Abstract
Background
There is a little or no data available on the natural Babesia bovis (B. bovis) infection in water buffaloes (Bubalus bubalis) comparing to the available one for cattle. This study was conducted to investigate the natural B. bovis infection in water buffaloes in comparison to crossbred cattle under field conditions in Egypt.
Methods:
A total of 35 buffaloes and cattle were clinically and laboratory investigated from March to June 2008. Twenty-nine buffaloes and cattle out of 35 were naturally infected with B. bovis and showed signs of bovine babesiosis. Three cows and three buffaloes showed no clinical signs and were free from external, internal, and blood parasites served as control group.
Results:
Babesia bovis-infected cattle showed typical signs of bovine babesiosis while B. bovis-infected buffaloes showed a milder form (less severe) of the clinical signs. Advanced cases of cattle showed dark brown to dark red (coffee-color) urine, hemoglobinuria and nervous manifestations while these manifestations were not detected in the infected buffaloes. Hematological changes in both species however, these changes were less significant in buffaloes than those reported in cattle.
Conclusion:
This paper documents the first description of natural B. bovis infection in water buffaloes which were found to be more likely to be tolerant than cattle to the natural clinical infection with B. bovis and its subsequent haematological changes. Our finding may lead to a better understanding of the disease pattern of B. bovis infection under field conditions in buffaloes.
Keywords: Babesia bovis, Natural infection, Water buffaloes, Clinical signs, Hematology
Introduction
Bovine babesiosis is economically the most important tick-borne disease of cattle worldwide including areas of Australia, Africa, South and Central America (Bock et al. 2004). In addition, the United States is continuously under threat of reintroduction of the vector and the disease (Bock et al. 2004). Under natural conditions, Babesia bovis transmitted by the tick Rhipicephalus microplus, although transmission may occur by other tick species (Papadopoulos et al. 1996). The life cycle of B. bovis has two phases. In the vertebrate host they multiply by merogony in erythrocytes while in ticks by sporogony (Susan and Asa 1999).
The disease is the most prevalent in tropical and subtropical countries, affecting cattle industries causing a major economic impact worldwide (Böse et al. 1995, Nayel et al. 2012). Costs due to babesiosis are the results of high mortality, ill-thrift, abortions, loss of milk/meat production and draft power and from control measures such as acaricide treatments, purchase of vaccines and therapeutics (Bock et al. 2004). It was estimated that losses and control of babesiosis and anaplasmosis in Kenya, Zimbabwe, Tanzania, South Africa, China, India, Indonesia and Philippines cost 5.1, 5.4, 6.8, 21.6, 19.4, 57.2, 3.1 and 0.6 million US dollars annually, respectively (Bock et al. 2004). Kaufmann (1996) reported that the mortality rates in cattle infected by B. bovis without treatment could reach 70–80%. The diagnosis of ruminant babesiosis is generally based upon the microscopic examination of Giemsa-stained blood smears and clinical signs in acute cases. Previous studies provide information on the relative susceptibility of various breeds of cattle to Babesia infection (Bock et al. 1997).
In Egypt, bovine babesiosis is caused mainly by B. bigemina and B. bovis and considered as the most important and endemic parasitic disease affecting cattle (Nagati 1947, Adham et al. 2009). Bovine babesiosis has a significant impact on meat and milk production and consequently, on livestock management (Adham et al. 2009). The rapidly changing patterns of demand for cattle and its products point to cattle production being an important and increasing component of the Egyptian agriculture economy which required improving cattle health. Egyptians farmers cross between the Holstein-Friesian breed and a native local breed known as Baladi cattle breed (Bos taurus) to improve production and disease resistance.
Water buffaloes (Bubalus bubalis) represent an important source of various human needs, such as meat, horns, hides, milk and milk products, leather, land plowing, and transportation of people and crops (Somparn et al. 2004). Due to the fact that water buffaloes are raised together with cattle, among which bovine babesiosis is highly prevalent (Iseki et al. 2010), they might be potential carriers for Babesia parasites. Babesia bovis infection was experimentally investigated in splenectomised buffaloes (Mahmoud and Abou-Zeina 2008). Yao et al. (1997) reported the clinical findings on buffaloes after experimental infection with cryopreserved B. bovis parasites. However, efforts to furnish information about natural infection with B. bovis in water buffaloes as well as crossbred cattle are necessary for better understanding of disease pattern under uncontrolled field conditions and subsequently, implementation the suitable policy for treatment and control.
The objective of the present study was to investigate the natural B. bovis infection in water buffaloes in comparison to crossbred cattle under field conditions in Egypt.
Materials and Methods
Animals and sampling
Blood samples were collected from the jugular vein into EDTA-containing tubes from 35 animals (23 cattle and 12 buffaloes) of both sexes and aged 2–5 years, and were originating from different villages: El-Aslogy, Shobk Basta and Tel Basta around Zagazig city, Sharkia province. These animals were divided into 2 groups, the field-exposed (diseased) group comprised of 20 cattle and 9 water buffaloes which was examined at the Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Zagazig University, during the period from March to June 2008 and resulted to have persistent fever, anemia and anorexia. The control group (3 cows and 3 buffaloes) was carefully examined clinically and parasitologically and found healthy and free from external, internal, and hemoparasites.
The common available foods for animals under the present study were mainly consisted of Barseem (Trifolium alexandrinum), rice or wheat straw and concentrate mixture (1–2 kg/head/day). Crossbred cattle were resulted from crossbreeding between the imported Holstein-Friesian breed cattle and Egyptian Baladi cattle breed (Bos taurus). Samples collection, handling and examination of cattle and buffaloes under the current study were done after approval of animals’ owners.
Clinical examination
Animals were subjected to clinical and hematological examinations at Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Zagazig University. The filed-exposed group showed various degrees of bovine babesiosis such as high fever (>40 °C), anorexia, hemoglobinuria (bloody urine), anemia, and jaundice. They were also infested with ticks to various degrees. The control group was examined thoroughly for presence of any abnormal clinical changes and external parasites, and was thoroughly examined by different laboratory techniques such as direct smear, flotation, sedimentation and Barmen’s techniques and blood film to confirm the absence of any internal parasites and/or hemoparasites (Rosenberger 1990).
Microscopic examination
Thin blood films were prepared immediately after taking the blood samples directly from the ear vein in the field to allow these smears to dry by air then fixed by using methanol for about 3–5min, allow them to dry by air after fixation step then stained with Giemsa stain diluted at 8% with distilled water for about 30–45min. They were dried by air and examined on Olympus microscope using oil immersion lens at x1000 magnification (Kelly 1984). Blood film was examined for B. bovis at 1/4–1/2 inch from the end of the film by visually scanning from one side of the film to other (cross–sectional method) to give constant and representative examination. Each blood film and at least twenty microscopic fields of each slide were examined twice before being considered negative.
Hemogram Parameters
Approximately 5ml of blood was taken from the jugular vein of all animals with a syringe containing EDTA. The blood samples were subjected to hematological parameters analysis (Schalm et al. 1975, Coles 1986), that is, red blood cell (RBC) and white blood cell (WBC) counts were made with improved Neubauer hacmocytometers, Hemoglobin concentration (Hb) by Sahli’s haemoglobinometer and packed cell volume (PCV%) by microhematocrit tubes. Differential WBC counts were performed on thin blood smears by the Battlement technique.
Statistical analysis
The obtained data were statistical analyzed by mean of computer based statistical program, SPSS (Borenstein et al. 1997). Data were analyzed using Student’s t-test to compare the mean data between groups. The results obtained were expressed as mean ±SD. Differences were considered statistically significant based on P< 0.05.
Results
Clinical Findings
Cattle infected with B. bovis showed typical clinical signs of babesiosis, Table 1. Briefly, highly rise in body temperature (40–41.5 °C), conjunctival and vaginal mucous membranes were anemic and the clinical severity was ranged from paleness in mild cases to severe yellow discoloration (icterus) in more progressive cases, dark brown to dark red (coffee-color) urine, hemoglobinuria was common sign in cattle with severe clinical manifestation and accelerated heart and respiratory rates. Some cases showed nervous manifestations in advanced stages such as incoordination and head pressing. Various degrees of tick infestations were present around groins, horns, Inter-mandibular space, and ears.
Table 1.
Clinical findings of Babaesia bovis (B. bovis)-infected cattle and buffaloes in comparison to control group under natural field conditions
| Parameters | Field-exposed animals (n= 29) | Control group (n= 6) | |
|---|---|---|---|
| B. bovis infected cattle (n= 20) | B. bovis infected buffloes (n= 9) | ||
| Temperature (°C) | 40.8 (40.3–41.4) | 40.6 (40.2–41.1) | 38.5 (38.1–38.8) |
| Appetite | anorexia | anorexia | Normal |
| Haemoglobinuria | Present in advanced acute cases | No haemoglobinuria | Straw yellow |
| Mucus membranes | Varied from paleness in mild cases to severe yellow discoloration in progressive ones | paleness and anemic of mucous membranes | Bright red, moist and no lesions |
| Icterus | Marked and characteristic | No | No |
| Nervous signs | Incoordination, head pressing | No | No |
| Body condition | Thin/emaciation and anemic | Weak to moderate | Good |
| Lymph nodes | Normal | Normal | Normal |
| Respiration | Exaggerated/accelerated | Exaggerated/accelerated | Normal |
| Giemsa-stained blood film | Intraerythrocytic piroplasms of B. bovis in the form of pyriform or pear-shaped Advanced cases showed severe haemolytic anemia with anisocytosis and poikilocytosis | Intraerythrocytic piroplasms of B. bovis in the form of pyriform or pear-shaped | No parasites, normal RBCs |
Water buffaloes infected with B. bovis showed a milder form (less severe) of clinical signs of infection in comparison to the clinical signs appeared on B. bovis-infected cattle. These clinical signs were in the form of highly rise in body temperature (40–41.5 °C), and conjunctival and vaginal mucous membranes were mainly anemic and pale in color and loss of body condition. Icterus, hemoglobinuria and nervous manifestations were not detected/observed in the affected buffaloes.
Hematological Findings
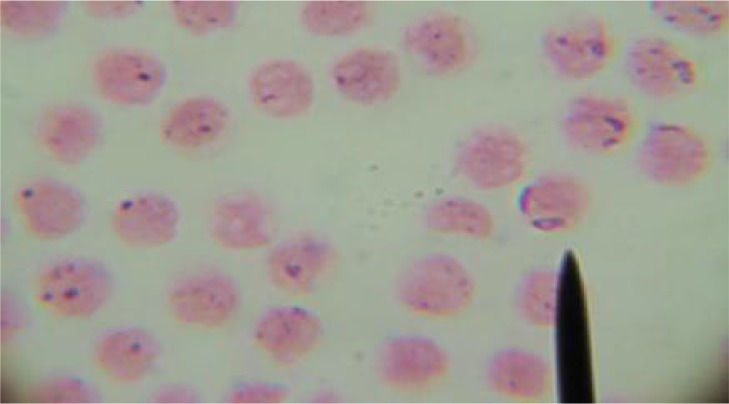
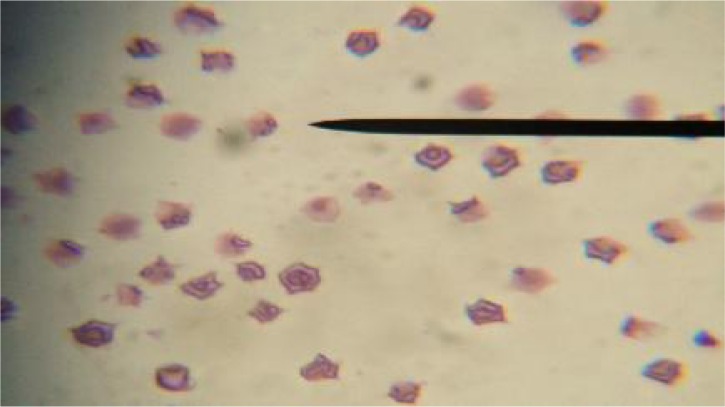
Giemsa-stained blood smears from B. bovis infected animals showed intra-erythrocytic piroplasms of B. bovis that were in the form of pyriform or pear-shaped, Fig. 1. Blood smears from B. bovis progressive cases of cattle showed severe hemolytic anemia with abnormalities in cell size (Anisocytosis) and cell shape (Poikilocytosis) of erythrocytes, Fig. 2. Giemsa-stained blood smears from B. bovis uninfected cattle and buffaloes showed no parasites or erythrocytic changes. The control group resulted to be healthy on clinical and laboratory examination and free from external, internal and hemoparasites.
Fig. 1.
Giemsa-stained blood smear showing intra-erythrocytic Pyriform (Pear-shape) of Babesia bovis in pairs
Fig. 2.
Giemsa-stained blood smear showing severe hemolytic anemia with abnormalities in cell size (Anisocytosis) and cell shape (Poikilocytosis) of erythrocytes from advanced cases of cattle naturally infected with Babesia bovis
The mean values of RBCs, hemoglobin amount, PCV %, WBCs, and differential leucocytic count are listed in Table 2. Briefly, the important findings can be summarized as follows; there is a clear significant difference in the haematological parameters between B. bovis-infected buffaloes and B. bovis-infected cattle in comparison to control group at P-value (≤ 0.01) and (≤ 0.001), respectively. Haematological changes for B. bovis-infected buffaloes were less significant than their changes for B. bovis-infected cattle.
Table 2.
Hemogram findings of B. bovis-infected cattle and buffaloes in comparison to control group under natural field conditions (mean ±S.E)
| Parameters | Field-exposed animals (n= 29) | Control (n= 6) | |
|---|---|---|---|
| B. bovis infected buffaloes (n= 9) | B. bovis infected cattle (n= 20) | ||
| RBCs 1 (106 /ml) | 3.4±0.1 a ** | 2.6±0.2 b *** | 6.9±0.9 c |
| Hb 2 (g/dl) | 7.3±0.2 a ** | 5.5±0.3 b *** | 12.7±1.2 c |
| PCV 3 (%) | 24.6±1.1 a ** | 18.1±1.5 b *** | 36±1.3 c |
| WBCs 4 (103 mul−1) | 7.3±0.2 a* | 7.2±0.5 a * | 9.3±0.5 b |
| Lymphocytes (103 mul−1) | 4.1±0.1 a* | 3.8±0.5 a * | 4.9±0.8 b |
| Monocytes (103 mul−1) | 0.46±0.07 a | 0.44±0.9 a | 0.46±0.01 a |
| Neutrophils (103 mul−1) | 3.1±0.4 a | 2.9±0.5 a | 3.2±0.6 a |
| Eosinophils (103 mul−1) | 0.2±0.01 a | 0.2±0.02 a | 0.2±0.04 a |
| Basophils (103 mul−1) | 0.02±0.001 a | 0.02±0.002 a | 0.02±0.003 a |
Values with different superscripts are significantly different from each other (
P≤ 0.05,
P≤ 0.01,
P≤ 0.001).
RBCs= Red Blood Cells,
Hb= hemoglobin,
PCV= packed cell volume,
WBCs= White Blood Cells
Discussion
Babesia bovis is one of the most important blood parasites affecting cattle and buffaloes and in its acute forms, it lowers the productive performance of the affected animals (Talkhan et al. 2010, Ziapour et al. 2011). Most of the previous studies described the clinical findings of B. bovis infection in cattle of different breeds. To the best of our knowledge, this is the first study which investigating thoroughly the clinical and hematological pictures of natural B. bovis infection in water buffaloes under uncontrolled field conditions. The reported clinical findings of B. bovis infection in cattle come in agreement with what was previously described by Brown and Torres (2008), Georgi et al. (1990) and Kaufmann (1996). The demonstrated high fever could be attributed as response to the effect of unspecific toxic substances produced during the metabolism of Babesia on thermoregulatory (Radostits et al. 2000).
It was notable that water buffaloes identified as B. bovis-infected showed a milder form (less severe) of clinical signs of B. bovis infection in comparison to the clinical signs appeared on B. bovis-infected cattle. This variation was represented in appearance of icterus, hemoglobinuria and nervous manifestations in clinically infected cattle while they were not reported in infected buffaloes. This finding may propose that buffaloes may have more tolerance to clinical infection with B. bovis than cattle. Tolerance means that the host is infected by the pathogen, but suffers little adverse effect (FAO 2007). It could be argued that buffaloes may have acquired natural immunity/tolerance to some extent against B. bovis infection. Genetic variations within the host between cattle and buffaloes may explain the variation in their susceptibility. This finding is inconsistent with the experimental findings by Yao et al. (1997) who found that B. bovis produces acute, often fatal, infections in buffaloes.
The proportion of buffaloes identified as B. bovis-infected was (31.1%) while the proportion of cattle identified as B. bovis-infected was (68.9%) within the same period of the study. This finding could suggest that water buffalos have more tendencies to be carriers (apparently healthy) than showing clinical manifestations. This finding supported by Ferreri et al. (2008) who noticed that water buffaloes seem to be unapparent carriers of the parasite.
The marked anemia and hemoglobinuria in cattle could be attributed to the severe haemolytic process associated the presence of Babesia piroplams inside the erythrocytes and destruction of large numbers of these erythrocytes by the parasite resulting in hemoglobinaemia and consequently hemoglobinuria (Georgi et al. 1990, Fujinaga 1981), the physical effect of parasite multiplication (Wright 1981), the increase of phagocytosis of erythrocytes by activated macrophages (Shoda et al. 2000, Court et al. 2001), the production of an anti-erythrocyte antibody (Goe’s et al. 2007) and the increase in the erythrocytic membrane permeability (Alkhalil et al. 2007).
Hematological findings showed a significant decrease in the RBCs, WBCs counts, Hb concentrations and PCV% in the B. bovis-infected animals in comparison to the control group, these observations were similar to what were reported by Col and Uslu (2007) and Durrani et al. (2006). It seems that the immune response to the babesial antigen causes a significant lymphocytosis. This comes in agreement with what was described previously by Schalm (2000). Hematological changes resulted from B. bovis infection in buffaloes are less significant than the hematological changes of B. bovis infection in cattle, table 2. This finding reflected clinically on B. bovis-infected buffaloes which showed a milder form of clinical picture of B. bovis infection than B. bovis-infected cattle.
The hemolytic anemia due to the breakdown of erythrocytes membranes leading to release of hemoglobin and manifested by the presence of free hemoglobin resulting in the discoloration of the plasma (Sowemimo-Coker 2002). Extensive lipid peroxidation in biological membranes causes disturbances of its structural integrity, loss of fluidity, decrease in membrane potential, and increased permeability to ions (Gutteridge 1995). These changes lead to rupture of the membrane and release of cell contents (Halliwell and Chirico 1993). Babesia parasite (Alkhalil et al. 2007), and B. bovis (Aikawa et al. 1985) dramatically alters the permeability of its host erythrocytes to various organic solutes. B. bovis infection is associated with impairment of blood parameters and subsequently, hematological examination may be a useful tool for confirmation the clinical diagnosis of bovine babesiosis.
Conclusion
Water buffaloes showed a milder form of B. bovis infection than cattle suggesting that buffaloes may be more tolerant to the clinical infection with B. bovis than cattle. Hematological changes as a result of B. bovis infection in buffaloes are less significant than hematological changes of B. bovis infection in cattle. B. bovis infection might be associated with severe clinical and hematological changes especially in cattle, which might be of bad prognosis.
With respect to the study population, future studies should consider a larger sample size for cattle and buffaloes for the robustness of the findings. Recent molecular techniques such as PCR showed many advantages with regard to the sensitivity and specificity for detection and surveillance of hemoparasites (Nayel et al. 2012, Hüe et al. 2013, Ybañez et al. 2013). Hence, it would be advisable for future studies to use such techniques for investigating the B. bovis infection in buffaloes.
Acknowledgments
This study was accomplished and funded by Faculty of Veterinary Medicine, Zagazig University, Egypt. Author is grateful to Prof Farouk Elbalkemy and Dr Ahmed Abdelaal, Department of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, for their support. Special thanks for Prof Ahmed Badwy, Department of Parasitology, Faculty of Veterinary Medicine, Zagazig University for his help in the lab examination. The author of this paper has not a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
References
- Adham FK, Abd-El-Samie EM, Gabre RM, El-Hussein H. Detection of tick blood parasites in Egypt using PCR assay I-Babesia bovis and Babesia bigemina. Parasitol Res. 2009;105(3):721–730. doi: 10.1007/s00436-009-1443-8. [DOI] [PubMed] [Google Scholar]
- Aikawa M, Rabbege J, Uni S, Ristic M, Miller LH. Structural alteration of the membrane of erythrocytes infected with Babesia bovis. Am J Trop Med Hyg. 1985;34(1):45–49. doi: 10.4269/ajtmh.1985.34.45. [DOI] [PubMed] [Google Scholar]
- Alkhalil A, Hill DA, Desai SA. Babesia and plasmodia increase host erythrocyte permeability through distinct mechanisms. Cell Microbiol. 2007;9(4):851–860. doi: 10.1111/j.1462-5822.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- Bock R, Jackson L, De Vos AJ, Jorgensen W. Babesiosis of cattle. Parasitol. 2004;129(Suppl):S247–269. doi: 10.1017/s0031182004005190. [DOI] [PubMed] [Google Scholar]
- Bock RE, De Vos AJ, Kingston TG, McLellan DJ. Effect of breed of cattle on innate resistance to infection with Babesia bovis, B. bigemina and Anaplasma marginale. . Aust Vet J. 1997;75(5):337–340. doi: 10.1111/j.1751-0813.1997.tb15706.x. Erratum in: [DOI] [PubMed] [Google Scholar]
- Borenstein M, Rothstein H, Cohen J. Sample power: Release 1.00. SPSS, Inc; Chicago, IL: 1997. [Google Scholar]
- Brown C, Torres A. USAHA Foreign Animal Diseases, Seventh Edition. Committee of Foreign and Emerging Diseases of the US Animal Health Association. Boca Publications Group, Inc; 2008. [Google Scholar]
- Col R, Uslu U. Changes in selected serum components in cattle naturally infected with Theileria annulata. Bull Vet Inst Pulawy. 2007;51:15–18. [Google Scholar]
- Coles EA. Veterinary Clinical Pathology. 4th ed. WB. Sunders Company; Philadelphia: 1986. [Google Scholar]
- Court RA, Jackson LA, Lee RP. Elevated anti-parasitic activity in peripheral blood monocytes and neutrophils of cattle infected with Babesia bovis. Int J Parasitol. 2001;31(1):29–37. doi: 10.1016/s0020-7519(00)00144-2. [DOI] [PubMed] [Google Scholar]
- Durrani AZ, Kamal N, Khan MS. Incidence of theileriosis and estimation of packed cell volume, total erythrocyte count and hemoglobin in buffaloes. J Anim Pl Sci. 2006;16(3–4):85–88. [Google Scholar]
- FAO. The state of the world’s animal genetic resources for food and agriculture. Rome: 2007. p. 101. Section E. ftp://ftp.fao.org/docrep/fao/010/a1250e/a1250e05.pdf Accessed May 14, 2012. [Google Scholar]
- Ferreri L, Benitez D, Dominguez M, Rodriguez A, Asenzo G, Mesplet M, Florin-Christensen M, Schnittger L. Water Buffalos as carriers of Babesia bovis in Argentina. Ann N Y Acad Sci. 2008;1149:149–151. doi: 10.1196/annals.1428.036. [DOI] [PubMed] [Google Scholar]
- Fujinaga T. Bovine Babesiosis in Japan clinical and clinicopathological studies on cattle experimentally infected with Babesia ovata. Jpn J Vet Sci. 1981;43:803–813. doi: 10.1292/jvms1939.43.803. [DOI] [PubMed] [Google Scholar]
- Georgi JR, Georgi ME, Theodrides VJ. Parasitology for Veterinarians. 5th ed. WB Saunders Company. Harcourt Brace Jovanovich, Inc; Philadelphia: 1990. [Google Scholar]
- Góes TS, Góes VS, Ribeiro MF, Gontijo CM. Bovine babesiosis: anti-erythrocyte antibodies purification from the sera of naturally infected cattle. Vet Immunol Immunop. 2007;116(3–4):215–218. doi: 10.1016/j.vetimm.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Gutteridge JMC. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41(12 Pt 2):1819–1828. [PubMed] [Google Scholar]
- Halliwell B, Chirico S. Lipid peroxidation: Its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57(5):715–725. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- Hüe T, Graille M, Mortelecque A, Desoutter D, Delathière J, Marchal C, Teurlai M, Barré N. Diagnostic methods used to monitor an outbreak of babesiosis (Babesia bovis) in a herd of feral cattle in New Caledonia. Aust Vet J. 2013;91(6):254–258. doi: 10.1111/avj.12059. [DOI] [PubMed] [Google Scholar]
- Iseki H, Zhou L, Kim C, Inpankaew T, Sununta C, Yokoyama N, Xuan X, Jittapalapong S, Igarashi I. Seroprevalence of Babesia infections of dairy cows in northern Thailand. Vet Parasitol. 2010;170(3–4):193–196. doi: 10.1016/j.vetpar.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Kaufmann J. Parasitic Infections of Domestic Animals, A diagnostic Manual. Birkhaüser Verlag; Basel: 1996. [Google Scholar]
- Kelly WG. Veterinary Clinical Diagnosis. 3rd ed. Bailliere Tindall; London: 1984. [Google Scholar]
- Mahmoud MS, Abou-Zeina HAA. Current State in the Serological Diagnosis of Babesiosis and Haematological Changes in Splenectomised Buffaloes. Global Veterinaria. 2008;2(5):271–281. [Google Scholar]
- Nagati HE. Some new and rare records of piroplasmosis with a list of the species of Babesia and Theileria so far recorded from Egypt. Vet Rec. 1947;59:145–147. [PubMed] [Google Scholar]
- Nayel M, El-Dakhly KM, Aboulaila M, Elsify A, Hassan H, Ibrahim E, Salama A, Yanai T. The use of different diagnostic tools for Babesia and Theileria parasites in cattle in Menofia, Egypt. Parasitol Res. 2012;111(3):1019–1024. doi: 10.1007/s00436-012-2926-6. [DOI] [PubMed] [Google Scholar]
- Papadopoulos B, Perié NM, Uilenberg G. Piroplasms of domestic animals in the Macedonia region of Greece. 1. Serological cross-reactions. Vet Parasitol. 1996;63(1–2):41–56. doi: 10.1016/0304-4017(95)00878-0. [DOI] [PubMed] [Google Scholar]
- Radostits OMC, Blood CCG, Hinchcliff KW. Veterinary Medicine. 9th Ed. WB Saunders; London: 2000. [Google Scholar]
- Rosenberger G. Clinical Examination of Cattle. Verlag Paul Parey; Berlin/Hamburg: 1990. [Google Scholar]
- Schalm OW. Classification and Laboratory evaluation of anaemia. In: Feldman BF, Zinkl JG, Jain NC, editors. Veterinary Hematology. 5th Ed. Lippincott Williams and Wilkins; Philadelphia: 2000. pp. 143–162. [Google Scholar]
- Schalm OW, Jain NC, Carroll EJ. Veterinary Haematology. 3rd Edition . Lea and Febiger; Philadelphia: 1975. [Google Scholar]
- Shoda LKM, Palmer GH, Florin-Christensen J, Florin-Christensen M, Godson DL, Brown WC. Babesia bovis stimulated macrophages express interleukin-1b, interleukin-12, tumor necrosis factor alpha, and nitric oxide and inhibit parasite replication in vitro. Infect Immun. 2000;68(9):5139–5145. doi: 10.1128/iai.68.9.5139-5145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somparn P, Gibb MJ, Markvichitr K, Chaiyabutr N, Thummabood S, Vajrabukka C. Analysis of climatic risk for cattle and buffalo production in northeast Thailand. Int J Biometeorol. 2004;49(1):59–64. doi: 10.1007/s00484-004-0206-6. [DOI] [PubMed] [Google Scholar]
- Sowemimo-Coker SO. Red blood cell hemolysis during processing. Transfus Med Rev. 2002;16(1):46–60. doi: 10.1053/tmrv.2002.29404. [DOI] [PubMed] [Google Scholar]
- Susan EA, Asa M. The Merk Veterinary Manual. 8th Ed. Merk and Co. Inc.; 1999. [Google Scholar]
- Talkhan OFA, Radwan MEI, Ali MA. Cattle babesiosis and associated biochemical alteration in Kalubyia Governorate. Nat Sci. 2010;12:24–27. [Google Scholar]
- Wright IG. Biochemical characteristics of Babesia and physiochemical reactions in the host. In: Babesiosis MR, Kreier JP, editors. Babesiosis. Academic Press, Inc; New York: 1981. pp. 171–205. [Google Scholar]
- Yao B, Zhao J, Ma L, Liu Z. Studies on the pathogenicity of Babesia bovis in water buffaloes after cryopreservation and resuscitation. Trop Anim Health Prod. 1997;29(4 Supp l):40S–42S. doi: 10.1007/BF02632916. [DOI] [PubMed] [Google Scholar]
- Ybañez AP, Sivakumar T, Ybañez RH, Vincoy MR, Tingson JA, Perez ZO, Gabotero SR, Buchorno LP, Inoue N, Matsumoto K, Inokuma H, Yokoyama N. Molecular survey of bovine vector-borne pathogens in Cebu, Philippines. Vet Parasitol. 2013 doi: 10.1016/j.vetpar.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Ziapour SP, Esfandiari B, Youssefi MR. Study of the prevalence of babesiosis in domesticated animals with suspected signs in Mazandaran province, north of Iran, during 2008. J Animal Vet Adv. 2011;10(6):712–714. [Google Scholar]