Abstract
Opioid receptors in the central nervous system are important modulators of itch transmission. In this study, we examined the effect of mixed-action opioid butorphanol on histamine itch, cowhage itch and heat pain in healthy volunteers. Using functional MRI, we investigated significant changes in cerebral perfusion to identify the critical brain centers mediating the antipruritic effect of butorphanol. Butorphanol suppressed the itch induced experimentally with histamine, reduced the intensity of cowhage itch by approximately 35%, and did not affect heat pain sensitivity. In comparison with the placebo, butorphanol produced a bilateral deactivation of claustrum, insula and putamen, areas activated during itch processing. Analysis of cerebral perfusion patterns of brain processing of itch vs. itch inhibition under the effect of the drug, revealed that the reduction of cowhage itch by butorphanol was correlated with changes in cerebral perfusion in the midbrain, thalamus, S1, insula and cerebellum. The suppression of histamine itch by butorphanol was paralleled by the activation of nucleus accumbens and septal nuclei, structures expressing high levels of kappa opioid receptors. In conclusion, important relays of the mesolimbic circuit were involved in the inhibition of itch by butorphanol and could represent potential targets for the development of antipruritic therapy.
INTRODUCTION
Recent advances in functional MRI have enabled the visualization of brain responses evoked by itch stimulation. Arterial Spin Labeling (ASL) is a suitable technique capable of capturing the long-lasting effect of itch on cerebral activity. ASL has evolved from a 2-dimensional to a 3-dimensional technique, such as the 3-D Grase (gradient echo and spin echo) - Propeller (periodically rotated parallel lines with enhanced reconstruction) used in this study (Tan et al, 2011). Previously, ASL was successfully employed to analyze and compare cerebral activations evoked by histamine and cowhage itches (Papoiu et al, 2012), or the mechanisms of itch relief provided by active vs. passive scratching (Papoiu et al, 2013). Moreover, ASL enables the comparative analysis of itch responses evoked in healthy individuals and chronic itch sufferers (Ishiuji et al, 2009; Papoiu et al, 2014).
Itch stimulation triggers a complex cerebral response manifested in multiple cortical and subcortical regions that process sensory-discriminative, cognitive, affective and memory-related dimensions of itch. Projected to the cortex by ventrobasal and posterior thalamic nuclei (Davidson et al, 2012), itch registers in the primary and secondary somatosensory areas (S1 and S2) and engages associative parietal regions of the supramarginal, angular gyri and precuneus. Itch stimulation activates insula, a salience and interoceptive center, and claustrum, a fast stimuli detector and multisensory integrator. The highly charged emotional aspect of itch translates into the activation of deep-seated areas of the cingulate cortex, amygdala and hippocampus, situated along the Papez circuit (Papez, 1937). Itch is a primary sensation that is not easily suppressible, which explains why many forms of itch, and chronic pruritus in particular, remain a clinical challenge. From a therapeutic perspective, the crucial question is which areas are involved in the formation of itch sensation, and among them, which could be amenable to medical intervention? In previous neuroimaging studies, clues have been sought to decipher a central mechanism for itch inhibition, using various interventions such as scratching, acupuncture or thermal modulation (Mochizuki et al, 2003; Yosipovitch et al, 2008; Vierow et al, 2009; Pfab et al, 2010; Papoiu et al, 2013; Napadow et al, 2014). Based on the findings of an fMRI study of active scratching, we proposed that the reward-related areas in the midbrain and ventral striatum encode not only the pleasurable aspect of scratching, but may hold the key to effectively mediate itch relief (Papoiu et al, 2013). This hypothesis is in analogy with the concept of pain and pleasure sharing a dual, but common pathway (Leknes & Tracey, 2008). Since reward-related areas express high levels of opioid receptors, an alternative experimental approach is to stimulate them by pharmacological means. The interaction of opioid signaling pathways with itch transduction mechanisms is a topic under active investigation (Kardon et al, 2014).
In this study we used butorphanol, a mixed action opioid with a pronounced κ receptor affinity, to study the mechanism underlying its antipruritic action. Butorphanol is FDA approved as an analgesic and is available as a nasal spray or an injectable formulation. Intranasal butorphanol has been used successfully to treat severe cases of chronic pruritus (Dawn and Yosipovitch, 2006), and administered epidurally, butorphanol blocks the itch induced by the epidural injection of morphine (Yokoyama et al, 2009).
Histamine and cowhage can be used experimentally in humans to induce itch sensations that are transmitted via distinct peripheral and spinothalamic pathways which synapse in subtly distinct thalamic nuclei (Davidson et al, 2007; 2012). Significant differences in their cortical processing have also been described (Papoiu et al, 2012). While histamine is the classical experimental pruritogen, the nonhistaminergic PAR2-mediated cowhage itch resembles more closely chronic pruritus of pathological origin. Previous studies have implicated PAR2 itch pathway in atopic eczema (Steinhoff et al, 2003) and stressed the lack of therapeutic efficacy of antihistamines in chronic pruritus. Therefore, it is of significant interest to investigate whether butorphanol has a differential effect in relieving these two forms of itch. In this study, we have used functional MRI to explore the underlying mechanism of butorphanol’s antipruritic action. Our aim was to identify the key regions in the brain that mediate the inhibition of itch.
RESULTS
1) Psychophysical effects on itch and heat pain perception
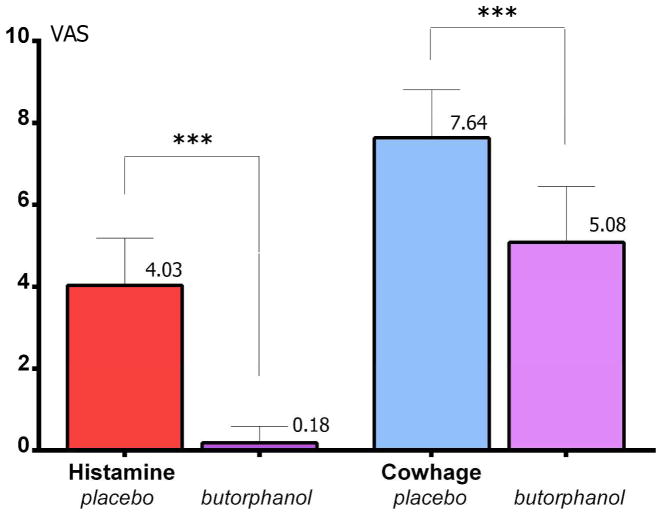
Butorphanol completely suppressed the itch induced experimentally with histamine (p<0.001), while it only reduced the intensity of cowhage itch by approximately 35% (p<0.001; paired t tests, two-tails, Bonferroni corrected for multiple comparisons; see Figure 2). Butorphanol did not alter sensitivity to heat pain, or the heat-pain associated unpleasantness. Therefore, at a 1 mg dose it appeared that the mixed-action opioid exerted a differential effect on these 3 sensory modalities.
Figure 2.
Psychophysical data. Butorphanol completely suppressed itch induced with histamine (p<0.001)***, and reduced the intensity of cowhage itch by approximately 35% (p<0.001)***.
2) Significant effects of butorphanol on cerebral activity
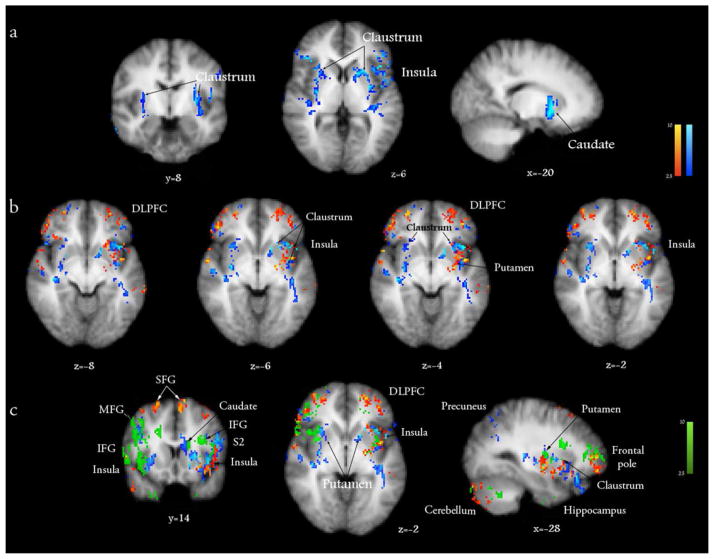
Butorphanol induced a significant deactivation of the claustrum, putamen, anterior and posterior insulae (Fig. 3a, in blue; Table 1), which were activated during itch processing by histamine and cowhage. The deactivating effect of butorphanol appeared to overlap to a substantial extent with the areas activated by histamine itch in the contralateral insula and claustrum (in red, Fig. 3b), but did not fully overlap (i.e. counter) the responses evoked by cowhage at the ipsilateral sites (in green, Fig. 3c). Butorphanol induced significant activations in the midbrain, in areas consistent with the location of VTA, raphé nucleus, substantia nigra, red nucleus and periaqueductal gray matter (PAG), as well as cerebellum, precuneus and thalamus, when analyzed in contrast to the placebo (Figure 4, Table 1).
Figure 3.
a) The effect of intranasal butorphanol (1 mg) on cerebral activity was analyzed in comparison to a placebo (0.9% intranasal saline). Butorphanol extensively deactivated bilaterally an area situated between the insular cortex and putamen, which coincides with the anatomical location of the claustrum, while also deactivating the left insular cortex and the putamen. b) The activations induced by histamine itch (red) are overlaid with the deactivations induced by butorphanol (blue; vs. placebo), displaying a significant conjunction in the contralateral insula, claustrum and putamen. c) Overlay of brain responses induced by histamine itch (red), cowhage itch (green) and the deactivations induced by butorphanol (blue). x, y, z - Montreal Neurological Institute (MNI) standard space coordinates. Z score > 2.3, p<0.05.
Table 1.
A) The main effects of intranasal butorphanol on cerebral perfusion, analyzed in contrast with a placebo. B) Principal areas mediating the antipruritic action of butorphanol on histamine itch, whose activation paralleled itch suppression. C) Results of GLM regression analysis identify deactivation areas correlated with the attenuation of cowhage itch by butorphanol (inhibited approximately 35%). MNI coordinates.
| Brain area | x | y | z | Z score |
|---|---|---|---|---|
| A1. Significant activations induced by butorphanol in comparison with placebo | ||||
| Midbrain | ||||
| VTA | 4 | −22 | −14 | 2.78 |
| 6 | −20 | −18 | 2.90 | |
| Red nucleus | 6 | −20 | 10 | 2.31 |
| Sb. nigra | 14 | −16 | −10 | 2.91 |
| Dorsal nucleus of raphé | 2 | −24 | −16 | 2.55 |
| PAG | 8 | −26 | −4 | 2.53 |
| Cerebellum (posterior lobe, uvula) | 8 | −66 | −36 | 2.76 |
| Semilunar lobule | 34 | −66 | −44 | 2.86 |
| Posterior lobe (cerebellar tonsil) | 18 | −46 | −44 | 2.59 |
| Precuneus (BA31) | −14 | −74 | 32 | 3.28 |
| −4 | −74 | 52 | 2.93 | |
| Cuneus | −4 | −84 | 26 | 3.44 |
| Thalamus (MDNc) | 4 | −18 | 6 | 2.70 |
| 6 | −18 | 4 | 2.76 | |
| A2. Significant deactivations induced by butorphanol | ||||
| Claustrum (L) | −36 | −6 | −4 | 2.87 |
| −38 | −10 | −4 | 3.01 | |
| −32 | −10 | 8 | 2.51 | |
| −32 | −10 | 10 | 2.44 | |
| −32 | −6 | 8 | 2.81 | |
| −30 | 18 | −6 | 3.28 | |
| Claustrum (R) | 32 | 18 | 4 | 3.01 |
| 30 | 20 | 4 | 2.77 | |
| 34 | 16 | −6 | 2.86 | |
| Anterior insula | −42 | −4 | −4 | 2.45 |
| −38 | 18 | 4 | 2.68 | |
| −38 | 16 | −8 | 3.84 | |
| −38 | 14 | −10 | 3.72 | |
| −36 | 16 | −6 | 3.41 | |
| Posterior insula | 42 | −22 | 2 | 3.01 |
| −38 | −2 | −4 | 2.96 | |
| Putamen | 26 | 10 | 4 | 2.39 |
| 22 | 8 | 4 | 2.36 | |
| −30 | −4 | 4 | 3.71 | |
| −20 | 18 | 4 | 2.98 | |
| −18 | 10 | 4 | 3.18 | |
| B. Areas significantly activated during the suppression of histamine itch by butorphanol | ||||
| Nc. accumbens (L) | −10 | 8 | −8 | 3.01 |
| −10 | 8 | −10 | 3.16 | |
| (R) | 6 | 6 | −6 | 2.43 |
| 8 | 8 | −4 | 2.31 | |
| Medial nucleus of septum | 2 | 8 | −4 | 3.02 |
| 2 | 10 | 0 | 3.27 | |
| 2 | 10 | −2 | 2.57 | |
| 2 | 8 | −2 | 3.08 | |
| Nuclei of the diagonal band of Broca | 4 | 10 | −6 | 2.35 |
| 4 | 6 | −6 | 2.55 | |
| 2 | 6 | −8 | 2.53 | |
| 2 | 8 | −6 | 2.99 | |
| −6 | 8 | −18 | 3.76 | |
| Basal nucleus of Meynert | −16 | 10 | −16 | 2.50 |
| −18 | 10 | −18 | 2.42 | |
| −14 | 10 | −18 | 2.66 | |
| Septal area of ACC (subgenual ACC; BA 25) | 2 | 18 | −6 | 3.05 |
| 2 | 18 | −14 | 2.92 | |
| C. Deactivation areas significantly correlated with the reduction of cowhage itch | ||||
| S1 | −34 | −32 | 58 | 3.26 |
| Insula | 38 | −6 | −12 | 2.31 |
| Claustrum | 38 | −8 | −10 | 2.66 |
| Midbrain (sb. nigra) | 12 | −22 | −12 | 3.04 |
| Midbrain (VTA) | 2 | −20 | −10 | 3.21 |
| 2 | −20 | −12 | 6.31 | |
| Midbrain (red nucleus) | −2 | −22 | −12 | 2.38 |
| Thalamus (medial dorsal nucleus) | −4 | −18 | 2 | 2.83 |
| Pulvinar | −22 | −26 | 4 | 2.92 |
| PCC | −2 | −18 | 38 | 4.83 |
| Uncus | −28 | −12 | −32 | 4.92 |
| Hippocampus | −30 | −12 | −30 | 6.10 |
| Parahippocampus | 34 | −12 | −22 | 4.79 |
| Amygdala | −16 | −12 | −16 | 3.31 |
| Cerebellum Culmen | −10 | −50 | −22 | 4.81 |
| Dentate gyrus | 18 | −58 | −22 | 4.87 |
| Declive | 20 | −76 | −22 | 4.14 |
| Fusiform gyrus | 48 | −50 | −10 | 3.00 |
Figure 4.
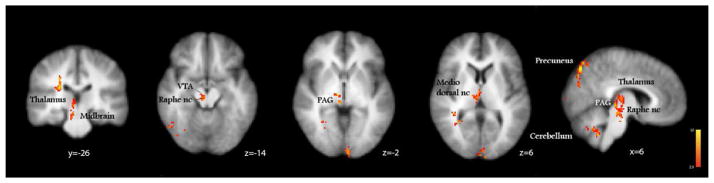
The brain activations induced by butorphanol, analyzed in comparison with placebo were found in the midbrain: ventral tegmental area (VTA), periaqueductal gray (PAG), raphé nucleus; in the thalamus, precuneus and cerebellum. x, y, z – MNI standard space coordinates.
3) Dissection of key brain areas mediating the antipruritic action of butorphanol on histamine itch
To identify the key brain areas involved in the mediation of the antipruritic effect of butorphanol, we have implemented a study design that allowed us to construct a stepwise contrast between active and control states, when itch was induced in the presence of the drug vs. placebo (Methods). This global analysis (performed for the whole-brain) revealed that nucleus accumbens (NAc), septal nuclei and the adjacent septal area of the anterior cingulate cortex were significantly activated during the inhibition of histamine itch (Figure 5, Table 1). These results suggest that suppression of histamine itch was significantly mediated by the activation of these formations. These results were not found in identical contrasts run for the effect of butorphanol on cowhage itch or heat pain (which did not yield significant results).
Figure 5.
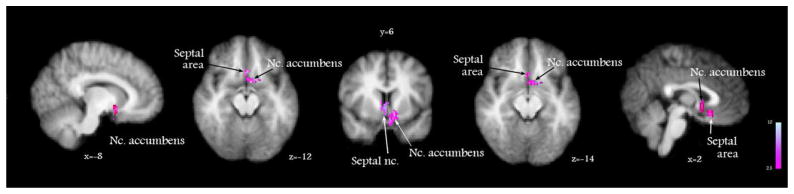
The cerebral mechanism underlying butorphanol’s suppression of histamine itch was analyzed by a General Linear Model paired t test (contrast) of perfusion weighted images between the following states: [(histamine + drug) vs. drug] vs. [(histamine + placebo) vs. placebo]. This analysis showed that the inhibition of histamine itch by butorphanol was paralleled by the activation of nucleus accumbens and septal nuclei. x, y, z – MNI standard space coordinates.
4) Brain responses correlated with the effect of butorphanol on cowhage itch
Since butorphanol was able to attenuate cowhage itch intensity by 35%, we were interested to investigate whether significant effects in brain perfusion were correlated with the reduction of itch, comparing (cowhage itch + drug) vs. (cowhage + placebo) conditions. Differences in cerebral perfusion observed for cowhage itch stimulation after butorphanol vs. placebo in S1, insula, thalamus, ventral tegmental area (VTA), posterior cingulate cortex (PCC), cerebellum and hippocampus, were significantly correlated with reduction of itch (Table 1, Fig. S1), consistent with a mechanism of butorphanol-induced deactivation. These areas were notably different from the ones identified during the inhibition of histamine itch, which suggests that the inhibition of these 2 itch modalities engages different cerebral networks.
DISCUSSION
In this study we used Arterial Spin Labeling fMRI and experimental itch induction to investigate the changes in cerebral perfusion occurring under the effect of butorphanol. This pharmacological fMRI study enabled us to trace meaningful perfusion changes underlying the antipruritic action of this drug in the brain. Due to the particular design of our study, contrast analyses enabled us to identify significant cerebral targets of the drug and to pinpoint key areas engaged during the suppression of histamine itch.
In order to characterize the effect of butorphanol on cerebral activity, we have analyzed the changes in CBF patterns elicited by this opioid drug in contrast with a placebo. Butorphanol significantly and extensively deactivated the claustrum, putamen and insula, areas previously described to be activated during itch processing. The brain activations induced by butorphanol implicated the midbrain (VTA, red nucleus, PAG), cerebellum and precuneus. Interestingly, we recently discovered these structures to be associated with itch relief and pleasurability provided by self-scratching (Papoiu et al, 2013).
The extensive deactivations induced in a contiguous area lying between the putamen and insula, bilaterally, along several coronal planes from z = 12 to z = −8 (MNI space coordinates), strikingly coincide with the anatomical location of the claustrum. Claustrum is a thin gray matter structure which expresses a very high density of κ-opioid receptors (“++++”), as shown by in situ hybridization studies in humans, and labeling studies in primates (Peckys et al, 1999; Sim-Selley et al, 1999). The significant deactivation we found is also consistent with the mechanism of action of opioids, which induce neuronal inhibition by hyperpolarization. Therefore, the present results tracing the effects of butorphanol by fMRI are in agreement with the structural data on the expression of its target receptors in the human brain, and with its known molecular mechanism. These findings support our previous observation that claustrum plays a significant role in itch processing (Papoiu et al, 2012). The high density pattern of expression of κ-opioid receptors in the claustrum is consistent across species, in primates and rodents (Meng et al, 1993; Mansour et al, 1994).
The complete inhibition of histamine itch by butorphanol was paralleled by significant activations which mapped to nucleus accumbens, septal nuclei, slightly extending laterally to the basal nucleus of Meynert, and to the adjacent septal area of subgenual ACC (BA 25), which suggests that the antipruritic action of butorphanol is mediated by these formations. The identification of structures within the human brain underlying the antipruritic effect of an opioid that showed clinical efficacy is to our knowledge previously unreported.
Nucleus accumbens (NAc) has been previously described to play a significant role in mediating opioid- and nociceptive stimulus-induced analgesia. In humans, NAc expresses a high density of μ, κ and δ opioid receptors at comparable levels (Peckys et al, 1999). A complex interplay between μ/ δ and κ receptors has been described in the modulation of antinociception mediated by NAc (Schmidt et al, 2002), and dopamine has been proposed as the critical mediator in accumbens-mediated antinociception (Altier & Stewart, 1998). In nucleus accumbens, μ-receptor activation leads to dopamine release (Yokoo et al, 1994), while κ-receptor activation decreases dopamine release (Bals-Kubik et al, 1993). Thus, a tempting hypothesis is that κ-mediated antipruritic action depends on decreasing dopamine release in nucleus accumbens. However, kappa-opioid mediated activation also decreases the release of glutamate and GABA from NAc, using different mechanisms (Hjelmstad and Fields, 2003). The κ opioid receptor sits on the presynaptic side of the excitatory synapses and dendrites of GABA-ergic spiny neurons of the NAc shell and could be regulated by glutamatergic inputs from the prefrontal cortex, amygdala or hippocampus (Schmidt et al, 2002). Of note, the septal area is directly interconnected with the hippocampus and amygdala.
Septal nuclei (SN) are discrete gray matter structures that include the medial nucleus of septum and the diagonal band of Broca, mostly composed of cholinergic, GABA-ergic and glutamatergic neurons. Notably, SN drive the hippocampal theta rhythm and are highly synchronized with hippocampal activity (Petsche et al, 1962; Vertes and Kocsis, 1997; Hangya et al, 2009). An interesting observation that could be relevant to itch processing is that sensory stimulation resets the pace of theta oscillations in the septal nuclei (Buzsáki et al, 1979). Neurons operating in phase-lock with the hippocampal theta oscillation were found in the ventral tegmentum and dorsal raphé nucleus (Bland 1986). These structures were implicated in the relief of itch induced by self-scratching (Papoiu et al, 2013), and were activated by butorphanol in this study (Figure 4). The medial septal region has been proposed to function as a node for the ascending brainstem pathways, sending inputs to the posterior cingulate, enthorinal cortex and hippocampus (Bland et al, 2000). Recent studies have implicated the medial septal nucleus in general anesthesia (Ma et al, 2002; Leung et al 2013, Tai et al, 2014). The septal area has also been associated with analgesia induced by acupuncture (Xiong and Zheng 1990; Zhao 2008). A classical electrophysiological experiment using implanted electrodes linked SN with reward processing and highly pleasurable experiences (Olds and Milner, 1954). The septal area of ACC is connected with NAc and has been associated with emotional-affective functions. Taken together, these findings suggest that a central mechanism of antipruriception is associated with the cerebral areas involved in reward processing.
Butorphanol activated the periaqueductal gray matter (PAG), VTA and the dorsal nucleus of raphé, areas also rich in opioid receptors, which may suggest that a descending modulatory pathway could be ultimately engaged to exert inhibitory action on spinal centers. In this context, the implication of the medial dorsal nucleus of the thalamus may indicate the co-operation of a gating mechanism in the modulation of itch intensity.
The effects of butorphanol on cerebral perfusion in the cerebellum and midbrain (VTA) were correlated with cowhage itch reduction. These areas are similar with the areas deactivated by self-scratching and correlated with the pleasurability of scratching, when itch was induced using cowhage (Papoiu et al, 2013). The responses observed in the thalamus are in agreement with the areas activated by cowhage itch, identified by antidromic stimulation (Davidson et al., 2012) or mapped by neuroimaging (Papoiu et al, 2012).
The lack of effect of intranasal butorphanol on heat pain is not entirely surprising, as this opioid has a limited clinical use as an analgesic. A previous study with the TRK-820 kappa agonist also showed a modest to inexistent analgesic action vs. heat pain, depending on the temperature tested (Endoh et al, 1999). The lack of effect on heat pain may stem from the weak μ-agonist properties of butorphanol, which could be suboptimal for analgesia at a 1 mg dose, and its dominant kappa-agonist action. Recent findings support the concept that kappa mediated effects centrally antagonize the μ-dependent analgesia (Pan, 1998).
To conclude, we identified distinct stations of the reward (mesolimbic) circuit as potential key centers mediating antipruritic action. Intriguingly, butorphanol exerted this complete suppression selectively on the histamine itch pathway. A regression analysis performed for the partial effect of butorphanol on cowhage itch yielded different areas correlated with itch attenuation, suggestive of mechanistic differences in the inhibition of these pathways. With the potential limitation that these differential responses may be dose dependent, our results appear to suggest that κ-opioid receptors are differentially involved in the processing of these itch modalities. The clinical applicability of these findings requires further study.
Despite certain limitations of functional brain imaging to establish causality and dissect cellular or molecular events, mechanistic inferences can be derived from the analysis of changes in cerebral perfusion patterns using symmetrical designs. Although functional MRI does not directly trace the effect of a drug on molecular targets, the results of this study support the notion that well-defined significant changes in perfusion can provide insight into the mechanism of action. These results suggest that the central inhibition of histamine itch is coupled with κ-opioid receptor signaling, leading to the activation of nucleus accumbens and septal nuclei, and to the deactivation of the claustrum. Butorphanol was less effective in relieving PAR2-induced itch and did not exert a significant effect on thermal nociception.
MATERIALS AND METHODS
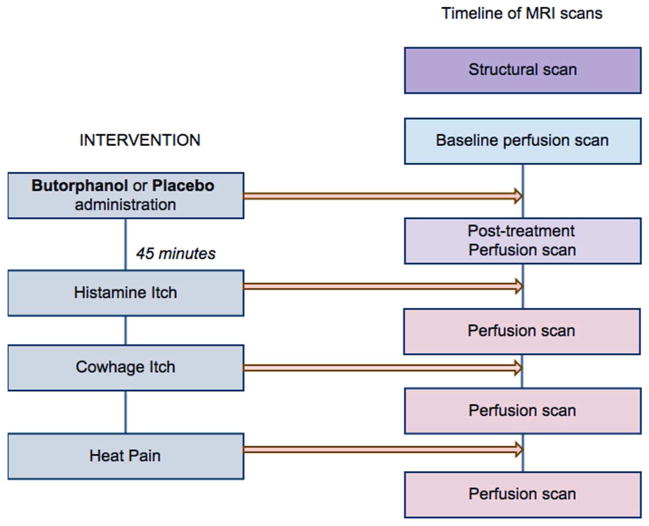
Functional MRI cerebral perfusion images were acquired by Arterial Spin Labeling, using a 3-D Grase-Propeller protocol (Tan et al, 2011). Perfusion images were acquired at rest prior to drug (or placebo) administration, and 40 minutes after drug/placebo to capture the effect on cerebral perfusion prior to itch/pain stimulation. Images were subsequently acquired immediately after itch/pain induction, after an interval of 45 minutes post-treatment (Fig. 1). This was carefully chosen to coincide exactly with the moment when the pharmacodynamic effect of butorphanol was at its peak (Davis et al, 2004).
Figure 1.
Timeline of experimental interventions and functional MRI sessions. Subjects were assigned to go into either the placebo or the butorphanol arm first, following a randomization scheme, and then they switched to the other arm. The two parts of the experiment were performed 7 days apart. The post-treatment perfusion scan was started 40 minutes after drug (or placebo) administration. The stimulation sequence using the three sensory modalities was randomized for each experiment. Perfusion scans following itch or pain interventions were started approximately 45 minutes post-drug (or placebo) administration, at the peak of the pharmacological action of intranasal butorphanol. Each perfusion ASL scan lasted 4 min 54 s.
Study design
This study employed a blinded, placebo controlled, randomized, crossover design. The placebo consisted of a 0.9 % isotonic saline solution delivered as an intranasal spray, using a standardized dispenser delivering a volume of 0.1 ml, in an identical manner with the dispenser delivering the pharmacologically active compound. Butorphanol was formulated as a 10 mg/ml solution (Apotex; Toronto, Ontario, Canada). A spray volume of 0.1 ml delivered a single dose of 1 mg butorphanol in one nostril. A screening visit was employed to investigate whether participants could detect perceptual or organoleptic differences between the two intranasal spray solutions administered in nebulized form, to assess potential side effects and tolerability, and to evaluate the effect of butorphanol on itch and heat pain. Study participants were not able to detect any differences between the two solutions.
Sequence and randomization
The study had two arms: a placebo arm and a butorphanol arm, which were crossed over. Subjects were assigned to go into either the placebo or the butorphanol part first, following a simple randomization scheme, and then they switched. These two parts of the experiment were performed on different dates, 7 days apart (Fig. 1). There was no repetition of the same stimulus in the same fMRI experiment. Sensory modalities were randomized for each experiment and were always given following the administration of the drug or placebo, starting 45 minutes post-administration. We did not observe any residual or order effects. Histamine and cowhage itch stimulation were performed on the dominant (right) forearm 5 cm apart, while heat pain was performed applying the Peltier thermode on the right lower extremity in the middle of the calf area, over the gastronecmius muscle. No significant psychophysical effects of butorphanol or of the placebo were observed on heat pain.
Itch and heat pain stimulation details are described in the Supplementary material.
Subjects
24 healthy volunteers, all right-handed, with ages between 21-35, participated in this study (11 males, 13 females; average age 26.5) and signed a written informed consent. The study was approved by the Internal Review Board of Wake Forest University Health Sciences and was conducted in accordance with the principles of Declaration of Helsinki. Subjects were free of skin or neurological disease and were not currently using centrally acting medications, antihistamines, corticosteroids or analgesics.
Psychophysical measures
Itch or heat pain intensity ratings were taken on a Visual Analog Scale (VAS) anchored between 0 (no sensation) and 10 (maximum, unbearable itch or pain).
Functional MRI
All experiments were carried out on a GE 1.5T TwinSpeed scanner (GE Healthcare, Milwaukee, WI) with an eight-channel phased array receive-only head coil (Invivo Devices, Gainesville, FL) for data collection. For technical details on fMRI sequence and image acquisition, see Supplementary Material.
Analysis of changes in cerebral blood perfusion (CBF) was performed with FSL (FMRIB, Oxford, UK). Each condition was first analyzed in contrast with two reference perfusion scans at baseline. Cerebral perfusion maps were analyzed using General Linear Model (GLM) using FEAT (FMRI expert analysis tool) of FSL 5.98, part of FMRIB’s Software Library (www.fmrib.ox.ac.uk/fsl), using as first-level analysis parameters: noise level: 0.66%, 12 degrees of freedom for registration to the high-resolution structural image; Z threshold for design efficiency 5.3; temporal smoothness 0.34; cluster threshold Z score > 2.3, p<0.05. Z (Gaussianised True/False) statistic images were thresholded using clusters determined by Z>2.3 and a corrected cluster significance threshold of p=0.05 (Worsley, 2001). Contrasts between different conditions were studied via paired t tests, with a statistical significance set at Z score > 2.3 and p < 0.05. Butorphanol effect on itch was analyzed by General Linear Model paired t tests of perfusion weighted images, as follows. First, we contrasted the CBF patterns of the itch condition (or no itch post-drug state in the case of histamine) with the control condition represented by drug (or placebo) alone, respectively. The CBF maps corresponding to these states were compared at the first level (e.g. histamine itch + drug) vs. (drug alone) and (histamine itch +placebo) vs. (placebo alone). At the second level, these two results were contrasted with each other via a paired t test:
[Histamine itch + drug) vs. (drug alone)] vs. [(Histamine itch + placebo) vs. (placebo alone)], in order to identify drug-specific effects that can provide insight into the mechanism of action. This design allowed us to use as controls for the dynamic effect of the drug (or placebo) in the presence of stimuli, the drug effect on perfusion alone (or the effect of the placebo). Regression analysis for the effect on butorphanol on cowhage-induced itch was performed in respect to drug’s effect on itch intensity. To identify brain areas where changes in cerebral perfusion were significantly correlated with changes in cowhage itch intensity, brain responses were contrasted in drug and placebo conditions, using as covariates of interest the ratio: ΔVAS (placebo − drug) / control VAS, which gave a proportional measure of drug’s effect, proportionally referenced to the amplitude of response in each subject. The contrast was set up as a pairwise t test, using Z threshold > 2.3, and p < 0.05, for a whole-brain analysis. Anatomical mapping of septal nuclei was performed according to the MNI coordinates described by Zaborszky et al (2008), Butler et al (2012–2014) and Li et al (2014).
Supplementary Material
Acknowledgments
This study was supported by National Institute of Health (NIAMS) grant 5R01AR055902 to G.Y.
Footnotes
Conflict of interest. The authors state no competing financial interests.
References
- Altier N, Stewart J. Dopamine receptor antagonists in the nucleus accumbens attenuate analgesia induced by ventral tegmental area substance P or morphine and by nucleus accumbens amphetamine. J Pharmacol Exp Ther. 1998;285:208–215. [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, et al. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Bland BH. Physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26:1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behavioural Brain Research. 2001;127(1):119–136. doi: 10.1016/s0166-4328(01)00358-8. [DOI] [PubMed] [Google Scholar]
- Bland BH. The medial septum: node of ascending brainstem hippocampal synchronizing pathways. In: Numan R, editor. The Behavioral Neuroscience of the Septal Region. Vol. 6. Springer Verlag; New York: 2000. pp. 115–145. [Google Scholar]
- Butler T, Zaborszky L, Pirraglia E, et al. Comparison of human septal nuclei MRI measurements using automated segmentation and a new manual protocol based on histology. Neuroimage. 2014;97:245–251. doi: 10.1016/j.neuroimage.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T, Zaborszky L, Wang X, et al. Septal nuclei enlargement in human temporal lobe epilepsy without mesial temporal sclerosis. Neurology. 2013;80(5):487–491. doi: 10.1212/WNL.0b013e31827f0ed7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T, Blackmon K, Zaborszky L, et al. Volume of the human septal forebrain region is a predictor of source memory accuracy. J Int Neuropsychol Soc. 2012;18(1):157–161. doi: 10.1017/S1355617711001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Grastyán E, Tveritskaya IN, et al. Hippocampal evoked potentials and EEG changes during classical conditioning in the rat. Electroencephalogr Clin Neurophysiol. 1979;47:64–74. doi: 10.1016/0013-4694(79)90033-6. [DOI] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, et al. Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol. 2012;108:1711–1723. doi: 10.1152/jn.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GA, Rudy AC, Archer SM, et al. Pharmacokinetics of butorphanol tartrate administered from single-dose intranasal sprayer. Am J Health Syst Pharm. 2004;61(3):261–266. doi: 10.1093/ajhp/61.3.261. [DOI] [PubMed] [Google Scholar]
- Dawn AG, Yosipovitch G. Butorphanol for treatment of intractable pruritus. J Am Acad Dermatol. 2006;54:527–531. doi: 10.1016/j.jaad.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tajima A, et al. Potent antinociceptive effects of TRK-820, a novel kappa-opioid receptor agonist. Life Sci. 1999;65(16):1685–1694. doi: 10.1016/s0024-3205(99)00417-8. [DOI] [PubMed] [Google Scholar]
- Ishiuji Y, Coghill RC, Patel TS, et al. Distinct patterns of brain activity evoked by histamine-induced itch reveal an association with itch intensity and disease severity in atopic dermatitis. Br J Dermatol. 2009;161(5):1072–80. doi: 10.1111/j.1365-2133.2009.09308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangya B, Borhegyi Z, Szilágyi N, et al. GABAergic neurons of the medial septum lead the hippocampal network during theta activity. J Neurosci. 2009;29(25):8094–8102. doi: 10.1523/JNEUROSCI.5665-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmstad GO, Fields HL. Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J Neurophysiol. 2003;89:2389–2395. doi: 10.1152/jn.01115.2002. [DOI] [PubMed] [Google Scholar]
- Kardon AP, Polgár E, Hachisuka J, et al. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron. 2014;82(3):573–586. doi: 10.1016/j.neuron.2014.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nature Rev Neurosci. 2008;9:314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- Leung LS, Ma J, Shen B, et al. Medial septal lesion enhances general anesthesia response. Exp Neurol. 2013;247:419–428. doi: 10.1016/j.expneurol.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Li CS, Ide JS, Zhang S, et al. Resting state functional connectivity of the basal nucleus of Meynert in humans: In comparison to the ventral striatum and the effects of age. Neuroimage. 2014;97:321–332. doi: 10.1016/j.neuroimage.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, et al. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350(3):412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Meng F, Xie GX, Thompson RC, et al. Cloning and pharmacological characterization of a rat kappa opioid receptor. Proc Natl Acad Sci USA. 1993;90:9954–9958. doi: 10.1073/pnas.90.21.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Li A, Loggia ML, et al. The brain circuitry mediating antipruritic effects of acupuncture. Cereb Cortex. 2014;24(4):873–882. doi: 10.1093/cercor/bhs363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Shen B, Stewart LS, et al. The septohippocampal system participates in general anesthesia. J Neurosci. 2002;22(2):RC200. doi: 10.1523/JNEUROSCI.22-02-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H1, Tashiro M, Kano M, et al. Imaging of central itch modulation in the human brain using positron emission tomography. Pain. 2003;105(1–2):339–346. doi: 10.1016/s0304-3959(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47(6):419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Pan ZZ. μ-Opposing actions of the kappa-opioid receptor. Trends Pharmacol Sci. 1998;19:94–98. doi: 10.1016/s0165-6147(98)01169-9. [DOI] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. J Neuropsychiatry Clin Neurosci. 1937;7(1):103–112. doi: 10.1176/jnp.7.1.103. 1937 republished in 1995 (Winter) [DOI] [PubMed] [Google Scholar]
- Papoiu ADP, Coghill RC, Kraft RA, et al. A tale of two itches. Common features and notable differences in brain activation evoked by cowhage and histamine induced itch. Neuroimage. 2012;59:3611–3623. doi: 10.1016/j.neuroimage.2011.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoiu ADP, Nattkemper LA, Sanders KM, et al. Brain’s reward circuits mediate itch relief. A functional MRI study of active scratching. PLoS One. 2013;8(12):e82389. doi: 10.1371/journal.pone.0082389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoiu AD, Emerson NM, Patel TS, et al. Voxel-based morphometry and arterial spin labeling fMRI reveal neuropathic and neuroplastic features of brain processing of itch in end-stage renal disease. J Neurophysiol. 2014 doi: 10.1152/jn.00827.2013. advance online publication; pii: jn.00827.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckys D, Landwehrmeyer GB. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience. 1999;88(4):1093–1135. doi: 10.1016/s0306-4522(98)00251-6. [DOI] [PubMed] [Google Scholar]
- Petsche H, Stumpf C, Gogolák G. The significance of the rabbit’s septum as a relay station between midbrain and the hippocampus: I. The control of hippocampus arousal activity by the septum cells. Electroencephalogr Clin Neurophysiol. 1962;14:202–211. doi: 10.1016/0013-4694(62)90030-5. [DOI] [PubMed] [Google Scholar]
- Pfab F, Valet M, Sprenger T, et al. Temperature modulated histamine-itch in lesional and nonlesional skin in atopic eczema - a combined psychophysical and neuroimaging study. Allergy. 2010;65(1):84–94. doi: 10.1111/j.1398-9995.2009.02163.x. [DOI] [PubMed] [Google Scholar]
- Schmidt BL, Tambeli CH, Levine JD, et al. μ/δ Cooperativity and opposing κ-opioid effects in nucleus accumbens-mediated antinociception in the rat. European Journal of Neuroscience. 2002;15:861–868. doi: 10.1046/j.1460-9568.2002.01915.x. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Daunais JB, Porrino LJ, et al. Mu and kappa1 opioid-stimulated [35S]guanylyl-5′-O-(gamma-thio)-triphosphate binding in cynomolgus monkey brain. Neuroscience. 1999;94:651–662. doi: 10.1016/s0306-4522(99)00344-9. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Neisius U, Ikoma A, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23:6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai SK, Ma J, Leung LS. Medial septal cholinergic neurons modulate isoflurane anesthesia. Anesthesiology. 2014;120(2):392–402. doi: 10.1097/ALN.0b013e3182a7cab6. [DOI] [PubMed] [Google Scholar]
- Tan H, Hoge WS, Hamilton CA, et al. 3D GRASE PROPELLER: improved image acquisition technique for arterial spin labeling perfusion imaging. Magn Reson Med. 2011;66(1):168–173. doi: 10.1002/mrm.22768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Vierow V, Fukuoka M, Ikoma A, et al. Cerebral representation of the relief of itch by scratching. J Neurophysiol. 2009;102(6):3216–3224. doi: 10.1152/jn.00207.2009. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. Chapter 14. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. Oxford University Press; 2001. [Google Scholar]
- Xiong K, Zheng P. The effect of the septal area in acupuncture analgesia. Zhen Ci Yan Jiu. 1990;15(1):1, 5, 12. [PubMed] [Google Scholar]
- Yokoo H, Yamada S, Yoshida M, et al. Effect of opioid peptides on dopamine release from nucleus accumbens after repeated treatment with methamphetamine. Eur J Pharmacol. 1994;256:335–338. doi: 10.1016/0014-2999(94)90560-6. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Yokoyama T, Nagao Y, et al. Treatment of epidural morphine induced pruritus with butorphanol. Masui. 2009;58(2):178–182. [PubMed] [Google Scholar]
- Yosipovitch G, Ishiuji Y, Patel TS, et al. The brain processing of scratching. J Invest Dermatol. 2008;128(7):1806–1811. doi: 10.1038/jid.2008.3. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Hoemke L, Mohlberg H, et al. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage. 2008;42:1127–1141. doi: 10.1016/j.neuroimage.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85(4):355–375. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.