Abstract
Objectives
To evaluate if diastolic pulmonary gradient (DPG) can predict survival in patients with pulmonary hypertension due to left heart disease (PH-LHD).
Background
Patients with combined post- and pre-capillary PH-LHD have worse prognosis than those with passive pulmonary hypertension. The transpulmonary gradient (TPG) and pulmonary vascular resistance (PVR) have commonly been used to identify high-risk patients. However, these parameters have significant shortcomings and do not always correlate with pulmonary vasculature remodeling. Recently, it has been suggested that DPG may be better marker, yet its prognostic ability in patients with cardiomyopathy has not been fully assessed.
Methods
A retrospective cohort of 1236 patients evaluated for unexplained cardiomyopathy at Johns Hopkins Hospital was studied. All patients underwent right heart catheterization and were followed until death, cardiac transplantation or the end of the study period (mean time 4.4 years). The relationships between DPG, TPG or PVR and survival in subjects with PH-LHD (n=469) were evaluated with Cox Proportional Hazards Regression and Kaplan Meier analyses.
Results
DPG was not significantly associated with mortality (HR 1.02; p=0.10) in PH-LHD whereas elevated TPG and PVR predicted death (HR 1.02, p=0.046 and HR 1.11, p=0.002, respectively). Similarly, DPG did not differentiate survivors from non-survivors at any selected cutpoints including a DPG of 7mmHg.
Conclusions
In this retrospective study of patients with cardiomyopathy and PH-LHD, an elevated DPG was not associated with worse survival.
Keywords: Diastolic pulmonary gradient, pulmonary hypertension, left heart disease, survival
Introduction
Patients with pulmonary hypertension (PH) due to left heart disease (defined as pulmonary capillary wedge pressure (PCWP) >15mmHg and mean pulmonary artery pressure (mPAP) ≥25mmHg) have worse prognosis compared to those without PH (1). Among those patients with PH, two phenotypes have been described: 1) a group of isolated post-capillary (IpcPH) or “passive” PH in which elevated pulmonary pressures are reversible and in proportion to increases in left atrial pressure, and 2) a group with “pre-capillary” component (combined post-capillary and pre-capillary PH) whose pulmonary hypertension is worse than can be fully explained by passive elevation secondary to elevated left atrial pressure. This latter group may have comorbid pulmonary vascular remodeling and therefore may demonstrate persistent PH after interventions to lower left sided filling pressures. The ability to accurately define and separate a high-risk subgroup has major implications in the management and outcomes of heart failure patients as those with combined post-capillary and pre-capillary PH (CpcPH) due to left heart disease (PH-LHD) have worse prognosis (1,2) and may not be suitable for cardiac transplantation (2).
In an effort to better characterize the two populations, several hemodynamic parameters have been used. A transpulmonary gradient (TPG: mPAP-PCWP) >12–15mmHg and a pulmonary vascular resistance (PVR: TPG/cardiac output) >2.5–3 Wood units (WU) have been used to describe patients with “out of proportion” or those with a pre-capillary component to PH (1). TPG however, is flow-dependent (3) and influenced by elevation in left atrial pressure (4), making it an unreliable marker of the pulmonary vascular contribution to PH-LHD. Although not without limitations, most favor PVR to identify high risk patients. Our group and others have shown that elevated PVR predicts outcomes in patients with PH-LHD better than TPG (5–7).
More recently diastolic pulmonary gradient (DPG: diastolic PAP minus PCWP) has been proposed to distinguish CpcPH from IpcPH (3,8). Elevated DPG (≥7mmHg) may be associated with pulmonary vascular remodeling and predict worse survival in individuals with elevated TPG and PH-LHD (9). We have previously shown, however, that DPG is not associated with death after heart transplant, which may call into question the assertion that DPG is a strong marker of intrinsic pulmonary vascular disease in PH-LHD (10). In this study, we sought to determine whether an elevated DPG predicted survival using a cohort of 1236 patients previously evaluated for unexplained cardiomyopathy (5).
Methods
Patients
Study subjects included inpatients and outpatients referred to the Johns Hopkins Hospital Cardiomyopathy Service for further evaluation of heart failure due to undiagnosed cardiomyopathy. All patients received treatment of their heart failure prior to undergoing right heart catheterization and biopsy. A total of 1236 patients were evaluated between December 1982 and December 1997 as previously described (11). All patients underwent extensive work up which included endomyocardial biopsy with right heart catheterization by a heart failure cardiologist and coronary angiography when indicated. After the evaluation, all patients were assigned a cause of cardiomyopathy. Age, gender, race, height and weight were recorded at the time of their initial evaluation. The patients were followed until death, cardiac transplantation or the end of the study period (January 1, 1998). Vital status was obtained from medical records and through a search of the Nation Death Index (12). The study was approved by the Joint Committee on Clinical Investigation at Johns Hopkins Hospital. All patients provided informed consent to use their data in the study.
Right heart catheterization
Patients underwent right heart catheterization by heart failure specialists at the Johns Hopkins catheterization laboratory with a balloon-tipped, flow-directed catheter placed into the right internal jugular vein. Hemodynamics were measured at the time of presentation before optimizing medical therapy. Cardiac output (CO) was determined as the mean of 3 to 5 separate measurements with the thermodilution method. Systemic arterial pressure was measured noninvasively. Mean right atrial pressure, systolic pulmonary artery pressure (sPAP), diastolic pulmonary artery pressure (dPAP), mean pulmonary artery pressure (mPAP), and pulmonary capillary wedge pressure (PCWP) were recorded at end expiration. Pulmonary vascular resistance (PVR) was calculated in Wood units as the difference between mPAP and PCWP divided by CO. Transpulmonary gradient (TPG) was calculated as the difference between mPAP and PCWP. Diastolic pulmonary gradient (DPG) was calculated as the difference between the dPAP and PCWP.
Statistical Analysis
Comparison of groups were performed with Mann-Whitney rank-sum test or, for multiple groups, by 1-way ANOVA. Categorical variables were compared with chi-squared test. Hazard ratios of death for DPG, TPG and PVR were estimated with Cox Proportional Hazards regression analysis in all patients with PH-LHD (PCWP >15mmHg and mPAP ≥25mmHg). The primary endpoint was death from all causes. Participants who underwent transplantation (n=36 of 469) were censored at the time of transplantation. Unadjusted and adjusted models for age, gender, race and body mass index were considered. For our sample size (n=469) and mortality rate (43%), we had adequate power (80%) to detect a 10% or smaller difference in the hazard of death for all of the evaluated hemodynamic parameters. While we might have been underpowered to detect smaller differences in the hazard of death, such small difference in mortality would argue against the use of these parameters to discriminate survivors. Survival was also estimated with the non-parametric methods of Kaplan and Meier and compared using the log-rank test. A p-value (two-tailed) of <0.05 was considered significant. Medians are presented with interquartile range. Statistical analyses were performed using STATA version 12 (Stata Corp, Texas) and SigmaPlot version 11.0 (Systat Software Inc, San Jose, CA).
Results
Study population
Among 1236 patients who were evaluated with a right heart catheterization for a new diagnosis of heart failure, 1174 had a complete set of hemodynamics. Most patients had diagnosis of a dilated cardiomyopathy. Of the 1174, 558 had an elevated PCWP >15mmHg. Of those, 469 had mPAP ≥25mmHg consistent with PH-LHD. 650 patients did not have PH (mPAP <25mmHg). Of the 1174 patients, 124 (10.6 %) had a DPG ≥7mmHg, and of those, 92 (74.2%) also had PH and 62 (50%) had PH-LHD. Therefore, 32 patients without PH (mPAP < 25mmHg) had a DPG ≥ 7 mmHg. In addition, 355 (30.2%) of all patients evaluated and 169 (36%) of the subjects with PH-LHD had a negative DPG value. The clinical characteristics and hemodynamics of those patients with a negative DPG are found in Supplemental table 1. On average, the negative DPG group had worse hemodynamics as evidenced by lower right and left ventricular stroke work index and higher PCWP.
The association between DPG, TPG, or PVR and death in PH-LHD
DPG was not significantly associated with mortality in unadjusted (HR 1.02; p=0.08) analysis or after adjusting for age, gender, race and body mass index (HR 1.02; p=0.10) (Table 1). TPG was associated with mortality in unadjusted (HR 1.02; p=0.03) and was borderline significant after adjustment (HR 1.02; p= 0.046). PVR predicted mortality in our cohort (unadjusted HR 1.13, p=0.002, adjusted HR: 1.11, p=0.002) (Table 1). Because DPG, TPG and PVR have different units, qualitative comparison of hazard ratios per unit change is difficult. Re-parameterization of markers by interquartile range allowed comparison between markers. The hazard of mortality appeared more similar in this context; however, the strength of association with re-parameterization is not changed and the association with mortality remained strongly significant for PVR, of borderline significance for TPG, and not significant for DPG.
Table 1.
Hazard of death in DPG, TPG, or PVR.
| Hazard Ratio (95% CI) per unit increase |
Hazard Ratio (95% CI) per interquartile increase |
p-value | |
|---|---|---|---|
| DPG | |||
| Unadjusted | 1.02 (1.00–1.05) | 1.15 (0.98–1.34) | 0.08 |
| Adjusted | 1.02 (1.00–1.05) | 1.14 (0.98–1.34) | 0.10 |
| TPG | |||
| Unadjusted | 1.03 (1.00–1.05) | 1.20 (1.02–1.41) | 0.03 |
| Adjusted | 1.02 (1.00–1.05) | 1.19 (1.00–1.40) | 0.046 |
| PVR | |||
| Unadjusted | 1.13 (1.06–1.20) | 1.29 (1.12–1.48) | <0.001 |
| Adjusted | 1.11 (1.04–1.19) | 1.25 (1.09–1.44) | 0.002 |
Adjusted model accounts for age, gender, race and body mass index
PH-LHD = Pulmonary Hypertension due to left heart disease. DPG = Diastolic Pulmonary Gradient. TPG = Transpulmonary Gradient. PVR = Pulmonary Vascular Resistance. CI: Confidence Intervals.
Survival in patients with PH-LHD and elevated DPG
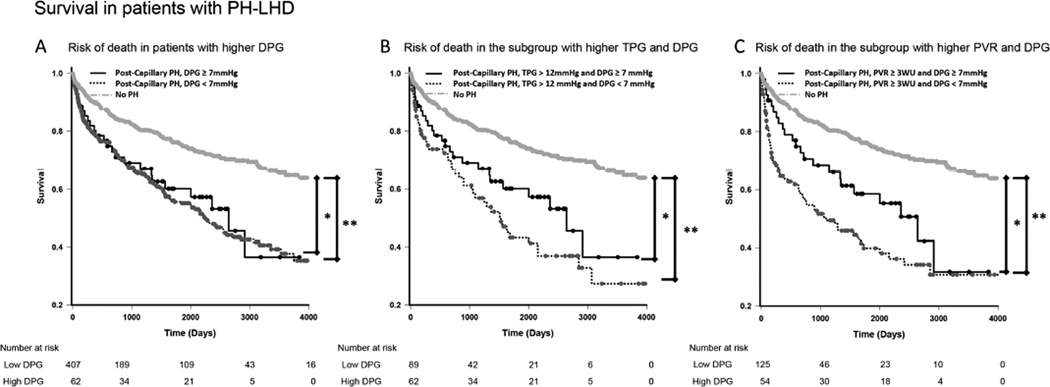
In keeping with the results of the Cox analysis, there was no statistical difference in mortality between high (defined as ≥1, ≥3, ≥5, ≥7, or ≥9mmHg) and low DPG groups (<1, <3, <5, <7, or <9mmHg) (Table 2). We further examined the cut-off of 7mmHg, which has previously been shown to be a surrogate marker for CpcPH (9) and has been proposed for clinical use (8). Demographic, diagnostic and hemodynamic data for those subjects (DPG<7 and DPG ≥ 7mmHg) as well as the 650 patients without PH are presented in Table 3. Demographic and heart failure diagnosis were similar between the high and low DPG groups. Compared with the lower DPG group (<7mmHg), patients with DPG ≥7mmHg had higher systemic and pulmonary artery pressures, higher right and left ventricular stroke work index and higher PVR. Patients with a lower DPG had a higher PCWP (26 vs. 22mmHg – p<0.001). No difference in survival between the two groups at a mean follow up time of 4.4 years was observed (Figure 1A).
Table 2.
Hazard of death for participants using a variety of commonly used DPG cut-offs.
| Hazard Ratio per interquartile range (95% CI) |
p-value | |
|---|---|---|
| DPG: Cut-off 1mmHg | ||
| (251 participants with high DPG, 218 with low DPG) | ||
| Unadjusted | 1.21 (0.92–1.61) | 0.18 |
| Adjusted | 1.20 (0.90–1.60) | 0.21 |
| DPG: Cut-off 3mmHg | ||
| (174 participants with high DPG, 295 with low DPG) | ||
| Unadjusted | 1.30 (0.98–1.73) | 0.07 |
| Adjusted | 1.28 (0.96–1.71) | 0.09 |
| DPG: Cut-off 5mmHg | ||
| (117 participants with high DPG, 352 with low DPG) | ||
| Unadjusted | 1.15 (0.84–1.58) | 0.40 |
| Adjusted | 1.19 (0.87–1.64) | 0.28 |
| DPG: Cut-off 7mmHg | ||
| (62 participants with high DPG, 407 with low DPG) | ||
| Unadjusted | 0.91 (0.60–1.38) | 0.66 |
| Adjusted | 0.93 (0.61–1.42) | 0.74 |
| DPG: Cut-off 9mmHg | ||
| (37 participants with high DPG, 432 with low DPG) | ||
| Unadjusted | 0.74 (0.43–1.28) | 0.28 |
| Adjusted | 0.75 (0.42–1.31) | 0.31 |
Adjusted model accounts for age, gender, race and body mass index
DPG = Diastolic Pulmonary Gradient. CI: Confidence Intervals.
Table 3.
Demographic, diagnostic and hemodynamic data of the different patient cohorts.
| DPG ≥7 (n=62) | DPG <7 (n=407) | p-value† | No PH (n=650) | p-value‡ | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 49.3 [39.5–58.5] | 49.0 [36.7–60.9] | 0.98 | 46.7 [35.4–57.4] | 0.11 |
| Height (m) | 1.75 [1.65–1.80] | 1.73 [1.68–1.83] | 0.08 | 1.73 [1.35–1.98] | 0.21 |
| Weight (kg) | 83.8 [68.7–98.0] | 79.0 [65.9–91.8] | 0.15 | 76.8 [63.6–89.0] | 0.017 |
| Body Mass Index (kg/m2) | 27.3 [22.9–30.7] | 26.1 [22.9–30.9] | 0.47 | 25.5 [22.4–29.1] | 0.021 |
| Female Gender | 18 (29) | 149 (37) | 0.31* | 274 (42.1) | 0.045* |
| Race | |||||
| Black | 29 (47) | 140 (35) | 208 (32) | ||
| Caucasian | 32 (52) | 257 (63) | 425 (66) | ||
| Other | 1 (2) | 8 (2) | 0.18* | 12 (2) | 0.24* |
| Diagnosis | |||||
| Idiopathic | 27 (44) | 205 (50) | 326 (50) | ||
| Coronary artery disease | 5 (8) | 41 (10) | 36 (6) | ||
| Myocarditis | 4 (6) | 28 (7) | 76 (12) | ||
| Toxic/Metabolic | 5 (8) | 19 (5) | 28 (4) | ||
| Other | 21 (34) | 114 (28) | 0.63* | 184 (28) | 0.033* |
| Hemodynamics | |||||
| Heart rate (beats per minute) | 94 [82–107] | 93 [80–106] | 0.69 | 84 [72–95] | <0.001 |
| Systemic Blood Pressure | |||||
| Systolic (mmHg) | 128 [105–151] | 117 [104–138] | 0.04 | 120 [107–137] | 0.09 |
| Diastolic (mmHg) | 82 [72.0–90.3] | 77.5 [68.0–86.0] | 0.01 | 73 [67.0–81.2] | <0.001 |
| Mean (mmHg) | 98.2 [87.2–111.3] | 90.7 [80.7–105.4] | 0.01 | 89.5 [81.0–100.0] | 0.001 |
| Systemic Vascular Resistance (Wood units) | 22.6 [16.3–30.1] | 20.6 [16.4–27.3] | 0.52 | 19.1 [15.2–23.1] | <0.001 |
| Left Ventricular Stroke Work Index (mmHg ml/m2) | 1521 [1208–2236] | 1342 [1033–1870] | 0.035 | 2316 [1766–3041] | <0.001 |
| Right Atrial Pressure (mmHg) | 13 [8.8–17.0] | 10.0 [7.0–15.0] | 0.048 | 4 [2.0–6.0] | <0.001 |
| Pulmonary Artery Pressures | |||||
| Systolic (mmHg) | 60.0 [51.5–70.0] | 52.0 [45.0–59.0] | <0.001 | 28 [23.0–33.0] | <0.001 |
| Diastolic (mmHg) | 31.5 [28.0–36.5] | 26.0 [22.0–30.0] | <0.001 | 11.5 [8.0–15.0] | <0.001 |
| Mean (mmHg) | 40.2 [36.7–48.8] | 34.0 [29.7–40.0] | <0.001 | 17 [13.7–10.7] | <0.001 |
| Pulmonary Capillary Wedge Pressure (mmHg) | 22.0 [18.0–27.0] | 26.0 [22.0–30.0] | <0.001 | 10 [7.0–13.0] | <0.001 |
| Cardiac Index (L/min/m2) | 1.90 [1.55–2.5] | 1.93 [1.60–2.35] | 0.96 | 2.4 [2.0–2.8] | <0.001 |
| Right Ventricular Stroke Work Index (mmHg ml/m2) | 604 [456–795] | 511 [354–665] | 0.001 | 369 [322–432] | <0.001 |
| Pulmonary Vascular Resistance (Wood units) | 4.7 [3.5–6.3] | 2.2 [1.4–3.3] | <0.001 | 1.37 [0.95–2.0] | <0.001 |
| Transpulmonary Gradient (mmHg) | 18.3 [16.3–21.8] | 8.3 [5.7–11.7] | <0.001 | 6.3 [4.7–8.3] | <0.001 |
| Diastolic Pulmonary Gradient (mmHg) | 9.0 [8.0–11.0] | 0 [−3.0–3.0] | <0.001 | 1 [−1.0–3.0] | <0.001 |
| RA/PCWP | 0.54 [0.42–0.69] | 0.40 [0.29–0.56] | <0.001 | 0.4 [0.25–0.57] | <0.001 |
No variable was missing in more than 3.3% of participants.
Data presented as median [interquartile range]
DPG ≥7 vs. DPG <7; Rank Sum test unless otherwise indicated
Comparison of all three groups; ANOVA unless otherwise indicated
Chi-square; () = Percentage
Figure 1. Kaplan-Meir survival curves in all patients evaluated for heart failure.
A. In patients with PH-LHD (mPAP ≥25mmHg and PCWP >15mmHg), higher DPG (≥7mmHg) failed to discriminate survivors. Patients without PH had better survival. B. In the subgroup of increased TPG, low DPG did not discriminate survivors. C. In subjects with PH-LHD and PVR ≥3WU, lower DPG showed a trend towards worse survival (P=0.051). * P <0.05, ** P <0.001; PH = Pulmonary Hypertension. DPG = Diastolic Pulmonary Gradient. TPG = Transpulmonary Gradient. PVR = Pulmonary Vascular Resistance. WU = Wood units.
Survival in patients with PH-LHD and elevated TPG or PVR
After exploring various TPG cut-off points (high defined as >6, >9, >12, or 15mmHg and low defined as ≤6, ≤9, ≤12, or ≤15mmHg), a TPG >9mmHg significantly differentiated survivors from non-survivors (Table 4). In a sub-cohort of patients with TPG >12 mmHg (n=151), higher DPG (≥7 mmHg) was not associated with increased mortality (Figure 1B).
Table 4.
Hazard of death for participants for a variety of commonly used TPG and PVR cut-offs.
| Hazard Ratio per interquartile range (95% CI) |
p-value | |
|---|---|---|
| TPG: Cut-off 6mmHg | ||
| (362 participants with high TPG, 107 with low TPG) | ||
| Unadjusted | 1.26 (0.90–1.78) | 0.18 |
| Adjusted | 1.29 (0.91–1.84) | 0.16 |
| TPG: Cut-off 9mmHg | ||
| (236 participants with high TPG, 233 with low TPG) | ||
| Unadjusted | 1.34 (1.01–1.77) | 0.04 |
| Adjusted | 1.34 (1.00–1.79) | 0.05 |
| TPG: Cut-off 12mmHg | ||
| (152 participants with high TPG, 317 with low TPG) | ||
| Unadjusted | 1.24 (0.92–1.66) | 0.14 |
| Adjusted | 1.19 (0.88–1.60) | 0.26 |
| TPG: Cut-off 15mmHg | ||
| (89 participants with high TPG, 380 with low TPG) | ||
| Unadjusted | 0.91 (0.60–1.38) | 0.66 |
| Adjusted | 0.93 (0.61–1.42) | 0.74 |
| PVR: Cut-off 2WU | ||
| (298 participants with high PVR, 171 with low PVR) | ||
| Unadjusted | 1.60 (1.18–2.18) | 0.003 |
| Adjusted | 1.48 (1.07–2.03) | 0.02 |
| PVR: Cut-off 2.5WU | ||
| 223 participants with high PVR, 246 with low PVR) | ||
| Unadjusted | 1.78 (1.34–2.36) | <0.001 |
| Adjusted | 1.59 (1.18–2.13) | 0.002 |
| PVR: Cut-off 3WU | ||
| 184 participants with high PVR, 285 with low PVR) | ||
| Unadjusted | 1.79 (1.35–2.36) | <0.001 |
| Adjusted | 1.57 (1.18–2.10) | 0.002 |
| PVR: Cut-off 3.5WU | ||
| (132 participants with high PVR, 337 with low PVR) | ||
| Unadjusted | 1.60 (1.18–2.18) | 0.003 |
| Adjusted | 1.48 (1.07–2.03) | 0.02 |
Adjusted model accounts for age, gender, race and body mass index
TPG = Transpulmonary Gradient. PVR = Pulmonary Vascular Resistance. WU= Wood units. CI: Confidence Intervals
All PVR cutpoints explored (low defined as <2, <2.5, <3, or < 3.5 and high defined as ≥2, ≥2.5, ≥3, or ≥3.5WU) predicted worse survival in the original cohort (Table 4). In exploratory models, PVR was considered as an effect modifier of the relationship between DPG or TPG and death. PVR did not significantly modify the association between TPG and death (p-interaction= 0.13). PVR did modify the association between DPG and death such that increasing DPG decreased the hazard of death at high levels of PVR (p-interaction=0.02; hazard ratio of the interaction term=0.98). Similarly, Figure 1C suggests that in subjects with PVR ≥3mmHg (n=179), those subjects with a low DPG (<7mmHg) trended towards worse survival compared with high DPG (≥7mmHg) (p=0.051). The number of participants at-risk in these exploratory subgroup analyses was relatively small and estimates of association may be unstable.
Removing patients with HIV diagnosis (who had an overall worse prognosis during this study period), those with an infiltrative disease (amyloid/sarcoid), and those with a diagnosis of restrictive cardiomyopathy left a cohort of 419 PH-LHD patients. DPG also did not predict survival in this cohort (Supplemental Table 2).
Discussion
In the present study, we used a well characterized, large cohort of patients previously evaluated by the cardiomyopathy service at Johns Hopkins Hospital with right heart catheterization and cardiac biopsy (5), to assess the ability of DPG to predict mortality. DPG used independently or in combination with elevated TPG or PVR and in either unadjusted or adjusted analyses, failed to predict mortality in patients with PH-LHD. Conversely, PVR was associated with decreased survival in all analyses in subjects with PH-LHD, similar to prior analyses (6,7,13).
In PH due to left heart disease, elevated left heart filling pressures are transmitted to the pulmonary veins and lead to increased diastolic PAP. Persistent pulmonary venous congestion results in endothelial dysfunction with decreased nitric oxide production, increase production of vasoactive factors (endothelin 1, angiotensin II etc) favoring vasoconstriction and may ultimately lead to irreversible remodeling of the pulmonary vasculature (8,14). Elevation in left atrial pressure also leads to increased vascular stiffness (decreased compliance). This results in an increased systolic PAP, and therefore mPAP, leading to elevation of TPG as well as PVR (4, 14). Both of these factors depend on the flow (cardiac output) (3). The diastolic PAP however is less sensitive to these effects, and therefore DPG (diastolic PAP minus PCWP) has been recommended as an alternative and more reliable marker of PH-LHD with a pre-capillary component (8).
The prognostic capability of DPG in patients with CpcPH was recently evaluated in a cohort of 1094 patients with PH-LHD. In this study by Gerges et al., participants with a TPG >12mmHg and a DPG ≥7mmHg had worse survival compared to those with a TPG ≤12mmHG and a DPG <7mmHg. In 18 of these participants, lung tissue was evaluated and participants with elevated DPG had advanced remodeling of the pulmonary vasculature (9). This study coupled with sound physiologic reasoning has led to the recent recommendations from the Fifth World Symposium on Pulmonary Hypertension that DPG be the sole discriminator of pre- and post-capillary PH in those with left heart disease (8). Our heart failure cohort was significantly different from the study population of Gerges et al. as it had relatively lower incidence of PH (44% vs. 91%) Our patients were also younger and were less likely to have an ischemic cardiomyopathy. When considering only the PH-LHD patients, the distribution of CpcPH (TPG > 12mmHg) was relatively similar (32 vs. 45%).
Using the United Network of Organ Sharing (UNOS) database, we recently demonstrated that elevated pre-transplant DPG had no association with post-transplant survival (10). These findings argued against DPG as a marker of clinically significant irreversible pulmonary vascular remodeling, although they did not necessarily exclude the possibility that DPG could predict outcomes in a heart failure population that did not undergo transplant. Unfortunately, the findings of our current study do not support the use of DPG in this regard. DPG was not associated with survival in any analysis and high DPG may have even been a marker of better prognosis in an exploratory subgroup of CpcPH with high PVR. The lack of association or even inverse association with mortality may be related to the important observation in our cohort that low DPG may have identified a sicker group of patients with a higher PCWP and lower systemic blood pressure. This was true in both the entire cohort of 1174 patients as well as those only with PH-LHD.
Despite its promise, the use of DPG has significant shortcomings and limitations. The DPG may be particularly susceptible to technical errors. Measurement of diastolic PAP, particularly when using fluid filled catheters, is subject to error from catheter motion artifacts. This likely accounts for the negative DPG values observed in our study as well as others. In a study of critically ill patients by Wilson and colleagues, the DPG was negative in 18.5% of the readings (15). Similar results have been reported after coronary artery bypass surgery (16). Moreover, in a classic investigation by Harvey et al., patients with left heart disease had a mean DPG of −2mmHg (17). Even small errors in the measurement of diastolic PAP or PCWP will have a major impact on the DPG given its relatively low absolute value. As previously highlighted by Ryan et al., the use of computerized mean PCWP pressures averaged throughout the respiratory cycle rather than end-expiratory measurements leads to an underestimation of the true PCWP, particularly in patients with higher intrathoracic pressures (18). In addition, inaccurate wedging of the pulmonary artery catheter can overestimate PCWP leading to falsely low DPG. Finally, DPG itself accounts for only a small proportion of total right ventricular load in patients with PH-LHD and therefore may not be necessarily associated with significant RV dysfunction. Right ventricular function is a well known prognosticator of outcomes in heart failure, and therefore, PVR may be a superior prognosticator because it includes flow assessment (19). However, even in patients with an elevated PVR, an elevated DPG was not associated with worse prognosis.
The use of DPG in PH is not new and it was extensively studied in previous decades (15, 20, 21). Many factors other than pulmonary vascular remodeling also affect the DPG. DPG is acutely elevated in several different clinical scenarios including hypoxemia in patients with ARDS and COPD (17,22,23), after coronary artery bypass surgery (15), and in sepsis due to acidosis, release of endotoxins, or microthrombi (17,24). Tachycardia, which is commonly encountered in individuals with LHD due to decreased cardiac output, tachyarrhythmias or inotropic support, also increases the DPG (25).
We acknowledge that our retrospective study has several limitations. First, our cohort included patients evaluated for unexplained cardiomyopathy with a broad representation of different heart failure pathologies, which may not necessarily represent the general heart failure population. Although this cohort consisted of patients with both preserved and reduced function, most patients had a diagnosis of a dilated cardiomyopathy, leaving open the possibility that DPG may have a prognostic ability in heart failure with preserved ejection fraction (HFpEF), or in a more select group of heart failure patients. Because the incidence of PH-LHD was relatively low in our population (44%), this could limit our power to detect a difference in survival between the low and high DPG groups. However, TPG, and in particular PVR, did discriminate survivors from non-survivors. The large number of patients with a negative DPG (assuming the negative DPG is the result of measurement error) could bias the results, as the actual DPG may have been elevated in these patients. If this limitation is true then this may speak to a real world limitation to the use of DPG since these measurements were all performed by heart failure cardiologists with significant experience in hemodynamic evaluations. It also remains possible that a very high DPG similar to those seen in idiopathic pulmonary artery hypertension (~20mmHg) (26), could predict survival. Nevertheless, those patients are quite rare in PH-LHD (in this analysis only 9 patients had a DPG>15mmHg and only 4 had a DPG>20mmHg). PVR and TPG may have influenced the decision of who was ultimately transplanted. In accordance with previous investigations on this topic, we censored participants who went on to require transplantation (n=36) at the time of transplantation. Censoring participants at the time of transplant could lead to an underestimation of mortality. Furthermore, information regarding medical therapies, echocardiography and other co-morbid conditions like COPD, smoking, sleep apnea, atrial fibrillation, renal failure etc. was not available and therefore their association with PH-LHD and survival could not be assessed. In addition, our analysis did not correct for multiple comparisons. Finally, hemodynamic data on response to vasodilators to evaluate the reversibility of PH was not routinely tested in this cohort.
In conclusion, our study shows that in a large cohort of patients with PH due to left heart disease, including those with ‘out-of-proportion’ (elevated TPG and PVR) PH, the diastolic pulmonary gradient did not discriminate survivors from non-survivors. Considering the technical limitations interfering with the accurate measurement of DPG and other clinical factors that affect the DPG aside from pulmonary vasculature remodeling, this work argues against the use of DPG as a marker of prognosis in patients with PH-LHD. Likewise, the routine use of DPG in diagnostic algorithms of PH-LHD is premature and requires further validation.
Supplementary Material
Abbreviations
- CpcPH
Combined post-capillary and pre-capillary PH
- CO
Cardiac output
- dPAP
diastolic Pulmonary artery pressure
- DPG
Diastolic pulmonary gradient
- IpcPH
Isolated post-capillary PH
- mPAP
mean Pulmonary artery pressure
- PCWP
Pulmonary capillary wedge pressure
- PH
Pulmonary hypertension
- PVR
Pulmonary vascular resistance
- RVSWI
Right ventricular stroke work index
- sPAP
systolic Pulmonary artery pressure
- TPG
Transpulmonary gradient
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No authors have relevant disclosures with industry.
References
- 1.Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation. 2012;126:975–990. doi: 10.1161/CIRCULATIONAHA.111.085761. [DOI] [PubMed] [Google Scholar]
- 2.Mehra MR, Jessup M, Gronda E, Costanzo MR. Rationale and process: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates--2006. J Heart Lung Transplant. 2006;25:1001–1002. doi: 10.1016/j.healun.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Naeije R, Vachiery JL, Yerly P, Vanderpool R. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J. 2013;41:217–223. doi: 10.1183/09031936.00074312. [DOI] [PubMed] [Google Scholar]
- 4.Tedford RJ, Hassoun PM, Mathai SC, et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation. 2012;125:289–297. doi: 10.1161/CIRCULATIONAHA.111.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappola TP, Felker GM, Kao WH, Hare JM, Baughman KL, Kasper EK. Pulmonary hypertension and risk of death in cardiomyopathy: patients with myocarditis are at higher risk. Circulation. 2002;105:1663–1668. doi: 10.1161/01.cir.0000013771.30198.82. [DOI] [PubMed] [Google Scholar]
- 6.Khush KK, Tasissa G, Butler J, McGlothlin D, De MT. Effect of pulmonary hypertension on clinical outcomes in advanced heart failure: analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) database. Am Heart J. 2009;157:1026–1034. doi: 10.1016/j.ahj.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction. JACC: Heart Failure. 2013;1:290–299. doi: 10.1016/j.jchf.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Vachiery JL, Adir Y, Barbera JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62:D100–D108. doi: 10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 9.Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in "out-of-proportion" pulmonary hypertension. Chest. 2013;143:758–766. doi: 10.1378/chest.12-1653. [DOI] [PubMed] [Google Scholar]
- 10.Tedford RJ, Beaty CA, Mathai SC, et al. Prognostic value of the pre-transplant diastolic pulmonary artery pressure to pulmonary capillary wedge pressure gradient (DPG) in cardiac transplant recipients with pulmonary hypertension. Journal of Heart and and Lung Transplantation. 2013 doi: 10.1016/j.healun.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 12.Boyle CA, Decoufle P. National sources of vital status information: extent of coverage and possible selectivity in reporting. Am J Epidemiol. 1990;131:160–168. doi: 10.1093/oxfordjournals.aje.a115470. [DOI] [PubMed] [Google Scholar]
- 13.Aronson D, Eitan A, Dragu R, Burger AJ. Relationship between reactive pulmonary hypertension and mortality in patients with acute decompensated heart failure. Circ Heart Fail. 2011;4:644–650. doi: 10.1161/CIRCHEARTFAILURE.110.960864. [DOI] [PubMed] [Google Scholar]
- 14.Guazzi M, Arena R. Pulmonary hypertension with left-sided heart disease. Nat Rev Cardiol. 2010;7:648–659. doi: 10.1038/nrcardio.2010.144. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RF, Beckman SB, Tyburski JG, Scholten DJ. Pulmonary artery diastolic and wedge pressure relationships in critically ill and injured patients. Arch Surg. 1988;123:933–936. doi: 10.1001/archsurg.1988.01400320019002. [DOI] [PubMed] [Google Scholar]
- 16.Heinonen J, Salmenpera M, Takkunen O. Increased pulmonary artery diastolic-pulmonary wedge pressure gradient after cardiopulmonary bypass. Can Anaesth Soc J. 1985;32:165–170. doi: 10.1007/BF03010044. [DOI] [PubMed] [Google Scholar]
- 17.Harvey RM, Enson Y, Ferrer MI. A reconsideration of the origins of pulmonary hypertension. Chest. 1971;59:82–94. doi: 10.1378/chest.59.1.82. [DOI] [PubMed] [Google Scholar]
- 18.Ryan JJ, Rich JD, Thiruvoipati T, Swamy R, Kim GH, Rich S. Current practice for determining pulmonary capillary wedge pressure predisposes to serious errors in the classification of patients with pulmonary hypertension. Am Heart J. 2012;163:589–594. doi: 10.1016/j.ahj.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 19.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 20.Harvey RM, Enson Y, Ferrer MI. A reconsideration of the origins of pulmonary hypertension. Chest. 1971;59:82–94. doi: 10.1378/chest.59.1.82. [DOI] [PubMed] [Google Scholar]
- 21.Sibbald WJ, Paterson NA, Holliday RL, Anderson RA, Lobb TR, Duff JH. Pulmonary hypertension in sepsis: measurement by the pulmonary arterial diastolic-pulmonary wedge pressure gradient and the influence of passive and active factors. Chest. 1978;73:583–591. doi: 10.1378/chest.73.5.583. [DOI] [PubMed] [Google Scholar]
- 22.Zapol WM, Snider MT. Pulmonary hypertension in severe acute respiratory failure. N Engl J Med. 1977;296:476–480. doi: 10.1056/NEJM197703032960903. [DOI] [PubMed] [Google Scholar]
- 23.Her C, Mandy S, Bairamian M. Increased pulmonary venous resistance contributes to increased pulmonary artery diastolic-pulmonary wedge pressure gradient in acute respiratory distress syndrome. Anesthesiology. 2005;102:574–580. doi: 10.1097/00000542-200503000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Marland AM, Glauser FL. Significance of the pulmonary artery diastolic-pulmonary wedge pressure gradient in sepsis. Crit Care Med. 1982;10:658–661. doi: 10.1097/00003246-198210000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Enson Y, Wood JA, Mantaras NB, Harvey RM. The influence of heart rate on pulmonary arterial-left ventricular pressure relationships at end-diastole. Circulation. 1977;56:533–539. doi: 10.1161/01.cir.56.4.533. [DOI] [PubMed] [Google Scholar]
- 26.Adir Y, Humbert M, Sitbon O, et al. Out-of-proportion pulmonary hypertension and heart failure with preserved ejection fraction. Respiration. 2013;85:471–477. doi: 10.1159/000339595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.