Abstract
BACKGROUND
Patients with small node-negative breast tumors, who are younger, or have human epidermal growth factor receptor 2 (HER2)-positive or triple negative breast cancer (TNBC) subtypes, are at increased recurrence risk. Concurrently, systemic treatment recommendations have evolved. Less is known about how frequently cytotoxic chemotherapy is given to these patients. Mastectomy rates have also increased. This study reports recent incidence of T1a,bN0M0 breast cancer and the characteristics associated with chemotherapy delivery and surgery selected.
PATIENTS AND METHODS
This retrospective cohort is comprised of invasive female breast cancers diagnosed with AJCC Stage T1a,bN0M0 during 2010–2012 from the Iowa Surveillance, Epidemiology and End Results (SEER) Cancer Registry. Chemotherapy use and surgery were identified by the registry. Univariate and multivariate analysis were performed to determine patient differences across subtype and factors associated with treatment.
RESULTS
The study included 1,687 patients. This represented 27.6% of all AJCC Stage I(a–c)-III breast cancer in 2010–2012, up from 18% in 1990 (P<0.0001). Of 1,456 patients with known subtype, 8.8% and 6.4% had HER2-positive and TNBC disease, respectively. Chemotherapy was given to 7.5% of women with T1aN0M0 and 12.7% of T1bN0M0 tumors. Likelihood of systemic treatment was associated with breast cancer subtype, tumor differentiation and age in a multivariate model. Mastectomy rate was 31.8%.
CONCLUSION
Small, node-negative breast cancers continue to grow significantly as a percent of invasive breast cancer diagnoses. In 2010–2012, in Iowa, systemic chemotherapy correlated with risk factors associated with recurrence: age, subtype, and tumor differentiation. Relatively high rates of mastectomy were seen.
Keywords: Breast Neoplasms, Antineoplastic Agents, Mastectomy, Small tumor, Cancer registry
INTRODUCTION
In the United States breast cancer is the most common cancer among women and their second leading cause of cancer-related death.1 In recent decades, due largely to widespread use of mammography the incidence of the smallest, early stage breast cancers, T1a,b lymph node-negative tumors (T1a,bN0M0), has increased.2–6
Women with small, unselected, node-negative breast cancers have been thought to have an excellent prognosis.7–10 However, some subgroups of patients with these smaller tumors are at increased risk of recurrence and death. Early series looking at patients with T1a,b breast cancers demonstrated a higher risk of recurrence or breast cancer-related mortality to be associated with young age, high tumor grade, adverse histologic features and negative hormone receptor status.11,12 Most recently, HER2-positive T1a,bN0M0 tumors have been shown to have higher recurrence rates.13–18 A meta-analysis of 764 patients found that HER2-positive patients with these small tumors were over four times more likely to relapse than their HER2-negative counterparts.19 With regard to small TNBC tumors, ≤ 1 cm in size, several series have reported worse outcomes for these women compared to those with hormone receptor positive disease.13,20 A single institution retrospective review however has suggested that with multimodality therapy these women may have a more favorable prognosis.21
For some of these very early stage tumors the recurrence risk is high enough that adjuvant therapy is warranted. Major guidelines have been modified in recent years to reflect the increased risk associated with T1bN0M0 tumors and recommend consideration of chemotherapy and trastuzumab, if appropriate. Deciding whether to treat these patients with systemic chemotherapy, and anti-HER2 therapy if needed, is an increasingly common clinical scenario. Most clinical trials however, excluded women with these early stage tumors, so limited high-level evidence is available to guide therapy. Retrospective reviews have often been single institution and extended back to the pre-trastuzumab era. Less is known about how population-based women with these small tumors have been treated during a recent time period.
As understanding of the varying prognoses for patients with T1a,bN0M0 breast cancer was evolving, surgical choices for these tumors were also changing. From 1993–1994 to 2003–2004 the rate of breast conserving therapy (BSC) for patients with T1a,bN0M0 tumors increased from 61% to 78% in a series of over 123,000 cases.3 However, more recent series from the United States suggest that mastectomy rates for tumors ≤2 cm are again climbing, concurrent with the overall trend of breast cancer patients increasingly electing mastectomy over breast conserving surgery (BCS).22,23
For the first time with 2010 breast cancer diagnoses, the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program required reporting of HER2 status. To better elucidate the most current trends in population-based treatment choices for women with these very small tumors, we report the chemotherapy use and surgical choice in women with T1a,bN0M0 breast cancer from the Iowa Cancer Registry, a long-standing SEER Registry, for breast cancers diagnosed in 2010–2012 by patient and tumor characteristics. We performed multivariate analyses to further elucidate the factors contributing to treatment decisions for this cohort of women.
PATIENTS AND METHODS
Patients were identified through the Iowa Cancer Registry and were eligible if they were female Iowa residents diagnosed with microscopically confirmed Stage T1aN0M0 or T1bN0M0 breast cancers between January 1, 2010 and December 31, 2012. Patients with T1mic (n=147) were excluded because there has been less discussion of the role of systemic chemotherapy in this population. The final study population included 1,687 women. This project was reviewed by the Institutional Review Board at the University of Iowa and determined to not be human subject research because of its de-identified data and minimal risk to patients.
The Iowa Cancer Registry records use of chemotherapy as part of first course therapy, but it does not record regimen or dose. Hospital-based medical records are the primary source for this information. Since chemotherapy can be delivered from a physician’s office, it is possible for this information to be incomplete, and consequently underreported. However, unlike some registries, the Iowa Cancer Registry staff does collect this information from oncologists’ offices and thus should have less underreporting.
The following SEER codes were used to define type of surgery: breast conserving surgery (20–24), mastectomy (30–80), other (90 and 99), and no surgery (0).24
We reviewed and collapsed the following invasive ICD-O-3 morphology codes25 into histology categories: ductal (8140, 8141, 8255, 8500, 8522, 8523, 8524, 8541, 8543), lobular (8520), and other (8000, 8010, 8022, 8032, 8046, 8200, 8201, 8211, 8260, 8401, 8480, 8501, 8503, 8504, 8507, 8510, 8530, 8540, 8575). The SEER Program classifies tumor grade as well differentiated, moderately differentiated, poorly differentiated, undifferentiated and unknown. Poorly differentiated and undifferentiated histologies were combined.
Estrogen receptor (ER) and progesterone receptor (PR) status has been reported in SEER since 1991. HER2 status was added with 2010 diagnoses. We created three, non-overlapping categories of subtype: 1) HER2-positive (regardless of hormone receptor (HR) status), 2) HR-positive and HER2-negative, and 3) Triple Negative Breast Cancer (TNBC). Patients with HER2-positive disease were categorized into one group in this analysis of systemic therapy use, as this is the primary driver of a chemotherapy decision regardless of HR status. Patients were considered HR-positive if either ER or PR was positive. Defined this way, patients were categorized only once. For the subtype analyses only, we excluded patients with 1) missing HER2 status and/or 2) missing ER and PR status.
Bivariate analyses were conducted using chi-squared tests for independence. In one set of analyses, we assessed patient and breast cancer characteristics by stage. For these we compared T1aN0M0 to T1bN0M0 and to those with higher stage disease (T1cN0, Stage II–III). Our previously stated inclusion and exclusion criteria were applied to the higher stage patients also. Those with missing values for a given characteristic were dropped from statistical analyses of that variable. Including them as a separate group did not significantly change the p-values. Tests were two-sided. Multivariate logistic regression was also applied to determine factors that influenced the use of chemotherapy. All analyses were conducted using STATA MP version 12.0 (STATA Corp, College Station, TX).
RESULTS
Patient and Tumor Characteristics
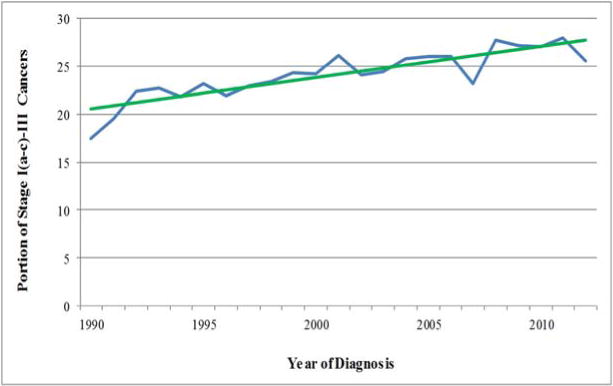
In 2010–2012, 6,103 women were diagnosed with AJCC Stage I(a–c) -III breast cancer in Iowa. Of these tumors, 519 (8.5%) and 1,168 (19.1%) were T1aN0M0 and T1bN0M0 respectively (Table 1). Small, ≤ 1 cm, node-negative tumors represent a growing percent of all non-metastatic invasive breast cancers diagnosed in Iowa since 1990 (Figure 1), having increased from 18.0% of diagnoses in 1990 to 27.6% in 2010–2012 (P<0.0001). The majority of T1aN0M0 (88.8%) and T1bN0M0 (90.7%) breast cancers diagnosed in 2010–12 occurred in women ≥ 50 years of age (Table 1). T1a,bN0M0 tumors represented 29.8% of Stage I(T1a-c)-III breast cancer diagnoses in this age group. Very early stage tumors were relatively rare among young patients. Only 18 of 226 (8.0%) of women ≤ 39 years of age who were diagnosed with breast cancer had T1a,bN0M0 disease at diagnosis.
Table 1.
Patient and Breast Cancer Characteristics by Stage at Diagnosis, Iowa, 2010–2012
| AJCC Stage
|
P valuea | |||
|---|---|---|---|---|
| T1aN0M0 | T1bN0M0 | All others (T1cN0, Stage II–III) | ||
|
| ||||
| N | 519 | 1,168 | 4,416 | |
|
| ||||
| Age at diagnosis | P<0.0001 | |||
| ≤39 | 5 (1.0%) | 13 (1.1%) | 208 (4.7%) | |
| 40–49 | 53 (10.2%) | 96 (8.2%) | 622 (14.1%) | |
| ≥50 | 461 (88.8%) | 1,059 (90.7%) | 3,586 (81.2%) | |
|
| ||||
| Tumor Grade | P<0.0001 | |||
| Well diff | 255 (49.1%) | 498 (42.6%) | 928 (21.0%) | |
| Moderately diff | 198 (38.2%) | 469 (40.2%) | 1,808 (41.0%) | |
| Poorly diff | 56 (10.8%) | 184 (15.8%) | 1,598 (36.2%) | |
| Unknown | 10 (1.9%) | 17 (1.4%) | 82 (1.8%) | |
|
| ||||
| Histology | P<0.0001 | |||
| Ductal | 447 (86.1) | 1,023 (87.6%) | 3,684 (83.4%) | |
| Lobular | 36 (6.9) | 78 (6.7%) | 485 (11.0%) | |
| Other | 36 (6.9) | 67 (5.7%) | 247 (5.6%) | |
Abbreviations: AJCC = American Joint Committee on Cancer; diff = differentiated
Calculated using χ2 test, “unknown” categories not included.
Figure 1.
T1a,bN0M0 Breast Cancer as a Portion of AJCC Stage I(a–c)–III Diagnoses in Iowa Women, 1990–2012
The majority of T1a,bN0M0 tumors, 44.6% of the cohort, were well differentiated. Poorly differentiated histology was reported in 14.2% of T1a,bN0M0 breast cancer and, as expected, represented a larger percent of more advanced tumors, 36.2% of Stage I(T1cN0)-III disease (P<0.0001). Most T1a,bN0M0 breast cancer was ductal histology. Lobular breast cancer was proportionally under-represented among smaller tumors, seen in 6.8% of T1a,bN0M0 breast cancer compared to 11.0% of Stage I(T1cN0)-III; (P≤0.0001).
Subtype information was missing for 233 (13.8%) of T1a,bN0M0 women (n=83 for T1aN0M0 and n=150 for T1bN0M0) and 559 (12.7%) of women with higher Stage I(T1cN0)-Stage III disease. The vast majority of those with unknown subtype were due to missing HER2 status (n=231 for T1a,bN0M0, n=558 for those with Stage I(T1cN0)-Stage III disease). For those with known subtype (n=1,454), T1a,bN0M0 breast cancer was predominately HR-positive (1,233 cases (84.8%)) followed by HER2-positive tumors (128 (8.8%)) and TNBC (93 (6.4%)) (Table 2). In this cohort, of those with known subtype, the majority of HER2-positive T1a,bN0M0 tumors were also HR-positive with 92 of these being both HR-positive and HER2-positive. For higher Stage I(T1cN0)-III breast cancer diagnoses in Iowa 2010–2012 with known subtype, the proportions of patients with HER2-positive and TNBC disease were larger, 14.0% and 14.4%, respectively (data not shown).
Table 2.
Patient and Tumor Characteristics for T1a,bN0M0 Breast Cancer by Subtype, Iowa, 2010–2012
| Subtypea | P valueb | |||
|---|---|---|---|---|
|
| ||||
| HER2+ | HR+ | TNBC | ||
|
| ||||
| N | 128 | 1,233 | 93 | |
|
| ||||
| Median age at diagnosis | 59 | 66 | 64 | P<0.0001 |
| Age at diagnosis | ||||
| ≤39 | 5 (3.9%) | 7 (0.6%) | 3 (3.2%) | |
| 40–49 | 22 (17.2%) | 100 (8.1%) | 7 (7.5%) | |
| ≥50 | 101 (78.9%) | 1,126 (91.3%) | 83 (89.3%) | |
|
| ||||
| AJCC Stage at diagnosis | P=0.021 | |||
| T1aN0M0 | 52 (40.6%) | 359 (29.1%) | 25 (26.9%) | |
| T1bN0M0 | 76 (59.4%) | 874 (70.9%) | 68 (73.1%) | |
|
| ||||
| Tumor grade | P<0.0001 | |||
| Well differentiated | 17 (13.3%) | 629 (51.0%) | 4 (4.3%) | |
| Moderately differentiated | 66 (51.6%) | 481 (39.0%) | 31 (33.3%) | |
| Poorly differentiated | 42 (32.8%) | 108 (8.8%) | 58 (62.4%) | |
| Unknown | 3 (2.3%) | 15 (1.2%) | 0 | |
|
| ||||
| Histology | P=0.110 | |||
| Ductal | 118 (92.2%) | 1,067 (86.5%) | 86 (92.5%) | |
| Lobular | 4 (3.1%) | 93 (7.6%) | 2 (2.1%) | |
| Other | 6 (4.7%) | 73 (5.9%) | 5 (5.4%) | |
|
| ||||
| Race | P=0.571 | |||
| Caucasian | 124 (96.9%) | 1,210 (98.1%) | 93 (100.0%) | |
| Non-Caucasian | 3 (2.3%) | 18 (1.5%) | 0 | |
| Unknown | 1 (0.8%) | 5 (0.4%) | 0 | |
Abbreviations: HER2 = human epidermal growth factor receptor 2; HR=estrogen or progesterone hormone receptor; TNBC=triple negative breast cancer; AJCC = American Joint Committee on Cancer
Subtype data missing for T1aN0M0 (n=83) and T1bN0M0 (n=150).
Calculated using χ2 test, “unknown” categories not included.
For those with subtype information, HER2-positive and TNBC T1a,bN0M0 tumors occurred more in younger patients. Median age for HER2-positive, HR-positive and TNBC was 59, 66 and 64, respectively. T1aN0M0 tumors comprised less than half of the T1a,bN0M0 tumors. Of the three, HER2-positive tumors had the largest proportion of T1aN0M0 relative to T1bN0M0 (40.6% vs. 59.4%,) and TNBC the smallest proportion (26.9% vs 73.1%); the proportion of each stage across subtype was statistically different from one another (P<0.021). Tumor grade also correlated with known breast cancer subtype, with proportionally more tumors of the TNBC and HER2postive phenotypes showing poor differentiation (P<0.0001).
Systemic and surgical treatment
Overall 39 of 519 (7.5%) and 148 of 1,168 (12.7%) of women with T1aN0M0 and T1bN0M0 tumors, respectively, received first course cytotoxic chemotherapy (Table 3). For T1aN0M0 tumors, 26.9% of women with HER2-positive disease received chemotherapy, and 4 of 25 (16.0%) women with T1aN0M0 TNBC received systemic treatment. The majority of women with T1bN0M0 HER2-positive and TNBC were treated with chemotherapy. On univariate analysis, there was a trend for younger women to receive chemotherapy. This achieved statistical significance for T1aN0M0 and T1bN0M0 as a whole, along with all T1bN0M0 subtypes. Tumor grade also correlated closely with the delivery of chemotherapy for each stage, although not for all subtypes within a given stage.
Table 3.
Chemotherapy Use by Patient and Tumor Characteristics and Breast Cancer Subtype, Iowa, 2010–2012
| All subtypes@ (including missing HER2) |
HER 2 Positive | HR Positive (either ER or PR positive) |
TNBC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemo | P value | Chemo | P value | Chemo | P value | Chemo | P value | |||||
| No | Yes | No | Yes | No | Yes | No | Yes | |||||
| T1aN0M0 | 480 | 39 | 38 | 14 | 344 | 15 | 21 | 4 | ||||
|
| ||||||||||||
| Age | ||||||||||||
| <50 | 47 (9.8%) | 11 (28.2%) | P<0.0001 | 6 (15.8%) | 5 (35.7%) | P=0.119 | 32 (9.3%) | 4 (26.7%) | P=0.028 | 2 (9.5%) | 0 | P=0.520 |
| ≥50 | 433 (90.2%) | 28 (71.8%) | 32 (84.2%) | 9 (64.3%) | 312 (90.7%) | 11 (73.3%) | 19 (90.5%) | 4 (100.0%) | ||||
|
| ||||||||||||
| Grade§ | ||||||||||||
| Well/mod diff | 39 (8.3%) | 17 (44.7%) | P<0.0001 | 8 (22.2%) | 7 (50.0%) | P=0.054 | 13 (3.8%) | 5 (35.7%) | P<0.0001 | 11 (62.4%) | 2 (50.0%) | P=0.930 |
| Poorly diff | 432 (91.7%) | 21 (55.3%) | 28 (77.8%) | 7 (50.0%) | 329 (96.2%) | 9 (64.3%) | 10 (47.6%) | 2 (50.0%) | ||||
|
| ||||||||||||
| T1bN0M0 | 1020 | 148 | 33 | 43 | 821 | 53 | 33 | 35 | ||||
|
| ||||||||||||
| Age | ||||||||||||
| <50 | 71 (7.0%) | 38 (25.7%) | P<0.0001 | 3 (9.1%) | 13 (30.2%) | P=0.025 | 56 (6.8%) | 15 (28.3%) | P<0.0001 | 0 | 8 (22.9%) | P=0.003 |
| ≥50 | 949 (93.0%) | 110 (74.3%) | 30 (90.9%) | 30 (69.8%) | 765 (93.2%) | 38 (71.7%) | 33 (100.0%) | 27 (77.1%) | ||||
|
| ||||||||||||
| Grade§ | ||||||||||||
| Well/mod diff | 108 (10.8%) | 76 (51.7%) | P<0.0001 | 8 (25.0%) | 19 (44.2%) | P=0.087 | 70 (8.6%) | 20 (38.5%) | P<0.0001 | 18 (54.5%) | 27 (77.1%) | P=0.049 |
| Poorly diff | 896 (89.2%) | 71 (48.3%) | 24 (75.0%) | 24 (55.8%) | 740 (91.4%) | 32 (61.5%) | 15 (45.5%) | 8 (22.9%) | ||||
Abbreviations: HER2 = human epidermal growth factor receptor 2; HR=estrogen or progesterone hormone receptor; TNBC=triple negative breast cancer; diff = differentiated
Subtype data missing for T1aN0M0 (n=83) and T1bN0M0 (n=150).
Grade data missing for T1aN0M0 (n=10) and T1bN0M0 (n=17).
Overall, 34.1% and 30.1% of women with T1aN0M0 and T1bN0M0 tumors respectively underwent mastectomy (Table 4). For both groups younger women were more likely to undergo mastectomy. All women ≤ 39 years of age with T1aN0M0 tumors elected mastectomy. Chemotherapy use also correlated with the choice of mastectomy over BCS on univariate analysis. Subtype predicted mastectomy for both T1aN0M0 and T1bN0M0 tumors, with women with HER2 positive and TNBC tumors more likely to undergo more extensive surgery.
Table 4.
Surgery Performeda by Age, Chemotherapy Use and Breast Cancer Subtype, Iowa, 2010–2012
| BCS | Mastectomy | P valueb | |
|---|---|---|---|
| T1aN0M0 | 337 | 174 | |
|
| |||
| Age | |||
| ≤39 | 0 | 5 (2.9%) | P=0.002 |
| 40–49 | 30 (8.9%) | 23 (13.2%) | |
| ≥50 | 307 (91.1%) | 146 (83.9%) | |
|
| |||
| Chemo | |||
| Yes | 20 (5.9%) | 19 (10.9%) | P=0.044 |
| No | 317 (94.1%) | 155 (89.1%) | |
|
| |||
| Subtypec | |||
| HER2+ | 22 (6.5%) | 30 (17.2%) | P<0.0001 |
| HR+ | 254 (75.4%) | 99 (56.9%) | |
| TNBC | 12 (3.6%) | 12 (6.9%) | |
|
| |||
| T1bN0M0 | 791 | 352 | |
|
| |||
| Age | |||
| ≤39 | 6 (0.8%) | 6 (1.7%) | P=0.001 |
| 40–49 | 51 (6.5%) | 43 (12.2%) | |
| ≥50 | 734 (92.8%) | 303 (86.1%) | |
|
| |||
| Chemo | |||
| Yes | 80 (10.1%) | 65 (18.5%) | P<0.0001 |
| No | 711 (89.9%) | 287 (81.5%) | |
|
| |||
| Subtypec | |||
| HER2+ | 43 (5.4%) | 33 (9.4%) | P<0.0001 |
| HR+ | 617 (78.0%) | 237 (67.3%) | |
| TNBC | 37 (4.7%) | 30 (8.5%) | |
Abbreviations: BCS = breast conserving surgery; Chemo = chemotherapy; HER2 = human epidermal growth factor receptor 2; HR=estrogen or progesterone hormone receptor; TNBC=triple negative breast cancer.
Those with no surgery or unknown surgery were dropped from analysis (n=8 for T1aN0M0; n=25 for T1bN0M0).
Calculated using χ2 test.
Subtype data missing for T1aN0M0 (n=83) and T1bN0M0 (n=150) and surgery data missing for HR+ T1aN0M0 (n=6), HR+ T1bN0M0 (n=20), TNBC T1bN0M0 (n=1), and TNBC T1bN0M0 (n=1). Both surgery and subtype data missing for T1aN0M0 (n=1), and T1bN0M0 (n=4).
Multivariate analysis demonstrated that age, tumor differentiation, tumor size (T1aN0M0 versus T1bN0M0) and breast cancer subtype were all independent factors that predicted use of chemotherapy (Table 5). Surgical choice was not significantly associated with chemotherapy in this analysis.
Table 5.
Multivariate Analysis of Factors that Predict Chemotherapya
| Odds Ratiob | P value | 95% CI | |
|---|---|---|---|
| Subtype | |||
| HR+ | Ref | ||
| HER2+ | 10.06 | P 0.0001 | [6.15–16.47] |
| TNBC | 5.58 | P<0.0001 | [3.14–9.91] |
| AJCC Stage at diagnosis | |||
| T1a | Ref | ||
| T1b | 2.41 | P<0.0001 | [1.48–3.92] |
| Surgery | |||
| BCS | Ref | ||
| Mastectomy | 1.10 | P=0.627 | [0.73–1.67] |
| Age at diagnosis | |||
| Age ≤39 | 7.60 | P=0.008 | [1.69–34.00] |
| Age 40–49 | Ref | ||
| Age ≥50 | 0.28 | P<0.0001 | [0.166–0.480] |
| Tumor grade | |||
| Well diff | Ref | ||
| Moderately diff | 1.76 | P=0.039 | [1.03–3.00] |
| Poorly diff | 6.67 | P<0.0001 | [3.78–11.79] |
Abbreviations: CI = confidence interval; BCS = breast conserving surgery; HER2 = human epidermal growth factor receptor 2; HR=estrogen or progesterone hormone receptor; TNBC=triple negative breast cancer; AJCC = American Joint Committee on Cancer; diff = differentiated
The sample used in this analysis include all T1a,bN0M0 tumors with non-missing data (n=424 for T1aN0M0; n=988 for T1bN0M0).
Adjusted for all other variables in the table.
DISCUSSION
In this population-based sample of patients diagnosed with breast cancer between 2010 and 2012, T1a,bN0M0 tumors represented 27.6% of all tumors Stage I(T1a-c)- III. This demonstrates a continued increasing secular trend in the percent of breast cancer that these small tumors represent, even over other contemporary series,11 suggesting that management of this disease will be a growing clinical concern. Earlier studies have linked the increase in small breast tumor diagnoses to the wide-spread implementation of screening mammography.4–6 However, the reasons for the more recent increases are less clear, and could perhaps be related to improved imaging techniques. The percent of HER2-positive and TNBC tumors seen are consistent with other series.18,26,27 The majority of these small HER2-positive tumors in Iowa were also HR-positive. This is consistent with other series looking at T1a,bN0M0 tumors.16,27,28 Broadly, treatment with chemotherapy correlated with factors associated with risk of systemic disease recurrence. Mastectomy rates for these small tumors were even higher than seen in other recent reported series.
In 2010 the National Comprehensive Cancer Network (NCCN) and in 2011 the St. Gallen Consensus Conference recommended consideration of chemotherapy and trastuzumab for T1bN0M0 HER2-positive tumors.29 In Iowa, more than half of all women with T1bN0M0 HER2-positive tumors diagnosed in 2010 through 2012 received systemic cytotoxic therapy. Chemotherapy use during this period of evolving guidelines and literature was influenced by size (T1bN0M0 vs. T1aN0M0), receptor status, grade and patient age. A recent European retrospective review reported on 900 patients treated between 2000–2009 with T1a-cN0M0 tumors of which 407 were T1a,bN0M0.26 The rate of chemotherapy treatment was lower in this Italian review for T1aN0M0 tumors (3.0%) and higher for T1bN0M0 tumors (27.2%) than that seen in our more recent Iowa data. NCCN recently reported chemotherapy delivery rates in T1a,bN0M0 tumors from their database from 2000–2009. More than 50% of patients treated at these institutions with T1a,bN0M0 HER2 positive or TNBC in 2009 received chemotherapy.30
A very small number of women with these very early stage HR-positive, HER2-negative tumors, do receive chemotherapy. The NCCN series reported chemotherapy rates in 2009 of 2% and 13% for HR-positive, HER2-negative T1aN0M0 and T1bN0M0 tumors, respectively. This compares with 4.2% and 6.1% in the Iowa data. These data do not capture possible risk associated with lymphovascular invasion, multifocal disease, genomic profiling or other clinical factors. Still, a small group of, likely lower risk, women were started on chemotherapy. Better understanding of factors contributing to these decisions could prevent toxicity and allow for cost-savings, if in fact these treatments were of limited benefit.
Reports from the United States, have shown the rate of mastectomy for small tumors, which had been decreasing until recent years, is now again increasing. A recent SEER review showed a trend of more mastectomies for patients with T1 tumors.31 Notably, Iowa had among the highest overall rates of mastectomy. European cohorts have different findings. Two Italian series reported lower rates of mastectomy. Gamucci et al. in a multi-center retrospective analysis of 900 patients with T1a-cN0M0 breast cancer in 2000–2009 reported a BCS rate of 81.8%, with local therapy not related to breast cancer sub-type.26 Cancello et al. in a single institution review of patients with T1a,bN0M0 tumors treated from1997–2005 found mastectomy rates of <10% for all subtypes except HER2 positive where the rate was 36.6%.13
In 2010–12 in Iowa, the decision to undergo mastectomy correlated on univariate analysis with treatment with cytotoxic chemotherapy for patients with T1a,bN0M0 tumors. Women who underwent, what may be perceived to be more aggressive surgical management, also received more aggressive systemic therapy. A recent series from Memorial Sloan Kettering of 194 women with T1N0M0 TNBC found a similar significant correlation between mastectomy and receipt of systemic chemotherapy.21 However this was not corroborated in our multivariate analysis.
There are limitations to our study. Treatment choices are reported for one geographic area, which is predominately rural and has less racial diversity than the United States population as a whole. Some groups of breast cancer subtype by treatment choice have small numbers, limiting our analysis and precision. The subtypes grouped here are in large categories. Breast cancer is more complex than this, with variation in natural history and treatment response within each subset. Also, there were patients with unknown HER2 or unknown hormone receptor status that were excluded from subtype analysis. Finally, since chemotherapy is also administered in physician offices, systemic therapy can be underreported to hospital-based cancer registries.32,33 This is the reason why, although the registries do collect data on systemic therapy, SEER does not routinely release this information in their public-use database. Iowa SEER however does collect data from physicians’ offices, although imperfections in this process could underestimate the frequency with which chemotherapy is actually delivered.
Still, untreated HER2-positive and TNBC T1a,bN0M0 breast cancers broadly carry higher risk for recurrence and death than other stage-matched subtypes. This analysis is the largest population-based series to date which reports incidence of T1a,bN0M0 breast cancer and current treatment status of women with these tumor subtypes.
CONCLUSION
Important outcomes data from SEER by subtype will follow in coming years. In the intervening time however, treatment for this group of patients with very small tumors will continue to evolve. Offering less toxic, and less morbid therapies, to this lower risk group, compared to more advanced stage counterparts, will be a priority. Large phase III trials to address the oncologic management of these women are unlikely to be undertaken, given the low frequency of the tumors for which chemotherapy is of benefit and the low event rate. Smaller phase II studies will offer some guidance. Prospective databases and improved molecular profiling techniques will also likely provide direction in the management of this increasingly frequent, clinical question.
Clinical Practice Points.
Small, T1a,b,N0M0 breast cancers represent a growing proportion of breast cancer diagnoses and thus management of these tumors is an increasingly frequent clinical question.
Recent, 2010–2012 registry, data show that higher-risk small tumors, T1bN0 (as compared with T1aN0), HER2-positive or TNBC, were more likely to be treated with chemotherapy.
Patient age, tumor differentiation, tumor size and breast cancer subtype are all independent predictors of chemotherapy use for T1a,bN0M0 breast cancers.
Breast cancer subtype, HER2-positive and TNBC, and young age are predictive of mastectomy in women with small, T1a,b, node-negative breast tumors.
Mastectomy rates for these small tumors continue to increase.
Acknowledgments
We would like to acknowledge Dan Olson for his efforts in preparing and providing Iowa Cancer Registry Data. This work was funded in part by the Iowa SEER contract no. HHSN261201300020I/HHSN26100001.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: All authors have no conflicts of interest.
Contributor Information
Mary C. Schroeder, Assistant Professor, Department of Pharmacy Practice and Science, College of Pharmacy, University of Iowa.
Charles F. Lynch, Professor, Department of Epidemiology, University of Iowa.
Taher Abu-Hejleh, Clinical Assistant Professor, Division of Hematology, Oncology and Blood and Marrow Transplantation, Department of Internal Medicine, Carver College of Medicine, University of Iowa.
Elizabeth A. Chrischilles, Professor and Marvin A. and Rose Lee Pomerantz Chair in Public Health, Department of Epidemiology, College of Public Health, University of Iowa.
Alexandra Thomas, Email: alex-thomas@uiowa.edu, Clinical Associate Professor, Division of Hematology, Oncology and Blood and Marrow Transplantation, Department of Internal Medicine, C32 GH, 200 Hawkins Drive, University of Iowa, Iowa City, IA 52242, (319) 356-2148 PHONE, (319) 353-8383 FAX.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: a cancer journal for clinicians. 2011 Jul-Aug;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Schootman M, Jeffe D, Reschke A, Aft R. The full potential of breast cancer screening use to reduce mortality has not yet been realized in the United States. Breast cancer research and treatment. 2004 Jun;85(3):219–222. doi: 10.1023/B:BREA.0000025410.41220.67. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy T, Stewart AK, Bilimoria KY, Patel-Parekh L, Sener SF, Winchester DP. Treatment trends and factors associated with survival in T1aN0 and T1bN0 breast cancer patients. Annals of surgical oncology. 2007 Oct;14(10):2918–2927. doi: 10.1245/s10434-007-9441-5. [DOI] [PubMed] [Google Scholar]
- 4.Luke C, Nguyen AM, Priest K, Roder D. Female breast cancers are getting smaller, but socio-demographic differences remain. Australian and New Zealand journal of public health. 2004 Aug;28(4):312–316. doi: 10.1111/j.1467-842x.2004.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 5.Fracheboud J, Otto SJ, van Dijck JA, et al. Decreased rates of advanced breast cancer due to mammography screening in The Netherlands. British journal of cancer. 2004 Aug 31;91(5):861–867. doi: 10.1038/sj.bjc.6602075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vacek PM, Geller BM, Weaver DL, Foster RS., Jr Increased mammography use and its impact on earlier breast cancer detection in Vermont, 1975–1999. Cancer. 2002 Apr 15;94(8):2160–2168. doi: 10.1002/cncr.10459. [DOI] [PubMed] [Google Scholar]
- 7.Arnesson LG, Smeds S, Fagerberg G. Recurrence-free survival in patients with small breast cancer. An analysis of cancers 10 mm or less detected clinically and by screening. The European journal of surgery = Acta chirurgica. 1994 May;160(5):271–276. [PubMed] [Google Scholar]
- 8.Lee AK, Loda M, Mackarem G, et al. Lymph node negative invasive breast carcinoma 1 centimeter or less in size (T1a,bNOMO): clinicopathologic features and outcome. Cancer. 1997 Feb 15;79(4):761–771. [PubMed] [Google Scholar]
- 9.Leitner SP, Swern AS, Weinberger D, Duncan LJ, Hutter RV. Predictors of recurrence for patients with small (one centimeter or less) localized breast cancer (T1a,b N0 M0) Cancer. 1995 Dec 1;76(11):2266–2274. doi: 10.1002/1097-0142(19951201)76:11<2266::aid-cncr2820761114>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Rosen PP, Groshen S, Kinne DW, Norton L. Factors influencing prognosis in node-negative breast carcinoma: analysis of 767 T1N0M0/T2N0M0 patients with long-term follow-up. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1993 Nov;11(11):2090–2100. doi: 10.1200/JCO.1993.11.11.2090. [DOI] [PubMed] [Google Scholar]
- 11.Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, et al. Overall survival and cause-specific mortality of patients with stage T1a,bN0M0 breast carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007 Nov 1;25(31):4952–4960. doi: 10.1200/JCO.2006.08.0499. [DOI] [PubMed] [Google Scholar]
- 12.Fisher ER, Costantino JP, Leon ME, et al. Pathobiology of small invasive breast cancers without metastases (T1a/b, N0, M0): National Surgical Adjuvant Breast and Bowel Project (NSABP) protocol B-21. Cancer. 2007 Nov 1;110(9):1929–1936. doi: 10.1002/cncr.23011. [DOI] [PubMed] [Google Scholar]
- 13.Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis in women with small (T1mic,T1a,T1b) node-negative operable breast cancer by immunohistochemically selected subtypes. Breast cancer research and treatment. 2011 Jun;127(3):713–720. doi: 10.1007/s10549-011-1465-7. [DOI] [PubMed] [Google Scholar]
- 14.Sanpaolo P, Barbieri V, Genovesi D, Fusco V, Ausili Cefaro G. Biologically effective dose and breast cancer conservative treatment: is duration of radiation therapy really important? Breast cancer research and treatment. 2012 Jul;134(1):81–87. doi: 10.1007/s10549-011-1932-1. [DOI] [PubMed] [Google Scholar]
- 15.Livi L, Meattini I, Saieva C, et al. Prognostic value of positive human epidermal growth factor receptor 2 status and negative hormone status in patients with T1a/T1b, lymph node-negative breast cancer. Cancer. 2012 Jul 1;118(13):3236–3243. doi: 10.1002/cncr.26647. [DOI] [PubMed] [Google Scholar]
- 16.Theriault RL, Litton JK, Mittendorf EA, et al. Age and survival estimates in patients who have node-negative T1ab breast cancer by breast cancer subtype. Clinical breast cancer. 2011 Oct;11(5):325–331. doi: 10.1016/j.clbc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park YH, Kim ST, Cho EY, et al. A risk stratification by hormonal receptors (ER, PgR) and HER-2 status in small (< or = 1 cm) invasive breast cancer: who might be possible candidates for adjuvant treatment? Breast cancer research and treatment. 2010 Feb;119(3):653–661. doi: 10.1007/s10549-009-0665-x. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009 Dec 1;27(34):5700–5706. doi: 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrelli F, Barni S. Role of HER2-neu as a prognostic factor for survival and relapse in pT1a-bN0M0 breast cancer: a systematic review of the literature with a pooled-analysis. Medical oncology. 2012 Dec;29(4):2586–2593. doi: 10.1007/s12032-012-0201-4. [DOI] [PubMed] [Google Scholar]
- 20.Kwon JH, Kim YJ, Lee KW, et al. Triple negativity and young age as prognostic factors in lymph node-negative invasive ductal carcinoma of 1 cm or less. BMC cancer. 2010;10:557. doi: 10.1186/1471-2407-10-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho AY, Gupta G, King TA, et al. Favorable prognosis in patients with T1a/T1bN0 triple-negative breast cancers treated with multimodality therapy. Cancer. 2012 Oct 15;118(20):4944–4952. doi: 10.1002/cncr.27480. [DOI] [PubMed] [Google Scholar]
- 22.Adepoju L, Wanjiku S, Brown M, et al. Effect of insurance payer status on the surgical treatment of early stage breast cancer: data analysis from a single health system. JAMA surgery. 2013 Jun;148(6):570–572. doi: 10.1001/jamasurg.2013.61. [DOI] [PubMed] [Google Scholar]
- 23.Habermann EB, Abbott A, Parsons HM, Virnig BA, Al-Refaie WB, Tuttle TM. Are mastectomy rates really increasing in the United States? Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010 Jul 20;28(21):3437–3441. doi: 10.1200/JCO.2009.27.6774. [DOI] [PubMed] [Google Scholar]
- 24.Surveillance Research, Program N. http://www.seer.cancer.gov/tools/codingmanuals/index.html. Accessed November, 12, 2013.
- 25.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. Third. Geneva: World Health Organization; 2000. [Google Scholar]
- 26.Gamucci T, Vaccaro A, Ciancola F, et al. Recurrence risk in small, node-negative, early breast cancer: a multicenter retrospective analysis. Journal of cancer research and clinical oncology. 2013 May;139(5):853–860. doi: 10.1007/s00432-013-1388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amar S, McCullough AE, Tan W, et al. Prognosis and outcome of small (<=1 cm), node-negative breast cancer on the basis of hormonal and HER-2 status. The oncologist. 2010;15(10):1043–1049. doi: 10.1634/theoncologist.2010-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curigliano G, Viale G, Bagnardi V, et al. Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009 Dec 1;27(34):5693–5699. doi: 10.1200/JCO.2009.22.0962. [DOI] [PubMed] [Google Scholar]
- 29.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2011 Aug;22(8):1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaz Duarte Luis RAO Ines Maria, Hughes Melissa E, Mamet Rizvan, Burstein Harold J, Edge Stephen B, Gonzalez-Angulo Ana M, Javid Sara H, Moy Beverly, Rugo Hope S, Theriault Richard L, Weeks Jane C, Lin Nancy U. Time trends in the use of adjuvant chemotherapy (CTX) and outcomes in women with T1N0 breast cancer (BC) in the National Comprehensive Cancer Network (NCCN) Journal of Clinical Oncology. 2013;31(15_suppl):1006. [Google Scholar]
- 31.Mahmood U, Hanlon AL, Koshy M, et al. Increasing national mastectomy rates for the treatment of early stage breast cancer. Annals of surgical oncology. 2013 May;20(5):1436–1443. doi: 10.1245/s10434-012-2732-5. [DOI] [PubMed] [Google Scholar]
- 32.Warren JL, Harlan LC. Can cancer registry data be used to study cancer treatment? Medical care. 2003 Sep;41(9):1003–1005. doi: 10.1097/01.MLR.0000086827.00805.B5. [DOI] [PubMed] [Google Scholar]
- 33.Malin JL, Kahn KL, Adams J, Kwan L, Laouri M, Ganz PA. Validity of cancer registry data for measuring the quality of breast cancer care. Journal of the National Cancer Institute. 2002 Jun 5;94(11):835–844. doi: 10.1093/jnci/94.11.835. [DOI] [PubMed] [Google Scholar]


