Abstract
Over the past century, socio-environmental evolution (e.g., reduced pathogenic load, decreased physical activity [PA], improved nutrition) led to cumulative increments in maternal energy resources (i.e., body mass, adiposity) and decrements in energy expenditure and metabolic control. These decrements reduced the competition between maternal and fetal energy demands and increased the availability of energy substrates to the intrauterine milieu. This perturbation of mother-conceptus energy partitioning stimulated fetal pancreatic beta-cell and adipocyte hyperplasia, thereby inducing an enduring competitive advantage of adipocytes over other tissues in the acquisition and sequestering of nutrient-energy via intensified insulin secretion and hyperplastic adiposity. At menarche, the competitive dominance of adipocytes was further amplified via hormone-induced adipocyte hyperplasia and weight-induced decrements in PA. These metabolic and behavioral effects were propagated progressively when obese, inactive, metabolically compromised women produced progressively larger, more inactive and metabolically compromised children. Consequently, the evolution of human energy metabolism was significantly altered. This phenotypic evolution was exacerbated by increments in the use of Caesarian sections that allowed both the larger fetuses and the metabolically compromised mothers who produced them to survive and reproduce. Thus, natural selection was iatrogenically rendered artificial selection, and the frequency of obese, inactive, metabolically compromised phenotypes increased in the global population. By the late 20th century, a metabolic tipping point was reached in which the post-prandial insulin response was so intense, the relative number of adipocytes so magnified, and inactivity so pervasive that the competitive dominance of adipocytes in the sequestering of nutrient-energy was inevitable, and obesity was unavoidable.
Keywords: Childhood, Obesity, Evolution, Socio-cultural, Environmental, Maternal Effects, Phenotype
Preface
The purpose of this paper is to provide a reinterpretation and synthesis of existing empirical evidence in support of a novel theory of the etiology of the childhood obesity epidemic. The foundational theses are, 1) that obesity is the consequence of the competitive dominance of adipocytes over other cell types in the acquisition and sequestering of nutrient-energy, and 2) that the childhood obesity epidemic is the result of non-genetic evolutionary processes altering the interplay between maternal energy resources (e.g., body mass, adiposity), maternal patterns of physical activity, and the ensuing metabolic sequelae of pregnancy that impact subsequent fetal outcomes.
Introduction
The current gene-centric paradigm of inheritance and evolution has limited explanatory or predictive power with respect to the ubiquity, rapidity, and unidirectional nature of the dramatic increase in the prevalence of obesity and other significant phenotypic changes exhibited by infants and children over the past century (e.g., increased height and head circumference, body mass, precocious menarche1–4). While it may be true that “nothing in biology makes sense except in the light of evolution,”5 for most of the 20th century, non-genetic vectors of inheritance and the evolutionary consequences of developmental dynamics leading to novel phenotypes were largely ignored.6–8 This a priori constraint on heritability and evolution has no empirical or theoretic foundation, yet because theory affects research, clinical practice, and public health policy, the exclusion of non-genetic pathways for the intergenerational transmission of obesity and high-risk phenotypes has been unproductive.9
As noted by Harris (1904) more than 100 years ago, “Natural selection may explain the survival of the fittest, but it cannot explain the arrival of the fittest.”10 Given the heterogeneity of environments into which an organism may be born and the fact that phenotype-environment interactions are the substrate upon which natural selection acts, evolutionary fitness (i.e., enhanced survival and reproduction) necessitates mechanisms by which the salient environmental exposures that generated the (successful) phenotype of the mother are translated to offspring (i.e., the “arrival of the fittest”10). Because considerable environmental changes commonly occur from one generation to the next, adaptive phenotypes will not necessarily be generated via genetic inheritance. As such, I assert that the “missing heritability”11 in the rapid phenotypic changes exhibited over the past century (i.e., inheritance not explained via gene-centric paradigms) will not be found in the genome and propose a novel conceptualization of inheritance in which non-genetic vectors of evolution (i.e., maternal effects, socio-environmental and phenotypic evolution) are the predominant causal elements in the recent rise in the prevalence of childhood obesity.
Conceptual Foundation
In this paper, I provide a reinterpretation and synthesis of existing evidence to support a novel theory of inheritance and the evolution of the childhood obesity epidemic: the Maternal Resources Hypothesis (MRH). Stated simply, the MRH posits that the childhood obesity epidemic is the result of non-genetic evolutionary processes over the past century leading to a metabolic tipping point in human energy metabolism in which adipocytes (i.e., fat cells) outcompete other cell types in the acquisition and sequestering of nutrient-energy. This competitive dominance was established and is maintained by the confluence of excess maternal resources (e.g., body mass, adiposity) and inactivity-induced decrements in metabolic control during pregnancy. Given the continuum of fetal metabolic dysfunction induced via the confluence of maternal resources, inactivity, and sedentarism, I posit that the most inactive and obese familial lines have evolved beyond this metabolic tipping point (e.g., non-Hispanic blacks, Pima Amerindians).12–15 For the majority of individuals in these groups, increasing obesity and metabolic dysfunction are inevitable without significant preconception and prenatal intervention.
For this novel conceptualization of inheritance, evolution, and the etiology of obesity, there are a number of essential, interrelated, and empirically supported arguments. First, all living cells compete for nutrient-energy,16 and the strategies used for the acquisition, storage, and use of nutrient-energy vary across cell types17 and contexts.18 Thus, if obesity is defined as an excessive storage of energy as lipid in adipocytes, then it can logically be viewed as the result of the competitive dominance of adipocytes over other cells, tissues, and organs in the acquisition and sequestering of nutrient-energy resources. Second, the recent competitive dominance of adipocytes in children (i.e., the childhood obesity epidemic) was established and is maintained and/or exacerbated by three parallel, reciprocal evolutionary processes: maternal effects (ME),19 phenotypic evolution (PE),20 and socio-environmental evolution (SEE).21,22
Operational Definitions
Table 123–32 provides operational definitions for the key terms used in this manuscript. The definitions are broad and encompass the multi-dimensional nature and interdisciplinary structure of my hypotheses, which link non-genetic evolutionary processes and observed epidemiologic trends in maternal phenotype to the physiological mechanisms driving the childhood obesity epidemic. Throughout this paper, the term “evolution” is used broadly and refers to progressive, unidirectional changes over time in the variable under examination. This definition subsumes changes in inherited characteristics over successive generations (i.e., descent with modification33) and more restricted uses (e.g., changes in allele frequencies). This use is inclusive of the inheritance of both biological and non-biological (i.e., abiotic) characteristics (e.g., an impoverished postnatal environment).
Table 1.
Operational Definitions
| Environment |
External: the totality of the biotic and abiotic factors that are independent of an organism but influence development. Internal: the totality of the anatomical, physiological, and metabolic constituents that form an organism. |
| Evolution | Progressive, unidirectional changes over time in the variable under examination; inclusive of changes in inherited characteristics over successive generations and the inheritance of biological and non-biological (i.e., abiotic) characteristics (e.g., environmental resources). |
| Inheritance/Heritability | The intergenerational transmission of social and biological traits, attributes, characteristics, and/or features. Inheritance may occur via non-genetic (e.g., physiologic, cultural), epigenetic, and genetic vectors. |
| Maternal Effects (ME) | ME are non-genetic vectors of inheritance (i.e., intergenerational transmission) in which maternal phenotype (e.g., age, body mass, metabolism, behavior) and extended phenotype23 directly induce rapid, phenotypic alterations in offspring, independent of genotype.24–28 |
| Nutrient-Energy | Energy derived from the consumption of food and beverages that is available for metabolic processes. |
| Nutrient Partitioning | The metabolic fate of consumed nutrient-energy (e.g., anabolism, storage, oxidation). Body composition, physical activity and hormonal status (e.g., puberty, menopause) are the primary determinants.29–31 |
| Phenotype | An organism’s observable characteristics or traits, including but not limited to its morphology, development, physiology, metabolism, behavior, and products of behavior.23 |
| Phenotypic evolution (PE) | Unidirectional, progressive alterations in ontogeny that are propagated over multiple, successive generations and may be quantified as the change over time in the population mean for the trait under examination (e.g., height, obesity). PE is driven by developmental plasticity and adaptations to environmental heterogeneity. Because natural selection operates directly on the level of phenotype, PE has direct evolutionary consequences and may be induced via genetic, epigenetic, or non-genetic pathways of inheritance.32 |
| Socio-environmental Evolution (SEE) | SEE is a progression of social and/or cultural practices that significantly alters behavior and/or the environments in which humans exist.21,22 SEE has direct evolutionary consequences because phenotype-environment interactions are the substrate upon which natural selection acts. In social species, conspecifics and the environmental context may have a greater impact on an individual’s survival and reproduction (i.e., evolutionary fitness) than his or her genome. |
Background for Key Concepts
Maternal Effects
ME are non-genetic vectors of inheritance (i.e., intergenerational transmission) in which maternal phenotype (e.g., age, body mass, metabolism, behavior) and extended phenotype (e.g., environmental modifications)23 induce rapid, phenotypic alterations in offspring, independent of genotype.24–28 As such ME represent a mechanism by which the environmental exposures that generated the phenotype of the mother are translated directly (via developmental plasticity34) into the phenotype of the offspring.24 ME may be induced via direct physiological effects on the fetus in utero35,36 and/or the transmission of behavior25,28 from mothers to infants and children via social learning, imitation, and operant and/or classical conditioning.37–40 ME are ubiquitous in nature25,26 and contribute significantly to the variation of phenotypes derived from any given genotype.24–28,41,42 ME are causal elements in ontogeny and phenotypic plasticity in response to environmental heterogeneity24 and are of evolutionary significance19,42 because they are an essential component in generating the substrate upon which natural selection operates (i.e., the phenotype).19,24,25,32,42 Within a permissive environment, ME may be cumulative43,44 and can produce a progressive acceleration or regression of both phenotypic and genotypic evolution, as well as effects that may be in direct contrast to traits favored by natural selection (i.e., non-adaptive).7,25,27,45 ME occur in two developmental contexts, the prenatal (i.e., intrauterine) and postnatal environments, and are a major driver of the other evolutionary processes: phenotypic evolution (PE) and socio-environmental evolution (SEE).
Phenotypic Evolution
PE is a unidirectional, progressive alteration in ontogeny that is propagated over multiple, successive generations and may be quantified as the change over time in the population mean for the trait under examination (e.g., height, obesity). As will be presented in detail in a later section, PE is neither mere phenotypic plasticity nor acute adaptations to environmental heterogeneity but the progressive intergenerational transmission of acquired characteristics over multiple successive generations. PE may occur in anatomic and/or physiologic traits (e.g., height, weight, size at birth, age at menarche, hyperplastic adiposity, organ mass and function) or behavioral traits (e.g., inactivity, sedentarism). Because natural selection operates directly on the level of phenotype, PE has direct evolutionary consequences and may be induced via genetic, epigenetic, or non-genetic pathways of inheritance.32
Socio-environmental Evolution
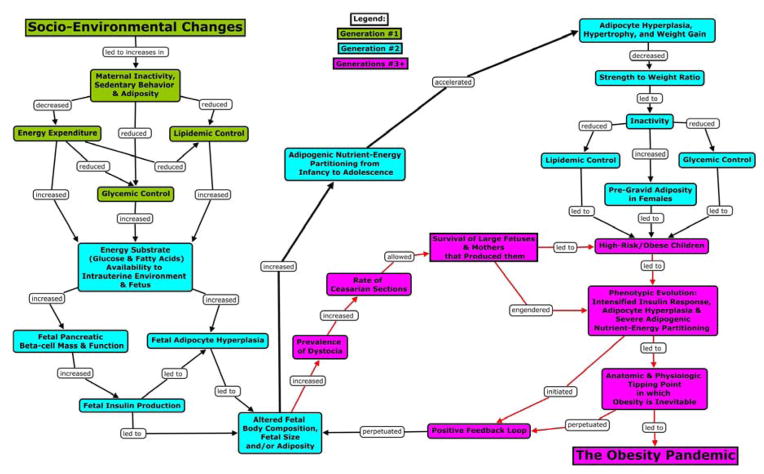
SEE is a progression of social and/or cultural practices that significantly alters behavior and/or the physical environments in which humans exist.21,22 It has been posited that SEE can be measured by a population’s “ability to utilize energy for human advancement or needs.”46 SEE occurs in multiple contexts such as social practices (e.g., healthcare) or changes in the physical environment (e.g., sanitation, food supply, labor and time-saving technologies, heating and air conditioning). SEE may be considered both process and product of numerous factors including both technological innovation21 and social learning and imitation (e.g., memes).47 Because SEE may affect the development of phenotype and significantly alter the environmental context and consequent phenotype-environment interactions, it has direct evolutionary consequences. In social species, conspecifics and the environmental context may have a greater impact on an individual’s survival than his or her genetic inheritance. SEE, PE, and ME can have reciprocal relationships as phenotype-environment interactions drive developmental dynamics that in turn drive the evolution of the social and environmental milieus. Figure 1 is a conceptual depiction of the MRH.
Figure 1.
Conceptual depiction of the MRH
The Maternal Resources Hypothesis (MRH)
The Recent Evolution of Human Energy Metabolism
Human metabolic, cardiovascular, and musculoskeletal systems evolved in environments in which survival necessitated prodigious amounts of physical exertion and high levels of energy expenditure.48 Evading predators, the hunting and gathering of food, and the literal “chopping wood and carrying water” of daily existence provided a wholesome dose of physical activity that obviated the need for deliberate exercise.49 Nevertheless, over the past few centuries, humans have become extremely adept at altering the environments in which they exist, and the evolution of their physical, social, and cultural milieus (i.e., SEE) has proceeded much more rapidly than genetic evolution.22 SEE has altered the evolution of human energy metabolism by inducing substantial decrements in the energy expenditure (EE) imposed by daily life,50 while improving both the quality and quantity of nutrient-energy availability.51 For example, as thermo-neutral environments became ubiquitous,52 the energy cost of thermoregulation declined, and improved sanitation (e.g., clean water, safer food)53 and vaccinations54 decreased the energy cost of supporting parasites (e.g., fleas)55 and resisting pathogens (e.g., communicable diseases, diarrheal infections).56 Together, these changes not only decreased EE, but dramatically curtailed periods of low energy consumption via reductions in both illness-induced and hyperthermic hypophagia.57
By gradually reducing the energy costs of survival and increasing nutrient-energy availability,53 SEE increased the energy available for development, growth, and reproduction. The positive energy balance facilitated by SEE led to the evolution of many human characteristics (i.e., PE). For example, improvements in health and nutrition over the last century have led to progressive and cumulative increases in height,1 body stature and mass,58 birth weight,59–61 organ mass,2,62 head circumference,3,63 and fat mass/adiposity.64 In concert with these increments has been a progressive global decline in the age at which adolescents attain sexual maturity, with breast development (i.e., thelarche) and menses (i.e., menarche) in girls and testicular development in boys beginning a year earlier in many populations.4 This PE has been ubiquitous and significant. A recent examination of the validity of the 1975 “Reference Man”65 for determining the safety of medication doses and occupational radiation exposure found that men and women in 2010 were heavier, taller, and had more fat and skeletal muscle mass and larger organ masses.65
Given that reproductive capacity is an essential facet of evolution, and in humans reproduction cannot occur without sufficient maternal resources (i.e., body mass, adiposity), these alterations in phenotype have non-genetic evolutionary consequences (i.e., they alter survival and reproductive success independent of changes in gene or allele frequency). Logically, these results are representative of PE because each of the aforementioned characteristics developed with a progressive, unidirectional linearity that was transmitted over successive generations. For example, from 1900 to 2000, the median height for Japanese boys and girls increased 20 cm and 19 cm at ages 13 and 11, respectively.1 These changes were neither mere developmental plasticity nor acute adaptations to improved nutrition and/or decreased energy expenditure via reductions in pathogen load. These changes in phenotype were indicative of a gradual, progressive, and enduring intergenerational transmission of greater stature over many generations that was robust to acute variations in environmental influences (e.g., food shortages).
The Late 20th Century and Increments in Maternal Resources
Until the middle of the 20th century, SEE and PE were adaptive, given that in most species, mothers with greater energy resources (i.e., physiologic or environmental) beget more robust offspring,41 and it is well-established that human mothers with adequate or ample physiologic and environmental resources produce healthier, more robust infants and children than women with fewer resources.51 Nevertheless, I posit that as the century drew to a close, sustained SEE and PE began driving maternal effects (ME) that led to the childhood obesity epidemic.
By the late 20th century, humans in industrialized nations were immersed in environments explicitly engineered to reduce manual labor,66–68 increase physical comfort (e.g., the ubiquity of chairs and thermo-neutral environments52), and afford passive entertainment.69 As a result, physical inactivity and sedentary pastimes (e.g., web-surfing, TV viewing) became both ubiquitous features of the post-industrial world51 and leading global risk factors for mortality and morbidity.70 Importantly, the confluence of passive transportation, spectator-based entertainment and decrements in occupational and household PA66,68,71 led to significant declines in PA energy expenditure (PAEE) and increments in sedentary behaviors in children, women, and mothers.66–68 From the 1960s to 2010, estimated maternal household PAEE decreased ~1,200–1,500 kcals/week, as the time spent in sedentary leisure (e.g., watching television) doubled to more than 2.5 hr/day.68 A majority of pregnant women currently spend >50% of their waking hours in sedentary behavior, and >15% of pregnant women spend more than 5 hr/day in leisure-time screen-based media use.72 Recent work suggests that by the 1990s, women and mothers allocated more time to screen-based media use (e.g., watching television) than to all forms of PA combined.68 In concert with the progressive increments in sedentarism, inactivity, and PAEE, were progressive decrements in population-level metabolic control73–75 and substantial increases in maternal pregravid obesity,76 gestational weight gain,77 and gestational diabetes (GDM).78
The Necessity of Physical Activity for Metabolic Health
Skeletal muscle (SM) activation via PA is an absolute requirement for metabolic health.79 Therefore, as mothers spent more time in sedentary behavior and the intensity, frequency, and volume of maternal PA decreased,67,68 there were marked reductions in SM activation and energy flux. Because SM is the principal tissue for both insulin-mediated glucose disposal17 and fatty acid oxidation18 and an essential element of energy metabolism,80 the progressive reductions in maternal PA and PAEE over the past century would result in progressive decrements in metabolic,17,29,31,81–83 glycemic,83–85 and lipidemic control.86–88 This loss of metabolic control led to both transient hyperglycemia (i.e., glycemic excursions) and hyperlipidemia,89–91 the former driven by reductions in insulin signaling resulting from replete myocyte glycogen stores,92,93 and the latter from reduced SM energy demands and consequent decrements in total fatty acid oxidation,86–88,94 increments in hepatic and adipocyte de novo lipogenesis,95–97 and lipid accumulation in adipose tissue.98,99
The Maternal Effects of Inactivity and Insulin Resistance
While inactivity has dire effects on human energy metabolism29,30,100,101 and health,70 given the recent SEE and PE, it is substantially more pathologic to pregnant women and their fetuses. Human pregnancy is characterized by numerous metabolic changes that promote the accretion of adipose tissue in concert with impaired insulin sensitivity and insulin resistance.102 As explained previously, SM is the principal tissue for glucose disposal, and normal pregnancies will exhibit a hormone-induced 40–60% reduction in insulin-mediated glucose disposal.103 This decrement in insulin sensitivity drives a 200–300% increase in insulin secretion to maintain maternal glycemic control.103 I posit that the progressive reductions in maternal PA and PAEE and consequent reductions in SM activation over the past half century act synergistically with the naturally occurring metabolic sequelae of pregnancy (i.e., hormone-induced insulin resistance, increased adiposity) to exacerbate the negative metabolic consequences of inactivity29,30,100,101 and drive fetal pathologies. The reductions in insulin sensitivity and increments in transient hyperglycemia and hyperlipidemia91 substantially increase the availability of energy substrates to the intrauterine environment. Because the human placenta evolved in a context of intense competition between maternal resources and fetal demands (i.e., low to moderate maternal body mass and adiposity in concert with moderate to high levels of maternal EE, PA, and PAEE104–106), the current context of high maternal resources in combination with low PA represents an evolutionary mismatch. Given that the partitioning of nutrient-energy between the mother and conceptus is a major determinant of fetal outcomes,107 the perturbation of the intrauterine milieu via the mismatch of increased maternal metabolic resources (e.g., body mass, adiposity) and inactivity driven decrements in PAEE has significant metabolic consequences for offspring.108
Excess intrauterine energy substrates stimulate the hypertrophy and hyperplasia of both pancreatic beta-cells35,109–113 and adipocytes,114–118 upregulate fetal fatty acid and glucose transporters,117 increase the direct free fatty acid uptake and storage as triglyceride in fetal adipocytes,119,120 alter myogenesis and increase collagen accumulation and crosslinking in fetal SM,121,122 and increase expression of enzymes mediating de novo lipogenesis.117 These points are critical. First, fetal adipose de novo fatty acid synthesis is a primary mechanism for the accumulation of lipid in fetal adipocytes.123 Second, maternal glucose is the major substrate for fetal lipogenesis, is highly correlated with newborn body fat,124 and is a predictor of the fat mass of pre-pubertal offspring.114 In the third trimester, maternal PA will be at its lowest point,125,126 and, therefore, maternal glycemic control will be at its nadir. Consequently, fetal lipogenesis and adipocyte hyperplasia will be maximized when compared to metabolically healthy (e.g., lean, active) mothers due to a number of processes. First, maternal hyperglycemic excursions will drive fetal hyperglycemia, which in turn results in fetal hyperinsulinemia (via enhanced beta cell mass and function) and drives growth factors that result in excessive fetal growth and adiposity.127–130 Second, maternal inactivity decreases maternal SM fatty acid oxidation and consequently promotes lipid transfer to the fetus by increasing the maternal–fetal fatty acid gradient.115
Given the strong inverse relationship between the oxidation of dietary fat in SM and obesity (i.e., obese individuals partition more fatty acids to storage as lipid, while lean individuals oxidize a greater relative amount131), the cumulative effect of alterations in fetal myogenesis and impaired SM morphology in concert with a greater number of adipocytes and increased pancreatic beta cell function (i.e., enhanced insulin secretion) produce metabolically compromised infants predisposed to life-long inactivity, metabolic dysfunction, and obesity due to the competitive dominance of adipocytes in the acquisition and sequestering of nutrient-energy.
Additionally, while SEE led to large and significant decrements in maternal activity and glycemic control, it also led to substantial declines in maternal smoking.132 Unfortunately, despite the maternal and fetal health benefits associated with reductions in tobacco use, the mild fetal hypoxia induced via smoking133 may have played a role in delaying the negative effects of inactivity on maternal glycemic control and consequent mother-conceptus energy partitioning by altering fetal glucose transporter regulation134 and growth.135
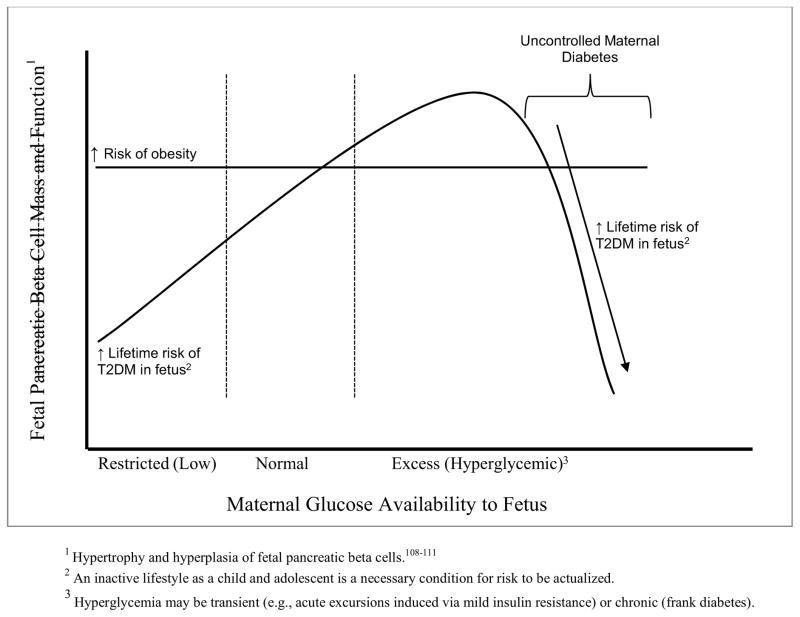
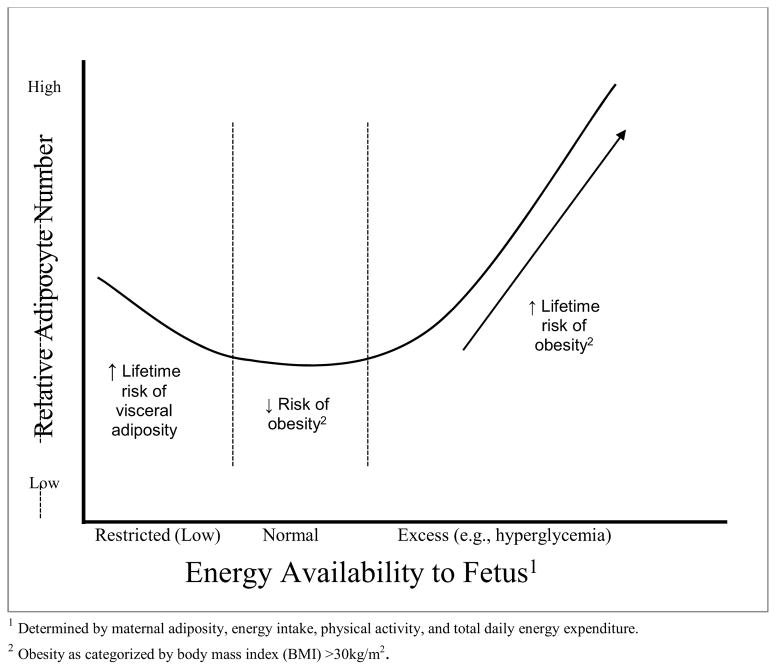
Figures 2 and 3 depict the hypothesized consequences of the perturbation of maternal-conceptus energy partitioning and fetal outcomes.
Figure 2. Hypothesized Consequences of Excess Maternal Glucose on Fetal Pancreatic beta-cell Function; T2DM = type II diabetes mellitus, CVD = cardiovascular disease.
Hypothesized Pancreatic β-cell Function and Maternal Glucose Availability to Fetus
Figure 3. Hypothesized Consequences of Excess Intrauterine Energy on Fetal Adipocyte Development.
Hypothesized Obesity Risk: Adipocyte Hyperplasia and Maternal Energy Available to Fetus
Counterfactual Support for the Maternal Resource Hypothesis
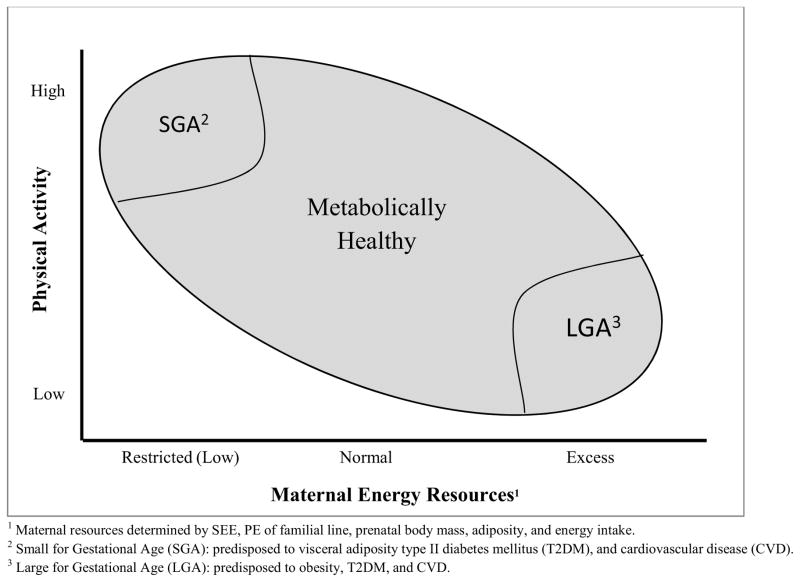
The aforementioned results are in direct contrast to women in non-industrialized nations who have not experienced similar SEE and PE over the past century. These women have relatively high levels of PA in concert with low energy resources (i.e., low body mass, adiposity, and nutrient-energy intake).136 Given that the evolutionary forces that induced increments in maternal energy resources and decrements in PA are not present, the net result is a decrease in the energy available to the intrauterine milieu. In the absence of maternal resources to buffer fetal demands,136 the competition between fetal energy requirements and maternal energy needs results in intrauterine growth restriction107 and associated pathologies.137 In congruence with the thrifty phenotype (i.e., Barker) hypothesis,138,139 the MRH posits that in the context of high levels of PA and low nutrient-energy intake, maternal myocytes and other metabolically active tissues (e.g., organs) outcompete both maternal adipocytes and fetal tissues for nutrient-energy. This results in the loss of maternal body mass and permanently alters fetal development and consequent energy metabolism while predisposing offspring to chronic non-communicable diseases (e.g., type II diabetes mellitus [T2DM], and cardiovascular disease [CVD]) when the post-natal environment permits low levels of PA in combination with adequate nutrition. Figure 4 depicts fetal outcomes as maternal resources and PA vary.
Figure 4. Hypothesized Consequences of Maternal Energy Balance on Fetal Development; SEE = Socio-Environmental Evolution, PE = Phenotypic Evolution, LGA = Large for Gestational Age, SMA = Small for Gestational Age.
Hypothesized Fetal Birth Outcomes: Physical Activity and Maternal Energy Resources
The MRH and the extant evidence suggest a continuum of metabolic control and mother-conceptus energy partitioning with both restricted136 and excess maternal resources140 pathologically altering the metabolic health of offspring.141 As such, the ideas presented herein subsume and extend both the Barker139 and Pedersen hypotheses,140 and offer a non-genetic mechanism for the intergenerational transmission of obese and other high-risk phenotypes. Stated simply, the MRH posits that the risk for obesity, T2DM, and CVD are propagated progressively via the interplay between maternal energy resources, maternal patterns of physical activity, and the ensuing metabolic sequelae of pregnancy.
Postnatal Maternal Effects
The intergenerational transmission of behavior is well accepted in social animals such as humans.142 Because the primary ecological niche of an infant is the social environment that caregivers create, the processes of postnatal ME provide non-genetic mechanisms by which the environmental exposures generated by the behavioral phenotype of the mother (or caregiver) directly alters the behavioral phenotype of infants and children. Numerous potential mechanisms have been posited including social learning, modeling (i.e., observational, operant, and/or classical conditioning.37–40,143–145 It is well established that a mother’s television (TV) viewing behaviors influence her progeny’s TV behaviors;37 therefore, as with the intergenerational transmission of smoking behavior,144 children who grow up with an inactive, sedentary caregiver may be more likely to be sedentary, inactive, and obese as adults.143,146 For example, if a woman develops the habit of breastfeeding while watching TV, her infant may associate the sights and sounds of the TV with feeding behavior. Given that maternal attention and feeding are powerful reinforcers,147 the process of classical conditioning may (metaphorically speaking) turn the TV into Pavlov’s dinner bell.145 The conjoined behaviors of feeding and TV viewing will be continuously reinforced when TV and food are used to control infant behavior (i.e., used as a babysitter).14,69
This conceptualization of the intergenerational transmission of inactivity and sedentary behavior is supported by research demonstrating strong relationships between mother-daughter BMI and obesogenic behaviors (e.g., eating in front of the TV).148 Maternal TV viewing and obesity are associated with greater infant TV exposure,146 with infants as young as three months old exposed to an average of > 2.5 hours of TV and/or videos daily, and nearly 40% of infants exposed to >3 hours of daily TV before 12 months of age.146 Having a TV in the bedroom is one of the most powerful predictors of childhood obesity,149 and large-scale epidemiologic studies have demonstrated that one of the strongest determinants of obesity and cardio-metabolic risk factors in later life was TV viewing in early life.150 In addition to the metabolic effects of postnatal ME, there are also cognitive effects. TV viewing before the age of three is associated with cognitive delays, decrements in language development, attentional issues, and sleep disorders.151
Screen-based Media as Caregiver (i.e., TV as Babysitter)
I posit that current obese phenotypes are predisposed at birth via prenatal ME, and that these predispositions are permanently entrenched by the infant’s and child’s early social environments. Over the past 50 years, the use of screen-based media has increased significantly,152 and by the late 1990s, mothers and children were spending the vast majority of their leisure time watching TV.69,148 Screen-based media (e.g., TV) is often used as a surrogate caregiver (i.e., “babysitter”)69 for precisely the same reason that it is detrimental to infants and children: it captures their attention and keeps them relatively immobile. In a non-media-enhanced world, the child will stimulate his or her nervous system via movement and “exploration” facilitated by the activation of SM. Because osteocytes, myocytes, and adipocytes share a common pool of progenitor cells, reduced PA leads to a reduction in the physiological resources (e.g., muscle development, strength, coordination) necessary for lifelong PA, and every kilocalorie of energy that is not used to build muscle and bone may be used to further increase adipocyte size and/or number.121,153 As such, the predisposition to obesity would be instantiated via accelerated hyperplastic adiposity, inactivity, decrements in the physiological resources necessary for movement (e.g., strength, coordination), and the initiation of a positive feedback loop that negatively alters health trajectories over successive generations via mother-daughter transmission.
Iatrogenic Artificial Selection
The excessive fetal growth induced via evolutionary processes has resulted in larger and fatter infants over the last few generations (e.g., increased neonate organ mass, head circumference, fat mass and birth weight2,3,60,63). Because the evolution of infant head circumference154 has progressed more quickly than the evolution of the birth canal,155 the prevalence of dystocia-related caesarian sections (i.e., surgically assisted births) has increased substantially.15,154,156 This SEE (i.e., progression of medical technology and practice) allowed both larger fetuses and the mothers who produced them to survive and reproduce, thereby increasing the frequency of metabolically compromised, obese phenotypes in the global population. As such, “natural selection” was iatrogenically and unintentionally rendered “artificial selection.” Support for the artificial selection of metabolically compromised infants is clearly evidenced by numerous facts: familial line is a major predictor of both dystocia157 and caesarian birth,158 childhood obesity has a strong relationship with cesarean birth,159 and, most importantly, the frequency of caesarian births is greatest in the population that is most inactive, sedentary, and obese (i.e., non-Hispanic black)13–15 and has had the largest increments in TV viewing over the past 50 years.160
Metabolic Tipping Point
The greatest declines in maternal activity (via our data67,68) occulted from the 1960s to the 1970s, although prior research suggests that the declines began earlier.161 This suggests that the female children of the increasingly inactive mothers of the 1950s through the 1970s would themselves be having metabolically compromised children and grandchildren 20 to 50 years later (i.e., from the early 1970s to late 2000s). As these metabolically compromised female children matured and transitioned through puberty, adipocyte number and mass were further exacerbated via the hormonal milieu162 and obesogenic environment (e.g., inactive caregivers producing inactive children and adolescents). When these women reproduced, the anatomic, physiologic, metabolic, and behavioral trajectories induced by the previous generation’s phenotype (i.e., the ME) were propagated progressively as the ontogeny of their offspring was initiated at a point further along the continuum of phenotypic plasticity (i.e., advanced baseline). This evolutionary process of accumulative maternal effects19 was facilitated by medicalized childbirth and led to anatomic, physiologic, metabolic, and behavioral tipping points that ensured an escalating competitive dominance of adipocytes in the acquisition and sequestering of nutrient-energy in many human sub-populations (e.g., African Americans). Within a few generations, the intensified post-prandial insulin response was so large (via enhanced beta-cell mass and function and inactivity-induced insulin resistance), the relative number of adipocytes so excessive, and inactivity so pervasive that the sequestering of nutrient-energy in adipocytes was inevitable and obesity was unavoidable.
Consequences of the MRH for Obesity Research
The majority of obesity research is based on the conceptual framework of energy balance derived from the first law of thermodynamics (FLT).163 The fundamental a priori assumption is that relative imbalances between nutrient-energy consumption and energy expenditure cause the excessive storage and sequestering of energy as lipid in adipocytes. This paradigm assumes a temporality that has no empirical foundation and merely provides a valid description of the increase in the storage and sequestering of energy (i.e., an analytic truth). As such, these paradigms offer no insight into the causal mechanisms or the temporal nature of the increase. I argue that because all tissues compete for energy, obesity is the result of adipocytes outcompeting other cells, tissues, and organs in post-prandial periods. The initial trajectory that engenders this competitive dominance of adipocytes (and consequent obesity) is initiated in utero due to maternal effects induced via reduced metabolic control leading to the confluence of an intensified insulin response (via enhanced beta-cell mass and function), decreased fatty acid oxidation via decrements in myogenesis and myocyte morphology, and the law of mass action (i.e., a larger relative number of fat cells disposing of a larger percentage of energy intake).
This conceptualization is strongly supported by extant research, given that increments in fat mass are a function of adiposity,164 adipocyte number is a primary determinant of obesity,165,166 and early development is a major determinant of adipocyte number.165 As such, the infant born to an inactive mother would be metabolically compromised via the confluence of the prenatal ME (e.g., adipocyte hyperplasia, reduced myogenesis) and the postnatal ME (e.g., learned inactivity). This hypothesis is strongly supported by the facts that the adipose tissue of young obese children differ both qualitatively and quantitatively from lean children167 and that adipocyte number increases throughout early development.168 Additionally, monozygotic twins concordant for birth weight exhibit similar adipocyte numbers, while in those discordant for birth weight, the smaller twin displays both lower body weight and adipocyte number.169 I posit that these results suggest an in utero “training effect” in which the chronic partitioning of energy to storage in adipose tissue induces numerous metabolic sequelae that lead to obesity via adipogenic nutrient partitioning and an exacerbated recruitment and differentiation of mesenchymal cells to mature adipocytes.170
Importantly, the increase in the storage and sequestering of nutrient-energy in adipocytes reduce the substrates and metabolic stimuli that inhibit hunger and appetitive processes (e.g., ATP/ADP ratio, hepatic energy flux, glucose and fatty acid oxidation).171,172 As such, this sequestration engenders a perception of fatigue173 (and consequent inactivity and inactivity-induced decrements in metabolic control), depression,174 decreased energy,173 and an accelerated development of hunger and consequent shorter inter-meal interval and/or increased energy density per meal. These phenomena result in a positive feedback loop that leads to excessive food and beverage consumption that exacerbates the vicious cycle of adipogenic nutrient-energy partitioning, increasing adiposity, decreased metabolic control, and obesity.
Logically, people do not develop excessive adiposity simply by being in positive energy balance; if this were true, the increases in muscle mass and parallel decreases in relative body fat as demonstrated by bodybuilders would be impossible. As such, the genesis of obesity is predicated on a greater allocation, storage, and sequestering of lipid in adipocytes as a function of adipocyte number, pancreatic beta-cell function (i.e., insulin secretion), and SM energy metabolism (i.e., glucose and fatty acid oxidation, glycogen synthesis).
Obesity as an Inherited, Chronic Condition
The MRH suggests that the energy metabolism of affected individuals is permanently altered in utero, and strategies such as reductions in energy intake (i.e., “dieting”) and other energy manipulations (e.g., exercise) will be offset, not by a regulatory mechanism per se, but by the fact that the nature of the nutrient-energy partitioning will not be altered via the loss of lipid content in the adipocytes or an increase in fatty acid oxidation by other tissues. Because it can be assumed that human energy metabolism evolved under intense selective pressures, it will be robust to acute perturbations. In other words, as long as the predisposing metabolic impairments exist, the individual will continue to store a greater relative amount of energy as lipid in adipocytes when compared to an individual with normal SM metabolism, pancreatic beta cell function, and adipocyte number. Hence, for the majority of individuals, obesity is a chronic condition of adipocyte dominance in the acquisition and sequestering of nutrient-energy that cannot be “cured” via “moving more and eating less.”
Practical Implications of the Maternal Resources Hypothesis
Given the breadth, scope, and strength of the evidence that supports the MRH, there are a number of practical implications. First, the acknowledgment that obesity is the result of non-genetic evolutionary forces and not gluttony and sloth175 may help to alter the moralizing and demoralizing social and scientific discourse that pervades both public and clinical settings. Second, the conceptual framework of tissues competing for nutrient-energy substrates has consequences for both the research community and clinicians. Future research may be most productive if funding is directed away from naïve examinations of energy balance per se and redirected to investigations of interventions that alter the competitive strategies of various tissues. From the standpoint of the clinician, accurate patient phenotyping (inclusive of family obstetric history and metabolic profiling) may allow the targeting of women most likely to be a part of populations that have evolved beyond the metabolic tipping point and therefore require significant preconception intervention.
Summary of the Maternal Resources Hypothesis
The MRH posits that the childhood obesity epidemic is the result of the evolutionary processes of ME, PE, and SEE leading to a metabolic tipping point in human energy metabolism in which adipocytes outcompete other cell types in the acquisition and sequestering of nutrient-energy. The recent competitive dominance of adipocytes was achieved via the confluence of multiple evolutionary processes. Over the last century, SEE and PE facilitated increments in maternal resources (e.g., body mass, adiposity), inactivity, and sedentarism that induced decrements in maternal metabolic control (e.g., insulin sensitivity). This PE pathologically increased the energy substrates available to fetuses, causing mothers to produce progressively larger, fatter, more inactive, and consequently more metabolically compromised and less physically fit176 offspring predisposed to chronic non-communicable diseases.177 Increments in the use of caesarian sections allowed the frequency of metabolically compromised female offspring in the population to increase. When these females reproduced, the ME of hyperplastic adiposity, intensified pancreatic beta cell function, altered SM myogenesis, and inactivity were progressively propagated to successive generations, thereby making obesity inevitable in many human familial lines. The consequences of the MRH suggest that recent evolutionary trends have not been adaptive,178 and that the evolutionary fitness (i.e., survival178 and reproduction179) of some human familial lines are in decline.
Conclusion
The MRH posits that obesity is the result of the competitive dominance of adipocytes over other tissues in the acquisition and sequestering of nutrient energy, and that the current population-wide dominance of adipocytes (i.e., the childhood obesity epidemic) is the result of non-genetic evolutionary processes altering the interplay between maternal energy resources, maternal patterns of physical activity, and the ensuing metabolic sequelae of pregnancy over multiple generations. Given that maternal metabolic control is a strong determinant of fetal metabolic outcomes and health (e.g., risk for obesity, T2DM, CVD), the health and wellbeing of future generations depend on policies and preconception interventions that can ameliorate the effects of more than a century of non-genetic evolutionary processes and overcome the current competitive dominance of adipocytes.
Acknowledgments
Support- This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number T32DK062710.
Abbreviations
- BMI
Body Mass Index
- CVD
Cardiovascular Disease
- ME
Maternal effects
- MRH
Maternal resources hypothesis
- PA
Physical activity
- PE
Phenotypic evolution
- SEE
Socio-environmental evolution
- T2DM
Type II Diabetes Mellitus
Footnotes
The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kagawa M, Tahara Y, Moji K, Nakao R, Aoyagi K, Hills AP. Secular changes in growth among Japanese children over 100 years (1900–2000) Asia Pac J Clin Nutr. 2011;20(2):180–189. [PubMed] [Google Scholar]
- 2.Thompson WS, Cohle SD. Fifteen-year retrospective study of infant organ weights and revision of standard weight tables. J Forensic Sci. 2004 May;49(3):575–585. [PubMed] [Google Scholar]
- 3.Karvonen M, Hannila ML, Saari A, Dunkel L. New Finnish reference for head circumference from birth to 7 years. Ann Med. 2012 Jun;44(4):369–374. doi: 10.3109/07853890.2011.558519. [DOI] [PubMed] [Google Scholar]
- 4.Euling SY, Herman-Giddens ME, Lee PA, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008 Feb;121 (Suppl 3):S172–191. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- 5.Dobzhansky T. Nothing in Biology Makes Sense Except in the Light of Evolution. American Biology Teacher. 1973;35:125–129. [Google Scholar]
- 6.Lickliter R. The origins of variation: evolutionary insights from developmental science. Advances in child development and behavior. 2013;44:193–223. doi: 10.1016/b978-0-12-397947-6.00007-6. [DOI] [PubMed] [Google Scholar]
- 7.Price TD, Qvarnstrom A, Irwin DE. The role of phenotypic plasticity in driving genetic evolution. Proc Biol Sci. 2003 Jul 22;270(1523):1433–1440. doi: 10.1098/rspb.2003.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noble D, Jablonka E, Joyner MJ, Muller GB, Omholt SW. Evolution evolves: physiology returns to centre stage. J Physiol. 2014 Jun 1;592(Pt 11):2237–2244. doi: 10.1113/jphysiol.2014.273151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyner MJ, Prendergast FG. Chasing Mendel: five questions for personalized medicine. J Physiol. 2014 Jun 1;592(Pt 11):2381–2388. doi: 10.1113/jphysiol.2014.272336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris JA. A New Theory of the Origin of Species. The Open Court. 1904;4:Article 1. [Google Scholar]
- 11.Llewellyn CH, Trzaskowski M, Plomin R, Wardle J. Finding the missing heritability in pediatric obesity: the contribution of genome-wide complex trait analysis. Int J Obes (Lond) 2013 Mar 26; doi: 10.1038/ijo.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz LO, Bennett PH, Ravussin E, et al. Effects of traditional and western environments on prevalence of type 2 diabetes in Pima Indians in Mexico and the U.S. Diabetes Care. 2006 Aug;29(8):1866–1871. doi: 10.2337/dc06-0138. [DOI] [PubMed] [Google Scholar]
- 13.Andersen RE, Crespo CJ, Bartlett SJ, Cheskin LJ, Pratt M. Relationship of physical activity and television watching with body weight and level of fatness among children: results from the Third National Health and Nutrition Examination Survey. JAMA. 1998 Mar 25;279(12):938–942. doi: 10.1001/jama.279.12.938. [DOI] [PubMed] [Google Scholar]
- 14.Sisson SB, Broyles ST. Social-ecological correlates of excessive TV viewing: difference by race and sex. J Phys Act Health. 2012 Mar;9(3):449–455. doi: 10.1123/jpah.9.3.449. [DOI] [PubMed] [Google Scholar]
- 15.Getahun D, Strickland D, Lawrence JM, Fassett MJ, Koebnick C, Jacobsen SJ. Racial and ethnic disparities in the trends in primary cesarean delivery based on indications. Am J Obstet Gynecol. 2009 Oct;201(4):422, e421–427. doi: 10.1016/j.ajog.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 16.Peters A. The selfish brain: Competition for energy resources. Am J Hum Biol. 2011 Jan-Feb;23(1):29–34. doi: 10.1002/ajhb.21106. [DOI] [PubMed] [Google Scholar]
- 17.Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Phys Endo Met. 1988 Dec 1;255(6):E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- 18.Aas V, Rokling-Andersen M, Wensaas AJ, Thoresen GH, Kase ET, Rustan AC. Lipid metabolism in human skeletal muscle cells: effects of palmitate and chronic hyperglycaemia. Acta Physiol Scand. 2005 Jan;183(1):31–41. doi: 10.1111/j.1365-201X.2004.01381.x. [DOI] [PubMed] [Google Scholar]
- 19.Badyaev AV. Maternal effects as generators of evolutionary change: a reassessment. Ann N Y Acad Sci. 2008;1133:151–161. doi: 10.1196/annals.1438.009. [DOI] [PubMed] [Google Scholar]
- 20.Arnold SJ. Constraints on Phenotypic Evolution. The American Naturalist. 1992;140:S85–S107. doi: 10.1086/285398. [DOI] [PubMed] [Google Scholar]
- 21.White LA. Energy and the Evolution of Culture. American Anthropologist. 1943;45(3):335–356. [Google Scholar]
- 22.Perreault C. The pace of cultural evolution. PLoS One. 2012;7(9):e45150. doi: 10.1371/journal.pone.0045150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawkins R. The Extended Phenotype. Oxford: Oxford University Press; 1982. [Google Scholar]
- 24.Wolf JB, Wade MJ. What are maternal effects (and what are they not)? Philosophical Transactions of the Royal Society B: Biological Sciences. 2009 Apr 27;364(1520):1107–1115. doi: 10.1098/rstb.2008.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mousseau TA, Fox CW. The adaptive significance of maternal effects. Trends in ecology & evolution. 1998 Oct 1;13(10):403–407. doi: 10.1016/s0169-5347(98)01472-4. [DOI] [PubMed] [Google Scholar]
- 26.Arnold SJ. Multivariate inheritance and evolution: A review of concepts. In: Boake CRB, editor. Quantitative genetic studies of behavioral evolution. Chicago: University of Chicago Press; 1994. pp. 17–48. [Google Scholar]
- 27.BERNARDO J. Maternal Effects in Animal Ecology. American Zoologist. 1996 Apr 1;36(2):83–105. [Google Scholar]
- 28.Broadhurst PL. Analysis of maternal effects in the inheritance of behaviour. Animal Behaviour. 1961 Jul;9(3–4):129–141. [Google Scholar]
- 29.Krogh-Madsen R, Thyfault JP, Broholm C, et al. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J Appl Physiol. 2010 May;108(5):1034–1040. doi: 10.1152/japplphysiol.00977.2009. [DOI] [PubMed] [Google Scholar]
- 30.Thyfault JP, Krogh-Madsen R. Metabolic disruptions induced by reduced ambulatory activity in free living humans. J Appl Physiol. 2011 Jun 2; doi: 10.1152/japplphysiol.00478.2011. [DOI] [PubMed] [Google Scholar]
- 31.Bergouignan A, Schoeller DA, Normand S, et al. Effect of physical inactivity on the oxidation of saturated and monounsaturated dietary Fatty acids: results of a randomized trial. PLoS Clin Trials. 2006;1(5):e27. doi: 10.1371/journal.pctr.0010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Day T, Bonduriansky R. A unified approach to the evolutionary consequences of genetic and nongenetic inheritance. Am Nat. 2011 Aug;178(2):E18–36. doi: 10.1086/660911. [DOI] [PubMed] [Google Scholar]
- 33.Darwin C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. London: John Murray; 1859. [Accessed 06/16/2013]. http://darwin-online.org.uk/content/frameset?itemID=F373&viewtype=text&pageseq=1. [Google Scholar]
- 34.Moczek AP, Sultan S, Foster S, et al. The role of developmental plasticity in evolutionary innovation. Proceedings of the Royal Society B: Biological Sciences. 2011 Jun 15; doi: 10.1098/rspb.2011.0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portha B, Chavey A, Movassat J. Early-life origins of type 2 diabetes: fetal programming of the beta-cell mass. Exp Diabetes Res. 2011;2011:105076. doi: 10.1155/2011/105076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gluckman PD, Hanson MA, Bateson P, et al. Towards a new developmental synthesis: adaptive developmental plasticity and human disease. Lancet. 2009 May 9;373(9675):1654–1657. doi: 10.1016/S0140-6736(09)60234-8. [DOI] [PubMed] [Google Scholar]
- 37.Notten N, Kraaykamp G, Konig RP. Family Media Matters: Unraveling the Intergenerational Transmission of Reading and Television Tastes. Sociological Perspectives. 2012;55(4):683–706. [Google Scholar]
- 38.Ricks MH. The Social Transmission of Parental Behavior: Attachment across Generations. Monographs of the Society for Research in Child Development. 1985;50(1/2):211–227. [Google Scholar]
- 39.Davison KK, Francis LA, Birch LL. Reexamining Obesigenic Families: Parents’ Obesity-related Behaviors Predict Girls’ Change in BMI. Obesity Research. 2005;13(11):1980–1990. doi: 10.1038/oby.2005.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skinner BF. Science and Human Behavior. New York: Macmillan; 1953. [Google Scholar]
- 41.Steiger S. Bigger mothers are better mothers: disentangling size-related prenatal and postnatal maternal effects. Proc Biol Sci. 2013;280(1766):20131225. doi: 10.1098/rspb.2013.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wade MJ. The Evolutionary Genetics of Maternal Effects. In: Mousseau TA, Fox CW, editors. Maternal Effects As Adaptations. New York: Oxford University Press; 1998. [Google Scholar]
- 43.Lande R, Kirkpatrick M. Selection response in traits with maternal inheritance. Genetical research. 1990 Jun;55(3):189–197. doi: 10.1017/s0016672300025520. [DOI] [PubMed] [Google Scholar]
- 44.Kirkpatrick M, Lofsvold D. The evolution of growth trajectories and other complex quantitative characters. Genome / National Research Council Canada = Genome / Conseil national de recherches Canada. 1989;31(2):778–783. doi: 10.1139/g89-137. [DOI] [PubMed] [Google Scholar]
- 45.Wells JC. The thrifty phenotype as an adaptive maternal effect. Biol Rev Camb Philos Soc. 2007 Feb;82(1):143–172. doi: 10.1111/j.1469-185X.2006.00007.x. [DOI] [PubMed] [Google Scholar]
- 46.MacCurdy GG. Human origins: A manual of prehistory. London: D. Appleton and Co; 1926. [Google Scholar]
- 47.Dawkins R. The Selfish Gene. 3. Oxford: Oxford University Press; 1976/2006. [Google Scholar]
- 48.Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004 Nov 18;432(7015):345–352. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- 49.Archer E, Blair SN. Physical activity and the prevention of cardiovascular disease: from evolution to epidemiology. Prog Cardiovasc Dis. 2011 May-Jun;53(6):387–396. doi: 10.1016/j.pcad.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Passmore R, Durnin JV. Human energy expenditure. Physiol Rev. 1955 Oct;35(4):801–840. doi: 10.1152/physrev.1955.35.4.801. [DOI] [PubMed] [Google Scholar]
- 51.WHO. Global Strategy on Diet, Physical Activity, and Health. Geneva: World Health Organization; 2004. [Google Scholar]
- 52.Kingma B, Frijns A, van Marken Lichtenbelt W. The thermoneutral zone: implications for metabolic studies. Front Biosci (Elite Ed) 2012;4:1975–1985. doi: 10.2741/e518. [DOI] [PubMed] [Google Scholar]
- 53.Semba RD. The Impact of Improved Nutrition on Disease Prevention. In: Ward JW, Warren C, editors. Silent Victories: The History and Practice of Public Health in Twentieth Century America. Oxford University Press; 2009. Oxford Scholarship Online. [Google Scholar]
- 54.Plotkin SL, Plotkin SA. A short history of vaccination. In: Plotkin SA, Orenstein WA, editors. Vaccines. Vol. 4. Philadelphia: WB Saunders; 2004. [Google Scholar]
- 55.Giorgi MS, Arlettaz R, Christe P, Vogel P. The energetic grooming costs imposed by a parasitic mite (Spinturnix myoti) upon its bat host (Myotis myotis) Proceedings of the Royal Society of London. Series B: Biological Sciences. 2001 Oct 7;268(1480):2071–2075. doi: 10.1098/rspb.2001.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009 Sep 12;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 57.Brown KH, Stallings RY, de Kanashiro HC, Lopez de Romana G, Black RE. Effects of common illnesses on infants’ energy intakes from breast milk and other foods during longitudinal community-based studies in Huascar (Lima), Peru. Am J Clin Nutr. 1990 Dec;52(6):1005–1013. doi: 10.1093/ajcn/52.6.1005. [DOI] [PubMed] [Google Scholar]
- 58.Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean body weight, height, and body mass index, United States 1960–2002. Adv Data. 2004 Oct 27;(347):1–17. [PubMed] [Google Scholar]
- 59.Ananth CV, Wen SW. Trends in fetal growth among singleton gestations in the United States and Canada, 1985 through 1998. Seminars in perinatology. 2002 Aug;26(4):260–267. doi: 10.1053/sper.2002.34772. [DOI] [PubMed] [Google Scholar]
- 60.Chike-Obi U, David RJ, Coutinho R, Wu SY. Birth weight has increased over a generation. Am J Epidemiol. 1996 Sep 15;144(6):563–569. doi: 10.1093/oxfordjournals.aje.a008966. [DOI] [PubMed] [Google Scholar]
- 61.Lahmann PH, Wills RA, Coory M. Trends in birth size and macrosomia in Queensland, Australia, from 1988 to 2005. Paediatr Perinat Epidemiol. 2009 Nov;23(6):533–541. doi: 10.1111/j.1365-3016.2009.01075.x. [DOI] [PubMed] [Google Scholar]
- 62.Shepard TH, Shi M, Fellingham GW, et al. Organ weight standards for human fetuses. Pediatr Pathol. 1988;8(5):513–524. doi: 10.3109/15513818809022307. [DOI] [PubMed] [Google Scholar]
- 63.Ounsted M, Moar VA, Scott A. Head circumference charts updated. Arch Dis Child. 1985 Oct;60(10):936–939. doi: 10.1136/adc.60.10.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olds TS. One million skinfolds: secular trends in the fatness of young people 1951–2004. Eur J Clin Nutr. 2009 Aug;63(8):934–946. doi: 10.1038/ejcn.2009.7. [DOI] [PubMed] [Google Scholar]
- 65.Later W, Bosy-Westphal A, Kossel E, Gluer CC, Heller M, Muller MJ. Is the 1975 Reference Man still a suitable reference? Eur J Clin Nutr. 2010 Oct;64(10):1035–1042. doi: 10.1038/ejcn.2010.125. [DOI] [PubMed] [Google Scholar]
- 66.Church TS, Thomas DM, Tudor-Locke C, et al. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One. 2011;6(5):e19657. doi: 10.1371/journal.pone.0019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Archer E, Lavie CJ, McDonald SM, et al. Maternal Inactivity: 45-Year Trends in Mothers’ Use of Time. Mayo Clinic Proceedings. 2013 Dec;88(12):1368–1377. doi: 10.1016/j.mayocp.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 68.Archer E, Shook RP, Thomas DM, et al. 45-Year Trends in Women’s Use of Time and Household Management Energy Expenditure. PLoS One. 2013;8(2):e56620. doi: 10.1371/journal.pone.0056620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taveras EM, Hohman KH, Price S, Gortmaker SL, Sonneville K. Televisions in the bedrooms of racial/ethnic minority children: how did they get there and how do we get them out? Clinical pediatrics. 2009 Sep;48(7):715–719. doi: 10.1177/0009922809335667. [DOI] [PubMed] [Google Scholar]
- 70.Lee, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012 Jul 21;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fisher EB, Fitzgibbon ML, Glasgow RE, et al. Behavior matters. Am J Prev Med. 2011 May;40(5):e15–30. doi: 10.1016/j.amepre.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evenson KR, Wen F. Prevalence and correlates of objectively measured physical activity and sedentary behavior among US pregnant women. Prev Med. 2011 Jul-Aug;53(1–2):39–43. doi: 10.1016/j.ypmed.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 73.Ioannou GN, Bryson CL, Boyko EJ. Prevalence and trends of insulin resistance, impaired fasting glucose, and diabetes. J Diabetes Complications. 2007 Nov-Dec;21(6):363–370. doi: 10.1016/j.jdiacomp.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 74.Li C, Ford ES, McGuire LC, Mokdad AH, Little RR, Reaven GM. Trends in hyperinsulinemia among nondiabetic adults in the U.S. Diabetes Care. 2006 Nov;29(11):2396–2402. doi: 10.2337/dc06-0289. [DOI] [PubMed] [Google Scholar]
- 75.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014 Apr 15;160(8):517–525. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heslehurst N, Ells LJ, Simpson H, Batterham A, Wilkinson J, Summerbell CD. Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36,821 women over a 15-year period. Bjog. 2007 Feb;114(2):187–194. doi: 10.1111/j.1471-0528.2006.01180.x. [DOI] [PubMed] [Google Scholar]
- 77.Helms E, Coulson CC, Galvin SL. Trends in weight gain during pregnancy: a population study across 16 years in North Carolina. Am J Obstet Gynecol. 2006 May;194(5):e32–34. doi: 10.1016/j.ajog.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 78.Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care. 2005 Mar;28(3):579–584. doi: 10.2337/diacare.28.3.579. [DOI] [PubMed] [Google Scholar]
- 79.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995 Feb 1;273(5):402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 80.Bergouignan A, Rudwill F, Simon C, Blanc S. Physical inactivity as the culprit of metabolic inflexibility: evidence from bed-rest studies. Journal of Applied Physiology. 2011 Oct;111(4):1201–1210. doi: 10.1152/japplphysiol.00698.2011. [DOI] [PubMed] [Google Scholar]
- 81.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988 Jun;37(6):667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 82.Ferraro R, Eckel R, Larson D, et al. Relationship between skeletal muscle lipoprotein lipase activity and 24-hour macronutrient oxidation. J Clin Invest. 1993 Jul;92(1):441–445. doi: 10.1172/JCI116586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mikus CR, Oberlin DJ, Libla JL, Taylor AM, Booth FW, Thyfault JP. Lowering physical activity impairs glycemic control in healthy volunteers. Med Sci Sports Exerc. 2012 Feb;44(2):225–231. doi: 10.1249/MSS.0b013e31822ac0c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kavouras SA, Panagiotakos DB, Pitsavos C, et al. Physical activity, obesity status, and glycemic control: The ATTICA study. Med Sci Sports Exerc. 2007 Apr;39(4):606–611. doi: 10.1249/mss.0b013e31803084eb. [DOI] [PubMed] [Google Scholar]
- 85.Short KR. Regulation of Glycemic Control by Physical Activity: A Role for Mitochondria? Diabetes. 2013 Jan 1;62(1):34–35. doi: 10.2337/db12-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berg A, Frey I, Baumstark MW, Halle M, Keul J. Physical activity and lipoprotein lipid disorders. Sports Med. 1994 Jan;17(1):6–21. doi: 10.2165/00007256-199417010-00002. [DOI] [PubMed] [Google Scholar]
- 87.Zderic TW, Hamilton MT. Physical inactivity amplifies the sensitivity of skeletal muscle to the lipid-induced downregulation of lipoprotein lipase activity. J Appl Physiol. 2006 Jan;100(1):249–257. doi: 10.1152/japplphysiol.00925.2005. [DOI] [PubMed] [Google Scholar]
- 88.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007 Nov;56(11):2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 89.Li C, Ford ES, McGuire LC, Mokdad AH, Little RR, Reaven GM. Trends in Hyperinsulinemia Among Nondiabetic Adults in the U.S. Diabetes Care. 2006 Nov 1;29(11):2396–2402. doi: 10.2337/dc06-0289. [DOI] [PubMed] [Google Scholar]
- 90.Cohen JD, Cziraky MJ, Cai Q, et al. 30-year trends in serum lipids among United States adults: results from the National Health and Nutrition Examination Surveys II, III, and 1999–2006. Am J Cardiol. 2010 Oct 1;106(7):969–975. doi: 10.1016/j.amjcard.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 91.Olmos PR, Rigotti A, Busso D, et al. Maternal hypertriglyceridemia: A link between maternal overweight-obesity and macrosomia in gestational diabetes. Obesity (Silver Spring) 2014 Jun 23; doi: 10.1002/oby.20816. [DOI] [PubMed] [Google Scholar]
- 92.Litherland GJ, Morris NJ, Walker M, Yeaman SJ. Role of glycogen content in insulin resistance in human muscle cells. J Cell Physiol. 2007 May;211(2):344–352. doi: 10.1002/jcp.20942. [DOI] [PubMed] [Google Scholar]
- 93.Jensen J, Rustad PI, Kolnes AJ, Lai YC. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front Physiol. 2011;2:112. doi: 10.3389/fphys.2011.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jensen MD. Fate of fatty acids at rest and during exercise: regulatory mechanisms. Acta Physiol Scand. 2003 Aug;178(4):385–390. doi: 10.1046/j.1365-201X.2003.01167.x. [DOI] [PubMed] [Google Scholar]
- 95.Blanc S, Normand S, Pachiaudi C, Fortrat JO, Laville M, Gharib C. Fuel homeostasis during physical inactivity induced by bed rest. J Clin Endocrinol Metab. 2000 Jun;85(6):2223–2233. doi: 10.1210/jcem.85.6.6617. [DOI] [PubMed] [Google Scholar]
- 96.Wilke MS, French MA, Goh YK, Ryan EA, Jones PJ, Clandinin MT. Synthesis of specific fatty acids contributes to VLDL-triacylglycerol composition in humans with and without type 2 diabetes. Diabetologia. 2009 Aug;52(8):1628–1637. doi: 10.1007/s00125-009-1405-9. [DOI] [PubMed] [Google Scholar]
- 97.Bellou E, Siopi A, Galani M, et al. Acute effects of exercise and calorie restriction on triglyceride metabolism in women. Med Sci Sports Exerc. 2013 Mar;45(3):455–461. doi: 10.1249/MSS.0b013e318278183e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hamilton MT, Areiqat E, Hamilton DG, Bey L. Plasma triglyceride metabolism in humans and rats during aging and physical inactivity. Int J Sport Nutr Exerc Metab. 2001 Dec;11 (Suppl):S97–104. doi: 10.1123/ijsnem.11.s1.s97. [DOI] [PubMed] [Google Scholar]
- 99.Slentz CA, Houmard JA, Kraus WE. Exercise, abdominal obesity, skeletal muscle, and metabolic risk: evidence for a dose response. Obesity (Silver Spring) 2009 Dec;17 (Suppl 3):S27–33. doi: 10.1038/oby.2009.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA. 2008 Mar 19;299(11):1261–1263. doi: 10.1001/jama.299.11.1259. [DOI] [PubMed] [Google Scholar]
- 101.Friedrichsen M, Mortensen B, Pehmoller C, Birk JB, Wojtaszewski JF. Exercise-induced AMPK activity in skeletal muscle: role in glucose uptake and insulin sensitivity. Mol Cell Endocrinol. 2013 Feb 25;366(2):204–214. doi: 10.1016/j.mce.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 102.Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular Mechanisms for Insulin Resistance in Normal Pregnancy and Gestational Diabetes. Diabetes Care. 2007 Jul 1;30(Supplement 2):S112–S119. doi: 10.2337/dc07-s202. [DOI] [PubMed] [Google Scholar]
- 103.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999 Apr;180(4):903–916. doi: 10.1016/s0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- 104.Power ML, Schulkin J. The Evolution of the Human Placenta. Baltimore, MD: Johns Hopkins University Press; 2012. [Google Scholar]
- 105.Dufour DL, Sauther ML. Comparative and evolutionary dimensions of the energetics of human pregnancy and lactation. American Journal of Human Biology. 2002;14(5):584–602. doi: 10.1002/ajhb.10071. [DOI] [PubMed] [Google Scholar]
- 106.Gluckman P, Hanson M. The Fetal Matrix: Evolution, Development and Disease. Cambridge, UK: Cambridge University Press; 2004. [Google Scholar]
- 107.WHO. PROMOTING OPTIMAL FETAL DEVELOPMENT: Report of a technical consultation. Geneva, Switzerland: World Health Organizatin; 2006. [Google Scholar]
- 108.Whitaker RC, Dietz WH. Role of the prenatal environment in the development of obesity. J Pediatr. 1998 May;132(5):768–776. doi: 10.1016/s0022-3476(98)70302-6. [DOI] [PubMed] [Google Scholar]
- 109.Martens GA, Pipeleers D. Glucose, regulator of survival and phenotype of pancreatic beta cells. Vitam Horm. 2009;80:507–539. doi: 10.1016/S0083-6729(08)00617-1. [DOI] [PubMed] [Google Scholar]
- 110.Steinke J, Driscoll SG. The extractable insulin content of pancreas from fetuses and infants of diabetic and control mothers. Diabetes. 1965 Sep;14(9):573–578. doi: 10.2337/diab.14.9.573. [DOI] [PubMed] [Google Scholar]
- 111.Kervran A, Guillaume M, Jost A. The endocrine pancreas of the fetus from diabetic pregnant rat. Diabetologia. 1978 Nov;15(5):387–393. doi: 10.1007/BF01219648. [DOI] [PubMed] [Google Scholar]
- 112.O’Dowd JF, Stocker CJ. Endocrine pancreatic development: impact of obesity and diet. Front Physiol. 2013;4:170. doi: 10.3389/fphys.2013.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Catalano PM, Drago NM, Amini SB. Longitudinal changes in pancreatic beta-cell function and metabolic clearance rate of insulin in pregnant women with normal and abnormal glucose tolerance. Diabetes Care. 1998 Mar;21(3):403–408. doi: 10.2337/diacare.21.3.403. [DOI] [PubMed] [Google Scholar]
- 114.Chandler-Laney PC, Bush NC, Rouse DJ, Mancuso MS, Gower BA. Maternal glucose concentration during pregnancy predicts fat and lean mass of prepubertal offspring. Diabetes Care. 2011 Mar;34(3):741–745. doi: 10.2337/dc10-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Herrera E, Amusquivar E. Lipid metabolism in the fetus and the newborn. Diabetes/Metabolism Research and Reviews. 2000;16(3):202–210. doi: 10.1002/1520-7560(200005/06)16:3<202::aid-dmrr116>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 116.Kalkhoff RK. Impact of maternal fuels and nutritional state on fetal growth. Diabetes. 1991 Dec;40 (Suppl 2):61–65. doi: 10.2337/diab.40.2.s61. [DOI] [PubMed] [Google Scholar]
- 117.Long NM, Rule DC, Zhu MJ, Nathanielsz PW, Ford SP. Maternal obesity upregulates fatty acid and glucose transporters and increases expression of enzymes mediating fatty acid biosynthesis in fetal adipose tissue depots. J Anim Sci. 2012 Jul;90(7):2201–2210. doi: 10.2527/jas.2011-4343. [DOI] [PubMed] [Google Scholar]
- 118.Dunlop M, Court JM. Lipogenesis in developing human adipose tissue. Early Hum Dev. 1978 Jul;2(2):123–130. doi: 10.1016/0378-3782(78)90004-x. [DOI] [PubMed] [Google Scholar]
- 119.Shadid S, Koutsari C, Jensen MD. Direct Free Fatty Acid Uptake Into Human Adipocytes In Vivo: Relation to Body Fat Distribution. 2007;56:1369–1375. doi: 10.2337/db06-1680. [DOI] [PubMed] [Google Scholar]
- 120.Szabo AJ, Szabo O. Placental free-fatty-acid transfer and fetal adipose-tissue development: an explantation of fetal adiposity in infants of diabetic mothers. Lancet. 1974 Aug 31;2(7879):498–499. doi: 10.1016/s0140-6736(74)92020-0. [DOI] [PubMed] [Google Scholar]
- 121.Tong JF, Yan X, Zhu MJ, Ford SP, Nathanielsz PW, Du M. Maternal obesity downregulates myogenesis and beta-catenin signaling in fetal skeletal muscle. Am J Physiol Endocrinol Metab. 2009 Apr;296(4):E917–924. doi: 10.1152/ajpendo.90924.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang Y, Zhao JX, Yan X, et al. Maternal obesity enhances collagen accumulation and cross-linking in skeletal muscle of ovine offspring. PLoS One. 2012;7(2):e31691. doi: 10.1371/journal.pone.0031691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kasser TR, Martin RJ, Allen CE. Effect of gestational alloxan diabetes and fasting on fetal lipogenesis and lipid deposition in pigs. Biology of the neonate. 1981;40(3–4):105–112. doi: 10.1159/000241478. [DOI] [PubMed] [Google Scholar]
- 124.Friis CM, Qvigstad E, Paasche Roland MC, et al. Newborn body fat: associations with maternal metabolic state and placental size. PLoS One. 2013;8(2):e57467. doi: 10.1371/journal.pone.0057467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Melzer K, Schutz Y, Boulvain M, Kayser B. Pregnancy-related changes in activity energy expenditure and resting metabolic rate in Switzerland. Eur J Clin Nutr. 2009 Oct;63(10):1185–1191. doi: 10.1038/ejcn.2009.49. [DOI] [PubMed] [Google Scholar]
- 126.Weissgerber TL, Wolfe LA, Davies GA, Mottola MF. Exercise in the prevention and treatment of maternal-fetal disease: a review of the literature. Appl Physiol Nutr Metab. 2006 Dec;31(6):661–674. doi: 10.1139/h06-060. [DOI] [PubMed] [Google Scholar]
- 127.Milner RD, Hill DJ. Fetal growth control: the role of insulin and related peptides. Clin Endocrinol (Oxf) 1984 Oct;21(4):415–433. doi: 10.1111/j.1365-2265.1984.tb03229.x. [DOI] [PubMed] [Google Scholar]
- 128.Pohlandt F, Heinze E, Fussganger F, Mayer V, Teller W. Insulin secretion in human neonates during longterm infusion of glucose. Acta endocrinologica Supplementum. 1973;173:122. doi: 10.1530/acta.0.072s122. [DOI] [PubMed] [Google Scholar]
- 129.Heinze E, Nguyen Thi C, Vetter U, Fussgänger RD. Interrelationship of Insulin and Somatomedin Activity in Fetal Rats. Neonatology. 1982;41(5–6):240–245. doi: 10.1159/000241557. [DOI] [PubMed] [Google Scholar]
- 130.Hill DJ, Sheffrin RA, Milner RD. Raised plasma somatomedin activity and cartilage metabolic activity (35S sulphate uptake in vitro) in the fetus of the mildly diabetic pregnant rat. Diabetologia. 1982 Sep;23(3):270–274. doi: 10.1007/BF00252854. [DOI] [PubMed] [Google Scholar]
- 131.Westerterp KR. Dietary fat oxidation as a function of body fat. Curr Opin Lipidol. 2009 Feb;20(1):45–49. doi: 10.1097/mol.0b013e3283186f6f. [DOI] [PubMed] [Google Scholar]
- 132.Singh GK, Kogan MD. Persistent socioeconomic disparities in infant, neonatal, and postneonatal mortality rates in the United States, 1969–2001. Pediatrics. 2007 Apr;119(4):e928–939. doi: 10.1542/peds.2005-2181. [DOI] [PubMed] [Google Scholar]
- 133.Sazak S, Kayiran SM, Paksoy Y. Umbilical cord serum erythropoietin levels and maternal smoking in pregnancy. The Scientific World Journal. 2012;2012:420763. doi: 10.1100/2012/420763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Baumann MU, Deborde S, Illsley NP. Placental glucose transfer and fetal growth. Endocrine. 2002 Oct;19(1):13–22. doi: 10.1385/ENDO:19:1:13. [DOI] [PubMed] [Google Scholar]
- 135.Miller HC, Hassanein K, Hensleigh PA. Fetal growth retardation in relation to maternal smoking and weight gain in pregnancy. Am J Obstet Gynecol. 1976 May 1;125(1):55–60. doi: 10.1016/0002-9378(76)90891-7. [DOI] [PubMed] [Google Scholar]
- 136.Prentice AM, Ward KA, Goldberg GR, et al. Critical windows for nutritional interventions against stunting. Am J Clin Nutr. 2013 May;97(5):911–918. doi: 10.3945/ajcn.112.052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Barker DJ, Lampl M, Roseboom T, Winder N. Resource allocation in utero and health in later life. Placenta. 2012 Nov;33 (Suppl 2):e30–34. doi: 10.1016/j.placenta.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 138.Barker DJ, Martyn CN, Osmond C, Hales CN, Fall CH. Growth in utero and serum cholesterol concentrations in adult life. BMJ. 1993 Dec 11;307(6918):1524–1527. doi: 10.1136/bmj.307.6918.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992 Jul;35(7):595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 140.Pedersen J. The pregnant diabetic and her newborn: problems and management. Copenhagen: Munksgaard; 1967/1977. [Google Scholar]
- 141.Wells JC. The thrifty phenotype: An adaptation in growth or metabolism? Am J Hum Biol. 2011 Jan-Feb;23(1):65–75. doi: 10.1002/ajhb.21100. [DOI] [PubMed] [Google Scholar]
- 142.Jablonka E, Lamb MJ. Precis of Evolution in Four Dimensions. Behav Brain Sci. 2007 Aug;30(4):353–365. doi: 10.1017/S0140525X07002221. discusssion 365–389. [DOI] [PubMed] [Google Scholar]
- 143.Franks PW, Ravussin E, Hanson RL, et al. Habitual physical activity in children: the role of genes and the environment. Am J Clin Nutr. 2005 Oct;82(4):901–908. doi: 10.1093/ajcn/82.4.901. [DOI] [PubMed] [Google Scholar]
- 144.Conrad KM, Flay BR, Hill D. Why children start smoking cigarettes: predictors of onset. British journal of addiction. 1992 Dec;87(12):1711–1724. doi: 10.1111/j.1360-0443.1992.tb02684.x. [DOI] [PubMed] [Google Scholar]
- 145.Pavlov IP. Conditional Reflexes. New York: Dover; 1927/1960. [Google Scholar]
- 146.Thompson AL, Adair LS, Bentley ME. Maternal characteristics and perception of temperament associated with infant TV exposure. Pediatrics. 2013 Feb;131(2):e390–397. doi: 10.1542/peds.2012-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Skinner BF. The phylogeny and ontogeny of behavior. Contingencies of reinforcement throw light on contingencies of survival in the evolution of behavior. Science. 1966 Sep 9;153(741):1205–1213. doi: 10.1126/science.153.3741.1205. [DOI] [PubMed] [Google Scholar]
- 148.Sonneville KR, Rifas-Shiman SL, Kleinman KP, Gortmaker SL, Gillman MW, Taveras EM. Associations of obesogenic behaviors in mothers and obese children participating in a randomized trial. Obesity (Silver Spring) 2012 Jul;20(7):1449–1454. doi: 10.1038/oby.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dennison BA, Erb TA, Jenkins PL. Television viewing and television in bedroom associated with overweight risk among low-income preschool children. Pediatrics. 2002 Jun;109(6):1028–1035. doi: 10.1542/peds.109.6.1028. [DOI] [PubMed] [Google Scholar]
- 150.Chen YY, Lee YS, Wang JP, et al. Longitudinal study of childhood adiposity and the risk of developing components of metabolic syndrome-the Da Qing children cohort study. Pediatr Res. 2011 Sep;70(3):307–312. doi: 10.1203/PDR.0b013e318225f8a9. [DOI] [PubMed] [Google Scholar]
- 151.Christakis DA, Zimmerman FJ, DiGiuseppe DL, McCarty CA. Early Television Exposure and Subsequent Attentional Problems in Children. Pediatrics. 2004 Apr 1;113(4):708–713. doi: 10.1542/peds.113.4.708. [DOI] [PubMed] [Google Scholar]
- 152.Robinson J. IT, TV and Time Displacement: What Alexander Szalai Anticipated but Couldn’t Know. Social Indicators Research. 2011;101(2):193–206. doi: 10.1007/s11205-010-9653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yan X, Zhu M, Dodson M, Du M. Developmental Programming of Fetal Skeletal Muscle and Adipose Tissue Development. Journal of Genomics. 2012;1:29–38. doi: 10.7150/jgen.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Taffel SM, Placek PJ, Liss T. Trends in the United States cesarean section rate and reasons for the 1980–85 rise. Am J Public Health. 1987 Aug;77(8):955–959. doi: 10.2105/ajph.77.8.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wells JC, DeSilva JM, Stock JT. The obstetric dilemma: an ancient game of Russian roulette, or a variable dilemma sensitive to ecology? Am J Phys Anthropol. 2012;149 (Suppl 55):40–71. doi: 10.1002/ajpa.22160. [DOI] [PubMed] [Google Scholar]
- 156.Elvander C, Hogberg U, Ekeus C. The influence of fetal head circumference on labor outcome: a population-based register study. Acta obstetricia et gynecologica Scandinavica. 2012 Apr;91(4):470–475. doi: 10.1111/j.1600-0412.2012.01358.x. [DOI] [PubMed] [Google Scholar]
- 157.Berg-Lekas ML, Hogberg U, Winkvist A. Familial occurrence of dystocia. Am J Obstet Gynecol. 1998 Jul;179(1):117–121. doi: 10.1016/s0002-9378(98)70260-1. [DOI] [PubMed] [Google Scholar]
- 158.Varner MW, Fraser AM, Hunter CY, Corneli PS, Ward RH. The intergenerational predisposition to operative delivery. Obstet Gynecol. 1996 Jun;87(6):905–911. doi: 10.1016/0029-7844(96)00064-6. [DOI] [PubMed] [Google Scholar]
- 159.Blustein J, Attina T, Liu M, et al. Association of caesarean delivery with child adiposity from age 6 weeks to 15 years. Int J Obes (Lond) 2013 Jul;37(7):900–906. doi: 10.1038/ijo.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Robinson JP, Godbey G. Time for life: The surprising ways Americans use their time. 2. University Park, PA: Pennysylvania State University Press; 1999. [Google Scholar]
- 161.Gershuny J, Robinson JP. Historical Changes in the Household Division of Labor. Demography. 1988;25(4):537–552. [PubMed] [Google Scholar]
- 162.Loomba-Albrecht LA, Styne DM. Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes. 2009 Feb;16(1):10–15. doi: 10.1097/med.0b013e328320d54c. [DOI] [PubMed] [Google Scholar]
- 163.Hebert JR, Allison DB, Archer E, Lavie CJ, Blair SN. Scientific decision making, policy decisions, and the obesity pandemic. Mayo Clin Proc. 2013 Jun;88(6):593–604. doi: 10.1016/j.mayocp.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Forbes GB. Body fat content influences the body composition response to nutrition and exercise. Ann N Y Acad Sci. 2000 May;904:359–365. doi: 10.1111/j.1749-6632.2000.tb06482.x. [DOI] [PubMed] [Google Scholar]
- 165.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008 Jun 5;453(7196):783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 166.Bjorntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord. 1996 Apr;20(4):291–302. [PubMed] [Google Scholar]
- 167.Knittle JL, Timmers K, Ginsberg-Fellner F, Brown RE, Katz DP. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J Clin Invest. 1979 Feb;63(2):239–246. doi: 10.1172/JCI109295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hager A, Sjostrm L, Arvidsson B, Bjorntorp P, Smith U. Body fat and adipose tissue cellularity in infants: a longitudinal study. Metabolism. 1977 Jun;26(6):607–614. doi: 10.1016/0026-0495(77)90082-8. [DOI] [PubMed] [Google Scholar]
- 169.Ginsberg-Fellner F. Growth of adipose tissue in infants, children and adolescents: variations in growth disorders. Int J Obes. 1981;5(6):605–611. [PubMed] [Google Scholar]
- 170.Sjostrom L, William-Olsson T. Prospective studies on adipose tissue development in man. Int J Obes. 1981;5(6):597–604. [PubMed] [Google Scholar]
- 171.Friedman MI. Control of energy intake by energy metabolism. 1995 Nov 1;62(5):1096S–1100. doi: 10.1093/ajcn/62.5.1096S. [DOI] [PubMed] [Google Scholar]
- 172.Mayer J. Correlation between metabolism and feeding behavior and multiple etiology of obesity. Bulletin of the New York Academy of Medicine. 1957 Nov;33(11):744–761. [PMC free article] [PubMed] [Google Scholar]
- 173.Wlodek D, Gonzales M. Decreased energy levels can cause and sustain obesity. J Theor Biol. 2003 Nov 7;225(1):33–44. doi: 10.1016/s0022-5193(03)00218-2. [DOI] [PubMed] [Google Scholar]
- 174.Kalarchian MA, Marcus MD. Psychiatric comorbidity of childhood obesity. International review of psychiatry (Abingdon, England) 2012 Jun;24(3):241–246. doi: 10.3109/09540261.2012.678818. [DOI] [PubMed] [Google Scholar]
- 175.Prentice AM, Jebb SA. Obesity in Britain - Gluttony or Sloth. Br Med J. 1995 Aug;311(7002):437–439. doi: 10.1136/bmj.311.7002.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gahche J, Fakhouri T, Carroll DD, Burt VL, Wang C, Fulton JE. Cardiorespiratory Fitness Levels Among U.S. Youth Aged 12–15 Years: United States, 1999–2004 and 2012; NCHS Data Brief No. 153. Hyattsville, MD: CDC; National Center for Health Statistics; 2014. [PubMed] [Google Scholar]
- 177.Carson V, Janssen I. Volume, patterns, and types of sedentary behavior and cardio-metabolic health in children and adolescents: a cross-sectional study. BMC Public Health. 2011;11:274. doi: 10.1186/1471-2458-11-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Olshansky SJ, Passaro DJ, Hershow RC, et al. A Potential Decline in Life Expectancy in the United States in the 21st Century. New England Journal of Medicine. 2005;352(11):1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 179.Martinez G, Daniels K, Chandra A. Fertility of men and women aged 15–44 years in the United States: National Survey of Family Growth, 2006–2010. Hyattsville, MD: National Center for Health Statistics; 2012. National health statistics reports; no. 51. [PubMed] [Google Scholar]