Abstract
Histone deacetylase 4 (HDAC4) is a critical negative regulator for chondrocyte hypertrophy by binding to and inhibiting Runx2, a critical transcription factor for chondrocyte hypertrophy. It is unclear how HDAC4 expression and stability are regulated during growth plate development. We report here that inhibition of mitogen-activated protein kinase (MAPK) p38 by dominant negative p38 or p38 inhibitor prevents HDAC4 degradation. Mutation of a potential caspase-2 and 3 cleavage site Asp289 stabilizes HDAC4 in chondrocytes. In contrast, constitutively active MAPK kinase 6 (constitutive activator of p38) transgenic mice exhibit decreased HDAC4 content in vivo. We also observed p38 stimulates caspase-3 activity in chondrocytes. Inhibition of p38 or caspases reduced HDAC4 degradation. HDAC4 inhibited Runx2 promoter activity in a dose-dependent manner and caspase inhibitors further enhanced this inhibition by preventing HDAC4 degradation. Overall, these results demonstrate that p38 promotes HDAC4 degradation by increasing caspase-mediated cleavage, which releases Runx2 from a repressive influence of HDAC4 and promotes the chondrocyte hypertrophy and bone formation.
Keywords: HDAC4, p38, caspase, inhibition, degradation
Introduction
Histone deacetylase 4 (HDAC4) plays a key role in the regulation of transcription, cell cycle, and development. HDAC4 activity alters chromosome structure and affects transcription factor access to DNA, as well as represses transcription when tethered to a promoter. Studies have shown that HDAC4 regulates bone and muscle development (Arnold et al., 2007; Lu et al., 2000) (McKinsey et al., 2000) (Miska et al., 1999; Miska et al., 2001). HDAC4 and Runx2 complexes have been shown to be critical for bone development. Complete loss of HDAC4 results in premature ossification of endochondral bone due to increased chondrocyte hypertrophy and postnatal lethality due to various defects (Vega et al., 2004). In contrast, overexpression of HDAC4 inhibits hypertrophy and differentiation, a result which mimics a Runx2 loss-of-function phenotype (Vega et al., 2004). Therefore, HDAC4 plays a key role in endochondral bone formation as a negative regulator for chondrocyte hypertrophy, and its stability is essential for controlling cell death and differentiation (Paroni et al., 2004). HDAC4 is highly expressed in pre-hypertrophic chondrocytes and its protein content is reduced in hypertrophic chondrocytes (Vega et al., 2004) (Guan et al., 2012), but it is not fully understood what regulates HDAC4 expression and stability during growth plate development.
Cernotta et al. found that serum starvation elicits a poly-ubiquitination and degradation of HDAC4 on serine sites for cell motility (Cernotta et al 2010). In cardiomyocytes, Backs et al. reported that protein kinase A (PKA) regulated proteolysis of HDAC4 through serine proteases (Backs et al., 2011; Shimizu et al., 2014). These studies indicate that the degradation of HDAC4 is complex and regulated by different pathways. Liu et al. reported that HDAC4 degradation is caspase-dependent (Liu et al., 2004), which may contribute to the distribution pattern of HDAC4 in hypertrophic chondrocytes. Caspases have been associated with the degradation of HDAC4 at the Asp289 site of HDAC4 in HeLa and IMR90-E1A cell lines (Liu et al., 2004) (Paroni et al., 2004). Mutation of Asp289 or treatment of caspase inhibitor could stabilize HDAC4(Liu et al., 2004) (Paroni et al., 2004). Increase of caspase activity has been documented in hypertrophic chondrocytes (Chrysis et al., 2002). We have previously shown simultaneous increase in mitogen-activated protein kinase p38 activity in hypertrophic chicken chondrocytes (Zhen et al., 2001). Previous studies showed that mitogen-activated protein kinase (MAPK) p38 mediates caspase-3 activity in the hippocampus under hypertonic stress (Niswander and Dokas, 2007) and during apoptosis induced by singlet oxygen in human leukemia cells (Zhuang et al., 2000). Thus, it is possible that p38 also mediates caspase activity in chondrocytes, and subsequently regulates HDAC4 degradation through caspases. In agreement with this hypothesis, HDAC4 protein content is remarkably decreased in hypertrophic chondrocytes compared with pre-hypertrophic chondrocytes (Guan et al., 2011) while both p38 activity and caspase activity are increased in this region (Chrysis et al., 2002; Zhen et al., 2001). To further test this hypothesis, we altered the intracellular levels of p38 and caspase activities in chondrocytes to determine if HDAC4 degradation is affected by alteration of p38 or caspase activity. If both p38 and caspases affect HDAC4 degradation in accordance to our hypothesis, we will investigate if p38 activates caspases and if mutation of the caspase cleavage site (D289E) could lead to resistance to caspase or p38 mediated HDAC4 degradation in chondrocytes. The results of these experiments will address the question of how HDAC4 is regulated in chondrocytes and help to better understand the mechanism of chondrocyte hypertrophy and bone formation.
Materials and Methods
Animals
Homozygous Col II/CAMKK6 transgenic (CAMKK6-TG) mice, and their wild type (WT) littermates were euthanized at 10 days of age (N=4). These samples (provided by Dr. Shunichi Murakami) were a subset of those used for a previous study and the increase of p38 activity has been validated in vivo (Zhang et al., 2006). All experiments were approved by the Animal Care and Use Committee of Rhode Island Hospital.
Primary Cell Culture
Primary chondrocyte cultures were established as described previously (Wei et al., 2010). Briefly, hypertrophic chondrocytes were obtained from the cephalic part of sterna cartilage from 17-day-old embryonic (E17) chickens in which the cephalic part of sterna cartilage contains early hypertrophic chondrocytes that just started to synthesize type X collagen (Chen et al., 1995). Cartilage pieces were incubated in Ham’s F-12 medium (Invitrogen, Grand Island, NY, USA) containing 0.1% testicular hyaluronidase, 0.3% collagenase and 0.1% trypsinase for 30 minunites at 37°C, and continued to incubate in fresh medium containing enzymes for 1 hour. Cells were collected by centrifugation and grew in F-12 medium supplemented with 10% fetal bovine serum (FBS, Invitrogen, Grand Island, NY, USA) at 37 °C. At about 80–90% confluence, cells were incubated with F12 medium with 0.5% FBS overnight before experiments.
Western blot
The CAMKK6 cDNA was generated by replacing both phosphorylation residues Ser207 and Thr211 by Glu, and the DNp38 cDNA was generated by replacing phosphorylation residues Thr180 and Tyr182 by Ala and Phe respectively (Raingeaud et al., 1996). Cells were transfected with pcDNA3 as control and construct containing HDAC4 (provided by Tony Kouzarides) (Miska et al., 2001), CAMKK6 and DNp38 (provided by Roger Davis) (Raingeaud et al., 1996), D289E (provided by Claudio Brancolini) (Paroni et al., 2004) at about 80% confluence and incubated for 48 hours at 37°C, with or without cycloheximide (25 ng/ml, Sigma-Aldrich, St Louis, MO, USA), which inhibits protein neosynthesis (Liu et al., 2004). Cells were washed with pre-chilled PBS 3 times and harvested with Complete Lysis-M buffer (Roche, Penzberg, Upper Bavaria, Germany). Lysate was transferred to ice and centrifuged for the supernatant of the homogenate. Equal amount of protein samples were separated on a 10% SDS-PAGE gel, transferred onto a nitrocellulose polyvinylidene difluoride membrane, and probed with primary antibodies against p38, phosphorylated p-38 (p-p38), HDAC4 (N-18), and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Detection and signal visualization were performed using the appropriate horseradish peroxidase conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and an ECL detection system (Pierce, Rockford, IL, USA). The experiments were repeated 3 times and results were similar. Averaged results were shown in the figure.
Histology and Immunofluorescence staining
Proximal tibia growth plate was harvested from P10 mice, immersed in 10% formalin for 24 hours, and decalcified in 20% EDTA solution (pH 7.2). 6-μm sections were mounted on slides. Standard Safranin-O staining was performed to visualize morphology with a Nikon Eclipse E800 microscope (Nikon, Tokyo, Japan). Immunofluorescence staining was performed to determine HDAC4 expression in vivo. Endogenous peroxidase was blocked by treating the sections with 3% hydrogen peroxide (Sigma-Aldrich, St Louis, MO, USA) in methanol (Sigma-Aldrich, St Louis, MO, USA) for 30 minutes. The sections were digested by 5 mg/ml hyaluronidase in PBS (Sigma-Aldrich, St Louis, MO, USA) for 20 minutes. The sections were analyzed by immuno-staining using a goat polyclonal antibody against HDAC4 (1:100 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C as published before (Guan et al., 2011). The secondary antibody, rhodamine-conjugated donkey-anti-goat IgG (H+L) (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) was applied for 1 hour. Normal goat IgG (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used in place of primary antibody as negative control. Slides were mounted and viewed under the Nikon Eclipse E800 microscope (Nikon, Tokyo, Japan). The intensity of HDAC4 signal was quantified using Elements imaging software (NIS Nikon Eclipse 90i, Melville, NY, USA) as the pixel unit of grey scale fluorescent signal. A representative result was shown in the figure.
Colorimetric Assay
Caspase-3 activity was determined using Caspase-3/CPP32 Colorimetric Assay Kit (Biovision, Milpitas, CA, USA). Briefly, hypertrophic chondrocytes were infected with culturing medium containing the Replication Competent Avian Splice (RCAS) vector or recombinant RCAS viruses expressing CAMKK6 and DN p38. After 48 hours, 1-5×106 cells were harvested with 50 μl of pre-chilled cell lysis buffer. Protein concentration in each cell lysate sample was determined using BCA reagent for protein quantification. Reaction buffer containing 10mM DTT which protects cysteine-containing proteins was added. Cell extracts were incubated with 5μl of 4mM tetrapeptide substrates (DEVD-pNA, caspase-3) at 37°C for 1-2 hours. The reaction was measured at 405 nm in the Spectra Max M2 Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). The experiments were repeated 3 times and the averaged results were shown in the figure.
Realtime PCR
The quantification of mRNA was performed by Realtime PCR using the QuantiTect SYBR Green PCR kit (Qiagen, Valencia, CA, USA) with the Bio-Rad CFX384 Realtime PCR Detection System (Bio-Rad, Hercules, CA, USA). Each reaction was performed in triplicate. Chicken HDAC4 primer assay was purchased from Qiagen. Amplification conditions were as follows: 2 minutes preincubation at 50°C, 10 minutes at 95°C for enzyme activation, and 40 cycles at 95°C denaturation for 10 seconds, 55°C annealing for 30 seconds and 72°C extension for 30 seconds. The comparative threshold cycle (Ct) method, i.e., 2−ΔΔCt method was used for the calculation of fold amplification (Wei et al., 2010). The experiments were repeated 3 times and similar results were obtained. Averaged results were shown in the figure.
Dual Luciferase Assay
To test the effect of HDAC4 on Runx2 activity, chicken chondrocytes were co-transfected with pcDNA3-Runx2-Luc reporter plasmid (100 ng), pcDNA3-HDAC4 plasmid (0.1 ng, encoding full length human HDAC4), and Renilla luciferase control plasmid (5 ng) per well in 12-well plates. After 24 hours, cells were subjected to caspase-2, 3 inhibitor treatments. Luciferase activity was assayed with the Dual-Glo Luciferase system (Promega, Madison, Wisconsin, USA) according to manufactory protocol.
Statistical Analysis
Data were expressed as means ± standard deviation (SD). Two-tailed paired t-tests were used to compare mRNA levels between the caspase inhibitor treated and control groups. A probability of <5% was considered significant. The Runx2 promoter assay were analyzed by one-way ANOVA with multiple pair-wise comparisons made by the Student-Newman-Keuls method (3 comparisons or more) at a rejection level of 5% unless otherwise noted.
Results and Discussion
Inhibition of p38 MAPK activity prevents HDAC4 degradation
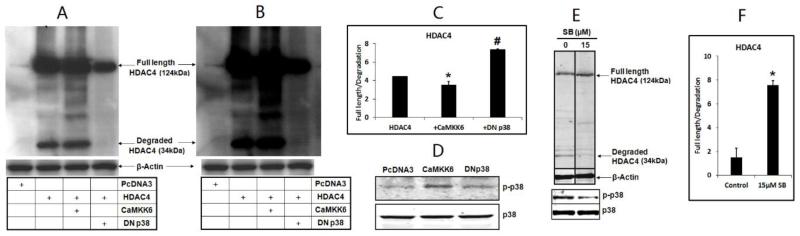
To determine whether p38 regulates HDAC4 degradation, we manipulated p38 activity by treating the cells with p38 inhibitor SB203580 or transfecting cells with dominant negative p38 (DN p38) or the constitutively active MAPK kinase 6 (CAMKK6). Cell lysate was assayed using western blot to examine the p38 kinase activity and HDAC4 degradation 2 days after transfection. Our data demonstrate that the inhibition of p38 by overexpressing DN p38 prevents HDAC4 degradation as indicated by decreasing the HDAC4 degradation fragment (34 kDa) compared to the empty vector control or CAMKK6 transfection (Fig. 1A and B), while increased p38 phosphorylation presents only in CAMKK6 transfected chondrocyotes but not DN p38 or empty vector control (Fig. 1D). Consistently, p38 inhibitor SB203580 also reduced the 34 kDa HDAC4 degradation fragment (Fig.1E and F). These observations strongly indicate that HDAC4 degradation is under the control of p38 MAPK.
Figure 1. Inhibition of p38 MAPK activity reduces HDAC4 degradation.
(A) Degraded HDAC4 band (34 kDa) was not detected in DN p38 treated cells, even after overexposure (B). (C) Intensity of the full length HDAC4/34 kDa degradation fragment was calculated and presented. *, #, p<0.05. (D) Western blot showed phosphorylation of p38 (p-p38) was induced by CAMKK but not DN p38. (E) Chicken chondrocytes were treated with p38 inhibitor SB203580 (15 μM) for 48 hours. Western blot showed that the degraded band (34 kDa) was decreased. (F) The intensity of full length HDAC4/degradation fragment was presented. *, p<0.05. SB203580 (15 μM) effectively inhibited p38 phosphorylation (E).
Mutation of caspase cleavage site stabilizes HDAC4 degradation in chondrocytes
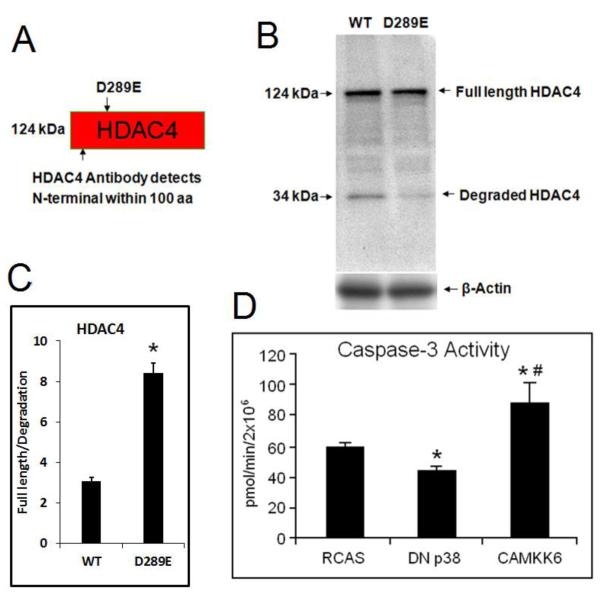
HDAC4 degradation is caspase-dependent (Liu et al., 2004). It has been reported that Asp289 is the primary cleavage site for caspase, and that mutation of Asp289 or treatment of caspase inhibitor could stabilize HDAC4 in human cancer lines (Liu et al., 2004) (Paroni et al., 2004). We also confirmed this finding in chondrocytes by showing that D289E mutants had less degradation fragment of HDAC4 when compared with wild types (34 kDa) (Fig. 2A-C). We noticed that HDAC4 degradation was significantly reduced but not completely disappeared (Fig. 2B), this is more likely due to the existence of the endogenous HDAC4 rather than incomplete prevention of degradation, because we also observed a very thin 34kDa band in the first lane of the Fig. 1B (without HDAC4 overexpression). This is consistent with the original publication from which we obtained the D289E mutant, HDAC4 degradation bands were significantly reduced, but not completely gone in the mutant as well (Paroni et al., 2004). Since we discovered that inhibition of p38 activity also prevents HDAC4 degradation (Fig. 1E and F), and it has been reported that increase of caspase and p38 activities co-exists in hypertrophic chondrocytes (Chrysis et al., 2002) (Zhen et al., 2001), we speculate that p38 mediates HDAC4 degradation at the Asp289 site through up-regulation of caspases. To test this possibility, we determined caspas-3 activity in treated cells in which p38 activities were manipulated. Caspase-3 activity assay showed that over-activation of p38 by CAMKK6 stimulates caspase-3 activity while inhibition of p38 by DN p38 has the opposite effect (Fig. 2D).
Figure 2. Mutation of Asp-289 stabilized HDAC4 and CaMKK6 induces capase-3 activity in chondrocytes.
(A) Schematic structure of HDAC4 revealed Asp 289 as the target of mutation to remove capase-2 and 3 cleavage site, and HDAC4 antibody detects both N-termimal degradation fragment and full length HDAC4. (B) Western blot shows that HDAC4 is cleaved in WT but the mutant D289 is resistant to degradation of HDAC4. The result indicates that the degradation of HDAC4 is related to ASP-289. (C) Intensity of full length HDAC4/degradation fragment was presented. *, p<0.05. (D) Caspase activity assays showed that over-activation of p38 by CAMKK6 stimulates caspase-3 activity while inhibition of p38 by DN p38 has the opposite effect when comparing to the empty RCAS control. Data are the means of three independent experiments, with S.D. indicated. *, p< 0.05 compared with control. #, p< 0.05 compared with DN p38.
Overactivation of p38 MAPK activity leads to decreased HDAC4 protein content in vivo
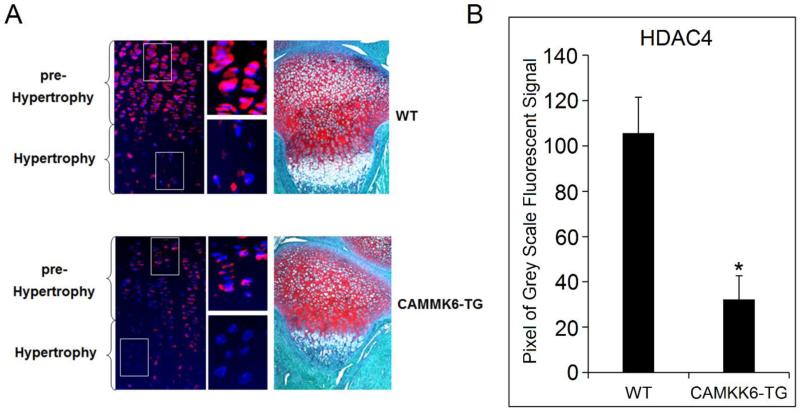
To further explore if p38 regulates HDAC4 in vivo, we examined the expression of HDAC4 in CAMKK6 transgenic mice (CAMKK6-TG) by immunofluorescent histochemistry. The expression pattern in the proximal tibia growth plate from 10 day postpartum (P10) mice was compared between CAMKK6-TG mice and their wild type (WT) litter mates. As expected, HDAC4 expression is decreased in the pre-hypertrophic chondrocytes in CAMKK6-TG mice compared to WT controls (Fig. 3). These samples were a subset of those used for a previous study and the increase in p38 activity has been validated in vivo (Zhang et al., 2006).
Figure 3. The expression of HDAC4 is dramatically decreased in the growth plate of CAMKK6 mice.
(A) Immunofluorescent histochemistry shows that in WT mice, HDAC4 is highly expressed in the pre-hypertrophic zone (red), but the expression is reduced in the hypertrophic zone. In contrast, CAMKK6 homozygote mice (CAMKK6-TG) exhibit a weaker HDAC4 staining in the pre-hypertrophic zone in comparison to the strong signal in WT mice. Magnified view of the pre-hypertrophic and hypertrophic chondrocytes from WT and CAMKK6-TG mice correspond to the boxed areas. Blue: Nucleus stained by DAPI. Red: HDAC4 stained by Rhoda mine. Purple: overlap of blue and red. Multiple animals were examined and consistent results were obtained (N=4). Safranin-O staining was used to show PG staining on the growth plate. (B) Intensity of the HDAC4 signal was quantified. CAMKK6-TG mice had much lower signal than WT controls. * p<0.05.
HDAC4 is stabilized by caspase-2, and -3 inhibitors
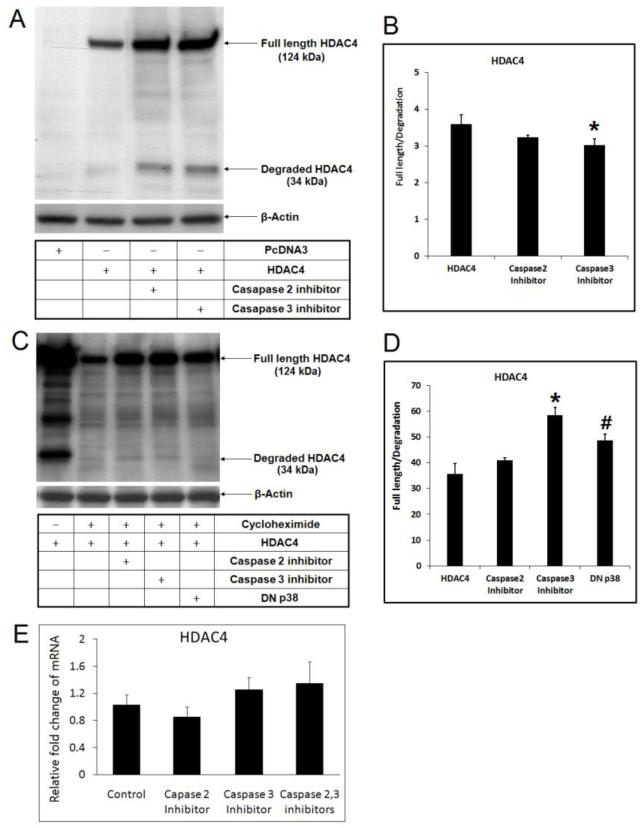
HDAC4 is unusually unstable and its degradation is caspase-dependent (Liu et al., 2004). Our hypothesis is that the elevated p38 activity in hypertrophic chondrocytes may induce HDAC4 degradation through induction of caspases. To test this possibility, we treated chicken chondrocytes with caspase-2 and 3 inhibitors, and found that full length HDAC4 is increased by the treatment (Fig. 4A). We noticed that although it is clear that the full length of HDAC4 is increased in the cells incubated with caspase inhibitors, the degradation fragment was also increased, and perhaps even to a greater extent (Fig. 4B). One of the possible explanations is that the accumulation of endogenous degradation due to the increase of the total amount of HDAC4. Another possible explanation is that the caspase inhibitors cannot stop the caspase activity completely.
Figure 4. HDAC4 is stabilized by caspase-2 or 3 inhibiters and by DN p38 MAPK.
(A) Full length HDAC4 is stabilized by inhibition of caspase-2 or 3 as shown by western. (B) Ratio of the full length HDAC4/degradation fragment was presented. *, p<0.05. (C) HDAC4 is stabilized by caspase-2 and caspase-3 inhibiters or by DN p38, and cycloheximide treatment excludes the variation of full length HDAC4 content due to protein neosynthesis. (D) Ratio of the full length HDAC4/degradation fragment was presented. *, #, p<0.05. (E) Realtime PCR excludes the possibility that caspase-2, 3 inhibitors may induce HDAC4 expression at the transcription level.
The protein pool of HDAC4 reflects accumulation, which is a combination of new protein synthesis and degradation. To eliminate the possibility that increased full length HDAC4 was caused by increased protein synthesis, we used cycloheximide to inhibit protein neosynthesis, and found that caspase inhibitors could still raise the amount of full length HDAC4 (Fig. 4C) and increase the ratio of full length HDAC4/degradation fragment (Fig. 4D), suggesting that the ratio was shifted from the 34 kDa fragment to the full length protein. To exclude the possibility that caspase inhibitors may increase HDAC4 transcription, HDAC4 mRNA was quantified by realtime PCR 24 hours after treating the cells with caspase inhibitors. In agreement with our speculation, realtime PCR result showed that caspase inhibitors did not significantly increase HDAC4 transcription (Fig. 4E).
Runx2 promoter activity is mediated by p38 MAPK/Caspases pathway through HDAC4
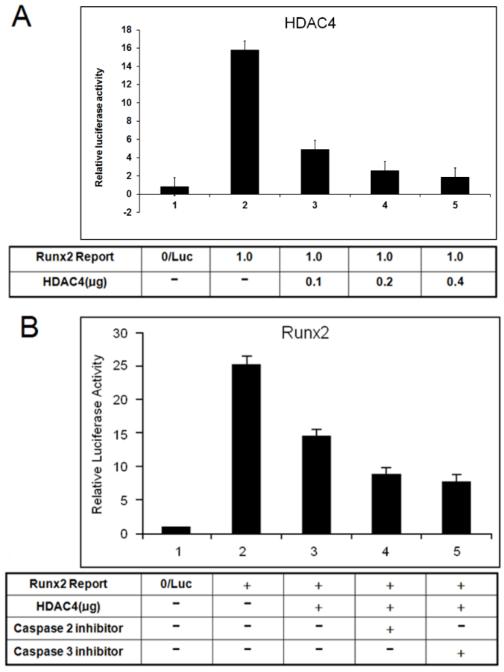
P38 MAPK induces caspase activity. We hypothesized that Runx2 expression depends on p38 activity/caspases pathway in hypertrophic chondrocytes through regulating HDAC4 protein levels. To test this hypothesis, we determined if Runx2 promoter activity is mediated by the p38 activity/caspases pathway through suppression of HDAC4 in hypertrophic chondrocytes. Runx2 promoter assay results showed that inhibition of Runx2 promoter activity by HDAC4 occurs in a dose-dependent manner in chondrocytes (Fig. 5A) and caspase-2 and 3 inhibitors further enhance HDAC4 repression on Runx2 promoter activity (Fig. 5B).
Figure 5. Caspases inhibitors further suppress Runx2 promoter activity by inhibiting degradation of HDAC4.
(A) Promoter assay showed that inhibition of Runx2 promoter activity presents an HDAC4-dose-dependent manner in chondrocytes. (B) HDAC4 inhibition of Runx2 promoter could be enhanced by caspases inhibitors via suppressing degradation of HDAC4.
Discussion
The majority of bones in the skeleton come from endochondral ossification, including the long bones and vertebrae. This process consists of mesenchymal condensation, chondrogenesis, chondrocyte maturation and hypertrophy, as well as vasculogenesis and osteoblast recruitment. HDAC4 regulates chondrocyte hypertrophy and endochondral bone formation by binding to and inhibiting the activity of transcriptional factor Runx2, which is essential to both chondrocyte and osteoblast differentiation (Vega et al., 2004) (Kang et al., 2005). Jeon et al. reported that HDAC4 deacetylates Runx2 and leads to protein degradation (Jeon et al., 2006). However, it is not clear how HDAC4 itself is regulated in chondrocytes.
To address this question, we first set out to find out what regulates HDAC4. Previous studies demonstrated that p38 MAPK signaling is required for hypertrophic chondrocyte differentiation (Stanton et al., 2004). The activity of p38 is elevated more than 10-fold in hypertrophic chondrocytes (Zhen et al., 2001), suggesting that it may play an important role in hypertrophic chondrocyte differentiation. However, it remains unclear how the p38 signaling pathway regulates chondrocyte hypertrophy. The simultaneous decline of HDAC4 levels in hypertrophic chondrocytes raises the question whether the decrease in HDAC4 is due to increased p38 activity. To answer this question, we used western blot to detect changes in HDAC4 profiles in chondrocytes in response to altered p38 activity. Because the amount of endogenous HDAC4 in chondrocytes may be low and this may create difficulties detecting a degradation band with western blotting, we employed exogenous HDAC4 to obtain a clearer degradation profile. Our data showed that HDAC4 degradation depends on p38 activity in vitro (Fig. 1) and in vivo (Fig. 3).
HDAC4 is not a direct target of p38, but caspase could provide a connection between HDAC4 and p38 because increased caspase and p38 activities have been found in hypertrophic chondrocytes (Chrysis et al., 2002) (Zhen et al., 2001). Previous studies have shown that as the central effecter of apoptosis, p38 is phosphorylated and subsequently activates the apoptotic gene caspase-3 when cells start apoptosis, suggesting activation of p38 is closely associated with caspase-3 (Wang et al., 2009) (Niswander and Dokas, 2007) (Zhuang et al., 2000) (Nakagami et al., 2001). In this study we also found that the elevated p38 activity induced by CAMKK6 increases caspase-3 activity in chicken hypertrophic chondrocytes (Fig. 2D), suggesting that caspase-3 activity is under control of p38. Our finding is consistent with previous findings (Wang et al., 2009) (Niswander and Dokas, 2007) (Zhuang et al., 2000) (Nakagami et al., 2001). HDAC4 degradation could be blocked by the inhibition of caspase activity (Liu et al., 2004). Caspase-2 and caspase-3 cleave HDAC4 in vitro and caspase-3 is critical for HDAC4 cleavage in vivo during UV-induced apoptosis (Paroni et al., 2004). Caspase-dependent processing of HDAC4 cleavage occurs at Asp 289 (Liu et al., 2004). In this study, we demonstrated that altered caspase activity could affect HDAC4 degradation (Fig. 4), and that mutation of the caspase cleavage site in HDAC4 (D289E) stabilized HDAC4 degradation (Fig. 2B and C). Thus, our results suggest that elevated p38 activity in hypertrophic chondrocytes may induce HDAC4 degradation through induction of caspases. Meanwhile, it is interesting to note that the 34 kDa HDAC4 degradation band is increased although full length band HDAC4 is preserved by treatment of caspase inhibitors (Fig. 4A and B). This observation implies that HDAC4 degradation at the site Asp289 may go through a caspase-dependent pathway, or may use an unknown bypass that is independent of caspase which would require further investigation. Backs et al. reported that blocking of PKA inhibited degradation of HDAC4, while blocking of other protetases had no affect (Backs et al., 2011; Shimizu et al., 2014). However, p38 and PKA function in different pathways (Suomalainen et al., 2001), thus it is unlikely that p38 mediates HDAC4 degradation through PKA. Ubiquitin mediates HDAC4 degradation through cleavage on serine site under serum starvation condition (Cernotta et al 2010), and there is no report indicating p38 is involved in this process. Thus, caspase seems to be the only connection between elevated p38 activity and increased HDAC4 degradation in chondrocytes. In addition to the experimental evidence supporting the HDAC4/Runx2 interaction provided in this manuscript and by Vega et al, 2004, we also showed HDAC4 inhibits Runx2 promoter in a dose-dependent manner (Li et al., 2014). Arnold et al. also showed that HDAC4 regulates the chondrocyte hypertrophy through MEF2 (Arnold et al., 2007). Thus, the collected evidences suggest that HDAC4 may target both MEF2 and Runx2 to regulate chondrocytes hypertrophy independently.
Overexpression of HDAC4 inhibits chondrocyte hypertrophy and differentiation, which mimics a Runx2 loss-of-function phenotype (Vega et al., 2004). HDAC4 is considered an inhibitor of Runx2 in the regulation of chondrocyte hypertrophy and endochondral bone formation (Vega et al., 2004) (Jeon et al., 2006; Kang et al., 2005). In this study, we used the promoter assay to demonstrate that HDAC4 directly binds with the promoter region of Runx2 and inhibits its transcription in a dose-dependent manner (Fig. 5). We further demonstrate that caspase inhibitors enhance HDAC4 inhibition on Runx2 promoter activity by preventing HDAC4 degradation. Taken together, these findings suggest that caspase can relieve the inhibition of endogenous Runx2 function by cleaving HDAC4.
In summary, we present the first evidence that p38 MAPK regulates HDAC4 degradation through caspases. Our findings suggest a model in which HDAC4 plays a central role in the signaling cascade, connecting the upstream p38-caspase-3 signaling pathway, and the downstream transcription regulator Runx2. This p38-caspase3-HDAC4-Runx2 signaling cascade releases HDAC4 inhibition of Runx2, which results in the hypertrophic state of chondrocytes and bone formation. These findings clarify the connection between p38 and HDAC4 in chondrocyte hypertrophy and contribute to a better understanding of the fundamental mechanism of bone development.
Highlights.
Inhibition of p38 by dominant negative p38 or inhibitors reduces HDAC4 degradation.
Mutation of caspase-2 and 3 cleavage site stabilizes HDAC4 in chondrocytes.
Constitutively activated p38 led to decreased HDAC4 content in vivo.
HDAC4 is stabilized by caspase-2 or 3 inhibiters.
Caspases inhibitors further suppress Runx2 promoter by stabilizing HDAC4.
Acknowledgements
The project was supported by Grant R01AR059142 from NIH/NIAMS and P20GM104937 from NIH/NIGMS, NSFC 81071495, 81171676 and 31271033, SXNSF 2011011042. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. The authors gratefully acknowledge Damson Lu for help with the quantity of western blot. The authors gratefully acknowledge Ericka M. Bueno, Ph.D. for help with the manuscript preparation and editorial services.
Abbreviations
- HDAC4
Histone deacetylase 4
- MAPK
mitogen-activated protein kinase
- DN p38
dominant negative p38
- CAMKK6
constitutively active MAPK kinase 6
- TG
transgenic
- WT
wild type
- E17
17-day-old embryonic
- P10
10 day postpartum
- FBS
fetal bovine serum
- RCAS
Replication Competent Avian Splice
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold MA, Kim Y, Czubryt MP, Phan D, McAnally J, Qi X, Shelton JM, Richardson JA, Bassel-Duby R, Olson EN. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Developmental Cell. 2007;12:377–389. doi: 10.1016/j.devcel.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Backs J, Worst BC, Lehmann LH, Patrick DM, Jebessa Z, Kreusser MM, Sun Q, Chen L, Heft C, Katus HA, Olson EN. Selective repression of MEF2 activity by PKA-dependent proteolysis of HDAC4. The Journal of cell biology. 2011;195:403–415. doi: 10.1083/jcb.201105063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Johnson DM, Haudenschild DR, Goetinck PF. Progression and recapitulation of the chondrocyte differentiation program: cartilage matrix protein is a marker for cartilage maturation. Dev Biol. 1995;172:293–306. doi: 10.1006/dbio.1995.0024. [DOI] [PubMed] [Google Scholar]
- Chrysis D, Nilsson O, Ritzen EM, Savendahl L. Apoptosis is developmentally regulated in rat growth plate. Endocrine. 2002;18:271–278. doi: 10.1385/ENDO:18:3:271. [DOI] [PubMed] [Google Scholar]
- Guan Y-J, Yang X, Wei L, Chen Q. MiR-365: a mechanosensitive microRNA stimulates chondrocyte differentiation through targeting histone deacetylase 4. FASEB Journal. 2011;25:4457–4466. doi: 10.1096/fj.11-185132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Chen Q, Yang X, Haines P, Pei M, Terek R, Wei X, Zhao T, Wei L. Subcellular relocation of histone deacetylase 4 regulates growth plate chondrocyte differentiation through Ca2+/calmodulin-dependent kinase IV. American Journal of Physiology - Cell Physiology. 2012;303:C33–40. doi: 10.1152/ajpcell.00348.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon E-J, Lee K-Y, Choi N-S, Lee M-H, Kim H-N, Jin Y-H, Ryoo H-M, Choi J-Y, Yoshida M, Nishino N, Oh B-C, Lee K-S, Lee YH, Bae S-C. Bone morphogenetic protein-2 stimulates Runx2 acetylation. Journal of Biological Chemistry. 2006;281:16502–16511. doi: 10.1074/jbc.M512494200. [DOI] [PubMed] [Google Scholar]
- Kang S, Jung M, Kim C-W, Shin DY. Inactivation of p38 kinase delays the onset of senescence in rabbit articular chondrocytes. Mechanisms of Ageing & Development. 2005;126:591–597. doi: 10.1016/j.mad.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Li P, Wei X, Guan Y, Chen Q, Zhao T, Sun C, Wei L. MicroRNA-1 regulates chondrocyte phenotype by repressing histone deacetylase 4 during growth plate development. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014 doi: 10.1096/fj.13-249318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Dowling M, Yang XJ, Kao GD. Caspase-mediated specific cleavage of human histone deacetylase 4. Journal of Biological Chemistry. 2004;279:34537–34546. doi: 10.1074/jbc.M402475200. [DOI] [PubMed] [Google Scholar]
- Lu J, McKinsey TA, Zhang CL, Olson EN. Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mol Cell. 2000;6:233–244. doi: 10.1016/s1097-2765(00)00025-3. [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Lu J, Olson EN. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature. 2000;408:106–111. doi: 10.1038/35040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska EA, Karlsson C, Langley E, Nielsen SJ, Pines J, Kouzarides T. HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO Journal. 1999;18:5099–5107. doi: 10.1093/emboj/18.18.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska EA, Langley E, Wolf D, Karlsson C, Pines J, Kouzarides T. Differential localization of HDAC4 orchestrates muscle differentiation. Nucleic Acids Research. 2001;29:3439–3447. doi: 10.1093/nar/29.16.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami H, Morishita R, Yamamoto K, Yoshimura SI, Taniyama Y, Aoki M, Matsubara H, Kim S, Kaneda Y, Ogihara T. Phosphorylation of p38 mitogen-activated protein kinase downstream of bax-caspase-3 pathway leads to cell death induced by high D-glucose in human endothelial cells. Diabetes. 2001;50:1472–1481. doi: 10.2337/diabetes.50.6.1472. [DOI] [PubMed] [Google Scholar]
- Niswander JM, Dokas LA. Hyperosmotic stress-induced caspase-3 activation is mediated by p38 MAPK in the hippocampus. Brain Research. 2007;1186:1–11. doi: 10.1016/j.brainres.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Paroni G, Mizzau M, Henderson C, Del Sal G, Schneider C, Brancolini C. Caspase-dependent regulation of histone deacetylase 4 nuclear-cytoplasmic shuttling promotes apoptosis. Molecular biology of the cell. 2004;15:2804–2818. doi: 10.1091/mbc.E03-08-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Molecular & Cellular Biology. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Nakatani T, He Z, Partridge NC. Parathyroid Hormone Regulates Histone Deacetylase (HDAC) 4 through Protein Kinase A-mediated Phosphorylation and Dephosphorylation in Osteoblastic Cells. The Journal of biological chemistry. 2014;289:21340–21350. doi: 10.1074/jbc.M114.550699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton LA, Sabari S, Sampaio AV, Underhill TM, Beier F. p38 MAP kinase signalling is required for hypertrophic chondrocyte differentiation. Biochemical Journal. 2004;378:53–62. doi: 10.1042/BJ20030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen M, Nakano MY, Boucke K, Keller S, Greber UF. Adenovirus-activated PKA and p38/MAPK pathways boost microtubule-mediated nuclear targeting of virus. The EMBO journal. 2001;20:1310–1319. doi: 10.1093/emboj/20.6.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, Meadows E, McAnally J, Pomajzl C, Shelton JM, Richardson JA, Karsenty G, Olson EN. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119:555–566. doi: 10.1016/j.cell.2004.10.024. see comment. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sun L, Xia C, Ye L, Wang B. P38MAPK regulates caspase-3 by binding to caspase-3 in nucleus of human hepatoma Bel-7402 cells during anti-Fas antibody- and actinomycin D-induced apoptosis. Biomedicine & Pharmacotherapy. 2009;63:343–350. doi: 10.1016/j.biopha.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Wei L, Kanbe K, Lee M, Wei X, Pei M, Sun X, Terek R, Chen Q. Stimulation of chondrocyte hypertrophy by chemokine stromal cell-derived factor 1 in the chondro-osseous junction during endochondral bone formation. Dev Biol. 2010;341:236–245. doi: 10.1016/j.ydbio.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Murakami S, Coustry F, Wang Y, Crombrugghe B. Constitutive activation of MKK6 in chondrocytes of transgenic mice inhibits proliferation and delays endochondral bone formation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:365–370. doi: 10.1073/pnas.0507979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen X, Wei L, Wu Q, Zhang Y, Chen Q. Mitogen-activated protein kinase p38 mediates regulation of chondrocyte differentiation by parathyroid hormone. Journal of Biological Chemistry. 2001;276:4879–4885. doi: 10.1074/jbc.M004990200. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Demirs JT, Kochevar IE. p38 mitogen-activated protein kinase mediates bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. Journal of Biological Chemistry. 2000;275:25939–25948. doi: 10.1074/jbc.M001185200. [DOI] [PubMed] [Google Scholar]