TO THE EDITOR
Laser ablation is a promising approach for minimally invasive removal of superficial and early nodular basal cell carcinomas (BCCs) (Smucler et al., 2008, 2012). Skin can be removed in μm-thin layers in a well-controlled manner, increasing preservation of the surrounding normal tissue. However, tissue is vaporized such that there is none available for immediate histopathological confirmation. The efficacy tends to be variable and recurrence rate (8.25%) low (Smucler et al., 2008), compared to that reported for Mohs surgery (2.1–3.5%) (Chren et al. 2011, 2013), excision (3.5–4.2%) and electrodessication and curettage (1.6–4.9%). Thus, the implementation of laser ablation for BCCs in a clinical setting is still limited.
A high-resolution nuclear-level optical imaging approach such as reflectance confocal microscopy (RCM) (Nori et al 2004, Guitera et al 2012) may guide laser ablation by detecting the presence or clearance of residual BCCs directly on the patient, to provide immediate histopathology-like feedback to improve the efficacy. Preliminary studies in human skin ex vivo with acetic acid for nuclear contrast (Sierra et al., 2013) and in vivo with aluminum chloride (Chen et al., 2014) revealed feasibility of imaging in post-ablated tissue. However, the imaging was of variable quality. Ablation was performed with a pulsed Erbium-doped Yttrium Aluminum Garnet (Er: YAG) laser, with pulse duration of ~250 μsec that produces an underlying zone of thermal coagulation of ~20 μm (Hohenleutner et al., 1997).
We therefore hypothesized that loss of tissue viability due to thermal coagulation must affect the repeatability and consistency of uptake of contrast agent and imaging of residual tumor. We may control thermal coagulation with optimal choice of ablation parameters (pulse duration, fluence, number of pulses, wavelength). This may subsequently allow repeatable and consistent uptake of contrast agent and imaging of residual nuclear morphology and detection of residual BCC tumors. In this Letter, we report the results of an extended study for determining optimal fluence and number of pulses for a given wavelength and pulse duration.
Fifty-eight discarded frozen-thawed BCC specimens from Mohs surgery were obtained under an IRB-approved protocol. The tissue was immersed in acetic acid (5%, 30 seconds) for brightening nuclear morphology (“acetowhitening”) using a previously described protocol (Patel et al., 2007). A reflectance confocal microscope (Vivascope 1500, Caliber Imaging and Diagnostics, Inc., Rochester, NY) was used to capture mosaics, displaying areas of 8mm × 8mm, of skin and to determine regions containing BCCs. A selected region containing BCCs was ablated with our Er: YAG laser (Sciton Profile, Palo Alto, CA; wavelength 2.94 μm, spot diameter 4mm), using fluences of 6.3, 12.5, 17.5 and 25.0 J/cm2 and number of passes 1 to 8. Each pass is a set of four independent pulses, separated by ~40 msec. All specimens were imaged, ablated, once again immersed in acetic acid, then imaged and finally processed for frozen histopathology. En face sections were prepared of the ablated surface that was imaged.
RCM mosaics were qualitatively evaluated against the corresponding histopathology for the appearance of nuclear, residual BCC tumor and surrounding dermal morphology. The evaluation showed that a total delivered fluence of up to 150 J/cm2 (maximum fluence 25 J/cm2 and 6 consecutive passes) allows repeatable and consistent uptake of contrast agent and RCM imaging. For higher total fluences, delivered in more than 6 consecutive number of passes without any tissue cooling in-between, the nuclear morphology appears amorphous and the residual tumor cannot be distinguished from the surrounding dermis. This must be due to the increase in thermal coagulation with increased number of passes (Hohenleutner et al., 1997, Walsh et al., 1989). However, in specimens in which ablation was performed with time interval of at least 1–2 seconds between multiple treatments (each consisting of maximum 6 consecutive passes), for passive cooling of the tissue, we can control thermal coagulation to allow the subsequent uptake of contrast agent and imaging. These observations were confirmed in the histopathology. (The limit of 6 warrants further investigation. Possibly, 6 may not be an intrinsic limit, and active cooling of the skin may allow more number of consecutive passes.)
To quantify the accuracy for detecting the clearance or presence of tumor after ablation, fifteen specimens were selected. We selected specimens with reasonably consistent initial conditions: (a) contained large tumors, and (b) treated with highest available fluence of 25J/cm2 and total of 1–10 passes, with no more than 6 consecutive passes per treatment. The presence or absence of tumor was evaluated in 30 half-mosaics (~5x magnification) against histopathology. The clearance rate, sensitivity and specificity were estimated. RCM imaging found an overall clearance rate of 40% compared to 23% by inspection of histopathology. Agreement between the confocal assessment for presence of residual tumor with histopathology was 77%. The preliminary measures of accuracy are 74% sensitivity and 86% specificity.
To mimic in vivo conditions, 10 additional specimens with intact stratum corneum were imaged and ablated with the intention of completely clearing tumor, using fluence of 25 J/cm2 and one treatment, each of 1–6 passes. The number of passes were selected based on the depth of the tumor as estimated with pre-ablation imaging (We have previously characterized depth of ablation per pass with fluence for this laser (Sierra et al., 2013)). After ablation, a RCM mosaic of the ablated surface was captured. Vertical frozen sections were then prepared from the superficial and deep margins of the ablated regions.
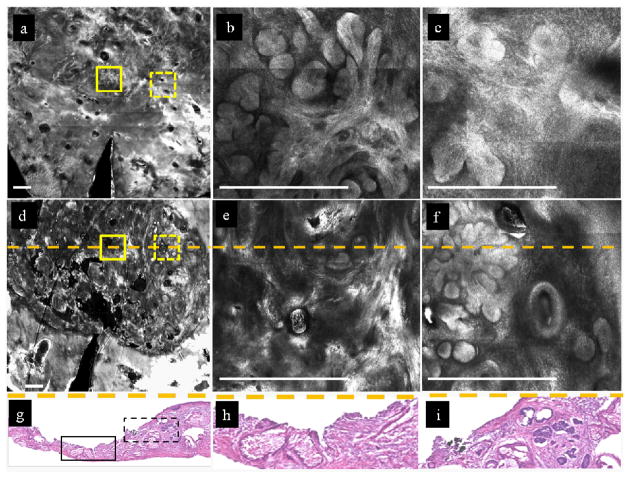
Figure 1 demonstrates the ability of RCM imaging to detect the presence and clearance of residual BCC tumor. Mosaics and images are shown of a specimen that was ablated through intact stratum corneum, with fluence of 25 J/cm2 and 6 passes. Vertical histopathology sections through the ablated region, confirmed the observations at the superficial and deep margins of the post-ablated wounds.
Figure 1.
RCM imaging detects nuclear morphology and the presence or clearance of residual BCC tumor in skin specimens ex vivo, after ablation through intact stratum corneum, with 6 passes at fluence of 25 J/cm2. Bar= 500 μm.
In Figure 1a, a pre-ablation mosaic at the dermal-epidermal junction (~130 μm depth) shows nodular BCCs (region inside both solid and dotted yellow squares). Enlarged views of the two regions within these solid and dotted squares (Figures 1b, 1c, respectively) show more clearly clusters of bright densely distributed nuclei and the nodular morphology of the tumors. Figure 1d shows a post-ablation mosaic. An enlarged view (figure 1e) of the area in the solid yellow square shows only dermal collagen and confirms clearance of tumor. By comparison, an enlarged view (figure 1f) of the region within the dashed yellow square shows clusters of densely distributed bright nuclei closer to the edge of the wound and indicates presence of residual tumor. Figure 1g shows a vertical frozen histopathology section through the wound, at the location of the dashed orange line in Figure 1d. The section confirms the clearance of tumor in the center of the wound (solid black rectangle which corresponds to the location of the dashed orange line within the solid yellow square in Figure 1d) and presence of residual tumor closer to the edge (dashed black rectangle which corresponds to the location of the dashed orange line within the dashed yellow square in Figure 1d). The pathology indicates the maximum depth of ablation to be ~160 μm and a thin layer of darker stained amorphous tissue (not obvious at low magnification) indicates a thermal coagulation zone of ~20–30 μm. Figures 1h and 1i show magnified views of the histopathology (corresponding to the location of the dashed orange line in Figure 1e and 1f), which further confirms, respectively, the clearance and presence of tumor.
For all 10 specimens, the histopathology sections confirm the observations in RCM mosaics regarding clearance of tumor or presence. The clearance, as intended, was seen in 9 specimens (true negatives) and the (unintended) presence in 1 (“false negative”). These initial results suggest that imaging may enable less invasive treatment via localized control on the depth of ablation, with potentially high negative predictive value. Furthermore, the estimation of lateral margins (not performed here, but feasible on patients (Pan et al., 2013)), in addition to depth, may improve the accuracy of ablation. However, the results highlight the current limitation of the imaging, which is mainly contrast (while resolution appears to be sufficient) for detectability of residual tumors. Further investigation and optimization of this approach for enhancement of tumor-to-dermis contrast is necessary.
Our work, together with other studies in vivo (Tannous et al., 2003, Nori et al. 2004; Scope et al., 2010; Guitera et al. 2012; Pan et al., 2013; Chen et al. 2014), suggests the potential possibility of peri-operative RCM imaging of superficial and early nodular BCCs to guide noninvasive diagnosis, pre-treatment detection of tumor margins, less invasive (ablative) treatment and post-treatment monitoring, directly on the patient. The ablation may be combined with other approaches such as debulking of tumor for enhancing the efficacy of treatment. Further clinical studies must be performed to rigorously test for accuracy (particularly, negative predictive value and recurrence rate for different subtypes of BCCs), combined with training for reading and interpretation of images
Acknowledgments
We thank the NIH for funding support (grant R01EB012466 from NIBIB’s Image-guided Interventions program).
Abbreviations
- RCM
Reflectance confocal microscopy
Footnotes
CONFLICT OF INTEREST
Three authors (HS, MC and C-SJCh) state no conflict of interest. One author (MR) owns equity in Caliber Imaging and Diagnostics (formerly, Lucid Inc).
References
- Chen ChJ, Sierra H, Cordova M, et al. Confocal microscopy guided laser ablation for superficial and early nodular basal cell carcinoma. JAMA Dermatol. doi: 10.1001/jamadermatol.2013.10225. (Published online May 14, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chren MM, Torres JS, Stuart SE, et al. Recurrence after treatment of nonmelanoma skin cancer: a prospective cohort study. Arch Dermatol. 2011;147(5):540–6. doi: 10.1001/archdermatol.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chren MM, Linos E, Torres JS, et al. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133(5):1188–96. doi: 10.1038/jid.2012.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitera P, Menzies SW, Longo C, et al. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases. J Invest Dermatol. 2012;132(10):2386–2394. doi: 10.1038/jid.2012.172. [DOI] [PubMed] [Google Scholar]
- Hohenleutner U, Hohenleutner S, Bäumler W, et al. Fast and effective skin ablation with an Er:YAG laser: determination of ablation rates and thermal damage zones. Lasers Surg Med. 1997;20(3):242–247. doi: 10.1002/(sici)1096-9101(1997)20:3<242::aid-lsm2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Nori S, Rius-Díaz F, Cuevas J, et al. Sensitivity and specificity of reflectance-mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: a multicenter study. J Am Acad Dermatol. 2004;51(6):923–930. doi: 10.1016/j.jaad.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Pan ZY, Lin JR, Cheng TT, et al. In vivo reflectance confocal microscopy of Basal cell carcinoma: feasibility of preoperative mapping of cancer margins. Dermatol Surg. 2012;38(12):1945–50. doi: 10.1111/j.1524-4725.2012.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel YG, Nehal KS, Aranda YI, et al. Confocal reflectance mosaicing of basal cell carcinomas in Mohs surgical skin excisions. J Biomed Opt. 2007;12(3):034027. doi: 10.1117/1.2750294. [DOI] [PubMed] [Google Scholar]
- Scope A, Mahmood U, Gareau DS, et al. In vivo reflectance confocal microscopy of shave biopsy wounds: feasibility of intra-operative mapping of cancer margins. Br J Dermatol. 2010;163(6):1218–1228. 1365–2133. doi: 10.1111/j.1365-2133.2010.10063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra H, Larson BA, Chen C, et al. Confocal microscopy to guide erbium:yttrium aluminum garnet laser ablation of basal cell carcinoma: an ex vivo feasibility study. J Biomed Opt. 2010;18(9):095001–095001. doi: 10.1117/1.JBO.18.9.095001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucler R, Vlk M. Combination of Er:YAG laser and photodynamic therapy in the treatment of nodular basal cell carcinoma. Lasers Surg Med. 2008;40(2):153–158. doi: 10.1002/lsm.20606. [DOI] [PubMed] [Google Scholar]
- Smucler R, Kriz M, Lippert J, et al. Ultrasound guided ablative-laser assisted photodynamic therapy of basal cell carcinoma (US-aL-PDT) Photomed Laser Surg. 2012;30(4):200–205. doi: 10.1089/pho.2011.3107. [DOI] [PubMed] [Google Scholar]
- Tannous Z, Torres A, González S. In vivo real-time confocal reflectance microscopy: a noninvasive guide for Mohs micrographic surgery facilitated by aluminum chloride, an excellent contrast enhancer. Dermatol Surg. 2003;29:839–46. doi: 10.1046/j.1524-4725.2003.29219.x. [DOI] [PubMed] [Google Scholar]
- Walsh JT, Flotte TJ, Anderson R, et al. Er:YAG laser ablation of tissue: effect of pulse duration and tissue type on thermal damage. Lasers Surg Med. 1989;9(4):314–326. doi: 10.1002/lsm.1900090403. [DOI] [PubMed] [Google Scholar]