Abstract
Cellular identity is established by genetic, epigenetic, and environmental factors that regulate organogenesis and tissue homeostasis. Although some flexibility in fate potential is beneficial to overall organ health, dramatic changes in cellular identity can have disastrous consequences. Emerging data within the field of pancreas biology are revising current beliefs about how cellular identity is shaped by developmental and environmental cues under homeostasis and stress conditions. Here, we discuss the changes occurring in cellular states upon fate modulation and address how our understanding of the nature of this fluidity is shaping therapeutic approaches to pancreatic disorders such as diabetes and cancer.
Introduction
In most tissues, a mature cell performing a specialized function represents a terminally differentiated cell that is restricted in potential, i.e., that may possess the capacity to replicate and expand the pool of like cells, but lacks the correct configuration of factors to produce a different cell type. On the other side of the spectrum, undifferentiated (or multipotent) stem cells are poised to respond to appropriate cues and differentiate into many different cell types. These cues include key transcription factors that exert a pivotal influence over cell lineage trajectory, epigenetic factors that affect the genetic framework and expression profile of the cell, and environmental factors, such as inflammation and changes in cellular metabolism that can trigger phenotypic changes. The progression from a stem/progenitor to a differentiated state was previously considered unidirectional; however, it is now evident that fate in differentiated cells is flexible (Cohen and Melton, 2011; Graf, 2011; Cherry and Daley, 2012). Defining pliability of cell fate or identity, therefore, has been a focus of regeneration research. Specifically in the pancreas, evidence has accumulated that most terminally differentiated cell types can change fate into other pancreatic cells, supporting the notion of cellular plasticity in differentiated cells. Gain in fate plasticity may be a strategic defense mechanism that allows differentiated pancreatic cells to rest and avoid injury or death caused by sustained stress. Here we evaluate emerging data and offer insight on the connection between different cellular states and perturbations and how the degree and type of insult presented to a cell may be an important determinant in whether a normally regulated defense mechanism can become a liability.
The pancreas, derived from the endodermal lineage, is composed of functionally distinct compartments that all originate from a common pool of progenitors. Exocrine acinar cells secrete digestive enzymes that are supplied to the gut through an elaborate ductal tree, and endocrine cells regulate blood glucose through secretion of hormones, including insulin and glucagon from β and α cells, respectively. Remarkably, such diverse functional capacities emerge from a common progenitor, prompting researchers to identify regulators of cellular identity within the pancreas during development and the mechanisms governing fate flexibility after differentiation is completed. Fate plasticity in the context of pancreatic cells can be defined as the ability of differentiated cells (exocrine and endocrine) to lose features that define the functional, mature state of the cells and to adopt features of other cell types within the same organ lineage. Most likely, such changes occur in a gradual way, with progressive loss of hallmark differentiation characteristics and increasing ability to express genes that mark alternate cell types. Accumulating examples that we will discuss in this Perspective have demonstrated that mature pancreatic cells can lose their terminally differentiated and defining functional characteristics to become dedifferentiated. This state may be transient and reversible; however, prolonged stress may convert such dedifferentiation toward different types of pancreatic diseases as a result of cellular transformation or functional senescence.
Understanding the triggers that encourage cellular transitions has also uncovered events of transdifferentiation, when a mature pancreatic cell can be converted into a pancreatic cell type of another lineage. This process may occur directly with no intermediary transition stage under cases of genetic reprogramming or forced expression of influential transcription factors or through intermediate stages as the mature cell progressively dedifferentiates to a multipotent-progenitor-like stage and then redifferentiates toward another cell lineage in cases of tissue injury. This Perspective will first discuss the genetic control of cellular fate within the pancreas and then the epigenetic regulation that affects identity. Next, a summary of artificial manipulations that have uncovered important roles for genetic factors in establishing cellular identity will be discussed, after which will follow a discussion of the normal response of cells to distinct stressors, such as injury. Finally, the Perspective will consider the pathological consequences of changes in cellular fate in the context of pancreatic diseases including diabetes and cancer. The distinct changes that occur in the pancreatic cells and the interconversion between fates are depicted in Figure 1 and Table 1 and will be referred to throughout the perspective.
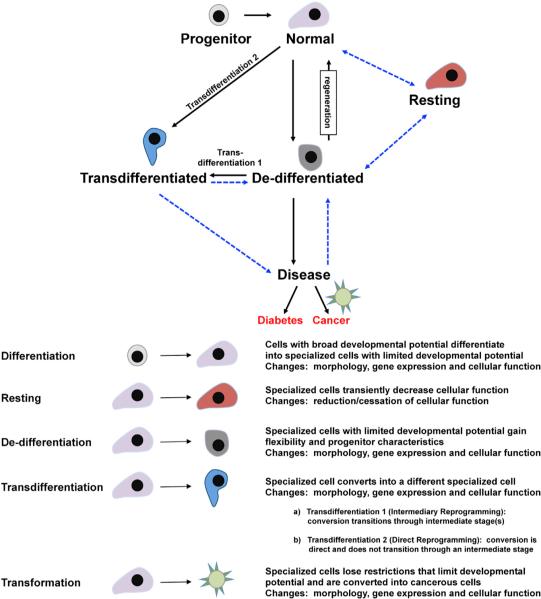
Figure 1. The Transition between Different Cellular States in Response to Genetic Manipulation or Injury and the Connection to Pancreatic Disease.
Schematic depicting the progressive cellular transitions that can potentially occur in response to insults and how these may contribute to the manifestation of different pancreatic disorders. A “Normal” cell can either change cellular identity to a novel fate, depicted as a “Transdifferentiated” cell, or lose functionality and become a “Dedifferentiated” cell. Reversal from a “Dedifferentiated” state has been observed in certain instances. Transition to a novel cellular phenotype (“Transdifferentiation”) could occur directly or through a dedifferentiated state. A hypothetical “Resting” state is also possible, wherein the cell ceases to function normally but retains key features of cellular identity and can presumably reverse back to a fully functional state. Prolonged stress, injury, or activation of oncogenic pathways can convert a “Dedifferentiated” cell into a diseased state, leading to pathogenesis. Such a state may also be achievable if the “Trans-differentiated” cell is unstable and amenable to further fate modulation. The reversibility of a diseased cell back to a “Normal” cell has great implications for therapy and remains to be established. Blue dashed arrows depict hypothetical fate changes. Detailed definitions of the different cellular states are noted.
Table 1.
Examples of Cellular Fate Change within the Pancreas
| Cellular Transition | Modifier | Mechanism of Action | Target Cell Identity |
Converted Cell Fate Identity |
Degree of Fate Change |
Setting | References |
|---|---|---|---|---|---|---|---|
| Dedifferentiation | pharmacological induction of metabolic and inflammatory injury | excessive digestive enzyme release | acinar cell | dedifferentiated acinar cell | ++ | in vivo | Jensen et al., 2005; Morris et al., 2010a; Folias et al., 2014 |
| genetic manipulation of transcription factors/signaling pathways and metabolic stress | repression of canonical β cell gene function | β cell | dedifferentiated β cell | ++ | in vivo, in vitro | Landsman et al., 2011; Talchai et al., 2012; Puri et al., 2013; Guo et al., 2013; Wang et al., 2014; Blum et al., 2014 | |
| Transdifferentiation: intermediary reprogramming | cellular injury due to pancreatic duct ligation | activation of an embryonic gene expression program | acinar cell | multipotent progenitor cell | +++ | in vivo | Pan et al., 2013 |
| genetic manipulation of transcription factors | transcriptional modulation of gene expression | β cell | α cell, δ cell | ++++ | in vivo | Talchai et al., 2012; Puri et al., 2013; Gao et al., 2014 | |
| toxin-mediated death of β cells, causing diabetes | transcriptional regulation of gene expression | α cell, δ cell | β cell | ++++ | in vivo | Thorel et al., 2010; Chera et al., 2014 | |
| Transdifferentiation: direct reprogramming | forced expression of key transcription factors | transcriptional regulation of gene expression | acinar cell | α cell, β cell, δ cell | ++++ | in vivo | Zhou et al., 2008; Li et al., 2014 |
| cytokine and growth factors | activation of STAT3 signaling | acinar cell | β cell | ++++ | in vivo, in vitro | Baeyens et al., 2006; Baeyens et al., 2009; Baeyens et al., 2014 | |
| modulation of epigenomic elements | derepression of the Arx promoter through DNA methylation | β cell | α cell | ++++ | in vivo | Dhawan et al., 2011; Papizan et al., 2011 | |
| Resting | physiological event | metabolic stress | β cell, acinar cell | β cell, acinar cell | + | in vivo | predicted |
| Transformation | oncogenic mutations in K-ras | activation of genetic/signaling programs | acinar cell, duct cell | PANIN, IPMN | +++++ | in vivo | Guerra et al., 2007; De La O et al., 2008; Morris et al., 2010a; Kopp et al., 2012; von Figura et al., 2014a |
Distinct modifiers can cause conversion of adult pancreatic cells into other differentiated cell types through multiple mechanisms.
Flexibility of Cellular Fate Is Uncovered through Manipulation of Developmental Programs
Cellular identities are created by sets of genetic, environmental, and epigenetic factors that define gene expression profiles uniquely associated with each cell fate and lineage (Huang, 2009; Cherry and Daley, 2012). Control over segregated gene expression is largely attributed to the tightly regulated expression patterns of transcription factors (Huang, 2009). Within the pancreas, an elegant transcriptional cascade has been identified that exerts such control on cellular identity (Wilson et al., 2003). During pancreatic development, a concert of transcriptional regulators leads to the specification of the exocrine (acinar and duct) and endocrine (α, β, ∂, epsilon, and PP) cell types while the cell transitions between different progenitor states (Figure 2). Many of the same regulators continue to modulate gene expression and cell fate in the adult organ long after differentiation states have been established (Arda et al., 2013). Researchers have thus turned to these factors to modulate cellular fate within the pancreas.
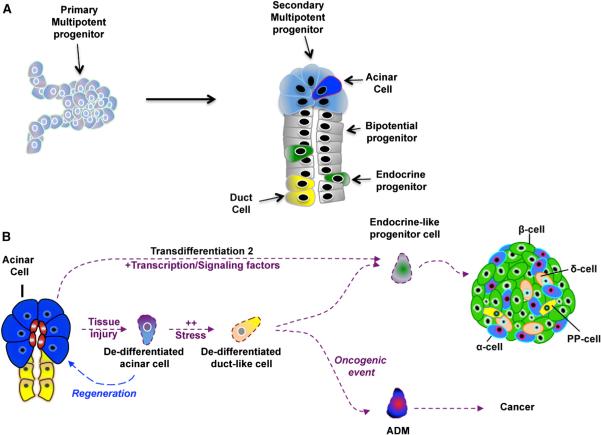
Figure 2. Acinar Dedifferentiation Is Reminiscent of Embryonic Multipotent Progenitor Cells.
(A) Schematic depicting how the different pancreatic lineages arise from a common multipotent progenitor cell that is most closely related to the acinar cell.
(B) Schematic indicating points where cellular stress (resulting from injury, expression of pivotal transcription factors, metabolic pressure, or inflammation) leads to the dedifferentiation of mature acinar cells. ADM, acinar-to-ductal metaplasia.
Pancreatic and duodenal homeobox 1 (Pdx1) is one such example, with a critical role in establishing cellular fate in the pancreas. It has been known for some time that Pdx1 is required for pancreas formation, as all compartments are derived from progenitor cells that express Pdx1 during early embryonic development (Gu et al., 2002). The absence of Pdx1 in both humans and mice results in pancreatic agenesis and a failure to generate duct, exocrine, or endocrine cell types (Jonsson et al., 1994; Offield et al., 1996; Stoffers et al., 1997b), indicating that Pdx1 is a master regulator for pancreas specification. A tremendous body of work has emerged to identify the exact activities of Pdx1 in the endocrine compartment and specifically in the insulin-producing β cell. High Pdx1 levels are maintained in a fully functional, mature β cell, and genetic deletion of Pdx1 in mouse models has clearly shown a requirement of this factor in maintenance of β cell fate and function (Ahlgren et al., 1998; Gannon et al., 2008; Gao et al., 2014). Loss of Pdx1 translates to a reduced capacity of the β cell to secrete insulin in response to elevated glucose, resulting in severe hyperglycemia. In humans, mutations in PDX1 have been linked to Maturity Onset Diabetes of the Young (MODY) (Stoffers et al., 1997a). One way Pdx1 exerts this effect is through binding directly to the insulin promoter, along with other transcriptional coactivators, to activate insulin transcription (Iype et al., 2005). It is therefore expected that a loss of Pdx1 leads to perturbed transcription of the insulin gene. Significantly, recent data demonstrate that Pdx1 actively represses genes that are associated with the α cell fate, providing new insights into how this factor impacts the maintenance of cellular identity (Gao et al., 2014). If Pdx1 is genetically deleted in adult β cells, mice suffer from severe hyperglycemia due to the loss of mature properties of β cells and the adoption of a transcriptional profile, ultrastructure, and physiological signature that more closely resembles that of an α cell. Interestingly, forced expression of Pdx1 in α cells did not convert these cells into β cells, but rather diverted these cells into a stable population of endocrine cells lacking any hormone expression (Yang et al., 2011). Thus, once the α cell program has been established, it appears that Pdx1 expression is unable to modify it toward the β cell fate. These analyses highlight the time- and context-dependent impact transcription factors can exert on cellular identity.
Endocrine fate specification within the embryonic pancreatic epithelium occurs by sequential activation and repression of specific genes. An example of such an interaction is evident by the inhibitory circuit of aristaless-related homeobox (Arx) and paired box gene 4 (Pax4), which play opposing roles during cellular differentiation. Arx promotes a cell and PP cell fates while Pax4 is involved in β/∂ cell differentiation (Collombat et al., 2003, 2005). Ectopic expression in mouse models has revealed the complex interplay of these factors in establishing cell fate.
Expression of Arx in α cells led to the appearance of α cell characteristics (Collombat et al., 2007), whereas forced expression of Pax4 converted embryonic α cells into β like cells with abnormal physiological responses (Collombat et al., 2009). Interestingly, deletion of Arx in α cells leads to loss of characteristics that define the α cell and appearance of β cell markers (Courtney et al., 2013; Wilcox et al., 2013), further supporting the notion that transcription factors simultaneously promote desirable fates while suppressing other fates within the same cell.
Due to the close developmental relationships shared by endocrine cells, many examples exist of fate conversion between the different endocrine cell types in addition to the ones described above. Specification of the endocrine lineage occurs through the combined action of numerous transcription factors (Pdx1, Nkx6.1, Nkx2.2, Sox9, Hnf6, Ngn3, Arx, Pax4, and others), and mature cell fate is maintained by a host of factors, including Pdx1, Nkx6.1, Pax6, Isl1, MafA, MafB, and NeuroD (Habener et al., 2005; Benitez et al., 2012; Mansouri, 2012). Deletion of Nkx6.1, an endocrine progenitor marker that is restricted to β cells during adulthood, can convert early-life β cells to somatostatin-expressing δ cells (Schaffer et al., 2013). Deletion of Pax6, a factor important in endocrine differentiation, causes adult islet cells to convert into ghrelin-expressing epsilon cells that are normally absent or low in postnatal islets (Hart et al., 2013). Concomitant deletion of Arx and Nkx2.2 perturbs fate specification by favoring the somatostatin/ghrelin fate at the expense of the α and β cells (Kordowich et al., 2011; Mastracci et al., 2011). Such genetic manipulations shed light on the requirement for several factors at various steps during fate spec-ification and after a differentiated state is established, and they point to the possibility of changing cell fate via approaches that manipulate the expression of transcriptional regulators.
Similar to that of the endocrine fate regulators, the identity of exocrine acinar cells also depends on a transcriptional cascade including Ptf1a, Mist1, and Nr5a2, among others. Ptf1a is expressed in progenitors that give rise to acinar cells and is maintained in differentiated cells (Kawaguchi et al., 2002; Lin et al., 2004; Beres et al., 2006; Holmstrom et al., 2011). The dosage of Ptf1a may play a role in determining fate outcome, because reduced activity of this transcription factor leads to expression of insulin in adult acinar cells (Hesselson et al., 2011). The observation that Ptf1a expression is not conducive to endocrine specification is suggested by an inhibitory interaction between Ptf1a and the proendocrine factor Nkx6.1 (Schaffer et al., 2010). Overexpression of Ptf1a in pancreatic progenitors represses Nkx6.1 expression and blocks endocrine differentiation. Thus, Ptf1a both promotes acinar cells and suppresses the endocrine program. Another factor important for maintenance of acinar identify and function is Mist1 (Direnzo et al., 2012). Mist1 knockout mice display the appearance of duct-like structures, and lineage tracing suggests that acinar cells convert to presumptive ducts upon loss of Mist1 (Pin et al., 2001). Similarly, Nr5a2 is also required for maintenance of acinar identity, and loss of Nr5a2 converts acinar cells to a duct-like state (von Figura et al., 2014b). Given the close physical relationship between acinar and ductal cells, it might not be surprising that perturbing the mature state of the acinar compartment leads to a conversion toward the ductal fate. The ready adoption of a duct-like fate by acinar cells is of great significance for understanding the mechanisms underlying the development of pancreatic ductal adenocarcinoma (PDA), and this will be discussed in detail later. Acinar cells also demonstrate the propensity to express markers of other lineages under specific conditions. Deletion of c-Myc in acinar cells during embryogenesis leads to conversion of the acinar lineage into adipocytes, as shown by lineage tracing (Bonal et al., 2009), suggesting a role for c-Myc in acinar differentiation.
Many attempts have been made to convert acinar cells into the endocrine lineage and specifically into β cells. In a landmark study, Melton and colleagues demonstrated activation of the β cell program upon ectopic expression of proendocrine genes Pdx1, MafA, and Ngn3 in acinar cells (Zhou et al., 2008). This conversion resulted in changes to cellular gene expression, as well as morphological and functional changes, without the presence of an observable transient intermediate serving as endocrine progenitor (Zhou et al., 2008). The important role of Ngn3 in suppressing exocrine fate and promoting endocrine fate was further proposed in a follow-up study with similar in vivo experiments (Li et al., 2014). Infecting acinar cells with the same three transcription factors led to the formation of ∂, α, or β cells, depending on the combination of factors employed. The authors concluded that proendocrine regulators exert effects in a temporal manner that first suppresses acinar fate and then establish an endocrine state (typified by the expression of the panendocrine gene Pax6) with subsequent induction of other endocrine genes. As discussed above, the common theme to emerge from such manipulations is that transcription factors serve activating and repressive functions concomitantly.
Less is known about the transcriptional regulation that maintains the ductal lineage within the exocrine pancreas. The ductal tree is composed of epithelial cells that express a distinct set of transcriptional regulators, including Sox9, Hnf6, and Hnf1b (Haumaitre et al., 2005; Pierreux et al., 2006; Zhang et al., 2009; Kopp et al., 2011; Delous et al., 2012; Manfroid et al., 2012). In the absence of a better description of the mature state of ducts cells, it is currently unclear what level of plasticity is expected from these differentiated cells. Overexpression of Ngn3 in duct cells isolated from human islets led to upregulated expression of some β cell genes, but also neural genes, suggesting that ectopic expression of Ngn3 is not sufficient for conversion of duct cells into bona fide β cells (Kopp et al., 2011; Swales et al., 2012). A recent paper shows that loss of a protein that participates in the recognition motif of a ubiquitin ligase in pancreatic ductal cells leads to activation of the endocrine program, as evidenced by an upregulation of Ngn3 and its downstream effectors (Sancho et al., 2014). Genetic deletion of Fbw7 leads to the stabilization of Ngn3 (normally a short-lived protein), and ductal cells adopt characteristics of β, α, and ∂ cells. Furthermore, ectopic expression of a stabilized form of Ngn3 (phospho mutant Ngn3-AA) in murine duct cells promotes transdifferentiation toward the β cell phenotype with low efficiency. While Ngn3-AA-expressing cells activate β cell markers, the response to glucose-stimulated insulin secretion is not extensive, further supporting the notion that factors other than Ngn3 are required for full conversion of duct cells into β cells (Sancho et al., 2014). The conversion frequency is low (~1%), suggesting that not all cells that are depleted of Fbw7 are equally amenable to such redirection of fate. Nevertheless, these data implicate Fbw7 as being critical for maintenance of ductal identity and provide another example of a regulator that is important in switching fates within the pancreas.
Centroacinar cells lie at the interface of acinar and terminal duct cells and have been considered candidates for pancreatic progenitor cells (Rovira et al., 2010; Cleveland et al., 2012). Furthermore, the gene expression profile of the centroacinar cell is poorly characterized aside from the expression of Sox9, a transcription factor required for endocrine specification (Seymour et al., 2007), and Hes1, an effector of the Notch pathway (Kopinke et al., 2012). Despite efforts to irreversibly mark centroacinar cells for lineage tracing, the contribution of this cell type to any other lineage in vivo remains controversial (Kopinke et al., 2011).
Epigenetic Regulation of Transcriptional Regulators Controls Cellular Identity
While transcription factors are most commonly involved in determining cellular identity, epigenetic regulation introduces additional layers of temporal and spatial control that can modify genomic information. Recent evidence suggests that the histone methylation signature of a cell can be an indicator of how easily the cell can be coaxed toward a different lineage. Global analysis of human islet cells revealed that many genes that are part of the β cell signature were bivalently marked in differentiated α cells (Bramswig et al., 2013). Such bivalency is best described in pluripotent cells, where developmental genes are silenced and yet poised for activation. Bivalency is conferred by concomitant trimethylation of histone H3 lysine 4 (H3K4, considered an active mark at the transcriptional start site and activated by the Trithorax complex) and histone H3 lysine 27 (H3K27, which transcriptionally represses through recruitment of the Polycomb group of proteins). Loss of either mark can activate or repress gene expression, allowing adoption of more than one developmental program in response to appropriate cues (Bernstein et al., 2006). These findings hint that the α cell may be amenable to expressing β cell-specific genes under the appropriate conditions. Treatment with a histone methyltransferase inhibitor led to coexpression of α and β cell genes in human islet cells (Bramswig et al., 2013), indicating that forced epigenetic manipulation can alter gene expression in vitro. Furthermore, transdifferentiation between endocrine cells subsequent to changes in genomic methylation has been observed in vivo. One example is through modulation of the methylation state of the Arx locus in differentiated β cells (Dhawan et al., 2011). A repressor complex that includes the homeodomain transcription factors Nkx2.2, Nkx6.1, a corepressor Groucho-3 (Grg3), and the de novo DNA methyltransferase Dnmt3a is recruited to the Arx locus to prevent expression during β cell replication (Papizan et al., 2011). Deletion of the DNA methyltransferase 1 (Dnmt1), which is responsible for maintaining DNA methylation upon replication, converts β cells into glucagon+ cells by derepressing the Arx promoter. Thus, transcriptional regulators and epigenetic modifiers collaborate to suppress the expression of Arx in the β cell, and loss of this regulation diverts β cell fate toward α cells.
Fate choice can also be modulated by acetylation and deacetylation of histones that in turn impacts gene expression. Both histone acetyltransferases and histone deacetylases (HDACs) play key roles in adoption of the appropriate pancreatic fate from progenitors during embryonic development (Haumaitre et al., 2008; Lenoir et al., 2011; Xu et al., 2011). Identification of small molecules that can specifically target histone modifiers permits us to test the role of these regulators in isolated cells. While treatment of the glucagon-producing α cell line αTC1 with HDAC inhibitors upregulated insulin expression, treatment of the β cell line βTC3 with these compounds led to either upregulation or downregulation of genes involved in insulin secretion, suggesting a complex regulation (Kubicek et al., 2012). Importantly, the study revealed that different classes of inhibitors could target distinct subsets of genes. Hydroxamic acids that inhibit HDAC1, HDAC2, HDAC3, HDAC6, and HDAC8 caused downregulation of a host of β cell regulatory genes (including Pdx1, Pax4, NeuroD, Nkx6.1, and Nkx2.2) as well as insulin, while orthoamino anilides, inhibiting HDAC1, HDAC2 and HDAC3, had no effect on these genes, but caused upregulation of MafB and Arx, resulting in increased glucagon expression in the β cell line. Thus, specific acetyltransferases allow modulation of endocrine cell fates, and the identification of compounds that target specific epigenetic pathways could be beneficial for fate modification.
An additional layer of epigenetic control is provided by micro-RNAs, small noncoding RNAs that silence gene expression through sequence-specific inhibition or degradation. Pancreas organogenesis relies on microRNA production (Lynn et al., 2007), and several microRNAs, including miR-375, are implicated in β cell function and gene expression (Poy et al., 2004, 2009; Kaspi et al., 2014) as well as exocrine tissue homeostasis (Prevot et al., 2013). In acinar cells, microRNAs repress expression of factors that may promote adoption of ductal fates (Prevot et al., 2013). Similarly, microRNAs in the β cell regulate expression of genes such as Mct1, which inhibits glucose-stimulated insulin secretion (Pullen et al., 2011). Further work will delineate how repression of undesirable fates by microRNAs contributes to maintenance of cellular identity and how manipulation of these small RNAs can allow cellular adoption of alternative fates.
Resting versus Dedifferentiated Cells: When Is Dedifferentiation Part of the Normal Cellular Phenotype?
The previous notion of terminal differentiation as a fixed state has been revised due to substantial evidence on the alterations in fully matured cells in response to certain stresses. Most attempts at changing cell fate involve overriding the regulatory mechanisms that are normally in place to control cell identity through artificial means. Thus, the following question arises: can this inherent plasticity be part of a cell's normal response to stress, or is it only identifiable under conditions of artificial genomic or epigenomic manipulation? Is there a narrow range of fate modification that a cell can tolerate as part of the normal physiological response? Are there distinct points of no return during changes in cellular differentiation, after which a switch back to the previous state becomes impossible? Much work lies ahead in order to fully uncover what buffering mechanisms are in place to allow a cell to maintain identity. Although increasing evidence suggests that cells do respond to challenges by transitioning to novel phenotypes, it remains to be determined whether the new differentiation state is equivalent to a cell that is formed during normal organogenesis or if it represents a new cell type.
A common cellular response to specific injuries or manipulations is the stimulation of cellular dedifferentiation (Figure 1). Currently, dedifferentiation is defined as a loss of the hallmark characteristic state that identifies a terminally differentiated cell. Cells may lose their normal mature state and become pliable to fate change, either inherently or through external stimulation. Alternatively, cells may dedifferentiate and cease to function but persist in an undefined or resting state while the insult persists. In the first instance, dedifferentiation is a transition step before the adoption of a novel identity, characterized by reemergence of factors that promote redirection of fate. In the second case, dedifferentiation could be viewed as an adaptive response to a stressful stimulus, such that the cell ceases normal activity to prohibit progression toward cellular dysfunction, culminating, in extreme cases, in cell death. This phenomenon may be viewed as a transient “resting” state for a cell to regroup and allow the insult to subside. Examples of both of these scenarios are suggested within the pancreas.
A substantial amount of evidence has demonstrated the ease with which pancreatic acinar cells dedifferentiate into cells resembling a duct-like phenotype in response to stresses such as injury or inflammation. Acinar cells undergo morphological changes, with a reduction in the expression of mature acinar markers concomitant with increased expression of factors normally expressed during embryonic development. Because the dedifferentiated cells exhibit qualities reminiscent of both a duct cell and a progenitor cell present during pancreatic development (including the expression of Sox9, Hnf6, Hes1, Pdx1, and Nestin), the term duct-like cell has been used to describe this population (Song et al., 1999; Jacquemin et al., 2003; Miyamoto et al., 2003; Jensen et al., 2005; Seymour et al., 2008; Morris et al., 2010a; Shi et al., 2013). The duct-like cells are not true progenitors, however, because they lack expression of other markers, including Hnf1b and Nkx6.1 (Jensen et al., 2005; Solar et al., 2009; Schaffer et al., 2010; Prévot et al., 2012).
A common notion is that acinar dedifferentiation may create a facultative progenitor cell capable of repopulating different types of pancreatic cells. During early development of the pancreas, multipotent progenitor cells residing at the tips of epithelial branches divide and leave behind progeny that differentiate into duct and endocrine cells that form the trunk of the branches (Figure 2A). The multipotent progenitors at the tips will eventually differentiate into acinar cells, suggesting that acinar cells are the most direct progeny of embryonic multipotent progenitor cells (Figure 2A) (Zhou et al., 2007; Solar et al., 2009). While controversy exists over whether an adult pancreatic stem cell exists, dedifferentiation of acinar cells into multipotent progenitor cells offers an attractive model for cell replacement in the adult pancreas. Indeed, acinar to duct-like dedifferentiation is observed under different models of pancreatic injury, including partial duct ligation (PDL) and chemically induced pancreatitis, perhaps suggestive of pancreatic cells adopting a more progenitor-like state that can help repopulate the organ after injury. For example, when the cholecystokinin (CCK) analog caerulein is used to pharmacologically stimulate acinar cells to secrete continuous and excessive amounts of digestive enzymes, acinar cells respond by shutting down expression of digestive enzymes and initiating expression of progenitor/stress factors (Jensen et al., 2005; Siveke et al., 2008; Morris et al., 2010a). The extent and degree of dedifferentiation is dependent upon the duration of injury, suggesting that this is a protective response that reduces the harmful effects of digestive enzymes on acinar cells and simultaneously generates a cell with progenitor-like qualities to repopulate the damaged pancreas. Notably, regeneration depends on interaction with immune cells that serve as critical regulators of acinar cell redifferentiation (Folias et al., 2014).
In another study of severe injury to the exocrine compartment, cells expressing endocrine progenitor markers localized to the ductal lining, suggesting that either a new progenitor cell is forming through the ductal lineage or acinar dedifferentiation may be enhanced in situations of intense pressure (Xu et al., 2008). Reprogramming of the ductal lineage to an endocrine fate has been controversial, because lineage tracing demonstrates that although Ngn3 is induced in duct cells upon PDL, the levels are insufficient to induce endocrine and β cell formation (Kopp et al., 2011). Ngn3 is an unstable protein, and one possibility for the failure of ductal cellular reprogramming could be rapid degradation of Ngn3. As noted above, Fbw7 was dramatically reduced upon induction of pancreatic injury, such as PDL, which might suggest that the extent of the damage (which would correlate with the reduction in Fbw7 and the consequent stabilization of Ngn3) may determine the ability of cells to undergo fate change (Sancho et al., 2014). A recent study wherein acinar cells were transiently labeled in adult mice prior to injury by PDL demonstrated that while most acinar cells died, some cells located at the tips acquired the molecular signature of embryonic multipotent progenitor cells present at the end of pancreatic branches (Pan et al., 2013). Multipotency was confirmed in these progenitors when they gave rise to exocrine acinar and duct cells, as well as cells of the endocrine pancreas (Figure 2B). This study supports the idea that adult acinar cells retain the potential to revert into progenitor-like cells with self-renewal and multipotent capacities. What remains unclear is whether all acinar cells have this capacity or whether the niche environment of the cell might limit or create increased multipotentiality. It is also important to consider the type of insult, because different models of injury provide divergent outcomes (Desai et al., 2007).
Dedifferentiation in the context of a diseased state has been observed in the endocrine compartment as well, although the physiological stressors are distinct from those in the exocrine system. In the presence of metabolic stress due to persistent hyperglycemia and insulin resistance, β cells are forced to function at supranormal levels. Such glucotoxicity leads to loss of cellular function and cell death (Butler et al., 2003; Poitout and Robertson 2008). However, different types of stresses, either genetic or metabolic, have all converged on a new look at dedifferentiation in the β cell as a potential cause for disease, and dedifferentiation has been recently introduced as an alternative fate to cell death in the context of type II diabetes (Figure 3). For example, β cell-specific deletion of the transcription factor Foxo1 during states of metabolic stress (including aging and pregnancy) results in reduced β cell mass that causes diabetes (Talchai et al., 2012). Intriguingly, lineage-tracing studies showed that β cells do not undergo apoptosis but dedifferentiate into cells that have lost the canonical β cell marker expression. These dedifferentiated β cells appeared to express stem cell markers, including Oct4, and could occasionally redifferentiate into other hormone-producing cell types (Talchai et al., 2012). Similar findings were reported in a mouse model of β cell inexcitability, wherein ectopic expression of a gain-of-function mutation in the KATP channel led to dedifferentiation in β cells (Wang et al., 2014). In both studies, progenitor markers, defined as genes normally absent from mature β cells but present in pancreas progenitors, were observed in the dedifferentiated β cells, while adoption of non-β cell fates by the de-differentiated cells was not observed upon sustained activation of the KATP channel. Thus, depending on the exact nature of the stressor, distinct outcomes are reached. The onset of dedifferentiation also had significantly different temporal requirements in each study. In the Talchai et al. study, diabetes onset was well into adulthood, and it presented with a progressive deterioration of β cell function, reminiscent of how the disease develops in humans. The dedifferentiation seen in the activated KATP context occurs rapidly, in that hyperglycemia is observed within 20 days after administration of tamoxifen. Thus, different perturbations may impact the timing and extent of β cell dedifferentiation. Similarly, oxidative stress modifies the cellular state, causing reduced activity of important transcription factors as a result of displacement from the nucleus (Guo et al., 2013). PDX1 and a small subset of key islet-enriched transcription factors were reduced in β cells from islets isolated from type II diabetic patients (Guo et al., 2013) and in rodents with chronic hyperglycemia (Harmon et al., 1999; Jonas et al., 1999). Interestingly, progenitor factors (NEUROGENIN 3, NANOG, POU5F1, and MYCL1) found in previous rodent studies were not detected in islets from humans with type II diabetes, suggesting that there may be species-specific differences in the stress response of islets (Guo et al., 2013). This study underscores the importance of subcellular localization of transcription factors, because gene expression levels would be less informative in such a scenario. Inappropriate activation of the embryonic Hedgehog signaling pathway in adult β cells also induces dedifferentiation and compromised cellular function, including reactivation of the progenitor markers Hes1 and Sox9 (Landsman et al., 2011). While transdifferentiation into other pancreatic cell types was not observed, a remarkable finding was that silencing of the Gli2 transgene responsible for sustained Hedgehog signaling activity allowed cells to redifferentiate into functional β cells (Landsman et al., 2011). These findings support the notion of reversibility of a dedifferentiated state upon cessation of the original stressor.
Figure 3. Endocrine Fate Conversion within the Islet.
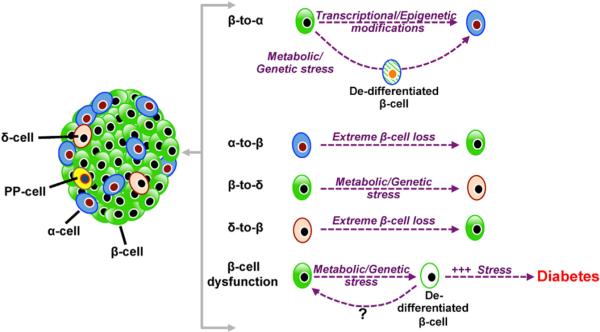
Fate change between the different endocrine cells is observed under different conditions of stress. This may occur either directly or through a dedifferentiated state. Continued stress on the β cell can lead to dedifferentiation that causes diabetes.
Lastly, alterations in the metabolic state of the cell can cause modifications in cellular identity. This is evident upon erroneous activation of the hypoxia response pathway that leads to β cell dedifferentiation, with concomitant activation of a subset of embryonic markers (Puri et al., 2013). In the majority of the studies, β cells appeared to persist in a dedifferentiated state, with only a fraction of the dedifferentiated cells demonstrating the ability to adopt alternative fates. Such prolonged dedifferentiation may be viewed as a mechanism that allows the cell to reduce the need to continuously produce insulin and thus achieve a resting state that at least temporarily might stave off cell death signaling. Evidence that such a state is reversible comes from the observation that introduction of exogenous insulin lowered the physiological hyperglycemia in KATP mutant animals and appeared to reverse the β cell dedifferentiation phenotype (Wang et al., 2014). A pressing question is whether such redifferentiation occurs under other stress conditions as well. It is likely that the extent of β cell dedifferentiation will determine how reversible this condition would be. In other words, impaired β cell functionality that might be observed during the early stages of human prediabetes or diabetes might be reversible if the underlying stressor that causes β cell dedifferentiation is removed. A detailed understanding of this process is an avenue worth pursuing, as it could have significant implications toward our understanding of the development and management of diabetes.
Collectively, manipulations within both the endocrine and exocrine compartments suggest that distinct pressures cause cells to first transition into a “resting” state (Figure 1), with down-regulation of factors that are creating stress, such as those promoting production of digestive enzymes in the acinar cell and insulin in the β cell. This initial decrease in expression of factors critical to the overall function of the cell may represent a transition period that occurs before dedifferentiation, wherein cells maintain normal cellular morphology and defining characteristics. If the pressure is too great or persistent enough, this resting state progresses further to an overt dedifferentiated state wherein the cell undergoes more dramatic changes in the expression of defining factors, as well as changes in morphological and functional features (Figure 1 and Figure 2B). It is reasonable to consider that both the resting and dedifferentiated states of a cell are transient and that the cell can be restored to normal if the pressures are relieved in time (Landsman et al., 2011). While reversibility of fate in dedifferentiated β cells is still a new concept, this idea is clearly evident in models wherein pancreatic acinar cells are able to regenerate after dedifferentiation in response to caerulein treatment (Jensen et al., 2005). However, it is important to consider that persistent and/or intense pressure can cause erosion of normal phenotypic and functional characteristics to a point where the cell undergoes permanent changes in cellular fate.
Is Dedifferentiation a Gateway for Transdifferentiation?
An interesting phenomenon in progressive dedifferentiation is the process of trans-differentiation (Figure 1). We define transdifferentiation or cellular reprogramming as the conversion of one specialized cell into a different cell lineage (Figure 1). Different methods, both naturally occurring and experimental manipulations, have resulted in the transdifferentiation of pancreatic cells in both the endocrine and exocrine compartments. This process of cellular reprogramming might occur via distinct routes (Figure 1). For example, intermediary transdifferentiation requires that a mature cell first dedifferentiate into a progenitor cell that can then give rise to a cell of a different origin (Transdifferentiation 1, Figure 1). A second scenario that is documented in the field is the conversion of cell fates without an observable or detectable intermediate step; this is termed direct reprograming (or Transdifferentiation 2, Figure 1).
An example of intermediary transdifferentiation can be seen when acinar cells dedifferentiate into a duct-like cell with exocrine and endocrine potential. So far, evidence suggests that pancreatic cells only give rise to other pancreatic lineages and not other tissues or germ layers. Even though the appearance of stem cell markers Oct4 and Nanog has been reported in dedifferentiated β cells (Talchai et al., 2012), there is no evidence that these cells can give rise to nonendocrine lineages despite the expression of pluripotency markers. It is conceivable that a dedifferentiated cell might retrace the normal developmental path toward a different, albeit closely related, cell type once it has reached the progenitor state.
Direct reprogramming of the exocrine pancreas into an endocrine population using exogenous factors demonstrates that cells can be prompted to directly transdifferentiate (Zhou et al., 2008; Li et al., 2014). Recent reports further demonstrated both in vitro (Baeyens et al., 2005, 2006, 2009) and in vivo (Baeyens et al., 2014) that acinar cells can be reprogrammed into β like cells in response to cytokine treatment with epidermal growth factor (EGF) and ciliary neurotrophic factor (CNTF). Notably, treatment with EGF and CNTF led to reprogramming of acinar cells in hyperglycemic diabetic mice by generating a functional α cell mass sufficient to restore and maintain normoglycemia in an indirect process dependent on Ngn3 and signaling through Stat3 (Baeyens et al., 2014). The authors do not report a dedifferentiation step in the process of converting the acinar cells into endocrine progenitors, furthering the notion that acinar cells directly (without transitioning through a dedifferentiation state) switch fate into the β cell lineage. While these studies provide evidence that cells originating from the acinar lineage can be reprogrammed into β cells capable of regulating glycemia to a certain extent, it remains unclear whether acinar-derived cells achieve a state identical to that of normal islet β cells.
As previously discussed, the endocrine pancreas also appears to have a significant ability to switch fates between different islet cell types. Ablation of β cells prompted the adjacent glucagon-expressing α cells and somatostatin-expressing ∂ cells to spontaneously acquire features of insulin-producing β cells (Thorel et al., 2010; Chera et al., 2014). Furthermore, conversion of β cells into α like cells after loss of Pdx1 showed no evidence for an endocrine progenitor state (described as the lack of expression of Arx or Ngn3), suggesting a direct conversion through a hybrid state in which cells display properties of both α and β cells (Gao et al., 2014). Reaggregation of dispersed human islets led to spontaneous conversion of β cells into α cells in an Arx-dependent manner, although the glucagon-expressing cells continue to express Pdx1 and Nkx6.1 (Spijker et al., 2013). This cell may resemble the multihormone-positive cell that can be generated from human embryonic stem cells during directed differentiation, wherein inappropriate cues can lead to simultaneous activation of β cell and α cell-specific genes (Rezania et al., 2011). Conversion of β cells into other endocrine cells subsequent to dedifferentiation has also been demonstrated (Talchai et al., 2012; Puri et al., 2013). Such fate conversion between endocrine cells is likely facilitated by the substantial similarity in genome-wide chromatin structure between the different endocrine cells. Thus, suppression of β cell identity might eliminate factors that normally repress non-β endocrine cell identities. Overall the idea that the identity of endocrine cells is fragile and can be disrupted in cases of metabolic stress has led to the theory that β cell dedifferentiation, rather than β cell death, is an underappreciated cause of β cell failure in diabetes (in type II and possibly type I diabetes). If this is the case, reversal of this process might be achievable by pushing dedifferentiated β cells or transdifferentiated endocrine cells back to their original functional mature state (Landsman et al., 2011).
Importantly, full transdifferentiation is currently difficult to establish because we are still trying to establish parameters to evaluate the extent of conversion. In general, it is not clear whether morphology, gene expression, epigenetic landscape, ultrastructure, or function of a transdifferentiated cell are identical to that of a cell formed during normal development. The most critical factors that need to be resolved to fully evaluate transdifferentiated cells are the extent of their maturity and the stability of the newly acquired characteristics. While examples clearly exist that describe direct reprograming without the requirement of an intermediate transition stage, future work will help resolve how these changes in cellular phenotypes differ from those of intermediary reprogramming or development.
Hijacking Flexibility for Irreversibility: The Path to Cancer
Pathological conditions that encourage fluidity in cellular plasticity and the reemergence of progenitor factors in both the endocrine and exocrine compartment can also render these cells susceptible to oncogenic transformation. In the absence of tumorigenic pressures, it is believed that partially differentiated cells prefer to revert back toward a more stable state of terminal differentiation. In the case of cellular transformation involving active oncogenes, the continuous change suggests that neoplastic cells have lost fate constraints and can adopt a permanently dedifferentiated state that promotes cancer formation.
A clear example of such plasticity being misdirected toward disease is in the etiology of pancreatic adenocarcinoma (PDA). Current data support the notion that PDA can arise from distinct neoplastic lesions, including pancreatic intraepithelial neoplasia (PanIN), mucinous cystic neoplasm (MCN), and intraductal papillary mucinous neoplasia (IPMN) (Feldmann et al., 2007; Matthaei et al., 2011). Determining the cellular source of PDA has been challenging in humans because the histological appearances and morphological features used to classify neoplasia and tumors do not necessarily predict the cell of origin. Nevertheless, an impressive body of research using animal models has been devoted to identifying mutations and characterizing the precursor lesions that lead to formation of PDA within the pancreas. Recent evidence points to both acinar and duct cells as potential sources of precancerous lesions (Carrière et al., 2007; Guerra et al., 2007; De La O et al., 2008; Habbe et al., 2008; Morris et al., 2010a; Kopp et al., 2012; Pylayeva-Gupta et al., 2012; Shi and Hruban 2012; von Figura et al., 2014a). Acinar cells undergo dedifferentiation into a duct-like state under certain types of injury, as described previously. Activated Kras (KrasG12D) has the ability to hijack the dedifferentiated cellular state to initiate formation of PanIN lesions in mouse models (Morris et al., 2010b; Kopp et al., 2012). Lineage tracing studies have demonstrated that KrasG12D can induce PanIN formation by destabilizing the acinar cell phenotype and initiating the transition of acinar cells into a duct-like state, an important event in tumor progression (Habbe et al., 2008; Kopp et al., 2012). Furthermore, the ductal factor Sox9 synergizes with Kras to accelerate acinar reprogramming, indicating that progression through a duct-like state may be a critical determinant of Kras-induced PanIN formation (Kopp et al., 2012).
Duct cells have also been suggested as a cellular source of origin for PDA. Specific deletion of the epigenetic regulator Brg1, an essential member of SWI/SNF complexes, renders duct cells sensitive to oncogenic Kras-driven neoplastic transformation, supporting the idea that duct cells can contribute to the formation of IPMN lesions (von Figura et al., 2014a). It remains unclear whether duct cells undergo a similar dedifferentiation step observed in acinar cells during or prior to Kras-driven transformation. It is also unknown whether dedifferentiation of both mature acinar cells and mature ductal cells can result in a similar dedifferentiated duct-like progenitor cell in both cases. Hypothetically, dedifferentiation of both cell types might lead to the formation of a similar intermediate progenitor cell, with the context-dependent genetic and environmental factors shaping the aggressiveness and nature of PDA development. Alternatively, acinar and duct-derived cancers retain information regarding their origin, suggesting profound differences despite similar morphology of the respective tumors. Independent of the cell of origin, factors like inflammation and metabolic stress, accompanied by a genetic predisposition, can catapult a dedifferentiated cell into a disease-causing trajectory that leads to transformation and changes in both cellular identity and behavior (Figure 1). Thus, in the presence of oncogenic mutations, fate plasticity proves to be detrimental.
Cancers of the endocrine pancreas called pancreatic neuroendocrine tumors (pNETs) can occur that contain either nonfunctional endocrine cells (maintaining an apparent endocrine cell identity, but not secreting any identifiable hormone) or functioning endocrine cells (cells that can secrete normal islet hormones, hormones characteristic of the fetal pancreas, or factors produced by islet cells at any stage of development) (Klöppel, 2011). Islet dedifferentiation and the acquisition of a different endocrine-like identity may also be the driving force that propels genetically predisposed cells into an oncogenic transformation. Currently the cell of origin of pNET in humans remains unknown. Manipulation of tumor suppressor genes in β and α cells induces insulinomas and glucagonomas, supporting the tumorigenic potential of these cells and an endocrine origin of pNET (Bertolino et al., 2003; Lu et al., 2010). However, it has also been suggested that pNET might actually arise from the exocrine pancreas, and recent data demonstrating endocrine potential from acinar cells support this idea, but whether this actually occurs in humans remains a mystery (Kamisawa et al., 2002; Vortmeyer et al., 2004). Summarily, it appears that risk factors such as chronic pancreatitis or genetic variants that render pancreatic cells more plastic may reduce the threshold for pancreatic dedifferentiation and increase the likelihood of the development of different types of pancreatic cancers.
Clinical Implications: How Are Therapeutic Strategies Changing?
Understanding that pancreatic cells are more fluid than previously expected and that the changes in cellular plasticity contribute to the manifestation of different pancreatic diseases promises a tremendous impact on therapeutic approaches. Importantly, identifying factors that promote, inhibit, or stabilize this process could have valuable implications for different treatment strategies for β cell replacement therapy, diabetes, and pancreatic cancer. In the mouse model of pancreatic cancer, removal of oncogenic Kras activity in established precursor lesions can revert the cells back to an acinar fate and reduce tumor burden (Collins et al., 2012; Ying et al., 2012), pointing to a need for the development of Kras inhibitors. If changes in cell fate are as reversible as they are readily created, then new therapeutics can target factors that inhibit dedifferentiation or promote redifferentiation depending on the physiological situation. Accomplishing this in a functional and controlled manner requires the detailed and progressive analysis of different cellular states during degenerative diseases, as well as the identification of biomarkers that can differentiate these distinct transition states.
The notion that cellular fates of both mature and immature cells can easily be altered by manipulation of specific factors emphasizes the need for us to identify the critical regulators that exert a pivotal influence over the differentiation fate of individual pancreatic cells. Such information is directly applicable to current β cell replacement strategies that are aimed at generating β cells for transplantation purposes from the differentiation of alternative cellular sources such as human embryonic stem cells. Identification of regulators that can modify, maintain, or erode cellular fate can lead to the application of such information to optimizing differentiation protocols that are geared toward generating vast quantities of β cells for replacement therapy. Additionally, different approaches in regenerative medicine designed to promote the transdifferentiation of pancreatic cell types into β cells are also dependent upon manipulating the expression of influential transcription factors or modifying the epigenetic landscape of cells to promote changes in cellular fate. Lastly, understanding how different factors can alter cellular differentiation increases our ability to perform sophisticated, and easily manipulated, disease modeling in human in vitro systems (Shang et al., 2014). These new ideas in cellular plasticity expand our ability to manipulate patient-specific cells that have gene mutations already identified. These cells can be used as a screening platform for drug discovery and for the exploration of how mutations affect the function of individual cells removed from a complex physiological system, and they can provide in vitro systems wherein human mutations can be corrected and investigated. Overall, it is becoming clear that understanding cellular plasticity and differentiation offers applicable therapeutic potential to pancreatic tissue injury and regeneration, diabetes, and pancreatic cancer.
Conclusion
Our understanding of the impact of cellular plasticity on tissue function and disease formation continues to evolve. Manipulations within embryonic progenitors during pancreas organogenesis have informed us of the critical players that regulate cellular identity. Applying such findings to mature, fully differentiated cells has revealed that pancreatic cells exhibit more fluidity in cellular fate than previously thought. Examples of such fate flexibility have also been noted in other tissues. For instance, dedifferentiation in response to injury in nerve cells promotes regeneration through replication, and transdifferentiation of B cells into macrophages can be induced by forced expression of key transcription factors (Jopling et al., 2011). In response to genetic factors, epigenetic factors, and environmental cues, cells can be prompted to transition through different stages. The propensity and extent of these cellular transitions is dependent upon the severity of exposure and receptivity of the cell to these stimuli. Naturally occurring pressures such as injury or metabolic or inflammatory stress can induce a more pliable cellular state (either a resting state or a dedifferentiated state) that can be transient or prolonged depending on the duration of the insult. A cell's adoption of a resting or dedifferentiated state can temporarily allow a suspension of the normal cellular functions as a preventive measure to circumvent injury or death. This idea is exemplified by the fragility of endocrine cells that can undergo dedifferentiation rather than apoptosis in cases of persistent metabolic stress (Talchai et al., 2012; Guo et al., 2013; Puri et al., 2013). Alternatively, dedifferentiation in response to stress may cause the emergence of cellular flexibility that permits a cell's transformation into a different cellular fate. This may lead the cell to undergo intermediary transdifferentiation, progressively converting into another terminally differentiated cell. Finally, pathological conditions that encourage fate fluidity and the reemergence of progenitor factors can also render pancreatic cells susceptible to oncogenic transformation. Identifying ways to harvest or inhibit this flexibility in a directed effort can improve different approaches in the prevention and treatment of pancreatic diseases. The idea that terminally differentiated cells retain an inherent plasticity substantially increases the opportunities of targets that can be used to restore tissue function in cases of disease and destruction.
ACKNOWLEDGMENTS
We thank Drs. Audrey Parent, Nilotpal Roy, and Holger A. Russ for critical reads of the manuscript. We thank The Leona M. and Harry B. Helmsley Charitable Trust (09PG-T1D018), the Juvenile Diabetes Research Foundation (JDRF 17-2013-380), and the NIH and NCI (R01CA172045) for supporting the work in the laboratory of M.H. and The Juvenile Diabetes Research Foundation (3-2011-95) for supporting the postdoctoral fellowship of A.E.F.
Footnotes
AUTHOR CONTRIBUTIONS
S.P., A.E.F., and M.H. conceived the general topics of the Perspective and cowrote the manuscript.
REFERENCES
- Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arda HE, Benitez CM, Kim SK. Gene regulatory networks governing pancreas development. Dev. Cell. 2013;25:5–13. doi: 10.1016/j.devcel.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens L, De Breuck S, Lardon J, Mfopou JK, Rooman I, Bouwens L. In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia. 2005;48:49–57. doi: 10.1007/s00125-004-1606-1. [DOI] [PubMed] [Google Scholar]
- Baeyens L, Bonné S, German MS, Ravassard P, Heimberg H, Bouwens L. Ngn3 expression during postnatal in vitro beta cell neogenesis induced by the JAK/STAT pathway. Cell Death Differ. 2006;13:1892–1899. doi: 10.1038/sj.cdd.4401883. [DOI] [PubMed] [Google Scholar]
- Baeyens L, Bonne S, Bos T, Rooman I, Peleman C, Lahoutte T, German M, Heimberg H, Bouwens L. Notch signaling as gatekeeper of rat acinar-to-beta-cell conversion in vitro. Gastroenterology. 2009;136:1750–1760. e1713. doi: 10.1053/j.gastro.2009.01.047. [DOI] [PubMed] [Google Scholar]
- Baeyens L, Lemper M, Leuckx G, De Groef S, Bonfanti P, Stangé G, Shemer R, Nord C, Scheel DW, Pan FC, et al. Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nat. Biotechnol. 2014;32:76–83. doi: 10.1038/nbt.2747. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Benitez CM, Goodyer WR, Kim SK. Deconstructing pancreas developmental biology. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a012401. http://dx.doi.org/10.1101/cshperspect.a012401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beres TM, Masui T, Swift GH, Shi L, Henke RM, MacDonald RJ. PTF1 is an organ-specific and Notch-independent basic helix-loop-helix complex containing the mammalian Suppressor of Hairless (RBP-J) or its paralogue, RBP-L. Mol. Cell. Biol. 2006;26:117–130. doi: 10.1128/MCB.26.1.117-130.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Bertolino P, Tong WM, Herrera PL, Casse H, Zhang CX, Wang ZQ. Pancreatic beta-cell-specific ablation of the multiple endocrine neoplasia type 1 (MEN1) gene causes full penetrance of insulinoma development in mice. Cancer Res. 2003;63:4836–4841. [PubMed] [Google Scholar]
- Blum B, Roose AN, Barrandon O, Maehr R, Arvanites AC, Davidow LS, Davis JC, Peterson QP, Rubin LL, Melton DA. Reversal of beta cell de-differentiation by a small molecule inhibitor of the TGFbeta pathway. eLife. 2014;3 doi: 10.7554/eLife.02809. http://dx.doi.org/10.7554/eLife.02809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonal C, Thorel F, Ait-Lounis A, Reith W, Trumpp A, Herrera PL. Pancreatic inactivation of c-Myc decreases acinar mass and transdifferentiates acinar cells into adipocytes in mice. Gastroenterology. 2009;136:309–319. e309. doi: 10.1053/j.gastro.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Bramswig NC, Everett LJ, Schug J, Dorrell C, Liu C, Luo Y, Streeter PR, Naji A, Grompe M, Kaestner KH. Epigenomic plasticity enables human pancreatic α to β cell reprogramming. J. Clin. Invest. 2013;123:1275–1284. doi: 10.1172/JCI66514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Carrière C, Seeley ES, Goetze T, Longnecker DS, Korc M. The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia. Proc. Natl. Acad. Sci. USA. 2007;104:4437–4442. doi: 10.1073/pnas.0701117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chera S, Baronnier D, Ghila L, Cigliola V, Jensen JN, Gu G, Furuyama K, Thorel F, Gribble FM, Reimann F, Herrera PL. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature. 2014;514:503–507. doi: 10.1038/nature13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry AB, Daley GQ. Reprogramming cellular identity for regenerative medicine. Cell. 2012;148:1110–1122. doi: 10.1016/j.cell.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland MH, Sawyer JM, Afelik S, Jensen J, Leach SD. Exocrine ontogenies: on the development of pancreatic acinar, ductal and centroacinar cells. Semin. Cell Dev. Biol. 2012;23:711–719. doi: 10.1016/j.semcdb.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DE, Melton D. Turning straw into gold: directing cell fate for regenerative medicine. Nat. Rev. Genet. 2011;12:243–252. doi: 10.1038/nrg2938. [DOI] [PubMed] [Google Scholar]
- Collins MA, Bednar F, Zhang Y, Brisset JC, Galbán S, Galbán CJ, Rakshit S, Flannagan KS, Adsay NV, Pasca di Magliano M. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J. Clin. Invest. 2012;122:639–653. doi: 10.1172/JCI59227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003;17:2591–2603. doi: 10.1101/gad.269003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P, Hecksher-Sørensen J, Broccoli V, Krull J, Ponte I, Mundiger T, Smith J, Gruss P, Serup P, Mansouri A. The simultaneous loss of Arx and Pax4 genes promotes a somatostatin-producing cell fate specification at the expense of the alpha- and beta-cell lineages in the mouse endocrine pancreas. Development. 2005;132:2969–2980. doi: 10.1242/dev.01870. [DOI] [PubMed] [Google Scholar]
- Collombat P, Hecksher-Sørensen J, Krull J, Berger J, Riedel D, Herrera PL, Serup P, Mansouri A. Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression. J. Clin. Invest. 2007;117:961–970. doi: 10.1172/JCI29115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney M, Gjernes E, Druelle N, Ravaud C, Vieira A, Ben-Othman N, Pfeifer A, Avolio F, Leuckx G, Lacas-Gervais S, et al. The inactivation of Arx in pancreatic α-cells triggers their neogenesis and conversion into functional β-like cells. PLoS Genet. 2013;9:e1003934. doi: 10.1371/journal.pgen.1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La O JP, Emerson LL, Goodman JL, Froebe SC, Illum BE, Curtis AB, Murtaugh LC. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc. Natl. Acad. Sci. USA. 2008;105:18907–18912. doi: 10.1073/pnas.0810111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delous M, Yin C, Shin D, Ninov N, Debrito Carten J, Pan L, Ma TP, Farber SA, Moens CB, Stainier DY. Sox9b is a key regulator of pancreaticobiliary ductal system development. PLoS Genet. 2012;8:e1002754. doi: 10.1371/journal.pgen.1002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, Leach SD, Stoffers DA. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J. Clin. Invest. 2007;117:971–977. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan S, Georgia S, Tschen SI, Fan G, Bhushan A. Pancreatic β cell identity is maintained by DNA methylation-mediated repression of Arx. Dev. Cell. 2011;20:419–429. doi: 10.1016/j.devcel.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Direnzo D, Hess DA, Damsz B, Hallett JE, Marshall B, Goswami C, Liu Y, Deering T, Macdonald RJ, Konieczny SF. Induced Mist1 expression promotes remodeling of mouse pancreatic acinar cells. Gastroenterology. 2012;143:469–480. doi: 10.1053/j.gastro.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann G, Beaty R, Hruban RH, Maitra A. Molecular genetics of pancreatic intraepithelial neoplasia. J. Hepatobiliary Pancreat. Surg. 2007;14:224–232. doi: 10.1007/s00534-006-1166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folias AE, Penaranda C, Su AL, Bluestone JA, Hebrok M. Aberrant innate immune activation following tissue injury impairs pancreatic regeneration. PLoS ONE. 2014;9:e102125. doi: 10.1371/journal.pone.0102125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon M, Ables ET, Crawford L, Lowe D, Offield MF, Magnuson MA, Wright CV. pdx-1 function is specifically required in embryonic beta cells to generate appropriate numbers of endocrine cell types and maintain glucose homeostasis. Dev. Biol. 2008;314:406–417. doi: 10.1016/j.ydbio.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, McKenna B, Li C, Reichert M, Nguyen J, Singh T, Yang C, Pannikar A, Doliba N, Zhang T, et al. Pdx1 maintains β cell identity and function by repressing an α cell program. Cell Metab. 2014;19:259–271. doi: 10.1016/j.cmet.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T. Historical origins of transdifferentiation and reprogramming. Cell Stem Cell. 2011;9:504–516. doi: 10.1016/j.stem.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Guerra C, Schuhmacher AJ, Cañamero M, Grippo PJ, Verdaguer L, Pérez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. Inactivation of specific β cell transcription factors in type 2 diabetes. J. Clin. Invest. 2013;123:3305–3316. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habbe N, Shi G, Meguid RA, Fendrich V, Esni F, Chen H, Feldmann G, Stoffers DA, Konieczny SF, Leach SD, Maitra A. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc. Natl. Acad. Sci. USA. 2008;105:18913–18918. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener JF, Kemp DM, Thomas MK. Minireview: transcriptional regulation in pancreatic development. Endocrinology. 2005;146:1025–1034. doi: 10.1210/en.2004-1576. [DOI] [PubMed] [Google Scholar]
- Harmon JS, Gleason CE, Tanaka Y, Oseid EA, Hunter-Berger KK, Robertson RP. In vivo prevention of hyperglycemia also prevents glucotoxic effects on PDX-1 and insulin gene expression. Diabetes. 1999;48:1995–2000. doi: 10.2337/diabetes.48.10.1995. [DOI] [PubMed] [Google Scholar]
- Hart AW, Mella S, Mendrychowski J, van Heyningen V, Kleinjan DA. The developmental regulator Pax6 is essential for maintenance of islet cell function in the adult mouse pancreas. PLoS ONE. 2013;8:e54173. doi: 10.1371/journal.pone.0054173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haumaitre C, Barbacci E, Jenny M, Ott MO, Gradwohl G, Cereghini S. Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc. Natl. Acad. Sci. USA. 2005;102:1490–1495. doi: 10.1073/pnas.0405776102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haumaitre C, Lenoir O, Scharfmann R. Histone deacetylase inhibitors modify pancreatic cell fate determination and amplify endocrine progenitors. Mol. Cell. Biol. 2008;28:6373–6383. doi: 10.1128/MCB.00413-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselson D, Anderson RM, Stainier DY. Suppression of Ptf1a activity induces acinar-to-endocrine conversion. Curr. Biol. 2011;21:712–717. doi: 10.1016/j.cub.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrom SR, Deering T, Swift GH, Poelwijk FJ, Mangelsdorf DJ, Kliewer SA, MacDonald RJ. LRH-1 and PTF1-L coregulate an exocrine pancreas-specific transcriptional network for digestive function. Genes Dev. 2011;25:1674–1679. doi: 10.1101/gad.16860911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. Reprogramming cell fates: reconciling rarity with robustness. BioEssays. 2009;31:546–560. doi: 10.1002/bies.200800189. [DOI] [PubMed] [Google Scholar]
- Iype T, Francis J, Garmey JC, Schisler JC, Nesher R, Weir GC, Becker TC, Newgard CB, Griffen SC, Mirmira RG. Mechanism of insulin gene regulation by the pancreatic transcription factor Pdx-1: application of pre-mRNA analysis and chromatin immunoprecipitation to assess formation of functional transcriptional complexes. J. Biol. Chem. 2005;280:16798–16807. doi: 10.1074/jbc.M414381200. [DOI] [PubMed] [Google Scholar]
- Jacquemin P, Lemaigre FP, Rousseau GG. The Onecut transcription factor HNF-6 (OC-1) is required for timely specification of the pancreas and acts upstream of Pdx-1 in the specification cascade. Dev. Biol. 2003;258:105–116. doi: 10.1016/s0012-1606(03)00115-5. [DOI] [PubMed] [Google Scholar]
- Jensen JN, Cameron E, Garay MV, Starkey TW, Gianani R, Jensen J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patanè G, Laybutt R, Bonner-Weir S, Weir GC. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J. Biol. Chem. 1999;274:14112–14121. doi: 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat. Rev. Mol. Cell Biol. 2011;12:79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- Kamisawa T, Tu Y, Egawa N, Ishiwata J, Tsuruta K, Okamoto A, Hayashi Y, Koike M, Yamaguchi T. Ductal and acinar differentiation in pancreatic endocrine tumors. Dig. Dis. Sci. 2002;47:2254–2261. doi: 10.1023/a:1020139328215. [DOI] [PubMed] [Google Scholar]
- Kaspi H, Pasvolsky R, Hornstein E. Could microRNAs contribute to the maintenance of β cell identity? Trends Endocrinol. Metab. 2014;25:285–292. doi: 10.1016/j.tem.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat. Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- Klöppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer. 2011;18(1):S1–S16. doi: 10.1530/ERC-11-0013. [DOI] [PubMed] [Google Scholar]
- Kopinke D, Brailsford M, Shea JE, Leavitt R, Scaife CL, Murtaugh LC. Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas. Development. 2011;138:431–441. doi: 10.1242/dev.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopinke D, Brailsford M, Pan FC, Magnuson MA, Wright CV, Murtaugh LC. Ongoing Notch signaling maintains phenotypic fidelity in the adult exocrine pancreas. Dev. Biol. 2012;362:57–64. doi: 10.1016/j.ydbio.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, Ma J, Sander M. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris JP, 4th, Pan FC, Akiyama H, Wright CV, Jensen K, et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:737–750. doi: 10.1016/j.ccr.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordowich S, Collombat P, Mansouri A, Serup P. Arx and Nkx2.2 compound deficiency redirects pancreatic alpha- and beta-cell differentiation to a somatostatin/ghrelin co-expressing cell lineage. BMC Dev. Biol. 2011;11:52. doi: 10.1186/1471-213X-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicek S, Gilbert JC, Fomina-Yadlin D, Gitlin AD, Yuan Y, Wagner FF, Holson EB, Luo T, Lewis TA, Taylor B, et al. Chromatin-targeting small molecules cause class-specific transcriptional changes in pancreatic endocrine cells. Proc. Natl. Acad. Sci. USA. 2012;109:5364–5369. doi: 10.1073/pnas.1201079109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman L, Parent A, Hebrok M. Elevated Hedgehog/Gli signaling causes beta-cell dedifferentiation in mice. Proc. Natl. Acad. Sci. USA. 2011;108:17010–17015. doi: 10.1073/pnas.1105404108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir O, Flosseau K, Ma FX, Blondeau B, Mai A, Bassel-Duby R, Ravassard P, Olson EN, Haumaitre C, Scharfmann R. Specific control of pancreatic endocrine β- and δ-cell mass by class IIa histone deacetylases HDAC4, HDAC5, and HDAC9. Diabetes. 2011;60:2861–2871. doi: 10.2337/db11-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Nakanishi M, Zumsteg A, Shear M, Wright C, Melton DA, Zhou Q. In vivo reprogramming of pancreatic acinar cells to three islet endocrine subtypes. eLife. 2014;3:e01846. doi: 10.7554/eLife.01846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JW, Biankin AV, Horb ME, Ghosh B, Prasad NB, Yee NS, Pack MA, Leach SD. Differential requirement for ptf1a in endocrine and exocrine lineages of developing zebrafish pancreas. Dev. Biol. 2004;270:474–486. doi: 10.1016/j.ydbio.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Lu J, Herrera PL, Carreira C, Bonnavion R, Seigne C, Calender A, Bertolino P, Zhang CX. Alpha cell-specific Men1 ablation triggers the transdifferentiation of glucagon-expressing cells and insulinoma development. Gastroenterology. 2010;138:1954–1965. doi: 10.1053/j.gastro.2010.01.046. [DOI] [PubMed] [Google Scholar]
- Lynn FC, Skewes-Cox P, Kosaka Y, McManus MT, Harfe BD, German MS. MicroRNA expression is required for pancreatic islet cell genesis in the mouse. Diabetes. 2007;56:2938–2945. doi: 10.2337/db07-0175. [DOI] [PubMed] [Google Scholar]
- Manfroid I, Ghaye A, Naye F, Detry N, Palm S, Pan L, Ma TP, Huang W, Rovira M, Martial JA, et al. Zebrafish sox9b is crucial for hepatopancreatic duct development and pancreatic endocrine cell regeneration. Dev. Biol. 2012;366:268–278. doi: 10.1016/j.ydbio.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri A. Development and regeneration in the endocrine pancreas. ISRN Endocrinol. 2012;2012:640956. doi: 10.5402/2012/640956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastracci TL, Wilcox CL, Arnes L, Panea C, Golden JA, May CL, Sussel L. Nkx2.2 and Arx genetically interact to regulate pancreatic endocrine cell development and endocrine hormone expression. Dev. Biol. 2011;359:1–11. doi: 10.1016/j.ydbio.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthaei H, Schulick RD, Hruban RH, Maitra A. Cystic precursors to invasive pancreatic cancer. Nat Rev. Gastroenterol. Hepatol. 2011;8:141–150. doi: 10.1038/nrgastro.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS, et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- Morris JP, 4th, Cano DA, Sekine S, Wang SC, Hebrok M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J. Clin. Invest. 2010a;120:508–520. doi: 10.1172/JCI40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JP, 4th, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat. Rev. Cancer. 2010b;10:683–695. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Pan FC, Bankaitis ED, Boyer D, Xu X, Van de Casteele M, Magnuson MA, Heimberg H, Wright CV. Spatiotemporal patterns of multi-potentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development. 2013;140:751–764. doi: 10.1242/dev.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papizan JB, Singer RA, Tschen SI, Dhawan S, Friel JM, Hipkens SB, Magnuson MA, Bhushan A, Sussel L. Nkx2.2 repressor complex regulates islet β-cell specification and prevents β-to-α-cell reprogramming. Genes Dev. 2011;25:2291–2305. doi: 10.1101/gad.173039.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierreux CE, Poll AV, Kemp CR, Clotman F, Maestro MA, Cordi S, Ferrer J, Leyns L, Rousseau GG, Lemaigre FP. The transcription factor hepatocyte nuclear factor-6 controls the development of pancreatic ducts in the mouse. Gastroenterology. 2006;130:532–541. doi: 10.1053/j.gastro.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Pin CL, Rukstalis JM, Johnson C, Konieczny SF. The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J. Cell Biol. 2001;155:519–530. doi: 10.1083/jcb.200105060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr. Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P, Stoffel M. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- Poy MN, Hausser J, Trajkovski M, Braun M, Collins S, Rorsman P, Zavolan M, Stoffel M. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc. Natl. Acad. Sci. USA. 2009;106:5813–5818. doi: 10.1073/pnas.0810550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévot PP, Simion A, Grimont A, Colletti M, Khalaileh A, Van den Steen G, Sempoux C, Xu X, Roelants V, Hald J, et al. Role of the ductal transcription factors HNF6 and Sox9 in pancreatic acinar-to-ductal metaplasia. Gut. 2012;61:1723–1732. doi: 10.1136/gutjnl-2011-300266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot PP, Augereau C, Simion A, Van den Steen G, Dauguet N, Lemaigre FP, Jacquemin P. Let-7b and miR-495 stimulate differentiation and prevent metaplasia of pancreatic acinar cells by repressing HNF6. Gastroenterology. 2013;145:668–678. e663. doi: 10.1053/j.gastro.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Pullen TJ, da Silva Xavier G, Kelsey G, Rutter GA. miR-29a and miR-29b contribute to pancreatic beta-cell-specific silencing of monocarboxylate transporter 1 (Mct1). Mol. Cell. Biol. 2011;31:3182–3194. doi: 10.1128/MCB.01433-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, Akiyama H, Hebrok M. VHL-mediated disruption of Sox9 activity compromises β-cell identity and results in diabetes mellitus. Genes Dev. 2013;27:2563–2575. doi: 10.1101/gad.227785.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezania A, Riedel MJ, Wideman RD, Karanu F, Ao Z, Warnock GL, Kieffer TJ. Production of functional glucagon-secreting α-cells from human embryonic stem cells. Diabetes. 2011;60:239–247. doi: 10.2337/db10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc. Natl. Acad. Sci. USA. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho R, Gruber R, Gu G, Behrens A. Loss of Fbw7 reprograms adult pancreatic ductal cells into α, δ, and β cells. Cell Stem Cell. 2014;15:139–153. doi: 10.1016/j.stem.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer AE, Freude KK, Nelson SB, Sander M. Nkx6 transcription factors and Ptf1a function as antagonistic lineage determinants in multipotent pancreatic progenitors. Dev. Cell. 2010;18:1022–1029. doi: 10.1016/j.devcel.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer AE, Taylor BL, Benthuysen JR, Liu J, Thorel F, Yuan W, Jiao Y, Kaestner KH, Herrera PL, Magnuson MA, et al. Nkx6.1 controls a gene regulatory network required for establishing and maintaining pancreatic Beta cell identity. PLoS Genet. 2013;9:e1003274. doi: 10.1371/journal.pgen.1003274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc. Natl. Acad. Sci. USA. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Dubois CL, Shih HP, Patel NA, Sander M. A dosage-dependent requirement for Sox9 in pancreatic endocrine cell formation. Dev. Biol. 2008;323:19–30. doi: 10.1016/j.ydbio.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang L, Hua H, Foo K, Martinez H, Watanabe K, Zimmer M, Kahler DJ, Freeby M, Chung W, LeDuc C, et al. β-cell dysfunction due to increased ER stress in a stem cell model of Wolfram syndrome. Diabetes. 2014;63:923–933. doi: 10.2337/db13-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Hruban RH. Intraductal papillary mucinous neoplasm. Hum. Pathol. 2012;43:1–16. doi: 10.1016/j.humpath.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Shi G, DiRenzo D, Qu C, Barney D, Miley D, Konieczny SF. Maintenance of acinar cell organization is critical to preventing Kras-induced acinar-ductal metaplasia. Oncogene. 2013;32:1950–1958. doi: 10.1038/onc.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siveke JT, Lubeseder-Martellato C, Lee M, Mazur PK, Nakhai H, Radtke F, Schmid RM. Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology. 2008;134:544–555. doi: 10.1053/j.gastro.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Solar M, Cardalda C, Houbracken I, Martín M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L, Ferrer J. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev. Cell. 2009;17:849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Song SY, Gannon M, Washington MK, Scoggins CR, Meszoely IM, Goldenring JR, Marino CR, Sandgren EP, Coffey RJ, Jr., Wright CV, Leach SD. Expansion of Pdx1-expressing pancreatic epithelium and islet neogenesis in transgenic mice overexpressing transforming growth factor alpha. Gastroenterology. 1999;117:1416–1426. doi: 10.1016/s0016-5085(99)70292-1. [DOI] [PubMed] [Google Scholar]
- Spijker HS, Ravelli RB, Mommaas-Kienhuis AM, van Apeldoorn AA, Engelse MA, Zaldumbide A, Bonner-Weir S, Rabelink TJ, Hoeben RC, Clevers H, et al. Conversion of mature human β-cells into glucagon-producing α-cells. Diabetes. 2013;62:2471–2480. doi: 10.2337/db12-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat. Genet. 1997a;17:138–139. doi: 10.1038/ng1097-138. [DOI] [PubMed] [Google Scholar]
- Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat. Genet. 1997b;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- Swales N, Martens GA, Bonné S, Heremans Y, Borup R, Van de Casteele M, Ling Z, Pipeleers D, Ravassard P, Nielsen F, et al. Plasticity of adult human pancreatic duct cells by neurogenin3-mediated reprogramming. PLoS ONE. 2012;7:e37055. doi: 10.1371/journal.pone.0037055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura G, Fukuda A, Roy N, Liku ME, Morris Iv JP, Kim GE, Russ HA, Firpo MA, Mulvihill SJ, Dawson DW, et al. The chromatin regulator Brg1 suppresses formation of intraductal papillary mucinous neoplasm and pancreatic ductal adenocarcinoma. Nat. Cell Biol. 2014a;16:255–267. doi: 10.1038/ncb2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura G, Morris JP, 4th, Wright CV, Hebrok M. Nr5a2 maintains acinar cell differentiation and constrains oncogenic Kras-mediated pancreatic neoplastic initiation. Gut. 2014b;63:656–664. doi: 10.1136/gutjnl-2012-304287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortmeyer AO, Huang S, Lubensky I, Zhuang Z. Non-islet origin of pancreatic islet cell tumors. J. Clin. Endocrinol. Metab. 2004;89:1934–1938. doi: 10.1210/jc.2003-031575. [DOI] [PubMed] [Google Scholar]
- Wang Z, York NW, Nichols CG, Remedi MS. Pancreatic β cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab. 2014;19:872–882. doi: 10.1016/j.cmet.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CL, Terry NA, Walp ER, Lee RA, May CL. Pancreatic α-cell specific deletion of mouse Arx leads to α-cell identity loss. PLoS ONE. 2013;8:e66214. doi: 10.1371/journal.pone.0066214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Scheel D, German MS. Gene expression cascades in pancreatic development. Mech. Dev. 2003;120:65–80. doi: 10.1016/s0925-4773(02)00333-7. [DOI] [PubMed] [Google Scholar]
- Xu X, D'Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Xu CR, Cole PA, Meyers DJ, Kormish J, Dent S, Zaret KS. Chromatin “prepattern” and histone modifiers in a fate choice for liver and pancreas. Science. 2011;332:963–966. doi: 10.1126/science.1202845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YP, Thorel F, Boyer DF, Herrera PL, Wright CV. Context-specific α- to-β-cell reprogramming by forced Pdx1 expression. Genes Dev. 2011;25:1680–1685. doi: 10.1101/gad.16875711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ables ET, Pope CF, Washington MK, Hipkens S, Means AL, Path G, Seufert J, Costa RH, Leiter AB, et al. Multiple, temporal-specific roles for HNF6 in pancreatic endocrine and ductal differentiation. Mech. Dev. 2009;126:958–973. doi: 10.1016/j.mod.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Dev. Cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]