Abstract
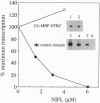
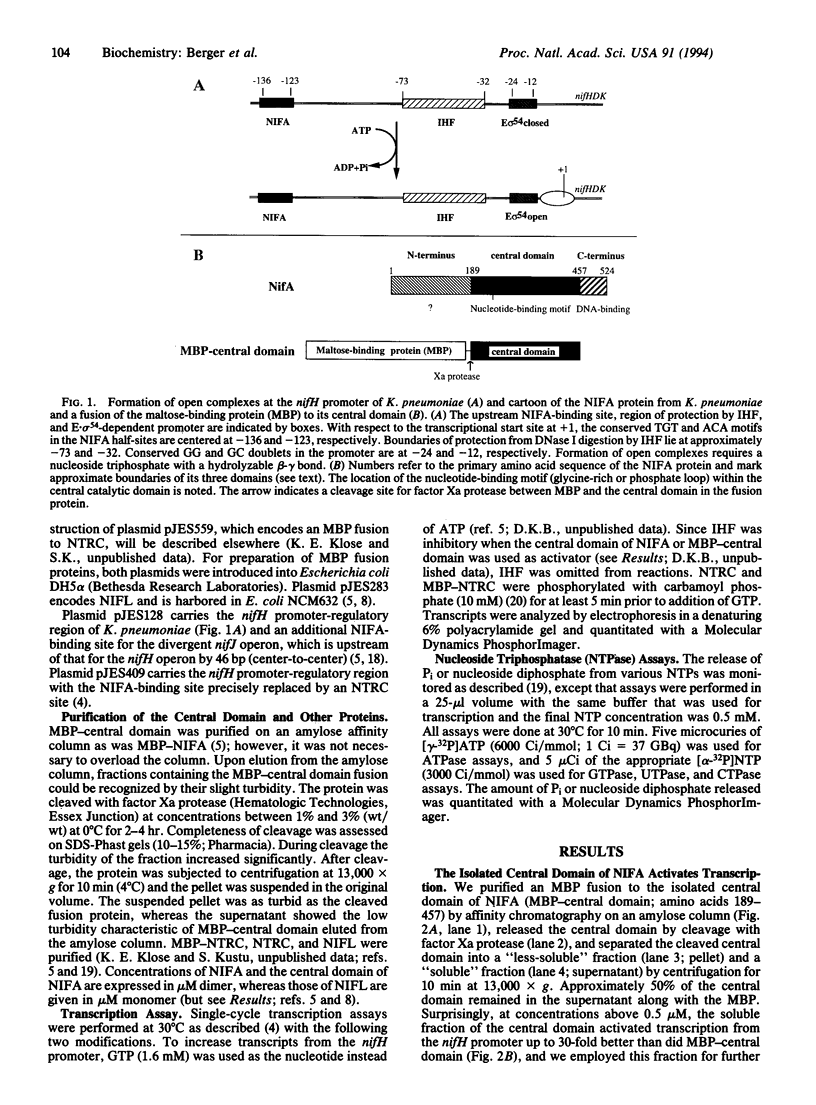
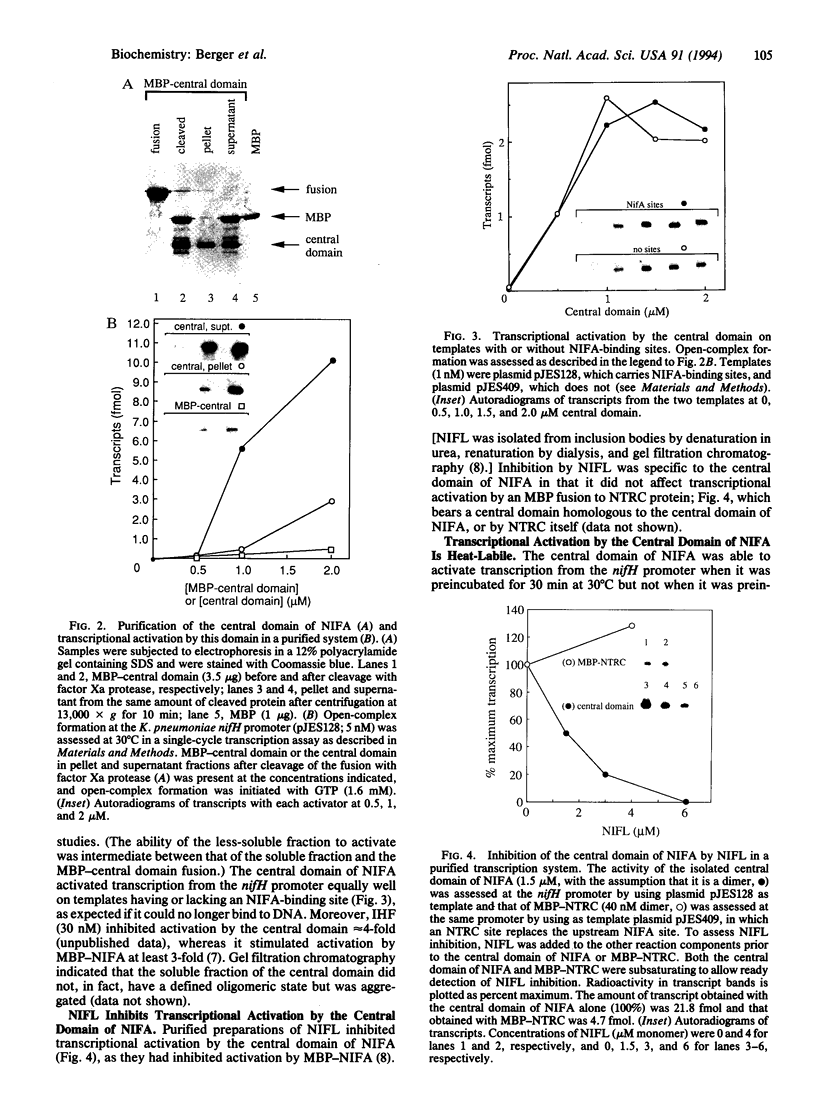
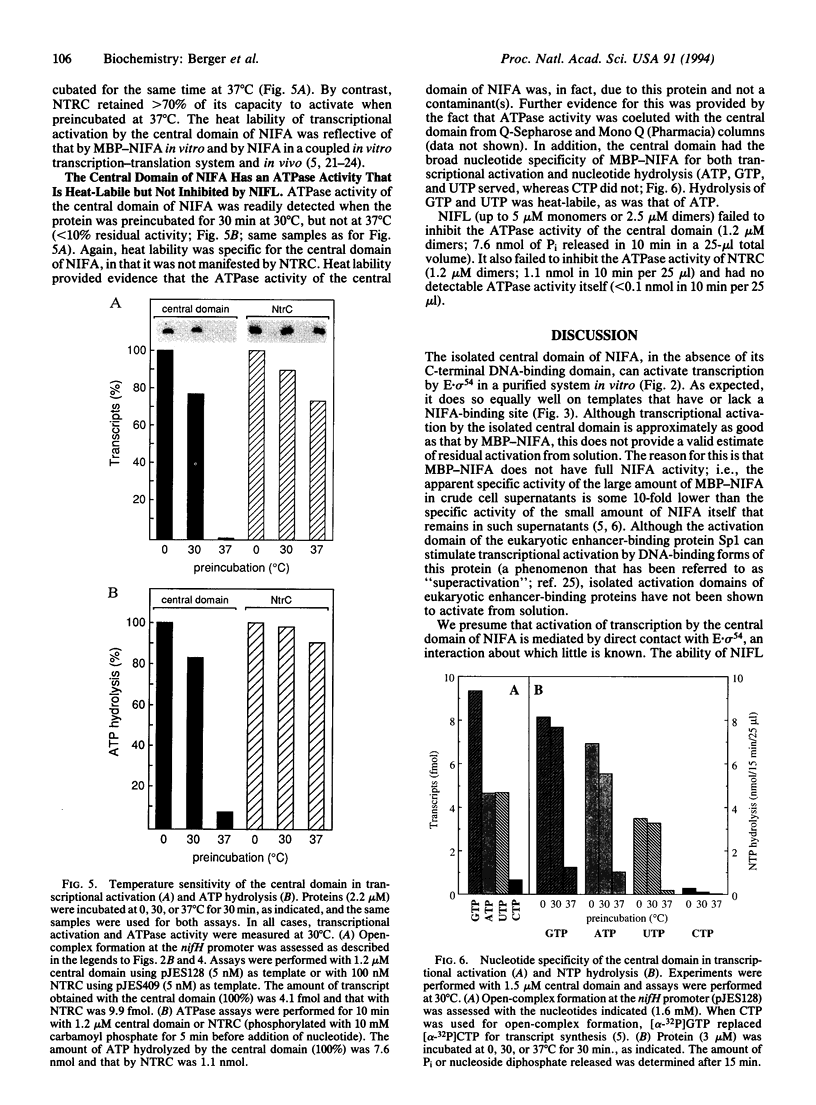
The NIFA protein of Klebsiella pneumoniae is required for transcription of all nif (nitrogen fixation) operons except the regulatory nifLA operon itself. NIFA activates transcription of nif operons by the alternative holoenzyme form of RNA polymerase, sigma 54-holoenzyme, in a nucleoside triphosphate (NTP)-dependent manner. NIFL antagonizes the action of NIFA in the presence of molecular oxygen or combined nitrogen. The NIFA protein of K. pneumoniae is composed of three domains: an N-terminal domain with unclear function, a central catalytic domain, and a C-terminal DNA-binding domain. We report that the isolated central domain of NIFA activates transcription in vitro and that this activation requires NTP with a hydrolyzable beta-gamma bond, as does activation by intact NIFA. Transcriptional activation by the isolated central domain has the heat lability characteristic of intact NIFA and is inhibited by NIFL. The central domain has an NTPase activity that is also heat-labile but is not inhibited by NIFL. Taken together, these results imply that NIFL interferes with contact between NIFA and sigma 54-holoenzyme.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cannon W., Buck M. Central domain of the positive control protein NifA and its role in transcriptional activation. J Mol Biol. 1992 May 20;225(2):271–286. doi: 10.1016/0022-2836(92)90921-6. [DOI] [PubMed] [Google Scholar]
- Drummond M. H., Contreras A., Mitchenall L. A. The function of isolated domains and chimaeric proteins constructed from the transcriptional activators NifA and NtrC of Klebsiella pneumoniae. Mol Microbiol. 1990 Jan;4(1):29–37. doi: 10.1111/j.1365-2958.1990.tb02012.x. [DOI] [PubMed] [Google Scholar]
- Drummond M., Whitty P., Wootton J. Sequence and domain relationships of ntrC and nifA from Klebsiella pneumoniae: homologies to other regulatory proteins. EMBO J. 1986 Feb;5(2):441–447. doi: 10.1002/j.1460-2075.1986.tb04230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellay R., Frey J., Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52(2-3):147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- Feng J., Atkinson M. R., McCleary W., Stock J. B., Wanner B. L., Ninfa A. J. Role of phosphorylated metabolic intermediates in the regulation of glutamine synthetase synthesis in Escherichia coli. J Bacteriol. 1992 Oct;174(19):6061–6070. doi: 10.1128/jb.174.19.6061-6070.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke H., Shanmugam K. T. Temperature control of nitrogen fixation in Klebsiella pneumoniae. Arch Microbiol. 1979;123(3):259–265. doi: 10.1007/BF00406659. [DOI] [PubMed] [Google Scholar]
- Hoover T. R., Santero E., Porter S., Kustu S. The integration host factor stimulates interaction of RNA polymerase with NIFA, the transcriptional activator for nitrogen fixation operons. Cell. 1990 Oct 5;63(1):11–22. doi: 10.1016/0092-8674(90)90284-l. [DOI] [PubMed] [Google Scholar]
- Huala E., Ausubel F. M. The central domain of Rhizobium meliloti NifA is sufficient to activate transcription from the R. meliloti nifH promoter. J Bacteriol. 1989 Jun;171(6):3354–3365. doi: 10.1128/jb.171.6.3354-3365.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huala E., Stigter J., Ausubel F. M. The central domain of Rhizobium leguminosarum DctD functions independently to activate transcription. J Bacteriol. 1992 Feb;174(4):1428–1431. doi: 10.1128/jb.174.4.1428-1431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. S., Berger D. K., Kustu S. Activity of purified NIFA, a transcriptional activator of nitrogen fixation genes. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2266–2270. doi: 10.1073/pnas.90.6.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morett E., Buck M. In vivo studies on the interaction of RNA polymerase-sigma 54 with the Klebsiella pneumoniae and Rhizobium meliloti nifH promoters. The role of NifA in the formation of an open promoter complex. J Mol Biol. 1989 Nov 5;210(1):65–77. doi: 10.1016/0022-2836(89)90291-x. [DOI] [PubMed] [Google Scholar]
- Morett E., Buck M. NifA-dependent in vivo protection demonstrates that the upstream activator sequence of nif promoters is a protein binding site. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9401–9405. doi: 10.1073/pnas.85.24.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morett E., Cannon W., Buck M. The DNA-binding domain of the transcriptional activator protein NifA resides in its carboxy terminus, recognises the upstream activator sequences of nif promoters and can be separated from the positive control function of NifA. Nucleic Acids Res. 1988 Dec 23;16(24):11469–11488. doi: 10.1093/nar/16.24.11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morett E., Kreutzer R., Cannon W., Buck M. The influence of the Klebsiella pneumoniae regulatory gene nifL upon the transcriptional activator protein NifA. Mol Microbiol. 1990 Aug;4(8):1253–1258. doi: 10.1111/j.1365-2958.1990.tb00704.x. [DOI] [PubMed] [Google Scholar]
- North A. K., Klose K. E., Stedman K. M., Kustu S. Prokaryotic enhancer-binding proteins reflect eukaryote-like modularity: the puzzle of nitrogen regulatory protein C. J Bacteriol. 1993 Jul;175(14):4267–4273. doi: 10.1128/jb.175.14.4267-4273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal E., Tjian R. Different activation domains of Sp1 govern formation of multimers and mediate transcriptional synergism. Genes Dev. 1991 Sep;5(9):1646–1656. doi: 10.1101/gad.5.9.1646. [DOI] [PubMed] [Google Scholar]
- Santero E., Hoover T. R., North A. K., Berger D. K., Porter S. C., Kustu S. Role of integration host factor in stimulating transcription from the sigma 54-dependent nifH promoter. J Mol Biol. 1992 Oct 5;227(3):602–620. doi: 10.1016/0022-2836(92)90211-2. [DOI] [PubMed] [Google Scholar]
- Santero E., Hoover T., Keener J., Kustu S. In vitro activity of the nitrogen fixation regulatory protein NIFA. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7346–7350. doi: 10.1073/pnas.86.19.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S. C., Xue Z. T., Kong Q. I., Wu Q. L. An open reading frame upstream from the nifH gene of Klebsiella pneumoniae. Nucleic Acids Res. 1983 Jun 25;11(12):4241–4250. doi: 10.1093/nar/11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D. S., Batut J., Klose K. E., Keener J., Kustu S. The phosphorylated form of the enhancer-binding protein NTRC has an ATPase activity that is essential for activation of transcription. Cell. 1991 Oct 4;67(1):155–167. doi: 10.1016/0092-8674(91)90579-n. [DOI] [PubMed] [Google Scholar]
- Wootton J. C., Drummond M. H. The Q-linker: a class of interdomain sequences found in bacterial multidomain regulatory proteins. Protein Eng. 1989 May;2(7):535–543. doi: 10.1093/protein/2.7.535. [DOI] [PubMed] [Google Scholar]
- Zhu J., Brill W. J. Temperature sensitivity of the regulation of nitrogenase synthesis by Klebsiella pneumoniae. J Bacteriol. 1981 Feb;145(2):1116–1118. doi: 10.1128/jb.145.2.1116-1118.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]