Abstract
Cancer patients undergoing chemotherapy treatment are advised to increase food intake to overcome the therapy-induced side effects, and weight loss. Dietary restriction is known to slow down the aging process and hence reduce age-related diseases such as cancer. Fasting or short-term starvation is more effective than dietary restriction to prevent cancer growth since starved cells switch off signals for growth and reproduction and enter a protective mode, while cancer cells, being mutated, are not sensitized by any external growth signals and are not protected against any stress. This phenomenon is known as differential stress resistance (DSR). Nutrient signaling pathways involving growth hormone/insulin-like growth factor-1 axis and its downstream effectors, play a key role in DSR in response to starvation controlling the other cell maintenance systems, such as autophagy and apoptosis, that are related to the tumorigenesis. Yeast cells lacking these effectors are better protected against oxidative stress compared to normal cells. In the same way, starvation protects many cell lines and mice against high-dose chemotherapeutic drugs. According to a series of studies, fasting results in overall reduction in chemotherapy side effects in cancer patients. Data shows that starvation-dependent differential chemotherapy is safe, feasible and effective in cancer treatment, but the possible side effects of starvation limit its efficacy. However, further studies and clinical trials may result in its implementation in cancer treatment.
Keywords: Starvation, Dietary restriction, Differential stress resistance, Differential chemotherapy, Nutrient-signaling pathways
Introduction
Cancer is a leading cause of death worldwide. Many types of cancer treatments are available, but all have associated side effects. These side effects are likely due to the genotoxicity of the therapeutic agents that kill both cancer and normal cells. Chemotherapy is the most widely recommended treatment for any cancer type. At first it was believed that chemotherapy drugs selectively killed cancer cells but it is now known that they also damage normal cells, resulting in dose dependent side effects such as nausea, fatigue, vomiting, hair loss, and, in severe cases, even death. Hence, there is a need for treatments that specifically destroy malignant cells, but not normal cells.
Low or no calorie intake is known to be effective against many diseases, specifically cancer. Different forms of food deprivation include dietary restriction (DR) or calorie restriction (CR), which may be acute or intermittent, fasting, and starvation. Short-term starvation is much more effective in protecting normal cells against chemotherapy drugs compared to dietary restriction.1
Starvation differentially protects normal cells but not cancer cells against oxidative stress and chemotherapy drugs, this phenomenon is termed differential stress resistance (DSR).2 Normally cell energy is balanced between cell growth and reproduction, and maintenance. During starvation cell energy is diverted to the maintenance systems but cancer cells, being mutated, show no response to stressed conditions like starvation and are selectively killed by chemotherapeutic drugs. A case report showed that short-term starvation of up to five days followed by chemotherapy is not only safe and feasible, but also helps to ameliorate chemotherapy related side-effects.3
This article addresses the physiological responses of the body against starvation, the effect of starvation on insulin, insulin-like growth factor-1 (IGF-1) and its downstream effectors and their conserved role in cellular protection, and compares of the efficacy of dietary restriction to that of the fasting or starvation. The correlation between starvation, autophagy, and cancer is also discussed and finally, the mechanism of differential chemotherapy.
Physiology of starvation
The human population has faced many great famines in the past, but the condition of fasting and even severe starvation is not limited to humans but includes microorganisms and other animals. To cope with such stressed conditions all organisms have evolved specific survival mechanisms. In normal conditions, glucose is the main source of energy for the metabolic reaction. During starvation, the process of gluconeogenesis forms glucose, using stored lipids and proteins. This glucose supply is limited and is used by the brain while the remaining energy requirement is fulfilled by ketone bodies (acetoacetate, β-hydroxybutyrate and acetone).4
Physiological responses to starvation are quite different for that of CR diets. During the first 10 hours of starvation, when the body utilizes any consumed food, stored glycogen becomes the main source of energy. When liver glycogen stores deplete, amino acids act as a source of energy. Eventually fatty acids and glycerol from adipose tissue serve as a major source of energy. If starvation continues for a week or more, fat derived β-hydroxybutyrate becomes the most abundant ketone in body while glucose production becomes very low. When fat stores are depleted rapid muscle degradation occurs and provides energy to the body through gluconeogenesis.5 Weight loss caused by starvation is initially intense and rapid but eventually becomes gradual. A study has shown that during the first week of starvation average weight loss is 0.9kg/day and reduces to 0.3kg/day by the third week.6 This data shows that short-term starvation is feasible and may be effective to prevent age-related diseases. Side effects of short-term starvation may include headaches, lightheadedness, nausea, weakness, and edema.7 It is important to keep in mind that starvation should not be administered to patients deficient in metabolic pathways. For example, patients with fructose 1,6-bisphosphate deficiency may experience hypoglycemia, acidosis, and failure to produce ketone bodies, the key source of energy during starvation.8
Starvation is likely to be the most effective with respect to cancer treatment based on previous studies. According to Raffaghello and collagues,9 DR may not be a feasible option as it has been shown to only delay cancer onset but does not stop its progression, it also causes chronic weight loss, delayed wound healing, and immunologic impairment. Furthermore, it takes a long time to become effective (weeks to months).10 Intermittent fasting/alternate day feeding is more advantageous than DR, as it provides greater stress resistance than daily DR of 30-40%, but it also requires two to three weeks to be effective.11 Lee and Longo have presented a comparison of DR and starvation in their study suggesting that starvation is more effective in starvation based differential chemotherapy.1
Starvation, autophagy and cancer
Autophagy is a conserved process of self-eating adopted by cells in response to starvation and other stressed conditions. It is a catabolic process, which involves the lysosomal breakdown of proteins and organelles for maintenance of cellular homeostasis and reduction of metabolic stress. Defected autophagy may cause diseases such as neurodegradation, liver failure, inflammatory bowel disease, aging, and most importantly cancer. Autophagy is a complex process that acts as a double-edged sword in tumorigenesis. Loss of autophagy may cause increased cellular proliferation and plays an important role in cancer cell survival mechanisms. Cancer cells demand high energy and nutrients for rapid cell growth. This demand may be fulfilled by high rates of aerobic glycolysis and glutaminolysis causing reactive oxygen species (ROS) production and oxidative stress in tumors leading to their own autophagy for survival. ROS may also stimulate autophagy in stromal cells and release metabolites by tumor associated fibroblasts (TAFs), which are utilized by neighboring cancer cells.12
Studies suggest that in mammals, starvation of essential nutrients and growth factors induces autophagy.13 Genes such as AMP-activated protein kinase (AMPK), mammalian target of rapamycin (mTOR), and TAFs, regulating aging and stress resistance, also regulate autophagy.13 Starvation based autophagy causes autophagosomes to engulf cytosol or organelles, which are then degraded by lysosomes and nutrients are released. In this process, autophagy-related gene 1 (ATG-1) plays an important role.14 Some oncogenes are reported to inhibit autophagy, through mTOR activation, reflecting that catabolic processes are suppressed in tumors to facilitate accumulation of cell mass.15 In contrast, many cancer cells show increased autophagy, which is mediated by ammonia production a by-product of glutaminolysis that occurs in the mitochondria of tumor cells undergoing the Warburg effect (the enhanced conversion of glucose to lactate by tumor cells).12,16 This ammonia then increases autophagy and protects cancer cells from apoptosis induced by tumor necrosis factor-α (TNF-α) and controls the tumor proliferation under metabolic stress.17
It has been reported that serum and growth factors induce Jun genes (expressing JunB and c-Jun proteins) which inhibit autophagy. Jun proteins are major components of oncogenic transcription factor AP-1, regulating cell cycle and tumorigenesis.18 During starvation JunB protein is repressed by down-regulation at both transcriptional and post-transcriptional levels by reduction in mTOR activity. Mice fibroblasts deficient in JunB exhibit increased autophagy. Low levels of JunB are found in many human cancer cell lines.18 Hence, starvation induced autophagy may protect normal cells and be fatal to cancer cells.19 These studies suggest that autophagy is another factor that may differentially effect cancer and normal cells under starvation conditions.
Starvation, nutrient signaling pathways, and growth factors
The starvation-induced physiological state is similar to that caused by mutated nutrient signaling pathways, which lead to decreased activity.11 DR is also helpful against age-related malfunctioning and reduces the risk factors of diabetes, cardiovascular diseases, and cancer in rodents, monkeys, and humans by inactivating one or more nutrient signaling pathways.20,21
Nutrient signaling pathways are fundamental to aging and its resulting problems. Conserved nutrient signaling pathways in different organisms reveal that they have evolved to overcome the periods of starvation.19 Deletion of TOR or Sch9 increases the lifespan of yeast several fold.22,23 In worms, flies, and mammals DR reduces the activity of nutrient signaling pathways through reduced levels of growth factors such as IGF-1. The role of TOR and S6K is conserved from yeast to mammals. Another pathway effecting the aging process, involves three proteins Ras, adenylate cyclase (AC), and protein kinase A (PKA).24,25 In yeast, starvation, induced by switching from glucose to water, doubles their lifespan.26,27 In response to glucose starvation Ras-AC-PKA and TOR-Sch9 signals are decreased, and there is increased transcription activity of zinc finger transcription factors Msn2 and Msn4.28,29 In Caenorhabditis elegans (C. elegans) reduced activity of the insulin/IGF-1 like signaling (IIS) pathway is involved in lifespan expansion.30 This expanded lifespan requires the forkhead box transcription factor (FOXO) DAF-16, which regulates the genes involved in cellular stress and detoxification of free radicals.31
In drosophila, the evolutionary role of IIS is conserved as in yeast and C. elegans.32 Dietary restriction in drosophila effects insulin peptides and transcript levels of insulin-like pepticle 5 (dilp5),33 and loss of FOXO can be compensated by other pathways such as the TOR pathway.34 In mice, mutations in growth hormone and IIS genes can increase their lifespan.35 Furthermore, growth hormone (GH) deficiency increases the expression of antioxidant enzymes and increases stress resistance in muscle cells and fibroblasts of mice.36 DR expands the lifespan of mice by up to 60% partially by reducing the occurrence of chronic diseases such as cancer.20 In the same way IGF-1 signaling deficiency may reduce the probability of spontaneous mutations and tumors in the kidneys and small intestine of DR mice.37 In individuals with GH-receptor deficiency diabetes and cancer are very rare, but they don’t reach older ages.38
Hormonal changes, as a result of dietary restriction, may include increased adiponectin, and reduced triiodothyronine, testosterone, insulin, cholesterol, C-reactive protein, blood pressure and intima media thickness of carotid arteries, which are risk factors for cardiovascular diseases and cancer.21,39
Inhibition of IGF-1 receptor is thought to enhance the killing of various cancer cell types. However, the role of IGF-1 signaling in differential protection is unknown. Studies reveal that starvation reduces levels of IGF-1 significantly. Short-term starvation of 72 hours reduces circulating IGF-1 by 70% and increases the level of IGF binding protein (IGFBP-1) an IGF-1 inhibitor, by 11-fold. Furthermore, glucose levels were reduced by 41% and GH levels were increased slightly. Mice with 70% reduced IGF-1 levels were protected against chemotherapy drugs.40 Hence, these nutrient signaling pathways are basic key regulators of starvation induced differential stress resistance in normal and cancer cells.
Starvation and stress resistance
Most organisms live in fluctuating environmental conditions, where food is not available all the time. During food deprivation, they have to undergo some harsh or stressed conditions such as UV radiation, heat and cold, so nature has evolved the physiology of organisms to handle these problems. Most organisms show increased stress resistance as a result of food deprivation.41 During starvation organisms withdraw energy from growth and reproduction and invest it in maintenance systems of the cell. Nutrient signaling pathways and stress resistance transcription factors are key regulators of growth, metabolism, and protection against oxidants and other toxins.9
Short-term starvation induces mitochondrial activity to counteract the energy depletion. CR and, specifically, glucose restriction results in increased mitochondrial respiration in yeast and worms.42-45 CR also enhances mitochondrial biogenesis and oxidative phosphorylation in rodents and increased respiratory capacity in mammalian cells.46,47 In contrast, mitochondrial dysfunction may result in age-related diseases such as diabetes, cancer, and neurodegeneration.48,49 In mitochondria ROS are produced as a by-product. Increased metabolic rate as a result of DR causes increased ROS production, which is a major determinant of life span.50 Increased ROS production induces a positive response that increases stress resistance, which results in long-term reduction of oxidative stress,51 that further increases life span and lowers the risk of cancer, diabetes and other age-related diseases.41,52 Hyperbaric and hyperthermic conditions in C. elegans and mice, respectively, mimic DR and trigger the same adaptive response for increased stress resistance.53
Deficiencies in these nutrient signaling pathways or growth factors pay protection to the cells against stressed conditions.54 Carbon or nitrogen starvation in Escherichia coli (E.coli) enhances the components of stress resistance, such as heat shock proteins.55-57 Yeast when switched from glucose to water had increased life span and protection against oxidative stress and heat shock.58 Furthermore, yeast cells deficient in nutrient signaling Ras/cAMP/PKA and TOR/S6K pathways had double the life span and increased stress resistance.58,59
Intermittent fasting in worms increases oxidative and heat stress resistance and extends life span by up to 56% through Ras homolog enriched in brain (RHEB-1) and TOR signaling pathways. These pathways are linked to DAF-16.60 In contrast, a high glucose concentration reduces life span possibly by decreasing DAF-16 activity.61 In Drosophila melanogaster, moderate DR along with amino acid restriction increases resistance against oxidative damage.62 The starvation dependent protection against oxidative stress is controlled by d4E-BP, located downstream to P13K/AKT/DFOXO3; it binds with elF4E suppressing translation. The pathway is necessary for stressed cells to divert energy from growth to protection.63
DR mice also show increased stress resistance. Fourty-eight to 60 hours of fasting enhances cellular protection in three different strains of mice against the oxidative effect of chemotherapy drug etoposide.1 Furthermore, 72 hours of starvation shields CD-1 mice from lethal doses of doxorubicin, which normally could cause death due to oxidative stress induced cardiotoxicity.40
Starvation and differential stress resistance
The basis of DSR is the differences between normal and cancer cells. These differences provide the opportunity for the fasting, and conserved nutrient signaling pathways to protect the normal cells against high-dose chemotherapy. Cancer cells are self-sufficient in growth signals and hence are insensitive to external growth signals.64
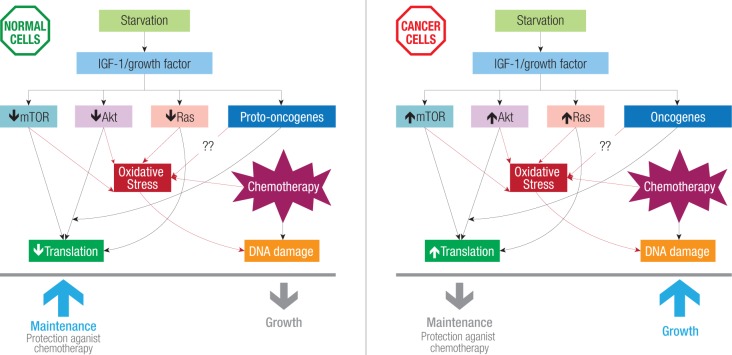
Normally all cells require growth factors for proper growth and reproduction whereas cancer cells are not affected by the absence or presence of growth factors.64 The self-sufficiency of cancer cells is mainly due to mutations in proto-oncogenes coding Ras, Akt, and mTOR [Figure 1] (the main components of nutrient signaling pathways). These mutated pathways enable cancer cells to proliferate independently of growth signals and show no response to the stressed conditions.64 Therefore, even in the presence of a stress such as fasting cancer cells go on proliferating and do not show any resistance against stress.
Figure 1.
Starvation-induced differential stress resistance. Starvation decreases serum insulin-like growth factor 1 (IGF-1) levels, and growth factors, which further affect downstream growth regulators mammalian target of rapamycin (mTOR), Akt and Ras. In cancer cells these regulators are activated in response to reduced IGF-1, increasing oxidative stress leading to DNA, damage and eventually cell death in the presence of chemotherapy drugs.
In cancer cells some proteins are either over expressed or absent, as a quarter of all cancers have a mutated form of Ras.65 Phosphatase and tensin homolog (PTEN), a tumor suppressor, and p53, a coordinator of stress resistance, are inactivated in cancer cells.66 Oncogenic mutations also cause the hyperactivation of IGF-1, Akt, Ras, mTOR and PKA in most human cancers. Reduced levels of IGF-1 are also important in starvation based DSR,40 possibly by negatively regulating the nutrient signaling pathway. There are many chemotherapy drugs that specifically target IGF-1 receptors, since it is commonly activated in variety of tumors.67
Cancer cells are dependent on glycolysis rather than oxidative phosphorylation (Warburg effect). This provides a clue that a starvation induced raise in ketone bodies may increase the sensitivity of cancer cells.11 Although glycolysis produces a low amount of adenosine tri-phosphate (ATP) compared to oxidative phosphorylation, it provides essential biosynthetic precursors required by fast growing cells.68 Moreover, cancer cell survival is amplified by increased glucose metabolism due to restriction of cytochrome-c mediated apoptosis in cancer cells.69 Hence, glucose starvation sensitizes cancer cells against various stresses and may induce apoptosis in cancer cells.
Studies reveal that 72 hours of starvation reduces glucose levels by 41% and IGF-1 levels by 70%, while IGFBP1 increases 11.4-fold.40 Mice fibroblast cells with deleted Igf-1 (R-) were highly protected compared to R+ cells, which were treated with doxorubicin after 24 hours of fasting and Igf-1 infusion to these cells reverses the protection effect.40
In mice, with subcutaneous breast cancer (4T1) it has been shown that two cycles of fasting alone was as effective as two cycles of cyclophosphamide treatment. The mice regained normal weight within five days of re-feeding.54 In another study DNA damage in two types of cancer cells due to fasting alone was greater than that for cells treated with chemotherapy alone. Fasting alone is effective in retardation of the growth of many cancer cell types, and in some cases equates the effect of chemotherapy drugs. However, fasting in combination with chemotherapy is more effective.54
Starvation and differential chemotherapy
As discussed earlier, short-term starvation differentially protects normal and cancer cells against oxidative stress. Raffaghello and his colleagues have worked extensively in this field and have shown that short-term starvation induces DSR against chemotherapy drugs in yeast and mice.1 In their study, yeast cells pre-starved for 48–60 hours were protected against oxidative stress and high doses of chemotherapy drugs compared to control. Another experiment using mice, showed that starved mice were protected against two to three times the maximum dose recommended for humans of the drug etoposide (Etopophos, Bristol-Myers Squibb), while 40% of the un-starved mice died and those remaining exhibited severe toxicity. A second group of mice were starved for 60 hours and then treated with a dose of etoposide four to five times the maximum dose recommended for humans. Mice in the control group died by day five, whereas no pre-starved mice died or showed toxicity. Starved mice lost 40% of their weight but regained it after one week of re-feeding. To investigate the effect of starvation on tumor cells they injected the mice with neuroblastoma-type malignant cells. The result was that the starved mice were more protected than control mice and lived longer when exposed to high dose chemotherapy.1
In a more recent study, Lee and his colleagues investigated the effect of fasting on cancer cell survival in the presence or absence of chemotherapeutic drugs.54 They repeated the experiments performed by Raffaghello et al, on yeast and found the same results. Furthermore, they found that starvation for 24 hours before and 24 hours after chemotherapy, sensitized 15 of 17 cancer cell lines against doxorubicin and cyclophosphamide. Fasting alone has the same effect on the tumor cell mass as that of the drug cyclophosphamide. In a mice model of human matastatic cancer, after 34 days of five fasting cycles (48 hours each), tumor size was less than half of that in normally fed mice. However, fasting cycles combined with cyclophosphamide treatment reduced tumor size to less than half of that achieved in treatments with cyclophosphamide or fasting alone. However, as a result of combined fasting and chemotherapy, DNA damage was increased 20-fold in both cancer cell types.
There is also evidence of starvation-based differential chemotherapy in humans. Ten volunteers with different types of cancers were starved for 48–140 hours before chemotherapy and five–56 hours after. Overall, all patients showed decreased side effects of chemotherapy. None of the patients reported side effects caused by the fasting itself.2 This suggests that starvation-based differential chemotherapy is a safe and feasible option for treatment of cancer.
Discussion
Chemotherapy is the most commonly used cancer treatment, but it has many side effects, which can be life threatening as chemotherapeutic drugs damage both cancerous and normal cells. Short-term starvation or fasting, differentially kills cancer cells but not the normal cells in response to chemotherapy drugs. Fasting for three to five days is not acceptable by most patients, in this case alternate day fasting, or DR with protein restriction specifically, may provide similar results.11,70 Furthermore, there is the option to reduce IGF-1 and its downstream effectors without fasting by using drugs or by down-regulating genes, which may reduce cancer progression or sensitize cancer cells against chemotherapy drugs by mimicking fasting.10,40 However, short-term starvation, as experimented in yeast and mice, has potential to differentially kill cancerous cells compared to other interventions. Hence, further studies and clinical trials are required to evaluate the efficacy of differential chemotherapy.
Conclusion
Cancer is one of the biggest health care issues world over, and there is great need to adopt better and effective strategies for cure and treatment of cancer with more emphases on early detection and prevention.71
Available studies indicate that fasting/starvation greatly reduces the side effects of chemotherapy and starvation-based differential chemotherapy is a safe, feasible and effective option in cancer treatment. However, only few studies have been reported so far, which are not conclusive, therefore, there is great need to do further studies and clinical trials so this treatment could be used in standard practice.
Disclosure
The authors declared no conflict of interests. No funding was received for this work.
References
- 1.Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene 2011. Jul;30(30):3305-3316. 10.1038/onc.2011.91 [DOI] [PubMed] [Google Scholar]
- 2.Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A 2008. Jun;105(24):8215-8220. 10.1073/pnas.0708100105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, et al. Fasting and cancer treatment in humans: A case series report. Aging (Albany NY) 2009. Dec;1(12):988-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahill jr GF. Fuel metabolism in starvation. Annual review of nature. 2006; 26: 1-22. [DOI] [PubMed]
- 5.Wang T, Hung CC, Randall DJ. The comparative physiology of food deprivation: from feast to famine. Annu Rev Physiol 2006;68:223-251. 10.1146/annurev.physiol.68.040104.105739 [DOI] [PubMed] [Google Scholar]
- 6.Kerndt PR, Naughton JL, Driscoll CE, Loxterkamp DA. Fasting: the history, pathophysiology and complications. West J Med 1982. Nov;137(5):379-399. [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson TJ, Runcie J, Miller V. Treatment of obesity by total fasting for up to 249 days. Lancet 1966. Nov;2(7471):992-996. 10.1016/S0140-6736(66)92925-4 [DOI] [PubMed] [Google Scholar]
- 8.van den Berghe G. Disorders of gluconeogenesis. J Inherit Metab Dis 1996;19(4):470-477. 10.1007/BF01799108 [DOI] [PubMed] [Google Scholar]
- 9.Raffaghello L, Safdie F, Bianchi G, Dorff T, Fontana L, Longo VD. Fasting and differential chemotherapy protection in patients. Cell Cycle 2010. Nov;9(22):4474-4476. 10.4161/cc.9.22.13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontana L, Partridge L, Longo VD. Extending healthy life span–from yeast to humans. Science 2010. Apr;328(5976):321-326. 10.1126/science.1172539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson JB, John S, Laub DR. Pretreatment with alternate day modified fast will permit higher dose and frequency of cancer chemotherapy and better cure rates. Med Hypotheses 2009. Apr;72(4):381-382. 10.1016/j.mehy.2008.07.064 [DOI] [PubMed] [Google Scholar]
- 12.Lozy F, Karantza V. Autophagy and cancer cell metabolism. Semin Cell Dev Biol 2012. Jun;23(4):395-401. 10.1016/j.semcdb.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell 2010. Oct;40(2):280-293. 10.1016/j.molcel.2010.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wrighton KH. Autophagy: Myosin II moves in on autophagosomes. Nat Rev Mol Cell Biol 2011. Feb;12(2):77. 10.1038/nrm3053 [DOI] [PubMed] [Google Scholar]
- 15.Maiuri MC, Tasdemir E, Criollo A, Morselli E, Vicencio JM, Carnuccio R, et al. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ 2009. Jan;16(1):87-93. 10.1038/cdd.2008.131 [DOI] [PubMed] [Google Scholar]
- 16.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the warberg effect: the metabolic requirments of cell proliferation. Science 2009;324:1183-1191 . 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eng CH, Abraham RT. Glutaminolysis yields a metabolic by-product that stimulates autophagy. Autophagy 2010. Oct;6(7):968-970. 10.4161/auto.6.7.13082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yogev O, Shaulian E. Jun proteins inhibit autophagy and induce cell death. Autophagy 2010. May;6(4):566-567. 10.4161/auto.6.4.11950 [DOI] [PubMed] [Google Scholar]
- 19.Mariño G, Kroemer G. Ammonia: a diffusible factor released by proliferating cells that induces autophagy. Sci Signal 2010;3(124):pe19. 10.1126/scisignal.3124pe19 [DOI] [PubMed] [Google Scholar]
- 20.Anderson RM, Shanmuganayagam D, Weindruch R. Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol 2009. Jan;37(1):47-51. 10.1177/0192623308329476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA 2007. Mar;297(9):986-994. 10.1001/jama.297.9.986 [DOI] [PubMed] [Google Scholar]
- 22.Kaeberlein M, Powers RW, III, Steffen KK, Westman EA, Hu D, Dang N, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 2005. Nov;310(5751):1193-1196. 10.1126/science.1115535 [DOI] [PubMed] [Google Scholar]
- 23.Wei M, Fabrizio P, Madia F, Hu J, Ge H, Li LM, et al. Tor1/Sch9-regulated carbon source substitution is as effective as calorie restriction in life span extension. PLoS Genet 2009. May;5(5):e1000467. 10.1371/journal.pgen.1000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science 2001. Apr;292(5515):288-290. 10.1126/science.1059497 [DOI] [PubMed] [Google Scholar]
- 25.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 2000. Sep;289(5487):2126-2128. 10.1126/science.289.5487.2126 [DOI] [PubMed] [Google Scholar]
- 26.Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, et al. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet 2008. Jan;4(1):e13. 10.1371/journal.pgen.0040013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longo VD, Ellerby LM, Bredesen DE, Valentine JS, Gralla EB. Human Bcl-2 reverses survival defects in yeast lacking superoxide dismutase and delays death of wild-type yeast. J Cell Biol 1997. Jun;137(7):1581-1588. 10.1083/jcb.137.7.1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medvedik O, Lamming DW, Kim KD, Sinclair DA. MSN2 and MSN4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in Saccharomyces cerevisiae. PLoS Biol 2007. Oct;5(10):e261. 10.1371/journal.pbio.0050261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaeberlein M, Burtner CR, Kennedy BK. Recent developments in yeast aging. PLoS Genet 2007. May;3(5):e84. 10.1371/journal.pgen.0030084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson TE. Caenorhabditis elegans 2007: the premier model for the study of aging. Exp Gerontol 2008. Jan;43(1):1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doonan R, McElwee JJ, Matthijssens F, Walker GA, Houthoofd K, Back P, et al. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev 2008. Dec;22(23):3236-3241. 10.1101/gad.504808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piper MD, Selman C, McElwee JJ, Partridge L. Separating cause from effect: how does insulin/IGF signalling control lifespan in worms, flies and mice? J Intern Med 2008. Feb;263(2):179-191. 10.1111/j.1365-2796.2007.01906.x [DOI] [PubMed] [Google Scholar]
- 33.Yan L, Vatner DE, O’Connor JP, Ivessa A, Ge H, Chen W, et al. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell 2007. Jul;130(2):247-258. 10.1016/j.cell.2007.05.038 [DOI] [PubMed] [Google Scholar]
- 34.Giannakou ME, Goss M, Partridge L. Role of dFOXO in lifespan extension by dietary restriction in Drosophila melanogaster: not required, but its activity modulates the response. Aging Cell 2008. Mar;7(2):187-198. 10.1111/j.1474-9726.2007.00362.x [DOI] [PubMed] [Google Scholar]
- 35.Bartke A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology 2005. Sep;146(9):3718-3723. 10.1210/en.2005-0411 [DOI] [PubMed] [Google Scholar]
- 36.Brown-Borg HM. Hormonal regulation of longevity in mammals. Ageing Res Rev 2007. May;6(1):28-45. 10.1016/j.arr.2007.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia AM, Busuttil RA, Calder RB, Dollé ME, Diaz V, McMahan CA, et al. Effect of Ames dwarfism and caloric restriction on spontaneous DNA mutation frequency in different mouse tissues. Mech Ageing Dev 2008. Sep;129(9):528-533. 10.1016/j.mad.2008.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shevah O, Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm IGF Res 2007. Feb;17(1):54-57. 10.1016/j.ghir.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 39.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A 2004. Apr;101(17):6659-6663. 10.1073/pnas.0308291101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, et al. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res 2010. Feb;70(4):1564-1572. 10.1158/0008-5472.CAN-09-3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free Radic Biol Med 2011. Jul;51(2):327-336. 10.1016/j.freeradbiomed.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 42.Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 2007. Oct;6(4):280-293. 10.1016/j.cmet.2007.08.011 [DOI] [PubMed] [Google Scholar]
- 43.Sharma PK, Agrawal V, Roy N. Mitochondria-mediated hormetic response in life span extension of calorie-restricted Saccharomyces cerevisiae. Age (Dordr) 2011. Jun;33(2):143-154. 10.1007/s11357-010-9169-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zuin A, Carmona M, Morales-Ivorra I, Gabrielli N, Vivancos AP, Ayté J, et al. Lifespan extension by calorie restriction relies on the Sty1 MAP kinase stress pathway. EMBO J 2010. Mar;29(5):981-991. 10.1038/emboj.2009.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 2002. Jul;418(6895):344-348. 10.1038/nature00829 [DOI] [PubMed] [Google Scholar]
- 46.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 2005. Oct;310(5746):314-317. 10.1126/science.1117728 [DOI] [PubMed] [Google Scholar]
- 47.López-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A 2006. Feb;103(6):1768-1773. 10.1073/pnas.0510452103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiederkehr A, Wollheim CB. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology 2006. Jun;147(6):2643-2649. 10.1210/en.2006-0057 [DOI] [PubMed] [Google Scholar]
- 49.Tatsuta T, Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J 2008. Jan;27(2):306-314. 10.1038/sj.emboj.7601972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol 1956. Jul;11(3):298-300. 10.1093/geronj/11.3.298 [DOI] [PubMed] [Google Scholar]
- 51.Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp Gerontol 2010. Jun;45(6):410-418. 10.1016/j.exger.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 52.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 2009. Jun;119(21):2789-2797. 10.1161/CIRCULATIONAHA.108.822403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masoro EJ. The role of hormesis in life extension by dietary restriction. Interdiscip Top Gerontol 2007;35:1-17. [DOI] [PubMed] [Google Scholar]
- 54.Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Montalvo AM et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4:124ra27. [DOI] [PMC free article] [PubMed]
- 55.Lee C, Raffaghello L, Longo VD. Starvation, detoxification, and multidrug resistance in cancer therapy. Drug Resist Updat 2012. Feb-Apr;15(1-2):114-122. 10.1016/j.drup.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matin A. The molecular basis of carbon-starvation-induced general resistance in Escherichia coli. Mol Microbiol 1991. Jan;5(1):3-10. 10.1111/j.1365-2958.1991.tb01819.x [DOI] [PubMed] [Google Scholar]
- 57.Hua Q, Yang C, Oshima T, Mori H, Shimizu K. Analysis of gene expression in Escherichia coli in response to changes of growth-limiting nutrient in chemostat cultures. Appl Environ Microbiol 2004. Apr;70(4):2354-2366. 10.1128/AEM.70.4.2354-2366.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, et al. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet 2008. Jan;4(1):e13. 10.1371/journal.pgen.0040013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei M, Fabrizio P, Madia F, Hu J, Ge H, Li LM, et al. Tor1/Sch9-regulated carbon source substitution is as effective as calorie restriction in life span extension. PLoS Genet 2009. May;5(5):e1000467. 10.1371/journal.pgen.1000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Honjoh S, Yamamoto T, Uno M, Nishida E. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature 2009. Feb;457(7230):726-730. 10.1038/nature07583 [DOI] [PubMed] [Google Scholar]
- 61.Lee SJ, Murphy CT, Kenyon C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab 2009. Nov;10(5):379-391. 10.1016/j.cmet.2009.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vigne P, Frelin C. Diet dependent longevity and hypoxic tolerance of adult Drosophila melanogaster. Mech Ageing Dev 2007. May-Jun;128(5-6):401-406. 10.1016/j.mad.2007.05.008 [DOI] [PubMed] [Google Scholar]
- 63.Tettweiler G, Miron M, Jenkins M, Sonenberg N, Lasko PF. Starvation and oxidative stress resistance in Drosophila are mediated through the eIF4E-binding protein, d4E-BP. Genes Dev 2005. Aug;19(16):1840-1843. 10.1101/gad.1311805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000. Jan;100(1):57-70. 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- 65.Medema RH, de Vries-Smits AM, van der Zon GC, Maassen JA, Bos JL. Ras activation by insulin and epidermal growth factor through enhanced exchange of guanine nucleotides on p21ras. Mol Cell Biol 1993. Jan;13(1):155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer 2011. Apr;11(4):289-301. 10.1038/nrc3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer 2004. Jul;4(7):505-518. 10.1038/nrc1387 [DOI] [PubMed] [Google Scholar]
- 68.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell, 4th edn. Garland Science2002, New York. [Google Scholar]
- 69.Vaughn AE, Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat Cell Biol 2008. Dec;10(12):1477-1483. 10.1038/ncb1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fontana L, Klein S, Holloszy JO. Long-term low-protein, low-calorie diet and endurance exercise modulate metabolic factors associated with cancer risk. Am J Clin Nutr 2006. Dec;84(6):1456-1462. [DOI] [PubMed] [Google Scholar]
- 71.Al-Moundhri M. The need for holistic cancer care framework: breast cancer care as an example. Oman Med J 2013. Sep;28(5):300-301. 10.5001/omj.2013.90 [DOI] [PMC free article] [PubMed] [Google Scholar]