Abstract
BACKGROUND
Hereditary diffuse leukoencephalopathy with spheroids (HDLS) is a devastating, hereditary white matter (WM) disorder with heterogenous neuropsychiatric features. We looked for CSF1R mutations in primary progressive multiple sclerosis (PPMS) patients and report the clinical features of a family with a novel CSF1R mutation.
METHODS
We sequenced CSF1R exons 12-22 in a cohort of 220 PPMS patients from the Swedish and Norwegian national MS registries.
RESULTS
One patient had a novel mutation, c.2562T>A; p.Asn854Lys, in the CSF1R gene. Her symptoms started at the age of 29 years with insidious onset of pyramidal weakness in the left leg. The cerebrospinal fluid (CSF) examination showed four IgG bands. An MRI performed 4 years after symptom onset demonstrated patchy deep WM lesions. She was diagnosed as having PPMS and treated with intramuscular interferon beta 1a. Due to slow disease progression, the development of memory decline, and cerebellar signs, she was given subcutaneous interferon beta 1a without any benefit. The updated pedigree indicated that 5 siblings also had the CSF1R gene mutation; one was diagnosed with PPMS. Six more distant relatives also had a neurological disorder; four were clinically diagnosed with PPMS.
CONCLUSIONS
Our study indicates that a chronic course of HDLS may mimic PPMS. Genetic testing for CSF1R mutations in PPMS cases with a positive family history of neurological disorders may establish the diagnosis of HDLS.
Keywords: hereditary diffuse leukoencephalopathy with spheroids, white matter lesions
Introduction
A mutation in the colony stimulating factor 1 receptor (CSF1R) gene has recently been discovered to cause the progressive brain white matter (WM) disorder - hereditary diffuse leukoencephalopathy with spheroids (HDLS) [1]. HDLS has a symptom onset that typically occurs at approximately 40 years of age; however, it can range from 18-72 years [1-3]. The phenotype and clinical course are divergent, leading to disease durations from 2-30 years from symptom onset to death [2-4]. HDLS is an autosomal dominant disorder, but de novo mutations are reported [1, 5]. Over 30 different mutations in the CSF1R gene have been discovered. All of the mutations are located in the intracellular tyrosine-kinase domain of the receptor, which is encoded by CSF1R exons 12 to 22 [1, 5-13]. Carriers of CSF1R mutations were clinically diagnosed as having primary progressive multiple sclerosis (PPMS), frontotemporal dementia, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), or atypical Parkinsonism [1, 14]. Since HDLS was initially described in Sweden [2] and because of our earlier research, we learned that CSF1R mutation carriers could be clinically diagnosed as having PPMS [1, 14]. We aimed to study Scandinavian PPMS cases from Swedish and Norwegian MS repositories to find out if additional CSF1R mutation carriers could be identified and if so, what was their clinical presentation.
Methods
PPMS Patients
A total of 220 PPMS patients were included in our study, and none of them had previously been evaluated for HDLS.
Ninety-five of these PPMS patients were included from the Genes and Environment in Multiple Sclerosis (GEMS) collections, which is a case-controlled study based on a questionnaire and a blood-sampling kit sent to patients identified by the Swedish MS register. The GEMS repository is located at the Karolinska University Hospital, which contains about 1000 PPMS patient samples. For this study we selected patients with a record of familial MS cases. There were 84 patients with a family history of MS. Additionally, we included 11 patients with insufficient (n=5) or no (n=6) information regarding familial occurrence in order to fill up the sequencing plate.
One hundred twenty-five of the total 220 PPMS patients were recruited from the Norwegian MS registry and Biobank, which contains clinical data but no family history. All patients were diagnosed by the neurologist practicing in the local MS unit that entered the patient into the registries.
The study was approved as a multicenter study by the Ethical Committees of the Karolinska Institute, Sweden, by the Research Ethical Committee of Gothenburg, Sweden, The Regional Committee for Medical and Health Research Ethics in Western Norway, and the Mayo Clinic Institutional Review Board. Informed consent was obtained from all patients.
Genetic analysis
Anonymized DNA samples were sent to the Mayo Clinic in Florida for screening of the CSF1R gene. We sequenced exons 12-22 in the CSF1R as previously described by Rademakers et al [1].
Family investigation
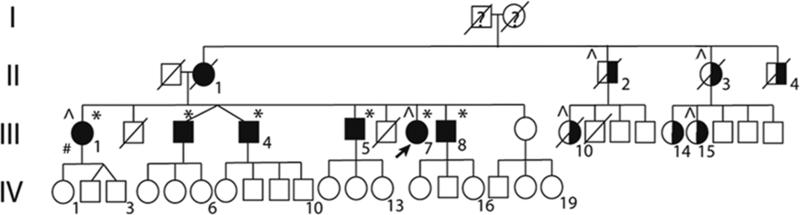
A pedigree was established, Figure 1a. The index patient and her six living siblings were prospectively investigated with clinical history, neurological examination, and an assessment of the HDLS symptoms using the Regional Functional Scoring System (RFSS), which is based on the Kurtzke Functional Systems [3] . The degree of neurologic impairment in multiple sclerosis (MS) was assessed by the expanded disability status scale (EDSS). A Mini-Mental State Examination (MMSE) was performed on all patients. Available medical records were reviewed. These examinations were conducted by two of the authors (CS and VK). Magnetic resonance imaging (MRI) was performed on a 1.5-Tesla scanner. Previous MRIs that were done on 1.5-Tesla MRI scanners were also reviewed. Cerebrospinal fluid (CSF) and blood samples were obtained. CSF specimens were analyzed for IgG and IgM anti-herpes simplex and anti-varicella virus antibodies, IgG and IgM anti-borrelia antibodies, total IgG, albumin, Link index of intrathecal IgG production, isoelectric focusing with immunoblot, CSF cell counts, cytology, β2-microglobulin, total-tau (T-tau), phosphorylated tau (P-tau), neurofilament light protein (NFL), glial fibrillary acidic protein (GFAP), and amyloid protein 1-42 (Aβ1-42), as described [3]. Blood and urine were analyzed with routine tests, including amino acids, VDRL, HIV and Borrelia serology. Assays of antinuclear antibody (ANA), anti-neutrophil cytoplasmic antibodies (ANCA), and rheumatoid arthritis (RA) were also performed.
Figure 1a. Pedigree of the CSF1R mutation family.
Abbreviations: Fully darkened symbols: Individuals with HDLS phenotype; *: Confirmed CSF1R mutation; ^: Previously diagnosed with MS; # Sibling (III:1) diagnosed with PPMS; Half-filled symbols: Individuals with neurological symptoms; A diagonal line through a symbol: Deceased persons not screened for the CSF1R mutation; Arrow: Index/Proband; Squares: Males; Circles: Females
Results
The clinical data of the 220 PPMS patients is shown in Table e-1 (online only). We identified a novel mutation, c.2562T>A; p.Asn854Lys, in the CSF1R gene. The index patient, subject III:7, was from the Swedish MS registry. Confirmative genetic testing was conducted at Centogene (Centogene GmbH, Rostock, Germany). The proband was informed of her CSF1R mutation carrier status through clinical and genetic counselling. She had eight siblings; six of them were alive. After initial contact by the proband, the siblings consented to clinical and genetic investigations (CS and VK). Of these, one had received a MS diagnosis, one had severe neuropsychiatric symptoms, three had undiagnosed neurological symptoms including one further with a suspected MS diagnosis, and one was healthy. Detailed clinical information for these individuals is presented in Table e-2 (online only) and appendix e-1 (online only). The proband and the five symptomatic siblings carried the same CSF1R mutation. The healthy sibling did not carry the mutation. Pedigree structure indicates an autosomal dominant inheritance (Figure 1a). MRI was performed on three of the mutation carriers, while three did not consent to the MRI examination (Figure 1b). CSF findings in two patients harbouring the mutation are displayed in Table e-3 (online-only). Two siblings had died from complications of drug abuse. Her distant family (uncles, aunts and cousins) were not willing to participate in our study; their carrier status is unknown. However, we have anecdotal information indicating that six had neurological symptoms, and four carry an MS diagnosis. There was no history of consanguinity.
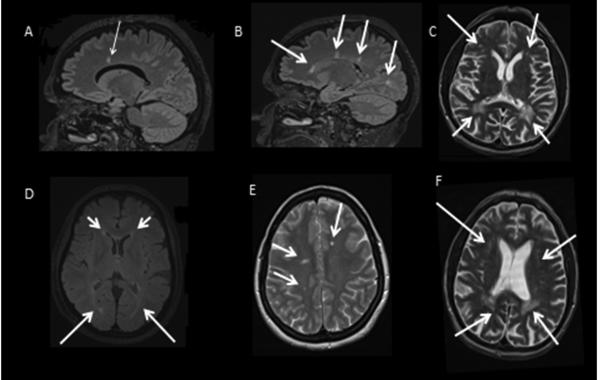
Figure 1b. MRIs of mutation carriers.
(A) Index patient, (sagittal T2-fluid attenuate inversion recovery (FLAIR) -weighted), one callosomarginal lesion (arrow) 4 years after symptom onset. (B) Patient III:1, (sagittal FLAIR-weighted), demonstrating three callosomarginal lesion 1 year after symptom onset and one white matter lesion (WML) deep in the occipital hemisphere. (C) Patient III: 8, (Axial T2-weighted), localized confluent biparietal WMLs (lower arrows) with more patchy deep WMLs in the bifrontal regions (upper arrows), 5 years after symptom onset. (D) Index patient, (Axial T2-FLAIR-weighted), prominent signal changes periventricular which extend into the deep white matter (lower arrows), but sparing the U-fibers, 11 years after symptom onset. (E) Patient III:1, (Axial FLAIR-weighted), asymmetric, localized, patchy deep WML (arrows) 1 year after symptom onset. (F) Patient III:8, (Axial T2-weighted), localized confluent biparietalt WML (lower arrow) with more patchy deep WMLs in the bifrontal regions (upper arrows) 5 years after symptom onset.
Discussion
Recently, mutations in the CSF1R gene were found to cause HDLS [1]. We previously reported HDLS patients who were misdiagnosed as having PPMS [14]. In another series of 95 MS patients none had CSF1R mutations [15]. No study has been performed in a series with PPMS and a family history of MS [15]. We conducted a screening investigation in PPMS patients from the Norwegian and Swedish MS registries to search for CSF1R gene mutation carriers. Among 220 Scandinavian PPMS patients, we identified one individual with a novel CSF1R mutation. This patient's symptoms were compatible with a PPMS diagnosis. The disease course started with a pyramidal syndrome followed by brain stem and cerebellar symptoms. Her MRI and the oligoclonal banding in her CSF indicated MS. A prospective investigation of the proband's family showed that five siblings harbored the same CSF1R mutation. These mutation carriers exhibited fatigue, cognitive decline, gait and balance difficulties. Their phenotype is consistent with that seen in other CSF1R mutation carriers. Interestingly, the twins (III:3 and III:4) had different phenotypes. Case III:3 had more psychiatric problems and a later onset of neurological deficits. Case III:4 had frontal lobe syndrome, which included depression, apathy, and a lack of initiative. He also neglected his personal hygiene .
Two patients with CSF1R mutations who received a PPMS diagnosis had a subtle disease progression. The mean age of onset for all six carriers was 41 years, compatible with both HDLS and PPMS. The course of the disease was insidious from onset with moderate accumulated disability during the observation time ranging from 3 to15 years. The most severely affected, patient III:8, was able to walk with support five years after symptom onset. No mutation carriers had the sub-acute course described in the original Swedish HDLS family, albeit the genetic status of this original HDLS family is currently unknown [3].
The proband fulfilled the revised McDonald criteria for MS [16]. MRI in the symptomatic sister showed asymmetric WMLs without contrast enhancements, more compatible with MS, Figure 1b and appendix e-1 online only. On the other hand, WMLs were more confluent in the severely-affected sibling, and thus more consistent with HDLS. The corpus callosum is usually affected in HDLS; however, in the early stages of the disease, the diagnostic specificity is low, and some genetic mutations might not be associated with this feature. In addition to subcortical lesions, the proband had one callosomarginal lesion, but her sister had three. We did not observe lesions with this specific localization in our previous studies. The MRI findings are known to vary in patients with different CSF1R mutations, but they are generally similar among patients carrying the same mutation [17].
The proband and her sister had obvious similarities with PPMS with regard to clinical symptoms, MRI results, and CSF findings. HDLS has been reported to masquerade as PPMS [1, 9, 13]. A sporadic HDLS patient with pyramidal symptoms had MRI results compatible with HDLS, but the CSF was normal [13]. Cognitive problems are seen in PPMS patients [18], but appear later in the disease course. In HDLS, cognitive dysfunction occurs earlier and is dominated by personality and behavioral changes. In both PPMS and HDLS there is subsequent multifocal neurological progression, although intention tremor is more disabling in PPMS [18]. In our case, the presence of left-sided facial hypoesthesia is typical for MS, but quadrant anopsia is not [3, 14, 18].
MS is a multifactorial disease with a clear genetic component [19], but the disease course is influenced by multifactorial factors. Could CSF1R gene mutations modify MS-associated neurodegeneration? Can CSF1R mutations predispose individuals to MS? Several GWAS studies performed in MS showed no association with the CSF1R gene mutations [20]. Even though potentially important to MS, the mutation frequency of the CSF1R mutation in MS is too low to make it detectable by GWAS. However, a similarity between HDLS and PPMS is evident in the neuropathology, characterized by extensive demyelination and numerous axonal spheroids [14, 21]. It is unknown whether the neuronal loss is a primary event or a secondary process of damage to the axons in both diseases. Axonal neurodegeneration, histologically resulting in spheroid formations, is increasingly accepted as the cause of primary and secondary progression with accumulated disability in MS and seems to be a likely explanation for HDLS [14, 22]. While the cause of HDLS, the CSF1R mutation, has now been identified, the etiology of MS remains unknown, but a complex combination of HLA polymorphisms, rare genetic variants, and environmental factors are thought to lead to disease development [19, 21].
Selective oligoclonal bands in the CSF may indicate that HDLS, at least with the p.Asn854Lys genetic mutation, is driven by an inflammatory process, leading to neurological deficits. The CSF1R gene encodes a tyrosine kinase transmembrane receptor that is expressed on the microglia. This receptor is essential for microglial development and has a broad range of functions, including inflammatory responses [1, 23]. Increased CSF- β2-microglobulin in two of our patients provides some support for an inflammatory process. Our HDLS cases had normal CSF cell counts, which is in opposition to typical MS. Elevation in the NFL suggests the destruction of large-calibre myelinated axons and the minimal elevation of T-tau protein levels suggests damage to the cortical neurons [24]. GFAP was also increased in our patients, suggesting astroglial cell damage. Both phospho-tau and Aβ1-42 levels were normal, indicating the absence of Alzheimer-like pathology [25]. These CSF findings confirm a neurodegenerative disorder with signs of inflammation. To our knowledge, this is the first time oligoclonal bands have been demonstrated in HDLS, but whether this is a specific finding in p.Asn854Lys CSF1R mutation carriers remains to be confirmed. Myelin is known to be extremely sensitive to inflammation, so the demyelinating component in our family may be more important in the pathogenesis of cases with the p.Asn854Lys CSF1R mutation. This is hypothetical, but with the increasing interest in microgliopathies, our findings are worthy of further investigation. Microglia are known to have a key role in the initiation of demyelination [23].
The clinical spectrum of HDLS is wide [1, 6, 14]. Therefore, clarification of the definition of HDLS with a CSF1R mutation is a necessity. The diagnostic tools listed in Table 1 are helpful for distinguishing between HDLS due to CSF1R mutations and PPMS. Our study indicates that a chronic course of HDLS might mimic PPMS clinically, radiologically, and in laboratory findings. This underscores the importance of excluding HDLS when diagnosing MS in patients with a family history that indicates a neurological disorder.
Table 1.
Suggested diagnostic tools for HDLS separating it from PPMS
| Diagnostic criteria | Features | HDLS | PPMS |
|---|---|---|---|
| 1. | Initial symptom with cognitive decline | Yes | No |
| 2. | Initial symptom with spastic-rigid paraparesis | No | Yes |
| 3. | MRI with severe WML related to the stage of disease without enhancement | Yes | No |
| 4. | Callosomarginal lesions | No | Yes |
| 5. | Predominant confluent WML in the frontoparietal areas | Yes | No |
| 6. | CSF pleocytosis | No | Yes/No |
| 7. | Visual tract involvement | Mostly retrochiasmatic | Mostly prechiasmatic |
| 8. | Similar neurological disorder running in the family | Yes, but sporadic cases exist | Yes/No |
Abbreviations: CSF= cerebrospinal fluid, WML= white matter lesions, OCB= oligoclonal bands
Recently, considerable progress has been made in terms of understanding WM disorders [26, 27]. However, further genetic and functional studies are required to expose the molecular mechanisms underlying neurodegeneration.
Supplementary Material
Acknowledgments
We thank all those who contributed samples and clinical data to the Norwegian MS Registry and Biobank and the Swedish MS Registry. Special thanks goes to Kelly Viola, ELS, at the Mayo Clinic for excellent help with editing and to Audrey J. Strongosky at the Mayo Clinic for help with the pedigree. Finally, the authors thank Drs. Shinsuke Fujioka and Jan Aasly for their assistance with data collection.
ZKW is partially supported by the NIH/NINDS P50 NS072187, Mayo Clinic Center for Regenerative Medicine, The Michael J. Fox Foundation for Parkinson's Research, and the gift from Carl Edward Bolch, Jr., and Susan Bass Bolch. CS is partially supported by Capio research- and Anna-Lisa och Bror Björnssons Founding 2012-2014.
RR receives research support from the NIH, the ALS Therapy Alliance, and the Consortium for Frontotemporal Degeneration Research. Dr. Rademakers further received honoraria for lectures or educational activities not funded by industry; she serves on the medical advisory board of the Association for Frontotemporal Degeneration, on the board of directors of the International Society for Frontotemporal Dementia and holds a patent on methods to screen for the hexanucleotide repeat expansion in the C9ORF72 gene.
Footnotes
Detailed descriptions of family members are provided in appendix e-1 (online only).
Disclosures
All other authors report no disclosures.
References
- 1.Rademakers R, Baker M, Nicholson AM, et al. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat Genet. 2011;44:200–205. doi: 10.1038/ng.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axelsson R, Roytta M, Sourander P, Akesson HO, Andersen O. Hereditary diffuse leucoencephalopathy with spheroids. Acta Psychiatr Scand Suppl. 1984;314:1–65. [PubMed] [Google Scholar]
- 3.Sundal C, Ekholm S, Nordborg C, et al. Update of the original HDLS kindred: divergent clinical courses. Acta Neurol Scand. 2011 doi: 10.1111/j.1600-0404.2011.01624.x. [DOI] [PubMed] [Google Scholar]
- 4.Sundal C, Fujioka S, Van Gerpen JA, et al. Parkinsonian features in hereditary diffuse leukoencephalopathy with spheroids (HDLS) and CSF1R mutations. Parkinsonism Relat Disord. 2013;19:869–877. doi: 10.1016/j.parkreldis.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kondo Y, Kinoshita M, Fukushima K, Yoshida K, Ikeda S. Early involvement of the corpus callosum in a patient with hereditary diffuse leukoencephalopathy with spheroids carrying the de novo K793T mutation of CSF1R. Intern Med. 2013;52:503–506. doi: 10.2169/internalmedicine.52.8879. [DOI] [PubMed] [Google Scholar]
- 6.Sundal C, Wszolek Z. CSF1R-Related Hereditary Diffuse Leukoencephalopathy with Spheroids. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews(R) Seattle (WA): 2012. [Google Scholar]
- 7.Ahmed R, Guerreiro R, Rohrer JD, et al. A novel A781V mutation in the CSF1R gene causes hereditary diffuse leucoencephalopathy with axonal spheroids. J Neurol Sci. 2013;332:141–144. doi: 10.1016/j.jns.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battisti C, Di Donato I, Bianchi S, et al. Hereditary diffuse leukoencephalopathy with axonal spheroids: three patients with stroke-like presentation carrying new mutations in the CSF1R gene. J Neurol. 2014;261:768–772. doi: 10.1007/s00415-014-7257-3. [DOI] [PubMed] [Google Scholar]
- 9.Inui T, Kawarai T, Fujita K, et al. A new CSF1R mutation presenting with an extensive white matter lesion mimicking primary progressive multiple sclerosis. J Neurol Sci. 2013;334:192–195. doi: 10.1016/j.jns.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Terasawa Y, Osaki Y, Kawarai T, et al. Increasing and persistent DWI changes in a patient with hereditary diffuse leukoencephalopathy with spheroids. J Neurol Sci. 2013;335:213–215. doi: 10.1016/j.jns.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Karle KN, Biskup S, Schule R, et al. De novo mutations in hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS). Neurology. 2013;81:2039–2044. doi: 10.1212/01.wnl.0000436945.01023.ac. [DOI] [PubMed] [Google Scholar]
- 12.Guerreiro R, Kara E, Le Ber I, et al. Genetic analysis of inherited leukodystrophies: genotype-phenotype correlations in the CSF1R gene. JAMA Neurol. 2013;70:875–882. doi: 10.1001/jamaneurol.2013.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saitoh BY, Yamasaki R, Hayashi S, et al. A case of hereditary diffuse leukoencephalopathy with axonal spheroids caused by a de novo mutation in CSF1R masquerading as primary progressive multiple sclerosis. Multiple Sclerosis. 2013;19:1367–1370. doi: 10.1177/1352458513489854. [DOI] [PubMed] [Google Scholar]
- 14.Sundal C, Lash J, Aasly J, et al. Hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS): A misdiagnosed disease entity. J Neurol Sci. 2012;314:130–137. doi: 10.1016/j.jns.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadovnick AD, Traboulsee AL, Lee JD, Ross JP, Bernales CQ, Vilarino-Guell C. Colony stimulation factor 1 receptor (CSF1R) is not a common cause of multiple sclerosis. Euro J Neurol. 2013;20:e115–116. doi: 10.1111/ene.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundal C, Van Gerpen JA, Nicholson AM, et al. MRI characteristics and scoring in HDLS due to CSF1R gene mutations. Neurology. 2012;79:566–574. doi: 10.1212/WNL.0b013e318263575a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skoog B, Runmarker B, Winblad S, Ekholm S, Andersen O. A representative cohort of patients with non-progressive multiple sclerosis at the age of normal life expectancy. Brain. 2012;135:900–911. doi: 10.1093/brain/awr336. [DOI] [PubMed] [Google Scholar]
- 19.Oksenberg JR. Decoding multiple sclerosis: an update on genomics and future directions. Exp Rev Neurotherapeutics. 2013;13:11–19. doi: 10.1586/14737175.2013.865867. [DOI] [PubMed] [Google Scholar]
- 20.Beecham AH, Patsopoulos NA, Xifara DK, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45:1353–1360. doi: 10.1038/ng.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. New Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 22.Stadelmann C. Multiple sclerosis as a neurodegenerative disease: pathology, mechanisms and therapeutic implications. Curr Opin Neurol. 2011;24:224–229. doi: 10.1097/WCO.0b013e328346056f. [DOI] [PubMed] [Google Scholar]
- 23.Benarroch EE. Microglia: Multiple roles in surveillance, circuit shaping, and response to injury. Neurology. 2013;81:1079–1088. doi: 10.1212/WNL.0b013e3182a4a577. [DOI] [PubMed] [Google Scholar]
- 24.Mattsson N, Blennow K, Zetterberg H. CSF biomarkers: pinpointing Alzheimer pathogenesis. Ann NY Acad Sci. 2009;1180:28–35. doi: 10.1111/j.1749-6632.2009.04944.x. [DOI] [PubMed] [Google Scholar]
- 25.Olsson B, Zetterberg H, Hampel H, Blennow K. Biomarker-based dissection of neurodegenerative diseases. Prog Neurobiol. 2011 doi: 10.1016/j.pneurobio.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 26.van der Knaap M, Valk J. Magnetic resonance of myelination and myelin disorders. 3 edn. Springer; New York: 2005. [Google Scholar]
- 27.Kohlschutter A, Bley A, Brockmann K, et al. Leukodystrophies and other genetic metabolic leukoencephalopathies in children and adults. Brain Dev. 2010;32:82–89. doi: 10.1016/j.braindev.2009.03.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.