Abstract
Background
The present study was designed to investigate the insecticidal efficacy of four different classes of insecticides: pyrethroids, organophosphates, phenyl-pyrazoles and neo-nicotenoids. One representative chemical from each class was selected to compare the toxicity: deltamethrin from pyrethroids, Dichlorovinyl Dimethyl Phosphate (DDVP) from organophosphates, fipronil from phenyl-pyrazoles and imidacloprid from neo-nicotenoids. The objective of this study was to determine which of these insecticides were most effective against American cockroach.
Methods:
These insecticides were tested for their LC50 values against Periplaneta americana under topical bioassay method, using different concentrations for each chemical.
Results:
Fipronil 2.5% EC was highly effective at all concentrations applied, while DDVP 50% EC was least toxic amongst all. One way analysis of variance confirmed significant differences between mortality of P. americana and different concentrations applied (P< 0.05).
Conclusion:
Locality differentiation is an important factor in determining the range of resistance between various localities, as all three localities behaved differently in terms of their levels of resistance.
Keywords: Insecticides, Periplaneta americana, Topical bioassay, Resistance, Efficacy
Introduction
Periplaneta americana (Linnaeus) is an obnoxious and grubby domestic pest of tropical countries of the world (Lee and Robinson 2001). Their aesthetically unappealing damage to household materials and stored products, and transmission of diseases makes them a high priority pest. It is considered as a commonly found pest of bakeries, restaurants and kitchens (Gadd and Raubenheimer 2000, Lee and Lee 2000). They are usually found in basements, steam tunnels, grocery stores and boiler rooms. They contaminate food in contact by carrying filth and microbial pathogens on their body and legs (Watanabe et al. 2003, Fasulo et al. 2005).
As they have unsanitary mode of life, cockroaches are potent vectors of infectious pathogens (Vythilingam et al. 1997). Several species of Enterobacteriaceae including Klebsiella spp., Salmonella spp. and Escherichia spp. can be harbored in cuticle of P. americana (Mpuchane et al. 2006). According to the findings of Barcay (2004), E. coli, Compylobacter spp., Streptococcus spp., Salmonella spp., Toxoplasma gondii, Staphylococcus spp. and Shigella spp. are some of the medically important species of pathogens for which P. americana serves as an important vector.
Moreover, they may cause allergic reactions to some people by carrying the eggs of parasitic worms. Some of these major allergic responses include asthma, itching, swelling of eyelids, dermatitis and severe respiratory problems (Sarinho et al. 2004, Gore and Schal 2007, Pechal et al. 2007). Several diseases such as cholera, pneumonia, anthrax, tetanus, diphtheria, tuberculosis, enteric fever, gastroenteritis and food poisoning may spread by P. americana (Lane et al. 2001, Morane 2002). Kinfu and Erko (2008) have reported in their studies that cockroaches are potential carriers of human intestinal parasites especially Ascaris lumbricoides, Entamoeba coli and E. histolytica. Besides mechanically transmitting a variety of diseases, it is also responsible for causing insectophobia in some people (Czajka et al. 2003).
The control of P. americana can be done by applying insecticides to the hiding and resting places in the form of insecticidal dusts and residual sprays. Chemical control has been the most popular and effective method so far Lee and Robinson (2001), but their control with insecticides is not a suitable approach because of several reasons; the most important of which is that they may develop resistance against certain frequently used insecticides (Tsai and Lee 2001).
Resistance against pesticides is a phenomenon, mainly dependant on genetic basis. Exposure of a population of pest to a certain pesticide results in development of resistance against that chemical (pesticide). Not necessarily all insects need to be killed during this phenomenon. Hemingway et al. (1993) have reported that cockroaches have developed resistance against many groups of insecticides especially pyrethroids.
Much of the work regarding insecticidal efficacy has been done on german cockroach, however, very little data is available with respect to american cockroach. Therefore, keeping in view the work carried out by various researchers, the present work was designed to investigate the insecticidal efficacy of four groups of insecticides (organophosphates, pyrethroids, phenyl-pyrazoles and neo-nicotenoids) on P. americana, and the susceptibility of adults to these insecticides through laboratory bioassay using topical application method. Considering all the facts which demonstrate that these insecticides work as strong agents for the chemical control of P. americana, the aims of this study were to:
Investigate the current status of insecticide resistance in different field populations of P. americana, that is useful to find out their extent of resistance against commonly used insecticides.
Evaluate the toxic efficacy of four insecticides and to compare the older groups of insecticides i.e. pyrethroids and organophosphates with that of relatively new compounds i.e. neo-nicotenoids and phenyl-pyrazoles. This information will be helpful for the end users to choose better options for the management of these insects in Pakistan.
Materials and Methods
Experimental site
The laboratory bioassays were conducted at the Entomology Research Laboratory, LCWU, Lahore, Pakistan.
Experimental insect
Keeping in view their unappealing damages to house-hold materials and medical importance among all urban pests, P. americana was selected as experimental insect for testing against commonly used insecticides. These were collected from kitchens, basements, bakeries, and sewerage manholes by hand catch and trapping methods. Based on the previous history of insecticidal spray frequency, three different localities were selected for the collection of cockroaches (Table 1). These far apart areas were chosen to determine the degree of variability of insecticide resistance in different field populations of P. americana.
Table 1.
Information on field collected P. americana (L.) localities used in toxicity bioassay (during 2011)
| Localities | Sources | n | Dates of collection |
|---|---|---|---|
| SMH (Locality 1) | Sewerage manholes from LCWU, Lahore | 160 | March 28–30, 2011 |
| KTCH (Locality 2) | Kitchens and basements from residential areas in Lahore cantonment and nearby localities | 185 | April 15–20, 2011 |
| KLPT (Locality 3) | Bakeries and food-courts from Kot Lakhpat area | 210 | May 21–26, 2011 |
Collection of P. americana (L)
The collection traps for cockroaches comprised of simple glass or plastic jars with butter, sugar and bread inside. The trapping jars were comprised of two parts: the upper inside of it was coated with petroleum jelly (Vaseline®) in order to avoid their escape and keep them entrapped till bioassay. The sliding lower part was used to separate them from trapping jars and cockroaches were transferred to the main collection containers placed in laboratory. Considering the nocturnal habitat of cockroaches and to provide their preferred hidden places of living, a dark paper was used to cover these containers externally. They were provided with wet bread, card-board and water till the onset of bioassay. The collection containers were held at specified laboratory conditions (temperature = 28 ± 2 ºC and relative humidity = 75± 3%). Following the methodologies described by Appel et al. (1983) and Abd-Elghafar et al. (1990), only adult males were selected for bioassays because of their uniform weights, as compared to that of females.
Chemicals
Following technical grade insecticides and chemicals were used during bioassay: Imidacloprid 5% SC, Fipronil 2.5% EC, Deltamethrin 2.5% SC, DDVP 50% EC (Ali Akbar Group of Chemicals, Lahore), Acetone (E-Merck, D-6100, Germany), Distilled water. The choice of these insecticides was based on the fact that these chemicals have not been tested against P. americana in Pakistan so far. Moreover, relatively new insecticides like imidacloprid and fipronil were also needed to be investigated for their toxicity, in order to determine whether they are effective against field populations of P. americana. Therefore, comparison between four groups of insecticides (in terms of LC50 values) was studied by selecting one representative from each insecticidal class.
All chemical were obtained from Ali Akber group of chemical industries, 1-KM Bhoptian Chowk Defence Road, and Off Raiwind Road, Lahore, Pakistan.
Preparation of stock solution
For toxicity tests, stock solutions for four insecticides imidacloprid 5% SC, fipronil 2.5% EC, deltamethrin 2.5% SC and DDVP 50% EC were 5.0, 2.0, 20.0 and 50.0µl/ml respectively. Known quantity from each insecticide was pipetted out and dissolved in acetone (solvent for dissolving these insecticides). Following formula was used for preparing concentrations.
For preparing 5.0 µl/ ml stock solution of imidacloprid 5% SC, 2.0 µl of it was pipetted out and dissolved in acetone to make final volume up to 20.0 ml. 2.0 µl/ ml stock solution of fipronil 2.5% EC was prepared by dissolving 1.6 µl of it in acetone to make final volume up to 20.0 ml. For preparing 20.0 µl/ ml stock solution of deltamethrin 2.5% SC, 16.0 µl of it was pipetted out and dissolved in acetone to make final volume up to 20 ml. For preparing 50.0 µl/ ml stock solution of DDVP 50% EC, 2.0 µl of it was pipetted out and dissolved in acetone up to final volume of 20.0 ml.
Serial dilutions
Using these stock solutions, further serial dilutions were prepared to obtain five concentrations for each insecticide. Serial dilutions for imidacloprid 5% SC were 1.0, 2.0, 3.0, 4.0 and 5.0µl/ml. Serial dilutions for fipronil 2.5% EC were 0.05, 0.25, 0.5, 1.0 and 2.0µl/ ml. Serial dilutions for deltamethrin 2.5% SC were 1.0, 1.5, 10.0, 15.0 and 20.0µl/ml. Serial dilutions for DDVP 50% EC were 5.0, 10.0, 30.0, 40.0 and 50.0µl/ ml.
Topical application method
Topical bioassay was used to treat the adult male P. americana according to the method described by Lee et al. (1996). Four preliminary tests were performed to find out the discriminating doses. Twenty insects were separated from collection containers and for easy handling during bioassay, they were exposed to 4 ºC for 1–2 minutes to keep them immobilized for few minutes. Using an insulin syringe serving as micro applicator, 1.0µl of pre-determined amount of each insecticide concentration was topically applied to the first segment of abdominal sternites. Five to seven doses of each insecticide having mortality range between 1 and 100% were determined.
No insecticide was applied to control group, they only received 1.0µl acetone. Clean autoclaved petri plates were used to maintain the treated cockroaches and they were provided with bread and moistened cotton plug as food source. Each plate was having ten treated cockroaches which were provided with same temperature and humidity conditions as that of colony. Freshly prepared concentrations were applied for the treatment, starting from low to high dilutions and three replicates were prepared for each concentration. Pair of forceps was used to properly orient the immobilized cockroaches. Mortality criterion was as follows: if a cockroach was unable to return back to its normal position within 2–3 minutes after being touched with forceps, it was considered dead and counted in mortality data.
Data evaluation and statistical analysis
The data obtained from this study was subjected to mean, standard error, regression analysis and one way analysis of variance. The LC50 values for each insecticide were obtained by using EPA Probit Analysis Program used for calculating LC/EC values (version 1.5). Regression analysis of variance was applied to express the trend of variation within each treatment by using Microsoft Office Excel 2007. Results with P< 0.05 were considered statistically significant for all comparisons.
Results
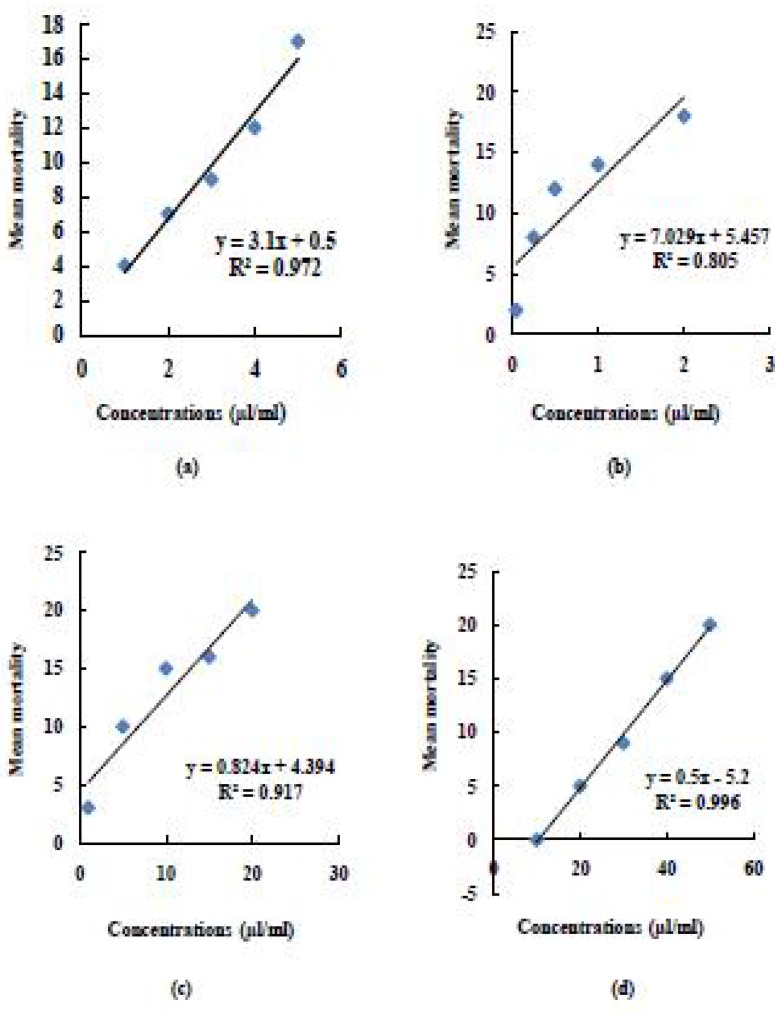
Table 2 represents the comparative toxicities of four insecticides against three localities of P. amercana (L). When different concentrations (1.0, 2.0, 3.0, 4.0 and 5.0µl/ ml) of imidacloprid 5% SC were tested against P. americana, variable range was obtained according to its respective localities. LC50 for SMH was 2.712 µl/ ml, whereas comparatively low values were recorded for KTCH and KLPT (1.966 µl/ ml and 1.318 µl/ ml respectively). The regression analysis of variance further confirmed that there was an increasing trend in mean mortality of insects after 48 hours of treatment [for SMH: b= 3.1± 0.9, F(1,4)= 106.78, P= 0.001, for KTCH: b= 3± 2.63, F(1,4)= 3.42E+ 32, P= 3.48E- 49 and for KLPT: b= 2.7± 0.1, F(1,4)= 729, P= 0.001) (Fig. 1–3(a)].
Table 2.
Comparative toxicities of four insecticides against three localities of P. americana (L) in topical application method
| Insecticides | Localities |
LC50 (µl/ml) |
95% confidence Limits |
Fit of probit line
|
RR | |||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Slope ± SE | χ2 (df) | P | ||||
| Imidacloprid 5% SC | SMH | 2.712 | 2.024 | 3.622 | 2.353±0.579 | 2.698 (4) | P< 0.001 | 2.00 |
| KTCH | 1.966 | 1.304 | 2.554 | 2.321±0.562 | 1.701 (4) | P< 0.001 | 1.50 | |
| KLPT | 1.318 | 0.714 | 1.771 | 2.419±0.585 | 3.091 (4) | P<0.001 | 1.00 | |
| Fipronil 2.5% EC | SMH | 0.362 | 0.220 | 0.554 | 1.552±0.294 | 0.435 (4) | P< 0.001 | 3.93 |
| KTCH | 0.176 | 0.092 | 0.275 | 1.508±0.289 | 1.960 (4) | P< 0.001 | 1.91 | |
| KLPT | 0.092 | 0.038 | 0.156 | 1.483±0.305 | 1.646 (4) | P< 0.001 | 1.00 | |
| Deltamethrin 2.5% SC | SMH | 4.145 | 2.540 | 5.879 | 1.938±0.352 | 3.252 (4) | P< 0.001 | 2 |
| KTCH | 2.500 | 1.226 | 3.874 | 1.632±0.321 | 2.951 (4) | P< 0.001 | 1.21 | |
| KLPT | 2.067 | 0.930 | 3.298 | 1.602±0.323 | 2.148 (4) | P< 0.001 | 1.00 | |
| DDVP 50% EC | SMH | 28.556 | 24.650 | 32.292 | 6.028±1.097 | 3.680 (4) | P< 0.001 | 1.42 |
| KTCH | 26.388 | 22.293 | 30.380 | 5.018±0.885 | 4.164 (4) | P< 0.001 | 1.31 | |
| KLPT | 20.138 | 16.119 | 23.900 | 4.035±0.695 | 6.600 (4) | P< 0.001 | 1.00 | |
Fig. 1.
Regression line of (a) Imidacloprid 5% SC (b) Fipronil 2.5% EC (c) Deltamethrin 2.5% SC and (d) DDVP 50% EC on mean mortality of SMH (locality 1) of P. americana (L)
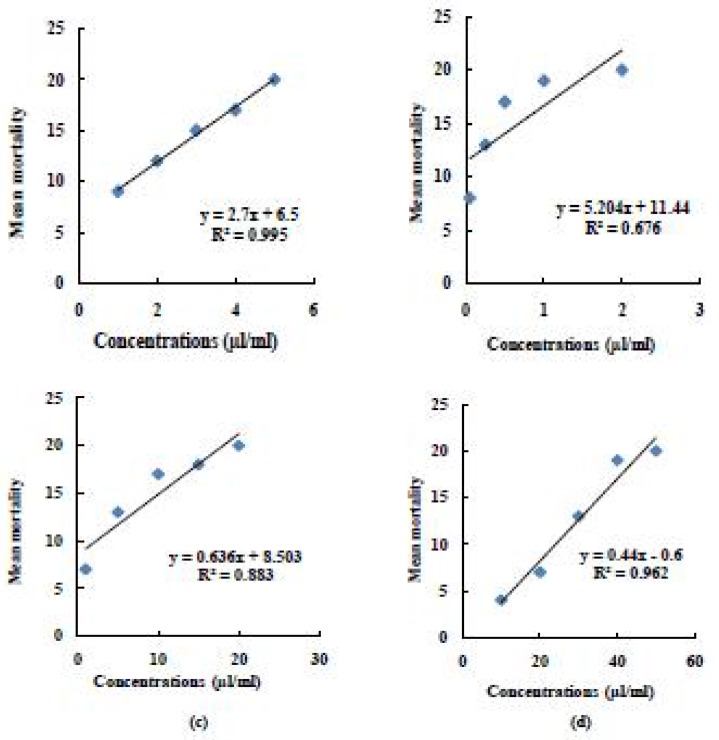
Fig. 3.
Regression line of (a) Imidacloprid 5% SC (b) Fipronil 2.5% EC (c) Deltamethrin 2.5% SC and (d) DDVP 50% EC on mean mortality of KLPT (locality 3) of P. americana (L)
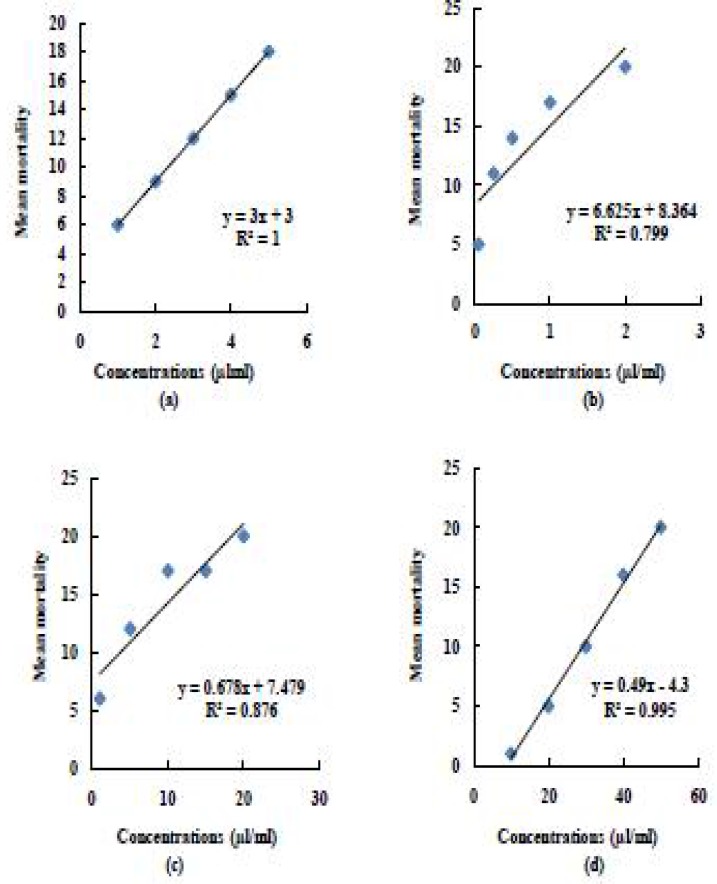
The concentrations used for fipronil 2.5% EC were 0.05, 0.25, 0.5, 1.0 and 2.0µl/ ml for all three localities tested. It is evident from results, that there is marked difference in toxicity of this insecticide. Localities 1, 2 and 3 were having their LC50 values 0.362, 0.176 and 0.092µl/ ml respectively. The regression analysis of variance further confirmed that there was an increasing trend in mean mortality of insects after 72 hours in topical application method [for SMH: b= 7.03± 9.63, F(1,4)= 12.46, P= 0.04, for KTCH: b= 6.63± 8.89, F(1,4)= 11.99, P= 0.04 and for KLPT: b= 5.20± 10.49, F(1,4)= 6.26, P= 0.08) (Figs 1–3(b)].
When different concentrations (1.0, 5.0, 10.0, 15.0 and 20.0 µl/ ml) of deltamethrin 2.5% SC were tested against P. americana, variable range was obtained according to its respective localities. It is evident from table 2, that LC50 for SMH was 4.145µl/ ml, whereas comparatively low values were recorded for KTCH and KLPT (2.500µl/ ml and 2.067µl/ ml respectively). The regression analysis of variance further confirmed that there was an increasing trend in mean mortality of insects at 48 hours post-treatment [for SMH: b= 0.82± 4.68, F(1,4)= 33.45, P= 0.01, for KTCH: b= 0.67± 4.98, F(1,4)= 21.32, P= 0.02 and for KLPT: b= 0.64± 4.12, F(1,4)= 22.70, P= 0.02) (Fig. 1–3(c)].
The concentrations used for DDVP 50% EC were 10.0, 20.0, 30.0, 40.0 and 50.0µl/ ml for all three localities tested. All three localities were having their LC50 values 28.556, 26.388 and 20.138 µl/ml respectively. The regression analysis of variance further confirmed that there was an increasing trend in mean mortality of insects after 48 hours in topical application method [for SMH: b= 0.5± 0.267, F(1,4)= 937.5, P= 7.65E-05, for KTCH: b= 0.49± 0.367, F(1,4)= 654.82, P= 0.000 and for KLPT: b= 0.44 ± 2.53, F(1,4)= 76.42, P= 0.003) (Fig. 1–3(d)].
Based on LC50 values, Imidacloprid, Deltamethrin and DDVP were having non-significant values, and tested insecticides were arranged according to following descending order of preferences:
Fipronil 2.5% EC> Imidacloprid 5% SC> Deltamethrin 2.5% SC> DDVP 50% EC.
Discussion
In various studies, researchers have paid attention to an effective and powerful strategy for control of cockroaches (Shafiqur-Rahman and Akter 2006, Sulaiman et al. 2007, Ahmad et al. 2010). Conventional pyrethroids and carbamates which are not much effective in pest management strategies have been replaced by new chemicals like neonicotenoids and phenyl-pyrazoles. Resistance against these new formulated insecticides has not been reported yet. Therefore, keeping in view the recent development in insecticides resistance management for the control of urban pests, this study was designed to monitor the insecticidal efficacy and resistance control strategy of these new chemicals and why these are considered as better options for cockroach control.
Among phenyl-pyrazole family of insecticides, fipronil is a relatively new insecticide that is found to be very effective against cockroaches and other household pests. Previous studies have also explained the strong neurotoxic effects of fipronil, which demonstrate that even a very low dose of it is suitable enough to attain desirable results. Studies conducted by Ahmad and Suliyat (2011) have reported that in the form of gel baits, it worked efficiently and even a very small amount (0.03%) of it was proved to be lethal against cockroaches. Similar results were obtained by Nasirian et al. (2006) who have reported that fipronil acts as powerful neuro-toxicant against german cockroach. Current investigation is in strong agreement with these findings, where low concentrations of fipronil were effective against adult P. americana. The toxicity data from this study explains that few hours after the topically applied fipronil, there was successive increase in mortality of treated cockroaches (same pattern observed in all three localities) that gradually got decreased up to 48 hours post treatment and then became stable after 72 hours of exposure.
Similar results were obtained by Holbrook et al. (2003) and Nasirian et al. (2006) who have reported that topical application is the most sensitive method in determining resistance ratio in field populations of cockroaches and even 0.01% of fipronil is toxic to field population. They have also reported that regression line curve of log dose is in accordance with mortality rates of treated individuals. Rettich and Stejskal (2008) have reported that in bait formulations, soluble concentration (SC) of fipronil is very effective against field populations of germen cockroach. American cockroach in our study has also found to be non-resistant against fipronil at low concentrations.
Among all the four insecticides tested, fipronil was proved to be highly effective. In various other findings, same conclusions have been reported. Scott and Klen (1997), Wang et al. (2004) and Rina et al. (2002) have presented similar results as in current study. Our findings are also parallel with those of Valles et al. (1997) who have concluded that fipronil is sufficient enough to kill pre-resistant populations even in nano-gram quantity. The most reasonable justification in this regard is perhaps fipronil being a new addition in phenyl-pyrazole class of insecticides, which has not been used frequently. That is why insects and agricultural pests have not yet developed resistance against this chemical. The results of present study have also revealed the fact that phenyl-pyrazole class is more toxic then organophosphates. DDVP, an effective member of organophosphates failed to control resistant field population of american cockroaches. Kaakeh et al. (1997) supported our findings, while concluding that organophosphates are not effective enough to combat susceptible and resistant populations of cockroaches.
In contrast to a few studies where organophosphates acted as neurotoxic agent against cockroaches, this chemical did not come up with satisfactory results in our study. The possible reason for this least toxic effectiveness might be the frequency and level of its application. This class is less favored by pest control industries, mainly because of its strong odor than pyrethroids (Lee and Lee 2004). In those parts of the world where such studies have proved organophosphate as effective class of insecticides, this chemical might not have been sprayed quite often, and its reduced frequency of application have resulted in emergence of susceptible populations of cockroaches, which are sensitive to this chemical. Unless and until, an insecticide is applied repeatedly again and again over a certain period of time, resistance does not develop. Whenever a chemical is sprayed quite often and its active ingredient is repeatedly exposed to pests’ species, it reduces its effectiveness and as a result, resistance against that insecticide gets developed gradually. Here in case of DDVP 50% EC, same phenomenon seems to have happened. This active ingredient is readily mixed in every other insecticidal spray from low to moderate quantity, it has almost lost its efficacy and the targeted species have become resistant against this insecticide.
Various researchers have reported the presence of high level resistance in insects against pyrethroids and have highlighted its control failure incidence (Zhai and Rhobinson 1996, Lee et al. 1996, Scharf et al. 1997, Lee et al. 1997, Lee 1998, Lee et al. 2000). The results of present study highlight the fact that among all four insecticides used, pyrethroid (deltamethrin 2.5% SC) exhibited moderate to high level of resistance against adult P. americana. The comparison of percentage mortality of these localities at different concentrations of deltamethrin reveals that there is a marked difference in range of effective concentrations for these localities.
Even very low concentration (1µl/ml) was found to be effective against localities 2 and 3 of P. americana, however, locality 1 remained almost ineffective (with almost 15% mortality rate comparable with other two). The highest concentration (20.0 µl/ml) ended up with expected results, killing 100% of the overall tested individuals. Similar results were obtained by Ballard et al. (1984), Scott et al. (1986), Scharf et al. (1996) and Lee at al. (1996) where either or two of the tested localities came up with 100% mortality rate at this concentration. The findings of Shafiqur-Rahman and Akter (2006), however, contradict our results proving that pyrethroids are highly effective against P. americana. Using cypermethrin and permethrin instead of deltamethrin, the treated individuals gave immediate mortality response after treatment with topical application.
This variation in susceptibility of different localities is because of their far apart localities from where they were collected. Locality 3, which proved to be highly susceptible in both toxicity bioassays, was collected from those areas which were not previously exposed to any insecticide (or their exposure level was comparatively lower to develop resistance). That is why when they were treated with deltamethrin, all of them showed mortality immediately after few hours. These results of current investigation strongly favor the concept given by Lee and Lee (2002), who have reported that pyrethroids exhibit low to moderate level of resistance. Similar conclusion has been made recently by Ahmad et al. (2009) and Nasirian et al. (2009) who have reported that resistance against pyrethroids have been developed in certain species of cockroaches. Same concepts of deltamethrin resistance have been put forward by Choo et al. (2000) and Chai and Lee (2010) who have reported heavy resistance for pyrethroids (deltamethrin and cypermethrin) against both american and german cockroaches.
Unlike conventional pyrethroids and organophosphates which are older compounds and have given rise to resistant localities of cockroaches, imidacloprid is a relatively new chemical which have not been tested for so long in Pakistan. Low usage and reduced application of imidacloprid has raised its significance in controlling urban pests effectively. It’s completely different mode of action from organophosphates and pyrethroids has made it a sensible option for pest control industries (Cole et al. 1993, Salgado 1997).
Imidacloprid was found to be slow responding insecticide during toxicity bioassays, and considerable results were achieved after 48 to 72 hours of treatment. Same case was reported by Appel and Tanley (2000). Regarding toxicity of imidacloprid, Kaakeh et al. (1997) observed its transient and rapid knockdown effect in a similar manner as recorded in our study. Among its different concentrations tested, the mortality response for all three localities was significantly different. This variable level of susceptibility is perhaps because of locality differentiation. Locality 3 might not be previously treated with imidacloprid or any other insecticide, which is why their individuals did not have resistant genes among them. Wei et al. (2001) have achieved similar results and presented the idea that cross resistance might be involved in this case. As the resistance ratio is quite low in this case, it is difficult to conclude that whether these localities are naturally resistant against imidacloprid or phenomenon of cross-resistance is involved.
Conclusion
In the light of above mentioned findings it can be suggested that resistance against insecticides is a common phenomenon and all tested localities of P. americana populations exhibited resistance against tested insecticides. It is therefore, mandatory to establish a sensitive method for a prior detection of resistant localities. The bioassay methods adopted in this study are suitable for meeting this criterion well. Moreover, dealing with discriminating doses requires extreme caution, because minor errors may possibly alter the end results and susceptible localities may wrongly be considered as resistant localities. Another important aspect while establishing the discriminating doses of insecticides is the selective choice of susceptible locality. There should be completely distinguished range of doses for resistant and susceptible localities while dealing with insecticides resistance.
Fig. 2.
Regression line of (a) Imidacloprid 5% SC (b) Fipronil 2.5% EC (c) Deltamethrin 2.5% SC and (d) DDVP 50% EC on mean mortality of KTCH (locality 2) of P. americana (L)
Acknowledgments
Our deepest acknowledgement is for the Head of Zoology Department, LCWU, for providing all the facilities to conduct research. The authors are grateful to the laboratory staff for providing necessary equipments and their nice cooperation throughout the research period. The authors declare that there is no conflict of interest.
References
- Abd-Elghafar SF, Appel AG, Mack TP. Toxicity of several insecticide formulations against adult German cockroaches (Dictyoptera: Blattellidae) J Econ Entomol. 1990;83:2290–2294. doi: 10.1093/jee/83.6.2290. [DOI] [PubMed] [Google Scholar]
- Ahmad I, Suliyat A. Development Of Fipronil Gel Bait Against German Cockroaches, Blattella germanica (Dictyoptera: Blattellidae) J Entomol. 2011;8(3):288–294. [Google Scholar]
- Ahmad I, Sriwahjuningsih SA, Putra RE, Permana AD. Monitoring pyrethroid resistance in field collected Blattella germanica Linn. (Dictyoptera: Blattellidae) in Indonesia. Entomol Res. 2009;39:114–118. [Google Scholar]
- Ahmed AS, Bashir NH, Assad YO. Susceptibility of Periplaneta americana (L.) (Orthoptera: Blattidae) population from wad medani (Sudan Gezira) to three public health insecticides. Rest Pest Manag Newsletter. 2010;19(2):8–14. [Google Scholar]
- Appel AG, Tanley MJ. Laboratory and field performance of imidacloprid gel bait against german cockroaches (Dictyoptera: Blattellidae) in Indonesia. Entomol Res. 2000;39:114–118. doi: 10.1603/0022-0493-93.1.112. [DOI] [PubMed] [Google Scholar]
- Appel AG, Reierson DA, Rust MK. Comparative water relations and temperature sensitivity of cockroaches. Comp Biochem Physiol. 1983;74:357–361. [Google Scholar]
- Ballard JB, Gold RE, Rauscher JD. Effectiveness of six insecticide treatment strategies in the reduction of German cockroach (Orthoptera: Blattellidae) populations in infested apartments. J Econ Entomol. 1984;77:1092–1094. [Google Scholar]
- Barcay SJ. Cockroaches. In: Mallis A, editor. Handbook of pest control. 9th ed. GIE Media; Cleveland, Ohio, United States: 2004. pp. 121–215. [Google Scholar]
- Chai RY, Lee CY. Insecticide Resistance Profiles and Synergism in Field Populations of the German Cockroach (Dictyoptera: Blattellidae) From Singapore. J Econ Entomol. 2010;103(2):460–471. doi: 10.1603/ec09284. [DOI] [PubMed] [Google Scholar]
- Choo LE, Tang CS, Pang FY, Ho SH. Comparison of two bioassay methods for determining deltamethrin resistance in German cockroaches (Blattodea: Blattellidae) J Econ Entomol. 2000;93:905–910. doi: 10.1603/0022-0493-93.3.905. [DOI] [PubMed] [Google Scholar]
- Cole LM, Nicholson RA, Casida JE. Action of Phenyl pyrazole Insecticides at the GABA-Gated Chloride Channel. Pestic Biochem Physiol. 1993;46:47–54. [Google Scholar]
- Czajka E, Pancer K, Kochman M, Gliniewiez A, Sawicka B, Rabczenko D. Characteristics of bacteria isolated from body surface of German cockroaches caught in hospitals. Przegl Epidemiol. 2003;57(4):655–662. [PubMed] [Google Scholar]
- Fasulo TR, Kern WH, Koehler PG, Short DE. Pests In and Around the Home. University of Florida; United States: 2005. pp. 124–126. [Google Scholar]
- Gadd CA, Raubenheimer D. Nutrient specific learning in an omnivorous insect: the American cockroach Periplaneta americana L. learns to associate dietary protein with the odors citral and carvone. J Insect Behav. 2000;13:851–864. [Google Scholar]
- Gore JC, Schal C. Cockroach Allergen Biology and Mitigation in the In door Environment. Annu Rev Entomol. 2007;52:439–463. doi: 10.1146/annurev.ento.52.110405.091313. [DOI] [PubMed] [Google Scholar]
- Holbrook G, Roebuck J, Moore C, Waldvogel M, Schal C. Origin and extent of resistance to fipronil in the german cockroach, Blattella germanica (L.) (Dictyoptera: Blattellidae) J Econ Entomol. 2003;96(5):1548–1558. doi: 10.1093/jee/96.5.1548. [DOI] [PubMed] [Google Scholar]
- Kinfu A, Erko B. Cockroaches as carriers of human intestinal parasites in two localities in Ethiopica. Trans R Soc Trop Med Hyg. 2008;102:1143–1147. doi: 10.1016/j.trstmh.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Kaakeh W, Reid BL, Bennett GW. Toxicity of fipronil to German and American cockroaches. Entomol Exp Appl. 1997;84:229–237. [Google Scholar]
- Lane HC, Montagne JL, Fauci AS. Bioterrorism: A clear and present danger. Nature Medicine. 2001;7:1271–1273. doi: 10.1038/nm1201-1271. [DOI] [PubMed] [Google Scholar]
- Lee CY. Control of insecticide-resistant German cockroaches, Blattella germanica (L.) (Dictyoptera: Blattellidae) in food-outlets with hydramethylnon- based bait stations. Trop Biomed. 1998;15:45–51. [Google Scholar]
- Lee CY, Lee LC. Influence of sanitary condition on the field performance of chlorpyrifos based baits against American cockroaches, Periplaneta americana (L.) (Dictyoptera: Blattidae) J Vect Ecol. 2000;25:218–221. [PubMed] [Google Scholar]
- Lee CY, Robinson W. Handbook of Malaysian Household and Structural Pests. Pest Control Association of Malaysia; Kuala Lumpur, Malaysia: 2001. pp. 96–108. [Google Scholar]
- Lee CY, Hemingway J, Yap HH, Chong NL. Biochemical characterization of insecticide resistance in the German cockroach, Blattella germanica, from Malaysia. Med Vet Entomol. 2000;14:11–18. doi: 10.1046/j.1365-2915.2000.00215.x. [DOI] [PubMed] [Google Scholar]
- Lee CY, Yap HH, Chong NL. Elevated esterase detection in insecticide resistant German cockroaches (Dictyoptera: Blattellidae) using modified Pasteur-Georghiou’s filter-paper method. Trap Biomed. 1997;14:81–86. [Google Scholar]
- Lee CY, Yap HH, Chong NL, Lee RS. Insecticide resistance and synergism in field-collected German cockroaches (Dictyoptera: Blattellidae) in Peninsular Malaysia. Bull Entomol Res. 1996;86:675–682. [Google Scholar]
- Lee KM, Lee CY. Prevalence of insecticide resistance in field-collected populations of the German cockroach, Blattella germanica (Linnaeus) (Dictyoptera: Blattellidae) in Peninsular Malaysia. Med Entomol. 2002;53(4):219–225. [Google Scholar]
- Lee LC, Lee CY. Insecticide resistance profiles and possible underlying mechanisms in German cockroaches, Blattella germanica (Linnaeus) (Dictyoptera: Blattellidae) from Peninsular Malaysia. Med Entomol Zool. 2004;55(2):77–93. [Google Scholar]
- Morane G. Threats in bioterrorism II: CDC category B and C agents. Emerg Med Clin N Am. 2002;20:311–330. doi: 10.1016/s0733-8627(01)00003-7. [DOI] [PubMed] [Google Scholar]
- Mpuchane S, Matsheka IM, Gashe BA, Allotey J, Murindamombe G, Mrema N. Microbiological studies of cockroaches from three localities in Gaborone, Botswana. Afr J Food Nutr Sci. 2006;6:56–59. [Google Scholar]
- Nasirian H, Ladonni H, Vatandoost H. Duration of Fipronil Topical Application Toxicity in Blattella germanica Field Population Strains. Pak J Biol Sci. 2006;9(5):800–804. [Google Scholar]
- Nasirian H, Ladonni H, Vatandoost H. Duration of Fipronil Topical Application Toxicity in Blattella germanica Field Population Strains. Pak J Biol Sci. 2009;9(5):800–804. [Google Scholar]
- Nasirian H, Ladonni H, Shayeghi M, Vatandoost H, Yaghoobi MR, Rassi Y, Abolhassani M, Abaei MR. Comparison of Permethrin and Fipronil Toxicity against German cockroach (Dictyoptera: Blattellidae) Strains. Iranian J Publ Health. 2006;35(1):63–67. [Google Scholar]
- Pechal JL, Austin J, Gold R, Tomberlin JK. Epidemiology and Spatial Relationships of Bacteria Associated with Periplaneta americana (L.) (Blattodea: Blattidae) in Central Texas. J Agric Urban Entomol. 2007;24(4):205–216. [Google Scholar]
- Rettich F, Stejskal V. Residual effect of Fipronil SC against insecticide resistant populations of the german cockroach. In: Robinson WH, Bajomi D, editors. Proceedings of the 6th International Conference on Urban Pests; 2008. pp. 501–504. [Google Scholar]
- Rina T, Tilak VW, Yadav JD, Dutta G. Efficacy of fipronil and propoxur in the control of German cockroaches (Dictyoptera: Blattellidae) J Com Dis. 2002;34:65–69. [PubMed] [Google Scholar]
- Salgado VL. The modes of action of spinosad and other insect control products. Down Earth. 1997;52:35–43. [Google Scholar]
- Sarinho ED, Schor MA, Rizzo JA. There are more asmatics in homes with high cockroach infestation. Braz J Biol Res. 2004;37:503–510. doi: 10.1590/s0100-879x2004000400007. [DOI] [PubMed] [Google Scholar]
- Scharf ME, Hemingway J, Reid BI, Small GJ, Bennett GW. Toxicological and biochemical characterization of insecticide resistance in a field collected strain of Blattella germanica (Dictyoptera: Blattellidae) J Econ Entomol. 1996;89:322–331. [Google Scholar]
- Scharf ME, Kaakeh W, Bennett GW. Changes in an insecticide resistant field-population of German cockroach following exposure to an insecticide mixture. J Econ Entomol. 1997;90:38–48. [Google Scholar]
- Shafiqur-Rahman AS, Akter MY. Toxicity of diazinon and cypermethrin against the American cockroach, Periplaneta americana (L.) Univ. J Zool Rajshahi. 2006;25:63–64. [Google Scholar]
- Sulaiman S, Muhammad AH, Othman H. Efficacy of hydramethylnon and fipronil get baits with laboratory and field strains of Periplaneta americana (L.) (Dictyoptera: Blattidae) in Malaysia. J Trop Med Parasitol. 2007;30:64–67. [Google Scholar]
- Tsai C, Lee H. Analysis of specific adaptation to a domicile habitat: A comparative study of two closely related cockroach species. J Med Entomol. 2001;38:245–252. doi: 10.1603/0022-2585-38.2.245. [DOI] [PubMed] [Google Scholar]
- Valles SM, Koehler PG, Brenner RJ. Antagonism of fipronil toxicity by piperonyl butoxide and S, S, S,-tributyl phosphorotrithioate in the German cockroach (Dictyoptera: Blattellidae) J Econ Entomol. 1997;90:1254–1258. [Google Scholar]
- Vythilingam I, Jeffery J, Oothuman P, Abdul Razak AR, Sulaiman A. Cockroaches from human dwellings: isolation of bacterial pathogens and control. Southeast Asian J Trop Med Pub Hlth. 1997;28:218–222. [PubMed] [Google Scholar]
- Wang C, Scharf M, Bennett G. Behavioral and physiological resistance of the German cockraoch to gel bait (Blattodea: Blattellidae) J Econ Entomol. 2004;97(6):2067–2072. doi: 10.1093/jee/97.6.2067. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Kobayashi Y, Sakura M, Matsumoto Y, Mizunami M. Classical olfactory conditioning in the cockroach Periplaneta americana. Zool Sci. 2003;20:1447–1454. doi: 10.2108/zsj.20.1447. [DOI] [PubMed] [Google Scholar]
- Wei Y, Appel AG, Moar WJ, Liu N. Pyrethroid resistance and cross-resistance in the German cockroach, Blatella germinca (L) Pest Manag Sci. 2001;57:1055–1059. doi: 10.1002/ps.383. [DOI] [PubMed] [Google Scholar]
- Zhai J, Robinson WH. Instability of cypermethrin resistance in a field population of the German cockroach (Orthoptera: Blattellidae) J Econ Entomol. 1996;89:332–336. doi: 10.1093/jee/85.2.348. [DOI] [PubMed] [Google Scholar]