd.2006.03.0029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Connect.Parkinson participating sites.(DOC 44 KB)
Additional file 2: Schedule of activities.(DOC 46 KB)


