Abstract
Background
Inflammation is thought to be involved in the pathogenesis of obstructive sleep apnea syndrome (OSAS). Hepcidin, a 25-kD peptide hormone produced by the liver, modulates acute inflammatory responses. This study aimed to determine the association of serum levels of hepcidin with the presence and severity of OSAS.
Material/Methods
We enrolled 184 patients with OSAS and 110 healthy subjects. Serum levels of hepcidin were evaluated using enzyme-linked immunosorbent assay (ELISA) method.
Results
OSAS patients had significantly higher serum hepcidin levels compared with healthy controls. Multivariable logistic regression analysis indicated that serum hepcidin levels were an independent determinant of the presence of OSAS (OR 1.224, 95% CI 1.159–1.292; P<0.001). Serum hepcidin levels were significantly elevated in severe OSAS patients compared with mild and moderate OSAS patients. Spearman correlation analysis revealed that serum hepcidin levels were correlated with the severity of OSAS. In addition, serum levels of hepcidin were correlated with apnea-hypopnea index (AHI) in patients with OSAS.
Conclusions
Elevated serum hepcidin levels are associated with the presence and severity of OSAS.
MeSH Keywords: Hepcidins; Inflammation; Obesity; Sleep Apnea, Obstructive
Background
Obstructive sleep apnea syndrome (OSAS) is characterized by repetitive collapse of the upper airway during sleep, resulting in obstruction of airflow and oxygen desaturation, which cause arousal from sleep [1]. There is a causal relationship between OSAS and obesity. OSAS has a prevalence of approximately 40% in obese individuals, and about 70% of OSAS patients are diagnosed as obese [2]. Another potential mechanism for OSAS is the activation of systemic inflammation. A variety of inflammatory cytokines such as soluble intercellular adhesion molecule-1 (sICAM-1), soluble vascular adhesion molecule 1 (sVCAM-1), monocyte chemoattractant protein 1 (MCP-1), and C-reactive protein (CRP) have been found to be associated with the development of OSAS [3].
Hepcidin, a liver-derived acute-phase reactant, regulates iron homeostasis by inhibiting the release of iron from enterocytes, macrophages, and hepatocytes [4]. As the key regulator of transmembrane iron transport, hepcidin plays a key role in the absorption of iron in the intestine, the mobilization of iron from hepatic stores, and iron recycling [5]. Hepcidin expression is up-regulated in response to iron stores, inflammation, and endoplasmic reticulum stress and is inhibited by anemia, erythropoiesis, hypoxia, and oxidative stress [6]. Hepcidin expression levels were found to be enhanced in both visceral and subcutaneous adipose tissue of obese patients, which points to the role of hepcidin in the pathogenesis of obesity [7]. Therefore, it has been speculated that hepcidin might be involved in the mechanism of OSAS.
The present study was designed to determine serum levels of hepcidin in OSAS patients and its relevance in the pathogenesis of OSAS.
Material and Methods
Patients
A consecutive 184 males with clinical symptoms of OSAS and who were examined by polysomnography (PSG) in our hospital was enrolled in this study. Patients were excluded if they had personal or family history of psychiatric disorders, history of alcohol or drug abuse, or any other significant medical illnesses such as diabetes mellitus, cancer, cardiovascular, cerebrovascular, pulmonary, or neuromuscular disease. We recruited 110 healthy volunteers without clinical symptoms of OSAS as the control group. The control group was matched with OSAS patients for age and body mass index (BMI).
The study was planned according to the ethics guidelines of the Helsinki Declaration and was approved by the Institutional Research Ethics Board of our hospital. All patients gave written informed consent regarding participation in this study.
Sleep study
All subjects underwent overnight polysomnography using standard techniques. The diagnosis of OSAS was established on the basis of clinical symptoms such as excessive daytime sleepiness, unexplained daytime fatigue, choking or gasping during sleep, and an apnea hypopnea index (AHI) of ≥5 events/h on PSG. According to AHI, there are 3 levels of OSAS: mild OSAS (AHI ≥5 and <15), moderate OSAS (AHI ≥15 and <30), and severe OSAS (AHI ≥30) [8]. Subjects with AHI <5 were considered as controls [8].
Measurements
Anthropometric (height, weight, and blood pressures), clinical, and laboratory analysis were performed. Venous blood was collected after a minimum of 10 h of absolute diet. Serum triglycerides (TG), serum total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were tested using an auto biochemistry instrument (Hitachi 7170, Tokyo, Japan). Serum hepcidin levels were determined using an ELISA (DRG Instruments GmbH, Marburg, Germany).
Statistical analysis
Statistical analysis was carried out using the SPSS version 13.0 software program (SPSS Inc., Chicago, IL). Data normality was analyzed using the Kolmogorov-Smirnov test. The results are expressed as means ± standard errors when the data are normally distributed, and are expressed as median (interquartile range) when the data was not normally distributed. Comparison of the characteristics between patients with OSAS and healthy controls were performed by unpaired t test or Mann-Whitney U test. Univariate analysis was performed and the variables with a P<0.10 were then entered into a backward stepwise multivariate logistic regression model to calculate the odds ratio (OR) values and 95% confidence intervals (CI) for the presence of OSAS. The Kruskal-Wallis test was used to compare the differences in serum hepcidin levels among mild, moderate, and severe OSAS patients. The correlation of serum hepcidin levels with the severity of OSAS was determined using Spearman correlation analysis. The correlation between serum hepcidin and AHI were analyzed using Spearman correlation analysis. P values less than 0.05 were considered to be statistically significant.
Results
Baseline clinical characteristics
The clinical and laboratory characteristics of OSAS patients and control subjects are presented in Table 1. OSAS patients showed higher levels of homeostasis model assessment of insulin resistance (HOMA-IR), TC, LDL-C, and AHI compared with healthy controls. There were no significant differences in other characteristics between the 2 groups.
Table 1.
Clinical and biochemical characteristics of OSAS patients and healthy controls.
| OSAS patients | Control | P value | |
|---|---|---|---|
| N | 184 | 110 | |
| Age (years) | 54.02±8.99 | 53.38±10.37 | 0.581 |
| BMI (Kg/m2) | 26.41±3.50 | 26.38±4.04 | 0.959 |
| SBP (mmHg) | 141.96±21.98 | 139.41±21.92 | 0.337 |
| DBP (mmHg) | 88.72±13.41 | 89.14±15.91 | 0.794 |
| HOMA-IR | 3.38±0.99 | 3.03±0.94 | 0.004 |
| TC (mmol/L) | 5.71±1.28 | 5.34±1.18 | 0.013 |
| TG (mmol/L) | 1.86±0.52 | 1.75±0.48 | 0.451 |
| LDL-C (mmol/L) | 3.80±1.05 | 3.41±1.00 | 0.002 |
| HDL-C (mmol/L) | 1.43±0.34 | 1.42±0.30 | 0.864 |
| AHI | 21.00 (15.00–30.00) | 2.00 (1.00–3.00) | <0.001 |
| Hepcidin (ng/mL) | 27.75 (22.39–31.57) | 20.88 (16.00–25.72) | <0.001 |
Serum hepcidin level in OSAS patients
Table 1 shows the serum levels of hepcidin between OSAS patients and healthy controls. OSAS patients showed significantly elevated serum hepcidin levels compared with healthy controls. Simple logistic regression analysis indicated that HOMA-IR (OR 1.442, 95% CI 1.123–1.852; P=0.004), TC (OR 1.280, 95% CI 1.050–1.560; P=0.014), LDL-C (OR 1.467, 95% CI 1.149–1.872; P=0.002), and hepcidin levels (OR 1.229, 95% CI 1.165–1.296; P<0.001) showed a trend (P<0.10) toward an association with the presence of OSAS (Table 2). All these parameters were then entered into a multivariate logistic regression model and the serum hepcidin levels remained associated with the presence of OSAS (OR 1.224, 95% CI 1.159–1.292; P<0.001) (Table 2).
Table 2.
Logistic regression Analysis for the presence of OSAS.
| Simple regression OR (95%CI) | P | Multiple regression OR (95%CI) | P | |
|---|---|---|---|---|
| Age (years) | 1.007 (0.982–1.032) | 0.580 | ||
| BMI (Kg/m2) | 1.002 (0.940–1.068) | 0.959 | ||
| SBP (mmHg) | 1.005 (0.994–1.016) | 0.336 | ||
| DBP (mmHg) | 0.998 (0.982–1.015) | 0.881 | ||
| HOMA-IR | 1.442 (1.123–1.852) | 0.004 | 1.391 (1.039–1.864) | 0.027 |
| TC (mmol/L) | 1.280 (1.050–1.560) | 0.009 | 0.904 (0.502–1.628) | 0.738 |
| TG (mmol/L) | 1.077 (0.888–1.308) | 0.451 | ||
| LDL-C (mmol/L) | 1.467 (1.149–1.872) | 0.002 | 1.532 (0.756–3.103) | 0.236 |
| HDL-C (mmol/L) | 1.066 (0.514–2.211) | 0.863 | ||
| Hepcidin (ng/mL) | 1.229 (1.165–1.296) | <0.001 | 1.224 (1.159–1.292) | <0.001 |
Serum hepcidin levels with the severity of OSAS
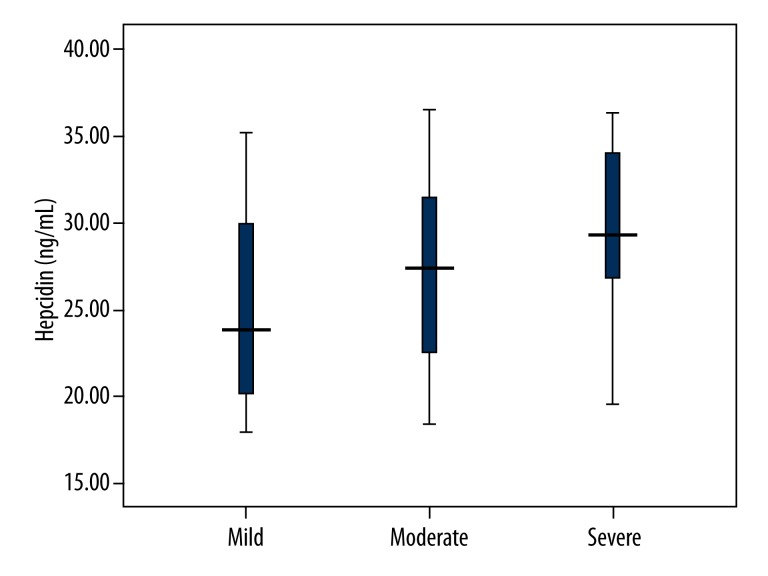
Serum hepcidin levels in mild, moderate, and severe OSAS patients are shown in Figure 1. Severe OSAS patients had significantly higher serum hepcidin levels compared with those in mild and moderate OSAS patients (P<0.001 and P=0.014, respectively). Furthermore, serum hepcidin levels were significantly elevated in moderate OSAS patients compared with mild OSAS patients (P=0.018). Spearman correlation analysis suggested that serum hepcidin levels were correlated with the severity of OSAS (r=0.293, P<0.001).
Figure 1.
Serum hepcidin levels in mild, moderate, and severe OSAS patients. Severe OSAS patients showed significantly higher levels of serum hepcidin compared with mild and moderate OSAS patients. In addition, Moderate OSAS patients had significantly higher serum hepcidin levels compared with mild OSAS patients.
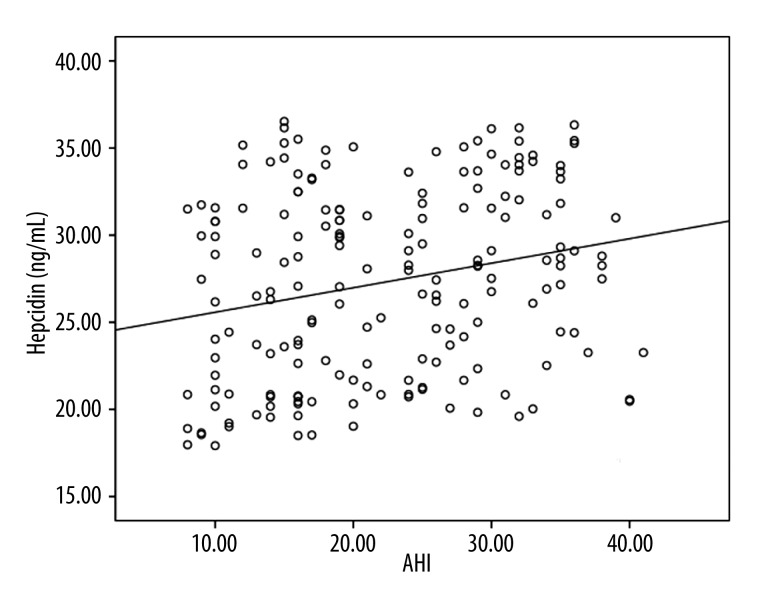
The association of serum hepcidin levels with AHI
Spearman correlation analysis showed that serum hepcidin levels in OSAS patients were correlated with AHI (r=0.239, P<0.001). Figure 2 shows the correlation between serum hepcidin levels and AHI.
Figure 2.
Correlation of serum hepcidin levels with AHI.
Discussion
In the present study, we found that OSAS patients had significantly higher serum hepcidin levels compared with healthy controls. Serum levels of hepcidin were significantly elevated in severe OSAS patients compared with mild and moderate OSAS patients. In addition, serum hepcidin levels were correlated with AHI. To the best of our knowledge, this is the first cross-sectional study to demonstrate the association of serum hepcidin levels with the presence and severity of OSAS.
OSAS is associated with significant cardiovascular morbidity and mortality [9]. OSAS significantly increases the risk of cardiovascular disease, including hypertension, heart failure, arrhythmia, coronary artery disease (CAD), stroke, and diabetes mellitus [10]. Therefore, it is essential to evaluate the risk of OSAS at an early stage and then to target strategies to prevent and treat OSAS. Nowadays, biomarkers are utilized in early disease diagnosis, prognosis of disease progress, and as therapy for many diseases. The present study revealed that serum hepcidin levels were significantly higher in OSAS patients compared with healthy controls, suggesting the potential role of hepcidin in the pathophysiology of OSAS. Recently, Abakay et al. also reported increased mean serum hepcidin levels in OSAS subjects compared to control subjects [11]. In addition, our results also found that serum hepcidin levels in severe OSAS patients were significantly elevated compared with those in mild and moderate OSAS patients. Serum hepcidin levels were correlated with the severity of OSAS. Therefore, serum hepcidin levels are suggested to be associated with the presence and severity of OSAS.
Besides the liver, hepcidin is also expressed in other tissues, including adipose tissue. Adipocytes are capable of producing hepcidin, probably triggered by inflammatory stimuli [12]. Recent studies have indicated that hepcidin is closely associated with obesity. The expression levels of hepcidin were found to be increased in adipose tissue of obese patients [7]. Obese children showed significantly elevated serum hepcidin levels compared with non-obese children [13]. Furthermore, serum hepcidin levels in obese premenopausal women were significantly decreased after 6 months of restrictive bariatric surgery, indicating the association of serum hepcidin with weight loss [14]. Obesity is considered as a major risk factor for OSAS [15]. The prevalence of OSAS in obese or severely obese subjects is nearly twice that of normal-weight subjects [16]. This indicates that hepcidin may be involved in the pathogenesis of OSAS by serving as a link between obesity and OSAS. However, the exact mechanism needs to be explored by further studies.
Inflammation has been indicated as a potential mechanism for OSAS. Elevated levels of various circulating inflammatory markers have been suggested to be associated with OSAS [3]. Hepcidin is a conserved 25-amino acid peptide identified as an acute inflammatory marker and the pivotal regulator of iron distribution in the body. Several inflammatory cytokines, such as interleukin-6 (IL-6), IL-1β, and tumor necrosis factor-α, have been shown to promote the expression of hepcidin [17]. Circulating hepcidin levels has also been correlated with acute phase proteins such as C-reactive protein (CRP) and alpha-1-acid-glycoprotein [18]. These results indicate that hepcidin may play an important role in inflammation. Hepcidin may be involved in the mechanism of OSAS through the inflammatory pathway.
The limitation of the present study should be considered. First, this was a cross-sectional study performed in a relatively small sample. Therefore, our findings should be validated by further longitudinal studies in a larger population sample. Second, we did not assess whether continuous positive airway pressure (CPAP) treatment has an effect on the serum levels of hepcidin.
Conclusions
This study showed that serum hepcidin levels were higher in OSAS patients compared with healthy controls. Serum hepcidin levels in severe OSAS patients were significantly higher compared with those in mild and moderate OSAS patients. Our findings suggest that elevated serum hepcidin levels are associated with the presence and severity of OSAS.
Footnotes
Declaration of interest
All authors have nothing to declare.
Source of support: Departmental sources
References
- 1.Zirlik S, Hauck T, Fuchs FS, et al. Leptin, obestatin and apelin levels in patients with obstructive sleep apnoea syndrome. Med Sci Monit. 2011;17(3):CR159–64. doi: 10.12659/MSM.881450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 3.Al Lawati N, Mulgrew A, Cheema R, et al. Pro-atherogenic cytokine profile of patients with suspected obstructive sleep apnea. Sleep Breath. 2009;13:391–95. doi: 10.1007/s11325-009-0259-1. [DOI] [PubMed] [Google Scholar]
- 4.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–10. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 5.Lou DQ, Lesbordes JC, Nicolas G, et al. Iron- and inflammation-induced hepcidin gene expression in mice is not mediated by Kupffer cells in vivo. Hepatology. 2005;41:1056–64. doi: 10.1002/hep.20663. [DOI] [PubMed] [Google Scholar]
- 6.Kuwahara R, Kofman AV, Landis CS, et al. The hepatic stem cell niche: identification by label-retaining cell assay. Hepatology. 2008;47:1994–2002. doi: 10.1002/hep.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bekri S, Gual P, Anty R, et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788–96. doi: 10.1053/j.gastro.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 8.American Academy of Sleep Medicine. International classification of sleep disorders, 2nd edition: Diagnostic and coding manual. Westchester, Illinois: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 9.Martinez D, Klein C, Rahmeier L, et al. Sleep apnea is a stronger predictor for coronary heart disease than traditional risk factors. Sleep Breath. 2012;16:695–701. doi: 10.1007/s11325-011-0559-0. [DOI] [PubMed] [Google Scholar]
- 10.Weiss JW, Launois SH, Anand A, Garpestad E. Cardiovascular morbidity in obstructive sleep apnea. Prog Cardiovasc Dis. 1999;41:367–76. doi: 10.1053/pcad.1999.0410367. [DOI] [PubMed] [Google Scholar]
- 11.Abakay O, Abakay A, Palanci Y, et al. Relationship between hepcidin levels and periodic limb movement disorder in patients with obstructive sleep apnea syndrome. Sleep Breath. 2014 doi: 10.1007/s11325-014-1028-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Coimbra S, Catarino C, Santos-Silva A. The role of adipocytes in the modulation of iron metabolism in obesity. Obes Rev. 2013;14:771–79. doi: 10.1111/obr.12057. [DOI] [PubMed] [Google Scholar]
- 13.Sanad M, Osman M, Gharib A. Obesity modulate serum hepcidin and treatment outcome of iron deficiency anemia in children: a case control study. Ital J Pediatr. 2011;37:34. doi: 10.1186/1824-7288-37-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tussing-Humphreys LM, Nemeth E, Fantuzzi G, et al. Decreased serum hepcidin and improved functional iron status 6 months after restrictive bariatric surgery. Obesity (Silver Spring) 2010;18:2010–16. doi: 10.1038/oby.2009.490. [DOI] [PubMed] [Google Scholar]
- 15.Sunitha C, Aravindkumar S. Obstructive sleep apnea: clinical and diagnostic features. Indian J Dent Res. 2009;20:487–91. doi: 10.4103/0970-9290.59457. [DOI] [PubMed] [Google Scholar]
- 16.Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137:711–19. doi: 10.1378/chest.09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaffney-Stomberg E, McClung JP. Inflammation and diminished iron status: mechanisms and functional outcomes. Curr Opin Clin Nutr Metab Care. 2012;15:605–13. doi: 10.1097/MCO.0b013e328357f63b. [DOI] [PubMed] [Google Scholar]
- 18.Park KH, Sawada T, Kosuge T, et al. Surgical inflammation induces hepcidin production after abdominal surgery. World J Surg. 2012;36:800–6. doi: 10.1007/s00268-012-1473-8. [DOI] [PubMed] [Google Scholar]