Abstract
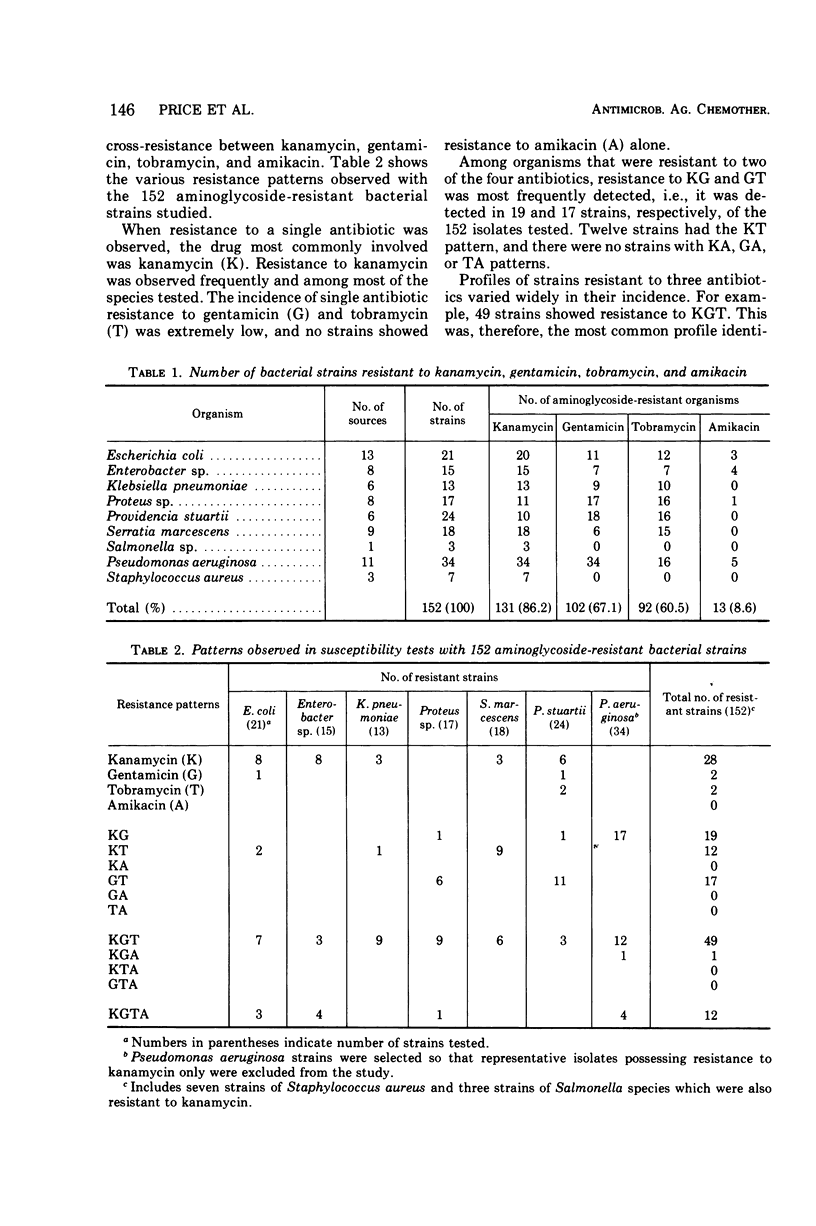
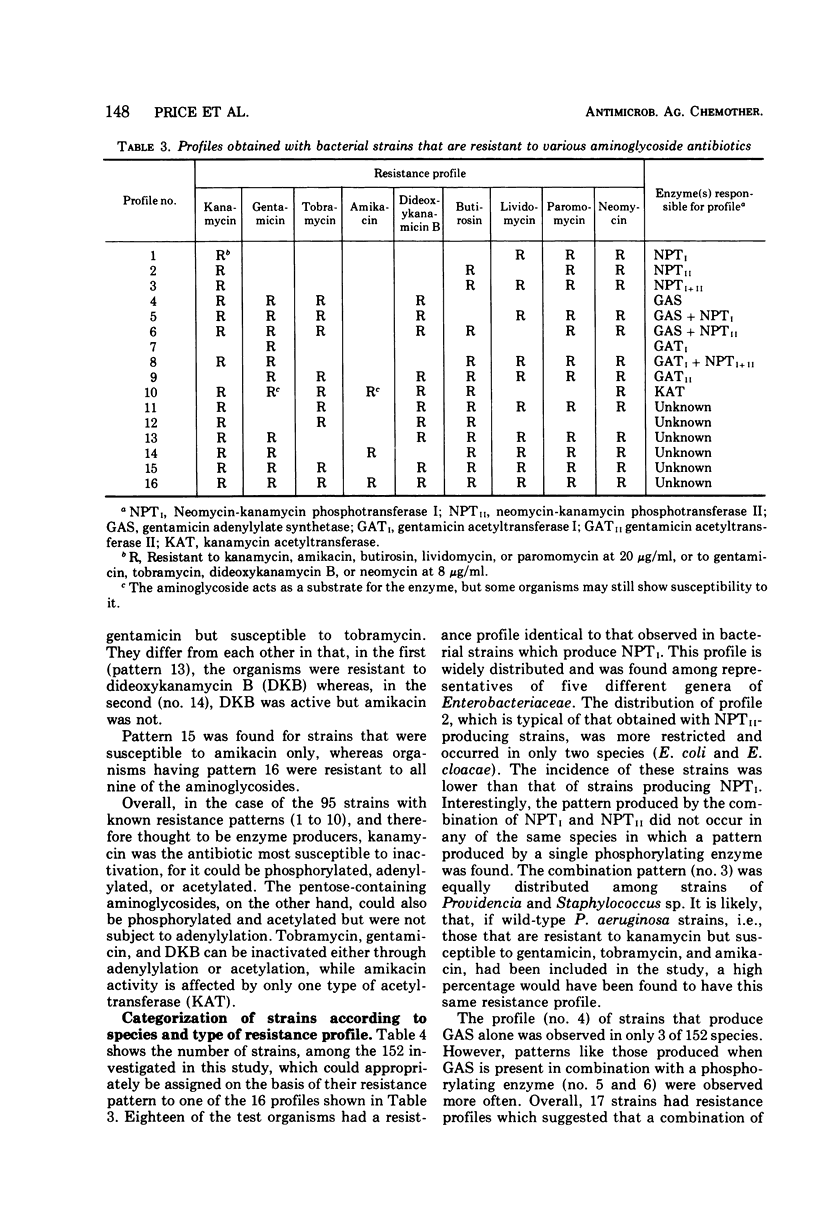
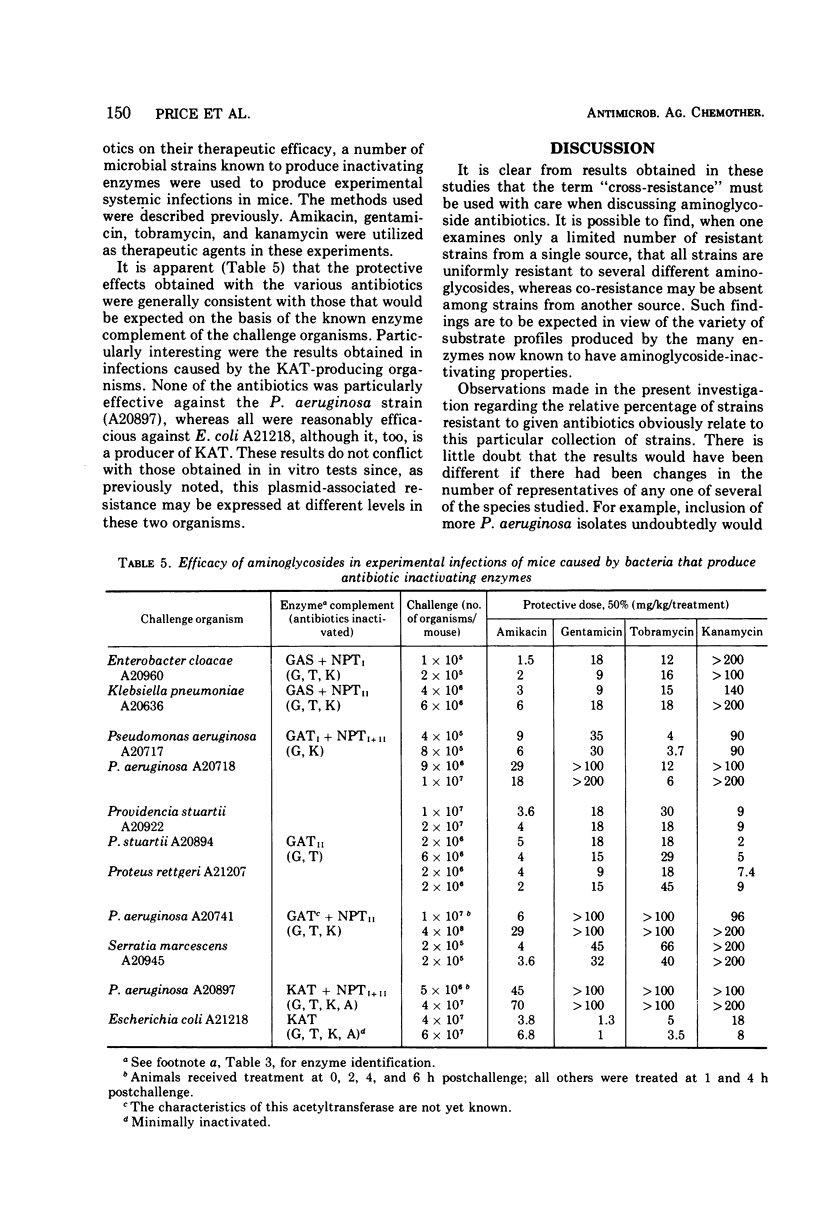
One hundred fifty-two bacterial strains that possess resistance to kanamycin A, gentamicin, or tobramycin, or to more than one of these antibiotics, were collected from various sources in Canada, Europe, Japan, and the United States. This collection was composed of Staphylococcus aureus and Pseudomonas aeruginosa and members of the Enterobacteriaceae family. Their susceptibility to BB-K8 (amikacin), a new broad-spectrum semisynthetic derivative of kanamycin A, and to the other agents, was determined on Mueller-Hinton Medium by the twofold agar dilution method. Test results revealed that 60.5% of the isolates were resistant to 8 μg of tobramycin per ml, 67.1% to 8 μg of gentamicin per ml, 86.2% to 20 μg of kanamycin A per ml, and only 8.6% to 20 μg of amikacin per ml. Of interest is the fact that the amikacin-resistant strains were generally resistant to all of the other aminoglycosides. The broad spectrum of amikacin was not totally unexpected, because the compound has been shown to be a poor substrate for most enzymes that inactivate other aminoglycosides through O-phosphorylation, O-adenylylation, or N-acetylation. A number of susceptibility profiles were obtained when the organisms were tested against a series of nine aminoglycosides. The majority of these profiles resembled those found for organisms that possess known mechanisms of enzymatic inactivation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK J., CALESNICK B., WILLIAMS D., WEINSTEIN M. J. PHARMACOLOGY OF GENTAMICIN, A NEW BROAD-SPECTRUM ANTIBIOTIC. Antimicrob Agents Chemother (Bethesda) 1963;161:138–147. [PubMed] [Google Scholar]
- Bakker A. J., Michel M. F. In vitro activity of gentamicin against common pathogenic bacteria. Chemotherapy. 1970 Mar;15(3):129–136. doi: 10.1159/000220676. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Davies J. R-factor mediated gentamicin resistance: A new enzyme which modifies aminoglycoside antibiotics. FEBS Lett. 1971 May 20;14(5):293–296. doi: 10.1016/0014-5793(71)80282-x. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Yamada T., Davies J. Enzymatic Adenylylation of Streptomycin and Spectinomycin by R-Factor-Resistant Escherichia coli. Infect Immun. 1970 Jan;1(1):109–119. doi: 10.1128/iai.1.1.109-119.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P., Stewart D. In vitro studies of BB-K8, a new aminoglycoside antibiotic. Antimicrob Agents Chemother. 1973 Aug;4(2):186–192. doi: 10.1128/aac.4.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P., Stewart D. In vitro studies of tobramycin. Antimicrob Agents Chemother. 1972 Sep;2(3):109–113. doi: 10.1128/aac.2.3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusch J. L., Barza M., Bergeron M. G., Weinstein L. Cross-resistance of Pseudomonas to gentamicin and tobramycin. Antimicrob Agents Chemother. 1972 Mar;1(3):280–281. doi: 10.1128/aac.1.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinska M., Benveniste R., Davies J., Daniels P. J., Weinstein J. Gentamicin resistance in strains of Pseudomonas aeruginosa mediated by enzymatic N-acetylation of the deoxystreptamine moiety. Biochemistry. 1972 Feb 29;11(5):761–765. doi: 10.1021/bi00755a013. [DOI] [PubMed] [Google Scholar]
- Brzezinska M., Davies J. Two enzymes which phosphorylate neomycin and kanamycin in Escherichia coli strains carrying R factors. Antimicrob Agents Chemother. 1973 Feb;3(2):266–269. doi: 10.1128/aac.3.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger L. M., Sanford J. P., Zweighaft T. Tobramycin: bacteriological evaluation. Am J Med Sci. 1973 Feb;265(2):135–142. [PubMed] [Google Scholar]
- Cabana B. E., Taggart J. G. Comparative pharmacokinetics of BB-K8 and kanamycin in dogs and humans. Antimicrob Agents Chemother. 1973 Apr;3(4):478–483. doi: 10.1128/aac.3.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe C. C., Sanders E. Is there complete cross-resistance of gram-negative bacilli to gentamicin and tobramycin? Antimicrob Agents Chemother. 1972 Nov;2(5):415–416. doi: 10.1128/aac.2.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curreri P. W., Bruck H. M., Lindberg R. B., Mason A. D., Jr, Pruitt B. A., Jr Providencia stuartii sepsis: a new challenge in the treatment of thermal injury. Ann Surg. 1973 Feb;177(2):133–138. doi: 10.1097/00000658-197302000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene V. E., Farrar W. E., Jr Tobramycin: in vitro activity and comparison with kanamycin and gentamicin. Antimicrob Agents Chemother. 1972 Apr;1(4):340–342. doi: 10.1128/aac.1.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion H. W., Woo P. W., Willmer N. E., Kern D. L., Onaga J., Fusari S. A. Butirosin, a new aminoglycosidic antibiotic complex: isolation and characterization. Antimicrob Agents Chemother. 1972 Aug;2(2):84–88. doi: 10.1128/aac.2.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi O., Ogura M., Tanaka N., Umezawa H. Inactivation of kanamycin, neomycin, and streptomycin by enzymes obtained in cells of Pseudomonas aeruginoa. Appl Microbiol. 1968 Sep;16(9):1276–1281. doi: 10.1128/am.16.9.1276-1281.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karney W., Holmes K. K., Turck M. Comparison of five aminocyclitol antibiotics in vitro against Enterobacteriaceae and Pseudomonas. Antimicrob Agents Chemother. 1973 Mar;3(3):338–342. doi: 10.1128/aac.3.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi H., Naito T., Nakagawa S., Fujisawa K. I. BB-K 8, a new semisynthetic aminoglycoside antibiotic. J Antibiot (Tokyo) 1972 Dec;25(12):695–708. doi: 10.7164/antibiotics.25.695. [DOI] [PubMed] [Google Scholar]
- Kirby W. M., Standiford H. C. Gentamicin: in vitro studies. J Infect Dis. 1969 Apr-May;119(4):361–363. doi: 10.1093/infdis/119.4-5.361. [DOI] [PubMed] [Google Scholar]
- Klastersky J., Daneau D., de Maertelaer V. Comparative study of tobramycin and gentamicin with special reference to anti-pseudomonas activity. Clin Pharmacol Ther. 1973 Jan-Feb;14(1):104–111. doi: 10.1002/cpt1973141104. [DOI] [PubMed] [Google Scholar]
- Kondo S., Okanishi M., Utahara R., Maeda K., Umezawa H. Isolation of kanamycin and paromamine inactivated by E. coli carrying R factor. J Antibiot (Tokyo) 1968 Jan;21(1):22–29. doi: 10.7164/antibiotics.21.22. [DOI] [PubMed] [Google Scholar]
- Kondo S., Yamamoto H., Naganawa H., Umezawa H. Isolation and characterization of lividomycin A inactivated by Pseudomonas aeruginosa and Escherichia coli carrying R factor. J Antibiot (Tokyo) 1972 Aug;25(8):483–484. doi: 10.7164/antibiotics.25.483. [DOI] [PubMed] [Google Scholar]
- Kunin C. M. Absorption, distribution, excretion and fate of kanamycin. Ann N Y Acad Sci. 1966 Jun 14;132(2):811–818. doi: 10.1111/j.1749-6632.1966.tb42999.x. [DOI] [PubMed] [Google Scholar]
- Lockwood W. R., Bower J. D. Tobramycin and gentamicin concentrations in the serum of normal and anephric patients. Antimicrob Agents Chemother. 1973 Jan;3(1):125–129. doi: 10.1128/aac.3.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers B. R., Hirschman S. Z. Pharmacologic studies on tobramycin and comparison with gentamicin. J Clin Pharmacol New Drugs. 1972 Aug-Sep;12(8):321–324. doi: 10.1002/j.1552-4604.1972.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Mori T., Kyotani Y., Watanabe I., Oda T. The structure of mannosylparomomycin (No. 2230-C). J Antibiot (Tokyo) 1972 May;25(5):317–319. doi: 10.7164/antibiotics.25.317. [DOI] [PubMed] [Google Scholar]
- Okanishi M., Kondo S., Suzuki Y., Okamoto S., Umezawa H. Studies on inactivation of kanamycin and resistances of E. coli. J Antibiot (Tokyo) 1967 Jul;20(3):132–135. [PubMed] [Google Scholar]
- Preston D. A., Wick W. E. Preclinical assessment of the antibacterial activity of nebramycin factor 6. Antimicrob Agents Chemother (Bethesda) 1970;10:322–327. [PubMed] [Google Scholar]
- Price K. E., Chisholm D. R., Misiek M., Leitner F., Tsai Y. H. Microbiological evaluation of BB-K 8, a new semisynthetic aminoglycoside. J Antibiot (Tokyo) 1972 Dec;25(12):709–731. doi: 10.7164/antibiotics.25.709. [DOI] [PubMed] [Google Scholar]
- Snelling C. F., Ronald A. R., Cates C. Y., Forsythe W. C. Resistance of gram-negative bacilli to gentamicin. J Infect Dis. 1971 Dec;124 (Suppl):S264–S270. doi: 10.1093/infdis/124.supplement_1.s264. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Yamamoto H., Yagisawa M., Kondo S., Takeuchi T. Letter: Kanamycin phosphotransferase. I. Mechanism of cross resistance between kanamycin and lividomycin. J Antibiot (Tokyo) 1973 Jul;26(7):407–411. doi: 10.7164/antibiotics.26.407. [DOI] [PubMed] [Google Scholar]
- Witchitz J. L., Chabbert Y. A. Résistance transférable à la gentamicine. II. Transmission et liaisons du caractère de résistance. Ann Inst Pasteur (Paris) 1972 Mar;122(3):367–378. [PubMed] [Google Scholar]
- Witchitz J. L. Plasmid-mediated gentamicin resistance not associated with kanamycin resistance in Enterobacteriaceae. J Antibiot (Tokyo) 1972 Oct;25(10):622–624. doi: 10.7164/antibiotics.25.622. [DOI] [PubMed] [Google Scholar]
- Yagisawa M., Naganawa H., Kondo S., Hamada M., Takeuchi T. Adenylyldideoxykanamycin B, a product of the inactivation of dideoxykanamycin B by Escherichia coli carrying R factor. J Antibiot (Tokyo) 1971 Dec;24(12):911–912. doi: 10.7164/antibiotics.24.911. [DOI] [PubMed] [Google Scholar]
- Yagisawa M., Naganawa H., Kondo S., Takeuchi T., Umezawa H. 6'-N-acetylation of 3',4'-dideoxykanamycin B by an enzyme in a resistant strain of Pseudomonas aeruginosa. J Antibiot (Tokyo) 1972 Aug;25(8):495–496. doi: 10.7164/antibiotics.25.495. [DOI] [PubMed] [Google Scholar]
- Yagisawa M., Yamamoto H., Naganawa H., Kondo S., Takeuchi T. A new enzyme in Escherichia coli carrying R-factor phosphorylating 3'-hydroxyl of butirosin A, kanamycin, neamine and ribostamycin. J Antibiot (Tokyo) 1972 Dec;25(12):748–750. doi: 10.7164/antibiotics.25.748. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Yagisawa M., Naganawa H., Kondo S., Takeuchi T. Kanamycin 6'-acetate and ribostamycin 6'-acetate, enzymatically inactivated products by Pseudomonas aeruginosa. J Antibiot (Tokyo) 1972 Dec;25(12):746–747. doi: 10.7164/antibiotics.25.746. [DOI] [PubMed] [Google Scholar]
- Yu P. K., Washington J. A., 2nd Comparative in vitro activity of three aminoglycosidic antibiotics: BB-K8, kanamycin, and gentamicin. Antimicrob Agents Chemother. 1973 Aug;4(2):133–139. doi: 10.1128/aac.4.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]



