Abstract
Background
Maternal depression is one of the most common prenatal complications. The consequences of fetal exposure to maternal depression are poorly understood. The aim of this study is to examine the association between fetal exposure to maternal depressive symptoms and cortical thickness in 6–9 year-old children.
Methods
A prospective, longitudinal study of maternal depressive symptoms at 19, 25 and 31 weeks gestation was followed by acquisition of a structural MRI scan in 81 children (86.1 ± 9.9 months).
Results
Significant (p<.01) cortical thinning in children primarily in the right frontal lobes was associated with exposure to prenatal maternal depression. The strongest association was at 25 weeks gestation; exposure to maternal depression at 25 gestational weeks was associated with cortical thinning in 19% of the whole cortex and 24% of the frontal lobes, primarily in the right superior, medial orbital and frontal pole regions of the prefrontal cortex (p<.01). The significant association between prenatal maternal depression and child externalizing behavior (p<.05) was mediated by cortical thinning in prefrontal areas of the right hemisphere.
Conclusions
The pattern of cortical thinning in children exposed to prenatal maternal depression is similar to patterns in depressed patients and in individuals with risk for depression. Exposure to prenatal depression coupled with subsequent cortical thinning was associated with presence of externalizing behavior in preadolescent children and may be prodromal markers of risk for dysphoria. Vulnerability to prenatal influences at 25 gestational weeks may result from the enormous growth and dramatic structural changes in the nervous system.
Keywords: Maternal Depression, Fetal Programming, Structural MRI, Externalizing Behavior, Childhood Risk, Prefrontal cortex
INTRODUCTION
When the quality of maternal care is compromised by postpartum depression, infants suffer pervasive and lingering negative health-related consequences[1–2] including poor emotional regulation[3–5] impaired cognitive, behavioral and motor development[3, 6–11] and mental health issues[3,12]. These associations with maternal postpartum depression are observed even if maternal depressive symptoms are subclinical[13–14]. Less is known about the consequences of maternal prenatal depression for child development. This is surprising because the rate of depressive symptoms during pregnancy is higher than it is postpartum. Estimates suggest that 13% to 40% of women struggle with symptoms of depression during their pregnancy making maternal depression one of the most common prenatal complications[15–17].
The great majority of studies on the effects of prenatal maternal mood on birth and infant/child outcomes have focused on fetal exposure to stress and anxiety[18–24]. Recent evidence, however, indicates that fetal exposure to antenatal depression may have larger effects on infant[25,26] and child outcomes[27] than either anxiety or postpartum depression[27–28]. For example, Barker et al[29] reported that prenatal maternal depression but not anxiety or postpartum depression, significantly increased the risk for externalizing behaviors and was associated with significantly lower verbal intelligence in a large sample (N=3298). In another large study (N=5029), prenatal but not postnatal depressive symptoms were associated with lower child intelligence[30]. Early exposures to maternal depression, including prenatally, were independently associated with externalizing behaviors and low social competence in adolescent boys[31].
There is evidence that neurological impairment, cortical thinning and reduced cortical volume, are associated with history of depression[32–33], current symptom severity in adults[32,34–36], cumulative exposure to maternal depression in children[37] and in individuals who are not ill but have familial risk for depression[32]. Patterns of cortical thinning among individuals reporting depressive symptoms have been broadly distributed[32,35–36,38] but primarily involve structures of the right hemisphere including the paracentral, frontal and cingulate regions[32,34,39]. The current study is the first prospective, longitudinal evaluation of the association between fetal exposure to maternal depressive symptoms, cortical thickness and behavioral problems in children. If fetal exposure to maternal depression is associated with eventual risk for depression, prodromal neurological markers in children may be present, especially in the right hemisphere and areas of the frontal cortex.
METHODS AND MATERIALS
Participants
English-speaking, healthy adult pregnant women with singleton pregnancies were recruited by 15 gestational weeks. Exclusion criteria: (i) tobacco, alcohol, or other drug use in pregnancy, (ii) uterine or cervical abnormalities, (iii) presence of conditions associated with neuroendocrine dysfunction. None of the women in this sample were or had been treated for psychiatric disorders. Two hundred and seventy-five women consented to participate in a follow-up study of their children. From this sample, we report on 81 children, 6–9 years, who completed artifact-free MRI scans. Parents and children gave informed (or affirmed) consent which was approved by the Institutional Review Board for protection of human subjects.
Assessments in pregnant women
Gestational age was determined by (i) last menstrual period (ii) early uterine size, and confirmed by obstetric ultrasonogram before 20 weeks[40]. Medical risk was defined as the presence of certain medical conditions in the index or previous pregnancies (e.g. vaginal bleeding, pregnancy-induced hypertension, anemia, infection(41). Ninety-three percent of the women reported one or fewer medical risks in the current or previous pregnancies and no woman in the study required hospitalization to manage risk. The sum of medical risk factors was calculated as an indicator of presence of any current or historical risk conditions[42].
Maternal depressive symptoms
Maternal depressive symptoms [Center for Epidemiological Studies Depression Scale (CES-D;[43,44]) were assessed for each woman at 19(±0.83, SD), 25(±0.9) and 31(±0.9) weeks gestation. Subjects indicated how often they had experienced each symptom of depression during the past week (Supplement). Levels of depressive symptoms at each visit and the average rating of depressive symptoms across gestation were used in the statistical analysis.
Current maternal depressive symptoms were assessed with the Beck Depression Inventory[45]. Prenatal depressive symptoms shared ~9–25% variance with current levels of maternal depression but were not associated with maternal prenatal medical risk, maternal age or education.
Assessments in children
All children had a stable neonatal course (Table 1) and were without known neonatal illness or congenital, chromosomal, or genetic anomalies. Participants had no evidence of neurological abnormalities in the newborn period. Children's structural MRI (sMRI) images were assessed by a neuroradiologist for anatomical appearance. Two children with abnormal scans (N=2) were not included in the final sample (N=81). At 6–9 years of age, no emotional or physical conditions were reported by the parents in a structured interview (46). For 88% of the children right hand was dominant (47).
Table 1.
Sociodemographic characteristics and birth outcomes
| MRI cohort (N=82) | Total Sample | |
|---|---|---|
| 30.2 (5.3) | ||
| Maternal Age | 30.6 (6.3) | |
|
| ||
| Race/ethnicity | 48% | |
| Non-Hispanic White | 54% | 25% |
| Hispanic White | 21% | 11% |
| African American | 10% | 8% |
| Asian | 7% | 8% |
| Other/Multi-Ethnic | 8% | |
|
| ||
| Annual household income | 20.9% | |
| $0 to $30,000 | 21.5% | 28.9% |
| $30,001 to $60,000 | 26.7% | 19.6% |
| $60,001 to $100,000 | 33.0% | 22.2% |
| Over $100,000 | 19.0% | |
|
| ||
| Maternal Depression | 1.71 (.58) | |
| 19 weeks gestation | 1.60 (.45) | 1.74 (.59) |
| 25 weeks gestation | 1.64 (.51) | 1.80 (.63) |
| 31 weeks gestation | 1.76 (.51) | |
|
| ||
| Maternal Education | 2.8% | |
| Less than high school | 1.3% | 14.5% |
| High School | 11.3% | 40.4% |
| Some College | 37.5% | 24.5% |
| Undergraduate degree | 40.0% | 17.8% |
| Graduate degree | 10.0% | |
|
| ||
| 5 minute APGAR | 9.0 (.2) | 8.9 (.5) |
|
| ||
| Primiparous | 55.6% | 48.0% |
|
| ||
| Child sex | 51.3 | |
| Male | 51.9% | 48.7 |
| Female | 48.1% | |
|
| ||
| Length of gestation (weeks) | 39.1 (1.6) (8.6% 34–36.9 weeks) | 38.9 (2.1) |
|
| ||
| Birth weight (grams) | 3476.5 (624.3) (4.9% <2500g) | 3363.3 (597.2) |
Child Behavioral Problems
Child affective and conduct disorder problems were measured by maternal report (Child Behavior Checklist; CBCL[48]). Responses to the DSM-oriented affective problems and conduct disorders scales were transferred to T-scores based on sex-specific reference tables.
sMRI Acquisition
The sMRI scan was acquired with a 3-T Philips Achieva system. Children wore earplugs and watched a movie while in the scanner. A high resolution T1 anatomical scan was acquired in the sagittal plane with 1mm3 isotropic voxel dimensions. An Inversion-Recovery Spoiled Gradient Recalled Acquisition (IR-SPGR) sequence with optimal parameters was applied: Repetition rate (TR)= 11ms, Echo Time (TE)= 3.3ms, Inversion Time (TI)= 1100ms, Turbo Field Echo factor (TFE)= 192, Number of slices: 150, no SENSE acceleration, Flip angle=18°, Shot interval (time from inversion pulse to the center of acquisition) = 2200ms.
Processing of MRI data
Cortical surface reconstruction and volumetric segmentation was performed with FreeSurfer (http://surfer.nmr.mgh.harvard.edu/). Streamlined image processing provided in this software package included; application of intensity normalization prior to segmentation to minimize errors in identifying the boundaries[49]; removal of non-brain tissues[50]; and transforming images into the Talairach space[50,51]. Pial and white matter surfaces were located by finding the highest intensity gradient[52–54]. Surface inflation was applied to each individual brain[55] and the inflated brains were registered to a spherical atlas. Cortical thickness was the closest distance from the gray matter/white matter surface to the pial surface at each vertex on the tessellated surface[53].
Cortical thickness and prenatal depression
Spatially normalized cortical thickness maps and maternal depression were entered into a linear regression model. The association between prenatal depression and cortical thickness was analyzed at every node on the cortical surface. Gestational age at birth, age of the child at testing, sex, handedness and current levels of maternal depression were included as covariates. A threshold of p< 0.05 was used for all statistical tests and outcomes were corrected for multiple comparisons using False Discovery Rate (FDR).
After FDR corrections, the number of vertices that were significantly associated with maternal depressive symptoms was determined and converted to percentage of the total in each region of the cortex. The same procedure was computed for the number of significant vertices in each lobe. For hemispheric and whole brain percentages, the procedure was the same except the total number of subcortical vertices was subtracted from the total.
RESULTS
Women whose children participated in the MRI study did not differ significantly on the sociodemographic characteristics (Table 1) from women in the general follow-up sample.
Prenatal depression and cortical thickness
The association between the average level of maternal depressive symptoms during pregnancy and child cortical thickness, adjusting for gestational age at birth, age of the child, sex, handedness, birth outcome and current maternal depressive symptoms (Supplement) is presented in Figure 1. The figure illustrates that 12% of the whole cortex is significantly thinner in children exposed to prenatal maternal depressive symptoms (Table 2; Average column). The effect is spread among the frontal (14%), parietal (12%) and the occipital cortices (16%). The right frontal lobe (20%) is most significantly associated with maternal prenatal depressive symptoms. Children exposed to higher levels of prenatal maternal depressive symptoms have a significantly thinner lateral surface of the right frontal lobe with cortical thinning in nearly 100% of the right frontal pole (Table 3). Thinner medial and lateral surfaces of the frontal cortex in the right hemisphere are associated with prenatal exposure to maternal depressive symptoms. The paracentral region of the medial frontal lobe is the only frontal area that is bilaterally thinner in children exposed to high levels of prenatal maternal depressive symptoms.
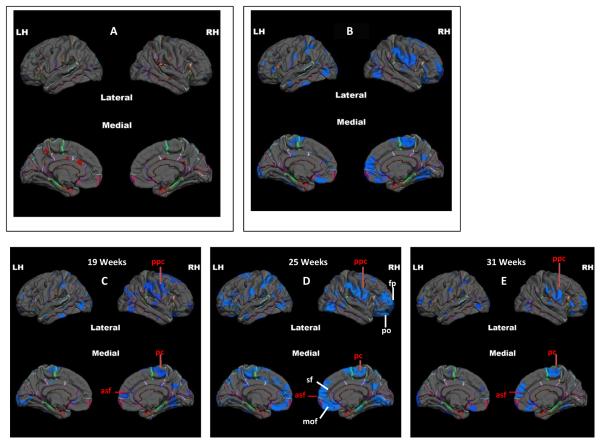
Figure 1.
Pial maps that illustrate the FDR corrected relation between cortical thickness in children and fetal exposure to maternal depression across gestation. Less than 0.7 percent of the cortex is thicker in children whose mother's reported higher levels of depressive symptoms (Figure 1A: thicker-hot colors) compared with massively thinner cortex in children exposed to higher levels of maternal prenatal depressive symptoms (Figure 1B: thinner-cool colors). Figures 1C–1E illustrate cortical thinning in children associated with fetal exposure to maternal depression at discrete gestational intervals. (LH=Left Hemisphere; RH= right hemisphere). Labels in red indicate major areas of thinning that are shared at the 3 gestational intervals (pcc=pre and post-central in the lateral view; pc= paracentral in the medial view; asf= anterior superiour frontal in the medial view). Labels in white indicate major areas that are significantly thinner and unique to fetal exposure to maternal depressive symptoms at 25 weeks gestation (mof= medial orbital frontal lobe; sf=superior frontal lobe; po=paraorbitalis; fp=frontal pole).
Table 2.
Percentage of whole cortex and the different cortical regions that are significantly thinner in children exposed as fetuses to maternal depressive symptoms across gestation (Average) and at three separate gestational intervals.
| Average | 19 Weeks | 25 Weeks | 31 Weeks | |
|---|---|---|---|---|
| Whole Brain | 12 | 8 | 19 | 7 |
| Frontal Cortex | 14 | 8 | 24 | 10 |
| Parietal Cortex | 12 | 10 | 19 | 5 |
| Temporal Cortex | 7 | 6 | 10 | 5 |
| Occipital Cortex | 16 | 14 | 20 | 9 |
| Cingulate/Limbic | 4 | 3 | 13 | 3 |
Table 3.
Percentage of structures of the frontal lobe that are thinner in children exposed to maternal depression averaged across gestation (top) and at 25 weeks gestation (bottom). The results are presented for the left and right hemisphere and for lateral and medial surfaces.
| AVERAGE ACROSS GESTATION | |||
|---|---|---|---|
| LH Medial Surface | Percent of Structure | RH Medial Surface | Percent of Structure |
| Orbital Frontal | 17 | Orbital Frontal | 40 |
| Paracentral | 32 | Paracentral | 35 |
| LH Lateral Surface | Percent of Structure | RH Lateral Surface | Percent of Structure |
| Superior Frontal | 6 | Superior Frontal | 19 |
| Rostral Middle Frontal | 6 | Rostral Middle Frontal | 17 |
| Frontal Pole | 0 | Frontal Pole | 99 |
| Pars Triangularis | 16 | Pars Triangularis | 32 |
| Parsorbitalis | 3 | Parsorbitalis | 30 |
| Lateral Orbital Frontal | 1 | Lateral Orbital Frontal | 13 |
| Parsopercularis | 0 | Parsopercularis | 7 |
| Caudal Middle Frontal | 4 | Caudal Middle Frontal | 9 |
| Precentral | 9 | Precentral | 19 |
| Insula | 0 | Insula | 11 |
| 25 WEEKS GESTATION | |||
|---|---|---|---|
| LH Medial Surface | Percent of Structure | RH Medial Surface | Percent of Structure |
| Medial Orbital Frontal | 47 | Medial Orbital Frontal | 74 |
| Paracentral | 54 | Paracentral | 23 |
| LH Lateral Surface | Percent of Structure | RH Lateral Surface | Percent of Structure |
| Superior Frontal | 26 | Superior Frontal | 26 |
| Rostral Middle Frontal | 17 | Rostral Middle Frontal | 37 |
| Frontal Pole | 0 | Frontal Pole | 100 |
| Pars Triangularis | 33 | Pars Triangularis | 45 |
| Parsorbitalis | 20 | Parsorbitalis | 44 |
| Lateral Orbital Frontal | 7 | Lateral Orbital Frontal | 29 |
| Parsopercularis | 9 | Parsopercularis | 20 |
| Caudal Middle Frontal | 3 | Caudal Middle Frontal | 21 |
| Precentral | 10 | Precentral | 21 |
| Insula | 3 | Insula | 12 |
There are broadly distributed significant associations between prenatal exposure to maternal depressive symptoms and cortical thinning at each gestational period. The association between fetal exposure to maternal depressive symptoms and cortical thinning was identical across gestation in the supramarginal, postcentral and paracentral areas (Figures 1C–E). The strongest association between cortical thinning and maternal depressive symptoms however, was at 25 weeks gestation (Tables 2 and 3). Nineteen percent of the whole cortex is thinner in children whose mothers report elevated levels of depression at 25 weeks gestation compared with 8 percent at 19 weeks and 7 percent at 31 weeks. The association between cortical thinning and fetal exposure to maternal depressive symptoms at 25 weeks is more bilateral and stronger in some regions than the associations with depressive symptoms averaged across gestation (Table 3). The effects at 25 weeks are strongest in the right hemisphere and in the frontal lobes.
Analysis within the major cortical lobes indicates that the medial and lateral surfaces of frontal areas, especially in the right hemisphere, are predominantly thinner in children exposed to maternal depressive symptoms at 25 gestational weeks. The entire lateral surface of the right frontal pole and 74% of the medial orbital frontal cortex are significantly thinner in children exposed to prenatal depressive symptoms at 25 weeks. Moreover the left medial frontal cortex (48–54% of the surface area) also is influenced by fetal exposure to maternal reports of depression. As presented in Table 3 (bottom panels), a large number of areas are thinner in association with maternal depression at 25 weeks.
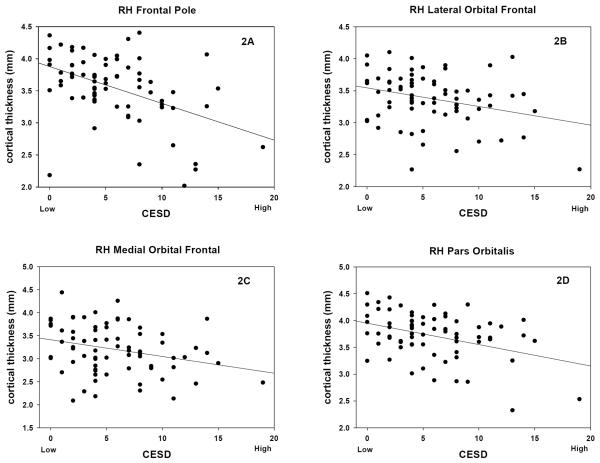
Several of many significant associations between maternal depressive symptoms at 25 weeks gestation and cortical thickness are illustrated in figure 2. Figure 2A shows the significant association between the thickness of the medial surface of the right frontal pole and levels of maternal depression at 25 weeks gestation. Figures 2B–D illustrate other areas of the frontal lobes that are significantly thinner in children who were exposed to higher levels of maternal depression at 25 weeks gestation.
Figure 2.
Scatter plots of cortical thickness in right frontal lobes of children with fetal exposure to maternal depressive symptoms (expressed as total score) at 25 weeks gestation. [RH Frontal Pole, r71 = −0.46, p<0.001; RH Lateral Orbital Frontal, r71 = −0.30, p=0.01; RH Medial Orbital Frontal, r71 = −0.28, p=0.01, RH Pars Orbitalis, r71 = −0.39, p<0.001]
Mediational analysis
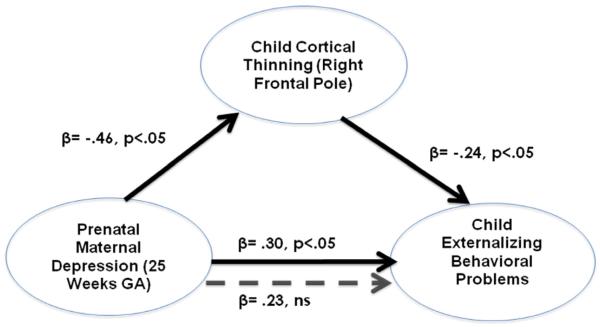
There were no significant relations between prenatal maternal depressive symptoms at the three gestational intervals and maternal ratings of childhood affective disorders (CBCL; adjusted for current levels of maternal depression). The non-significant correlations ranged from 0.16 to −0.06 (p>.15 to p> .84). None of the children were rated (range 50–70) in the clinically impaired (>70) range. After controlling for current maternal affect, higher levels of prenatal maternal depression were associated with child externalizing problems. Because exposure to prenatal maternal depressive symptoms was associated with both child externalizing behavior (range 33–66) and cortical thinning in frontal regions, we evaluated whether cortical thinning mediated[56] the association between exposure to prenatal depression and externalizing problems. Figure 3 illustrates that the significant association between levels of prenatal depression and child externalizing behavior at 25 weeks is mediated by cortical thinning in the right frontal pole. Moreover, when the prefrontal regions that were most strongly (r's >.23, p's <.05) associated with prenatal maternal depression at 25 weeks (right frontal pole, right lateral orbital frontal cortex and the right medial orbital frontal cortex) are included in the model, the relation between prenatal depression and child behavior was no longer significant (B=0.17, p=.19). This final model accounts for 12% of child behavioral problems and supports the argument that the association between maternal depressive symptoms and child behavioral problems is partially explained by the effects of fetal exposures on the development of the prefrontal cortex.
Figure 3.
Illustrates the mediation of prenatal maternal depression on child externalizing behavior by child cortical thinning in the right frontal pole. The mediational hypothesis is tested following the four-step suggestions of Baron & Kenny(56). Mediation is supported because (i) prenatal maternal depressive symptoms (at 25 weeks GA) is associated significantly (solid arrows) with cortical thinning and; (ii) with child externalizing behavior; (iii) child cortical thinning is associated with child externalizing behavior and; (iv) the association between prenatal maternal depressive symptoms and child externalizing behavior is not significant (doted arrow) after controlling for cortical thinning.
DISCUSSION
This is the first report of persisting neurological consequences for children born to mothers who reported symptoms of depression during pregnancy. Significant associations between maternal reports of prenatal depressive symptoms and cortical thickness in children were observed in >12% of the whole cortex. The largest associations between prenatal maternal depression and cortical thickness were in the prefrontal, the medial postcentral and the lateral ventral pre and post central regions of the right hemisphere. Meditational analysis indicated that thinning in prefrontal regions may underlie the association between levels of prenatal depression and child externalizing problems. There was no evidence the cortex was thicker in association with prenatal maternal depression.
The cortical areas significantly associated with maternal depressive symptoms at each gestational interval included the pre- and postcentral regions of the right hemisphere. The precentral area is the primary motor cortex and is responsible for executing movements through connections to the spinothalamic tract. The postcentral region is a topographically organized, functionally defined area responsible for integrating somatosensory information such as touch and proprioception. Neither motor nor somatosensory functions were assessed in the children included in the current study. There is fMRI evidence however, that depression is associated with activity of the “medial” cortex. This area is less active in depressed patients when they are asked to self-reflect but more active in tasks without self-reference[57]. It is possible that the similar patterns of cortical thinning observed at each gestational interval reflect a neurological index of familial or genetic risk associated with maternal depression.
The strongest association between maternal depressive symptoms and cortical thickness was observed at 25 gestational weeks. Fetal exposure to maternal depressive symptoms at 25 weeks was associated with cortical thinning in 19% of the whole brain and 24% of the frontal lobes. Cortical thinning was significantly associated with degree of maternal depression in the superior, medial orbital and frontal pole regions of the prefrontal cortex primarily in the right hemisphere only at 25 weeks. There is considerable overlap between the findings observed here and the reports of thinning in the pre and post central, frontal and prefrontal areas of the right hemisphere in young patients with “attenuated” syndromes[58] and in a mixed group of children and adults with familial risk for major depression but without a history of depression or current depressive symptoms[32]. Cortical thinning has been reported in adults[34,36], adolescents and young adults[58] and in children with depression. Although there are differences across studies, in general there is agreement that depression is associated with region-specific cortical thinning, reduced volume[32,34,58–59] and less activity[60,61] of the frontal and prefrontal cortex, especially in the right hemisphere.
Meta-analysis of fMRI studies have implicated reduced activity in the frontal areas of depressed patients[62,63] and this association was independent of medication use or current depressive state[64]. Peterson and Weissman[33] concluded that a profile of cortical thinning constituted a brain-based endophenotype for depression because a thinner cortex was observed in high risk individuals who have never had or been treated for depression or anxiety. Our findings complement these conclusions. First, there are areas in which the association of depression and cortical thinning overlap at all three gestational intervals. This is plausible support that these overlapping areas may represent familial risk for depression without evidence of current clinically significant symptoms in the mother or child. Second, the unique profile observed at 25 gestational weeks may reflect the independent contribution to neurological risk that is associated with fetal exposure to maternal depressive symptoms and the physiological changes that may affect the developing fetal brain. Third, the observed effects remained after controlling for current levels of maternal depression reducing the possibility that postnatal factors associated with maternal emotional state might influence children's cortical structure. The distribution of prenatal and current levels of maternal depression was skewed in the euthymic direction further eliminating the argument that only clinically significant maternal depressive symptoms influence child outcome. Fourth, none of the children in our study had clinically significant affective symptoms. The association between fetal exposure to maternal depressive symptoms and cortical thickness was not related to current childhood affective symptoms.
There is substantial evidence that fetal exposures to a variety of psychobiological stressors at specific intervals during gestation are associated with distinctive patterns of behavior and brain structures that persist into preadolescence[65–67]. We have reported that the behavioral consequences of fetal exposure to maternal depression are different and independent from the patterns from fetal exposure to maternal reports of anxiety associated with pregnancy[25,68]. Moreover, we reported that fetal exposure to pregnancy specific anxiety only at 19 weeks gestation is associated with decreased cortical volume (voxel-based morphology) in areas extending from the frontal to occipital regions[21]. The evidence reported here generally supports the previous finding that fetal exposure to maternal psychosocial stress (depression in this case) is associated with decreased cortical thickness or volume. The new findings are that the association between depression and cortical thickness were observed at each gestational interval and that exposure at 25 weeks gestation was a sensitive period for the fetus resulting in broad cortical patterns of thinning.
The precise mechanism by which the pregnant woman communicates depressive symptoms to her fetus is unknown, but one possibility is that prenatal depression exposes the fetus to dysregulated levels of maternal stress hormones. We did not discover a significant association between maternal depressive symptoms and maternal salivary cortisol levels assessed a single time at each gestational interval in our sample (Supplement). However, we are reluctant to draw the conclusion based on our methods that maternal cortisol is not an important signal to the fetus of maternal depressive state. For instance, morning salivary cortisol levels are higher in patients diagnosed with depression than they are in never-depressed individuals[69–71] and different forms of depression (melancholic and atypical) is associated either with hyper- or hypoactivation of the HPA axis[72]. Hyperactivity of the HPA axis in major depression is one of the most reliably reported neurobiological characteristics of affective disorders[73] and when depression is linked with elevated or temporally dysregulated cortisol secretion, the symptoms are more severe[74]. Symptoms of depression can be induced in experimental animals by intracerebroventricular injection of corticotropin-releasing-hormone (CRH) and depression is a frequent side effect of glucocorticoid treatment and is a symptom of Cushing's syndrome in humans[75]. Fetal exposure to maternal depression is associated with increased methylation of the glucocorticoid receptor gene in neonates and with increased HPA responses to stress or adversity[76]. The plausibility of a connection between maternal depression and the maternal HPA axis is supported by findings that infants born to women with symptoms of depression, even in the absence of major depression, have significant ACTH elevation at birth[77]. These results suggest that fetal exposure to maternal affect may be communicated to the fetus through the maternal stress system that establishes a developmental trajectory which includes reduced cortical thickness during preadolescence.
There are substantial linkages between the stress system and the areas of the brain associated with fetal exposure to maternal depression. A corticolimibic network is implicated in emotional regulation in depressive disorders and areas of the prefrontal region including dorsal and ventral lateral prefrontal cortex, the medial prefrontal cortex, and especially the orbitofrontal cortex, play an inhibitory role over subcortical areas in healthy subjects[61]. There is strong evidence that the fetal prefrontal cortex is sensitive to maternal distress[78], is involved in the regulation of stress hormone secretion[79] and has an abundance of glucocorticoid receptors[80]. Fetal exposure to elevated levels or dysregulation of cortisol alters limbic structures in animal models and humans including reduction of hippocampal size[81], cortical thinning in the rostral anterior cingulate[82], neuronal loss in the dentate gyrus[83] and increased volume in the amygdala[84]. Thus there is strong evidence that the neurological consequences of fetal exposure to maternal depression can be transmitted through the maternal stress system.
One significant finding from this study was that cortical thinning in children was most strongly associated with fetal exposure to maternal depressive symptoms at 25 weeks gestation. There is evidence that ~25 weeks gestation is a sensitive interval for the effects of maternal depression on fetal development. Both motor and mental development among infants was associated with maternal prenatal reports of depression[18] and this effect was strongest at 25 weeks gestation. Maternal levels of CRH at 25 weeks gestation predict infant temperament[85] and this stress signal[86] and other stress hormones[87] at 25 weeks predict maternal postpartum depression. All major stress hormones increase across gestation, and the most rapid changes occur around late second and early third trimester[18]. The human fetus may be especially vulnerable to rapid changes in maternal stress signals at 25 weeks gestation because there are crucial developments, such as expression of structural asymmetries in the nervous system through midgestation that are essential for subsequent neurological function[88]. There is enormous growth of the fetal nervous system during midgestation; between gestational weeks 20–24, axons form synapses with the cortical plate organizing cortical circuits[89,90] and by gestational week 28, the proliferation of neurons is 40% greater than in the adult[90–92]. All primary sulci are formed during fetal life with development of the superior frontal, rhinal, temporal and pre- and postcentral sulci complete by 25 to 26 weeks gestation[93]. The molecular level of the human nervous system, especially in the frontal lobes, is shaped during midgestation by regressive apoptotic mechanisms[94]. Cell decline in Layer 1 during this time of enormous growth, is due to cell death in contrast to cell migration which occurs later. Transcriptome studies of the human brain indicate that the majority of the genes (>76%)[88] are expressed in many brain regions and the largest temporal-spatial differences in gene expression across the brain are observed before birth[95]. There is approximately two-fold greater gene expression in the fetal brain (primarily the frontal lobe) compared with the adult[88] and some genes are expressed at midgestation and then decline by early childhood[95]. The fetal brain at midgestation is rapidly developing and because of these critical changes it is a likely target for organizing and disorganizing programming influences.
Cortical thinning in children may reflect accelerated maturation. We reported that widespread cortical thinning in preadolescent children was associated with advancing age[96]. There is separate evidence that exposure to psychological adversity early in development is associated with accelerated maturation influencing viability[97], growth and reproduction[98–100]. Our findings are adjusted for age so a strictly maturational influence on the nervous system can be ruled out. Functionally, cortical thinning may reflect the density of connections between neurons, decreased dendritic arbor of neurons or decreased myelination of axons that lie within the grey matter[101] each of which is related to a significant impairment in information processing and may be a risk factor for cognitive and emotional problems[102]. Inattention and poor visual memory have been reported in individuals at risk for depression and who had patterns of cortical thinning similar to those observed in this study[32].
Our finding of increased incidence of externalizing problems in children exposed to prenatal maternal depression is consistent with reports that prenatal maternal depression significantly increased the risk for externalizing behaviors in toddlers[29] and adolescents[31]. Moreover, the association between prenatal exposure to maternal depression and externalizing problems was partially mediated by cortical thinning in the frontal lobes. When the contribution of cortical thinning in the frontal lobes was included in the meditational model, the significant association between prenatal depression and externalizing problems disappeared. This argues that prenatal depression influences cortical thinning with the consequence of in an increased risk for externalizing problems in the children.
LIMITATIONS and IMPLICATIONS
The women in this study were not clinically depressed and their self-reported symptoms were within a narrow range. It is unknown if the associations between maternal depressive symptoms and child neurological integrity would be stronger in a clinical population. Also, it is unknown if the consequences of fetal exposure to maternal depression has clinically significant consequences for the child but we are currently following these children to assess this possibility. Despite these limitations, it is important to recognize that assessment and intervention in pregnant women with symptoms of prenatal depression will reduce the risk of postpartum depression and protect the developing fetal nervous system. Because the pattern of cortical thinning observed in children with prenatal exposure to maternal depressive symptoms is similar to the pattern exhibited by individuals who are clinically depressed it is plausible that the pattern may be a prodromal risk factor for later-life-affective problems. Children considered at risk for affective and conduct disorders because of maternal emotional problems during pregnancy can be indentified and provided preventative treatment.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by National Institute of Health grants NS-41298, HD-51852 and HD-28413 to CAS and Conte Center award MH-96889. The funding sources did not contribute to the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
We are grateful for the expert assistance of compensated staff, Kendra Leak, BA (participant recruitment, scheduling), Cheryl Crippen, PhD, (data collection, preliminary data analysis), Christina Canino, MA, (data collection, database management), Natalie Hernandez, BA, (subject recruitment, data collection) and Mariann Howland, BA, (manuscript preparation) and to the families who participated in our studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The results reported here were included as part of the Maternal Stress in Pregnancy symposium presented at the 166th Annual Meeting of the American Psychiatric Association, May, 2013.
FINANCIAL DISCLOSURES. All authors collaborated in the collection and analysis of the data and reviewed and edited iterations of the manuscript. The principal investigator is independent of any commercial funder and had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Fihrer I, McMahon CA, Taylor AJ. The impact of postnatal and concurrent maternal depression on child behaviour during the early school years. Journal of Affective Disorders. 2009;119:116–23. doi: 10.1016/j.jad.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Kurstjens S, Wolke D. Effects of maternal depression on cognitive development of children over the first 7 years of life. Journal of Child Psychology and Psychiatry. 2001;42:623–36. [PubMed] [Google Scholar]
- 3.Conroy S, Pariante CM, Marks MN, Davies HA, Farrelly S, Schacht R, Moran P. Maternal psychopathology and infant development at 18 months: The impact of maternal personality disorder and depression. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51:51–61. doi: 10.1016/j.jaac.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 4.McMahon C, Barnett B, Kowalenko N, Tennant C, Don N. Postnatal depression, anxiety and unsettled infant behaviour. Aust N Z J Psychiatry. 2001;35:581–8. doi: 10.1080/0004867010060505. [DOI] [PubMed] [Google Scholar]
- 5.Zelkowitz P, Papageorgiou A, Bardin C, Wang T. Persistent maternal anxiety affects the interaction between mothers and their very low birthweight children at 24 months. Early Human Development. 2009;85:51–58. doi: 10.1016/j.earlhumdev.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Cornish AM, McMahon CA, Ungerer JA, Barnett B, Kowalenko N, Tennant C. Postnatal depression and infant cognitive and motor development in the second postnatal year: The impact of depression chronicity and infant gender. Infant Behavior and Development. 2005;28:407–417. [Google Scholar]
- 7.Murray L, Arteche A, Fearon P, Halligan S, Croudace T, Cooper P. The effects of maternal postnatal depression and child sex on academic performance at age 16 years: A developmental approach. J Child Psychol Psychiatry. 2010;51:1150–9. doi: 10.1111/j.1469-7610.2010.02259.x. [DOI] [PubMed] [Google Scholar]
- 8.Brennan PA, Hammen C, Andersen MJ, Bor W, Najman JM, Williams GM. Chronicity, severity, and timing of maternal depressive symptoms: Relationships with child outcomes at age 5. Developmental Psychology. 2000;36:759. doi: 10.1037//0012-1649.36.6.759. [DOI] [PubMed] [Google Scholar]
- 9.Murray L, Sinclair D, Cooper P, Ducournau P, Turner P, Stein A. The socioemotional development of 5-year-old children of postnatally depressed mothers. J Child Psychol Psychiatry. 1999;40:1259–71. [PubMed] [Google Scholar]
- 10.Hay DF, Kumar R. nterpreting the effects of mothers' postnatal depression on children's intelligence: A critique and re-analysis. Child Psychiatry Hum Dev. 1995;25:165–81. doi: 10.1007/BF02251301. [DOI] [PubMed] [Google Scholar]
- 11.Hay DF, Pawlby S, Sharp D, Asten P, Mills A, Kumar R. Intellectual problems shown by 11-year-old children whose mothers had postnatal depression. J Child Psychol Psychiatry. 2001;42:871–89. doi: 10.1111/1469-7610.00784. [DOI] [PubMed] [Google Scholar]
- 12.Verbeek T, Bockting CLH, van Pampus MG, Ormel J, Meijer JL, Hartman CA, Burger H. Postpartum depression predicts offspring mental health problems in adolescence independently of parental lifetime psychopathology. Journal of Affective Disorders. 2012;136:948–954. doi: 10.1016/j.jad.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 13.Moehler E, Kagan J, Parzer P, Brunner R, Reck C, Wiebel A, et al. Childhood behavioral inhibition and maternal symptoms of depression. Psychopathology. 2007;40:446–52. doi: 10.1159/000107429. [DOI] [PubMed] [Google Scholar]
- 14.Marcus Y, Altman-Gueta H, Wolff Y, Gurevitz M. Rubisco mutagenesis provides new insight into limitations on photosynthesis and growth in Synechocystis PCC6803. J Exp Bot. 2011;62:4173–82. doi: 10.1093/jxb/err116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, et al. Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex. 2001;11:552–57. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- 16.Gerardin P, Wendland J, Bodeau N, Galin A, Bialobos S, Tordjman S, et al. Depression during pregnancy: Is the developmental impact earlier in boys? A prospective case-control study. J Clin Psychiatry. 2011;72:378–87. doi: 10.4088/JCP.09m05724blu. [DOI] [PubMed] [Google Scholar]
- 17.Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–26. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandman CA, Davis EP, Glynn LM. Prescient human fetuses thrive. Psychological Science. 2012;23:93–100. doi: 10.1177/0956797611422073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development. 2010;81:131–48. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blair MM, Glynn LM, Sandman CA, Davis EP. Prenatal maternal anxiety and early childhood temperament. Stress. 2011;14:644–51. doi: 10.3109/10253890.2011.594121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buss C, Davis EP, Muftuler LT, Head K, Sandman CA. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6-9-year-old children. Psychoneuroendocrinology. 2010;35:141–53. doi: 10.1016/j.psyneuen.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunkel Schetter C. Stress processes in pregnancy and preterm birth. Current Directions in Psychological Science. 2009;18:204–9. [Google Scholar]
- 23.Dunkel Schetter C. Psychological science on pregnancy: Stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol. 2011;62:531–58. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- 24.Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: How and why? Journal of Child Psychology and Psychiatry. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:737–46. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- 26.Davis EP, Snidman N, Wadhwa PD, Dunkel Schetter C, Glynn L, Sandman CA. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy. 2004;6:319–31. [Google Scholar]
- 27.Rotenberg S, McGrath JJ, Roy-Gagnon MH, Tu MT. Stability of the diurnal cortisol profile in children and adolescents. Psychoneuroendocrinology. 2012;37:1981–9. doi: 10.1016/j.psyneuen.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerr DC, Leve LD, Harold GT, Natsuaki MN, Neiderhiser JM, Shaw DS, Reiss D. Influences of biological and adoptive mothers' depression and antisocial behavior on adoptees' early behavior trajectories. J Abnorm Child Psychol. 2013;41:723–34. doi: 10.1007/s10802-013-9711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barker ED, Jaffee SR, Uher R, Maughan B. The contribution of prenatal and postnatal maternal anxiety and depression to child maladjustment. Depression and Anxiety. 2011;28:696–702. doi: 10.1002/da.20856. [DOI] [PubMed] [Google Scholar]
- 30.Evans J, Melotti R, Heron J, Ramchandani P, Wiles N, Murray L, Stein A. The timing of maternal depressive symptoms and child cognitive development: A longitudinal study. Journal of Child and Adolescent Psychiatry. 2011;53:632–40. doi: 10.1111/j.1469-7610.2011.02513.x. [DOI] [PubMed] [Google Scholar]
- 31.Korhonen M, Luoma I, Salmelin R, Tamminen T. A longitudinal study of maternal prenatal, postnatal and concurrent depressive symptoms and adolescent well-being. Journal of Affective Disorders. 2012;136:680–92. doi: 10.1016/j.jad.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Peterson BS, Warner V, Bansal R, Zhu H, Hao X, Liu J, et al. Cortical thinning in persons at increased familial risk for major depression. Proceedings of the National Academy of Sciences. 2009;106:6273–8. doi: 10.1073/pnas.0805311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson BS, Weissman MM. A brain-based endophenotype for major depressive disorder. Annual Review of Medicine. 2011;62:461–74. doi: 10.1146/annurev-med-010510-095632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu PC, Chen LF, Hsieh JC, Bai YM, Li CT, Su TP. Regional cortical thinning in patients with major depressive disorder: A surface-based morphometry study. Psychiatry Research:Neuroimaging. 2012;202:206–13. doi: 10.1016/j.pscychresns.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Fallucca E, MacMaster FP, Haddad J, Easter P, Dick R, May G, et al. Distinguishing between major depressive disorder and obsessive-compulsive disorder in children by measuring regional cortical thickness. Archives of General Psychiatry. 2011;68:527–33. doi: 10.1001/archgenpsychiatry.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyoo IK, Sung YH, Dager SR, Friedman SD, Lee JY, Kim SJ, et al. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disorders. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- 37.Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences. 2011;108:14324–9. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tollenaar MS, Beijers R, Jansen J, Riksen-Walraven JM, de Weerth C. Maternal prenatal stress and cortisol reactivity to stressors in human infants. Stress. 2011;14:53–65. doi: 10.3109/10253890.2010.499485. [DOI] [PubMed] [Google Scholar]
- 39.Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H. A meta analysis of neurofunctional imaging studies of emotion and cognition in major depression. NeuroImage. 2012;61:677–85. doi: 10.1016/j.neuroimage.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 40.O'Brien GD, Queenan JT, Campbell S. Assessment of gestational age in the second trimester by real-time ultrasound measurement of the femur length. Am J Obstet Gynecol. 1981;139:540–5. doi: 10.1016/0002-9378(81)90514-7. [DOI] [PubMed] [Google Scholar]
- 41.Hobel CJ. Identification of the patient at risk. In: Schwartz RH, Schneider J, editors. Perinatal medicine: Management of the high risk fetus and neonate. Williams & Wilkins; Baltimore: 1982. pp. 3–28. [Google Scholar]
- 42.Hobel CJ, Youkeles L, Forsythe A. Prenatal and intrapartum high-risk screening. II. Risk factors reassessed. Am J Obstet Gynecol. 1979;135:1051–6. doi: 10.1016/0002-9378(79)90735-x. [DOI] [PubMed] [Google Scholar]
- 43.Santor DA, Coyne JC. Shortening the CES-D to improve its ability to detect cases of depression. Psychological Assessment. 1997;9:233–43. [Google Scholar]
- 44.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement. 1977;1:385–401. [Google Scholar]
- 45.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty five years of evaluation. Clinical psychology review. 1988;8:77–100. [Google Scholar]
- 46.Armstrong J, Goldstein L. MacArthur Foundation Research Network on Psychopathology and Development, University of Pittsburgh. 2003. Manual for the MacArthur health and behavior questionnaire (HBQ 1.0) [Google Scholar]
- 47.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 48.Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms and profiles: An integrated system of multi-informant assessment. University of Vermont, Reaserch Center for Children, Youth, & Families; Burlington, VT: 2001. [Google Scholar]
- 49.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 50.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat D, et al. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 51.Fischl B, Salat D, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 52.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 53.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. Journal of Cognitive Neuroscience. 1993;5:162–76. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- 55.Fischl B, Sereno MI, Tootell RBH, Dale AM. High resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8:272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 57.Johnson SB, Blum RW, Giedd JN. Adolescent maturity and the brain: The promise and pitfalls of neuroscience research in adolescent health policy. J Adolesc Health. 2009;45:216–21. doi: 10.1016/j.jadohealth.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lagopoulos J, Hermens DF, Naismith SL, Scott EM, Hickie IB. Frontal lobe changes occur early in the course of affective disorders in young people. BMC Psychiatry. 2012;12:1–7. doi: 10.1186/1471-244X-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sacher J, Neumann J, Fünfstück T, Soliman A, Villringer A, Schroeter ML. Mapping the depressed brain: A meta analysis of structural and functional alterations in major depressive disorder. Journal of Affective Disorders. 2012;140:142–48. doi: 10.1016/j.jad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 60.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: A meta-analysis and new integration of baseline activation and neural response data. American Journal of Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- 61.Vizueta N, Rudie JD, Townsend JD, Torrisi S, Moody TD, Bookheimer SY, Altshuler LL. Regional fMRI hypoactivation and altered functional connectivity during emotion processing in nonmedicated depressed patients with bipolar II disorder. The American Journal of Psychiatry. 2012;169:831–40. doi: 10.1176/appi.ajp.2012.11030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diener SJ, Wessa M, Ridder S, Lang S, Diers M, Steil R, Flor H. Enhanced stress analgesia to a cognitively demanding task in patients with posttraumatic stress disorder. Journal of Affective Disorders. 2012;136:1247–51. doi: 10.1016/j.jad.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 63.Fu CHY, Steiner H, Costafreda SG. Predictive neural biomarkers of clinical response in depression: A meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiology of Disease. 2013;52:75–83. doi: 10.1016/j.nbd.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 64.van Tol MJ, van der Wee NJ, van den Heuvel OA, Nielen MM, Demenescu LR, Aleman A, et al. Regional brain volume in depression and anxiety disorders. Archives of General Psychiatry. 2010;67:1002–11. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- 65.Sandman CA, Davis EP, Buss C, Glynn LM. Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology. 2012;5:675–690. doi: 10.1159/000327017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sandman CA, Davis EP. Neurobehavioral risk is associated with gestational exposure to stress hormones. Expert Review of Endocrinology & Metabolism. 2012;7:445–459. doi: 10.1586/eem.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sandman CA, Davis EP. Gestational stress influences cognition and behavior. Future Neurology. 2010;5:675–90. [Google Scholar]
- 68.Davis EP, Sandman CA. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology. 2012;37:1224–33. doi: 10.1016/j.psyneuen.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knorr U, Vinberg M, Kessing LV, Wetterslev J. Salivary cortisol in depressed patients versus control persons: A systematic review and meta analysis. Psychoneuroendocrinology. 2010;35:1275–86. doi: 10.1016/j.psyneuen.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 70.Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom Med. 2011;73:114–26. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- 71.Foland-Ross LC, Hardin MG, Gotlib IH. Neurobiological markers of familial risk for depression. Curr Top Behav Neurosci. 2013;14:181–206. doi: 10.1007/7854_2012_213. [DOI] [PubMed] [Google Scholar]
- 72.Schutter DJ. The cerebello hypothalamic–pituitary–adrenal axis dysregulation hypothesis in depressive disorder. Medical Hypotheses. 2012;79:779–83. doi: 10.1016/j.mehy.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 73.Behnken A, Bellingrath S, Symanczik JP, Rieck MJ, Zavorotnyy M, Domschke K, et al. Associations between cognitive performance and cortisol reaction to the DEX/CRH test in patients recovered from depression. Psychoneuroendocrinology. 2013;38:447–54. doi: 10.1016/j.psyneuen.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 74.Brown ES, Varghese FP, McEwen BS. Association of depression with medical illness: Does cortisol play a role? Biol Psychiatry. 2004;55:1–9. doi: 10.1016/s0006-3223(03)00473-6. [DOI] [PubMed] [Google Scholar]
- 75.Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: Focus on the human hypothalamus. Brain Research Reviews. 2008;57:531–53. doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 77.Marcus SM, Lopez JF, McDonough S, MacKenzie MJ, Flynn HA, Neal CR, et al. Depressive symptoms during pregnancy: Impact on neuroendocrine and neonatal outcomes. Infant Behavior and Development. 2011;34:26–34. doi: 10.1016/j.infbeh.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barbazanges A, Piazza PV, Le Moal M, Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci. 1996;16:3943–9. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, et al. Deactivation of the limbic system during acute psychosocial stress: Evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;63:234–40. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 80.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 81.Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB, Farrel EB. Brain damage induced by prenatal exposure to dexamethasone in fetal macaques. I. Hippocampus. Brain Research Developmental Brain Research. 1990;53:157–67. doi: 10.1016/0165-3806(90)90002-g. [DOI] [PubMed] [Google Scholar]
- 82.Davis EP, Sandman CA, Buss C, Wing DA, Head K. Fetal Glucocorticoid Exposure Is Associated with Preadolescent Brain Development. Biological Psychiatry. 2013;74:647–655. doi: 10.1016/j.biopsych.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tauber SC, Schlumbohm C, Schilg L, Fuchs E, Nau R, Gerber J. Intrauterine exposure to dexamethasone impairs proliferation but not neuronal differentiation in the dentate gyrus of newborn common marmoset monkeys. Brain Pathology. 2006;16:209–17. doi: 10.1111/j.1750-3639.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E1312–9. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davis EP, Townsend EL, Gunnar MR, Guiang SF, Lussky RC, Cifuentes RF, Georgieff MK. Antenatal betamethasone treatment has a persisting influence on infant HPA axis regulation. Journal of Perinatology. 2006;26:147–53. doi: 10.1038/sj.jp.7211447. [DOI] [PubMed] [Google Scholar]
- 86.Yim IS, Glynn LM, Dunkel-Schetter C, Hobel CJ, Chicz-DeMet A, Sandman CA. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry. 2009;66:162–9. doi: 10.1001/archgenpsychiatry.2008.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yim IS, Glynn LM, Schetter CD, Hobel CJ, Chicz-Demet A, Sandman CA. Prenatal beta-endorphin as an early predictor of postpartum depressive symptoms in euthymic women. J Affect Disord. 2010;125:128–33. doi: 10.1016/j.jad.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson MB, Kawasawa YI, Mason CE, Krsnik Ž, Coppola G, Bogdanović D, et al. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62:494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kostovic I, Judas M, Rados M, Hrabac P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 2002;12:536–44. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- 90.Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 91.Becker LE, Armstrong DL, Chan F, Wood MM. Dendritic development in human occipital cortical neurons. Brain Res. 1984;315:117–24. doi: 10.1016/0165-3806(84)90083-x. [DOI] [PubMed] [Google Scholar]
- 92.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 93.Nishikuni K, Ribas GC. Study of fetal and postnatal morphological development of the brain sulci. J Neurosurg Pediatr. 2012;11:1–11. doi: 10.3171/2012.9.PEDS12122. [DOI] [PubMed] [Google Scholar]
- 94.Spreafico R, Arcelli P, Frassoni C, Canetti P, Giaccone G, Rizzuti T, et al. Development of layer I of the human cerebral cortex after midgestation: Architectonic findings, immunocytochemical identification of neurons and glia, and in situ labeling of apoptotic cells. The Journal of Comparative Neurology. 1999;410:126–42. doi: 10.1002/(sici)1096-9861(19990719)410:1<126::aid-cne11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 95.Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, et al. Spatio-temporal transcriptome of the human brain. Nature. 2011;478:483–9. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muftuler LT, Davis EP, Buss C, Head K, Hasso AN, Sandman CA. Cortical and subcortical changes in typically developing preadolescent children. Brain Research. 2011;1399:15–24. doi: 10.1016/j.brainres.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Denver RJ. Environmental stress as a developmental cue: Corticotropin-releasing hormone is a proximate mediator of adaptive phenotypic plasticity in amphibian metamorphosis. Hormones and Behavior. 1997;31:169–79. doi: 10.1006/hbeh.1997.1383. [DOI] [PubMed] [Google Scholar]
- 98.Coall DA, Chisholm JS. Evolutionary perspectives on pregnancy: Maternal age at menarche and infant birth weight. Soc Sci Med. 2003;57:1771–81. doi: 10.1016/s0277-9536(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 99.Coall DA, Chisholm JS. Reproductive development and parental investment during pregnancy: Moderating influence of mother's early environment. Am J Hum Biol. 2010;22:143–53. doi: 10.1002/ajhb.20965. [DOI] [PubMed] [Google Scholar]
- 100.Nettle D, Coall DA, Dickins TE. Birthweight and paternal involvement predict early reproduction in British women: Evidence from the National Child Development Study. Am J Hum Biol. 2010;22:172–9. doi: 10.1002/ajhb.20970. [DOI] [PubMed] [Google Scholar]
- 101.Lyall AE, Shi F, Geng X, Woolson S, Li G, Wang L, et al. Dynamic development of regional cortical thickness and surface area in early childhood. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu027. Epub ahead of print, PMID24591525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jacobs B, Schall M, Prather M, Kapler E, Driscoll L, Baca S, et al. Regional dendritic and spine variation in human cerebral cortex: A quantitative golgi study. Cerebral Cortex. 2001;11:558–71. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.