Abstract
Microabstract
The kinetics and relationships between testosterone (T) and prostate-specific antigen (PSA) during the first off-treatment interval in men with non-metastatic prostate cancer treated prospectively on a clinical trial of intermittent androgen deprivation were analyzed. Time to PSA rise and time to PSA rise after first T>50 ng/dL were prognostic for progression to castration-resistance.
Background
Intermittent androgen deprivation (IAD) represents an alternative to continuous AD with quality-of-life benefit and no evidence of inferior overall survival for non-metastatic prostate cancer. Early markers of prognosis for men treated with IAD have not been described.
Methods
Men with non-metastatic prostate cancer were treated with 9 months of leuprolide and flutamide followed by a variable “off-treatment” interval; AD was resumed when prostate specific antigen (PSA) reached a pre-specified value (1 ng/mL - radical prostatectomy; 4 ng/mL - intact prostate). Cycles were repeated until castration-resistance (CRPC), defined as two PSA rises with testosterone (T) ≤50 ng/dL. We evaluated kinetics and relationships of PSA and T levels, focusing on times to rise in each level, during the first off-treatment interval. Associations with CRPC and prostate cancer mortality (PCM) were estimated using Cox proportional hazards models controlling for age and Gleason score.
Results
Each 30-day increase in time to PSA rise was associated with a 21% reduction in the risk of developing CRPC (95% CI, 3–36%; P=0.02). Longer time (≥60 days) to PSA rise after rise to T >50 ng/dL was associated with a 71% reduction in the risk of developing CRPC (95% CI, 92% reduction to 2% inflation; P=0.05). Time to first T >50 ng/dL and PSA doubling time were not prognostic for progression to CRPC. No time interval was prognostic for PCM.
Conclusion
During the first off-treatment interval of IAD, longer times to PSA rise overall and after T >50 ng/dL were associated with reduced risk of developing CRPC.
Keywords: Intermittent Androgen Deprivation, Castration-resistant, Prostate Cancer, Prostate-specific Antigen, Testosterone, Prognosis
INTRODUCTION
Due to issues surrounding the morbidity and cost of continuous androgen deprivation (CAD), multiple randomized studies of intermittent AD (IAD) vs. CAD have been performed.1, 2 Overall survival and time to castration resistance have been similar, but there is evidence of less toxicity for those treated with IAD. Recently, a large trial of IAD vs. CAD for men who underwent radiation for prostate cancer revealed no difference in overall survival, with more prostate cancer related deaths for those receiving IAD yet more non-prostate cancer associated deaths for those receiving CAD.1 These findings suggest IAD should be a standard of care for men treated with AD in this setting.
There are few markers prognostic for progression to castration-resistant prostate cancer (CRPC) or prostate cancer mortality (PCM). This is particularly true for men undergoing IAD. We have previously shown that, when a pre-determined prostate-specific antigen (PSA) threshold is used in a prospective trial of IAD, the duration from the end of AD to reaching that threshold (defining the first off-treatment interval), is prognostic for time to CRPC and death.3 However, this prognostic time interval requires following patients for long periods of time until the PSA threshold is met and AD is resumed for a second treatment cycle. An earlier biologic and prognostic marker would be useful both in the clinic and to stratify risk for future clinical trials. For this reason, we evaluated times to testosterone (T) and PSA rises, times between these events, and PSA doubling time (PSAdt) during the first off-treatment interval of IAD as potential prognostic time intervals for time to CRPC and PCM in men with non-metastatic castration-sensitive prostate cancer in our prospective trial of IAD.
PATIENTS AND METHODS
Study Design
A prospective trial of IAD for men with non-metastatic prostate cancer with either locally advanced or biochemical relapse of prostate cancer after failure of primary therapy was initiated to study the time to CRPC as well as numerous physiological, emotional, and cognitive effects of AD.3, 4 Key eligibility requirements included a histological diagnosis of prostate cancer, at least 2 consecutive rises in PSA at least 2 weeks apart, original American Urological Association stage A2-D1, no detectable metastasis by bone and computed tomography scans, Eastern Cooperative Oncology Group performance status 0 or 1, and pre-treatment T >100 ng/dL. Late enrollment was permitted as long as the duration of therapy was <10 months. Patients could have received prior AD for neoadjuvant, adjuvant, or salvage settings as long as the duration of AD was ≤3 months and it was completed ≥1 year before study entry. Each patient signed written informed consent approved by the University of Washington institutional review board.
Initial treatment consisted of 9 months of combined AD with flutamide at 250 mg three times daily and a luteinizing hormone-releasing hormone agonist (see Figure 1). Patients with toxicity from flutamide were switched to bicalutamide 50 mg daily. At the end of the 9 months of treatment induction, AD was stopped provided that the PSA value was ≤1 ng/mL and not rising. PSA levels were measured monthly throughout IAD therapy, while T was measured quarterly during the 9-month on-treatment intervals and monthly during off-treatment intervals. When the PSA reached an arbitrary, pre-specified threshold of 1 ng/mL for those after radical prostatectomy and 4 ng/mL for those with an intact prostate (radiation or primary AD), a new cycle was initiated with another 9 months of combined AD. All patients continued cycling on and off therapy until the development of CRPC, defined as 2 serial rises in PSA while on AD with T <50 ng/dL.
Figure 1.
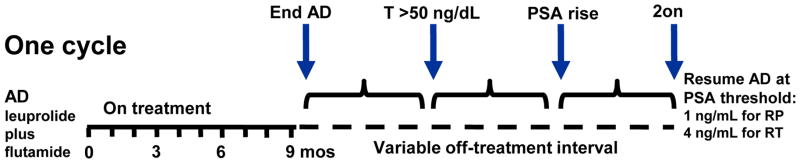
Study schema illustrates one full cycle of IAD. Specific time points of interest are designated with arrows. Cycles are repeated until the development of castration-resistance or death.
Four time intervals of interest during the first off-treatment interval were analyzed in this exploratory analysis: time from completion of AD to first T>50 ng/dL, time from first T>50 to PSA rise of ≥0.1 ng/mL, time from completion of AD to PSA rise of ≥0.1 ng/mL, and PSA doubling time (PSAdt) (see Figure 1). PSAdt was calculated using the first three PSA measurements starting from PSA rise in the Memorial Sloan-Kettering online nomogram.5 To be eligible for this analysis, patients must have had a sufficient number of measurements for determining at least one of these four time intervals of interest and a T recovery to >100 ng/dL during the first off-treatment interval to eliminate the possibility of chronic castration without therapy.
Statistical Methods
Cox proportional hazards models were used to quantify associations between the time intervals of interest and times to CRPC or PCM, with times to CRPC or PCM defined beginning at the last time point used to construct the marker. For example, in the model to quantify association between time to T >50 ng/dL and time to CRPC, time to CRPC begins when T >50 ng/dL. Based on preliminary graphical examination, all time intervals of interest entered the models as continuous measures with the exception of time from T >50 ng/dL to PSA rise, which was dichotomized as <60 days versus ≥60 days. In addition to the time interval of interest, all models controlled for age at study entry and grade category (dichotomized as Gleason score ≤3+4 versus ≥4+3).
Following standard graphical diagnostic evaluation of model performance and formal testing of the proportional hazards assumption using Schoenfeld residuals, we constructed plots illustrating predictions of corresponding models with each time interval binarized at the median (except for T > 50 ng/dL to PSA rise, which we continued to binarize at 60 days). For illustrative purposes, we adjusted for mean age (66 years) and the more common grade category (Gleason score ≤3+4).
RESULTS
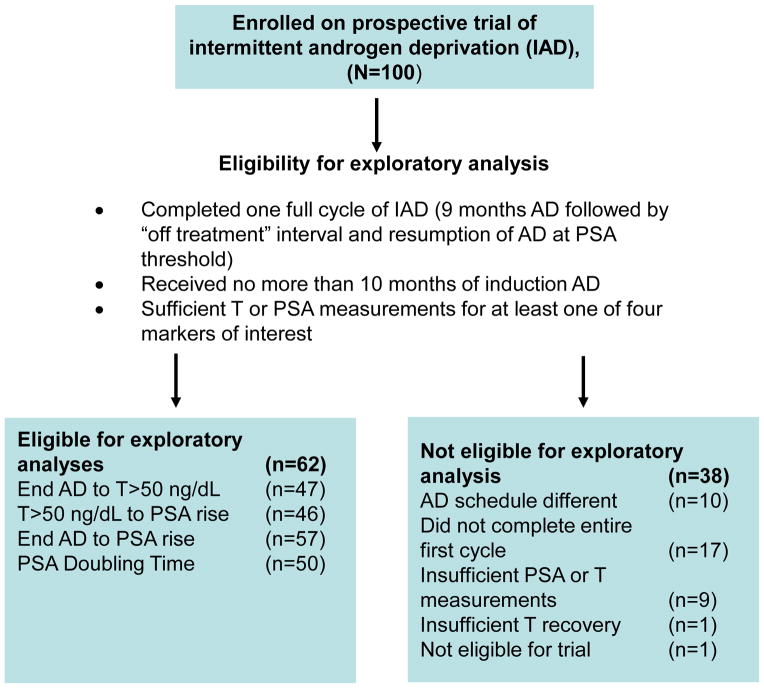
Between June 1996 and September 2006, 100 patients were accrued to the IAD trial. Sixty-two patients were eligible for this exploratory analysis (see Figure 2). Characteristics of the eligible patients are presented in Table 1. At diagnosis, the median age was 61.2 (range 48.3 to 74.8) years with a median PSA of 9.7 (range 3.9 to 130.5) ng/mL. The median Gleason score was 3+4 (range 6 to 9). Forty-seven patients underwent radical prostatectomy, 14 patients received definitive radiation therapy and one patient received primary AD. Of the 14 patients who received radiation, 13 experienced biochemical recurrence, fulfilling the contemporary Phoenix criteria.6 Median time from primary treatment to biochemical recurrence was 3.3 (range 0.5 to 14.6) years.
Figure 2.
Consort diagram displays rationale for selection of the 62 patients from the clinical trial for this exploratory analysis.
Table 1.
Patient Characteristics
| At Initial Diagnosis | (range) |
|---|---|
| Median Age, years | 61.2 (48.3–74.8) |
| Gleason Score | |
| 6 | 12 |
| 3+4 | 20 |
| 4+3 | 14 |
| 8 | 9 |
| 9 | 4 |
| Unknown | 3 |
| Median PSA, ng/mL | 9.7 (3.9–130.5) |
| Race | |
| Caucasian | 61 |
| Asian | 1 |
| Primary Treatment | |
| Radical Prostatectomy | 47 |
| Radiation Therapy | 14 |
| Primary AD | 1 |
| At Study Entry | |
| Median Age, years | 66.2 (51.2–81.1) |
| Baseline PSA, ng/mL | 3.9 (0.46–152.4) |
| Baseline T, ng/dL* | 350 (140–640) |
| Median Time to BR, years | 2.9 (0.5–14.6) |
| Prior AD | |
| None | 55 |
| Neoadjuvant | 2 |
| Adjuvant | 5 |
49/62 patients had baseline testosterone levels; missing values due to late enrollment
Abbreviations: AD – Androgen deprivation, BR – Biochemical Recurrence, PSA – Prostate-specific Antigen, T - Testosterone
At study entry, the median age was 66.2 (range 51.2 to 81.1) years with median baseline pre-study PSA and T levels of 3.9 (range 0.46 to 152.4) ng/mL and 350 (range 140 to 640) ng/dL respectively (see Table 1). Fifty-two patients achieved a PSA nadir of <0.1 ng/mL during the first treatment cycle, whereas the remaining 10 patients had a median PSA nadir of 0.2 (range 0.1 to 0.4) ng/mL. During the first off-treatment interval, the median time of recovery to first T >50 ng/dL was 3.1 (range 0 to 5.1) months. The median time to PSA rise was 4.5 (range 0.9 to 92.3) months and the median time to PSA rise after first T >50 ng/dL was 1.0 (range −0.9 to 88.5) months. The median duration of the first off-treatment interval was 9.5 (range 3.4 to 47.5+) months. Five patients are still in their first off-treatment interval because they have not yet reached the PSA threshold for initiating the second cycle of IAD; 3 of these patients still have an undetectable PSA); for these patients times to CRPC and PCM were censored at the date of the last clinic visit. The median PSAdt during the first off-treatment period starting from the first PSA rise was 1.2 (range 0 to 8.3) months.
Of 62 patients, 40 are alive (23 remain on IAD, 11 have CRPC, and 6 are off study) and 22 have died. Of the 22 patients who died, 13 (59%) patients died because of progressive CRPC and nine (41%) died of other causes. Thirty-nine patients came off study at some point after completing the first full cycle: 29 patients due to progression to CRPC and 10 for other reasons. After the first full cycle, median time to CRPC was 4.0 (range 0.5 to 8.6) years, and median time to death was 6.6 (range 2.9 to 13.0) years. Median times from primary treatment to CRPC and to death were 9.7 (range 4.0 to 21.0) years and 12.8 (range 5.5 to 21.6) years, respectively.
Results of the Cox proportional hazard models are presented in Table 2. There is moderate evidence that time to PSA rise and time to PSA rise after T >50 ng/dL were each associated with time to CRPC after controlling for age at study entry and Gleason category (p=0.02 and 0.05, respectively). Each 30-day increase in the time to PSA rise was associated with a 21% decrease in the risk of CRPC (95% confidence interval [CI] 3% to 36%). If a patient had a time to PSA rise of ≥60 days after their first T >50 ng/dL, this was associated with a 71% decrease in the risk of CRPC (95% CI 92% decrease to 2% increase). A sensitivity analysis showed that the association was similar for patients who had a time to PSA rise of ≥30 days after their first T > 50ng/dL (57% decrease, 95% CI 85% decrease to 28% increase) and for patients who had a time to PSA rise of ≥90 days after their first T >50ng/dL (69% decrease, 95% CI 93% decrease to 37% increase). Associations between time to T >50 ng/mL and PSAdt and time to CRPC are consistent with clinically important prognostic markers (each 30-day increase in time to T >50 ng/dL is associated with 32% decrease in the risk of CRPC and each 30-day increase in PSAdt is associated with a 39% decrease in the risk of CRPC); however, we have insufficient power to conclude statistical significance. No time intervals were associated with PCM. Figure 3 illustrates Kaplan-Meier survival curves predicted from the Cox models for time to CRPC and time to PCM for each time interval of interest.
Table 2.
Multivariate survival analyses using Cox proportional hazard models for time to castration-resistant prostate cancer and prostate cancer mortality controlling for age at study entry and Gleason category.
| Castration-resistant prostate cancer | ||||
|---|---|---|---|---|
| Marker | n | HR | 95% CI | p-value |
| End AD to T>50 ng/dL | 44 | 0.68 | (0.41, 1.13) | 0.13 |
| End AD to PSA rise | 54 | 0.79 | (0.64, 0.97) | 0.02 |
| T>50 to PSA rise | 43 | 0.29 | (0.08, 1.02) | 0.05 |
| PSA doubling time | 47 | 0.61 | (0.36, 1.04) | 0.07 |
| Prostate cancer mortality | ||||
|---|---|---|---|---|
| Marker | n | HR | 95% CI | p-value |
| End AD to T>50 ng/dL | 47 | 1.42 | (0.59, 3.41) | 0.44 |
| End AD to PSA rise | 57 | 0.94 | (0.72, 1.22) | 0.65 |
| T>50 to PSA rise | 46 | 0.45 | (0.08, 2.41) | 0.35 |
| PSA doubling time | 50 | 0.58 | (0.25, 1.35) | 0.20 |
Abbreviations: End AD – End of initial 9 months of androgen deprivation, HR - Hazard Ratio, CI - Confidence Interval, T – Testosterone, PSA – Prostate-specific Antigen
Figure 3.
Kaplan-Meier curves illustrating time to castration-resistant prostate cancer (left panels) and prostate cancer mortality (right panels) predicted by four time intervals (panel rows) above (gray) and below (black) the marker median values or, for time from T >50 ng/dL to PSA rise, <60 days (gray) versus ≥60 days (black).
DISCUSSION
Recent randomized phase 3 trial data supports the concept that IAD is not inferior in terms of survival when compared to CAD for men with biochemically recurrent disease after definitive or salvage radiation.1 Patients on IAD have a net improved quality of life with fewer hot flashes, reduced sexual side effects, and long-term preservation of bone mineral density.1, 7–10 Given the significant heterogeneity in long-term response to IAD, prognostic markers that distinguish those with indolent versus aggressive disease would be useful. Patients with early PSA rise could be considered for novel clinical trial designs since our data demonstrates that they have a worse prognosis if they simply continue on intermittent ADT. Those patients with late PSA rise might require less therapy and be apt to experience mortality from other causes, rather than prostate cancer. Therefore, early identification of these patients may not only stratify risk groups but could also affect future treatment.
Previously, we have shown that the duration of the first off-treatment interval is prognostic for both time to CRPC and death in this patient population.3 Drawbacks to using the duration of the first off-treatment interval as a prognostic factor are that it requires waiting for the off-treatment interval to end and it does not take into account the biologic relationship between T and PSA. Knowing that PSA changes over time have been shown to be an important prognostic factor in other prostate cancer disease states11–16 and hoping to find earlier indicators of prognosis than the duration of the first off-treatment interval, we sought to evaluate the kinetics and relationships of PSA and T rise during the first off-treatment period.
Our analysis demonstrated that there is a significant association between a longer time to PSA rise during the first off-treatment interval and a longer time to CRPC. This is not surprising, given our prior results that defined the duration of the first off-treatment interval as prognostic for both CRPC and death. However, the time to PSA rise occurs at an earlier time point and can be used to inform subsequent treatment decisions. Our analysis also showed that those with a longer time (e.g., ≥60 days) to PSA rise after first T>50 ng/dL experience a longer time to CRPC. This is an important finding since it confirms that a delay in PSA rise with a non-castrate T level is “good” and longer time from first T>50 ng/dL to PSA rise has improved outcomes.
While it is intuitive that a greater interval between T and PSA rise would lead to both improved quality of life and long-term outcomes, this is the first study in which this has been shown. However, the explanation for this is unclear. A simple explanation is that patients with a smaller overall tumor burden require greater overall stimulation from T to produce enough PSA to reach the threshold to start the next IAD cycle. Another more biologically relevant explanation relies on the known intra-tumoral production of androgens.17, 18 It is possible that those tumors that respond very quickly to T with a rise in PSA are tumors that are already producing androgens, requiring very little exogenous T stimulation to produce and increase PSA, and hence are closer to the clinical definition of CRPC. Conversely, those tumors that are making little to no androgens rely more on testicular sources of T before the neoplastic cells are stimulated and PSA begins to rise.
Numerous studies have reported that PSAdt after primary treatment 11, 12, 19, 20, biochemical recurrence13, 15, 21, and in the castration-resistant22, 23 and metastatic disease states16, 24 are associated with poorer prognosis. While an increased PSAdt is associated with a clinically important reduction in time to CRPC or PCM in our data, these relationships did not meet statistical significance due to inadequate patient numbers. One challenge in this analysis is that PSAdt may not be accurate when calculated at very low PSA values. Additionally, PSAdt is usually calculated to indirectly measure tumor growth at steady state androgen levels. It may be challenging to determine the impact of PSAdt in a setting when testosterone levels are actively rising.
A number of reasons may have contributed to the lack of prognostic significance in some of our evaluations. Our patient population size was likely insufficient to unearth a marker of marginal or moderate significance. Furthermore, the long duration of time and heterogeneity in the course of subsequent treatments following the development of CRPC, as well as the small number of patients who suffered PCM (n=13), limited our statistical power to demonstrate prognostic time intervals for PCM. Finally, studying prognostic time intervals during IAD is challenging because some patients progress during the first treatment cycle of AD while others may remain chronically castrate in the off-treatment period after only one treatment cycle of AD.
In this trial, time to first PSA rise during the first off-treatment interval was prognostic for progression to CRPC. Hence, the use of PSA and T during the first off-treatment interval of IAD for evaluating prognostic potential is reasonable. Examination of these parameters from a larger data set, such as the phase 3 PR7 trial, would be of interest. If this data were to be validated, one might consider these evaluations when making decisions about when to resume AD on an intermittent schedule: those who have good risk characteristics may be able to defer AD beyond the empiric PSA threshold defined in this trial, while those with poor risk characteristics should be considered for innovative clinical trials.
CLINICAL PRACTICE POINTS
IAD is a viable alternative for men with biochemically recurrent prostate cancer, however because of heterogeneity in long-term response to IAD, clinicians require prognostic markers to risk stratify those with indolent versus aggressive disease. The duration of the first off-treatment interval has been previously shown to be prognostic for both time to CRPC and death in this patient population. However, waiting for the first off-treatment interval to end does not take into account the biologic relationship between T and PSA and requires waiting for the PSA to rise to a preset threshold.
This analysis shows that there is a significant association between longer times to first PSA rise during the first off-treatment interval and longer times to CRPC. Also, those with a longer time (e.g., ≥60 days) to PSA rise after first T>50 ng/dL experience a longer time to CRPC.
Initial rise in PSA is a much earlier time point than waiting for the entire first off cycle to end and provides the clinician and patient much earlier information about prognosis. This information can also be used for stratification and selection of patients with more aggressive disease for clinical trial intervention using novel therapies and identification of patients who may require less therapy. Connections between T and PSA have not been previously explored for prognostic potential and our results confirm that a disconnect between T and PSA is desired. Optimally, patients on IAD will have improvement of AD side effects due to recovering T while maintaining an undetectable PSA.
Acknowledgments
Funding: Funded in part by SPORE NCI P50 CA097186, Drive Fore the Cure Northwest, DoD Prostate Cancer Research Program W81XWH-09-1-0144
The authors would like to thank Ruth Etzioni, Ph.D., for her thoughtful insight in review of our manuscript.
Footnotes
Prior Presentations: Presented in part at the 2012 Genitourinary Cancer Symposium, San Francisco, CA, Feb 2012, J Clin Oncol 30, 2012 (suppl 5; abstr 99) and 2012 American Society of Clinical Oncology Annual Meeting, Chicago, IL, Jun 2012, J Clin Oncol 30:4557A, 2012.
Clinical trials registry number: NCT00223665
CONFLICT OF INTEREST
Dr. Celestia S. Higano
Industry financial relationships over the past 3 years
Consultant for: AbbVie, Algeta, Amgen, Astellas, Bayer, BHR Pharma, Dendreon, Endo/Orion, Ferring, Fresenius, Genentech, Johnson&Johnson, Medivation, Novartis and Pfizer
Involvement in Research: Algeta, Amgen, Aragon, AstraZeneca, Bayer, Cougar Technology, Dendreon, Exelixis, Genentech, ImClone, Johnson&Johnson, Medivation, Millennium, Novartis, OncoGenex, Sanofi-Aventis and Teva Pharmaceuticals
Dr. Evan Y. Yu:
Industry financial relationships over the past 3 years:
Consultant for: Amgen, Astellas, Bayer, Dendreon, Janssen, Medivation, Millennium, Seattle Genetics
Involvement in Research: Agensys, Astellas, Astrazeneca, Bristol-Myers Squibb, Dendreon, GTx, Imclone, Janssen, Medivation, Novartis, OncoGeneX
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crook JM, O'Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895–903. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Leval J, Boca P, Yousef E, et al. Intermittent versus continuous total androgen blockade in the treatment of patients with advanced hormone-naive prostate cancer: results of a prospective randomized multicenter trial. Clin Prostate Cancer. 2002;1:163–171. doi: 10.3816/cgc.2002.n.018. [DOI] [PubMed] [Google Scholar]
- 3.Yu EY, Gulati R, Telesca D, et al. Duration of first off-treatment interval is prognostic for time to castration resistance and death in men with biochemical relapse of prostate cancer treated on a prospective trial of intermittent androgen deprivation. Journal of clinical oncology : J Clin Oncol. 2010;28:2668–2673. doi: 10.1200/JCO.2009.25.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherrier MM, Aubin S, Higano CS. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non-metastatic prostate cancer. Psychooncology. 2009;18:237–247. doi: 10.1002/pon.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Center MSKC. [accessed April 18, 2012];Prostate Cancer Nomograms:PSA Doubling Time. Available from URL: http://nomograms.mskcc.org/Prostate/PsaDoublingTime.aspx.
- 6.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Bruchovsky N, Klotz L, Crook J, Phillips N, Abersbach J, Goldenberg SL. Quality of life, morbidity, and mortality results of a prospective phase II study of intermittent androgen suppression for men with evidence of prostate-specific antigen relapse after radiation therapy for locally advanced prostate cancer. Clin Genitourin Cancer. 2008;6:46–52. doi: 10.3816/CGC.2008.n.008. [DOI] [PubMed] [Google Scholar]
- 8.Sato N, Akakura K, Isaka S, et al. Intermittent androgen suppression for locally advanced and metastatic prostate cancer: preliminary report of a prospective multicenter study. Urology. 2004;64:341–345. doi: 10.1016/j.urology.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 9.Spry NA, Kristjanson L, Hooton B, et al. Adverse effects to quality of life arising from treatment can recover with intermittent androgen suppression in men with prostate cancer. Eur J Cancer. 2006;42:1083–1092. doi: 10.1016/j.ejca.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Yu EY, Kuo KF, Gulati R, et al. Long-term dynamics of bone mineral density during intermittent androgen deprivation for men with nonmetastatic, hormone-sensitive prostate cancer. J Clin Oncol. 2012;30:1864–1870. doi: 10.1200/JCO.2011.38.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351:125–135. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 12.D'Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Prostate specific antigen doubling time as a surrogate end point for prostate cancer specific mortality following radical prostatectomy or radiation therapy. J Urol. 2004;172:S42–46. doi: 10.1097/01.ju.0000141845.99899.12. discussion S46–47. [DOI] [PubMed] [Google Scholar]
- 13.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 14.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 15.Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291:1325–1332. doi: 10.1001/jama.291.11.1325. [DOI] [PubMed] [Google Scholar]
- 16.Zagars GK, Pollack A. Kinetics of serum prostate-specific antigen after external beam radiation for clinically localized prostate cancer. Radiother Oncol. 1997;44:213–221. doi: 10.1016/s0167-8140(97)00123-0. [DOI] [PubMed] [Google Scholar]
- 17.Mohler JL, Titus MA, Wilson EM. Potential prostate cancer drug target: bioactivation of androstanediol by conversion to dihydrotestosterone. Clin Cancer Res. 2011;17:5844–5849. doi: 10.1158/1078-0432.CCR-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Surrogate end point for prostate cancer specific mortality in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;173:1572–1576. doi: 10.1097/01.ju.0000157569.59229.72. [DOI] [PubMed] [Google Scholar]
- 20.D'Amico AV, Moul JW, Carroll PR, Sun L, Lubeck D, Chen MH. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst. 2003;95:1376–1383. doi: 10.1093/jnci/djg043. [DOI] [PubMed] [Google Scholar]
- 21.Sandler HM, Dunn RL, McLaughlin PW, Hayman JA, Sullivan MA, Taylor JM. Overall survival after prostate-specific-antigen-detected recurrence following conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2000;48:629–633. doi: 10.1016/s0360-3016(00)00717-3. [DOI] [PubMed] [Google Scholar]
- 22.Semeniuk RC, Venner PM, North S. Prostate-specific antigen doubling time is associated with survival in men with hormone-refractory prostate cancer. Urology. 2006;68:565–569. doi: 10.1016/j.urology.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 23.Shulman MJ, Karam JA, Benaim EA. Prostate-specific antigen doubling time predicts response to deferred antiandrogen therapy in men with androgen-independent prostate cancer. Urology. 2004;63:732–736. doi: 10.1016/j.urology.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Loberg RD, Fielhauer JR, Pienta BA, et al. Prostate-specific antigen doubling time and survival in patients with advanced metastatic prostate cancer. Urology. 2003;62 (Suppl 1):128–133. doi: 10.1016/j.urology.2003.10.026. [DOI] [PubMed] [Google Scholar]