Abstract
Ultraviolet (UV) damage to the skin leads to the release of noncoding RNA (ncRNA) from necrotic keratinocytes that activates toll-like receptor 3 (TLR3). This release of ncRNA triggers inflammation in the skin following UV damage. Recently, TLR3 activation was also shown to aid wound repair and increase expression of genes associated with permeability barrier repair. Here, we sought to test if skin barrier repair after UVB damage is dependent on the activation of TLR3. We observed that multiple ncRNAs induced expression of skin barrier repair genes, that the TLR3 ligand Poly (I:C) also induced expression and function of tight junctions, and that the ncRNA U1 acts in a TLR3-dependent manner to induce expression of skin barrier repair genes. These observations were shown to have functional relevance as Tlr3−/− mice displayed a delay in skin barrier repair following UVB damage. Combined, these data further validate the conclusion that recognition of endogenous RNA by TLR3 is an important step in the program of skin barrier repair.
INTRODUCTION
Excessive exposure to UV causes damage to the skin resulting in painful sunburn and skin cancer. In the year 2000, ultraviolet (UV) light exposure was linked to 60,000 deaths worldwide and significant morbidity (>1.5 million disability-adjusted life years lost) (Lucas et al. 2006). Among the several forms of injury caused by UV light exposure, disruption of the skin permeability barrier must be responded to by subsequent repair (Haratake et al. 1997; Holleran et al. 1997). Previous studies have demonstrated that skin barrier repair is initiated following changes in the epidermal calcium gradient (Lee et al. 1992; Menon et al. 1992). Disruption of the calcium gradient results in changes in gene expression, epidermal lipid metabolism, and lamellar body secretion that help restore permeability barrier function to damaged skin. For example, disruption of the skin barrier caused by UVB exposure results in increases in lipid metabolism and lamellar body dynamics (Haratake et al. 1997; Holleran et al. 1997), and low doses of UVB induced epidermal lipid synthesis enzymes and antimicrobial peptides (Hong et al. 2008). Thus, although the antimicrobial and permeability barriers of the skin are often thought of as separate systems, many studies have shown that injury to the skin stimulates production of both structural and antimicrobial components of the barrier. This interaction demonstrates that the permeability and antimicrobial barriers of the skin are co regulated and dependent on one another (Dorschner et al. 2001; Aberg et al. 2007, 2008; Schauber et al. 2007; Ahrens et al. 2011).
While calcium sensing is instrumental in the barrier repair process, additional cellular mediators play key roles in this process (summarized in these reviews (Feingold et al. 2007; Feingold and Denda 2012) and primary articles (Jensen et al. 1999; Komuves et al. 2000; Ye et al. 2002; Hachem et al. 2003, 2006; Wang et al. 2004; Man et al. 2004; Schmuth et al. 2004; Lim et al. 2007; Demerjian et al. 2008; Sokabe et al. 2010; Mihara et al. 2011; Kida et al. 2012)). However, although many regulators of skin barrier repair have been investigated, the mechanisms that regulate skin barrier repair following UVB exposure are incompletely described.
Recently, the inflammatory response to UV damage was shown to be partially dependent on toll-like receptor 3 (TLR3) and its downstream signaling adaptor molecule TIR-domain-containing adapter-inducing interferon-β (TRIF) that acts by detection of the release of endogenous snRNA (Bernard et al. 2012). These observations were consistent with similar findings that TLR3 can sense necrosis of mammalian cells (Kariko et al. 2004b; Cavassani et al. 2008; Lai et al. 2009) and can influence wound repair (Lin et al. 2011, 2012), but are a departure from the classically known role of this pattern recognition receptor as being responsible for effective immune responses to viral double stranded RNA (dsRNA) (Kawai and Akira 2008; Dunlevy et al. 2010). Recently, it was also observed that activation of TLR3 induced the expression of genes in human keratinocytes that participate in formation of the physical barrier of the skin (Borkowski et al. 2013). In this study we hypothesized that TLR3 is physiologically relevant to the barrier repair response after UV damage. We demonstrate that release of endogenous RNA and the subsequent activation of TLR3 is necessary to permit restoration of the skin permeability barrier function after UVB injury.
RESULTS
UVB damaged keratinocytes stimulate genes important for the skin barrier
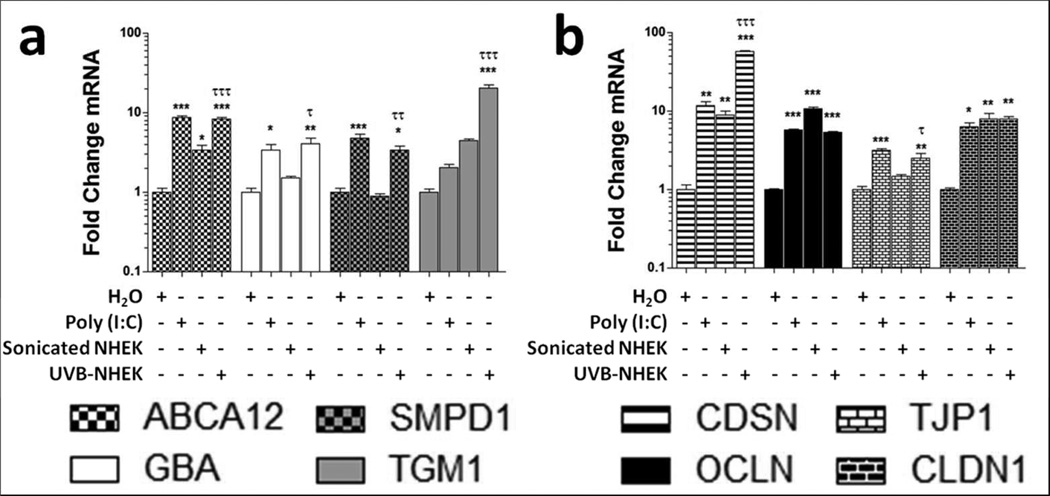
To detect whether products of UVB-damaged keratinocytes trigger expression of genes involved in skin barrier repair, we exposed cultured normal human epidermal keratinocytes (NHEK) to 15 mJ/cm2 UVB and then transferred these irradiated cells to nonirradiated NHEK cultures. The exposure of NHEK to the products of UVB-damaged keratinocytes caused significant increases in mRNA abundance of ATP-binding cassette sub-family A member 12 (ABCA12), glucocerebrosidase (GBA), acid sphingomyelinase (SMPD1), and transglutaminase 1 (TGM1) (Figure 1a).
Figure 1. UVB-damaged keratinocyte products stimulate genes important for skinbarrier.
Normal human keratinocytes were treated with either 1 μg/ml Poly (I:C), sonicated keratinocytes, or UVB-treated keratinocytes for 24 hours. Real-time PCR was used to quantify mRNA levels and fold change values are calculated relative and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression for (a) lipid transport (ABCA12), lipid metabolism (GBA and SMPD1), transglutaminase-1 (TGM1) and (b) desmosome (CDSN) and tight junction (OCLN,TJP1, and CLDN1) transcripts. Data are mean +/− SEM, n = 3, and are representative of at least three independent experiments. * = P < 0.05, ** = P < 0.01, *** = P < 0.001 compared to control. τ = P < 0.05, τ τ = P < 0.01, τ τ τ = P < 0.001 comparing sonicated to UVB treated NHEK treatments. One-way ANOVA with Bonferroni post test.
These increases in mRNA were significantly higher than NHEK cultures that were exposed to sonicated, non-irradiated NHEK although increases in ABCA12 mRNA were also observed following treatment with sonicated NHEK (Figure 1a). Desmosomes and tight junctions play an important role in forming a functional skin barrier (Furuse et al. 2002; Leclerc et al. 2009). To determine whether these components of the skin barrier were affected by dsRNA or UVB-damaged NHEK products, we measured the transcript abundance of the genes corneodesmosin (CDSN), occludin (OCLN), tight junction protein 1 (TJP1), and claudin 1 (CLDN1) after treatments with Poly (I:C), sonicated NHEK, and UVB-damaged NHEK. We observed that Poly (I:C)- and UVB-treated NHEK applied to NHEK cultures, stimulated significant increases in CDSN, OCLN, TJP1, and CLDN1 mRNA (Figure 1b). Sonicated NHEK significantly increased mRNA levels of CDSN, OCLN, and CLDN1 (Figure 1b). Only CDSN and TJP1 mRNA were induced significantly more in UVB-treated NHEK treatments compared to sonicated NHEK treatments (Figure 1b).
In order to assess the global effects of dsRNA on desmosomes and tight junctions in keratinocytes, we examined data from a previously performed microarray in which NHEK were treated for 24 hours with 1 μg/ml Poly (I:C) (Borkowski et al. 2013). Examination of this microarray data revealed that CDSN, periplakin 1 (PKP1), desmocolin 2 (DSC2), OCLN, CLDN4, CLDN7, and CLDN23 were all significantly increased (Borkowski et al. 2013). In order to validate the microarray results, we performed real-time PCR for desmosomal and tight junctional genes. In NHEK treated for 24 hours with 1 μg/ml Poly (I:C), we observed significant increases in desmoglein 1 (DSG1), DSG3, CDSN, plakophilin 1 (PKP1), desmoplakin (DSP), junction plakoglobin (JUP), desmocolin 1 (DSC1), OCLN, TJP1, CLDN1, CLDN 4, CLDN 7, CLDN 11, and CLDN 23 (Table 1).
Table 1. Desmosome and tight junction genes affected by Poly (I:C)-treatment.
Data in table represent real-time PCR and microarray fold change data from normal human epidermal keratinocytes (NHEK) treated with 1 μg/ ml Poly (I:C) versus control, water-treated NHEK.
| Gene name | Fold change (real-time PCR) | +/−SD | t-test |
|---|---|---|---|
| DSG1 | 4.61 | 1.548 | * |
| DSG3 | 4.78 | 0.535 | *** |
| CDSN | 126.25 | 10.740 | *** |
| PKP1 | 9.26 | 0.939 | *** |
| DSP | 3.21 | 0.120 | *** |
| JUP | 6.28 | 0.996 | *** |
| DSC1 | 4.05 | 0.415 | ** |
| DSC2 | 2.05 | 0.186 | ns |
| OCLN | 5.68 | 0.339 | *** |
| TJP1 | 3.19 | 0.237 | *** |
| CLDN1 | 6.26 | 1.484 | ** |
| CLDN4 | 9.75 | 0.667 | *** |
| CLDN5 | 1.13 | 0.680 | ns |
| CLDN7 | 8.11 | 1.609 | ** |
| CLDN11 | 0.19 | 0.027 | ** |
| CLDN23 | 9.00 | 2.291 | ** |
P < 0.05,
P < 0.01,
P < 0.001 compared to control. Two-tailed t-test.
Poly (I:C) increases tight junction function in keratinocytes
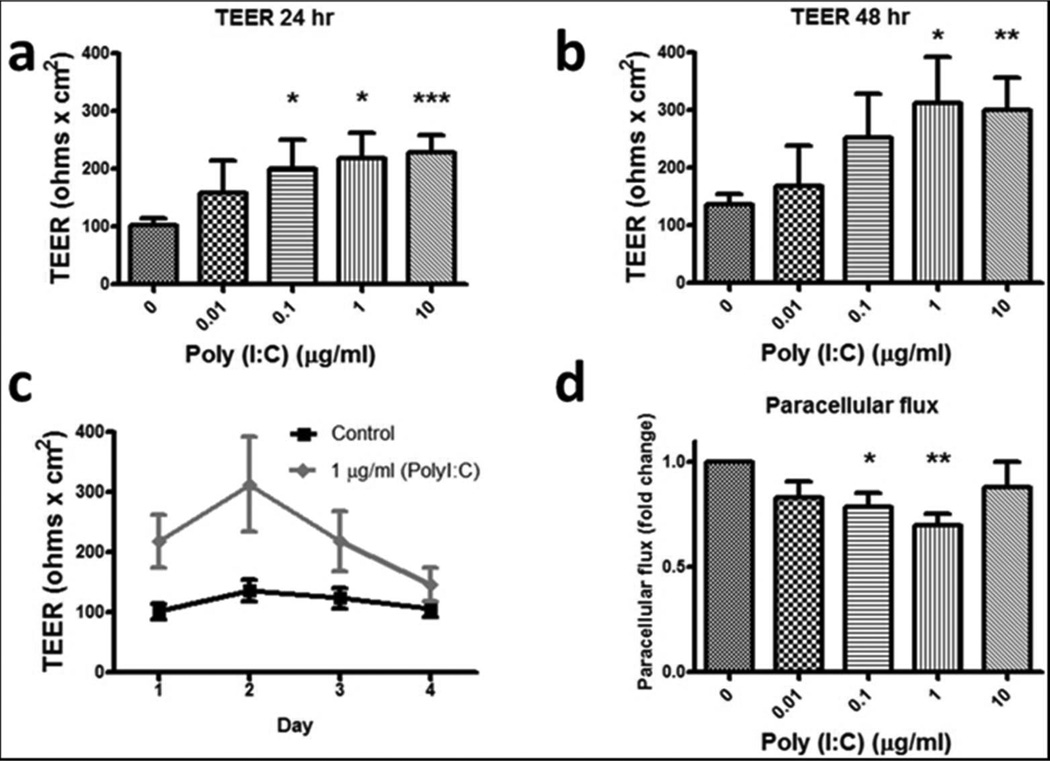
As we observed increased mRNA for genes associated with tight junctions following treatment of NHEK cultures with both Poly (I:C) and UVB-damaged NHEK products, we next evaluated the function of tight junctions in response to dsRNA. In this set of experiments, NHEK were grown to confluence in normal keratinocyte media in 24-well inserts prior to being differentiated (De Benedetto et al. 2011; Kuo et al. 2013). Differentiating, partially stratified keratinocytes were then treated with Poly (I:C) and transepithelial electrical resistance (TEER) values were measured using an EVOMX voltohmmeter (World Precision Instruments, Sarasota, FL). We observed that Poly (I:C) treatment led to dose-dependent increases in TEER readings at 24 and 48 hours after treatment (Figure 2a and 2b). The initial increase in TEER values stimulated by Poly (I:C) diminished over time and was no longer significantly different than control samples by day 4 (Figure 2c).
Figure 2. Poly (I:C)-treatment increases tight junction function in keratinocytes.
Transepithelial electrical resistance (TEER) was measured in confluent differentiated primary human keratinocytes grown in transwell inserts that were treated with various concentrations of Poly (I:C) for 24 hours (a) and 48 hours (b). (c) Time course data of TEER values. (d) Paracellular flux was measured 30 minutes after addition of fluorescein sodium to differentiated keratinocytes that were treated with various concentrations of Poly (I:C) for 48 hours. Data are mean +/− SEM, n = 3–8, and are representative of at least three independent experiments. * = P < 0.05, ** = P < 0.01, *** = P < 0.001. One-tailed t-test.
Another method used to assess tight junction function is measurement of paracellular flux of fluorescein sodium through differentiated keratinocytes. In this experiment, keratinocytes were grown to confluence and differentiated as previously described and Poly (I:C) was added and allowed to incubate for 48 hours. Fluorescein sodium was then added to the upper chamber and that which passed through the differentiated keratinocytes was measured in the bottom chamber after 30 minutes. We observed that doses of 0.1 and 1 μg/ml Poly (I:C) significantly decreased the paracellular flux of fluorescein sodium across differentiated keratinocytes (Figure 2d).
TLR3 activation is required for U1 RNA-induced changes in skin barrier gene expression
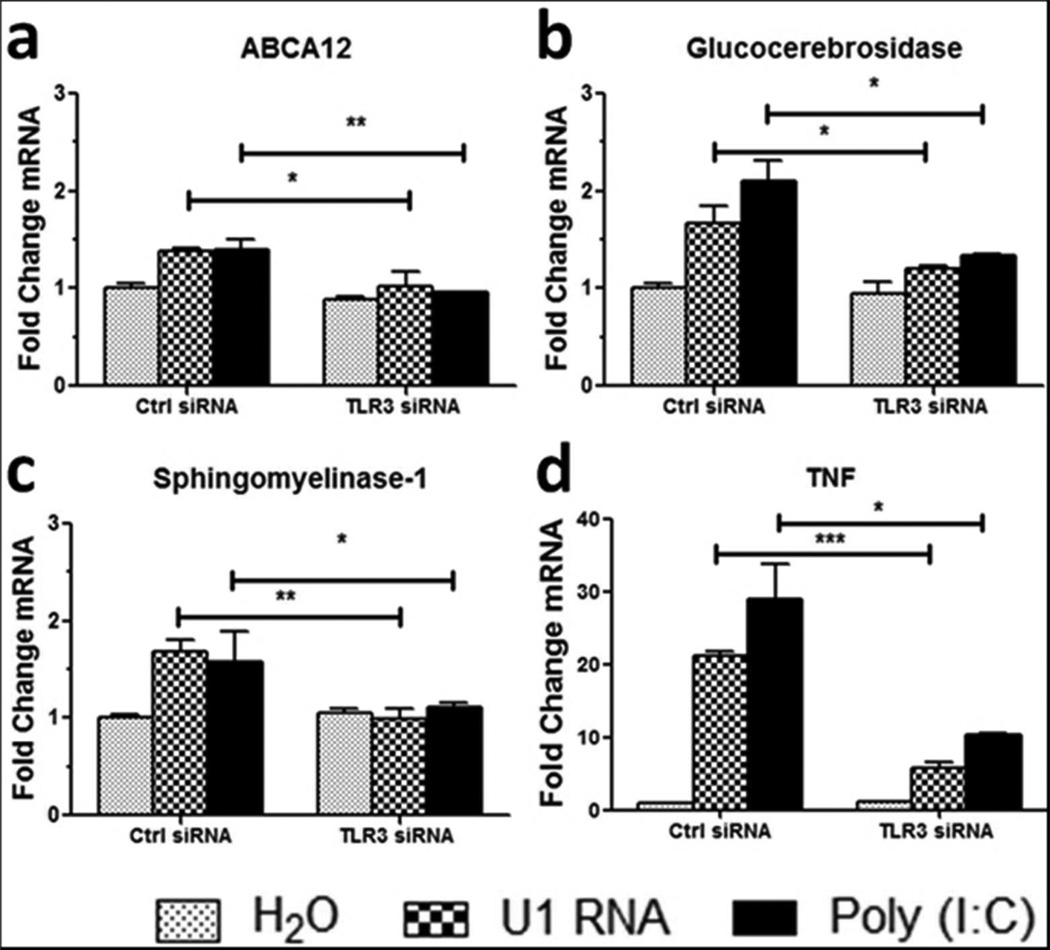
U1 spliceosomal RNA (U1 RNA) is a noncoding, small nuclear RNA (snRNA) that is increased in UVB-treated keratinocytes and stimulates inflammation in human keratinocytes and mouse skin in a TLR3-dependent manner (Bernard et al. 2012). In order to determine the effects of U1 RNA and TLR3 activation on skin barrier genes, TLR3 was knocked down using small interfering RNA (siRNA) and NHEK were then treated with 1 μg/ml U1 RNA for 24 hours. U1 RNA caused a significant increase in transcripts of ABCA12, GBA, SMPD1 and TNFα, while the induction of these genes was significantly decreased in NHEK pretreated with TLR3 siRNA (Figure 3).
Figure 3. U1 RNA stimulates skin barrier genes in a TLR3-dependent manner.
TLR3 was silenced in normal human epidermal keratinocytes (NHEKs) for 48 hours before treatment with 1 μg/ml U1 RNA or 1 μg/ml Poly(I:C) for 24 hours. Real-time PCR was used to quantify (a) ABCA12, (b) GBA, (c) SMPD1, and (d) TNFα mRNA levels and fold change values are calculated relative to and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. Data are mean +/− SEM, n = 3, and are representative of at least three independent experiments. * = P < 0.05, ** = P < 0.01, *** = P < 0.001. Two-tailed t-test.
Multiple snRNAs can stimulate skin barrier and inflammatory cytokine gene expression
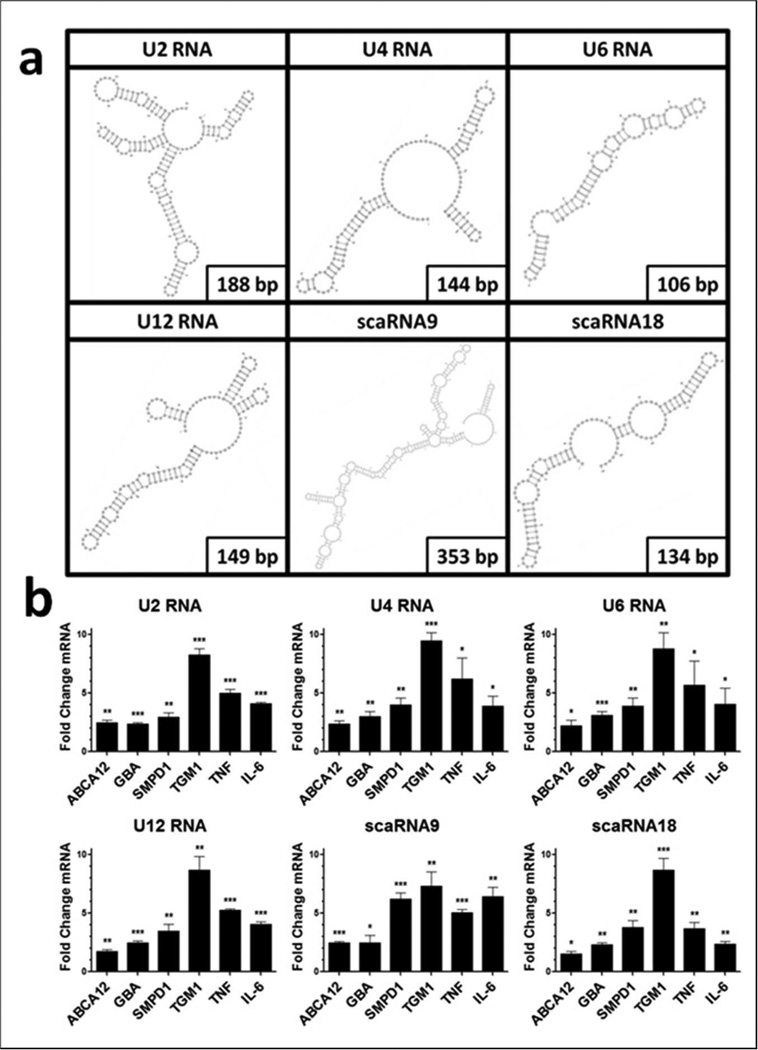
TLR3 is activated by dsRNA and it has been proposed that the double stranded stem loops of U1 RNA serve as the TLR3 ligand (Bernard et al. 2012). However, in addition to U1, numerous other noncoding snRNAs were observed to have increases in their read frequency after UVB exposure (Bernard et al. 2012). To determine whether additional ncRNAs could act in a similar manner to U1, we synthesized the spliceosomal RNAs U2, U4, U6, and minor spliceosomal RNA U12 (U2 RNA, U4 RNA, U6 RNA, and U12 RNA), as well as the small Cajol Body-specific RNAs 9 and 18 (scaRNA9 and scaRNA18). All of these snRNAs are predicted to contain double stranded regions using RNAfold software and the VARNA applet (Figure 4a) (Gruber et al. 2008; Blin et al. 2009). Treatment of NHEK for 24 hours with these synthetic snRNAs also resulted in an increase in mRNA abundance of ABCA12, GBA, SMPD1, TGM1, TNFα, and IL-6 (Figure 4b).
Figure 4. small nuclear RNAs stimulate skin barrier genes.
(a) Structures of snRNA species generated using RNAfold and VARNA applet. (b) Normal human epidermal keratinocytes were treated with 1 μg/ml in vitro transcribed snRNAs for 24 hours in the presence of a Dharmafect 1. Real-time PCR was used to quantify mRNA levels and fold change values are calculated relative to and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression and then to NHEK that have been treated with a control in vitro transcribed RNA. Data are mean +/Ȓ SEM, n = 3, and are representative of at least three independent experiments. * = P < 0.05, ** = P < 0.01, *** = P < 0.001. Two-tailed t-test.
Tlr3−/− mice display a barrier repair defect after UVB-induced barrier disruption
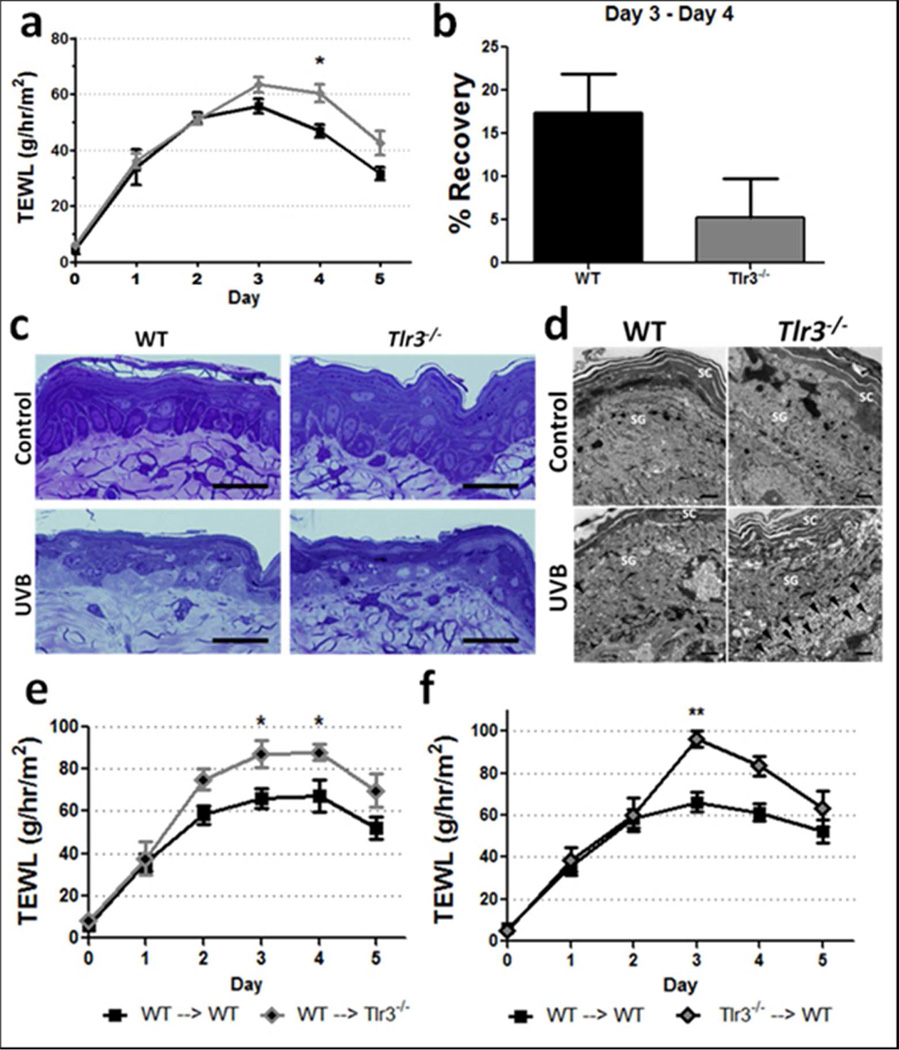
The capacity of UVB irradiated NHEK and dsRNAs to alter the expression of genes involved in barrier repair and increase NHEK tight junction function prompted us to directly test whether TLR3 influences permeability barrier function after UVB injury. Tlr3−/− mice and WT controls were exposed to a single 5 kJ/m2 dose of UVB as previously described (Bernard et al. 2012), and transepidermal water loss (TEWL) was examined to evaluate the kinetics of permeability barrier disruption and repair. This high dose of UVB has been reported to cause apoptosis and necrosis in cell culture and cause permeability barrier disruption in mice (Haratake et al. 1997; Lai et al. 2009; Uchida et al. 2010). Although levels of TEWL in WT and Tlr3−/− mice were similar over the first 3 days following UVB light exposure, Tlr3−/− mice displayed elevated and prolonged high levels of TEWL with significantly higher TEWL at Day 4 (Figure 5a). WT mice exhibited a 3.3-fold faster recovery between days 3 and 4 (p = 0.055) than Tlr3−/− mice (Figure 5b). Interestingly, permeability barrier disruption caused by a chemical depilatory reagent or by tape stripping did not have a significantly different effect on barrier disruption or repair in Tlr3−/− or Trif−/− mice, respectively, when compared to WT mice (Supplementary Figure 1). 24 hours after UVB exposure, no gross morphological differences were detected between WT and Tlr3−/− mice in semi-thin Toluidine blue stained sections (Figure 5c). However, transmission electron microscopy images demonstrated that Tlr3−/− mice display more abundant vacuolization of cells subjacent to the first layer of the stratum granulosum in comparison to WT mice (Figure 5d). Interestingly, 80% of Tlr3−/− mice exhibited chronic non-healing wounds at 8 and 16 weeks following a single acute 5 kJ/m2 dose of UVB. Photographs of these mice reveal that 4 of 5 Tlr3−/− mice failed to completely reepithelialize at both 8 and 16 weeks after UVB exposure (Supplementary Figure 2).
Figure 5. Tlr3−/− mice exhibit delayed barrier repair following UV-treatment.
(a) TEWL values were measured daily for 5 days in WT and Tlr3−/− mice exposed to 5 kJ/m2 UVB. Data are mean +/− SEM, n = 3 WT. n = 5 Tlr3−/− , and are representative of at least two independent experiments. * = P < 0.05, Two-way ANOVA. (b) Barrier recovery between day 3 and 4. One-tailed t-test. Skin was harvested from mice 24 hours after treatment with 5 kJ/m2 UVB. (c) Toluidine blue stained ultrathin sections. Scale bar = 20 μm (d) Transmission electron microscopy images of UVB-treated skin of WT and Tlr3−/− mice. Scale bar = 1 μm. (e+f) Mice were lethally irradiated and subsequently reconstituted with bone marrow 7 weeks prior to UVB irradiation and TEWL measurements. Data are mean +/− SEM, n = 6–8, and are representative of at least two independent experiments. * = P < 0.05, ** = P < 0.01 Two-way ANOVA.
Although our in vitro data described a keratinocyte response that may be responsible for skin barrier repair, we also sought to determine whether other cell types, either resident or migratory to the skin, contributed to the permeability barrier repair defect observed in Tlr3−/− mice. In order to assess relative contributions of Tlr3 present either on resident or migratory bone marrow derived cells in the skin, WT and Tlr3−/− mice were lethally irradiated and reconstituted with either WT or Tlr3−/− bone marrow in order to create chimeric mice. Control mice (WT → WT and Tlr3−/− → Tlr3−/−) showed similar differences in skin barrier repair after UVB induced barrier disruption as previously shown. When WT bone marrow was injected into Tlr3−/− mice (WT → Tlr3−/−), the barrier defect observed in Tlr3−/− mice was not rescued, and TEWL levels were significantly higher than WT → WT mice at days 3 and 4 (Figure 5e). Conversely, when Tlr3−/− bone marrow was injected into WT mice (Tlr3−/− → WT), TEWL levels were also significantly higher than WT → WT mice at day 3 (Figure 5f). In order to explore the differences in response to UVB radiation, we assessed the levels of 25 common cytokines and chemokines in the skin 24 hours after UVB treatment. We observed that significantly lower amounts of IL-5, RANTES, IL-15, and GM-CSF were present in Tlr3−/− → WT mice skin when compared to WT → WT mice. No other significant differences in cytokine and chemokine levels were observed between control (WT → WT) and other groups (Table 2).
Table 2. Cytokine levels in mouse skin 24 hours after UVB exposure.
Data in table represents presence cytokines/chemokines present in mouse skin 24 hours after exposure to 5 kJ/m2 UVB. Mean values are pg of analyte/mg of mouse skin. One-Way ANOVA. n = 3–5 mice/group. P values are from One-Way ANOVA in comparison to WT → WT group. Abbreviations: SEM, standard error of the mean; N/A, not applicable.
| WT -> WT | WT ->Tlr3−/− | Tlr3−/−-> WT | Tlr3−/−-> Tlr3−/− | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokin e |
Mean | SEM | P- valu e |
Mean | SEM | P- valu e |
Mean | SEM | P- valu e |
Mean | SEM | P- valu e |
| IL-5 | 72.08 | 8.41 | N/A | 40.82 | 9.34 | >0.05 | 25.50 | 5.76 | 0.006 | 62.35 | 11.06 | >0.05 |
| RANTES | 6.73 | 1.78 | N/A | 6.46 | 1.66 | >0.05 | ND | - | 0.014 | 7.54 | 0.90 | >0.05 |
| IL-15 | 278.50 | 83.73 | N/A | 120.48 | 30.37 | >0.05 | 63.25 | 10.93 | 0.036 | 130.23 | 39.14 | >0.05 |
| GM-CSF | 21.30 | 3.42 | N/A | 16.74 | 1.83 | >0.05 | 11.25 | 1.12 | 0.039 | 20.09 | 3.05 | >0.05 |
| G-CSF | 135.15 | 68.55 | N/A | 46.60 | 16.09 | >0.05 | 129.47 | 56.93 | >0.05 | 22.16 | 7.75 | >0.05 |
| IFN-gamma | 2.04 | 1.28 | N/A | 3.71 | 0.97 | >0.05 | 2.04 | 1.28 | >0.05 | 6.18 | 0.77 | >0.05 |
| IL-1a | 615.10 | 209.18 | N/A | 459.99 | 35.51 | >0.05 | 400.23 | 99.95 | >0.05 | 519.37 | 29.16 | >0.05 |
| IL-1b | 153.55 | 59.82 | N/A | 247.61 | 42.51 | >0.05 | 222.95 | 95.60 | >0.05 | 439.99 | 26.89 | >0.05 |
| IL-2 | ND | - | N/A | ND | - | >0.05 | ND | - | >0.05 | ND | - | >0.05 |
| IL-4 | ND | - | N/A | ND | - | >0.05 | ND | - | >0.05 | ND | - | >0.05 |
| IL-6 | 149.85 | 37.16 | N/A | 78.78 | 18.37 | >0.05 | 67.17 | 15.82 | >0.05 | 68.28 | 8.68 | >0.05 |
| IL-7 | ND | - | N/A | ND | - | >0.05 | 1.16 | 1.16 | >0.05 | 2.34 | 2.34 | >0.05 |
| IL-9 | 453.23 | 11.02 | N/A | 470.46 | 23.58 | >0.05 | 448.80 | 21.08 | >0.05 | 442.86 | 22.47 | >0.05 |
| IL-10 | 14.59 | 3.96 | N/A | 12.49 | 6.31 | >0.05 | 8.55 | 5.06 | >0.05 | 11.71 | 1.11 | >0.05 |
| IL-12(p40) | 19.07 | 4.01 | N/A | 21.42 | 5.54 | >0.05 | 14.84 | 6.19 | >0.05 | 30.18 | 7.63 | >0.05 |
| IL-12(p70) | 0.95 | 0.95 | N/A | ND | - | >0.05 | ND | - | >0.05 | ND | - | >0.05 |
| IL-13 | ND | - | N/A | ND | - | >0.05 | ND | - | >0.05 | ND | - | >0.05 |
| IL-17 | ND | - | N/A | ND | - | >0.05 | ND | - | >0.05 | ND | - | >0.05 |
| IP-10 | 317.53 | 65.21 | N/A | 354.87 | 73.58 | >0.05 | 191.12 | 78.09 | >0.05 | 463.12 | 110.37 | >0.05 |
| KC | 317.53 | 65.21 | N/A | 354.87 | 73.58 | >0.05 | 191.12 | 78.09 | >0.05 | 463.12 | 110.37 | >0.05 |
| MCP-1 | 254.87 | 16.79 | N/A | 202.96 | 45.18 | >0.05 | 120.94 | 41.32 | >0.05 | 208.80 | 23.98 | >0.05 |
| MIP-1a | 92.91 | 45.63 | N/A | 59.36 | 15.52 | >0.05 | 44.27 | 14.93 | >0.05 | 87.52 | 23.94 | >0.05 |
| MIP-1b | 81.69 | 33.94 | N/A | 46.46 | 16.02 | >0.05 | 26.65 | 11.08 | >0.05 | 69.35 | 36.84 | > 0.05 |
| MIP-2 | 850.93 | 728.66 | N/A | 171.57 | 73.77 | >0.05 | 267.11 | 168.62 | >0.05 | 172.70 | 58.77 | >0.05 |
| TNFa | 4.56 | 1.97 | N/A | 1.17 | 1.17 | >0.05 | 1.17 | 1.17 | >0.05 | 5.83 | - | >0.05 |
DISCUSSION
TLR3 activation has classically been described in the context of immunity as a mechanism for detecting viruses (Kawai and Akira 2008; Dunlevy et al. 2010), though recent evidence has revealed that it serves an additional role to sense injury (Cavassani et al. 2008; Lai et al. 2009; Lin et al. 2011, 2012; Bernard et al. 2012). Herein we describe how TLR3 acts to promote expression of skin barrier repair genes after UV injury, and that snRNAs or Poly (I:C) can initiate a similar response in keratinocytes. Furthermore, mice lacking Tlr3 demonstrate a decreased capacity to restore permeability barrier function. Though triggers of skin barrier repair had previously been described to involve sensing a disturbed calcium gradient in the epidermis (Lee et al. 1992; Menon et al. 1992), the results reported in this manuscript support the hypothesis that TLR3 serves as an additional sensor of skin damage following UVB exposure. Furthermore, as skin barrier disruption is often delayed 48–72 hours following acute UVB exposure (Haratake et al. 1997; Holleran et al. 1997), TLR3 activation during this time may serve as a mechanism for accelerating skin barrier repair.
Injury to the skin results in a breakdown of both the permeability barrier to small molecules and the immune defense barrier against infection. UVB exposure causes many molecular changes, including damage to keratinocytes resulting in both apoptotic and necrotic forms of cell death. Non-apoptotic forms of cell death trigger greater cytokine release from keratinocytes (Lai et al. 2009), likely through the release of cellular contents that present as damage associated molecular patterns (DAMPs) to numerous pattern recognition receptors (PRRs) present in or on neighboring cells (Kaczmarek et al. 2013). Additionally, U1 RNA, a single stranded, ncRNA is released from necrotic cells following UV damage, and TLR3 detects this mammalian RNA (Bernard et al. 2012). In the present study, products from UVB-damaged keratinocytes that induce cytokine responses also enhanced mRNA expression of skin barrier, desmosome, and tight junction genes. Additionally, synthetic U1 RNA stimulated expression of the skin barrier genes in a TLR3 dependent manner. These data demonstrate that the endogenous products of UVB damaged keratinocytes promote skin barrier repair gene expression through TLR3.
While tight junctions and desmosomes may not be directly responsible for maintaining permeability barrier function in the skin, they can create a barrier to ions, macromolecules and water flux (Kirschner et al. 2013), and play an important role in maintaining a functional skin barrier (Kirschner and Brandner 2012). Numerous skin diseases result from either mutations in adhesional proteins or autoimmunity to components of the desmosome or tight junction (Lai-Cheong et al. 2007). This manuscript reports that Poly (I:C), a ligand of TLR3, increases tight junction gene expression and function in human keratinocytes. In a physiological setting, damage to the epidermis typically affects more than just the stratum corneum. Thus it follows that sensing a damage signal such as dsRNA promotes formation of multiple components of the epidermis that help restore permeability barrier function and the mechanical integrity of the epidermis. Our data gives a more complete description of genes that are affected by TLR3 activation following UVB damage and suggests that multiple mechanisms may be responsible for the delayed repair observed in Tlr3−/− mice.
We hypothesized that U1 RNA was one of many endogenous ncRNAs that could potentially stimulate TLR3 by recognition of its double-stranded regions. To identify these additional ncRNAs we synthesized and studied a number of other candidate ncRNAs from a list of ncRNAs that were present in increased frequency in keratinocytes damaged by UVB (Bernard et al. 2012). These ncRNAs were specifically selected because the secondary structures of these snRNAs were predicted to contain double stranded regions which might activate TLR3. Double stranded RNA of at least 21 base pairs in length has been demonstrated to activate TLR3 (Kariko et al. 2004a). The stem-loop structures of the snRNAs that we synthesized satisfy this requirement and were able to activate TLR3. While this response to these ncRNAs has not been previously shown, the activation of TLR3 by self RNA has previously been demonstrated and it is likely that other endogenous ncRNAs exist within the cell that have the potential to activate TLR3. Previous studies demonstrated that U1 RNA must first be exposed to UVB in order to stimulate TLR3 (Bernard et al. 2012). In contrast, in our studies, treatment of the in vitro transcribed RNA with UVB had no effect on its potential to activate TLR3-dependent gene expression. It remains to be determined whether changes in RNA structure are caused by direct UVB exposure or whether other yet to be determined changes occur that make endogenous ncRNA more immunostimulatory.
In order to determine whether TLR3 was important for permeability barrier repair, Tlr3−/− mice were treated with a single high dose of UVB to induce permeability barrier disruption. The initial kinetics of barrier disruption were similar in WT and Tlr3−/− mice. However, Tlr3−/− mice displayed a prolonged elevation in TEWL values in comparison to WT, suggesting that these mice have a defect in permeability barrier repair. While tape stripping and chemical depilation may serve as important models for investigating permeability repair, they were not sufficient to demonstrate the importance of Tlr3 in barrier repair. This is most likely due to the fact that barrier disruptions of this type instantly disrupt the calcium gradient in the epidermis through removal of outer layers of the cornified envelope without causing cell death that could result in the release of cellular contents such as ncRNAs.
While it is known that wound healing is delayed in Tlr3−/− mice (Lin et al. 2011, 2012), this manuscript presents evidence that permeability barrier repair is affected by TLR3 deficiency. Although the deficiency in permeability barrier repair may play a role in the wound healing phenotype of Tlr3−/− mice, it is not sufficient to explain this phenotype and future studies will aim to more completely characterize these findings.
While much of our data describes keratinocyte specific changes in response to TLR3 activation, we sought to determine whether other cell types in the skin contribute to permeability barrier repair, as it is known that many myeloid derived cells migrate to sites of injury and are important for skin barrier disruption and repair (Haratake et al. 1997; Holleran et al. 1997). To determine the effect of Tlr3 present on bone marrow derived immunocytes on skin barrier repair, we injected WT bone marrow into Tlr3−/− mice prior to administering UVB-induced barrier disruption. The Tlr3-dependent skin barrier defect in these WT → Tlr3−/− mice was not rescued, and appeared to be exacerbated, as significant differences in TEWL values were observed earlier than in traditional Tlr3−/− mice. This finding demonstrates that Tlr3 present on keratinocytes or other radio-resistant cells is required for proper skin barrier repair, as Tlr3 present on only myeloid cells was not sufficient to restore normal skin barrier repair. Conversely, when Tlr3−/− bone marrow was injected into WT mice, we also observed a higher TEWL value at day 3 in comparison to WT → WT mice. This later finding is not surprising and demonstrates that Tlr3 on bone marrow derived cells is also required for proper skin barrier repair. Since these mice display deficiencies in certain cytokines (IL-5, RANTES, IL-15, and GM-CSF) following UVB exposure, it would be interesting to determine whether these cytokines affect permeability barrier repair. Thus, we conclude that Tlr3 on both resident skin cells and infiltrating myeloid cells is required for proper permeability barrier repair. Future studies should focus on the specific contributions of each cell type to permeability barrier repair.
In recent years, innate immune receptors, such as toll-like receptors have been shown to be responsible for more than just pathogen detection and clearance. Their role in recognizing and responding to DAMPs has become increasingly evident with many diseases being identified as being exacerbated or caused by PRR activation (Rosin and Okusa 2011). Prior studies have demonstrated that UV exposure stimulates an enhanced antimicrobial barrier. This report now shows how a pathway previously associated with anti-viral immune responses also stimulates repair of the permeability barrier. Indeed, the cutaneous immunological and physical barriers are closely interrelated and should be thought of as one. Future work should focus on the downstream signaling pathways of TLR3 that activate genes that promote skin barrier homeostasis and repair as well as the relative contributions of other cell types in the skin that could potentially influence this essential process.
MATERIALS & METHODS
UVB irradiation
NHEKs were irradiated with UVB at 15 mJ cm−2, using Spectronics handheld UVB lamps with two 8W bulbs (312 nm) as previously described (Lai et al. 2009). Dosimetry was performed using a digital ultraviolet radiometer by Solartech Inc. UVB-irradiated cells were collected 24 hours after exposure by pooling cells scraped from wells and floating cells., 600,000 of these “UV damaged” cells were then added to 200,000 NHEKs grown to 80% confluence. Nonirradiated NHEKs were lysed using a tip sonicator (30% power for 10 seconds) and used as controls. For mouse skin irradiation, hair was shaved and chemically depilated from the back, and 96 h later, the hairless skin was exposed to UVB (5 kJ/m2).
In vitro transcription of snRNA
snRNA was generated using Ampliscribe™ T7-Flash™ Transcription Kit from (Epicentre©, an Illumina© company, Madison, WI). Templates used for reactions were gel purified PCR products from the following primer pairs found in the supplementary methods.
Transepidermal Water Loss
Transepidermal water loss (TEWL) was measured using a TEWAMETER TM300 (C & K, Cologne, Germany). TEWL was measured prior to UVB barrier disruption and every 24 hours for 5 days.
Mice
Sex-matched C57BL/6 wild-type controls, male and female TLR3-deficient mice on a C57BL/6 background, and TRIF-deficient mice on a C57BL/6 background were housed at the University Research Center at the University of California, San Diego (UCSD). All animal experiments were approved by the UCSD Institutional Animal Care and Use Committee.
Bone Marrow Reconstitution
6 week old mice were administered antibiotics (200 mg Sulfamethoxazole and 40 mg Trimethoprim) (Hi-Tech Pharmacal, Amityville, NY) in the drinking water 1 day prior to lethal irradiation. Mice were placed in a cesium source irradiator (J.L. Shepherd & Associates, San Fernando, CA), and exposed to 10 Gy (1000 Rad) of total body γ-irradiation. The following day, bone marrow was isolated from the femur and tibia of 10 week old mice. 6*106 cells were injected suborbitally into lethally irradiated mice. Mice were allowed to recover 6 weeks before experimentation. Antibiotics were continued for 14 days after reconstitution with cages and water changed every other day during this time.
Supplementary Material
ACKNOWLEDGMENTS
We thank Eric L Greidinger at the University of Miami, Miller School of Medicine, Miami, Florida for kindly donating U1 RNA. We thank Y Jones and T Meerloo at the UCSD EM Core for processing samples and helpful discussion and Dr. Marilyn Farquhar for helpful discussion of EM images.
This work was supported by US National Institutes of Health (NIH) grants R01-AR052728, NIH R01-AI052453 and R01 AI0833358 to R.L.G., the UCSD Training in Immunology Grant 5T32AI060536-05 and UCSD Dermatologist Investigator Training Program Grant 1T32AR062496-01 supporting A.W.B., the NIEHS Training Grant ES007148 and the NIEHS Center Grant ES005022 supporting J.J.B.
Abbreviations used
- UV
ultraviolet
- TLR3
toll-like receptor 3
- ncRNA
noncoding RNA
- snRNAs
small nuclear RNAs
- dsRNA
double-stranded RNA
- NHEK
normal human epidermal keratinocytes
- TJ
tight junction
- TEER
transepithelial electrical resistance
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Aberg KM, Man M-Q, Gallo RL, et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008;128:917–925. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg KM, Radek KA, Choi E-H, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007;117:3339–3349. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens K, Schunck M, Podda G-F, et al. Mechanical and metabolic injury to the skin barrier leads to increased expression of murine β-defensin-1, -3, and -14. J Invest Dermatol. 2011;131:443–452. doi: 10.1038/jid.2010.289. [DOI] [PubMed] [Google Scholar]
- De Benedetto A, Rafaels NM, McGirt LY, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127:773–86. e1–e7. doi: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JJ, Cowing-Zitron C, Nakatsuji T, et al. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat Med. 2012;18:1286–1290. doi: 10.1038/nm.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin G, Denise A, Dulucq S, et al. Alignments of RNA structures. IEEE/ACM Trans Comput Biol Bioinform. 2009;7:309–322. doi: 10.1109/TCBB.2008.28. [DOI] [PubMed] [Google Scholar]
- Borkowski AW, Park K, Uchida Y, et al. Activation of TLR3 in keratinocytes increases expression of genes involved in formation of the epidermis, lipid accumulation, and epidermal organelles. J Invest Dermatol. 2013;133:2031–2040. doi: 10.1038/jid.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavassani KA, Ishii M, Wen H, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerjian M, Hachem J-P, Tschachler E, et al. Acute modulations in permeability barrier function regulate epidermal cornification: role of caspase-14 and the protease-activated receptor type 2. Am J Pathol. 2008;172:86–97. doi: 10.2353/ajpath.2008.070161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorschner RA, Pestonjamasp VK, Tamakuwala S, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117:91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- Dunlevy F, McElvaney N, Greene C. TLR3 Sensing of Viral Infection. Open Infect Dis J. 2010;4:1–10. [Google Scholar]
- Feingold KR, Denda M. Regulation of permeability barrier homeostasis. Clin Dermatol. 2012;30:263–268. doi: 10.1016/j.clindermatol.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Feingold KR, Schmuth M, Elias PM. The regulation of permeability barrier homeostasis. J Invest Dermatol. 2007;127:1574–1576. doi: 10.1038/sj.jid.5700774. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AR, Lorenz R, Bernhart SH, et al. The Vienna RNA websuite. Nucleic Acids Res. 2008;36:W70–W74. doi: 10.1093/nar/gkn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem JP, Crumrine D, Fluhr J, et al. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003;121:345–353. doi: 10.1046/j.1523-1747.2003.12365.x. [DOI] [PubMed] [Google Scholar]
- Hachem J-P, Houben E, Crumrine D, et al. Serine protease signaling of epidermal permeability barrier homeostasis. J Invest Dermatol. 2006;126:2074–2086. doi: 10.1038/sj.jid.5700351. [DOI] [PubMed] [Google Scholar]
- Haratake A, Uchida Y, Schmuth M. UVB-induced alterations in permeability barrier function: roles for epidermal hyperproliferation and thymocyte-mediated response. J Invest Dermatol. 1997;108:769–775. doi: 10.1111/1523-1747.ep12292163. [DOI] [PubMed] [Google Scholar]
- Holleran WM, Uchida Y, Halkier-Sorensen L, et al. Structural and biochemical basis for the UVB-induced alterations in epidermal barrier function. Photodermatol Photoimmunol Photomed. 1997;13:117–128. doi: 10.1111/j.1600-0781.1997.tb00214.x. [DOI] [PubMed] [Google Scholar]
- Hong SP, Kim MJ, Jung M-Y, et al. Biopositive effects of low-dose UVB on epidermis: coordinate upregulation of antimicrobial peptides and permeability barrier reinforcement. J Invest Dermatol. 2008;128:2880–2887. doi: 10.1038/jid.2008.169. [DOI] [PubMed] [Google Scholar]
- Jensen JM, Schütze S, Förl M, et al. Roles for tumor necrosis factor receptor p55 and sphingomyelinase in repairing the cutaneous permeability barrier. J Clin Invest. 1999;104:1761–1770. doi: 10.1172/JCI5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Karikó K, Bhuyan P, Capodici J, et al. Exogenous siRNA mediates sequence-independent gene suppression by signaling through toll-like receptor 3. Cells Tissues Organs. 2004a;177:132–138. doi: 10.1159/000079987. [DOI] [PubMed] [Google Scholar]
- Karikó K, Ni H, Capodici J, et al. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004b;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- Kida N, Sokabe T, Kashio M, et al. Importance of transient receptor potential vanilloid 4 (TRPV4) in epidermal barrier function in human skin keratinocytes. Pflugers Arch Eur J Physiol. 2012;463:715–725. doi: 10.1007/s00424-012-1081-3. [DOI] [PubMed] [Google Scholar]
- Kirschner N, Brandner JM. Barriers and more: functions of tight junction proteins in the skin. Ann N Y Acad Sci. 2012;1257:158–166. doi: 10.1111/j.1749-6632.2012.06554.x. [DOI] [PubMed] [Google Scholar]
- Kirschner N, Rosenthal R, Furuse M, et al. Contribution of tight junction proteins to ion, macromolecule, and water barrier in keratinocytes. J Invest Dermatol. 2013;133:1161–1169. doi: 10.1038/jid.2012.507. [DOI] [PubMed] [Google Scholar]
- Kömüves LG, Hanley K, Lefebvre AM, et al. Stimulation of PPARalpha promotes epidermal keratinocyte differentiation in vivo. J Invest Dermatol. 2000;115:353–360. doi: 10.1046/j.1523-1747.2000.00073.x. [DOI] [PubMed] [Google Scholar]
- Kuo I-H, Carpenter-Mendini A, Yoshida T, et al. Activation of epidermal toll-like receptor 2 enhances tight junction function: implications for atopic dermatitis and skin barrier repair. J Invest Dermatol. 2013;133:988–998. doi: 10.1038/jid.2012.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Di Nardo A, Nakatsuji T, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 2009;15:1377–1382. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai-Cheong JE, Arita K, McGrath Ja. Genetic diseases of junctions. J Invest Dermatol. 2007;127:2713–2725. doi: 10.1038/sj.jid.5700727. [DOI] [PubMed] [Google Scholar]
- Leclerc Ea, Huchenq A, Mattiuzzo NR, et al. Corneodesmosin gene ablation induces lethal skin-barrier disruption and hair-follicle degeneration related to desmosome dysfunction. J Cell Sci. 2009;122:2699–2709. doi: 10.1242/jcs.050302. [DOI] [PubMed] [Google Scholar]
- Lee SH, Elias PM, Proksch E, et al. Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J Clin Invest. 1992;89:530–538. doi: 10.1172/JCI115617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SW, Hong SP, Jeong SW, et al. Simultaneous effect of ursolic acid and oleanolic acid on epidermal permeability barrier function and epidermal keratinocyte differentiation via peroxisome proliferator-activated receptor-?? J Dermatol. 2007;34:625–634. doi: 10.1111/j.1346-8138.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- Lin Q, Fang D, Fang J, et al. Impaired wound healing with defective expression of chemokines and recruitment of myeloid cells in TLR3-deficient mice. J Immunol. 2011;186:3710–3717. doi: 10.4049/jimmunol.1003007. [DOI] [PubMed] [Google Scholar]
- Lin Q, Wang L, Lin Y, et al. Toll-like receptor 3 ligand polyinosinic:polycytidylic acid promotes wound healing in human and murine skin. J Invest Dermatol. 2012;132:2085–2092. doi: 10.1038/jid.2012.120. [DOI] [PubMed] [Google Scholar]
- Lucas R, McMichael T, Smith W, et al. Solar ultraviolet radiation: global burden of disease from solar ultraviolet radiation. In: Pruss-Ustun A, Zeeb H, Mathers C, et al., editors. Environ. Burd. Dis. Ser. No. 13. Geneva: WHO Document Production Services; 2006. 87 pp. [Google Scholar]
- Man MQ, Fowler AJ, Schmuth M, et al. Peroxisome-Proliferator-Activated Receptor (PPAR)-gamma activation stimulates keratinocyte differentiation. J Invest Dermatol. 2004;123:305–312. doi: 10.1111/j.0022-202X.2004.23235.x. [DOI] [PubMed] [Google Scholar]
- Menon GK, Elias PM, Lee SH, et al. Localization of calcium in murine epidermis following disruption and repair of the permeability barrier. Cell Tissue Res. 1992;270:503–512. doi: 10.1007/BF00645052. [DOI] [PubMed] [Google Scholar]
- Mihara H, Boudaka A, Sugiyama T, et al. Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J Physiol. 2011;589:3471–3482. doi: 10.1113/jphysiol.2011.207829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol. 2011;22:416–425. doi: 10.1681/ASN.2010040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, Coda AB, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuth M, Haqq CM, Cairns WJ, et al. Peroxisome proliferator-activated receptor (PPAR)-beta/delta stimulates differentiation and lipid accumulation in keratinocytes. J Invest Dermatol. 2004;122:971–983. doi: 10.1111/j.0022-202X.2004.22412.x. [DOI] [PubMed] [Google Scholar]
- Sokabe T, Fukumi-Tominaga T, Yonemura S, et al. The TRPV4 channel contributes to intercellular junction formation in keratinocytes. J Biol Chem. 2010;285:18749–18758. doi: 10.1074/jbc.M110.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Houben E, Park K, et al. Hydrolytic pathway protects against ceramide-induced apoptosis in keratinocytes exposed to UVB. J Invest Dermatol. 2010;130:2472–2480. doi: 10.1038/jid.2010.153. [DOI] [PubMed] [Google Scholar]
- Wang X-P, Schunck M, Kallen K-J, et al. The interleukin-6 cytokine system regulates epidermal permeability barrier homeostasis. J Invest Dermatol. 2004;123:124–131. doi: 10.1111/j.0022-202X.2004.22736.x. [DOI] [PubMed] [Google Scholar]
- Ye J, Garg A, Calhoun C, et al. Alterations in cytokine regulation in aged epidermis: implications for permeability barrier homeostasis and inflammationIIL-1 gene family. Exp Dermatol. 2002;11:209–216. doi: 10.1034/j.1600-0625.2002.110303.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.