Abstract
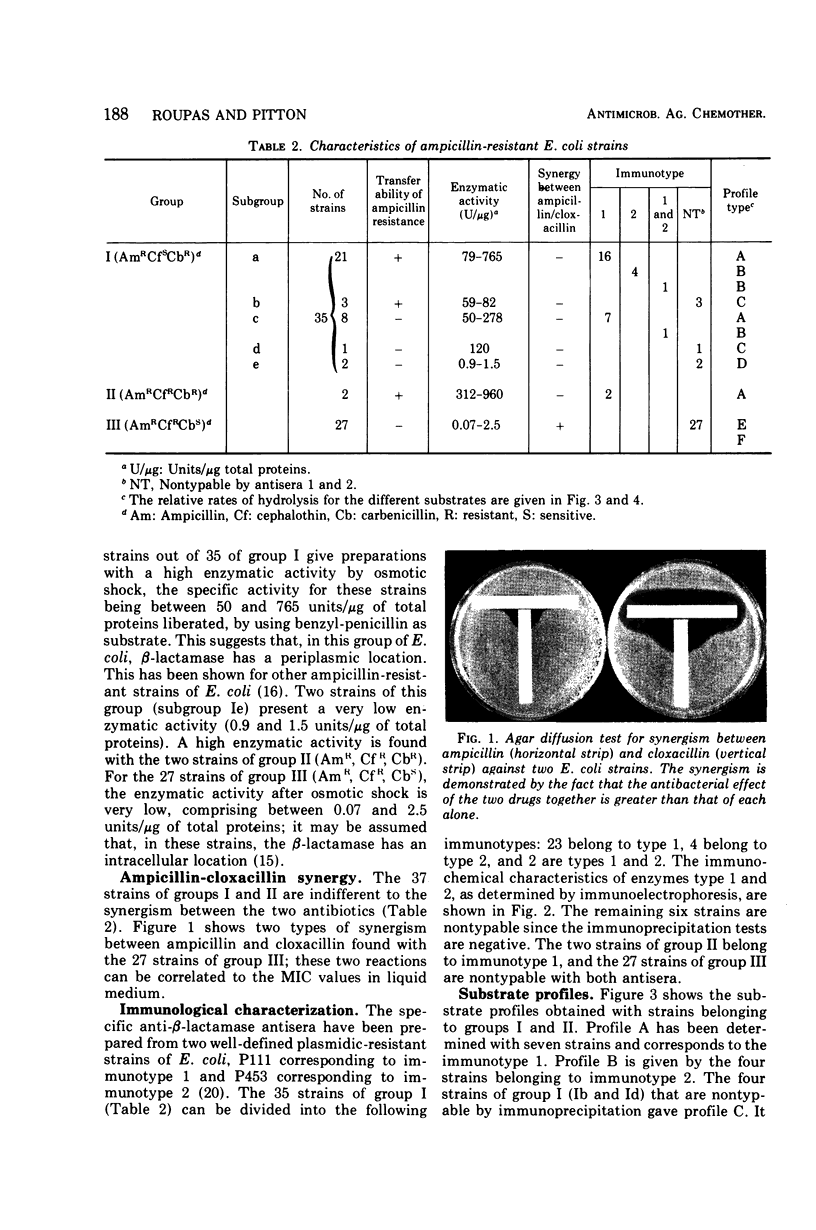
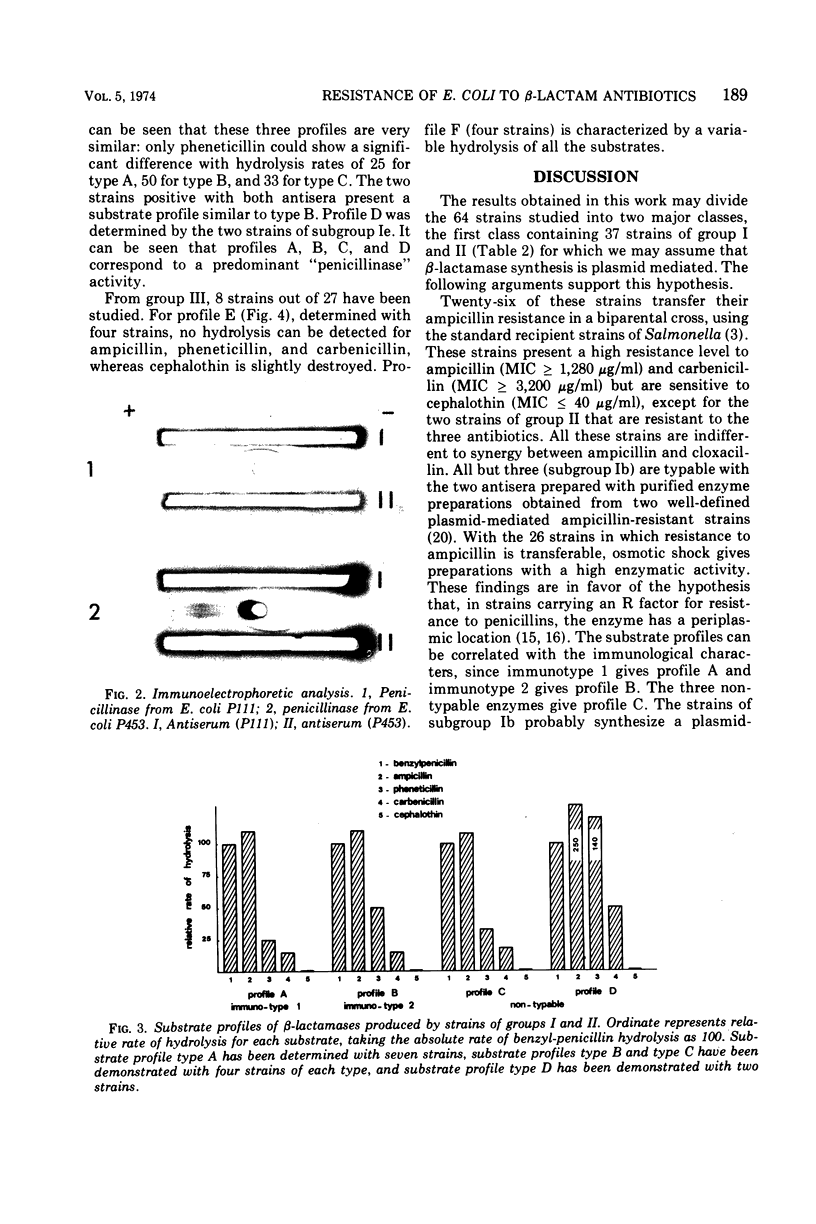
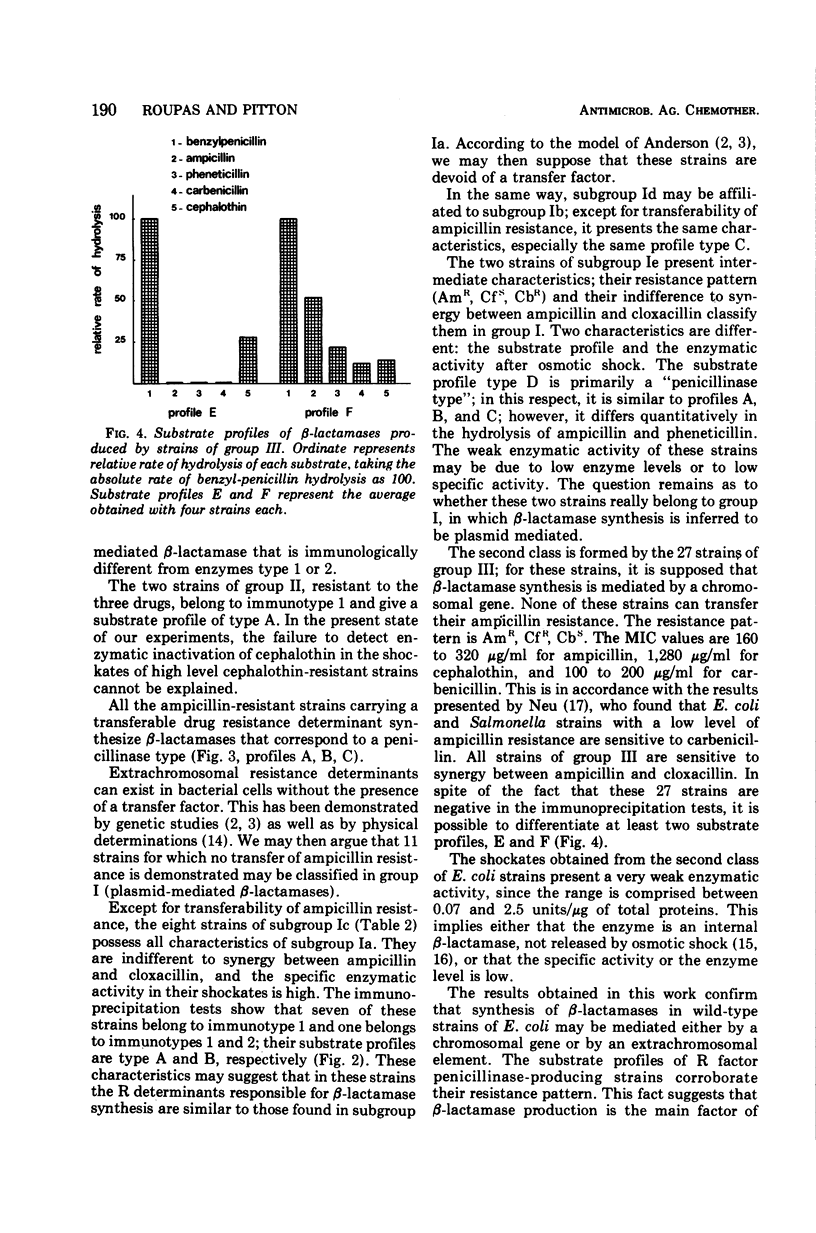
Sixty-four ampicillin-resistant strains of Escherichia coli were studied. Six characters were examined: (i) resistance to ampicillin, cephalothin, and carbenicillin, (ii) synergy between ampicillin and cloxacillin, (iii) level of β-lactamase activity after osmotic shock, (iv) transferability of ampicillin resistance, (v) immunological characterization of the enzyme, and (vi) determination of substrate profiles. One class of strains was found in which synthesis of β-lactamase is inferred to be plasmid mediated; these strains are highly resistant to ampicillin and carbenicillin, sensitive to cephalothin, do not show synergism between ampicillin and cloxacillin, and reveal a high enzymatic activity after osmotic shock. A second class is formed by strains for which β-lactamase synthesis is inferred to be chromosomal; these strains present a low resistance level to ampicillin, are sensitive to carbenicillin and resistant to cephalothin, show a synergism between ampicillin and cloxacillin, and reveal a very low enzymatic activity after osmotic shock. These characters may be used to differentiate periplasmic and cell-bound β-lactamases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acred P., Sutherland R. Antibacterial activities of combinations of ampicillin and cloxacillin. Antimicrob Agents Chemother (Bethesda) 1966;6:53–58. [PubMed] [Google Scholar]
- Anderson E. S., Lewis M. J. Characterization of a transfer factor associated with drug resistance in Salmonella typhimurium. Nature. 1965 Nov 27;208(5013):843–849. doi: 10.1038/208843a0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S. The ecology of transferable drug resistance in the enterobacteria. Annu Rev Microbiol. 1968;22:131–180. doi: 10.1146/annurev.mi.22.100168.001023. [DOI] [PubMed] [Google Scholar]
- Anderson F. M., Datta N., Shaw E. J. R factors in hospital infection. Br Med J. 1972 Jul 8;3(5818):82–85. doi: 10.1136/bmj.3.5818.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONIFAS V. Détermination de l'association synergique binaire d'antibiotes et de sulfamides. Experientia. 1952 Jun 15;8(6):234–235. doi: 10.1007/BF02170729. [DOI] [PubMed] [Google Scholar]
- Bach J. A., Buono N., Chisholm D., Price K. E., Pursiano T. A., Gourevitch A. In vitro and in vivo synergism of mixtures of penicillins. Antimicrob Agents Chemother (Bethesda) 1966;6:328–336. [PubMed] [Google Scholar]
- Bornside G. H. Synergistic antibacterial activity of ampicillin-cloxacillin mixtures against Proteus morganii. Appl Microbiol. 1968 Oct;16(10):1507–1511. doi: 10.1128/am.16.10.1507-1511.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Kontomichalou P. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature. 1965 Oct 16;208(5007):239–241. doi: 10.1038/208239a0. [DOI] [PubMed] [Google Scholar]
- Farrar W. E., Jr, O'Dell N. M., Krause J. M. Use of penicillinase-resistant penicillins to increase the susceptibility of gram-negative bacteria to antibiotics. Ann Intern Med. 1967 Oct;67(4):733–743. doi: 10.7326/0003-4819-67-4-733. [DOI] [PubMed] [Google Scholar]
- HAMILTON-MILLER J. M., SMITH J. T. INHIBITION OF PENICILLINASES FROM GRAM-POSITIVE AND GRAM-NEGATIVE BACTERIA BY SUBSTRATE ANALOGUES. Nature. 1964 Mar 7;201:999–1001. doi: 10.1038/201999a0. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. The demonstration and significance of synergism between -lactam antibiotics. J Med Microbiol. 1971 May;4(2):227–237. doi: 10.1099/00222615-4-2-227. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linström E. B., Boman H. G., Steele B. B. Resistance of Escherichia coli to penicillins. VI. Purification and characterization of the chromosomally mediated penicillinase present in ampA-containing strains. J Bacteriol. 1970 Jan;101(1):218–231. doi: 10.1128/jb.101.1.218-231.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milliken C. E., Clowes R. C. Molecular structure of an R factor, its component drug-resistance determinants and transfer factor. J Bacteriol. 1973 Feb;113(2):1026–1033. doi: 10.1128/jb.113.2.1026-1033.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. Effect of beta-lactamase location in Escherichia coli on penicillin synergy. Appl Microbiol. 1969 Jun;17(6):783–786. doi: 10.1128/am.17.6.783-786.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Swarz H. Resistance of Escherichia coli and Salmonella typhimurium to carbenicillin. J Gen Microbiol. 1969 Nov;58(3):301–305. doi: 10.1099/00221287-58-3-301. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The surface localization of penicillinases in Escherichia coli and Salmonella typhimurium. Biochem Biophys Res Commun. 1968 Jul 26;32(2):258–263. doi: 10.1016/0006-291x(68)90378-1. [DOI] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Pitton J. S. Mechanisms of bacterial resistance to antibiotics. Ergeb Physiol. 1972;65:15–93. doi: 10.1007/3-540-05814-1_2. [DOI] [PubMed] [Google Scholar]
- Pollack M., Charache P., Nieman R. E., Jett M. P., Reimhardt J. A., Hardy P. H., Jr Factors influencing colonisation and antibiotic-resistance patterns of gram-negative bacteria in hospital patients. Lancet. 1972 Sep 30;2(7779):668–671. doi: 10.1016/s0140-6736(72)92084-3. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Jack G. W., Sykes R. B. Mechanisms of drug resistance. The beta-lactamases of gram-negative bacteria including pseudomonads. Ann N Y Acad Sci. 1971 Jun 11;182:243–257. doi: 10.1111/j.1749-6632.1971.tb30661.x. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- SABATH L. D., ABRAHAM E. P. SYNERGISTIC ACTION OF PENICILLINS AND CEPHALOSPORINS AGAINST PSEUDOMONAS PYOCYANEA. Nature. 1964 Dec 12;204:1066–1069. doi: 10.1038/2041066a0. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., McCall C. E., Steigbigel N. H., Finland M. Synergistic penicillin combinations for treatment of human urinary-tract infections. Antimicrob Agents Chemother (Bethesda) 1966;6:149–155. [PubMed] [Google Scholar]
- Sawai T., Mitsuhashi S., Yamagishi S. Drug resistance of enteric bacteria. XIV. Comparison of beta-lactamases in gram-negative rod bacteria resistant to alpha-aminobenzylpenicillin. Jpn J Microbiol. 1968 Dec;12(4):423–434. doi: 10.1111/j.1348-0421.1968.tb00415.x. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Nordström K. Microiodometric determination of beta-lactamase activity. Antimicrob Agents Chemother. 1972 Feb;1(2):94–99. doi: 10.1128/aac.1.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]





